Integrated GPS-Enabled Physical Activity and Dietary Interventions Versus Physical Activity Alone for Obesity Control: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
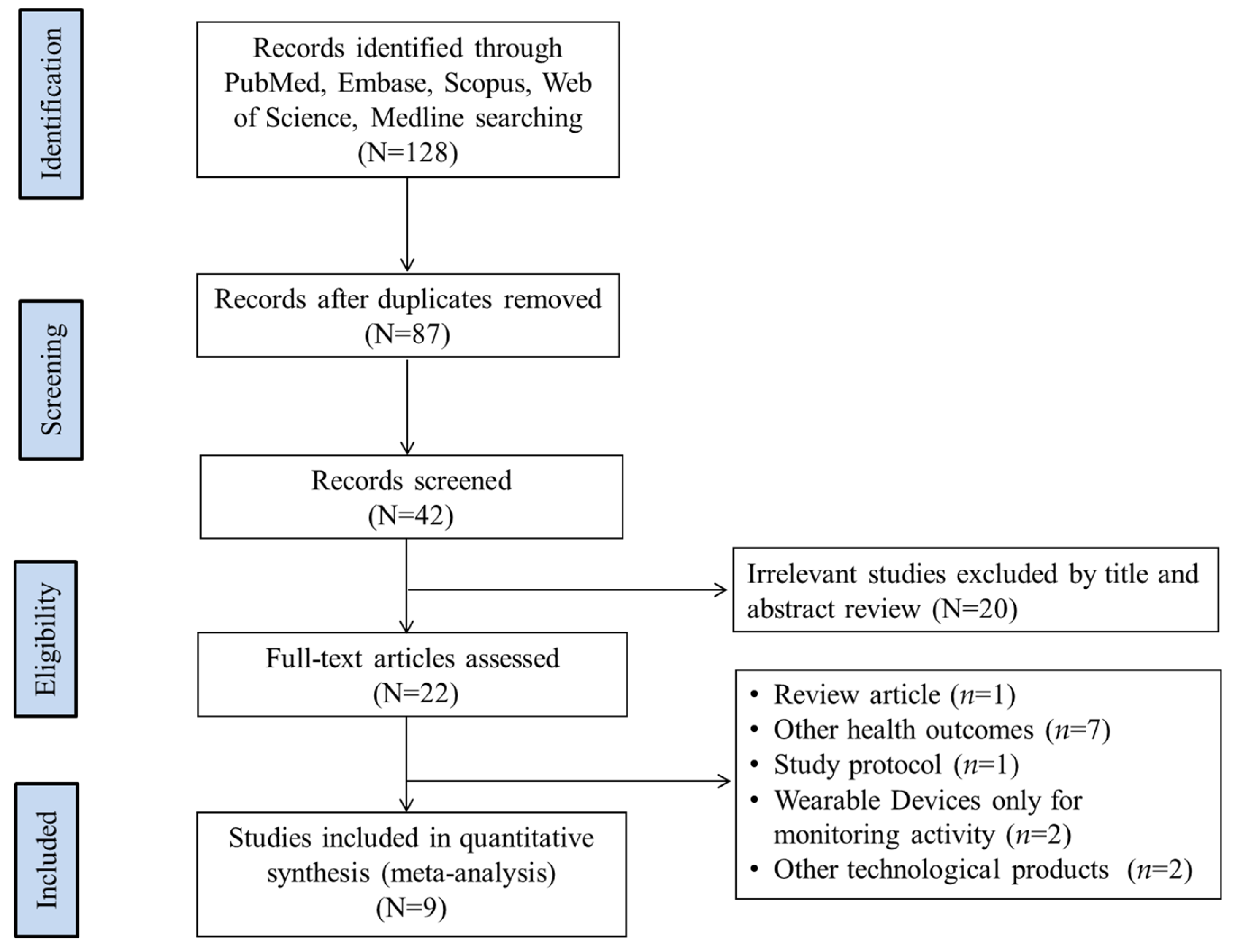
2.2. Study Selection
2.3. Data Extraction and Study Quality Assessment
2.3.1. Data Extraction
2.3.2. Study Quality Assessment
2.4. Data Analysis
3. Results
3.1. Main Study Characteristics and Findings
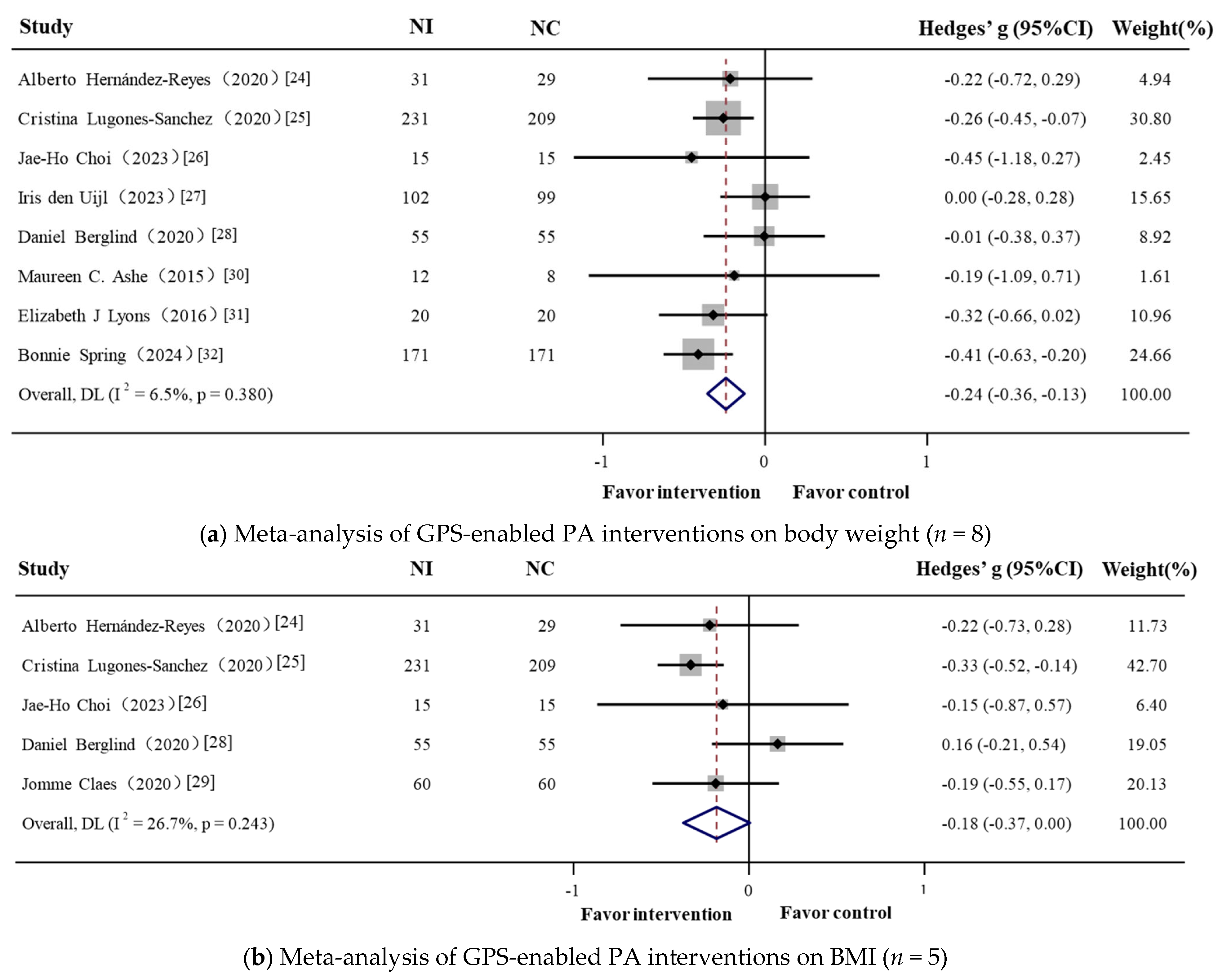
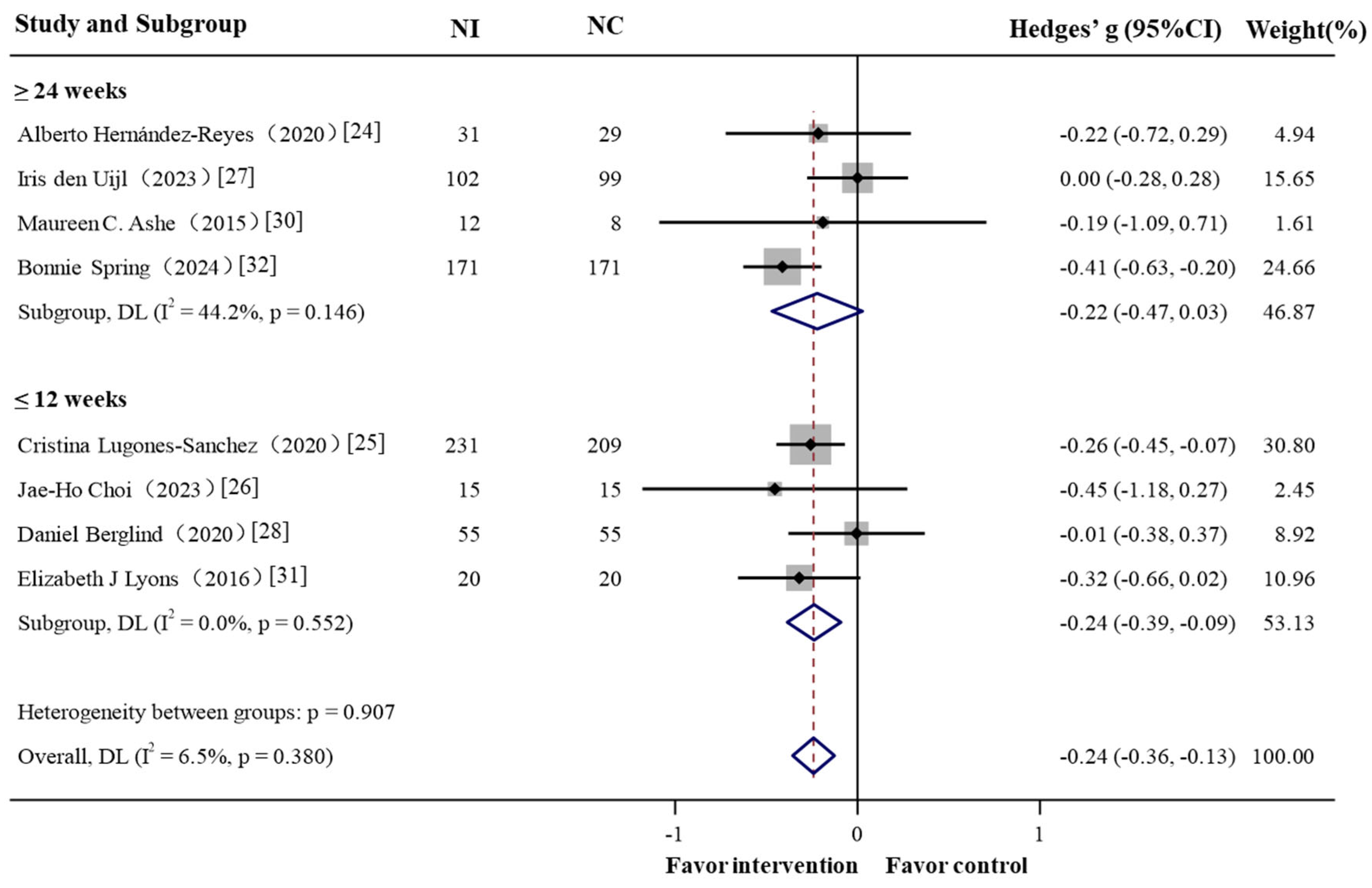
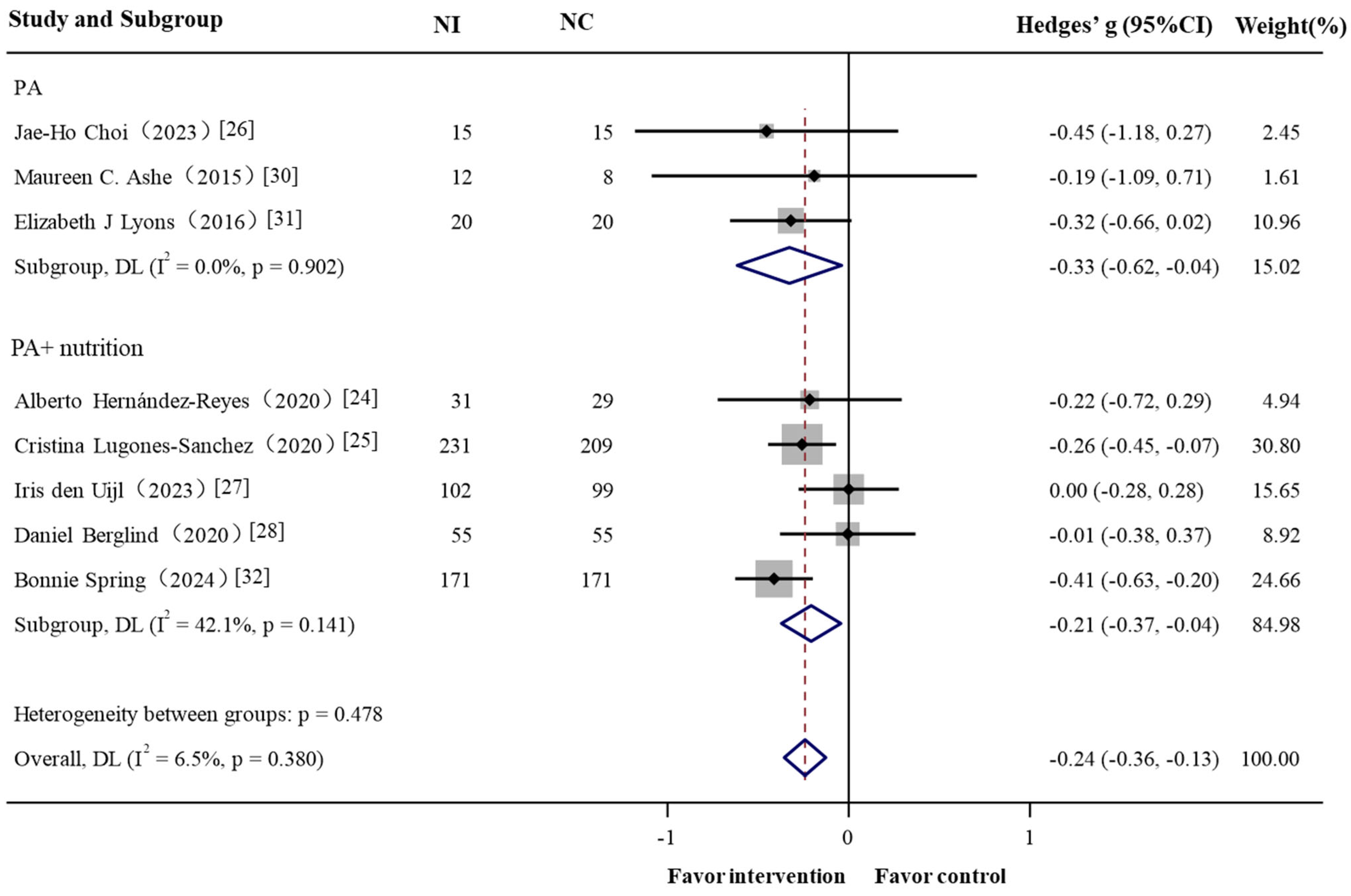
3.2. Meta-Analysis: GPS-Enabled Effects on Obesity-Related Outcomes
3.2.1. Overall
3.2.2. Subgroup Analysis
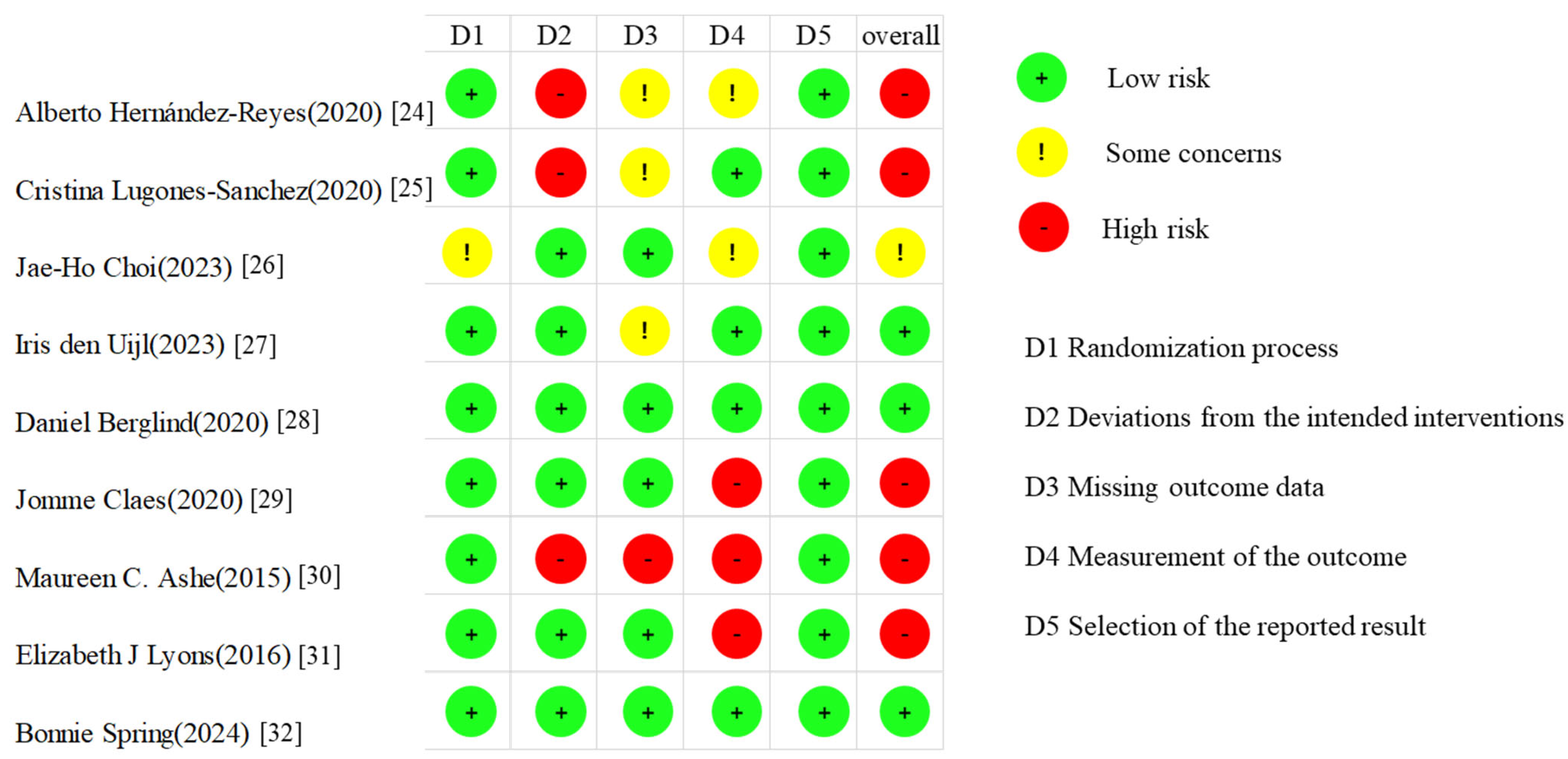
3.3. Risk of Bias Assessment
3.4. Sensitivity Analyses
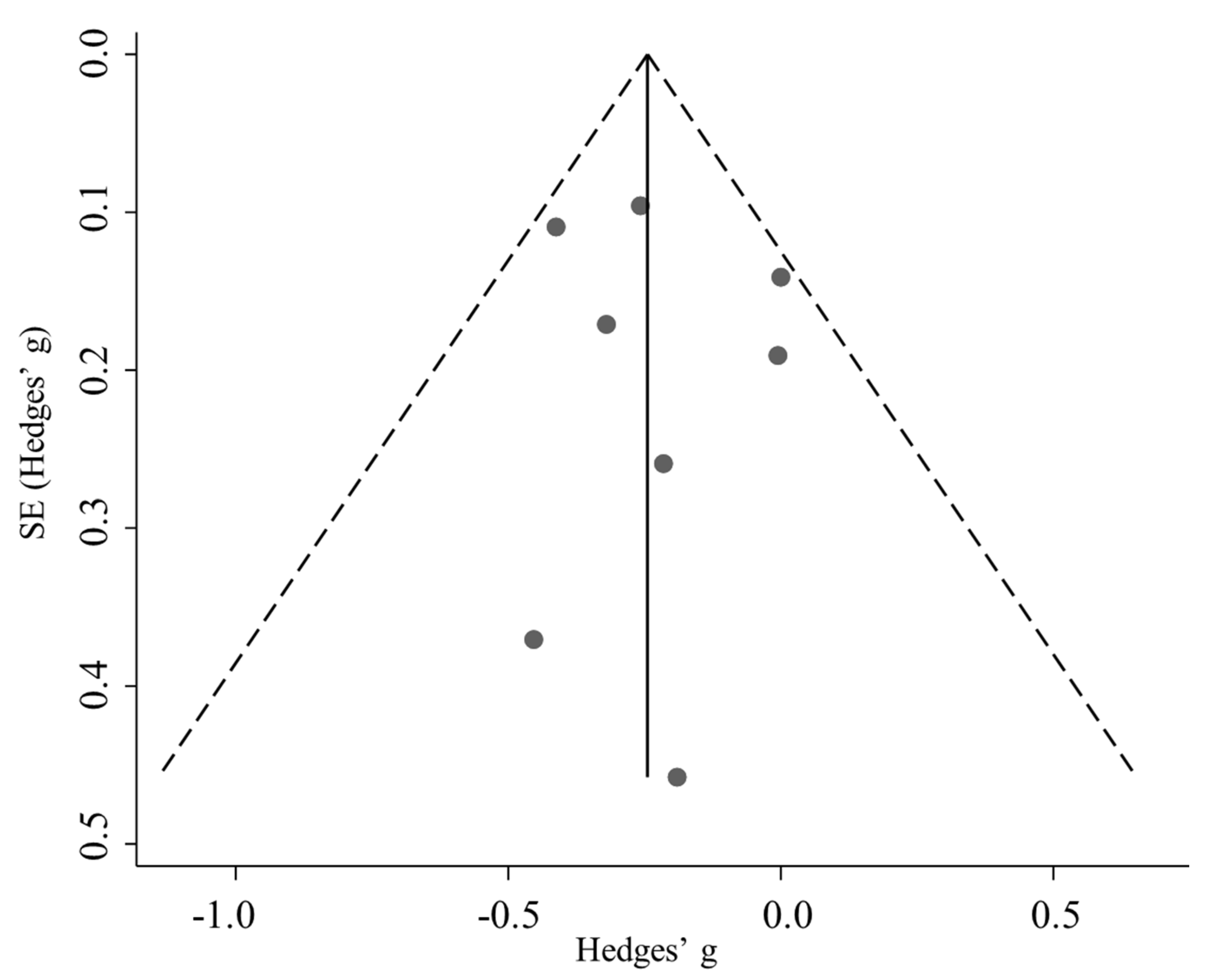
3.5. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PA | Physical Activity |
| IG | Intervention Group |
| CG | Control Group |
| RCT | Random Control Trails |
| ES | Effect Size |
| BW | Body Weight |
| BMI | Body Mass Index |
| BFP | Body Fat Percentage |
| WHR | Waist-to-hip Ratio |
| BFM | Body Fat Mass |
| NR | No Report |
| NA | No Application |
Appendix A
| Study | PA and Dietary Intervention | Effect of Intervention on Weight Status | Key Findings | |||||
|---|---|---|---|---|---|---|---|---|
| PA | Diet | BW | BMI | BFP | WHR | BFM | ||
| Alberto Hernández-Reyes (2020) [24] | Push notifications: exercise recommendations; app: specific functionalities: self-monitoring of weight at home, gamification, or prescription of PA | Push notifications: health tips, such as nutritional properties of specific foods | → | → | ↓ | NR | NR | The intervention group achieved significantly greater fat mass loss compared to the control group, though weight and BMI reductions were similar between groups. |
| Cristina Lugones-Sanchez (2020) [25] | Counselling: gave advice on physical activity App and smart band: record daily physical activity | Counselling: gave advice on healthy diet App: food intake daily | ↓ | ↓ | → | → | → | The mHealth intervention combining a smartphone app and smart band demonstrated greater reductions in weight, BMI, body fat percentage, and fat mass compared to standard counseling alone. No significant changes were observed in waist-to-hip ratio. |
| Jae-Ho Choi (2023) [26] | App and smart band: track physical activity data; mHealth system: exercise interventions | NA | → | → | ↓ | → | ↓ | The 12-week mHealth exercise intervention significantly reduced body fat percentage and fat mass in obese women but did not significantly affect body weight, BMI, or waist-to-hip ratio. |
| Iris den Uijl (2023) [27] | Group intervention: aerobic training with mainly non-weight-bearing exercises App and activity monitor: track physical activity data | Group intervention: nutrition education by dietician | → | NR | NR | NR | NR | The intervention demonstrated short-term (3 month) improvements in weight loss and physical activity compared to standard CR, but these benefits were not sustained long-term. |
| Daniel Berglind (2020) [28] | App: step tracking, home-based bodyweight exercise | App: food photograph | → | ↓ | NR | NR | → | Both the app-based and supervised exercise interventions showed comparable improvements in waist circumference and fat mass, with no significant between-group differences in weight or BMI after 12 weeks. |
| Jomme Claes (2020) [29] | PATHway system: encourage to achieve the PA goal, activity, and monitor activity data | NA | NR | → | → | → | NR | The intervention helped maintain stable cardiovascular risk factors, including body weight, BMI, body fat percentage, and waist-hip ratio, while these measures showed unfavorable trends in the usual care group over six months. No significant between-group differences were observed in absolute changes, though the intervention group demonstrated better stability in metabolic health markers. |
| Maureen C. Ashe (2015) [30] | Individualized physical activity prescription; activity monitor: provides immediate feedback on activities and monitor activity data | NA | ↓ | NR | NR | NR | NR | The intervention group showed significant improvements in weight and diastolic blood pressure compared to the control group, suggesting that reducing sedentary behavior and increasing daily activity may positively influence body composition and cardiovascular health. |
| Elizabeth J Lyons (2016) [31] | Activity monitor and app: set step goals, idle alert, and monitor activity data | NA | → | NR | → | NR | NR | The intervention showed small but favorable effects on weight and body composition (BMI, body fat), though changes were not statistically significant. |
| Bonnie Spring (2024) [32] | App and activity monitor: monitor activity data and automated feedback | App: self-reported diet | ↓ | NR | NR | NR | NR | Participants using the wireless feedback system (WFS) with coaching achieved greater weight loss and BMI reduction compared to WFS alone, though no significant differences were observed in step-up interventions for non-responders. |
| Sample Size | Number of Studies | Meta-Analytic Effect Size | Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| Effect Size (95% CI) | Z-Value | p-Value | I2 (%) | Q | p-Value a | |||
| A. BW | ||||||||
| Total | 1243 | 8 | −0.241 (−0.356, −0.127) | −4.133 | <0.001 | 6.5% | 7.49 | 0.380 |
| Intervention type | ||||||||
| PA | 90 | 3 | −0.328 (−0.616, −0.039) | −2.228 | 0.026 | 0.0% | 0.21 | 0.902 |
| PA + diet | 1153 | 5 | −0.208 (−0.372, −0.044) | −2.481 | 0.013 | 42.1% | 6.91 | 0.141 |
| Gender | ||||||||
| Male and female | 1133 | 5 | −0.224 (−0.381, −0.067) | −2.791 | 0.005 | 44.0% | 7.14 | 0.128 |
| Only female | 110 | 3 | −0.275 (−0.653, 0.102) | −1.429 | 0.153 | 0.0% | 0.32 | 0.853 |
| Age * | ||||||||
| ≤60 | 1183 | 6 | −0.221 (−0.372, −0.070) | −2.877 | 0.004 | 31.2% | 7.26 | 0.202 |
| >60 | 60 | 2 | −0.304 (−0.618, 0.010) | −1.899 | 0.058 | 0.0% | 0.07 | 0.791 |
| Intervention period | ||||||||
| ≤3 month | 623 | 4 | −0.239 (−0.386,−0.092)- | −3.182 | 0.001 | 0.0% | 2.10 | 0.552 |
| ≥6 month | 620 | 4 | 0.221 (−0.469, 0.026) | −1.751 | 0.080 | 44.2% | 5.38 | 0.146 |
| B. BMI | ||||||||
| Total | 760 | 5 | −0.185 (−0.375, 0.005) | −1.911 | 0.056 | 26.7% | 5.46 | 0.243 |
| Intervention type | ||||||||
| PA | 150 | 2 | −0.182 (−0.504, 0.141) | −1.105 | 0.269 | 0.0% | 0.01 | 0.919 |
| PA + diet | 610 | 3 | −0.152 (−0.477, 0.172) | −0.920 | 0.358 | 62.8% | 5.38 | 0.068 |
| Gender | ||||||||
| Male and female | 670 | 3 | −0.153 (−0.440, 0.134) | −1.044 | 0.296 | 63.1% | 5.42 | 0.067 |
| Only female | 90 | 2 | −0.199 (−0.614, 0.215) | −0.941 | 0.347 | 0.0% | 0.03 | 0.866 |
| Age * | ||||||||
| ≤60 | 640 | 4 | −0.161 (−0.424, 0.102) | −1.202 | 0.229 | 44.7% | 5.42 | 0.143 |
| >60 | 120 | 1 | −0.190 (−0.551, 0.171) | −1.033 | 0.302 | - | - | - |
| Intervention period | ||||||||
| ≤3 month | 580 | 3 | 0.163 (−0.211, 0.538) | −0.699 | 0.485 | 63.1% | 5.42 | 0.066 |
| ≥6 month | 180 | 2 | −0.202 (−0.496, 0.093) | −1.343 | 0.179 | 0.0% | 0.01 | 0.914 |
| C. BFP | ||||||||
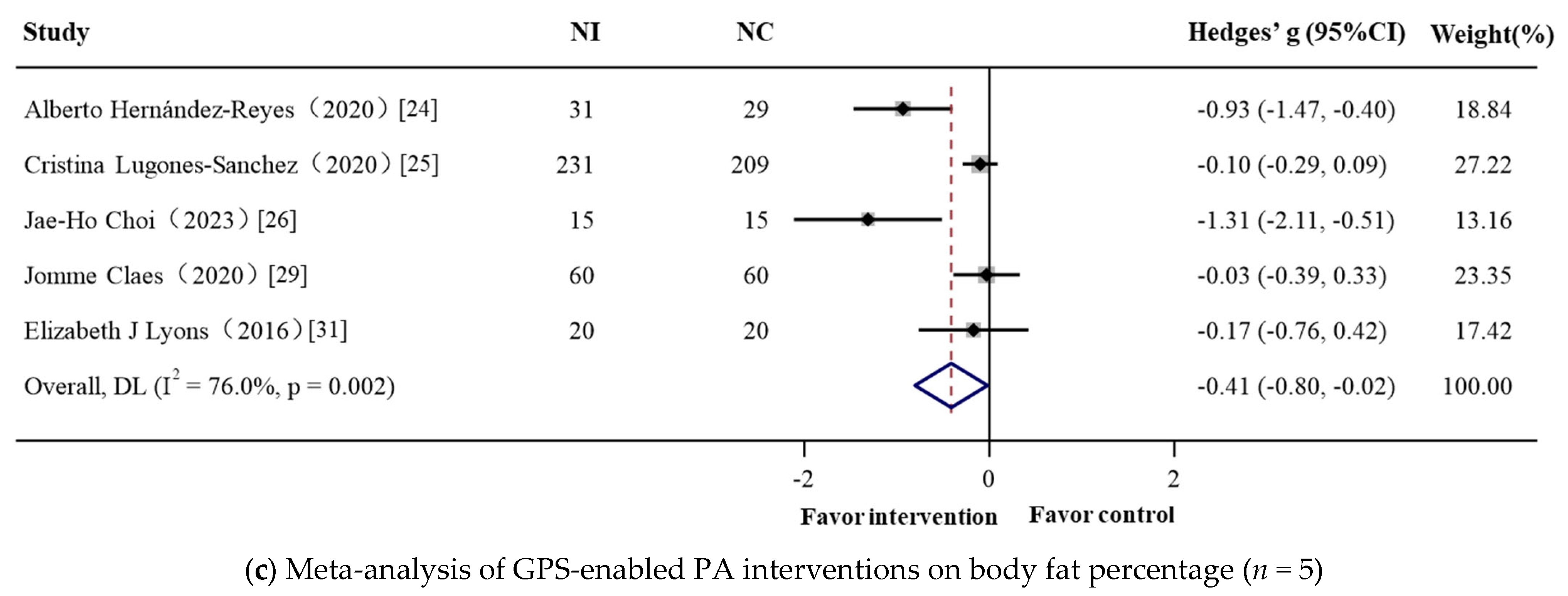
| Total | 690 | 5 | −0.412 (−0.804, −0.020) | −2.059 | 0.039 | 76.0% | 16.66 | 0.002 |
| By type of intervention | ||||||||
| PA | 190 | 3 | −0.425 (−1.091, 0.240) | −1.253 | 0.210 | 75.8% | 8.27 | 0.016 |
| PA + diet | 500 | 2 | −0.477 (−1.292, 0.338) | −1.147 | 0.251 | 88.0% | 8.34 | 0.004 |
| Gender | ||||||||
| Male and female | 600 | 3 | −0.090 (−0.250, 0.070) | −1.103 | 0.270 | 0.0% | 0.18 | 0.912 |
| Only female | 90 | 2 | −1.051 (−1.495, −0.606) | −4.631 | <0.001 | 0.0% | 0.59 | 0.441 |
| Age * | ||||||||
| ≤60 | 530 | 3 | −0.716 (−1.492, 0.061) | −1.807 | 0.071 | 87.1% | 15.56 | <0.001 |
| >60 | 160 | 2 | −0.067 (−0.374, 0.240) | −0.430 | 0.667 | 0.0% | 0.16 | 0.692 |
| Intervention period | ||||||||
| ≤3 month | 510 | 3 | −0.427 (−1.032, 0.178) | −1.385 | 0.166 | 76.2% | 8.39 | 0.015 |
| ≥6 month | 180 | 2 | −0.459 (−1.344, 0.425) | −1.017 | 0.309 | 86.8% | 7.56 | 0.006 |
| Study | ES | [95% Conf. Interval] | I2 (%) | p |
|---|---|---|---|---|
| A. BW | ||||
| Alberto Hernández-Reyes (2020) [24] | −0.238 | −0.369, −0.106 | 19.8 | 0.279 |
| Cristina Lugones-Sanchez (2020) [25] | −0.226 | −0.382, −0.069 | 19.6 | 0.280 |
| Jae-Ho Choi (2023) [26] | −0.232 | −0.357, −0.107 | 16.3 | 0.306 |
| Iris den Uijl (2023) [27] | −0.288 | −0.405, −0.171 | 0.0 | 0.684 |
| Daniel Berglind (2020) [28] | −0.266 | −0.379, −0.154 | 0.0 | 0.449 |
| Maureen C. Ashe (2015) [30] | −0.238 | −0.397, −0.180 | 19.7 | 0.279 |
| Elizabeth J Lyons (2016) [31] | −0.225 | −0.360, −0.091 | 17.5 | 0.296 |
| Bonnie Spring (2024) [32] | −0.188 | −0.312, −0.063 | 0.0 | 0.631 |
| B. BMI | ||||
| Alberto Hernández-Reyes (2020) [24] | −0.162 | −0.401, 0.076 | 45.1 | 0.141 |
| Cristina Lugones-Sanchez (2020) [25] | −0.070 | −0.291, 0.150 | 0.0 | 0.509 |
| Jae-Ho Choi (2023) [26] | −0.175 | −0.399, 0.049 | 44.6 | 0.144 |
| Daniel Berglind (2020) [28] | −0.287 | −0.443, −0.133 | 0.0 | 0.872 |
| Jomme Claes(2020) [29] | −0.161 | −0.424, 0.102 | 44.7 | 0.143 |
| C. BFP | ||||
| Alberto Hernández-Reyes (2020) [24] | −0.257 | −0.615, 0.101 | 66.0 | 0.032 |
| Cristina Lugones-Sanchez (2020) [25] | −0.558 | −1.136,0.021 | 77.7 | 0.004 |
| Jae-Ho Choi (2023) [26] | −0.256 | −0.558, 0.076 | 66.5 | 0.030 |
| Jomme Claes(2020) [29] | −0.562 | −1.116, −0.007 | 80.8 | 0.001 |
| Elizabeth J Lyons (2016) [31] | −0.483 | −0.960, −0.006 | 82.0 | <0.001 |
| BW | BMI | BFP | |
|---|---|---|---|
| p (Egger’s test) | 0.698 | 0.201 | 0.154 |
| p (Begg’s test) | 0.902 | 0.462 | 0.221 |
References
- World Obesity Atlas 2025. Available online: https://data.worldobesity.org/publications/?cat=23 (accessed on 5 April 2025).
- Wing, R.R.; Phelan, S. Long-term weight loss maintenance. Am. J. Clin. Nutr. 2005, 82, 222S–225S. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, E.; Van Velthoyen, M.H.; Meinert, E. Long-Term Weight Management Using Wearable Technology in Overweight and Obese Adults: Systematic Review. JMIR Mhealth Uhealth 2020, 8, e13461. [Google Scholar] [CrossRef] [PubMed]
- McDonough, D.J.; Su, X.; Gao, Z. Health wearable devices for weight and BMI reduction in individuals with overweight/obesity and chronic comorbidities: Systematic review and network meta-analysis. Br. J. Sports Med. 2021, 55, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Breasail, M.O.; Biswas, B.; Smith, M.D.; Mazhar, M.K.A.; Tenison, E.; Cullen, A.; Lithander, F.E.; Roudaut, A.; Henderson, E.J. Wearable GPS and Accelerometer Technologies for Monitoring Mobility and Physical Activity in Neurodegenerative Disorders: A Systematic Review. Sensors 2021, 21, 8261. [Google Scholar] [CrossRef]
- Cullen, A.; Mazhar, M.K.A.; Smith, M.D.; Lithander, F.E.; Breasail, M.O.; Henderson, E.J. Wearable and Portable GPS Solutions for Monitoring Mobility in Dementia: A Systematic Review. Sensors 2022, 22, 3336. [Google Scholar] [CrossRef]
- Hall, K.D.; Sacks, G.; Chandramohan, D.; Chow, C.C.; Wang, Y.C.; Gortmaker, S.L.; Swinburn, B.A. Obesity 3 Quantification of the effect of energy imbalance on bodyweight. Lancet 2011, 378, 826–837. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. Obesity 1 The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Appel, L.J.; Clark, J.M.; Yeh, H.-C.; Wang, N.-Y.; Coughlin, J.W.; Daumit, G.; Miller, E.R., III; Dalcin, A.; Jerome, G.J.; Geller, S.; et al. Comparative Effectiveness of Weight-Loss Interventions in Clinical Practice. N. Engl. J. Med. 2011, 365, 1959–1968. [Google Scholar] [CrossRef]
- Johns, D.J.; Hartmann-Boyce, J.; Jebb, S.A.; Aveyard, P.; Behavioural Weight Management Review Group. Diet or Exercise Interventions vs Combined Behavioral Weight Management Programs: A Systematic Review and Meta-Analysis of Direct Comparisons. J. Acad. Nutr. Diet. 2014, 114, 1557–1568. [Google Scholar] [CrossRef]
- Sharma, M. Behavioural interventions for preventing and treating obesity in adults. Obes. Rev. 2007, 8, 441–449. [Google Scholar] [CrossRef]
- Burke, L.E.; Wang, J.; Sevick, M.A. Self-Monitoring in Weight Loss: A Systematic Review of the Literature. J. Am. Diet. Assoc. 2011, 111, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Abraham, C.; Whittington, C.; McAteer, J.; Gupta, S. Effective Techniques in Healthy Eating and Physical Activity Interventions: A Meta-Regression. Health Psychol. 2009, 28, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Michie, S.; Yardley, L.; West, R.; Patrick, K.; Greaves, F. Developing and Evaluating Digital Interventions to Promote Behavior Change in Health and Health Care: Recommendations Resulting From an International Workshop. J. Med. Internet Res. 2017, 19, e232. [Google Scholar] [CrossRef] [PubMed]
- Marquet, O.; Hirsch, J.A.; Kerr, J.; Jankowska, M.M.; Mitchell, J.; Hart, J.E.; Laden, F.; Aaron Hipp, J.; James, P. GPS-based activity space exposure to greenness and walkability is associated with increased accelerometer-based physical activity. Environ. Int. 2022, 165, 107317. [Google Scholar] [CrossRef]
- Liu, W.; Chambers, T.; Clevenger, K.A.; Pfeiffer, K.A.; Rzotkiewicz, Z.; Park, H.; Pearson, A.L. Quantifying time spent outdoors: A versatile method using any type of global positioning system (GPS) and accelerometer devices. PLoS ONE 2024, 19, e0299943. [Google Scholar] [CrossRef]
- Middleton, K.M.R.; Patidar, S.M.; Perri, M.G. The impact of extended care on the long-term maintenance of weight loss: A systematic review and meta-analysis. Obes. Rev. 2012, 13, 509–517. [Google Scholar] [CrossRef]
- Taku, K.; Yoshida, Y.; Omori, T. Practice guideline of evidence-based medicine: Preferred reporting items for systematic reviews and meta-analyses (the PRISMA statement). Inf. Process. Manag. 2011, 54, 254–266. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Cochrane Handbook for Systematic Reviews of Interventions (Version 6.3). Available online: https://training.cochrane.org/handbook (accessed on 2 April 2025).
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Hernandez-Reyes, A.; Camara-Martos, F.; Molina Recio, G.; Molina-Luque, R.; Romero-Saldana, M.; Moreno Rojas, R. Push Notifications From a Mobile App to Improve the Body Composition of Overweight or Obese Women: Randomized Controlled Trial. JMIR Mhealth Uhealth 2020, 8, e13747. [Google Scholar] [CrossRef] [PubMed]
- Lugones-Sanchez, C.; Antonia Sanchez-Calavera, M.; Repiso-Gento, I.; Adalia, E.G.; Ignacio Ramirez-Manent, J.; Agudo-Conde, C.; Rodriguez-Sanchez, E.; Angel Gomez-Marcos, M.; Recio-Rodriguez, J.I.; Garcia-Ortiz, L.; et al. Effectiveness of an mHealth Intervention Combining a Smartphone App and Smart Band on Body Composition in an Overweight and Obese Population: Randomized Controlled Trial (EVIDENT 3 Study). JMIR Mhealth Uhealth 2020, 8, e21771. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Kim, S.-W.; Seo, J.; Sun, Y.; Jung, W.-S.; Park, H.Y.; Kim, J.; Lim, K. Effects of a Mobile-Health Exercise Intervention on Body Composition, Vascular Function, and Autonomic Nervous System Function in Obese Women: A Randomized Controlled Trial. J. Multidiscip. Healthc. 2023, 16, 1601–1615. [Google Scholar] [CrossRef] [PubMed]
- den Uijl, I.; van den Berg-emons, R.J.G.; Sunamura, M.; Lenzen, M.J.; Stam, H.J.; Boersma, E.; Tenbult-van Limpt, N.C.C.W.; Kemps, H.M.C.; Geleijnse, M.L.; ter Hoeve, N. Effects of a Dedicated Cardiac Rehabilitation Program for Patients With Obesity on Body Weight, Physical Activity, Sedentary Behavior, and Physical Fitness: The OPTICARE XL Randomized Controlled Trial. Phys. Ther. 2023, 103, pzad055. [Google Scholar] [CrossRef]
- Berglind, D.; Yacaman-Mendez, D.; Lavebratt, C.; Forsell, Y. The Effect of Smartphone Apps Versus Supervised Exercise on Physical Activity, Cardiorespiratory Fitness, and Body Composition Among Individuals With Mild-to-Moderate Mobility Disability: Randomized Controlled Trial. JMIR Mhealth Uhealth 2020, 8, e14615. [Google Scholar] [CrossRef]
- Claes, J.; Cornelissen, V.; McDermott, C.; Moyna, N.; Pattyn, N.; Cornelis, N.; Gallagher, A.; McCormack, C.; Newton, H.; Gillain, A.; et al. Feasibility, Acceptability, and Clinical Effectiveness of a Technology-Enabled Cardiac Rehabilitation Platform (Physical Activity Toward Health-I): Randomized Controlled Trial. J. Med. Internet Res. 2020, 22, e14221. [Google Scholar] [CrossRef]
- Ashe, M.C.; Winters, M.; Hoppmann, C.A.; Dawes, M.G.; Gardiner, P.A.; Giangregorio, L.M.; Madden, K.M.; McAllister, M.M.; Wong, G.; Puyat, J.H.; et al. “Not just another walking program”: Everyday Activity Supports You (EASY) model-a randomized pilot study for a parallel randomized controlled trial. Pilot Feasibility Stud. 2015, 1, 4. [Google Scholar] [CrossRef]
- Lyons, E.J.; Swartz, M.C.; Lewis, Z.H.; Martinez, E.; Jennings, K. Feasibility and Acceptability of a Wearable Technology Physical Activity Intervention With Telephone Counseling for Mid-Aged and Older Adults: A Randomized Controlled Pilot Trial. JMIR Mhealth Uhealth 2017, 5, e28. [Google Scholar] [CrossRef]
- Spring, B.; Pfammatter, A.F.; Scanlan, L.; Daly, E.; Reading, J.; Battalio, S.; McFadden, H.G.; Hedeker, D.; Siddique, J.; Nahum-Shani, I. An Adaptive Behavioral Intervention for Weight Loss Management. JAMA 2024, 332, 21–30. [Google Scholar] [CrossRef]
- Brown, B.B.; Wilson, L.; Tribby, C.P.; Werner, C.M.; Wolf, J.; Miller, H.J.; Smith, K.R. Adding maps (GPS) to accelerometry data to improve study participants’ recall of physical activity: A methodological advance in physical activity research. Br. J. Sports Med. 2014, 48, 1009–1010. [Google Scholar] [CrossRef]
- Gordon, B.A.; Bruce, L.; Benson, A.C. Physical activity intensity can be accurately monitored by smartphone global positioning system ‘app’. Eur. J. Sport Sci. 2016, 16, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Dac Minh Tuan, N.; Lecoultre, V.; Sunami, Y.; Schutz, Y. Assessment of Physical Activity and Energy Expenditure by GPS Combined With Accelerometry in Real-Life Conditions. J. Phys. Act. Health 2013, 10, 880–888. [Google Scholar] [CrossRef]
- Allahbakhshi, H.; Conrow, L.; Naimi, B.; Weibel, R. Using Accelerometer and GPS Data for Real-Life Physical Activity Type Detection. Sensors 2020, 20, 588. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Felty, C.; Johnston, C.S. Adherence to Diet Applications Using a Smartphone Was Associated With Weight Loss in Healthy Overweight Adults Irrespective of the Application. J. Diabetes Sci. Technol. 2017, 11, 184–185. [Google Scholar] [CrossRef]
- Braem, C.I.R.; Pasman, W.J.; van den Broek, T.J.; Caspers, M.P.M.; Jagers, F.L.P.W.; Yavuz, U.S.; Hermens, H.J.; Veltink, P.H.; Wopereis, S. The association of physical activity, heart rate and sleep from an activity tracker with weight loss during a 6-month personalized combined lifestyle intervention: A retrospective analysis. BMC Digit. Health 2025, 3, 8. [Google Scholar] [CrossRef]
- Tuominen, M.; Suorsa, K.; Pentti, J.; Koski, P.; Stenholm, S.; Leskinen, T. The Impact of a 12-Month Activity Tracker Intervention on Activity Behavior Across Body Mass Index Subgroups Among Recent Retirees: Post Hoc Analysis of a Randomized Controlled Trial. J. Phys. Act. Health 2021, 18, 1563–1569. [Google Scholar] [CrossRef]
- James, P.; Hart, J.E.; Hipp, J.A.; Mitchell, J.A.; Kerr, J.; Hurvitz, P.M.; Glanz, K.; Laden, F. GPS-Based Exposure to Greenness and Walkability and Accelerometry-Based Physical Activity. Cancer Epidemiol. Biomark. Prev. 2017, 26, 525–532. [Google Scholar] [CrossRef]
- Pasanen, S.; Halonen, J.I.; Pulakka, A.; Kestens, Y.; Thierry, B.; Brondeel, R.; Pentti, J.; Vahtera, J.; Leskinen, T.; Stenholm, S. Contexts of sedentary time and physical activity among ageing workers and recent retirees: Cross-sectional GPS and accelerometer study. BMJ Open 2021, 11, e042600. [Google Scholar] [CrossRef]
- Holliday, K.M.; Howard, A.G.; Emch, M.; Rodriguez, D.A.; Rosamond, W.D.; Evenson, K.R. Deriving a GPS Monitoring Time Recommendation for Physical Activity Studies of Adults. Med. Sci. Sports Exerc. 2017, 49, 939–947. [Google Scholar] [CrossRef]
- del Rosario, M.B.; Redmond, S.J.; Lovell, N.H. Tracking the Evolution of Smartphone Sensing for Monitoring Human Movement. Sensors 2015, 15, 18901–18933. [Google Scholar] [CrossRef]
- Hyun, A.-H. Effect of Real-Time Online High-Intensity Interval Training on Physiological and Physical Parameters for Abdominally Obese Women: A Randomized Pilot Study. Appl. Sci. 2021, 11, 12129. [Google Scholar] [CrossRef]
- Hurvitz, P.M.; Moudon, A.V.; Kang, B.; Saelens, B.E.; Duncan, G.E. Emerging technologies for assessing physical activity behaviors in space and time. Front. Public Health 2014, 2, 2. [Google Scholar] [CrossRef]
- Direito, A.; Carraca, E.; Rawstorn, J.; Whittaker, R.; Maddison, R. mHealth Technologies to Influence Physical Activity and Sedentary Behaviors: Behavior Change Techniques, Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Behav. Med. 2017, 51, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Direito, A.; Jiang, Y.; Whittaker, R.; Maddison, R. Apps for IMproving FITness and Increasing Physical Activity Among Young People: The AIMFIT Pragmatic Randomized Controlled Trial. Ann. Behav. Med. 2015, 17, e210. [Google Scholar] [CrossRef] [PubMed]
- Brickwood, K.-J.; Watson, G.; O’Brien, J.; Williams, A.D. Consumer-Based Wearable Activity Trackers Increase Physical Activity Participation: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2019, 7, e11819. [Google Scholar] [CrossRef]
- Tobin, K.; Heidari, O.; Volpi, C.; Sodder, S.; Duncan, D. Use of geofencing interventions in population health research: A scoping review. BMJ Open 2023, 13, e069374. [Google Scholar] [CrossRef]
- King, A.C.; Hekler, E.B.; Grieco, L.A.; Winter, S.J.; Sheats, J.L.; Buman, M.P.; Banerjee, B.; Robinson, T.N.; Cirimele, J. Harnessing Different Motivational Frames via Mobile Phones to Promote Daily Physical Activity and Reduce Sedentary Behavior in Aging Adults. PLoS ONE 2013, 8, e62613. [Google Scholar] [CrossRef]
- Bent, B.; Goldstein, B.A.; Kibbe, W.A.; Dunn, J.P. Investigating sources of inaccuracy in wearable optical heart rate sensors. NPJ Digit. Med. 2020, 3, 18. [Google Scholar] [CrossRef]
- Dombrowski, S.U.; Sniehotta, F.F.; Avenell, A.; Johnston, M.; MacLennan, G.; Araujo-Soares, V. Identifying active ingredients in complex behavioural interventions for obese adults with obesity-related co-morbidities or additional risk factors for co-morbidities: A systematic review. Health Psychol. Rev. 2012, 6, 7–32. [Google Scholar] [CrossRef]
- Krenn, P.J.; Titze, S.; Oja, P.; Jones, A.; Ogilvie, D. Use of Global Positioning Systems to Study Physical Activity and the Environment A Systematic Review. Am. J. Prev. Med. 2011, 41, 508–515. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Rotunda, W.; Rains, C.; Jacobs, S.R.; Ng, V.; Lee, R.; Rutledge, S.; Jackson, M.C.; Myers, K. Weight Loss in Short-Term Interventions for Physical Activity and Nutrition Among Adults With Overweight or Obesity: A Systematic Review and Meta-Analysis. Prev. Chronic Dis. 2024, 21, E21. [Google Scholar] [CrossRef] [PubMed]
- Baumel, A.; Muench, F.; Edan, S.; Kane, J.M. Objective User Engagement With Mental Health Apps: Systematic Search and Panel-Based Usage Analysis. J. Med. Internet Res. 2019, 21, e14567. [Google Scholar] [CrossRef] [PubMed]
- Perski, O.; Blandford, A.; West, R.; Michie, S. Conceptualising engagement with digital behaviour change interventions: A systematic review using principles from critical interpretive synthesis. Transl. Behav. Med. 2017, 7, 254–267. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Egger, M.; Smith, G.D. Systematic reviews in health care—Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001, 323, 101–105. [Google Scholar] [CrossRef]
- Chaput, J.-P.; Tremblay, A. Does short sleep duration favor abdominal adiposity in children? Int. J. Pediatr. Obes. 2007, 2, 188–191. [Google Scholar] [CrossRef]
- Kwan, M.-P. The Uncertain Geographic Context Problem. Ann. Assoc. Am. Geogr. 2012, 102, 958–968. [Google Scholar] [CrossRef]
| Study | Country | Population | Sample Size (n) | Mean Age (years) | BMI (Mean ± SD) (kg/m2) | Sex N (%) | Intervention Design |
|---|---|---|---|---|---|---|---|
| Alberto Hernández-Reyes (2020) [24] | Spain | Adult women with obesity or classed as overweight | 60 | 41.5 | 31.8 ± 5.3 | IG: 31 female (100.0%) CG: 29 female (100.0%) | Approach of intervention: PA + diet Intervention period: 26 weeks Control group: PA prescription and recommendation: without app to self-monitoring or push notifications Intervention group: push notifications with exercise recommendations and diet tips; app with specific functionalities: self-monitoring of weight at home, gamification, or prescription of PA. |
| Cristina Lugones-Sanchez (2020) [25] | Spain | Adult women with obesity or classed as overweight | 440 | IG: 47.4 CG: 48.8 | IG: 32.7 ± 3.3 CG: 32.9 ± 3.4 | IG: 161 female (69.7%); 70 male (30.3%) CG: 144 female (65.7%); 65 male (34.3%) | Approach of intervention: PA + diet Intervention period: 12 weeks Control group: counselling (5 min) on diet and PA; without app or smart band; single session only. Intervention group: counselling (5 min) on diet and PA; record daily physical activity and food intake daily using app and smart band |
| Jae-Ho Choi (2023) [26] | Republic of Korea | Adult women | 30 | IG: 39.7 CG: 39.2 | IG: 25.5 ± 4.3 CG: 26.0 ± 4.6 | IG: 15 female (100.0%); CG: 15 female (100.0%) | Approach of intervention: PA Intervention period: 12 weeks Control group: None Intervention group: exercise interventions using the mHealth system; app and smart band to track physical activity data |
| Iris den Uijl (2023) [27] | The Netherlands | Adults with obesity and coronary artery disease or nonvalvular atrial fibrillation | 201 | IG: 59.0 CG: 59.2 | IG: 34.4 ± 4.7 CG: 34.1 ± 4.6 | IG: 52 female (33.3%); 68 male (66.7%) CG: 21 female (21.2%); 78 male (78.8%) | Approach of intervention: PA + diet Intervention period: 48 weeks Control group: aerobic training with mainly weight-bearing exercises; without activity tracker, weekly sessions by a dietitian Intervention group: aerobic training with mainly non-weight-bearing exercises and nutrition education by dietician; app and activity monitor to track physical activity data |
| Daniel Berglind (2020) [28] | Sweden | Adults with mobility disability | 110 | IG: 35.6 CG: 34.5 | IG: 26.3 ± 5.7 CG: 27.2 ± 5.2 | IG: 47 female (85.0%); 8 male (15.0%) CG: 43 female (78.0%) 12 male (12.0%) | Approach of intervention: PA + diet Intervention period: 12 weeks Control group: 12-week supervised aerobic/strength training; lifestyle coaching (three sessions); without apps or wearable devices Intervention group: three consultation sessions; using apps to track steps and home-based bodyweight exercise; using food photography app to monitor diet |
| Jomme Claes (2020) [29] | Belgium and Ireland | Adults with CVD | 120 | 61.4 | 27.9 ± 4.5 | IG: 11 female (18.3%); 49 male (81.7%) CG: 11 female (18.3%); 49 male (81.7%) | Approach of intervention: PA Intervention period: 24 weeks Control group: verbal lifestyle advice; without app or remote support Intervention group: PATHway system, including PA planning, PA intervention, and monitor activity data |
| Maureen C. Ashe (2015) [30] | Canada | Inactive adult women | 20 | IG: 63.1 CG: 64.8 | IG: 32.9 ± 6.8 CG: 26.9 ± 6.8 | IG: 8 female (100.0%) CG: 12 female (100.0%) | Approach of intervention: PA Intervention period: 24 weeks Control group: monthly non-exercise education sessions; without PA prescription or Fitbit; without exercise professional contact Intervention group: activity monitor to record daily step counts, distance walked, and provides immediate feedback on activities; individualized physical activity prescription; education and incentives |
| Elizabeth J. Lyons (2016) [31] | USA | Adults with obesity or classed as overweight | 40 | 61.48 | 30.3 ± 3.5 | IG: 17 female (85.0%) CG: 17 female (85.0%) | Approach of intervention: PA Intervention period: 12 weeks Control group: None Intervention group: activity monitor and app to set step goals and monitor activity data; consultation; social interaction |
| Bonnie Spring (2024) [32] | USA | Adults with obesity or classed as overweight | 342 | IG: 40.9 CG: 40.2 | IG: 34.5 ± 4.4 CG: 34.3 ± 4.3 | IG: 153 female (76.1%); 48 male (23.9%) CG:152 female (76.4%); 47 male (23.6%) | Approach of intervention: PA + diet Intervention period: 48 weeks Control group: WFS: app and activity monitor with automated feedback to monitor activity data and self-reported diet; without coaching; re-randomization for nonresponses Intervention group: WFS: app and activity monitor with automated feedback to monitor activity data and self-reported diet; with coaching |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Zhang, S.; Sun, X.; Sun, Z.; Peng, W.; Shi, L.; Gou, B.; Wang, Y. Integrated GPS-Enabled Physical Activity and Dietary Interventions Versus Physical Activity Alone for Obesity Control: A Systematic Review and Meta-Analysis. Nutrients 2025, 17, 1886. https://doi.org/10.3390/nu17111886
Fan Y, Zhang S, Sun X, Sun Z, Peng W, Shi L, Gou B, Wang Y. Integrated GPS-Enabled Physical Activity and Dietary Interventions Versus Physical Activity Alone for Obesity Control: A Systematic Review and Meta-Analysis. Nutrients. 2025; 17(11):1886. https://doi.org/10.3390/nu17111886
Chicago/Turabian StyleFan, Yu, Sichen Zhang, Xiaomin Sun, Zhaozhang Sun, Wen Peng, Lin Shi, Bo Gou, and Youfa Wang. 2025. "Integrated GPS-Enabled Physical Activity and Dietary Interventions Versus Physical Activity Alone for Obesity Control: A Systematic Review and Meta-Analysis" Nutrients 17, no. 11: 1886. https://doi.org/10.3390/nu17111886
APA StyleFan, Y., Zhang, S., Sun, X., Sun, Z., Peng, W., Shi, L., Gou, B., & Wang, Y. (2025). Integrated GPS-Enabled Physical Activity and Dietary Interventions Versus Physical Activity Alone for Obesity Control: A Systematic Review and Meta-Analysis. Nutrients, 17(11), 1886. https://doi.org/10.3390/nu17111886







