Critical Review on the Anti-Tumor Activity of Bioactive Compounds from Edible and Medicinal Mushrooms over the Last Five Years
Abstract
1. Introduction
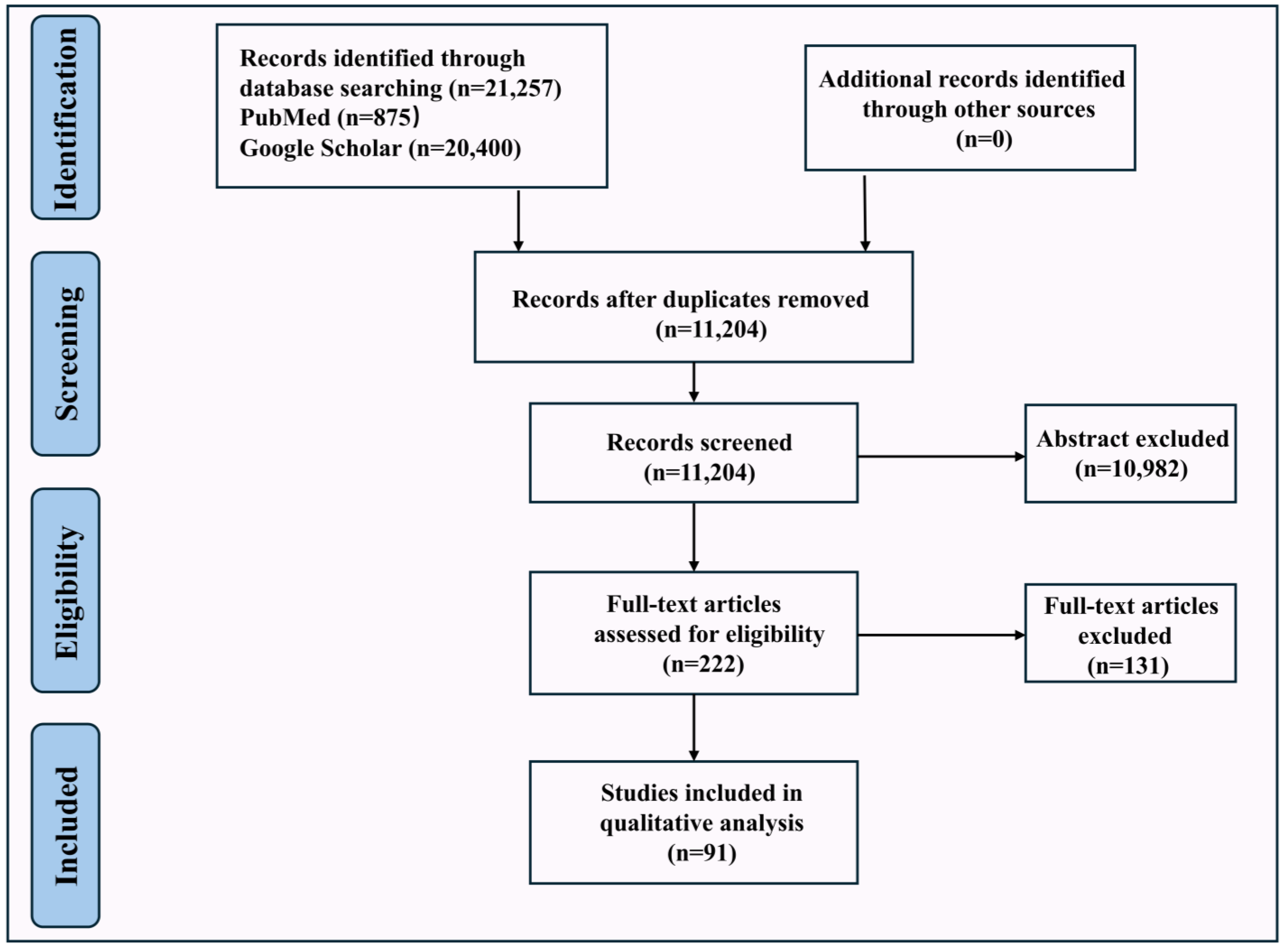
2. Methodology
2.1. Information Sources and Search Strategy
2.2. Eligibility and Exclusion Criteria
3. Summary of Research on the Anti-Cancer Properties of Active Compounds in Edible/Medicinal Mushrooms
3.1. Schizophyllum commune
3.1.1. Occurrence and Ecology
3.1.2. Anti-Cancer Properties of Bioactive Components from S. commune
3.2. Trametes versicolor
3.2.1. Occurrence and Ecology
3.2.2. Anti-Cancer Properties of Bioactive Components from T. versicolor
3.3. Grifola frondosa
3.3.1. Occurrence and Ecology
3.3.2. Anti-Cancer Properties of Bioactive Components from G. frondosa
3.4. Ganoderma lucidum
3.4.1. Occurrence and Ecology
3.4.2. Anti-Cancer Properties of Bioactive Components from G. lucidum
3.5. Lentinula edodes
3.5.1. Occurrence and Ecology
3.5.2. Anti-Cancer Properties of Bioactive Components from L. edodes
3.6. Laetiporus sulphureus
3.6.1. Occurrence and Ecology
3.6.2. Anti-Cancer Properties of Bioactive Components from L. sulphureus
3.7. Boletus edulis
3.7.1. Occurrence and Ecology
3.7.2. Anti-Cancer Properties of Bioactive Components from B. edulis
3.8. Phellinus igniarius
3.8.1. Occurrence and Ecology
3.8.2. Anti-Cancer Properties of Bioactive Components from P. igniarius
4. Conclusions and Perspective
4.1. Conclusions
4.2. Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef] [PubMed]
- Miao, K.; Liu, W.; Xu, J.; Qian, Z.; Zhang, Q. Harnessing the power of traditional Chinese medicine monomers and compound prescriptions to boost cancer immunotherapy. Front. Immunol. 2023, 14, 1277243. [Google Scholar] [CrossRef] [PubMed]
- Condello, M.; Meschini, S. Role of natural sntioxidant products in colorectal cancer fisease: A focus on a natural compound derived from Prunus spinosa, Trigno Ecotype. Cells 2021, 10, 3326. [Google Scholar] [CrossRef]
- Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; Jaén, C.R.; et al. Vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer: US preventive services task force recommendation statement. Jama 2022, 327, 2326–2333. [Google Scholar] [CrossRef]
- Ba, D.M.; Ssentongo, P.; Beelman, R.B.; Muscat, J.; Gao, X.; Richie, J.P. Higher mushroom consumption is associated with lower risk of cancer: A systematic review and meta-analysis of observational studies. Adv. Nutr. 2021, 12, 1691–1704. [Google Scholar] [CrossRef]
- Motta, F.; Gershwin, M.E.; Selmi, C. Mushrooms and immunity. J. Autoimmun. 2021, 117, 102576. [Google Scholar] [CrossRef]
- Ahmad, R.; Riaz, M.; Khan, A.; Aljamea, A.; Algheryafi, M.; Sewaket, D.; Alqathama, A. Ganoderma lucidum (Reishi) an edible mushroom; a comprehensive and critical review of its nutritional, cosmeceutical, mycochemical, pharmacological, clinical, and toxicological properties. Phytother. Res. 2021, 35, 6030–6062. [Google Scholar] [CrossRef]
- Gariboldi, M.B.; Marras, E.; Ferrario, N.; Vivona, V.; Prini, P.; Vignati, F.; Perletti, G. Anti-cancer potential of rdible/medicinal mushrooms in breast cancer. Int. J. Mol. Sci. 2023, 24, 10120. [Google Scholar] [CrossRef]
- Gao, H.; Shi, D.; Yin, C.; Fan, X.; Cheng, X.; Qiao, X.; Liu, C.; Hu, G.; Yao, F.; Qiu, J.; et al. A highly branched glucomannan from the fruiting body of Schizophyllum commune: Structural characteristics and antitumor properties analysis. Int. J. Biol. Macromol. 2024, 282, 137460. [Google Scholar] [CrossRef]
- Jian, L.; Zhicheng, H. Polysaccharide peptide induced colorectal cancer cells apoptosis by down-regulating EGFR and PD-L1 expression. Iran. J. Pharm. Res. 2022, 21, e123909. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, R.; Liu, F.; Ng, T.B. Purification and characterization of a novel protein with activity against non-small-cell lung cancer in vitro and in vivo from the edible mushroom Boletus edulis. Int. J. Biol. Macromol. 2021, 174, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Larypoor, M. Investigation of HER-3 gene expression under the influence of carbohydrate biopolymers extract of shiitake and reishi in MCF-7 cell line. Mol. Biol. Rep. 2022, 49, 6563–6572. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lu, J.L.; Liao, W.F.; Long, Y.R.; Zhang, X.; Zhu, Q.; Lu, H.L.; Hao, G.Y.; Ding, K.; Sun, J.H.; et al. GFPBW1, a β-glucan from Grifola frondosa as vaccine adjuvant: APCs activation and maturation. Acta Pharmacol. Sin. 2024, 45, 2394–2404. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, C.; Xu, T.; Xiang, X.; Amakye, W.K.; Zhao, Z.; Yao, M.; Zhu, Y.; Ren, J. A water polysaccharide-protein complex from Grifola frondosa inhibit the growth of subcutaneous but not peritoneal colon tumor under fasting condition. Mol. Nutr. Food Res. 2024, 68, e2400023. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Sheng, Z.; Fang, Z.; Fu, Y.; Wang, N.; Yang, B.; Xu, B. Latest research progress on anti-microbial effects, mechanisms of action, and product developments of dietary flavonoids: A systematic literature review. Trends Food Sci. Tech. 2024, 156, 104839. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Xu, B. Insights into the anticancer effects of galangal and galangin: A comprehensive review. Phytomedicine 2024, 135, 156085. [Google Scholar] [CrossRef]
- Sun, T.K.; Huang, W.C.; Sun, Y.W.; Deng, J.S.; Chien, L.H.; Chou, Y.N.; Jiang, W.P.; Lin, J.G.; Huang, G.J. Schizophyllum commune Reduces Expression of the SARS-CoV-2 Receptors ACE2 and TMPRSS2. Int. J. Mol. Sci. 2022, 23, 14766. [Google Scholar] [CrossRef]
- Zheng, S.X.; Chen, J.P.; Liang, R.S.; Zhuang, B.B.; Wang, C.H.; Zhang, G.L.; Shi, S.S.; Chen, J. Schizophyllum commune fruiting body polysaccharides inhibit glioma by mediating ARHI regulation of PI3K/AKT signalling pathway. Int. J. Biol. Macromol. 2024, 279, 135326. [Google Scholar] [CrossRef]
- Ekowati, N.; Mumpuni, A.; Ratnaningtyas, N.I.; Maharning, A.R. Compounds detection and inhibition activity of chloroform and ethyl acetate extracts of Schizophyllum commune on some cancer cell types. Biodiversitas 2020, 21, 5865–5871. [Google Scholar] [CrossRef]
- Alqurashi, Y.E.; Almalki, S.G.; Ibrahim, I.M.; Mohammed, A.O.; Abd El Hady, A.E.; Kamal, M.; Fatima, F.; Iqbal, D. Biological synthesis, characterization, and therapeutic potential of S. commune-mediated gold nanoparticles. Biomolecules 2023, 13, 1785. [Google Scholar] [CrossRef] [PubMed]
- Ansari, E.; Alvandi, H.; Kianirad, S.; Hatamian-Zarmi, A.; Mokhtari-Hosseini, Z.B. Research progress on production and biomedical applications of Schizophyllan as a tailor-made polysaccharide: A review. Carbohydr. Polym. 2025, 348, 122770. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Trametes versicolor (Synn. Coriolus versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef]
- Ajibola, O.O.; Nolasco-Hipolito, C.; Carvajal-Zarrabal, O.; Salleh, S.F.; Adeyinka, G.C.; Adefegha, S.A.; Ahmmed, M.K.; Sumaiya, K.; Thomas, R. Turkey tail mushroom (Trametes versicolor): An edible macrofungi with immense medicinal properties. Curr. Opin. Food Sci. 2024, 58, 101191. [Google Scholar] [CrossRef]
- He, Z.; Lin, J.; He, Y.; Liu, S. Polysaccharide-peptide from trametes versicolor: The potential medicine for colorectal cancer treatment. Biomedicines 2022, 10, 2841. [Google Scholar] [CrossRef]
- Lowenthal, R.; Taylor, M.; Gidden, J.A.; Heflin, B.; Lay, J.O., Jr.; Avaritt, N.; Tackett, A.J.; Urbaniak, A. The mycelium of the Trametes versicolor synn. Coriolus versicolor (Turkey tail mushroom) exhibit anti-melanoma activity in vitro. Biomed. Pharmacother. 2023, 161, 114424. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Newburg, D.S. Musarin, a novel protein with tyrosine kinase inhibitory activity from Trametes versicolor, inhibits colorectal cancer stem cell growth. Biomed. Pharmacother. 2021, 144, 112339. [Google Scholar] [CrossRef]
- Njue, A.W.; Omolo, J.; Ramos, R.S.; Santos, C.B.R.; Kimani, N.M. Ergostanes from the mushroom Trametes versicolor and their cancer cell inhibition: In vitro and in silico evaluation. Steroids 2024, 212, 109511. [Google Scholar] [CrossRef]
- Miletić, D.; Turło, J.; Podsadni, P.; Sknepnek, A.; Szczepańska, A.; Klimaszewska, M.; Malinowska, E.; Lević, S.; Nedović, V.; Nikšić, M. Production of bioactive selenium enriched crude exopolysaccharides via selenourea and sodium selenite bioconversion using Trametes versicolor. Food Biosci. 2021, 42, 101046. [Google Scholar] [CrossRef]
- Yang, C.L.-H.; Chik, S.C.-C.; Lau, A.S.-Y.; Chan, G.C.-F. Coriolus versicolor and its bioactive molecule are potential immunomodulators against cancer cell metastasis via inactivation of MAPK pathway. J. Ethnopharmacol. 2023, 301, 115790. [Google Scholar] [CrossRef]
- Jędrzejewski, T.; Pawlikowska, M.; Sobocińska, J.; Wrotek, S. Protein-bound polysaccharides from Coriolus Versicolor fungus disrupt the crosstalk between breast cancer cells and macrophages through inhibition of angiogenic cytokines production and shifting tumour-associated macrophages from the M2 to M1 subtype. Cell Physiol. Biochem. 2020, 54, 615. [Google Scholar]
- Jędrzejewski, T.; Sobocińska, J.; Maciejewski, B.; Slovakova, M.; Wrotek, S. Enhanced anti-cancer potential: Investigating the combined effects with Coriolus versicolor extract and phosphatidylinositol 3-kinase inhibitor (LY294002) In Vitro. Int. J. Mol. Sci. 2025, 26, 1556. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.; Mehmood, T.; Kumar, S.; Altaf, U.; Sharma, Y.P. 3 wild medicinal mushrooms. In Wild Mushrooms and Health: Diversity, Phytochemistry, Medicinal Benefits, and Cultivation; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Camilleri, E.; Blundell, R.; Baral, B.; Karpiński, T.M.; Aruci, E.; Atrooz, O.M. Unveiling the full spectrum of maitake mushrooms: A comprehensive review of their medicinal, therapeutic, nutraceutical, and cosmetic potential. Heliyon 2024, 10, e30254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dong, X.; Ji, H.; Yu, J.; Liu, A. Preparation and structural characterization of acid-extracted polysaccharide from Grifola frondosa and antitumor activity on S180 tumor-bearing mice. Int. J. Biol. Macromol. 2023, 234, 123302. [Google Scholar] [CrossRef]
- Wan, C.; Xu, Y.-Y.; Chen, L.; Liu, W.; Wang, X.; Gu, Q.; Zhou, T. Anti-tumor and immunomodulatory activities of a novel polysaccharide from Grifola frondosa prepared by hydrogen peroxide/vitamin C-assisted extraction. J. Food Meas. Charact. 2024, 18, 7402–7417. [Google Scholar] [CrossRef]
- Huo, Y.; Ding, W.-J.; Liu, Y.-R.; Li, Z.-T.; Dai, K.-Y.; Liu, C.; Ji, H.-Y.; Liu, A.-J. Selenochemical modification of low molecular weight polysaccharides from Grifola frondosa and the mechanism of their inhibitory effects on gastric cancer cells. Int. J. Biol. Macromol. 2024, 269, 131812. [Google Scholar] [CrossRef]
- González, A.; Atienza, V.; Montoro, A.; Soriano, J.M. Use of Ganoderma lucidum (Ganodermataceae, Basidiomycota) as radioprotector. Nutrients 2020, 12, 1143. [Google Scholar] [CrossRef]
- Yin, Z.; Liang, Z.; Li, C.; Wang, J.; Ma, C.; Kang, W. Immunomodulatory effects of polysaccharides from edible fungus: A review. Food Sci. Hum. Wellness 2021, 10, 393–400. [Google Scholar] [CrossRef]
- Wen, L.; Sheng, Z.; Wang, J.; Jiang, Y.; Yang, B. Structure of water-soluble polysaccharides in spore of Ganoderma lucidum and their anti-inflammatory activity. Food Chem. 2022, 373, 131374. [Google Scholar] [CrossRef]
- Han, W.; Chen, H.; Zhou, L.; Zou, H.; Luo, X.; Sun, B.; Zhuang, X. Polysaccharides from Ganoderma Sinense-rice bran fermentation products and their anti-tumor activities on non-small-cell lung cancer. BMC Complement. Med. 2021, 21, 169. [Google Scholar] [CrossRef]
- de Camargo, M.R.; Inacio, K.K.; Frazon, T.F.; Amôr, N.G.; Corrêa, L.E.; Costa, F.C.; Quagliato, E.N.; Rodini, C.O.; Lara, V.S. Ganoderma lucidum polysaccharides associated with 5-Fluorouracil impair OSCC tumorigenesis in vitro. Pharmacol. Res. Mod. Chin. Med. 2023, 9, 100310. [Google Scholar] [CrossRef]
- Cancemi, G.; Caserta, S.; Gangemi, S.; Pioggia, G.; Allegra, A. Exploring the therapeutic potential of ganoderma lucidum in cancer. J. Clin. Med. 2024, 13, 1153. [Google Scholar] [CrossRef] [PubMed]
- Jędrzejewski, T.; Pawlikowska, M.; Sobocińska, J.; Wrotek, S. COVID-19 and cancer diseases-The potential of Coriolus versicolor mushroom to combat global health challenges. Int. J. Mol. Sci. 2023, 24, 4864. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jiang, L.; Zhang, Z.; He, Y.; Teng, Y.; Li, J.; Yuan, S.; Pan, Y.; Liang, H.; Yang, H. Pancreatic cancer cell apoptosis is induced by a proteoglycan extracted from Ganoderma lucidum. Oncol. Lett. 2021, 21, 34. [Google Scholar] [CrossRef]
- Rahimnia, R.; Akbari, M.R.; Yasseri, A.F.; Taheri, D.; Mirzaei, A.; Ghajar, H.A.; Farashah, P.D.; Baghdadabad, L.Z.; Aghamir, S.M.K. The effect of Ganoderma lucidum polysaccharide extract on sensitizing prostate cancer cells to flutamide and docetaxel: An in vitro study. Sci. Rep. 2023, 13, 18940. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Q.; Lv, B.; Li, N.; Bian, X.; Chen, L.; Kong, M.; Shen, Y.; Zheng, W.; Zhang, J. Ganoderma lucidum polysaccharide supplementation significantly activates T-cell-mediated antitumor immunity and enhances anti-PD-1 immunotherapy efficacy in colorectal cancer. J. Agric. Food Chem. 2024, 72, 12072–12082. [Google Scholar] [CrossRef]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef]
- Hsu, W.-H.; Qiu, W.-L.; Tsao, S.-M.; Tseng, A.-J.; Lu, M.-K.; Hua, W.-J.; Cheng, H.-C.; Hsu, H.-Y.; Lin, T.-Y. Effects of WSG, a polysaccharide from Ganoderma lucidum, on suppressing cell growth and mobility of lung cancer. Int. J. Biol. Macromol. 2020, 165, 1604–1613. [Google Scholar] [CrossRef]
- Qiu, W.; Hsu, W.; Tsao, S.; Tseng, A.; Lin, Z.; Hua, W.; Yeh, H.; Lin, T.; Chen, C.; Chen, L. Wsg, a glucose-rich polysaccharide from Ganoderma lucidum, combined with cisplatin potentiates inhibition of lung cancer in vitro and in vivo. Polymers 2021, 13, 4353. [Google Scholar] [CrossRef]
- Fang, L.; Zhao, Q.; Guo, C.; Guo, D.; Li, Z.; Xu, J.; Guo, C.; Sang, T.; Wang, Y.; Chen, J. Removing the sporoderm from the sporoderm-broken spores of Ganoderma lucidum improves the anticancer and immune-regulatory activity of the water-soluble polysaccharide. Front. Nutr. 2022, 9, 1006127. [Google Scholar] [CrossRef]
- Lee, X.J.; Lim, H.N.; Gowthaman, N.; Rahman, M.B.A.; Abdullah, C.A.C.; Muthoosamy, K. In-situ surface functionalization of superparamagnetic reduced graphene oxide–Fe3O4 nanocomposite via Ganoderma lucidum extract for targeted cancer therapy application. Appl. Surf. Sci. 2020, 512, 145738. [Google Scholar] [CrossRef]
- Zheng, D.; Zhao, J.; Tao, Y.; Liu, J.; Wang, L.; He, J.; Lei, J.; Liu, K. pH and glutathione dual responsive nanoparticles based on Ganoderma lucidum polysaccharide for potential programmable release of three drugs. Chem. Eng. J. 2020, 389, 124418. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Zhang, A.; Liu, W.; Wang, X.; Zhang, F.; Linhardt, R.J.; Lin, Z.; Sun, P. Sustained release of Ganoderma lucidum antitumor drugs using a sandwich structured material prepared by electrospinning. J. Drug Deliv. Sci. Technol. 2021, 64, 102627. [Google Scholar] [CrossRef]
- Shin, M.-J.; Chae, H.-J.; Lee, J.W.; Koo, M.H.; Kim, H.-J.; Seo, J.B.; YanIllia, S.; Park, S.H.; Lo, H.E.; Kim, S.-H. Lucidumol A, purified directly from Ganoderma lucidum, exhibits anticancer effect and cellular inflammatory response in colorectal cancer. Evid.-Based Complement. Altern. Med. 2022, 2022, 7404493. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, S.; Peng, B.; Tan, D.; Wu, M.; Wei, J.; Wang, Y.; Luo, H. Ganoderma lucidum: A comprehensive review of phytochemistry, efficacy, safety and clinical study. Food Sci. Hum. Wellness 2024, 13, 568–596. [Google Scholar] [CrossRef]
- Liu, C.; Guo, M.; Wang, J.; Sun, Y.; Bian, Y.; Xu, Z. Prevalence and diversity of mycoviruses occurring in Chinese Lentinula edodes germplasm resource. Virology 2023, 582, 71–82. [Google Scholar] [CrossRef]
- Roszczyk, A.; Turło, J.; Zagożdżon, R.; Kaleta, B. Immunomodulatory properties of polysaccharides from Lentinula edodes. Int. J. Mol. Sci. 2022, 23, 8980. [Google Scholar] [CrossRef]
- Park, H.-J.; Boo, S.; Park, I.; Shin, M.S.; Takahashi, T.; Takanari, J.; Homma, K.; Kang, I. AHCC®, a standardized extract of cultured Lentinula edodes mycelia, promotes the anti-tumor dffect of dual immune checkpoint blockade effect in murine colon cancer. Front. Immunol. 2022, 13, 875872. [Google Scholar] [CrossRef]
- Islam, S.; Kitagawa, T.; Baron, B.; Kuhara, K.; Nagayasu, H.; Kobayashi, M.; Chiba, I.; Kuramitsu, Y. A standardized extract of cultured Lentinula edodes mycelia downregulates cortactin in gemcitabine-resistant pancreatic cancer cells. Oncol. Lett. 2021, 22, 654. [Google Scholar] [CrossRef]
- Yamashita, S.-N.; Tanaka, Y.; Kitagawa, T.; Baron, B.; Tokuda, K.; Paudel, D.; Nakagawa, K.; Ohta, T.; Hamada, J.-I.; Kobayashi, M. Down-regulating effect of a standardized extract of cultured lentinula edodes mycelia on cortactin in prostate cancer cells is dependent on malignant potential. Anticancer. Res. 2023, 43, 1159–1166. [Google Scholar] [CrossRef]
- Imam, K.M.S.U.; Xie, Y.; Liu, Y.; Wang, F.; Xin, F. Extraction, isolation, and identification of cytotoxic secondary metabolites from shiitake mushroom 808 Lentinula edodes (Berk.). ACS Food Sci. Technol. 2021, 1, 551–558. [Google Scholar] [CrossRef]
- Gorska, S.; Maksymiuk, A.; Turło, J. Selenium-containing polysaccharides—Structural diversity, biosynthesis, chemical modifications and biological activity. Appl. Sci. 2021, 11, 3717. [Google Scholar] [CrossRef]
- Yehia, R. Evaluation of the biological activities of β-glucan isolated from Lentinula edodes. Lett. Appl. Microbiol. 2022, 75, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.; Rutckeviski, R.; Villalva, M.; Abreu, H.; Soler-Rivas, C.; Santoyo, S.; Iacomini, M.; Smiderle, F.R. Isolation and comparison of α-and β-D-glucans from shiitake mushrooms (Lentinula edodes) with different biological activities. Carbohydr. Polym. 2020, 229, 115521. [Google Scholar] [CrossRef]
- Vetchinkina, E.; Fomin, A.; Navolokin, N.; Shirokov, A. Proteins and polysaccharides from vegetative mycelium of medicinal basidiomycete Lentinus edodes display cytotoxicity towards human and animal cancer cell lines. Int. J. Biol. Macromol. 2022, 195, 398–411. [Google Scholar] [CrossRef]
- Din, S.R.U.; Nisar, M.A.; Ramzan, M.N.; Saleem, M.Z.; Ghayas, H.; Ahmad, B.; Batool, S.; Kifayat, K.; Guo, X.; Huang, M. Latcripin-7A from Lentinula edodes C91-3 induces apoptosis, autophagy, and cell cycle arrest at G1 phase in human gastric cancer cells via inhibiting PI3K/Akt/mTOR signaling. Eur. J. Pharmacol. 2021, 907, 174305. [Google Scholar] [CrossRef]
- Kaleta, B.; Zielniok, K.; Roszczyk, A.; Turło, J.; Zagożdżon, R. Selenopolysaccharide Isolated from Lentinula edodes Mycelium Affects Human T-Cell Function. Int. J. Mol. Sci. 2024, 25, 11576. [Google Scholar] [CrossRef]
- Roszczyk, A.; Zych, M.; Zielniok, K.; Krata, N.; Turło, J.; Klimaszewska, M.; Zagożdżon, R.; Kaleta, B. The effect of novel selenopolysaccharide isolated from lentinula edodes mycelium on human T lymphocytes activation, proliferation, and cytokines synthesis. Biomolecules 2022, 12, 1900. [Google Scholar] [CrossRef]
- Kaleta, B.; Roszczyk, A.; Zych, M.; Kniotek, M.; Zagożdżon, R.; Klimaszewska, M.; Malinowska, E.; Pac, M.; Turło, J. Selective biological effects of selenium-enriched polysaccharide (Se-Le-30) isolated from Lentinula edodes mycelium on human immune cells. Biomolecules 2021, 11, 1777. [Google Scholar] [CrossRef]
- Kałucka, M.; Roszczyk, A.; Klimaszewska, M.; Kaleta, B.; Drelich, E.; Błażewicz, A.; Górska-Jakubowska, S.; Malinowska, E.; Król, M.; Prus, A.M. Optimization of Se-and Zn-Enriched Mycelium of Lentinula edodes (Berk.) Pegler as a Dietary Supplement with Immunostimulatory Activity. Nutrients 2023, 15, 4015. [Google Scholar] [CrossRef]
- Górska-Jakubowska, S.; Klimaszewska, M.; Podsadni, P.; Kaleta, B.; Zagożdżon, R.; Górska, S.; Gamian, A.; Strączek, T.; Kapusta, C.; Cieślak, M. Selenium-containing exopolysaccharides isolated from the culture medium of Lentinula edodes: Structure and biological activity. Int. J. Mol. Sci. 2021, 22, 13039. [Google Scholar] [CrossRef] [PubMed]
- Adamska, I. The possibility of using sulphur shelf fungus (Laetiporus sulphureus) in the food industry and in medicine-A review. Foods 2023, 12, 1539. [Google Scholar] [CrossRef] [PubMed]
- Jen, C.I.; Lu, M.K.; Lai, M.N.; Ng, L.T. Sulfated polysaccharides of Laetiporus sulphureus fruiting bodies exhibit anti-breast cancer activity through cell cycle arrest, apoptosis induction, and inhibiting cell migration. J. Ethnopharmacol. 2024, 321, 117546. [Google Scholar] [CrossRef] [PubMed]
- Jen, C.I.; Ng, L.T. Physicochemical properties of different sulfated polysaccharide components from Laetiporus sulphureus and their anti-proliferative effects on MDA-MB-231 breast cancer cells. J. Fungi 2024, 10, 457. [Google Scholar] [CrossRef]
- Petrović, J.; Glamočlija, J.; Ilić-Tomić, T.; Soković, M.; Robajac, D.; Nedić, O.; Pavić, A. Lectin from Laetiporus sulphureus effectively inhibits angiogenesis and tumor development in the zebrafish xenograft models of colorectal carcinoma and melanoma. Int. J. Biol. Macromol. 2020, 148, 129–139. [Google Scholar] [CrossRef]
- Hassan, K.; Matio Kemkuignou, B.; Stadler, M. Two new triterpenes from basidiomata of the medicinal and rdible mushroom, Laetiporus sulphureus. Molecules 2021, 26, 7090. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, F.; Ng, T.B. Interrelationship among paraptosis, apoptosis and autophagy in lung cancer A549 cells induced by BEAP, an antitumor protein isolated from the edible porcini mushroom Boletus edulis. Int. J. Biol. Macromol. 2021, 188, 313–322. [Google Scholar] [CrossRef]
- Meng, T.; Yu, S.-S.; Ji, H.-Y.; Xu, X.-M.; Liu, A.-J. A novel acid polysaccharide from Boletus edulis: Extraction, characteristics and antitumor activities in vitro. Glycoconj. J. 2021, 38, 13–24. [Google Scholar] [CrossRef]
- Quero, J.; Paesa, M.; Morales, C.; Mendoza, G.; Osada, J.; Teixeira, J.A.; Ferreira-Santos, P.; Rodríguez-Yoldi, M.J. Biological properties of boletus edulis extract on Caco-2 cells: Antioxidant, anticancer, and anti-inflammatory effects. Antioxidants 2024, 13, 908. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Balik, M.; Muszyńska, B. Selected species of the genus Phellinus–chemical composition, biological activity, and medicinal applications. Chem. Biodivers. 2021, 18, e2100609. [Google Scholar] [CrossRef]
- Wang, J.H.; Wang, J.J.; Ju, T.Y.; Huang, Y.X.; Yuan, L.X.; Luo, Y.H.; Chen, Y.J.; Wang, Z.B. Analysis of phellinus igniarius effects on gastric cancer cells by atomic force microscopy. Micron 2023, 164, 103376. [Google Scholar] [CrossRef] [PubMed]
- Boateng, I.D.; Guo, Y.-Z.; Yang, X.-M. Extraction, purification, structural characterization, and antitumor effects of water-soluble intracellular polysaccharide (IPSW-1) from Phellinus igniarius mycelia. J. Agric. Food Chem. 2024, 72, 19721–19732. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Chen, N.; Zhang, T.; Fang, Q.; Hu, Y.; Tao, J.; Lin, H. Phellinus igniarius polysaccharides induced mitochondrial apoptosis of hepatic carcinoma by enhancing reactive oxygen species-mediated AKT/p53 signalling pathways. Nat. Prod. Res. 2024, 38, 1748–1752. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, P.; Hou, Y.; Liu, Z.; Zhang, X.; Li, N. Osmundacetone modulates mitochondrial metabolism in non-small cell lung cancer cells by hijacking the glutamine/glutamate/α-KG metabolic axis. Phytomedicine 2022, 100, 154075. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, Z.; Mao, H.; Hu, P.; Li, X. Isolation and structure elucidation of polysaccharides from fruiting bodies of mushroom Coriolus versicolor and evaluation of their immunomodulatory effects. Int. J. Biol. Macromol. 2021, 166, 1387–1395. [Google Scholar] [CrossRef]
- Li, X.; Zhu, J.; Wang, T.; Sun, J.; Guo, T.; Zhang, L.; Yu, G.; Xia, X. Antidiabetic activity of Armillaria mellea polysaccharides: Joint ultrasonic and enzyme assisted extraction. Ultrason. Sonochemistry 2023, 95, 106370. [Google Scholar] [CrossRef]
- Li, G.; Liu, X.; Cong, S.; Deng, Y.; Zheng, X. A novel serine protease with anticoagulant and fibrinolytic activities from the fruiting bodies of mushroom Agrocybe aegerita. Int. J. Biol. Macromol. 2021, 168, 631–639. [Google Scholar] [CrossRef]
- Cantoral, A.; Collado-López, S.; Betanzos-Robledo, L.; Lamadrid-Figueroa, H.; García-Martínez, B.A.; Ríos, C.; Díaz-Ruiz, A.; Mariscal-Moreno, R.M.; Téllez-Rojo, M.M. Dietary risk assessment of cadmium exposure through commonly consumed foodstuffs in Mexico. Foods 2024, 13, 3649. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Kapoor, B.; Jain, S.K.; Gowthamarajan, K.; Zacconi, F.; Chellappan, D.K.; Gupta, G.; et al. Development of mushroom polysaccharide and probiotics based solid self-nanoemulsifying drug delivery system loaded with curcumin and quercetin to improve their dissolution rate and permeability: State of the art. Int. J. Biol. Macromol. 2021, 189, 744–757. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, M.; Huang, D. Dietary organosulfur-Containing compounds and their health-promotion mechanisms. Annu. Rev. Food Sci. Technol. 2022, 13, 287–313. [Google Scholar] [CrossRef]
- Lin, J.; Mao, Y.; Mai, L.; Li, G.; Liu, H.; Peng, S.; Wang, D.; Li, Q.; Yu, Z.; Yuan, J.; et al. Accelerating the humification of mushroom waste by regulating nitrogen sources composition: Deciphering mechanism from bioavailability and molecular perspective. Chemosphere 2024, 349, 140816. [Google Scholar] [CrossRef]
- Duan, X.P.; Qin, B.D.; Jiao, X.D.; Liu, K.; Wang, Z.; Zang, Y.S. New clinical trial design in precision medicine: Discovery, development and direction. Signal Transduct. Target. Ther. 2024, 9, 57. [Google Scholar] [CrossRef]

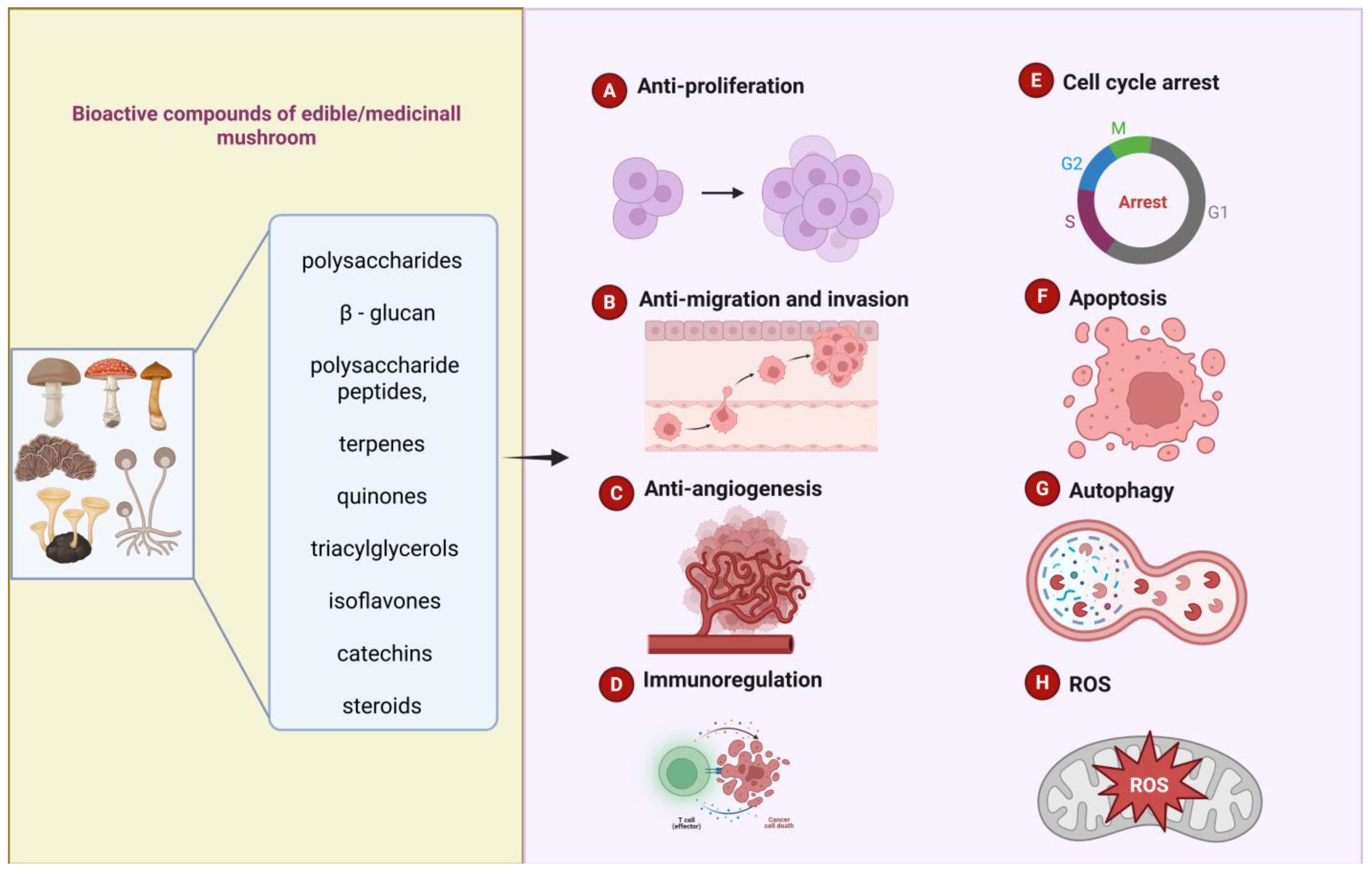
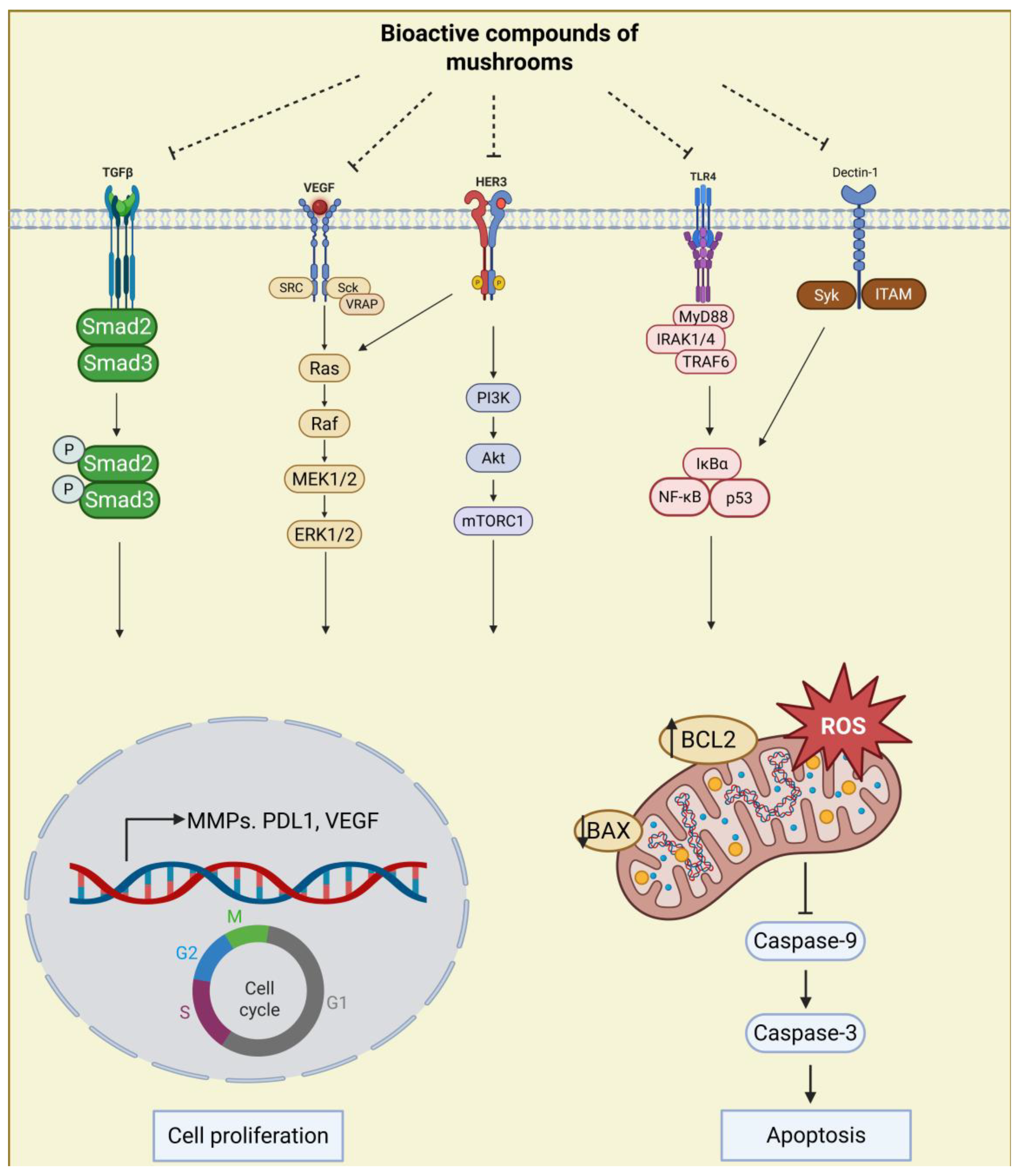
| Mushroom | Bioctive Compounds | Effect | Pathways | Targets | Ref. |
|---|---|---|---|---|---|
| S. commune | SCFP (fruiting body polysaccharide) | Anti-glioma effect; immunomodulation | PI3K/Akt | ↑Apoptosis | [19] |
| S. commune | SCP-1 (mucomannan) | Anti-lung cancer | PI3K/Akt/mTOR, Bax/Bcl-2 | Cell cycle arrest (S phase), ↑Apoptosis | [10] |
| S. commune | Gold nanoparticles (AuNPs) | Anti-lung cancer | ROS-mediated apoptosis | ↑ROS, ↓Cell viability (A549 cells) | [21] |
| S. commune | SPG (schizophyllan, β-glucan) | Immunomodulatory, drug delivery aid | Dectin-1/NF-κB | ↑Cytokines, ↑Macrophage and NK cell activity | [22] |
| S. commune | Mixed extract (terpenoids, flavonoids, alkaloids) | Anti-proliferative | Not specified | HeLa, MCF-7, T47D, WiDr cell inhibition | [20] |
| T. versicolor | PSK, PSP | Broad-spectrum anti-tumor activity | EGFR, PD-L1 | ↓EGFR, ↓PD-L1, ↓STAT3, ↓p-STAT, ↓c-Jun | [23,25] |
| T. versicolor | PSP | Anti-colorectal cancer | EGFR signaling pathway | EGFR↓, c-Jun↓, STAT3↓, p-STAT↓, PD-L1↓,‘ apoptosis↑ | [11] |
| T. versicolor | Musarin (12 kDa peptide) | Anti-CSC in colorectal cancer | EGFR-Raf signaling pathway | ↓EGFR phosphorylation, CSC marker inhibition | [27] |
| T. versicolor | Mycelium ethanol extract | Anti-melanoma | Autophagy/apoptosis pathways | ↑LC3-II, ↑MHC-II, ↑PD-L1, ↑PARP cleavage, ↓Migration | [26] |
| T. versicolor | Steroids (compound 1–5) | Anti-lung, colon, melanoma cancer | Not specified | Broad cytotoxicity on cancer cell lines | [28] |
| G. frondosa | GFPBW1 (β-glucan) | Anti-melanoma, adjuvant effect | Dectin-1/Syk/NF-κB signaling pathway | ↑NF-κB; macrophage activation | [14] |
| G. frondosa | PPC (GFG-4) | Anti-liver cancer | TLR4/NF/κB signaling pathway, gut microbiota modulation | ↑Muribaculaceae, Bacillus; ↓Lactic acid bacteria | [15] |
| G. frondosa | GFP-A | Immunomodulation Tumor inhibition | Not specified | ↑TNF-α, IL-2, IFN-γ; ↑NK cell, macrophage and lymphocyte activity | [35] |
| G. frondosa | GFP1 | Anti-lung cancer | P53/NF/κB signaling pathway, oxidative stress/apoptosis | ↑ROS, ↓MMP, ↑Caspase-3/8/9, ↑p65 phosphorylation | [36] |
| G. frondosa | Se-LMW-GFP | Anti-gastric cancer | Mitochondrial and death receptor | ↑Apoptosis proteins, ↑ROS, ↓MMP, cell cycle arrest in G1 | [37] |
| G.lucidum | GLPS (polysaccharides) | Anti-OSCC (oral squamous cell carcinoma) | CSC/EMT signaling | ↓CSC markers, ↓EMT markers | [42] |
| G.lucidum | FYGL (proteoglycan) | Anti-pancreatic cancer | ROS/autophagy | ↑ROS, ↓autophagy | [45] |
| G. lucidum | GLP + flutamide/docetaxel | Enhanced prostate cancer therapy | Multiple | ↓OPN, VEGF-c, Snail, E-cadherin, KLK2 | [46] |
| G.lucidum | GLP | Anti-colorectal cancer | TLR4/MyD88/NF-κB | ↓TLR4, ↓NF-κB, ↑SCFA, ↓endotoxemia | [48] |
| G.lucidum | GLP + anti-PD-1 | Enhanced immunotherapy | Immune checkpoint modulation | ↑anti-PD-1 efficacy | [47] |
| G.lucidum | GLP | Anti-lung cancer | Akt/ERK1/2/FAK/Smad2 | ↓phosphorylation, ↓EGFR and TGF-β receptors (lysosomal/proteasomal degradation) | [49] |
| G.lucidum | GLP + cisplatin | Synergistic lung cancer inhibition | Not specified | ↑apoptosis, ↓metastasis | [50] |
| G.lucidum | RSGLP vs. BSGLP | Anti-breast/colon/liver/lung cancers | Anti-inflammatory | ↓COX-2, IL-1β, iNOS, TNF-α | [51] |
| G. lucidum | Lucidumol A | Anti-colorectal cancer | Inflammation | COX-2↓, iNOS↓ | [55] |
| G. lucidum | GLE (extract) | Targeted drug delivery | Not specified | Cytotoxic to A549, carrier for rGO-Fe₃O₄/Quercetin | [52] |
| G.lucidum | GLP (RCGDDH NPs) | Anti-tumor nano-delivery system | MMP-9 inhibition | ↓MMP-9, dual pH/redox-responsive delivery | [53] |
| G. lucidum | GLT + GLP (film system | In vitro anti-cancer activity | Not specified | Effective against SGC-7901, A549, Hela, Caco-2 | [54] |
| G. lucidum | GL extract/spore products | Clinical adjuvant (lung/breast cancer) | Immunomodulation | ↑NK cells, ↑CD4/CD8 ratio, ↑QoL | [56] |
| L. edodes | AHCC® (mycelial extract) | Enhanced ICB therapy (anti-tumor effect) | Immunomodulation, microbiome | ↑Perforin, ↑Ki-67 (CD8+ T cells), ↑Ruminococcaceae | [59] |
| L. edodes | AHCC® | Anti-metastatic (pancreatic cancer) | Migration/invasion suppression | ↓Cortactin (CTTN), no change in actin | [60] |
| L. edodes | AHCC® | Anti-metastatic (prostate cancer) | Migration/invasion suppression | ↓Cortactin (LNCaP.FGC, DU145), no effect in PC-3 | [61] |
| L. edodes | Latcripin-7A (LP-7A, peptide) | Anti-gastric cancer | PI3K/Akt/mTOR signaling | ↑Apoptosis, ↑Autophagy, G1 arrest | [67] |
| L. edodes | Glycoprotein fractions | Cytotoxicity toward cancer cell | Not specified | ↓Metabolic activity in A549, HeLa, Hep-2, SPEV-2, C6 cells | [66] |
| L. edodes | Polysaccharides | Anti-breast cancer | p53/HER-3 signaling | ↑p53, ↓HER-3 | [13] |
| L. edodes | Aqueous extract + ILA | Anti-lung cancer | Not specified | Inhibition of A549 cells (via indole-3-lactic acid) | [62] |
| L. edodes | β-glucan | Anti-breast cancer | Immune modulation | ↓IL-1β, ↓IL-6, inhibition of topoisomerase I | [64] |
| T. versicolor | SMCV | Anti-glioblstoma | Anti-cancer effect and anti-invasive ability of T98G cells | ↓TNF-α, ↓MMP3 | [30] |
| T. versicolor | Polysaccharides | Enhance the function of RAW 264.7 macrophages | Immune modulation | ↑iNOS, ↑TNF-α | [86] |
| T. versicolor | PBP | Tumor microenvironment modulation | Macrophage polarization (M2 → M1) | ↑IL-6, ↑TNF-α, ↓IL-10, ↓TGF-β | [31] |
| L. sulphureus | Sulfated polysaccharides (SPS) | Anti-breast cancer | Cell cycle arrest, apoptosis | ↓CDK4, ↓Cyclin D1, ↑p21, G0/G1 arrest | [74,75] |
| L. sulphureus | Lectin (LSL) | Anti-colorectal cancer, anti-melanoma | Anti-angiogenesis, anti-metastasis | ↓VEGF, ↓neovascularization, comparable to sunitinib, no effect on neutrophils | [76] |
| L. sulphureus | Triterpenoids (laetiporins C and D) | Anti-breast cancer | Cell proliferation inhibition | Not specified; general antiproliferative activity on MCF-7 | [77] |
| B. edulis | BEAP (boletus edulis anti-tumor protein) | Anti-lung cancer, anti-metastatic, induces apoptosis, G1 arrest, enhances autophagy | MAPK, mTOR/AMPK, Bax/Bcl-2, caspase-3/-9, DRAM | Bax↑, Bcl-2↓, Cytochrome c↑, Caspase-3/9↑, CDK4↓, E2F↓, Vinculin↓, LC3-II↑, P62↑, Alix↓ | [12] |
| B. edulis | BEP (boletus edulis polysaccharide) acidic cold-water soluble polysaccharide | Anti-lung cancer, induces apoptosis, S and G0/G1 arrest, liver cancer | Mitochondrial apoptosis pathway | ↑Bax/Bcl-2, ↑cytochrome c, ↑caspase-3, cell cycle arrest (S, G0/G1) | [78] |
| B. edulis | BE extract (trehalose, phenolic compounds, minerals) | Anti-colon cancer, induces apoptosis and autophagy, G0/G1 arrest, antioxidant, anti-inflammatory | ROS signaling, apoptosis, autophagy | ↑ROS, ↓COX-2, ↓iNOS, ↑caspase-3, ↓MMP, | [79] |
| P. igniarius | IPSW-1 (intracellular polysaccharide) | Anti-liver cancer (HepG2) | Autophagy and Apoptosis-related pathways | ↓MMP-7, ↓RhoA, ↑LC3-II, ↓Bcl-2, ↑Bax, ↑Cleaved caspase-3 | [83] |
| P. igniarius | PIP (polysaccharide) | Anti-liver cancer (HepG2) | AKT/p53 signaling pathway | ↓p-AKT, ↓Bcl-2, ↑p53, ↑Cytochrome c, ↑Bax, ↑Cleaved caspase-3 | [84] |
| P. igniarius | Osmundacetone (OSC) | Anti-lung cancer (H460, A549) | GLUD1/glutamine metabolism axis | ↓GLUD1, inhibition of glutamine/glutamate/α-KG axis, ↓OXPHOS | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górska-Jakubowska, S.; Wu, Y.; Turło, J.; Xu, B. Critical Review on the Anti-Tumor Activity of Bioactive Compounds from Edible and Medicinal Mushrooms over the Last Five Years. Nutrients 2025, 17, 1887. https://doi.org/10.3390/nu17111887
Górska-Jakubowska S, Wu Y, Turło J, Xu B. Critical Review on the Anti-Tumor Activity of Bioactive Compounds from Edible and Medicinal Mushrooms over the Last Five Years. Nutrients. 2025; 17(11):1887. https://doi.org/10.3390/nu17111887
Chicago/Turabian StyleGórska-Jakubowska, Sandra, Yingzi Wu, Jadwiga Turło, and Baojun Xu. 2025. "Critical Review on the Anti-Tumor Activity of Bioactive Compounds from Edible and Medicinal Mushrooms over the Last Five Years" Nutrients 17, no. 11: 1887. https://doi.org/10.3390/nu17111887
APA StyleGórska-Jakubowska, S., Wu, Y., Turło, J., & Xu, B. (2025). Critical Review on the Anti-Tumor Activity of Bioactive Compounds from Edible and Medicinal Mushrooms over the Last Five Years. Nutrients, 17(11), 1887. https://doi.org/10.3390/nu17111887








