A Murine Model of High Dietary Histamine Intake: Impact on Histamine Contents and Release in Neural and Extraneural Tissues
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics
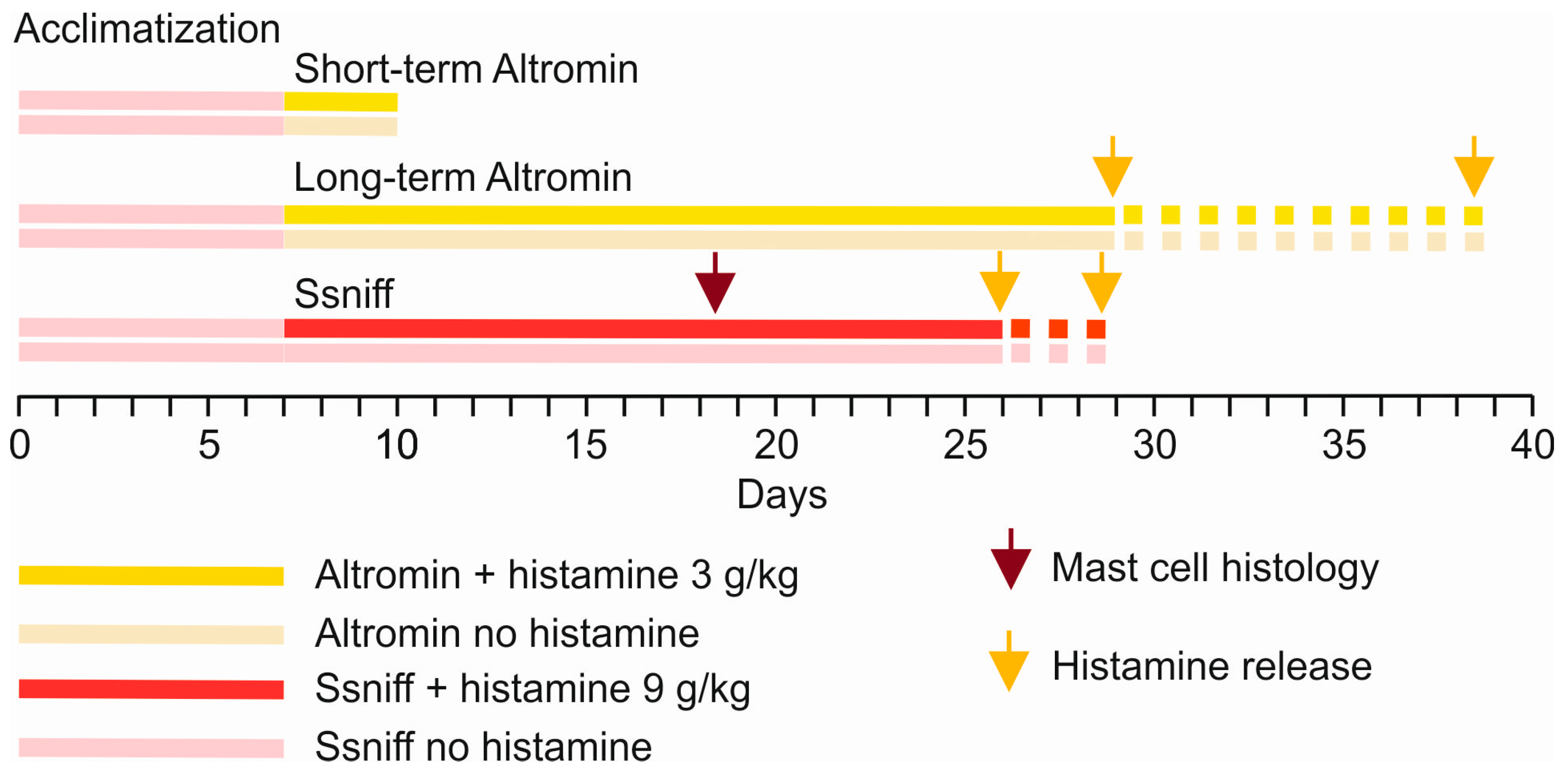
2.2. Low and High Dietary Histamine Fed Mice
2.3. Tissue Sample Preparation
2.4. Histamine Release Experiments
2.5. Histamine Concentration Assessment
2.6. Toluidine Blue Staining of Dura Mater
2.7. Calculations and Statistics
3. Results
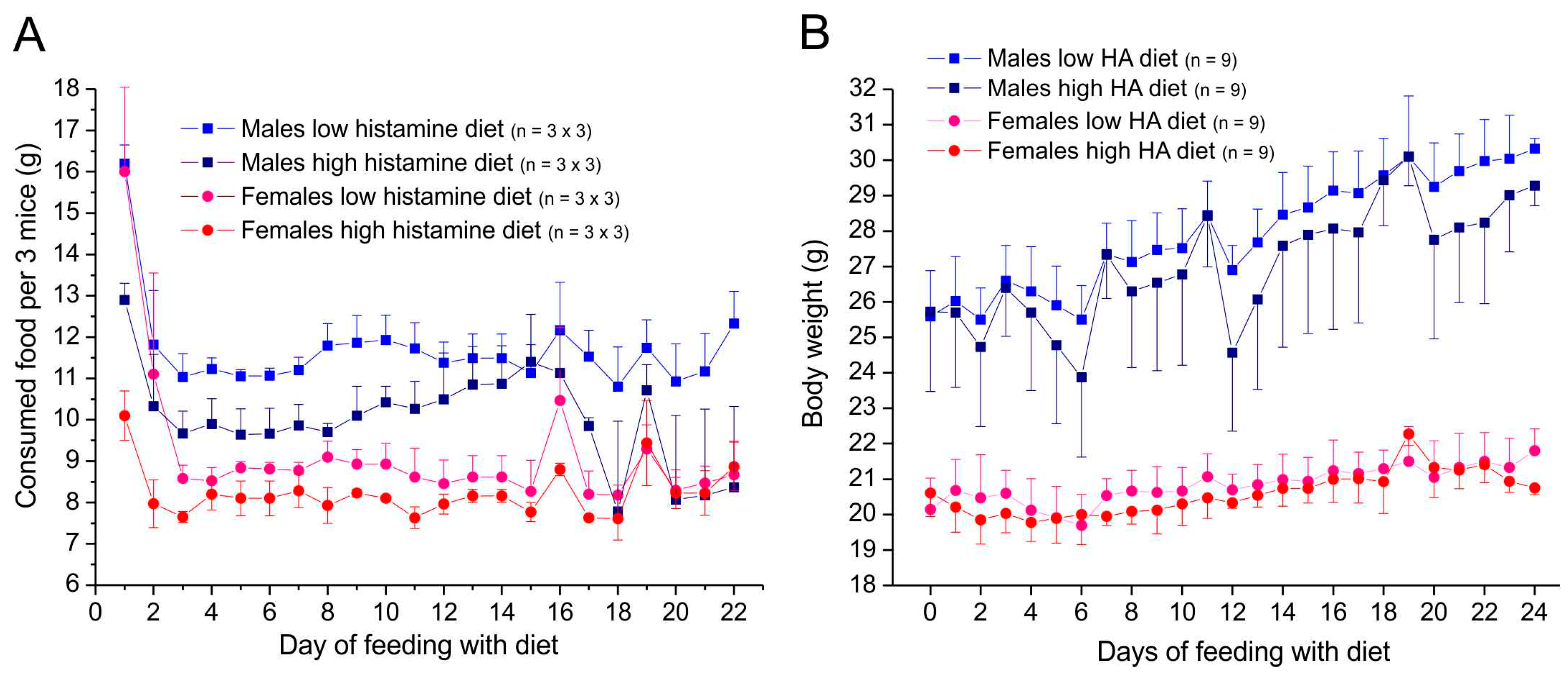
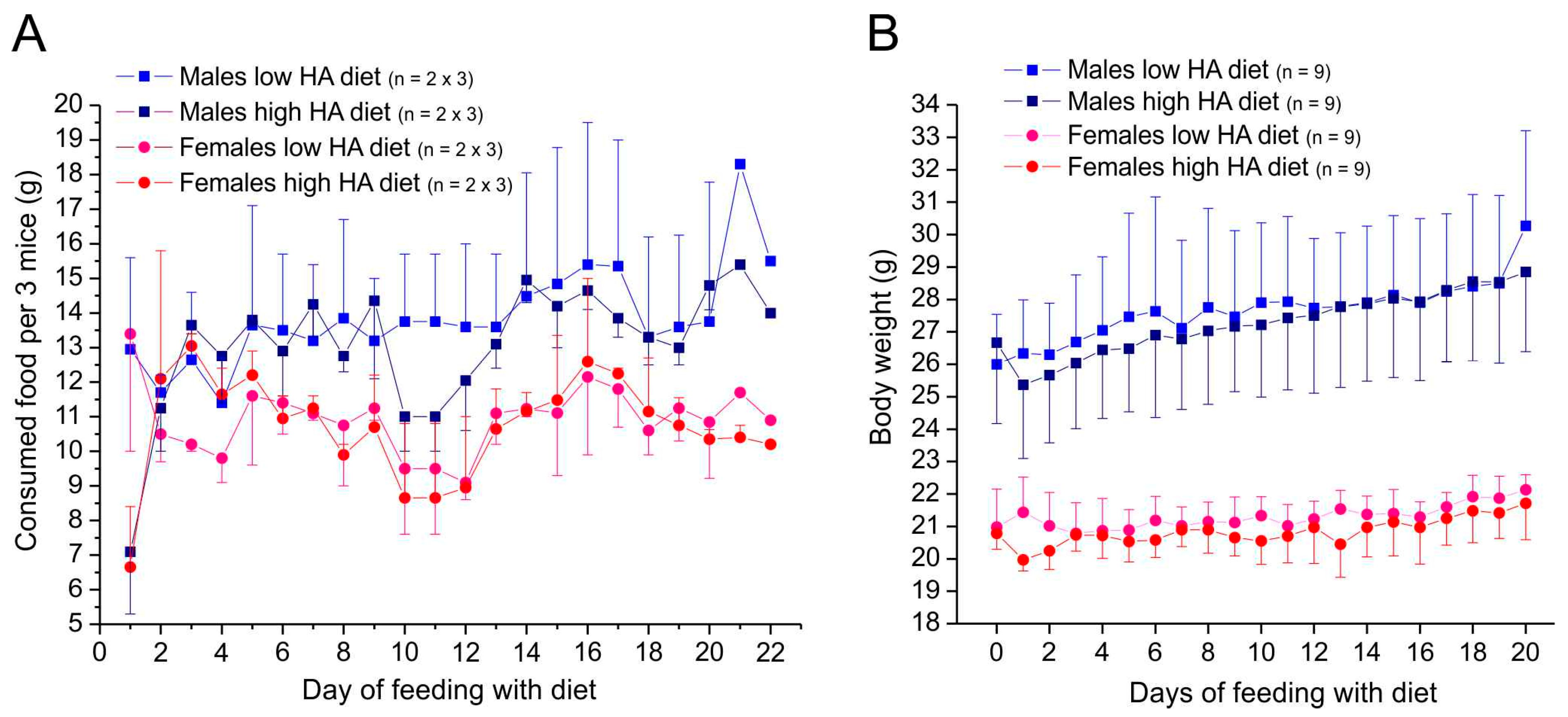
3.1. Condition, Food Consumption, and Changes in Body Weight
3.1.1. Animals Supplied with Altromin for 3–4 Weeks
3.1.2. Animals Supplied with Ssniff for About 3 Weeks
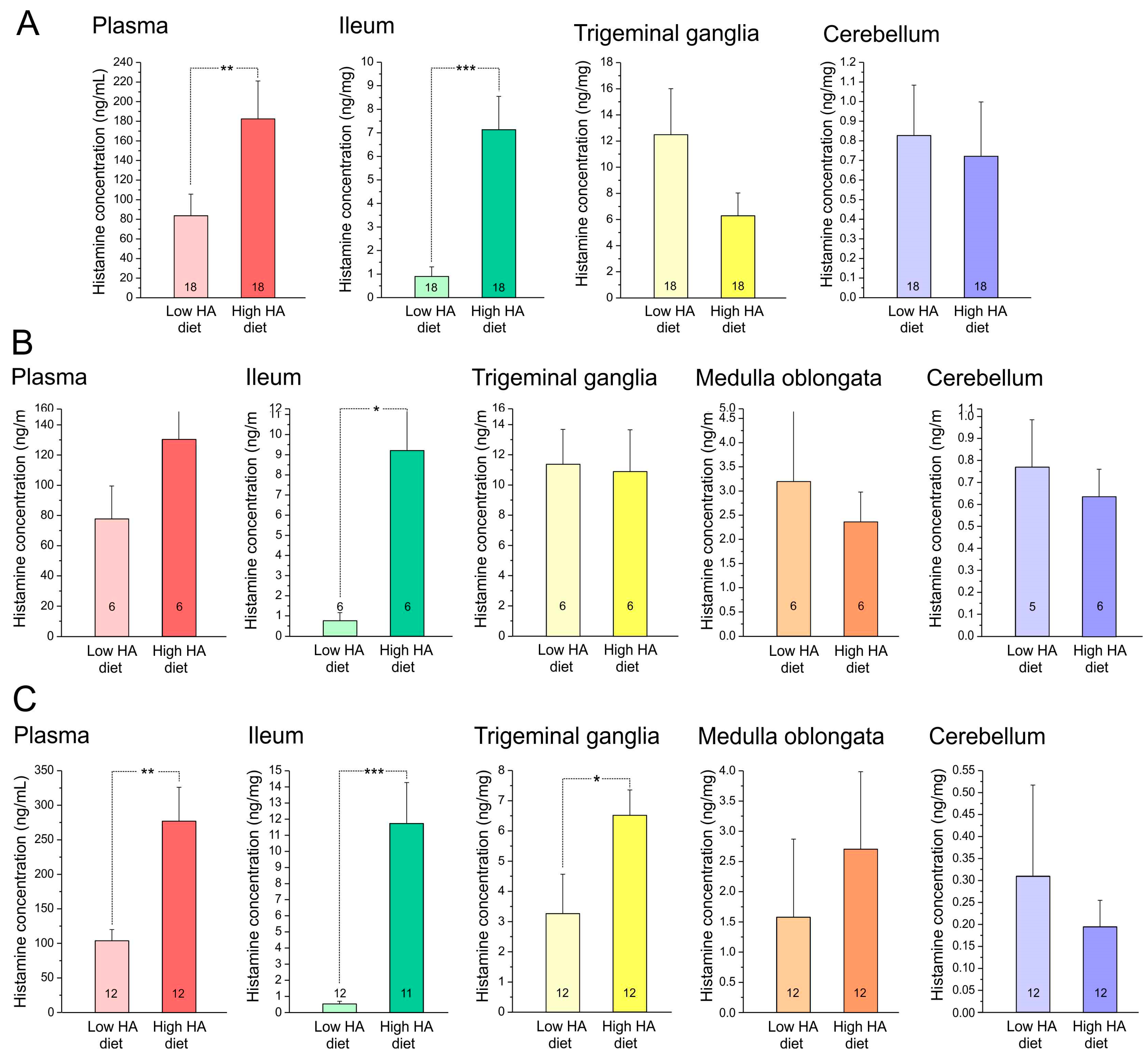
3.2. Histamine Content of Blood Plasma, Ileum, Trigeminal Ganglia, and Cerebellum
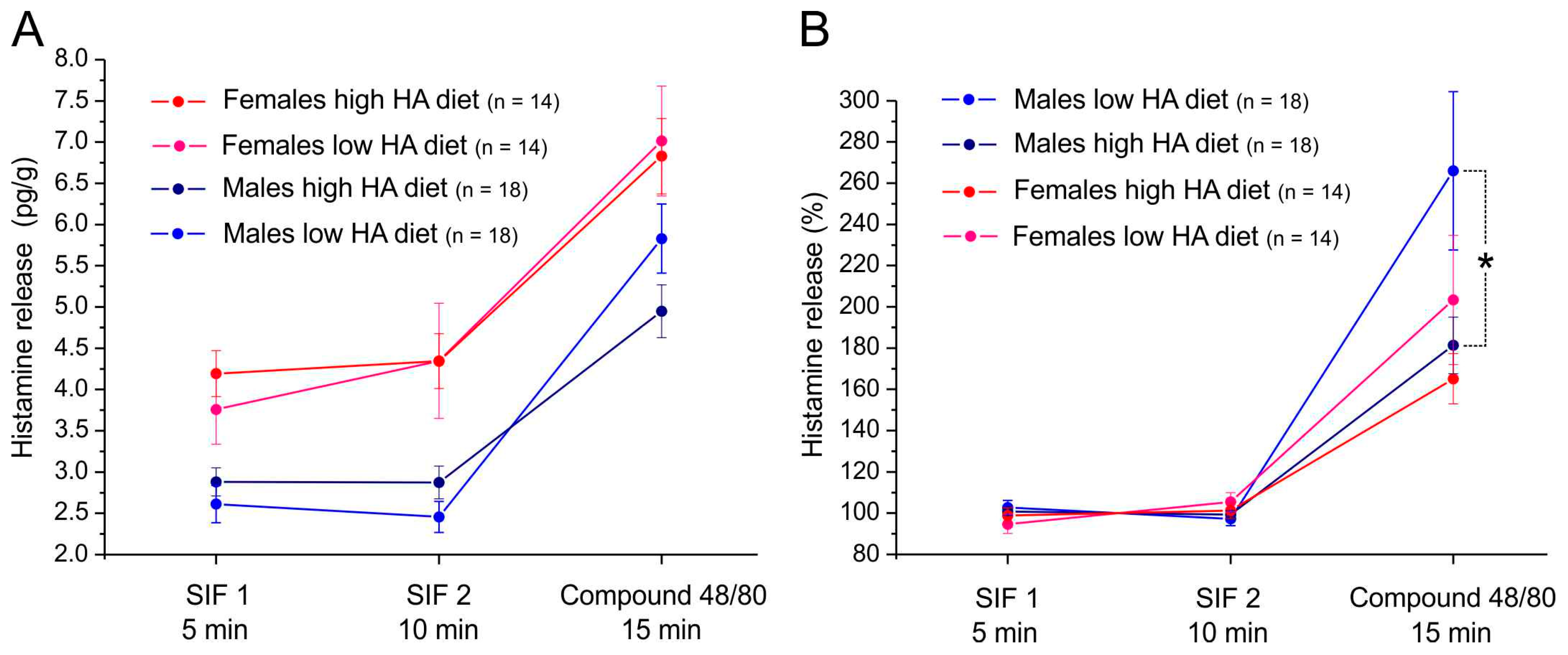
3.3. Histamine Release from Mouse Dura Mater
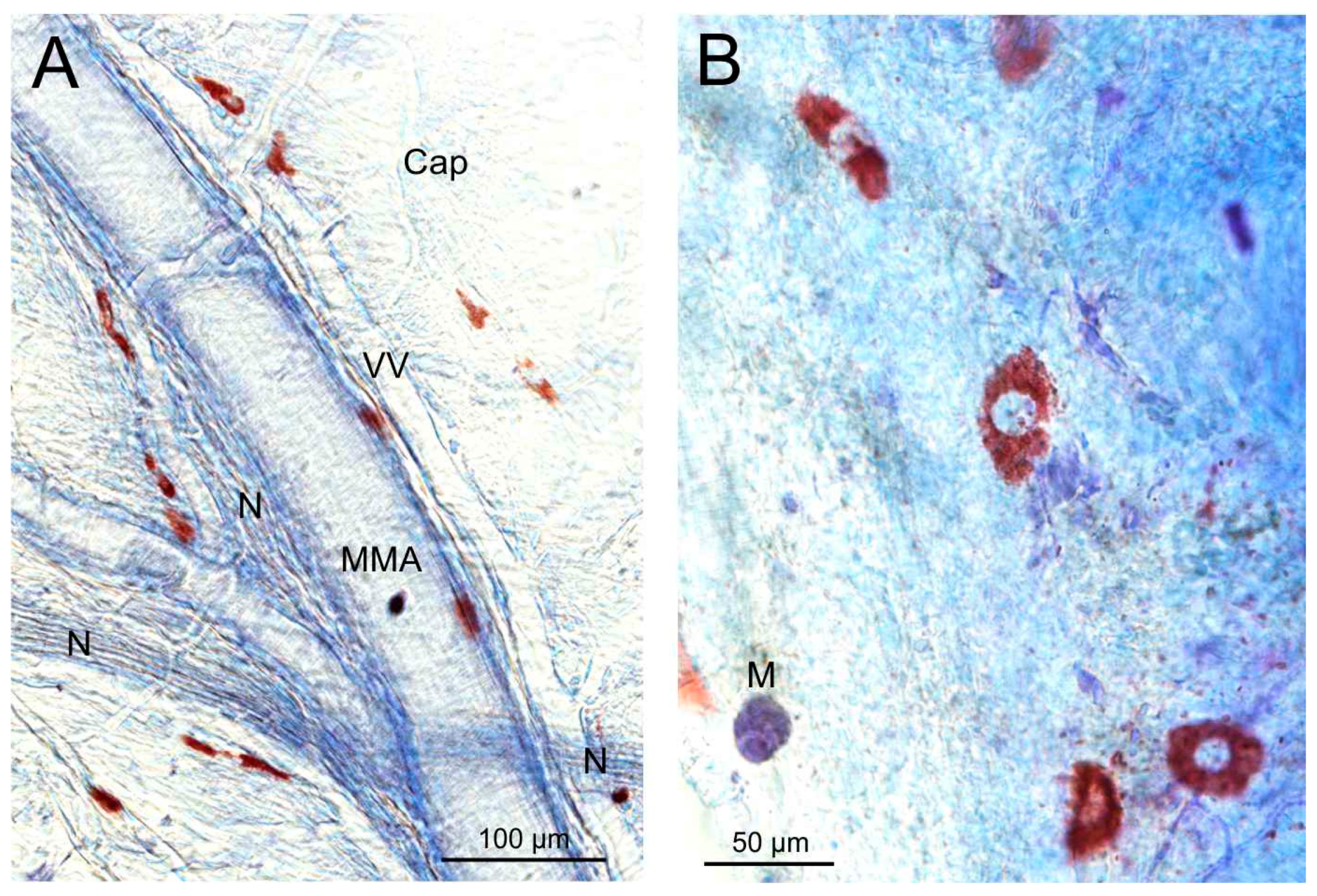
3.4. Toluidine Blue Staining of Mast Cells in Mouse Dura Mater
4. Discussion
4.1. Impact of High Histamine on Animal Conditions
4.2. Histamine Levels in Different Tissues
4.3. Histamine Uptake into Tissues
4.4. Mast Cells in the Dura Mater
4.5. Histamine Release from the Dura Mater
4.6. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Zhang, X.; Jin, H.; Chen, L.; Ji, J.; Zhang, Z. Histamine Intolerance-A Kind of Pseudoallergic Reaction. Biomolecules 2022, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Latorre-Moratalla, M.; Vidal-Carou, M.D.C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Hrubisko, M.; Danis, R.; Huorka, M.; Wawruch, M. Histamine Intolerance-The More We Know the Less We Know. A Review. Nutrients 2021, 13, 2228. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, S.; Imark, C.; Kneubühl, M. Biogenic amines in foods: Histamine and food processing. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. Al 1999, 48, 296–300. [Google Scholar] [CrossRef]
- Komericki, P.; Klein, G.; Reider, N.; Hawranek, T.; Strimitzer, T.; Lang, R.; Kranzelbinder, B.; Aberer, W. Histamine intolerance: Lack of reproducibility of single symptoms by oral provocation with histamine: A randomised, double-blind, placebo-controlled cross-over study. Wien. Klin. Wochenschr. 2011, 123, 15–20. [Google Scholar] [CrossRef]
- Schnedl, W.J.; Enko, D. Histamine Intolerance Originates in the Gut. Nutrients 2021, 13, 1262. [Google Scholar] [CrossRef]
- Millichap, J.G.; Yee, M.M. The diet factor in pediatric and adolescent migraine. Pediatr. Neurol. 2003, 28, 9–15. [Google Scholar] [CrossRef]
- Izquierdo-Casas, J.; Comas-Basté, O.; Latorre-Moratalla, M.L.; Lorente-Gascón, M.; Duelo, A.; Vidal-Carou, M.C.; Soler-Singla, L. Low serum diamine oxidase (DAO) activity levels in patients with migraine. J. Physiol. Biochem. 2018, 74, 93–99. [Google Scholar] [CrossRef]
- Worm, J.; Falkenberg, K.; Olesen, J. Histamine and migraine revisited: Mechanisms and possible drug targets. J. Headache Pain. 2019, 20, 30. [Google Scholar] [CrossRef]
- De Mora, F.; Messlinger, K. Is calcitonin gene-related peptide (CGRP) the missing link in food histamine-induced migraine? A review of functional gut-to-trigeminovascular system connections. Drug Discov. Today 2024, 29, 103941. [Google Scholar] [CrossRef]
- Dzienis-Koronkiewicz, E.; ØvrebØ, K.; Gislason, H.; Waldum, H.L.; Svanes, K. Gastric mucosal exposure to histamine in rats is followed by absorption of histamine but not mucosal hyperemia. Hepatogastroenterology 2002, 49, 290–296. [Google Scholar] [PubMed]
- Pár, G.; Szekeres-Barthó, J.; Buzás, E.; Pap, E.; Falus, A. Impaired reproduction of histamine deficient (histidine-decarboxylase knockout) mice is caused predominantly by a decreased male mating behavior. Am. J. Reprod. Immunol. 2003, 50, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, K.; Fukagawa, K.; Fukagawa, T.; Noguchi, H.; Kakuma, T.; Sakata, T.; Yoshimatsu, H. Hypothalamic neuronal histamine mediates the thyrotropin-releasing hormone-induced suppression of food intake. J. Neurochem. 2007, 103, 1102–1110. [Google Scholar] [CrossRef]
- Jørgensen, E.A.; Vogelsang, T.W.; Knigge, U.; Watanabe, T.; Warberg, J.; Kjaer, A. Increased susceptibility to diet-induced obesity in histamine-deficient mice. Neuroendocrinology 2006, 83, 289–294. [Google Scholar] [CrossRef]
- Flores, V.; Spicer, A.B.; Sonsalla, M.M.; Richardson, N.E.; Yu, D.; Sheridan, G.E.; Trautman, M.E.; Babygirija, R.; Cheng, E.P.; Rojas, J.M.; et al. Regulation of metabolic health by dietary histidine in mice. J. Physiol. 2023, 601, 2139–2163. [Google Scholar] [CrossRef]
- Buhner, S.; Schemann, M. Mast cell-nerve axis with a focus on the human gut. Biochim. Biophys. Acta 2012, 1822, 85–92. [Google Scholar] [CrossRef]
- Vogel, P.; Janke, L.; Gravano, D.M.; Lu, M.; Sawant, D.V.; Bush, D.; Shuyu, E.; Vignali, D.A.A.; Pillai, A.; Rehg, J.E. Globule Leukocytes and Other Mast Cells in the Mouse Intestine. Vet. Pathol. 2018, 55, 76–97. [Google Scholar] [CrossRef]
- Engevik, K.A.; Hazzard, A.; Puckett, B.; Hoch, K.M.; Haidacher, S.J.; Haag, A.M.; Spinler, J.K.; Versalovic, J.; Engevik, M.A.; Horvath, T.D. Phylogenetically diverse bacterial species produce histamine. Syst. Appl. Microbiol. 2024, 47, 126539. [Google Scholar] [CrossRef] [PubMed]
- Kaliner, M.; Shelhamer, J.H.; Ottesen, E.A. Effects of infused histamine: Correlation of plasma histamine levels and symptoms. J. Allergy Clin. Immunol. 1982, 69, 283–289. [Google Scholar] [CrossRef]
- Karer, M.; Rager-Resch, M.; Haider, T.; Petroczi, K.; Gludovacz, E.; Borth, N.; Jilma, B.; Boehm, T. Diamine oxidase knockout mice are not hypersensitive to orally or subcutaneously administered histamine. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. Al 2022, 71, 497–511. [Google Scholar] [CrossRef]
- Sakurai, E.; Sakurai, E.; Watanabe, T.; Yanai, K. Uptake of L-histidine and histamine biosynthesis at the blood-brain barrier. Inflamm. Res. Off J. Eur. Histamine Res. Soc. Al 2009, 58 (Suppl. S1), 34–35. [Google Scholar] [CrossRef] [PubMed]
- Alstadhaug, K.B. Histamine in migraine and brain. Headache 2014, 54, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Abou, Y.Z.; Adam, H.M.; Stephen, W.R. Concentration of histamine in different parts of the brain and hypophysis of rabbit: Effect of treatment with histidine, certain other amino acids and histamine. Br. J. Pharmacol. 1973, 48, 577–589. [Google Scholar] [CrossRef]
- Lozeva, V.; Tarhanen, J.; Attila, M.; Männistö, P.T.; Tuomisto, L. Brain histamine and histamine H3 receptors following repeated L-histidine administration in rats. Life Sci. 2003, 73, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, S.; Salvatore, C.A.; Johansson, S.; Chen, T.-B.; Zeng, Z.; Edvinsson, L. Localization of CGRP.; CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res. 2015, 1600, 93–109. [Google Scholar] [CrossRef]
- Dimlich, R.V.; Keller, J.T.; Strauss, T.A.; Fritts, M.J. Linear arrays of homogeneous mast cells in the dura mater of the rat. J. Neurocytol. 1991, 20, 485–503. [Google Scholar] [CrossRef]
- Dimitriadou, V.; Rouleau, A.; Tuong, M.T.; Newlands, G.; Miller, H.; Luffau, G.; Schwartz, J.-C.; Garbarg, M. Functional relationships between sensory nerve fibers and mast cells of dura mater in normal and inflammatory conditions. Neuroscience 1997, 77, 829–839. [Google Scholar] [CrossRef]
- Liao, H.; Peng, X.; Ge, Y.; Liang, Y.; Yin, Y.; Li, J.; Lin, L.; Wang, J.; Li, Y.; Li, L. Novel reactivation and degranulation of mast cells. Biomed. Pharmacother. 2020, 127, 110157. [Google Scholar] [CrossRef]
| Diet Group | Histidine Content (mg/kg) | Histamine Content (g/kg) | Feeding Period (Days) | Group Size (n) Males (M) Females (F) | Experiments Histamine (HA) |
|---|---|---|---|---|---|
| Altromin C1072 Control | 4.675 | 0 | 22–32 | 9 M/9 F | HA content |
| 9 M/7 F | HA release | ||||
| 3 | 3 M/3 F | HA content | |||
| Altromin C1072 Test | 4.675 | 3 | 22–32 | 9 M/9 F | HA content |
| 9 M/7 F | HA release | ||||
| 3 | 3 M/3 F | HA content | |||
| Ssniff Control | 5800 | 0 | 19–22 | 6 M/6 F | HA content |
| Ssniff Test | 5800 | 9 | 19–22 | 6 M/6 F | HA content |
| Ssniff Control | 5800 | 0 | 10–12 | 2 M/1 F | Mast cells |
| Ssniff Test | 5800 | 9 | 10–12 | 2 M/2 F | Mast cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuhn, A.; Schramm, J.; Vogler, B.; Dux, M.; de Mora, F.; Messlinger, K. A Murine Model of High Dietary Histamine Intake: Impact on Histamine Contents and Release in Neural and Extraneural Tissues. Nutrients 2025, 17, 1851. https://doi.org/10.3390/nu17111851
Kuhn A, Schramm J, Vogler B, Dux M, de Mora F, Messlinger K. A Murine Model of High Dietary Histamine Intake: Impact on Histamine Contents and Release in Neural and Extraneural Tissues. Nutrients. 2025; 17(11):1851. https://doi.org/10.3390/nu17111851
Chicago/Turabian StyleKuhn, Annette, Jana Schramm, Birgit Vogler, Mária Dux, Fernando de Mora, and Karl Messlinger. 2025. "A Murine Model of High Dietary Histamine Intake: Impact on Histamine Contents and Release in Neural and Extraneural Tissues" Nutrients 17, no. 11: 1851. https://doi.org/10.3390/nu17111851
APA StyleKuhn, A., Schramm, J., Vogler, B., Dux, M., de Mora, F., & Messlinger, K. (2025). A Murine Model of High Dietary Histamine Intake: Impact on Histamine Contents and Release in Neural and Extraneural Tissues. Nutrients, 17(11), 1851. https://doi.org/10.3390/nu17111851





