Vitamin D in the Transition from Acute to Chronic Pain: A Systematic Review
Abstract
1. Introduction
2. Methods
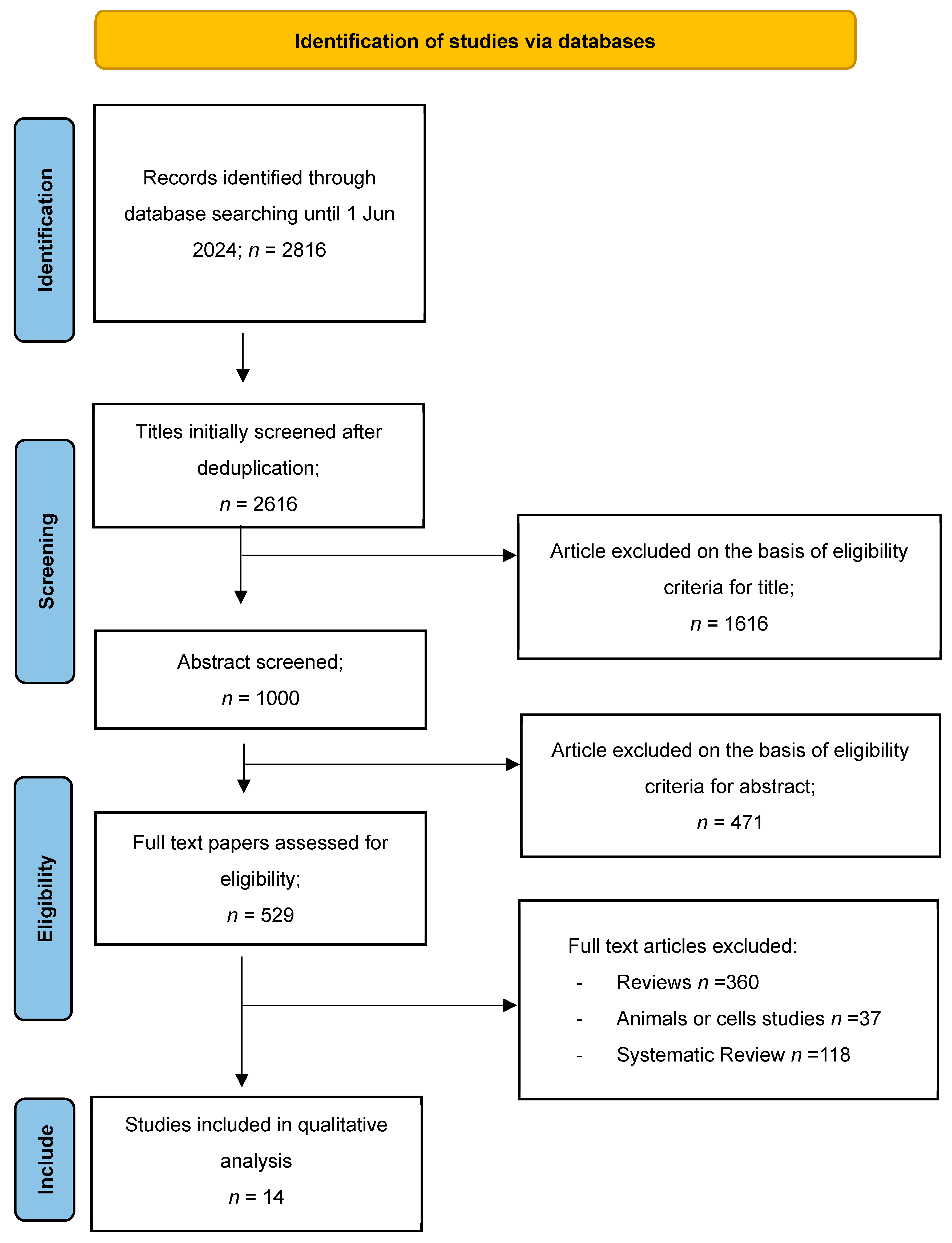
2.1. Paper Location and Selection
2.2. Study Selection and Data Extraction
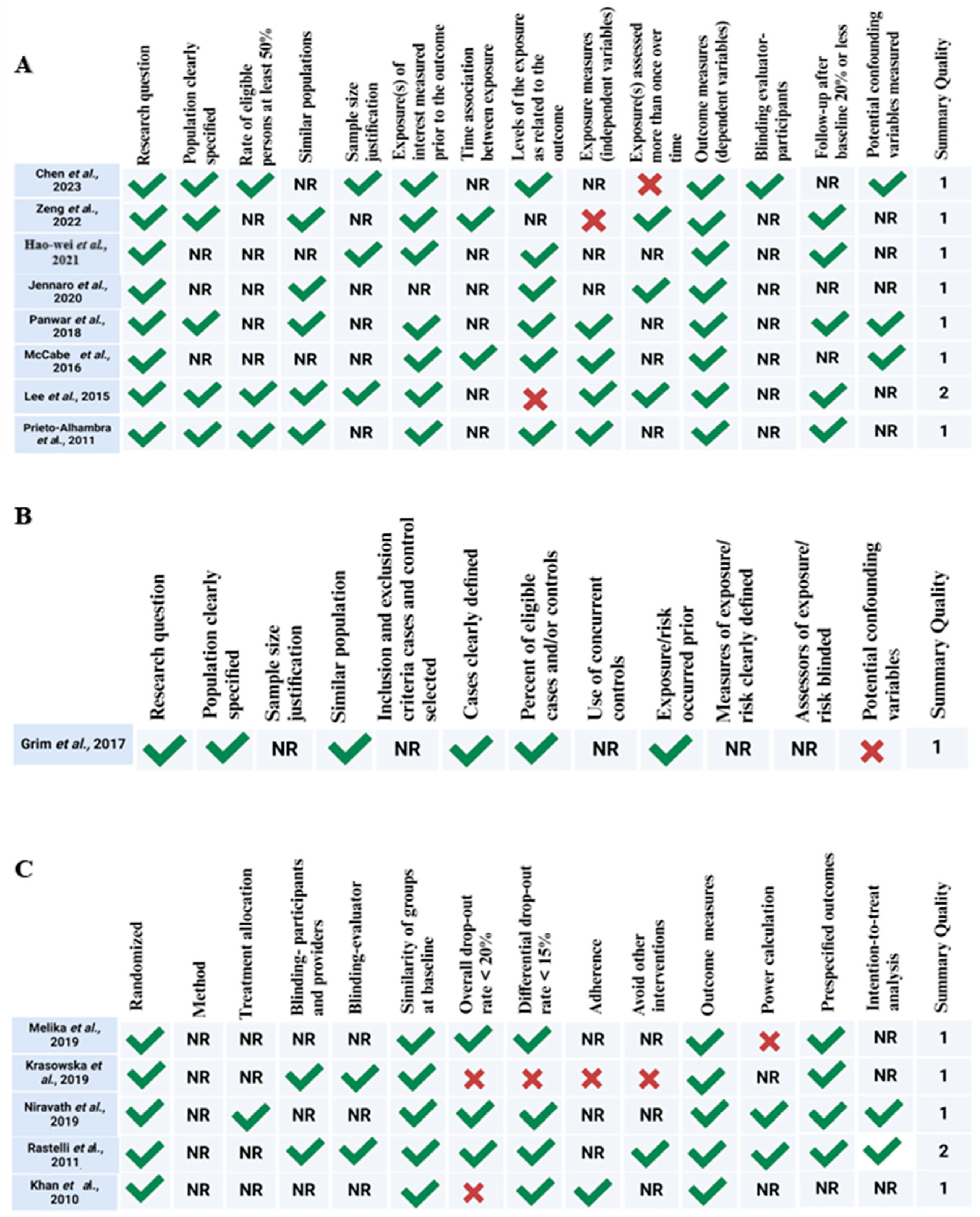
2.3. Quality Assessment and Risk of Bias Assessment
3. Results
3.1. Data Collection
3.2. Characteristics of the Studies Included
| Study/ Year | Type of Study | Country | Population | Mean, Age, Years (SD/IC) | Sex | n | Intervention/ Duration | Levels of VD | Pain Test Used |
|---|---|---|---|---|---|---|---|---|---|
| Chen et al., 2023 [38] | Prospective-retrospective study | USA | Patients with early-stage breast cancer with paclitaxel | 51.1 y ± 9.9 | F | 1191 | NR | 1.57 (1.14–2.15) IC | Sensory CIPN |
| Zeng et al., 2022 [30] | Retrospective cohort study | China | Patients who underwent elective non-cardiac thoracic surgery | Group 1 57.5 y ± 13.1 Group 2 58.8 y ± 11.8 | F = 74 M = 61 | 135 Group 1 = 73 Group 2 = 62 | 3 months | Group 1 low 25(OH)D levels (<30 nmol/L) Group 2 25(OH)D levels (>30 nmol/L) | Pain scores (numerical rating scale) |
| Hao-Wei et al., 2021 [42] | Cross-sectional study | China | Non-specific acute lower back pain (Ns-ALCP) and non-specific-chronic lower back pain patients (Ns-CLBP) | 63.42 ± 11.26 years, with a range of 33 to 80 years. | NR | 198 Ns-ALCP = 60 Ns-CLBP = 78 Control = 60 | NR | Ns-ALBP 21.44 ± 8.46 Ns-CLBP 18.25 ± 8.05 (ng/mL) Control 25.70 ± 10.04 | VAS scale |
| Jennaro et al., 2020 [39] | Prospective observation clinical study | USA | Patients with stage I–III breast cancer receiving weekly paclitaxel | 47.6 y (28–59 IC) 54.6 y (34–71 IC) | F | 37 | Duration 12 weeks | VD deficiency (defined as <20 ng/mL) was identified in 41% (15/37) of assessed patients | CIPN20 |
| Panwar et al., 2018 [31] | Prospective, observational, triple arm, case and control study | India | Patients with lower back pain of duration ≥ 6 weeks (CLBP), patients with subacute lower back pain (SLBP) and controls. | CLBP 40.40 y ± 13.638 SLBP 40.69 y ± 14.469 Control 37.95 y ± 15.255 | CLBP M = 104 F = 146 SLBP M = 82 F = 95 Control M = 112 F = 136 | 675 CLBP = 250 SLBP = 177 Control = 248 | 3 months | CLBP 20.36 ± 12.569 SLBP 21.42 ± 13.209 Controls 20.84 ± 6.931 | BMcGill Pain Map VAS scale MODQ |
| Grim et al., 2017 [32] | Case and control | Czech Republic | Patients with breast carcinoma from the undergoing chemotherapy based on 80 mg/m2 paclitaxel on a weekly basis (12 cycles) | 56 y ± 12.2 | M = 10% F = 90% | 70 | Duration 12 weeks Evaluation 1 after 4 weeks Evaluation 2 End treatment 12 weeks | Before chemotherapy Without NP 38.08 ± 15.6 nmol/L With NPz 26.94 nmol/L ± 8.5 During chemotherapy (4 week) Without NP 37.44 nmol/L ± 19.9 With NP 24.28 nmol/L ± 8.9 After chemotherapy (12 week) Without NP 42.33 nmol/L ± 9.6 With NP 31.4 nmol/L ± 10.4 | MNSI |
| McCabe et al., 2016 [37] | Multicenter/longitudinal study | Europe | Population sample of middle age and elderly men | aged 40–79 | M | 2736 | Participants were invited to attend repeat assessment after a mean interval of 4.3 years (range 3–5.7 years). | 25(OH)D–quintiles (ng/mL) 1. ≥36.3 2. 26.7–36.2 3. 20.7–26.6 4. 15.6–20.6 5. <15.6 25(OH)2D–quintiles (pg/mL) 1. ≥72.5 2. 62.2–72.5 3. 55.2–62.0 4. 45.4–55.0 5. <45.4 | Painful sites |
| Lee et al., 2015 [33] | Longitudinal cohort study | Hong Kong | Patients after surgery knee arthroplasty | 62–73 y | F = 153 M = 61 | 214 | 3 months | 25(OH)D levels <30 nmol/L >30 nmol/L–<50 nmol/L >50 nmol/L | Pain scale WOMAC EQ-5D-VAS |
| Study/Year | Type of Study | Country | Population | Mean, Age, Years (SD/IC) | Sex | n | Intervention/ Duration | Levels of VD | Pain Test Used |
|---|---|---|---|---|---|---|---|---|---|
| Melika et al., 2019 [34] | Randomized clinical trial | Iran | Adult patients with diagnosed brain tumor with serum level of 25 (OH) VD ≤ 20 ng/dL | VD 48.2 ± 15.3 y PL 44.3 ± 15.2 y | M = 30 F = 30 | 60 VD = 30 PL = 30 | 300,000 IU VD 2- to 14-day interval before surgery | Preoperatory VD = 15.9 ± 3.8 PL = 14.5 ± 3.6 | VAS scale |
| Krasowska et al., 2019 [35] | Double-blind randomization study | Poland | Patients undergoing posterior lumbar interbody fusion (PLIF) followed by rehabilitation. | VD 41.92 ± 2.97 PL 47.33 ± 2.15 | VD M = 9 F = 9 PL M = 9 F = 12 | 39 VD = 18 PL = 21 | VD (3200 IU dose of VD/day for 5 weeks) and placebo group (PL) | The initial serum 25(OH)D3 (nmol/L) VD 46.63 ± 1.69 PL 55.71 ± 4.11 after 5 weeks VD 75.03 ± 3.03 PL 53.62 ± 3.07 After surgery VD normal PL decrease levels | VAS scale |
| Niravath et al. (2019) [40] | Randomized control trial | USA | Post-menopausal women who were beginning adjuvant aromatase inhibitor therapy | 64 y (44–82 y) | F = 93 | 93 High dose = 46 Standard dose = 47 | Standard-dose VD3 (800 IU daily for 52 weeks), or high-dose VD3 (50,000 IU weekly for 12 weeks, followed by 2000 IU daily for 40 weeks) | Baseline vitamin D levels averaged 24.2 ng/mL in the standard-dose group and 21.7 ng/mL in the high-dose group. After 12 weeks, levels rose to 29.3 ng/mL and 50 ng/mL, respectively. | HAQ-II |
| Prieto-Alhambra et al. (2011) [43] | Prospective cohort study | Spain | Women with breast cancer who were women starting aromatase inhibitor therapy | VD < 30 ng/mL 2.63 (8.77) VD > 30 ng/mL 60.20 (9.64) | F | 284 VD < 30 ng/mL = 251 VD > 30 ng/mL = 33 | All received daily VD (800 IU) with calcium. Women with baseline VD concentration < 30 ng/mL also received 16,000 IU of D3 orally every 2 weeks. 3 months | Following baseline assessment, 89.7% (260 participants) were vitamin D deficient (<30 ng/mL), with 18.5% (48 individuals) showing severe deficiency (<10 ng/mL) | VAS scale |
| Rastelli et al. (2011) [36] | Randomized control trial | USA | Patients with musculoskeletal pain in women receiving adjuvant anastrozole improves aromatase inhibitor | 61.5 (8.4) | F = 57% | 57 VD = 28 PL = 29 | 50,000 IU VD2 weekly for 8 weeks then monthly for 4 months; or 50,000 IU VD2 weekly for 16 weeks then monthly for 2 months. | Stratum A = 20–29 ng/mL Stratum B = 10–19 ng/mL | BPI-SF FIQ HAQ-DI |
| Khan et al. (2010) [41] | Randomized, placebo-controlled trial | USA | Women with early-stage, receptor-positive, invasive breast cancer who were candidates for adjuvant aromatase inhibitor therapy | Mean age 56 y | F = 60 | 60 | Group 1: VD (high dose) Group 2 (control): VD (standard dose) 50,000 IU of VD weekly (16 weeks) | <40 ng/mL vit D = 47 >40 ng/mL vit D = 13 | HAQII |
3.3. Quality Assessment and Risk of Bias Assessment
3.4. Topic Identification
3.4.1. Topic 1: VD Levels and Its Relationship with Transition from Acute to Chronic Pain
3.4.2. Topic 2: The Impact of VD Supplementation on the Transition from Acute to Chronic Pain
4. Discussion
Limitation of the Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| VD | Vitamin D |
| MySLR | My Systematic Literature Review |
| LDA | Latent Dirichlet Allocation (algorithm for topic modeling) |
| NRS | Numeric Rating Scale |
| VAS | Visual Analog Scale |
| BPI | Brief Pain Inventory |
| BPI-SF | Brief Pain Inventory–Short Form |
| MODQ | Modified Oswestry Disability Questionnaire |
| MNSI | Michigan Neuropathy Screening Instrument |
| WOMAC | Western Ontario and McMaster Universities Osteoarthritis Index |
| CIPN | Chemotherapy-induced peripheral neuropathy |
| CIPN20 | Chemotherapy-Induced Peripheral Neuropathy 20-item Quality of Life Questionnaire |
| FIQ | Fibromyalgia Impact Questionnaire |
| HAQ | Health Assessment Questionnaire |
| HAQ-DI | Health Assessment Questionnaire–Disability Index |
| AI | Aromatase inhibitor |
| AIMSS | Aromatase Inhibitor-Associated Musculoskeletal Symptom |
| PL | Placebo |
| IU | International Units |
| 25(OH)D | 25-hydroxyvitamin D (a marker for Vitamin D status) |
| NP | Neuropathic pain |
| CWP | Chronic widespread pain |
| NAAA | N-Acylethanolamine acid amidase |
| SRE | Summary Risk of Bias Evaluation |
References
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Ilari, S.; Nucera, S.; Passacatini, L.C.; Scarano, F.; Macrì, R.; Caminiti, R.; Ruga, S.; Serra, M.; Giancotti, L.A.; Lauro, F.; et al. Exploring the Role of Bergamot Polyphenols in Alleviating Morphine-Induced Hyperalgesia and Tolerance through Modulation of Mitochondrial SIRT3. Nutrients 2024, 16, 2620. [Google Scholar] [CrossRef]
- Ilari, S.; Nucera, S.; Passacatini, L.C.; Caminiti, R.; Mazza, V.; Macrì, R.; Serra, M.; Scarano, F.; Malafoglia, V.; Palma, E.; et al. SIRT1: A likely key for future therapeutic strategies for pain management. Pharmacol. Res. 2025, 213, 107670. [Google Scholar] [CrossRef]
- Marcianò, G.; Siniscalchi, A.; Di Gennaro, G.; Rania, V.; Vocca, C.; Palleria, C.; Catarisano, L.; Muraca, L.; Citraro, R.; Evangelista, M.; et al. Assessing Gender Differences in Neuropathic Pain Management: Findings from a Real-Life Clinical Cross-Sectional Observational Study. J. Clin. Med. 2024, 13, 5682. [Google Scholar] [CrossRef]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef]
- Borsook, D.; Youssef, A.M.; Simons, L.; Elman, I.; Eccleston, C. When pain gets stuck: The evolution of pain chronification and treatment resistance. Pain 2018, 159, 2421–2436. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Nikolajsen, L.; Rice, A.S.C. Transition from acute to chronic pain: A misleading concept? Pain 2022, 163, e985–e988. [Google Scholar] [CrossRef]
- Paice, J.A. Chronic treatment-related pain in cancer survivors. Pain 2011, 152, S84–S89. [Google Scholar] [CrossRef]
- Ilari, S.; Lauro, F.; Giancotti, L.A.; Malafoglia, V.; Dagostino, C.; Gliozzi, M.; Condemi, A.; Maiuolo, J.; Oppedisano, F.; Palma, E.; et al. The Protective Effect of Bergamot Polyphenolic Fraction (BPF) on Chemotherapy-Induced Neuropathic Pain. Pharmaceuticals 2021, 14, 975. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Ji, R.-R.; Chamessian, A.; Zhang, Y.-Q. Pain regulation by non-neuronal cells and inflammation. Science 2016, 354, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Marchand, F.; Perretti, M.; McMahon, S.B. Role of the immune system in chronic pain. Nat. Rev. Neurosci. 2005, 6, 521–532. [Google Scholar] [CrossRef]
- Joseph, E.K.; Levine, J.D. Mitochondrial electron transport in models of neuropathic and inflammatory pain. Pain 2006, 121, 105–114. [Google Scholar] [CrossRef]
- Bennett, G.J.; Doyle, T.; Salvemini, D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat. Rev. Neurol. 2014, 10, 326–336. [Google Scholar] [CrossRef]
- Fotio, Y.; Jung, K.-M.; Palese, F.; Obenaus, A.; Tagne, A.M.; Lin, L.; Rashid, T.I.; Pacheco, R.; Jullienne, A.; Ramirez, J. NAAA-regulated lipid signaling governs the transition from acute to chronic pain. Sci. Adv. 2021, 7, eabi8834. [Google Scholar] [CrossRef]
- Price, T.J.; Basbaum, A.I.; Bresnahan, J.; Chambers, J.F.; De Koninck, Y.; Edwards, R.R.; Ji, R.-R.; Katz, J.; Kavelaars, A.; Levine, J.D.; et al. Transition to chronic pain: Opportunities for novel therapeutics. Nat. Rev. Neurosci. 2018, 19, 383–384. [Google Scholar] [CrossRef]
- Stevans, J.M.; Delitto, A.; Khoja, S.S.; Patterson, C.G.; Smith, C.N.; Schneider, M.J.; Freburger, J.K.; Greco, C.M.; Freel, J.A.; Sowa, G.A. Risk factors associated with transition from acute to chronic low back pain in US patients seeking primary care. JAMA Netw. open 2021, 4, e2037371. [Google Scholar] [CrossRef]
- Friedman, B.W.; Abril, L.; Naeem, F.; Irizarry, E.; Chertoff, A.; McGregor, M.; Bijur, P.E.; Gallagher, E.J. Predicting the transition to chronic pain 6 months after an emergency department visit for acute pain: A prospective cohort study. J. Emerg. Med. 2020, 59, 805–811. [Google Scholar] [CrossRef]
- Snyder, D.L.; Girgis, G.; Abd-Elsayed, A. Perioperative Pain Management-Introduction. In Perioperative Pain Management: A Clinical Guide; Springer: Cham, Switzerland, 2024; pp. 3–6. [Google Scholar]
- Hruschak, V.; Cochran, G. Psychosocial predictors in the transition from acute to chronic pain: A systematic review. Psychol. Health Med. 2018, 23, 1151–1167. [Google Scholar] [CrossRef]
- Habib, A.M.; Nagi, K.; Thillaiappan, N.B.; Sukumaran, V.; Akhtar, S. Vitamin D and its potential interplay with pain signaling pathways. Front. Immunol. 2020, 11, 820. [Google Scholar] [CrossRef]
- Alonso-Pérez, J.L.; Martínez-Pérez, I.; Romero-Morales, C.; Abuín-Porras, V.; López-Bueno, R.; Rossettini, G.; Leigheb, M.; Villafañe, J.H. Relationship Between Serum Vitamin D Levels and Chronic Musculoskeletal Pain in Adults: A Systematic Review. Nutrients 2024, 16, 4061. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gopal, H.; Khamkar, K.; Prajapati, P.; Mendiratta, N.; Misra, A.; Vaidya, B.; Abrol, A. Vitamin D deficiency as the primary cause of musculoskeletal complaints in patients referred to rheumatology clinic: A clinical study. Indian J. Rheumatol. 2012, 7, 199–203. [Google Scholar] [CrossRef]
- Prakash, S.; Rathore, C.; Makwana, P.; Dave, A.; Joshi, H.; Parekh, H. Vitamin D Deficiency in Patients with Chronic Tension-Type Headache: A Case-Control Study. Headache J. Head Face Pain 2017, 57, 1096–1108. [Google Scholar] [CrossRef]
- Helde-Frankling, M.; Björkhem-Bergman, L. Vitamin D in Pain Management. Int. J. Mol. Sci. 2017, 18, 2170. [Google Scholar] [CrossRef]
- Radbakhsh, S.; Abrego-Guandique, D.M.; Bacchetti, T.; Aghaee-Bakhtiari, S.H.; Mahmoudi, A.; Manteghi, A.A.; Bazyari, M.J.; Cione, E.; Ferretti, G.; Sahebkar, A. Direct hybridization and bioinformatics analysis of circulating microRNAs in patients with Alzheimer’s disease under intravenous trehalose treatment. Brain Res. 2025, 1857, 149607. [Google Scholar] [CrossRef]
- Saraceno, G.F.; Abrego-Guandique, D.M.; Cannataro, R.; Caroleo, M.C.; Cione, E. Machine Learning Approach to Identify Case-Control Studies on ApoE Gene Mutations Linked to Alzheimer’s Disease in Italy. BioMedInformatics 2024, 4, 600–622. [Google Scholar] [CrossRef]
- Abrego-Guandique, D.M.; Bonet, M.L.; Caroleo, M.C.; Cannataro, R.; Tucci, P.; Ribot, J.; Cione, E. The Effect of Beta-Carotene on Cognitive Function: A Systematic Review. Brain Sci. 2023, 13, 1468. [Google Scholar] [CrossRef]
- Abrego-Guandique, D.M.; Saraceno, G.F.; Cannataro, R.; de Burnside, M.M.; Caroleo, M.C.; Cione, E. Apolipoprotein E and Alzheimer’s Disease in Italian Population: Systematic Review and Meta-Analysis. Brain Sci. 2024, 14, 908. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, X.; Li, C.; Shi, H. Preoperative Vitamin D Level is Associated with Acute Pain After Video-Assisted Thoracoscopic Surgery: A Retrospective Cohort Study. J. Pain Res. 2022, 15, 3189–3196. [Google Scholar] [CrossRef]
- Panwar, A.; Valupadas, C.; Veeramalla, M.; Vishwas, H.N. Prevalence of vitamin D deficiency in chronic and subacute low back pain patients in India: A triple-arm controlled study. Clin. Rheumatol. 2018, 37, 1367–1374. [Google Scholar] [CrossRef]
- Grim, J.; Ticha, A.; Hyspler, R.; Valis, M.; Zadak, Z. Selected risk nutritional factors for chemotherapy-induced polyneuropathy. Nutrients 2017, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Chan, S.K.C.; Samy, W.; Chiu, C.H.; Gin, T. Effect of Hypovitaminosis D on Postoperative Pain Outcomes and Short-Term Health-Related Quality of Life After Knee Arthroplasty: A Cohort Study. Medicine 2015, 94, e1812. [Google Scholar] [CrossRef] [PubMed]
- Hajimohammadebrahim-Ketabforoush, M.; Shahmohammadi, M.; Khoundabi, B.; Shariatpanahi, Z.V. Effect of Vitamin D Supplementation on Postcraniotomy Pain After Brain Tumor Surgery: A Randomized Clinical Trial. World Neurosurg. 2019, 130, e105–e111. [Google Scholar] [CrossRef]
- Krasowska, K.; Skrobot, W.; Liedtke, E.; Sawicki, P.; Flis, D.J.; Dzik, K.P.; Libionka, W.; Kloc, W.; Kaczor, J.J. The preoperative supplementation with Vitamin D attenuated pain intensity and reduced the level of pro-inflammatory markers in patients after posterior lumbar interbody fusion. Front. Pharmacol. 2019, 10, 527. [Google Scholar] [CrossRef]
- Rastelli, A.L.; Taylor, M.E.; Gao, F.; Armamento-Villareal, R.; Jamalabadi-Majidi, S.; Napoli, N.; Ellis, M.J. Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): A phase II, double-blind, placebo-controlled, randomized trial. Breast Cancer Res. Treat. 2011, 129, 107–116. [Google Scholar] [CrossRef]
- McCabe, P.S.; Pye, S.R.; Beth, J.M.; Lee, D.M.; Tajar, A.; Bartfai, G.; Boonen, S.; Bouillon, R.; Casanueva, F.; Finn, J.D.; et al. Low vitamin D and the risk of developing chronic widespread pain: Results from the European male ageing study. BMC Musculoskelet. Disord. 2016, 17, 32. [Google Scholar] [CrossRef]
- Chen, C.S.; Zirpoli, G.; Barlow, W.E.; Budd, G.T.; McKiver, B.; Pusztai, L.; Hortobagyi, G.N.; Albain, K.S.; Damaj, M.I.; Godwin, A.K.; et al. Vitamin D Insufficiency as a Risk Factor for Paclitaxel-Induced Peripheral Neuropathy in SWOG S0221. J. Natl. Compr. Cancer Netw. 2023, 21, 1172–1180. [Google Scholar] [CrossRef]
- Jennaro, T.S.; Fang, F.; Kidwell, K.M.; Smith, E.M.L.; Vangipuram, K.; Burness, M.L.; Griggs, J.J.; Van Poznak, C.; Hayes, D.F.; Henry, N.L.; et al. Vitamin D deficiency increases severity of paclitaxel-induced peripheral neuropathy. Breast Cancer Res. Treat. 2020, 180, 707–714. [Google Scholar] [CrossRef]
- Niravath, P.; Hilsenbeck, S.G.; Wang, T.; Jiralerspong, S.; Nangia, J.; Pavlick, A.; Ademuyiwa, F.; Frith, A.; Ma, C.; Park, H.; et al. Randomized controlled trial of high-dose versus standard-dose vitamin D3 for prevention of aromatase inhibitor-induced arthralgia. Breast Cancer Res. Treat. 2019, 177, 427–435. [Google Scholar] [CrossRef]
- Khan, Q.J.; Reddy, P.S.; Kimler, B.F.; Sharma, P.; Baxa, S.E.; O’Dea, A.P.; Klemp, J.R.; Fabian, C.J. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res. Treat. 2010, 119, 111–118. [Google Scholar] [CrossRef]
- Hao-Wei, X.; Shu-Bao, Z.; Yu-Yang, Y.; Chen, H.; Hu, T.; Shan-Jin, W. Relationship between vitamin D and nonspecific low back pain may be mediated by inflammatory markers. Pain Physician 2021, 24, E1015. [Google Scholar]
- Prieto-Alhambra, D.; Javaid, M.K.; Servitja, S.; Arden, N.K.; Martinez-García, M.; Diez-Perez, A.; Albanell, J.; Tusquets, I.; Nogues, X. Vitamin D threshold to prevent aromatase inhibitor-induced arthralgia: A prospective cohort study. Breast Cancer Res. Treat. 2011, 125, 869–878. [Google Scholar] [CrossRef]
- Chen, X.; Ji, Y.; Liu, R.; Zhu, X.; Wang, K.; Yang, X.; Liu, B.; Gao, Z.; Huang, Y.; Shen, Y.; et al. Mitochondrial dysfunction: Roles in skeletal muscle atrophy. J. Transl. Med. 2023, 21, 503. [Google Scholar] [CrossRef]
- Ersoy, S.; Kesiktas, F.N.; Sirin, B.; Bugdayci, D.; Paker, N. The effect of vitamin D treatment on quality of life in patients with fibromyalgia. Irish J. Med. Sci. 2024, 193, 1111–1116. [Google Scholar] [CrossRef]
- Simonetti, M.; Mauceri, D. Cellular and Molecular Mechanisms Underlying Pain Chronicity. Cells 2023, 12, 1126. [Google Scholar] [CrossRef]
- Pak, D.J.; Yong, R.J.; Kaye, A.D.; Urman, R.D. Chronification of Pain: Mechanisms, Current Understanding, and Clinical Implications. Curr. Pain Headache Rep. 2018, 22, 9. [Google Scholar] [CrossRef]
- Chapman, C.R.; Vierck, C.J. The Transition of Acute Postoperative Pain to Chronic Pain: An Integrative Overview of Research on Mechanisms. J. Pain 2017, 18, 359.e1–359.e38. [Google Scholar] [CrossRef]
- Epping-Jordan, J.E.; Wahlgren, D.R.; Williams, R.A.; Pruitt, S.D.; Slater, M.A.; Patterson, T.L.; Grant, I.; Webster, J.S.; Atkinson, J.H. Transition to chronic pain in men with low back pain: Predictive relationships among pain intensity, disability, and depressive symptoms. Health Psychol. 1998, 17, 421–427. [Google Scholar] [CrossRef]
- Gatchel, R.J.; Bevers, K.; Licciardone, J.C.; Su, J.; Du, Y.; Brotto, M. Transitioning from Acute to Chronic Pain: An Examination of Different Trajectories of Low-Back Pain. Healthcare 2018, 6, 48. [Google Scholar] [CrossRef]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef]
- Zuqui-Ramírez, M.A.; Belalcazar-López, V.M.; Urenda-Quezada, A.; González-Rebatu y González, A.; Sander-Padilla, J.G.; Lugo-Sánchez, L.A.; Rodríguez-Vázquez, I.C.; Rios-Brito, K.F.; Arguedas-Núñez, M.M.; Canales-Vázquez, E. Multimodal Analgesia Approach in Acute Low Back Pain Management: A Phase III Study of a Novel Analgesic Combination of Etoricoxib/Tramadol. Pain Ther. 2024, 13, 1511–1528. [Google Scholar] [CrossRef] [PubMed]
- Shipton, E.A.; Shipton, E.E. Vitamin D and Pain: Vitamin D and Its Role in the Aetiology and Maintenance of Chronic Pain States and Associated Comorbidities. Pain Res. Treat. 2015, 2015, 904967. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R.; Holick, M.F. Chapter 57B-The IOM—Endocrine Society Controversy on Recommended Vitamin D Targets: In Support of the Endocrine Society Position. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: New York, NY, USA, 2018; pp. 1091–1107. ISBN 978-0-12-809965-0. [Google Scholar]
- Glare, P.; Aubrey, K.; Gulati, A.; Lee, Y.C.; Moryl, N.; Overton, S. Pharmacologic management of persistent pain in cancer survivors. Drugs 2022, 82, 275–291. [Google Scholar] [CrossRef]
- Soens, M.A.; Sesso, H.D.; Manson, J.E.; Fields, K.G.; Buring, J.E.; Lee, I.-M.; Cook, N.R.; Kim, E.; Bubes, V.; Dushkes, R.; et al. The effect of vitamin D and omega-3 fatty acid supplementation on pain prevalence and severity in older adults: A large-scale ancillary study of the VITamin D and OmegA-3 triaL (VITAL). Pain 2024, 165, 635–643. [Google Scholar] [CrossRef]
- Grant, W.B.; Wimalawansa, S.J.; Pludowski, P.; Cheng, R.Z. Vitamin D: Evidence-Based Health Benefits and Recommendations for Population Guidelines. Nutrients 2025, 17, 277. [Google Scholar] [CrossRef]
| Study/Year | Results |
|---|---|
| Chen et al., 2023 [38] | Of 1191 women, those with pre-treatment VD insufficiency (33.3%) had a higher risk of severe CIPN (20.7% vs. 14.2%; OR 1.57; 95% CI: 1.14–2.15; p = 0.005). |
| Zeng et al., 2022 [30] | Multivariable analysis showed that patients with low VD levels had a significantly higher risk of acute moderate–severe post-operative pain (OR 2.44; 95% CI: 1.18–5.04; p = 0.016), though pain scores at 3 months did not differ by vitamin D status. |
| Hao-Wei et al., 2021 [42] | Spearman’s analysis showed a significant negative correlation between vitamin D and IL-6 levels in both Ns-ALBP (r = −0.158, p = 0.027) and Ns-CLBP groups (r = −0.426, p < 0.001). VD levels also negatively correlated with VAS scores in Ns-CLBP patients (r ≈ −0.31, p < 0.001), but not in those with Ns-ALBP (p > 0.05). |
| Jennaro et al., 2020 [39] | Patients with VD deficiency showed a greater increase in neuropathic pain (36 ± 23 vs. 16 ± 16; p = 0.003) and a non-significant trend toward higher risk of treatment disruption (OR 2.98; 95% CI: 0.72–12.34; p = 0.16). Multivariable analysis confirmed an inverse association between baseline vitamin D levels and pain severity (β = −0.04; p = 0.02). |
| Panwar et al., 2018 [31] | While overall vitamin D deficiency rates were similar across CLBP, SLBP, and controls (~51%), both CLBP and SLBP groups had a significantly higher proportion of individuals with moderate to severe deficiency (≤16 ng/mL) compared to controls (CLBP: 43.6%, SLBP: 43.5%, controls: 20.1%; p < 0.001 and p = 0.001, respectively). This suggests a potential link between more severe vitamin D deficiency and chronic or subacute low back pain. |
| Grim et al., 2017 [32] | The key finding was that pre-chemotherapy vitamin D supplementation may offer neuroprotection against CIPN, as patients who developed CIPN consistently had lower 25(OH)D levels during the study period. |
| McCabe et al., 2016 [37] | At follow-up, 6.5% of participants developed new chronic widespread pain (CWP), while 24.9% remained pain-free. After adjusting for confounders, individuals in the lowest quintile of 25(OH)D (<15.6 ng/mL) had a nearly twofold increased risk of developing CWP compared to those in the highest quintile (≥36.3 ng/mL) (OR = 1.93; 95% CI: 1.0–3.6). |
| Lee et al., 2015 [33] | Patients with preoperative vitamin D deficiency were more likely to experience moderate-to-severe persistent postoperative pain (13.8% vs. 5.9%; p = 0.05). This deficiency was significantly associated with increased risk of persistent pain (OR 2.64; 95% CI: 1.03–6.77; p = 0.04). |
| Study/Year | Results |
|---|---|
| Melika et al., 2019 [34] | Did not find any significant effect of VD. However, a longer time before the operative time had an insignificantly lower pain score. |
| Krasowska et al., 2019 [35] | No significant difference in pain intensity was observed between VD and placebo groups after 5 weeks of supplementation. However, both groups showed a marked reduction in VAS scores following surgery and rehabilitation (p < 0.006 to p < 0.0001). Pain improvement was more pronounced in the VD group compared to placebo, both post-surgery (VAS: 2.83 ± 0.51 vs. 3.29 ± 0.37) and after rehabilitation (1.28 ± 0.29 vs. 2.62 ± 0.47). |
| Niravath et al. (2019) [40] | High-dose VD effectively increased serum levels, but it did not significantly impact the incidence of aromatase inhibitor-induced arthralgia. Additionally, neither baseline nor 12-week VD levels predicted the development of this condition. |
| Prieto-Alhambra et al. (2011) [43] | Following supplementation, half of the patients did not reach adequate 25(OH)D levels by 3 months. Overall, joint pain increased (mean change: +1.16 ± 2.66; p < 0.001), but this increase was significantly less in those who achieved ≥40 ng/mL of 25(OH)D (p = 0.02), with a lower risk of developing arthralgia (OR 0.12; 95% CI: 0.03–0.40). Achieving this target level may help prevent aromatase inhibitor-induced arthralgia, though higher loading doses are needed for those initially deficient. |
| Rastelli et al. (2011) [36] | At 2 months, patients receiving high-dose VD (HDD) showed significantly greater improvements in pain scores compared to placebo, including FIQ pain (p = 0.0045), BPI worst pain (p = 0.04), average pain (p = 0.0067), pain severity (p = 0.04), and pain interference (p = 0.034). The beneficial effects of HDD on aromatase inhibitor-associated musculoskeletal symptoms (AIMSSs) were more pronounced in Stratum B than Stratum A across all time points. |
| Khan et al. (2010) [41] | At baseline, the median 25(OH)D level was 27 ng/mL (range: 9–61 ng/mL). VD deficiency (≤20 ng/mL) was present in 30% of participants, while an additional 33% had insufficient levels (21–31 ng/mL). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrego-Guandique, D.M.; Ilari, S.; Nucera, S.; Passacatini, L.C.; Cione, E.; Cannataro, R.; Gallelli, L.; Caroleo, M.C.; Mollace, V.; Muscoli, C. Vitamin D in the Transition from Acute to Chronic Pain: A Systematic Review. Nutrients 2025, 17, 1912. https://doi.org/10.3390/nu17111912
Abrego-Guandique DM, Ilari S, Nucera S, Passacatini LC, Cione E, Cannataro R, Gallelli L, Caroleo MC, Mollace V, Muscoli C. Vitamin D in the Transition from Acute to Chronic Pain: A Systematic Review. Nutrients. 2025; 17(11):1912. https://doi.org/10.3390/nu17111912
Chicago/Turabian StyleAbrego-Guandique, Diana Marisol, Sara Ilari, Saverio Nucera, Lucia Carmela Passacatini, Erika Cione, Roberto Cannataro, Luca Gallelli, Maria Cristina Caroleo, Vincenzo Mollace, and Carolina Muscoli. 2025. "Vitamin D in the Transition from Acute to Chronic Pain: A Systematic Review" Nutrients 17, no. 11: 1912. https://doi.org/10.3390/nu17111912
APA StyleAbrego-Guandique, D. M., Ilari, S., Nucera, S., Passacatini, L. C., Cione, E., Cannataro, R., Gallelli, L., Caroleo, M. C., Mollace, V., & Muscoli, C. (2025). Vitamin D in the Transition from Acute to Chronic Pain: A Systematic Review. Nutrients, 17(11), 1912. https://doi.org/10.3390/nu17111912









