L-Theanine Mitigates Chronic Alcoholic Intestinal Injury by Regulating Intestinal Alcohol and Linoleic-Arachidonic Acid Metabolism in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
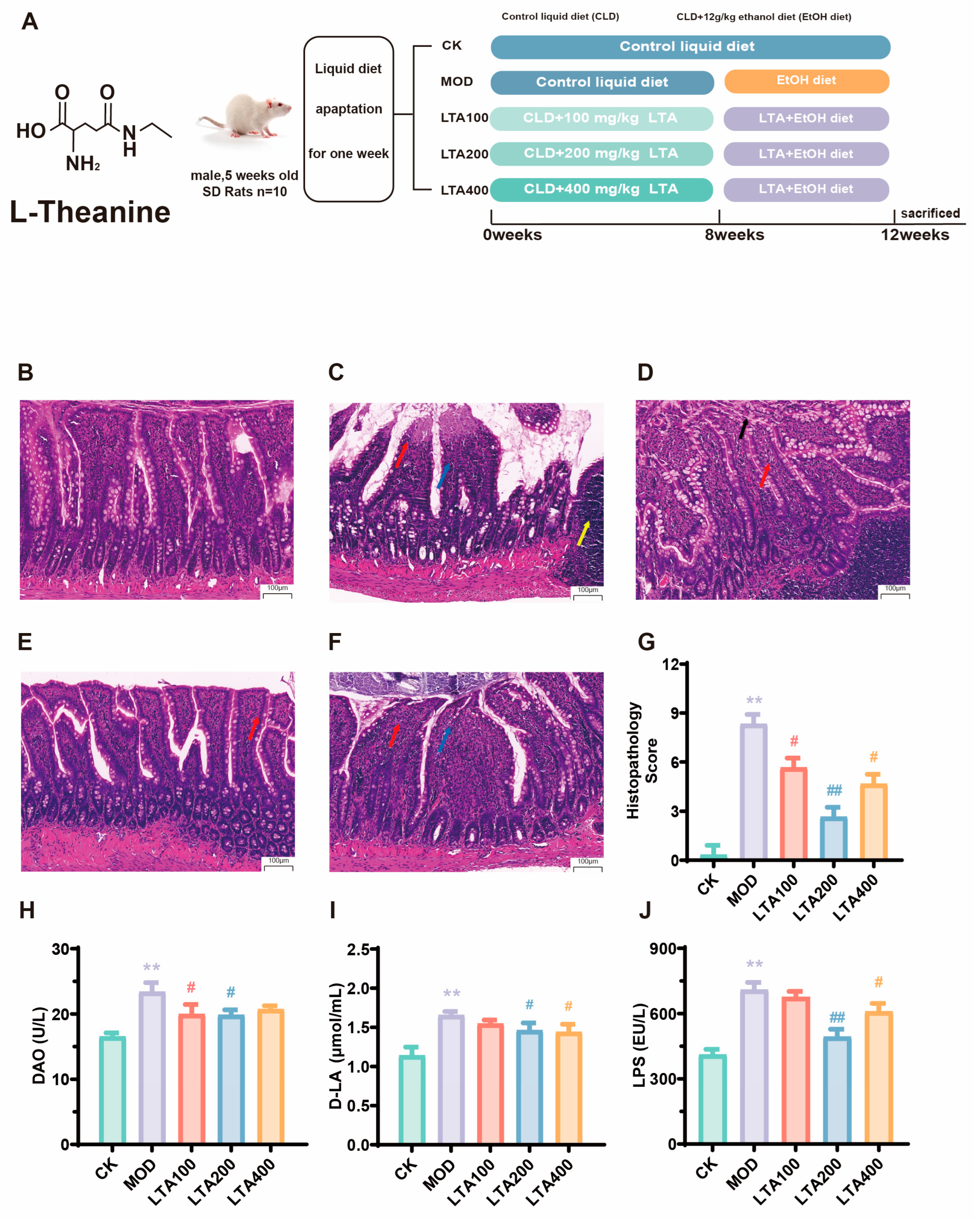
2.3. Establishment of Chronic Alcoholic Intestinal Injury Model in Rats
2.4. Treatments
2.5. Histopathological Assessment
2.6. Biochemical Assessment of Serum and Intestinal Tissues
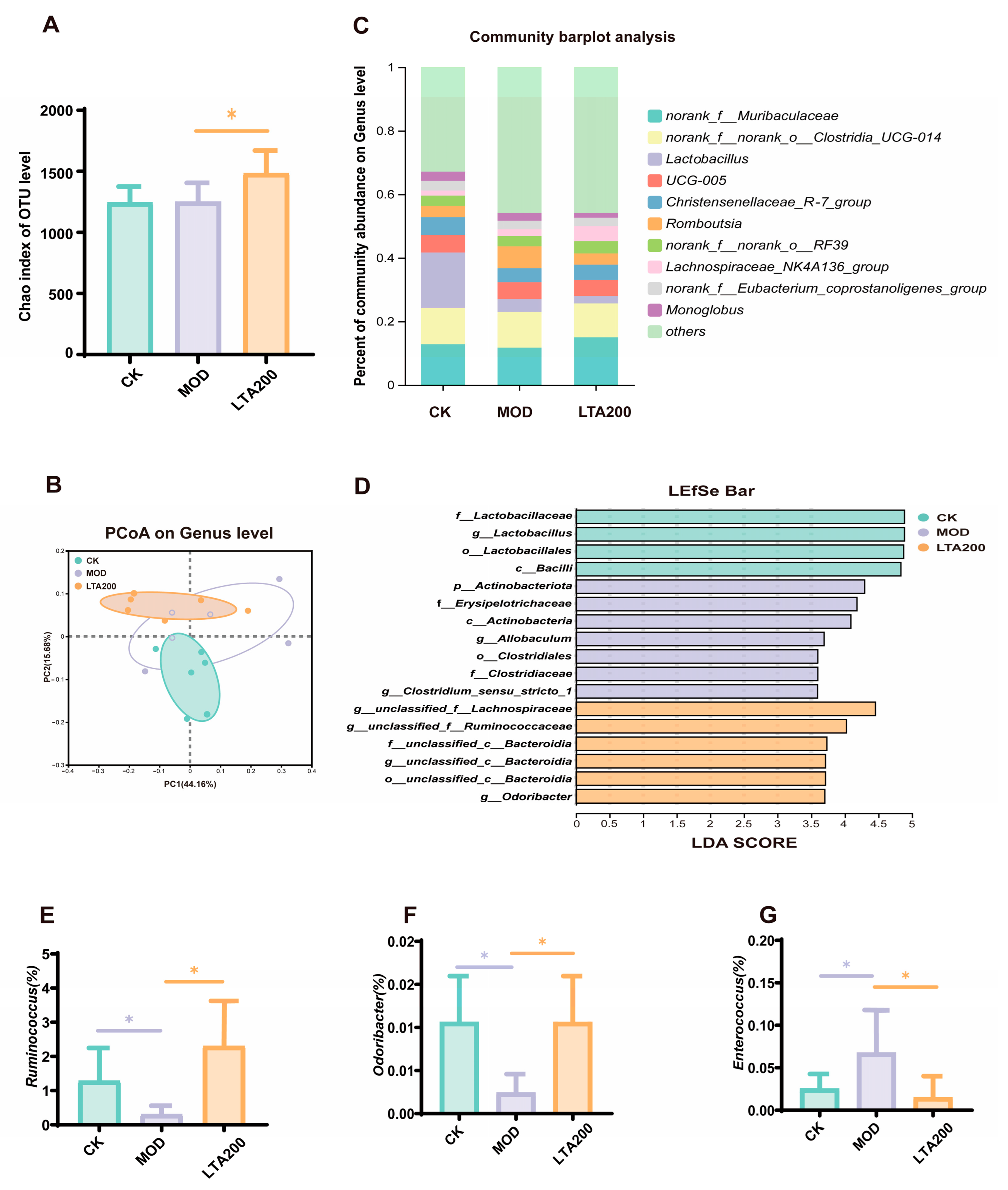
2.7. 16S rRNA-Based Intestine Microbiota Determination
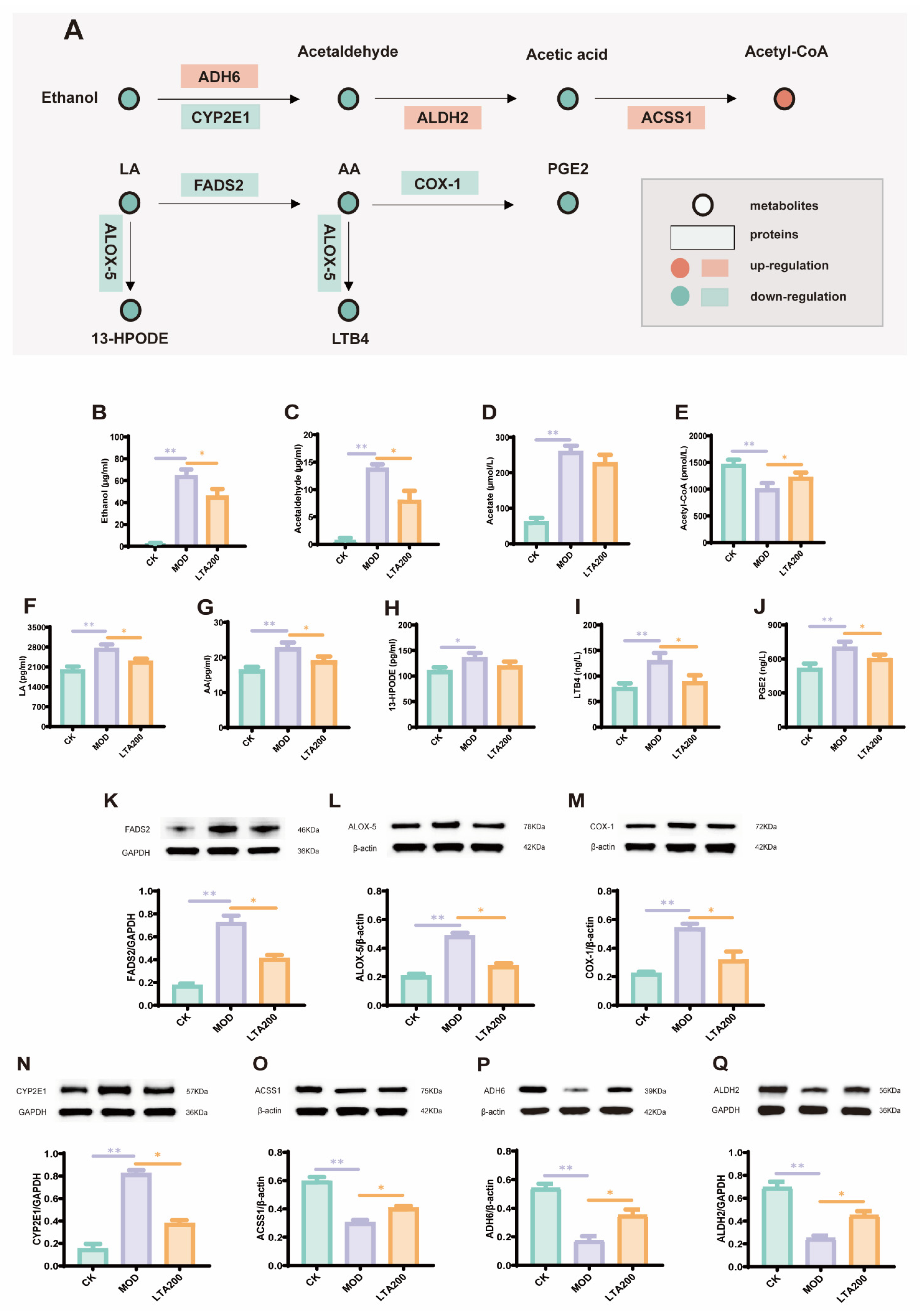
2.8. LC/MS-Based Intestine Metabolomics
2.9. Detection of Key Metabolites in Serum and Intestine
2.10. Quantitative Real-Time PCR
2.11. Western Blotting
2.12. Statistical Analysis
3. Results
3.1. Effect of LTA on Duodenal Pathological Damage and Permeability in Chronic Alcoholic Intestinal Injury Rats
3.2. LTA Ameliorates Inflammatory Infiltration and Oxidative Stress in the Intestine of Chronic Alcoholic Intestinal Injury Rats
3.3. Effects of LTA on Intestinal Microbiota in Rats with Chronic Alcoholic Intestinal Injury
3.4. Effects of LTA on Intestinal Metabolites in Rats with Chronic Alcoholic Intestinal Injury
3.5. LTA Regulates mRNA Expression of Intestinal Alcohol Metabolism and the LA-AA Metabolic Pathway
3.6. LTA Regulates the Expression of Key Proteins and Metabolites in the Alcohol and LA-AA Metabolic Pathways in the Intestines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De, G.G.; Costanzo, S. Alcohol and Health: Praise of the J Curves. J. Am. Coll. Cardiol. 2017, 70, 923. [Google Scholar]
- Millwood, I.Y.; Im, P.K.; Bennett, D.; Hariri, P.; Yang, L.; Du, H.; Kartsonaki, C.; Lin, K.; Yu, C.; Chen, Y. Alcohol intake and cause-specific mortality: Conventional and genetic evidence in a prospective cohort study of 512 000 adults in China. Lancet Public Health 2023, 8, e956–e967. [Google Scholar] [CrossRef]
- Fu, Y.; Mackowiak, B.; Lin, Y.-H.; Maccioni, L.; Lehner, T.; Pan, H.; Guan, Y.; Godlewski, G.; Lu, H.; Chen, C. Coordinated action of a gut–liver pathway drives alcohol detoxification and consumption. Nat. Metab. 2024, 6, 1380–1396. [Google Scholar] [CrossRef] [PubMed]
- Quertemont, E. Genetic polymorphism in ethanol metabolism: Acetaldehyde contribution to alcohol abuse and alcoholism. Mol. Psychiatry 2004, 9, 570. [Google Scholar] [CrossRef]
- Liu, M.Y.; Xu, K.H.; Liu, S.; Xiao, W.J. Protective Effect and Mechanism of l-Theanine on Acute Alcoholic Liver Injury in Mice. Mol. Nutr. Food Res. 2024, 68, 2400766. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Q.; Wu, S.; Sun, X.; Yin, R.; Ouyang, Z.; Yin, H.; Wei, Y. Amelioration of ethanol-induced oxidative stress and alcoholic liver disease by in vivo RNAi targeting Cyp2e1. Acta Pharm. Sin. B 2023, 13, 3906–3918. [Google Scholar] [CrossRef]
- Cho, Y.E.; Kim, D.K.; Seo, W.; Gao, B.; Yoo, S.H.; Song, B.J. Fructose Promotes Leaky Gut, Endotoxemia and Liver Fibrosis through CYP2E1-Mediated Oxidative and Nitrative Stress. Hepatology 2019, 73, 2180. [Google Scholar] [CrossRef]
- Haber, P.S.; Kortt, N.C. Alcohol use disorder and the gut. Addiction 2021, 116, 658–667. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Chen, H.-P.; Kuo, H.-Y.; Fan, P.-H.; Wu, L.-L.; Wu, C.-Y. Mo1211 Metabolic Switching, Gut Integrity and Host-Microbiota Interaction in Alcohol-Induced Intestinal Injury. Gastroenterology 2024, 166, S-980. [Google Scholar] [CrossRef]
- Seitz, H.K. In Memoriam Professor Jean Pierre von Wartburg (1931 to 2017). Alcohol. Clin. Exp. Res. 2017, 41, 1244–1245. [Google Scholar] [CrossRef]
- Xu, W.; Liu, A.-X.; Liu, K.-H.; Zhang, S.; Gong, Z.-H.; Xiao, W.-J. l-Theanine Alleviates Ulcerative Colitis by Regulating Colon Immunity via the Gut Microbiota in an MHC-II-Dependent Manner. J. Agric. Food Chem. 2024, 72, 19852–19868. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, H.; Zhang, Y.; Goh, B.; Bao, B.; Mello, S.S.; Sun, X.; Zheng, W.; Gazzaniga, F.S.; Wu, M. Gut microbial fatty acid isomerization modulates intraepithelial T cells. Nature 2023, 619, 837–843. [Google Scholar] [CrossRef] [PubMed]
- De Libero, G.; Mori, L. Novel insights into lipid antigen presentation. Trends Immunol. 2012, 33, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Gür, Z.T.; Çalışkan, B.; Garscha, U.; Olgaç, A.; Schubert, U.S.; Gerstmeier, J.; Werz, O.; Banoglu, E. Identification of multi-target inhibitors of leukotriene and prostaglandin E2 biosynthesis by structural tuning of the FLAP inhibitor BRP-7. Eur. J. Med. Chem. 2018, 150, 876–899. [Google Scholar] [CrossRef]
- Crittenden, S.; Goepp, M.; Pollock, J.; Robb, C.T.; Smyth, D.J.; Zhou, Y.; Andrews, R.; Tyrrell, V.; Gkikas, K.; Adima, A. Prostaglandin E2 promotes intestinal inflammation via inhibiting microbiota-dependent regulatory T cells. Sci. Adv. 2021, 7, eabd7954. [Google Scholar] [CrossRef]
- Wang, B.; Liu, S.; Lin, L.; Xu, W.; Gong, Z.; Xiao, W. The protective effect of l-theanine on the intestinal barrier in heat-stressed organisms. Food Funct. 2024, 15, 3036–3049. [Google Scholar] [CrossRef]
- Qiu, Z.; Xiang, L.; Han, Y.; Zhang, B.; Qiao, X.; Zheng, Z.; Xiao, H. Structure-anti-inflammatory activity relationship of garlic fructans in mice with dextran sulfate sodium-induced colitis: Impact of chain length. Carbohydr. Polym. 2024, 346, 122582. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, S.; Liu, Z.; Lv, H.; Cai, X.; Zhong, R.; Chen, L.; Zhang, H. Baicalin restore intestinal damage after early-life antibiotic therapy: The role of the MAPK signaling pathway. Pharmacol. Res. 2024, 204, 107194. [Google Scholar] [CrossRef]
- Türkzü, D.; Anlier, N. L-theanine, unique amino acid of tea, and its metabolism, health effects, and safety. Crit. Rev. Food Sci. Nutr. 2017, 57, 1681–1687. [Google Scholar] [CrossRef]
- Ben, P.; Zhang, Z.; Zhu, Y.; Xiong, A.; Gao, Y.; Mu, J.; Yin, Z.; Luo, L. l-Theanine attenuates cadmium-induced neurotoxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Neurotoxicology 2016, 57, 95–103. [Google Scholar] [CrossRef]
- Lin, L.; Zeng, L.; Liu, A.; Peng, Y.; Yuan, D.; Zhang, S.; Li, Y.; Chen, J.; Xiao, W.; Gong, Z. l-Theanine regulates glucose, lipid, and protein metabolism via insulin and AMP-activated protein kinase signaling pathways. Food Funct. 2020, 11, 1798–1809. [Google Scholar] [CrossRef]
- Dai, H.; Chen, X.; He, J.; Zheng, P.; Luo, Y.; Yu, B.; Chen, D.; Huang, Z. Dietary L-theanine supplementation improves antioxidant capacity and lipid metabolism in finishing pigs. J. Funct. Foods 2023, 110, 105831. [Google Scholar] [CrossRef]
- Liu, S.; Wang, B.; Lin, L.; Xu, W.; Gong, Z.H.; Xiao, W.J. L-Theanine alleviates heat stress through modulation of gut microbiota and immunity. J. Sci. Food Agric. 2024, 104, 2059–2072. [Google Scholar] [CrossRef]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef]
- Wang, H.; Shen, H.; Seo, W.; Hwang, S. Experimental models of fatty liver diseases: Status and appraisal. Hepatol. Commun. 2023, 7, e00200. [Google Scholar] [CrossRef]
- Food and Drug Administration. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; Center for Drug Evaluation and Research (CDER): Beltsville, MD, USA, 2005; Volume 7. [Google Scholar]
- Qu, Q.Y.; Song, X.Y.; Lin, L.; Gong, Z.H.; Xu, W.; Xiao, W.J. L-Theanine Modulates Intestine-Specific Immunity by Regulating the Differentiation of CD4+ T Cells in Ovalbumin-Sensitized Mice. J. Agric. Food Chem. 2022, 70, 14851–14863. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Gu, L.; Liu, X.; Yan, X.; Bi, X.; Fan, X.; Zhou, J.; Lu, S.; Luo, L.; Yin, Z. L-theanine prevents progression of nonalcoholic hepatic steatosis by regulating hepatocyte lipid metabolic pathways via the CaMKKβ-AMPK signaling pathway. Nutr. Metab. 2022, 19, 29. [Google Scholar] [CrossRef]
- Wang, G.; Ma, F.; Xie, K.; Li, X.; Tan, X.; Xia, Y.; Wang, Y.; Dong, J. Liensinine alleviates mouse intestinal injury induced by sepsis through inhibition of oxidative stress, inflammation, and cell apoptosis. Int. Immunopharmacol. 2024, 127, 111335. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chu, H.; Hu, L.; Li, Z.; Yang, L.; Hou, X. The role of NADPH oxidase 1 in alcohol-induced oxidative stress injury of intestinal epithelial cells. Cell Biol. Toxicol. 2022, 39, 2345–2364. [Google Scholar] [CrossRef]
- Cuesta, C.M.; Pascual, M.; Pérez-Moraga, R.; Rodríguez-Navarro, I.; García-García, F.; Ureña-Peralta, J.R.; Guerri, C. TLR4 Deficiency Affects the Microbiome and Reduces Intestinal Dysfunctions and Inflammation in Chronic Alcohol-Fed Mice. Int. J. Mol. Sci. 2021, 22, 12830. [Google Scholar] [CrossRef]
- Zhou, S.-K.; Xu, J.-D.; Gao, X.-Q.; Zhang, R.-J.; Cheng, F.-F.; Yao, W.-F.; Zhang, Y.; Geng, T.; Zhang, L. Fructus Jujubae cooperated with water-expelling members in Shizao decoction alleviated intestinal injury and malignant ascites by modulating gut microbiota and metabolic homeostasis. Phytomedicine 2024, 133, 155895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, C.; Chen, K.; Zhao, X.; Geng, Z. Effect of l-theanine on growth performance, intestinal development and health, and peptide and amino acid transporters expression of broilers. J. Sci. Food Agric. 2020, 100, 1718–1725. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Jin, S.; Huang, S.; Liu, Y. Vitamin D 3 attenuates cisplatin-induced intestinal injury by inhibiting ferroptosis, oxidative stress, and ROS-mediated excessive mitochondrial fission. Food Funct. 2022, 13, 10210–10224. [Google Scholar] [CrossRef] [PubMed]
- Meena, A.S.; Shukla, P.K.; Bell, B.; Giorgianni, F.; Caires, R.; Fernández-Peña, C.; Beranova, S.; Aihara, E.; Montrose, M.H.; Chaib, M. TRPV6 channel mediates alcohol-induced gut barrier dysfunction and systemic response. Cell Rep. 2022, 39, 110937. [Google Scholar] [CrossRef]
- Yi, Z.; Liu, X.; Liang, L.; Wang, G.; Xiong, Z.; Zhang, H.; Song, X.; Ai, L.; Xia, Y. Antrodin A from Antrodia camphorata modulates the gut microbiome and liver metabolome in mice exposed to acute alcohol intake. Food Funct. 2021, 12, 2925–2937. [Google Scholar] [CrossRef]
- Gubernatorova, E.O.; Tumanov, A.V. Tumor necrosis factor and lymphotoxin in regulation of intestinal inflammation. Biochemistry 2016, 81, 1309–1325. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Ni, Z.; Wei, Y.; Hou, K.; Valencak, T.G.; Wang, H. Extracellular vesicles of Bacteroides uniformis induce M1 macrophage polarization and aggravate gut inflammation during weaning. Mucosal Immunol. 2024, 17, 793–809. [Google Scholar] [CrossRef]
- Chen, L.; Xiao, W.-J.; Yan, Q.-X.; Gong, Z.-H.; Zhang, S.; Zeng, L.; Yang, M.; Zhou, Y.-H. Protective effects of L-theanine on rats with dextran sulfate sodium-induced inflammatory bowel disease. Arch. Pharmacal Res. 2020, 43, 821–862. [Google Scholar] [CrossRef]
- Saraiva, M.; O’garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Du, Y.; Li, L.; Gong, C.; Li, T.; Xia, Y. The diversity of the intestinal microbiota in patients with alcohol use disorder and its relationship to alcohol consumption and cognition. Front. Psychiatry 2022, 13, 1054685. [Google Scholar] [CrossRef]
- Bai, Y.; Ma, K.; Li, J.; Ren, Z.; Zhang, J.; Shan, A. Lactobacillus rhamnosus GG ameliorates DON-induced intestinal damage depending on the enrichment of beneficial bacteria in weaned piglets. J. Anim. Sci. Biotechnol. 2022, 13, 90. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, Z.; Fan, B.; Wang, L.; Liu, M.; An, Z.; Zhao, X. A comparative study of the effects of different fucoidans on cefoperazone-induced gut microbiota disturbance and intestinal inflammation. Food Funct. 2021, 12, 9087–9097. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Oh, D.Y.; Bandyopadhyay, G.; Lagakos, W.S.; Talukdar, S.; Osborn, O.; Johnson, A.; Chung, H.; Maris, M.; Ofrecio, J.M. LTB4 causes macrophage–mediated inflammation and directly induces insulin resistance in obesity. Nat. Med. 2015, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, S. Are Fried Foods Unhealthy? The Dietary Peroxidized Fatty Acid, 13-HPODE, Induces Intestinal Inflammation In Vitro and In Vivo. Antioxidants 2020, 9, 926. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Zhao, Q.; Chen, M. Homocysteine Promotes Intestinal Inflammation in Colitis Mice Through the PGE2/STAT3 Signaling Pathway. Dig. Dis. Sci. 2024, 69, 3742–3752. [Google Scholar] [CrossRef]
- Gong, M.; Duan, H.; Wu, F.; Ren, Y.; Gong, J.; Xu, L.; Lu, F.; Wang, D. Berberine Alleviates Insulin Resistance and Inflammation. Front. Pharmacol. 2021, 12, 722360. [Google Scholar] [CrossRef]
- Pandey, S.C.; Malovic, E. Gut–liver highway of ALDH2 in drinking. Nat. Metab. 2024, 6, 1202–1203. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Tan, S.; Yang, J.; Dang, X.; Liu, K.; Gong, Z.; Xiao, W. L-Theanine Mitigates Chronic Alcoholic Intestinal Injury by Regulating Intestinal Alcohol and Linoleic-Arachidonic Acid Metabolism in Rats. Nutrients 2025, 17, 1943. https://doi.org/10.3390/nu17111943
Gu J, Tan S, Yang J, Dang X, Liu K, Gong Z, Xiao W. L-Theanine Mitigates Chronic Alcoholic Intestinal Injury by Regulating Intestinal Alcohol and Linoleic-Arachidonic Acid Metabolism in Rats. Nutrients. 2025; 17(11):1943. https://doi.org/10.3390/nu17111943
Chicago/Turabian StyleGu, Jiayou, Simin Tan, Jiahao Yang, Xuhui Dang, Kehong Liu, Zhihua Gong, and Wenjun Xiao. 2025. "L-Theanine Mitigates Chronic Alcoholic Intestinal Injury by Regulating Intestinal Alcohol and Linoleic-Arachidonic Acid Metabolism in Rats" Nutrients 17, no. 11: 1943. https://doi.org/10.3390/nu17111943
APA StyleGu, J., Tan, S., Yang, J., Dang, X., Liu, K., Gong, Z., & Xiao, W. (2025). L-Theanine Mitigates Chronic Alcoholic Intestinal Injury by Regulating Intestinal Alcohol and Linoleic-Arachidonic Acid Metabolism in Rats. Nutrients, 17(11), 1943. https://doi.org/10.3390/nu17111943







