A Comparative Analysis of the Use of Low-Calorie and No-Calorie Sweeteners in Food Products Without and With Added Sugar on the Polish Market
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection
- (1)
- The product was available for retail sale in Poland during the study period;
- (2)
- The product label contained clear information about the presence or absence of added sugar and/or sweeteners;
- (3)
- The product belonged to one of the food categories described below.
2.2. Product Classification
2.3. Data Processing
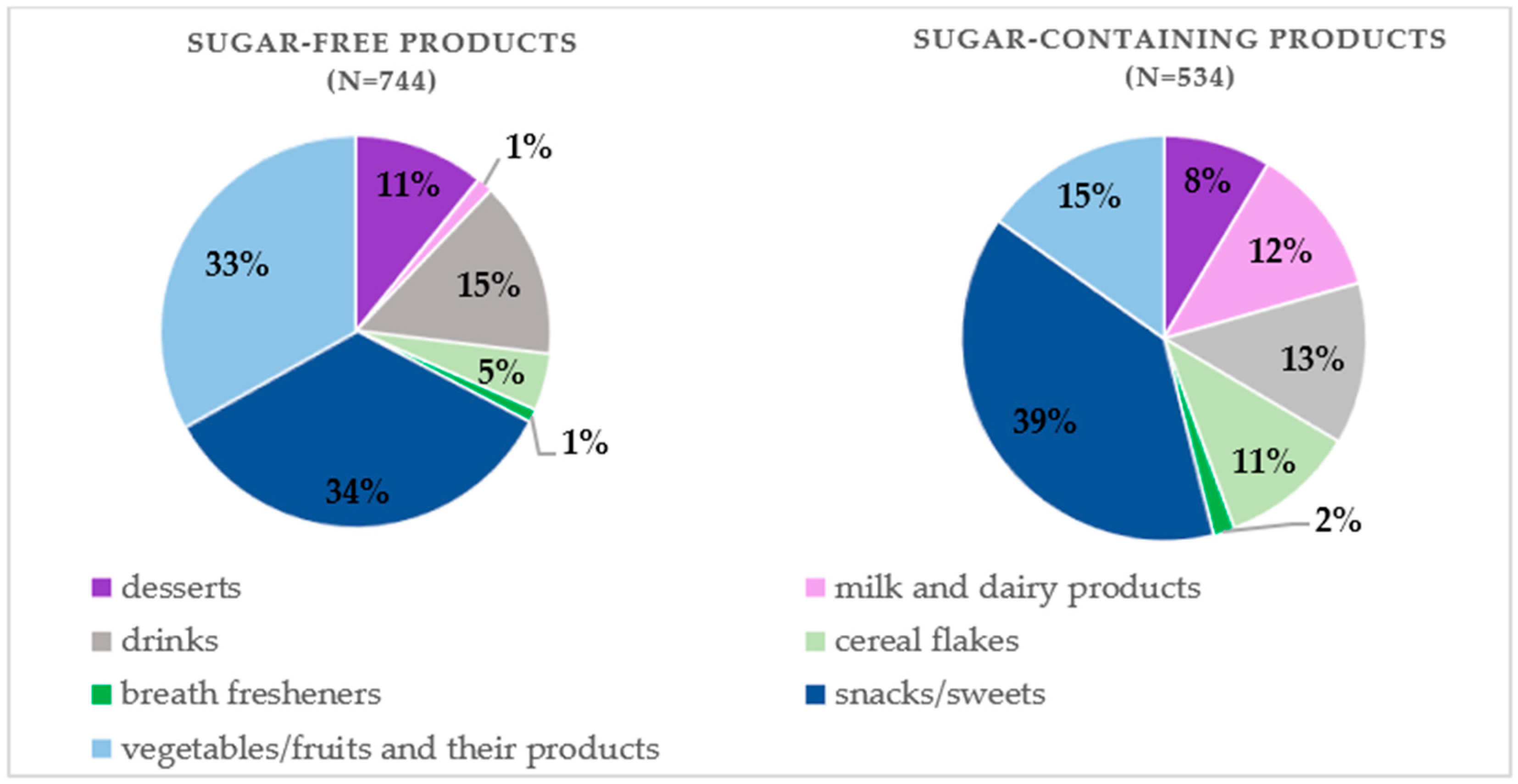
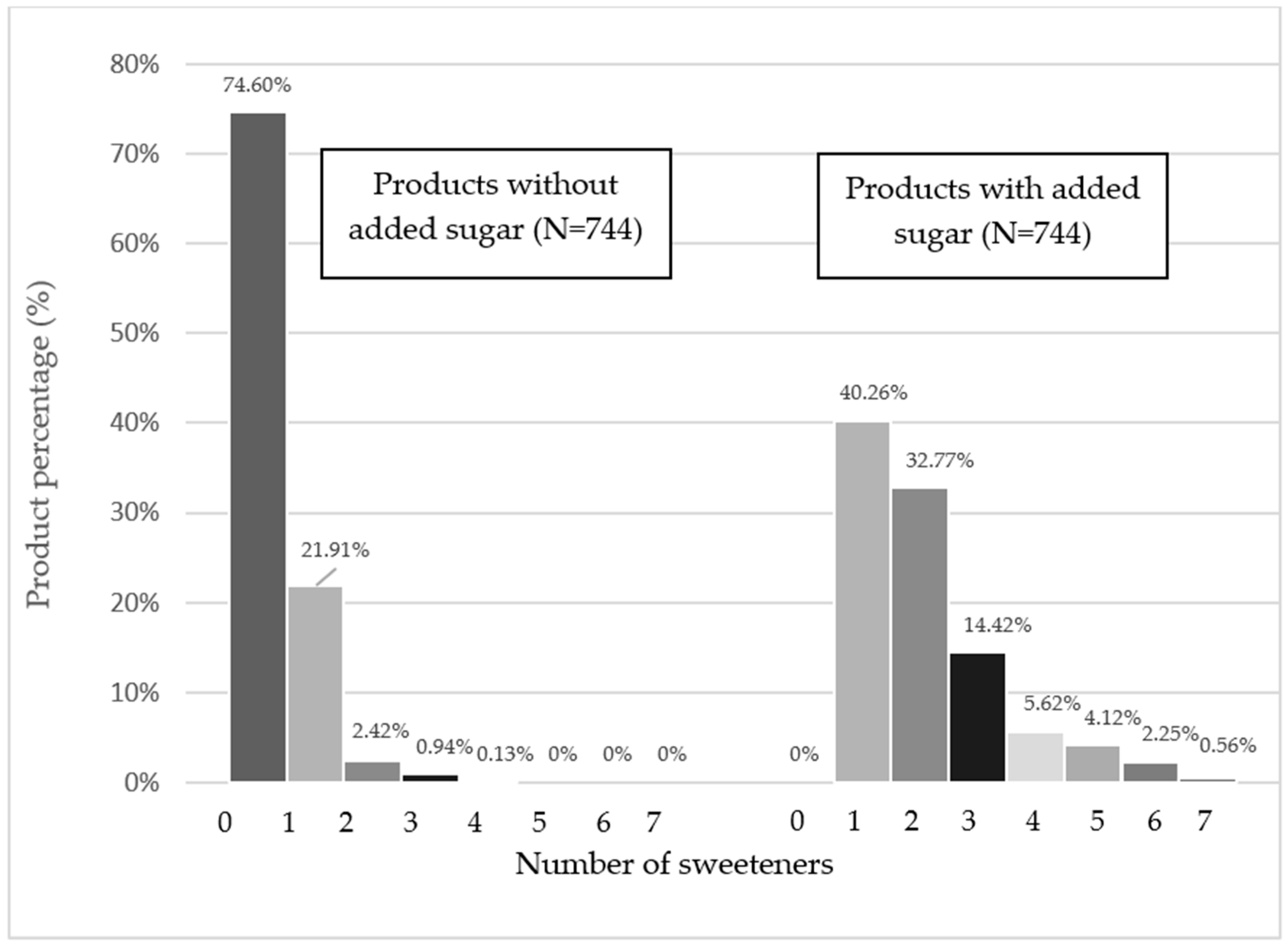
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LNCSs | Low- and No-Calorie Sweeteners |
| ADI | Acceptable Daily Intake |
References
- Gauthier, E.; Milagro, F.I.; Navas-Carretero, S. Effect of low-and non-calorie sweeteners on the gut microbiota: A review of clinical trials and cross-sectional studies. Nutrition 2024, 117, 112237. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.S.H.; Zhu, R.; Kjølbæk, L.; Raben, A. Effect of Non- and Low-Caloric Sweeteners on Substrate Oxidation, Energy Expenditure, and Catecholamines in Humans—A Systematic Review. Nutrients 2023, 15, 2711. [Google Scholar] [CrossRef] [PubMed]
- Maluly, H.D.B.; Johnston, C.; Giglio, N.D.; Schreiner, L.L.; Roberts, A.; Abegaz, E.G. Low- and No- Calorie Sweeteners (LNCS): Critical evaluation of their safety and health risks. Food Sci. Technol. 2020, 40, 1–10. [Google Scholar] [CrossRef]
- Abbeele, P.V.; Poppe, J.; Deyaert, S.; Laurie, I.; Gravert, T.K.O.; Abrahamsson, A.; Baudot, A.; Karnik, K.; Risso, D. Low-no-calorie sweeteners exert marked compound-specific impact on the human gut microbiota ex vivo. Int. J. Food Sci. Nutr. 2023, 74, 630–644. [Google Scholar] [CrossRef]
- Dunford, E.K.; Taillie, L.S.; Miles, D.R.; Eyles, H.; Tolentino-Mayo, L.; Ng, S.W. Non-Nutritive Sweeteners in the Packaged Food Supply—An Assessment across 4 Countries. Nutrients 2018, 10, 257. [Google Scholar] [CrossRef]
- Mora, M.R.; Dando, R. The sensory properties and metabolic impact of natural and synthetic sweeteners. Compr. Rev. Food Saf. 2021, 20, 1554–1583. [Google Scholar] [CrossRef]
- Te Morenga, L.; Mallard, S.; Mann, J. Dietary sugars and body weight: Systematic review and meta-analyses of randomised controlled trials and cohort studies. BMJ 2012, 346, e7492. [Google Scholar] [CrossRef]
- González-Rodríguez, M.; Redruello-Requejo, M.; Samaniego-Vaesken, M.L.; Montero-Bravo, A.; Puga, A.M.; Partearroyo, T.; Varela-Moreiras, G. Low- and No-Calorie Sweetener (LNCS) Presence and Consumption among the Portuguese Adult Population. Nutrients 2021, 13, 4186. [Google Scholar] [CrossRef]
- Hafner, E.; Pravst, I. The Sharp Rise in the Use of Low- and No-Calorie Sweeteners in Non-Alcoholic Beverages in Slovenia: An Update Based on 2020 Data. Front. Nutr. 2021, 8, 778178. [Google Scholar] [CrossRef]
- Barraj, L.; Bi, X.; Tran, N. Screening level intake estimates of low and no-calorie sweeteners in Argentina, Chile, and Peru. Food Addit. Contam. Part A 2021, 38, 1995–2011. [Google Scholar] [CrossRef]
- Orku, S.E.; Suyen, G.; Bas, M. The effect of regular consumption of four low- or no-calorie sweeteners on glycemic response in healthy women: A randomized controlled trial. Nutrition 2022, 106, 111885. [Google Scholar] [CrossRef] [PubMed]
- Hafner, E.; Hribar, M.; Hristov, H.; Kušar, A.; Žmitek, K.; Roe, M.; Pravst, I. Trends in the Use of Low and No-Calorie Sweeteners in Non-Alcoholic Beverages in Slovenia. Foods 2021, 10, 387. [Google Scholar] [CrossRef]
- Redruello-Requejo, M.; González-Rodríguez, M.; Samaniego-Vaesken, M.D.L.; Montero-Bravo, A.; Partearroyo, T.; Varela-Moreiras, G. Low-and no-calorie sweetener (LNCS) consumption patterns amongst the Spanish adult population. Nutrients 2021, 13, 1845. [Google Scholar] [CrossRef]
- Bielaszka, A.; Kardas, M.; Kiciak, A.; Szczepanska, E.; Grajek, M.; Jastrzebska, A.; Kardas, J.; Grochowska-Niedworok, E. Wykorzystanie stewii jako zamiennika cukru przez osoby dorosłe. Bromat Chem. Toksykol. 2016, 49, 450–454. [Google Scholar]
- O, B.Y.S.; Coyle, D.H.; Dunford, E.K.; Wu, J.H.Y.; Louie, J.C.Y. The Use of Non-Nutritive and Low-Calorie Sweeteners in 19,915 Local and Imported Pre-Packaged Foods in Hong Kong. Nutrients 2021, 13, 1861. [Google Scholar] [CrossRef]
- Barraj, L.; Scrafford, C.; Bi, X.; Tran, N. Intake of low and no-calorie sweeteners (LNCS) by the Brazilian population. Food Addit. Contam. Part A 2021, 38, 181–194. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. Off. J. Eur. Union 2008, L354, 16–53. [Google Scholar]
- Samaniego-Vaesken, M.L.; Ruiz, E.; Partearroyo, T.; Aranceta-Bartrina, J.; Gil, A.; Gonzalez-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G. Added Sugars and Low- and No-Calorie Sweeteners in a Representative Sample of Food Products Consumed by the Spanish ANIBES Study Population. Nutrients 2018, 10, 1265. [Google Scholar] [CrossRef]
- Figueiredo, L.S.; Scapin, T.; Fernandes, A.C.; Proença, R.P.C. Where are the low-calorie sweeteners? An analysis of the presence and types of low-calorie sweeteners in packaged foods sold in Brazil from food labelling. Public Health Nutr. 2017, 21, 447–453. [Google Scholar] [CrossRef]
- Sambra, V.; López-Arana, S.; Cáceres, P.; Abrigo, K.; Collinao, J.; Espinoza, A.; Valenzuela, S.; Carvajal, B.; Prado, G.; Peralta, R.; et al. Overuse of Non-caloric Sweeteners in Foods and Beverages in Chile: A Threat to Consumers’ Free Choice? Front. Nutr. 2020, 7, 68. [Google Scholar] [CrossRef]
- Buffini, M.; Goscinny, S.; Van Loco, J.; Nugent, A.P.; Walton, J.; Flynn, A.; Gibney, M.J.; McNulty, B.A. Dietary intakes of six intense sweeteners by Irish adults. Food Addit. Contam. Part A 2018, 35, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Le Donne, C.; Mistura, L.; Goscinny, S.; Janvier, S.; Cuypers, K.; D’Addezio, L.; Sette, S.; Catasta, G.; Ferrari, M.; Piccinelli, R. Assessment of dietary intake of 10 intense sweeteners by the Italian population. Food Chem. Toxicol. 2017, 102, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Samaniego-Vaesken, M.L.; Partearroyo, T.; Cano, A.; Urrialde, R.; Varela-Moreiras, G. Novel database of declared low- and no-calorie sweeteners from foods and beverages available in Spain. J. Food Compos. Anal. 2019, 82, 103234. [Google Scholar] [CrossRef]
- Sylvetsky, A.C.; Rother, K.I. Trends in the consumption of low-calorie sweeteners. Physiol. Behav. 2016, 164, 446–450. [Google Scholar] [CrossRef]
- Yen, S.H.Y.; Barrett, E.; Coyle, D.H.; Wu, J.H.Y.; Louie, J.C.Y. The distribution and co-occurrence of food additives in pre-packaged foods in Hong Kong. Food Control 2024, 158, 110210. [Google Scholar] [CrossRef]
- Eisenreich, A.; Gurtler, R.; Schafer, B. Heating of food containing sucralose might result in the generation of potentially toxic chlorinated compounds. Food Chem. 2020, 321, 126700. [Google Scholar] [CrossRef] [PubMed]
- Kujałowicz, A. Charakterystyka wybranych cukrów i słodzików. Tutoring Gedanensis 2022, 7, 25–40. [Google Scholar] [CrossRef]
- Shaher, S.A.A.; Mihailescu, D.F.; Amuzescu, B. Aspartame Safety as a Food Sweetener and Related Health Hazards. Nutrients 2023, 15, 3627. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the re-evaluation of butylated hydroxyanisole–BHA (E 320) as a food additive. EFSA J. 2011, 9, 2392. [Google Scholar] [CrossRef]
- World Health Organization. Use of Non-Sugar Sweeteners: WHO Guideline Summary; World Health Organization: Geneva, Switzerland, 2023; Available online: https://iris.who.int/handle/10665/375565 (accessed on 23 April 2024).
- Probst, Y.C.; Dengate, A.; Jacobs, J.; Louie, J.C.; Dunford, E.K. The major types of added sugars and non-nutritive sweeteners in a sample of Australian packaged foods. Public Health Nutr. 2017, 20, 3228–3233. [Google Scholar] [CrossRef]
- Hu, X.; Song, L.; Yang, Y.; Wang, L.; Li, Y.; Miao, M. Biosynthesis, structural characteristics and prebiotic properties of maltitol-based acceptor products. J. Funct. Foods 2021, 78, 104374. [Google Scholar] [CrossRef]
- Shen, P.; Walker, G.D.; Yuan, Y.; Reynolds, C.; Reynolds, E.C. Polyols and remineralisation of enamel subsurface lesions. J. Dent. 2017, 66, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Godswill, A.C.; Kate, E.C. Current developments in sugar alcohols: Chemistry, nutrition, and health concerns of sorbitol, xylitol, glycerol, arabitol, inositol, maltitol, and lactitol. Int. J. Adv. Acad. Research. Sci. Technol. Eng. 2019, 5, 1–33. [Google Scholar]
- Plaza-Diaz, J.; Pastor-Villaescusa, B.; Rueda-Robles, A. Plausible Biological Interactions of Low- and Non-Calorie Sweeteners with the Intestinal Microbiota: An Update of Recent Studies. Nutrients 2020, 12, 1153. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Sáez-Lara, M.J.; Gil, A. Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical Trials. Adv. Nutr. 2019, 10, 31–48. [Google Scholar] [CrossRef]
- Saraiva, A.; Carrascosa, C.; Raheem, D.; Ramos, F.; Raposo, A. Maltitol: Analytical Determination Methods, Applications in the Food Industry, Metabolism and Health Impacts. Int. J. Env. Res. Public Health 2020, 17, 5227. [Google Scholar] [CrossRef]
- Lenhart, A.; Chey, W.D. A Systematic Review of the Effects of Polyols on Gastrointestinal Health and Irritable Bowel Syndrome. Adv. Nutr. 2017, 8, 587–596. [Google Scholar] [CrossRef]
- Wan, Z.; Khubber, S.; Dwivedi, M.; Misra, N.N. Strategies for lowering the added sugar in yogurts. Food Chem. 2021, 344, 128573. [Google Scholar] [CrossRef]
- Janakiram, C.; Kumar, C.V.D.; Joseph, J. Xylitol in preventing dental caries: A systematic review and meta analyses. J. Nat. Sci. Biol. Med. 2017, 8, 16–21. [Google Scholar] [CrossRef]
- Szynal, K.; Polaniak, R.; Górski, M.; Grajek, M.; Ciechowska, K.; Grochowska-Niedworok, E. Processed Food and Food Additives in the Context of Dysbiosis and Its Health Consequences. Postępy Mikrobiol. 2021, 60, 223–230. [Google Scholar] [CrossRef]
- Nettleton, J.E.; Reimer, R.A.; Shearer, J. Reshaping the gut microbiota: Impact of low calorie sweeteners and the link to insulin resistance? Physiol. Behav. 2016, 164, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Lobach, A.R.; Roberts, A.; Rowland, I.A. Assessing the in vivo data on low/no-calorie sweeteners and the gut microbiota. Food Chem. Toxicol. 2019, 124, 385–399. [Google Scholar] [CrossRef]
- Rozporządzenie Ministra Zdrowia z Dnia 22 Listopada 2010 r. w Sprawie Dozwolonych Substancji Dodatkowych. Dz. U., 232, Item 1525; 2010; pp. 15876–15989. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20102321525 (accessed on 10 November 2024).
- Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and Repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004 Text with EEA Relevance. Available online: https://eur-lex.europa.eu/eli/reg/2011/1169/oj/eng (accessed on 10 November 2024).
- Luo, X.; Arcot, J.; Gill, T.; Louie, J.C.Y.; Rangan, A. A review of food reformulation of baked products to reduce added sugar intake. Trends Food Sci. Technol. 2019, 86, 412–425. [Google Scholar] [CrossRef]
- Talevi, A. Potential medicinal effects and applications of stevia constituents. Phytochem. Rev. 2022, 21, 161–178. [Google Scholar] [CrossRef]
- Samuel, P.; Ayoob, K.T.; Magnuson, B.A.; Wölwer-Rieck, U.; Jeppesen, P.B.; Rogers, P.J.; Rowland, I.; Mathews, R. Stevia Leaf to Stevia Sweetener: Exploring Ist Science, Benefits, and Future Potential. J. Nutr. 2018, 148, 1186–1205. [Google Scholar] [CrossRef]
- Peteliuk, V.; Rybchuk, L.; Bayliak, M.; Storey, K.B.; Lushchak, O. Natural sweetener stevia rebaudiana: Functionalities, health benefits and potential risks. EXCLI J. 2021, 20, 1412–1430. [Google Scholar] [PubMed]
- Fattore, E.; Botta, F.; Agostoni, C.; Bosetti, C. Effects of free sugars on blood pressure and lipids: A systematic review and meta-analysis of nutritional isoenergetic intervention trials. Am. J. Clin. Nutr. 2017, 105, 42–56. [Google Scholar] [CrossRef]
- Prinz, P. The role of dietary sugars in health: Molecular composition or just calories? Eur. J. Clin. Nutr. 2019, 73, 1216–1223. [Google Scholar] [CrossRef]
- Grembecka, M. Sugar alcohols—Their role in the modern world of sweeteners: A review. Eur. Food Res. Technol. 2015, 241, 1–14. [Google Scholar] [CrossRef]
- Miller, P.E.; Perez, V. Low-calorie sweeteners and body weight and composition: A meta-analysis of randomized controlled trials and prospective cohort studies. Am. J. Clin. Nutr. 2014, 100, 765–777. [Google Scholar] [CrossRef]
| Score | |||
|---|---|---|---|
| No. | Criterion | 0 | 1 |
| 1. | Presence of added sucrose | NO | YES |
| 2. | Presence of added fructose | NO | YES |
| 3. | Presence of added glucose | NO | YES |
| 4. | Presence of added lactose | NO | YES |
| 5. | Presence of added lactase | NO | YES |
| 6. | Presence of added maltodextrin | NO | YES |
| 7. | Presence of added isomaltulose | NO | YES |
| 8. | Presence of added sugar | NO | YES |
| 9. | Presence of added glucose syrup | NO | YES |
| 10. | Presence of added sucrose syrup | NO | YES |
| 11. | Presence of added maltose syrup | NO | YES |
| 12. | Presence of added glucose–fructose syrup | NO | YES |
| 13. | Presence of added rice syrup | NO | YES |
| 14. | Presence of added date syrup | NO | YES |
| 15. | Presence of added caramel syrup | NO | YES |
| 16. | Presence of added malt extract | NO | YES |
| 17. | Presence of added honey | NO | YES |
| 18. | Presence of added maple syrup | NO | YES |
| 19. | Presence of added agave syrup | NO | YES |
| 20. | Presence of added molasses | NO | YES |
| 21. | Presence of added acesulfame K | NO | YES |
| 22. | Presence of added aspartame | NO | YES |
| 23. | Presence of added cyclamic acid and/or its salt: sodium and calcium | NO | YES |
| 24. | Presence of added saccharin and its salts: sodium, potassium and calcium | NO | YES |
| 25. | Presence of added sucralose | NO | YES |
| 26. | Presence of added thaumatin | NO | YES |
| 27. | Presence of added neohesperidin DC | NO | YES |
| 28. | Presence of added steviol glycosides | NO | YES |
| 29. | Presence of added neotame | NO | YES |
| 30. | Presence of added aspartame and/or acesulfame salt | NO | YES |
| 31. | Presence of added sorbitol and/or sorbitol syrup | NO | YES |
| 32. | Presence of added mannitol | NO | YES |
| 33. | Presence of added xylitol | NO | YES |
| 34. | Presence of added erythritol | NO | YES |
| 35. | Presence of added maltitol and/or maltitol syrup | NO | YES |
| 36. | Presence of added lactitol | NO | YES |
| 37. | Presence of added isomalt | NO | YES |
| Presence of Low- and No-Calorie Sweeteners | p | Presence of Intense Artificial Sweeteners | p | Presence of Steviol Glycosides | p | Presence of Polyols | p | |
|---|---|---|---|---|---|---|---|---|
| Products without added sugar (N = 744) | 33.9% | <0.0001 | 16.8% | 0.005 | 4.2% | 0.082 | 22.9% | <0.0001 |
| Products with added sugar (N = 534) | 20.0% | 11.2% | 2.4% | 6.7% |
| Product Group | Presence of Intensive Artificial Sweeteners | p | Presence of Steviol Glycosides | p | Presence of Polyols | p | |
|---|---|---|---|---|---|---|---|
| Desserts | No added sugar (N = 81) | 53.1% (N = 43) | <0.0001 | 6.2% (N = 5) | 0.0849 | 13.6% (N = 11) | 0.035 |
| With added sugar (N = 46) | 0% (N = 0) | 0% (N = 0) | 2.2% (N = 1) | ||||
| Milk and dairy products | No added sugar (N = 10) | 10% (N = 1) | 0.01 | 0% (N = 0) | - | 0% (N = 0) | - |
| With added sugar (N = 64) | 0% (N = 0) | 0% (N = 0) | 0% (N = 0) | ||||
| Drinks | No added sugar (N = 109) | 25.7% (N = 28) | 0.0007 | 1.8% (N = 2) | 0.0002 | 1.8% (N = 2) | 0.6279 |
| With added sugar (N = 69) | 50.7% (N = 35) | 17.4% (N = 12) | 2.9% (N = 2) | ||||
| Cereal flakes | No added sugar (N = 35) | 5.7% (N = 2) | 0.0661 | 0% (N = 0) | - | 28.6% (N = 10) | <0.0001 |
| With added sugar (N = 58) | 0% (N = 0) | 0% (N = 0) | 0% (N = 0) | ||||
| Breath-fresheners | No added sugar (N = 8) | 100% (N = 8) | <0.0001 | 0% (N = 0) | - | 100% (N = 8) | <0.0001 |
| With added sugar (N = 9) | 0% (N = 0) | 0% (N = 0) | 0% (N = 0) | ||||
| Sweets and snacks | No added sugar (N = 255) | 4.7% (N = 12) | 0.0016 | 7.8% (N = 20) | <0.0001 | 42.8% (N = 109) | <0.0001 |
| With added sugar (N = 207) | 0% (N = 0) | 0% (N = 0) | 15.9% (N = 33) | ||||
| Vegetables, fruits and their products | No added sugar (N = 246) | 13% (N = 32) | 0.0002 | 1.6% (N = 4) | 0.7973 | 12.2% (N = 30) | 0.0034 |
| With added sugar (N = 81) | 30.9% (N = 25) | 1.2% (N = 1) | 1.2% (N = 1) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kołodziejczyk, A.; Nowak, J. A Comparative Analysis of the Use of Low-Calorie and No-Calorie Sweeteners in Food Products Without and With Added Sugar on the Polish Market. Nutrients 2025, 17, 1899. https://doi.org/10.3390/nu17111899
Kołodziejczyk A, Nowak J. A Comparative Analysis of the Use of Low-Calorie and No-Calorie Sweeteners in Food Products Without and With Added Sugar on the Polish Market. Nutrients. 2025; 17(11):1899. https://doi.org/10.3390/nu17111899
Chicago/Turabian StyleKołodziejczyk, Aleksandra, and Justyna Nowak. 2025. "A Comparative Analysis of the Use of Low-Calorie and No-Calorie Sweeteners in Food Products Without and With Added Sugar on the Polish Market" Nutrients 17, no. 11: 1899. https://doi.org/10.3390/nu17111899
APA StyleKołodziejczyk, A., & Nowak, J. (2025). A Comparative Analysis of the Use of Low-Calorie and No-Calorie Sweeteners in Food Products Without and With Added Sugar on the Polish Market. Nutrients, 17(11), 1899. https://doi.org/10.3390/nu17111899





