Daily Administration of Agmatine Reduced Anxiety-like Behaviors and Neural Responses in the Brains of Male Mice with Persistent Inflammation in the Craniofacial Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Persistent Craniofacial Inflammatory Model (CFA Mice)
2.3. Experimental Design
2.4. Agmatine (AGM) Treatment
2.5. Behavioral Tests
2.5.1. Elevated Plus Maze (EPM) Test
2.5.2. Dark–Light (DL) Test
2.5.3. Social Interaction (SI) Test
2.5.4. Open Field (OF) Test
2.5.5. Novel Object Recognition (NOR) Test
2.6. Immunohistochemical (IHC) Experiments
2.6.1. Preparations
2.6.2. IHC Procedures
2.6.3. Data Analysis
2.7. Statistical Analysis
2.8. Metabolomic Analysis of Sake Lees Extract
3. Results
3.1. Anxiety-like Behaviors
3.1.1. Elevated Plus Maze (EPM) Test
- Non-CFA mice (Figure 2B, left panel)
- AGMt Group
- b.
- AGMp Group
- CFA mice (Figure 2B, right panel)
- AGMt Group
- b.
- AGMp Group
3.1.2. Dark–Light Room (DL) Test
- Non-CFA mice (Figure 2D, left panel)
- 2.
- CFA mice (Figure 2D, right panel)
- AGMt Group
- b.
- AGMp Group
3.1.3. Open Field (OF) Test
- Time spent in the center area (Figure 3B)
- Non-CFA mice
- AGMt Group
- AGMp Group
- b.
- CFA mice
- AGMt Group
- AGMp Group
- 2
- Total movement distance on the OF (Figure 3C)
- Non-CFA mice
- AGMt Group
- AGMp Group
- b.
- CFA mice
- AGMt Group
- AGMp Group
3.1.4. Social Interaction (SI) Test
- 1
- Non-CFA mice
- AGMt group (Figure 4B)
- b.
- AGMp Group (Figure 4C)
- 2
- CFA mice
- AGMt group (Figure 4D)
- b.
- AGMp group (Figure 4E)
3.1.5. Novel Object Recognition (NOR) Test
- 1
- Non-CFA mice (Supplement Figure S2)
- 2
- CFA mice (Figure 5B)
- Vehicle group
- b.
- AGMt group
- c.
- AGMp group
3.2. Immunohistochemistry (IHC)
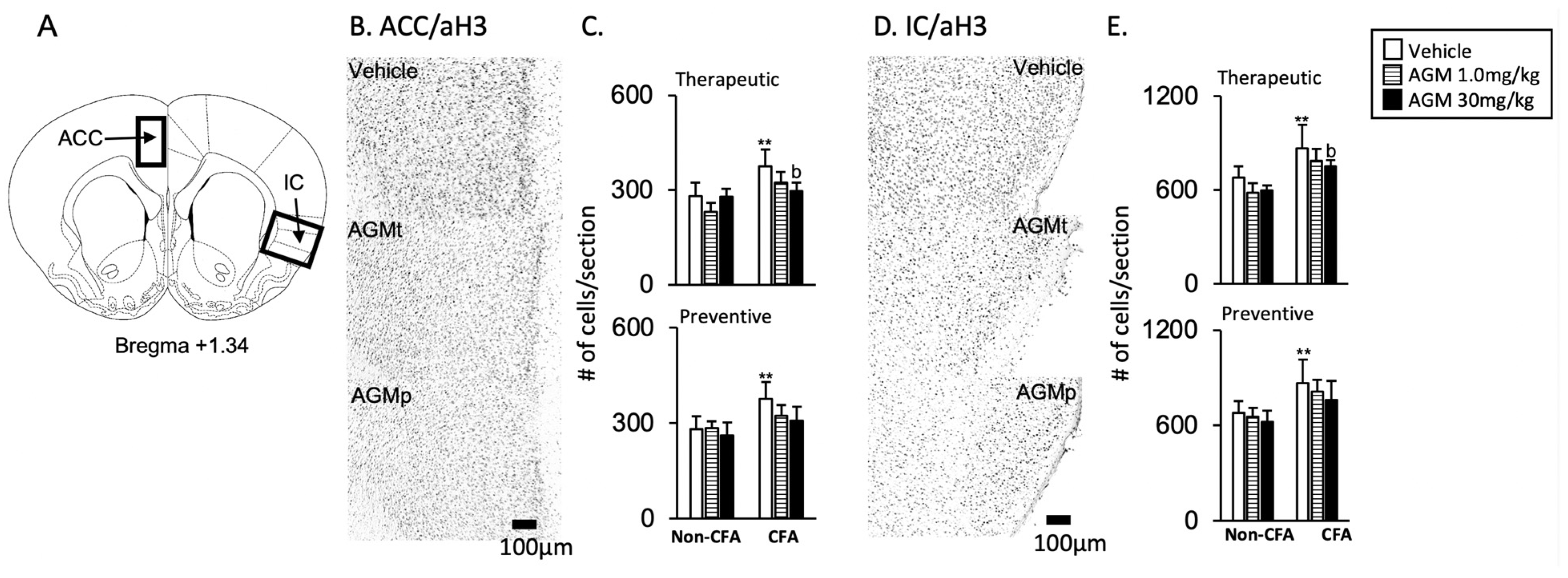
3.2.1. ACC and IC
- 1
- Acetylation histone H3 immunoreactivity
- AGMt (Figure 6C,E)
- b.
- AGMp (Figure 6C,E)
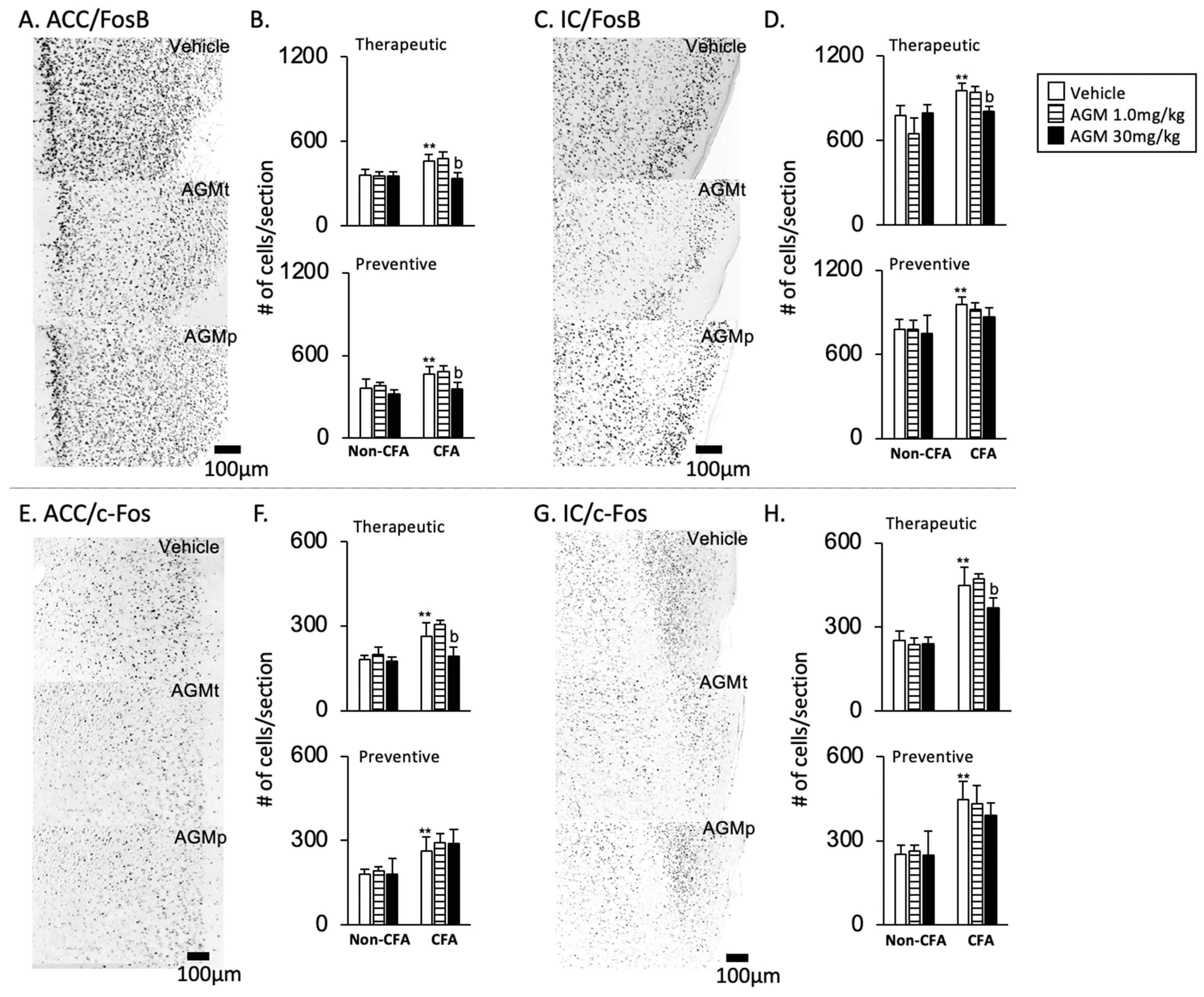
- 2
- FosB and c-Fos immunoreactivities
- FosB (Figure 7A–D)
- b.
- c-Fos (Figure 7E–H)
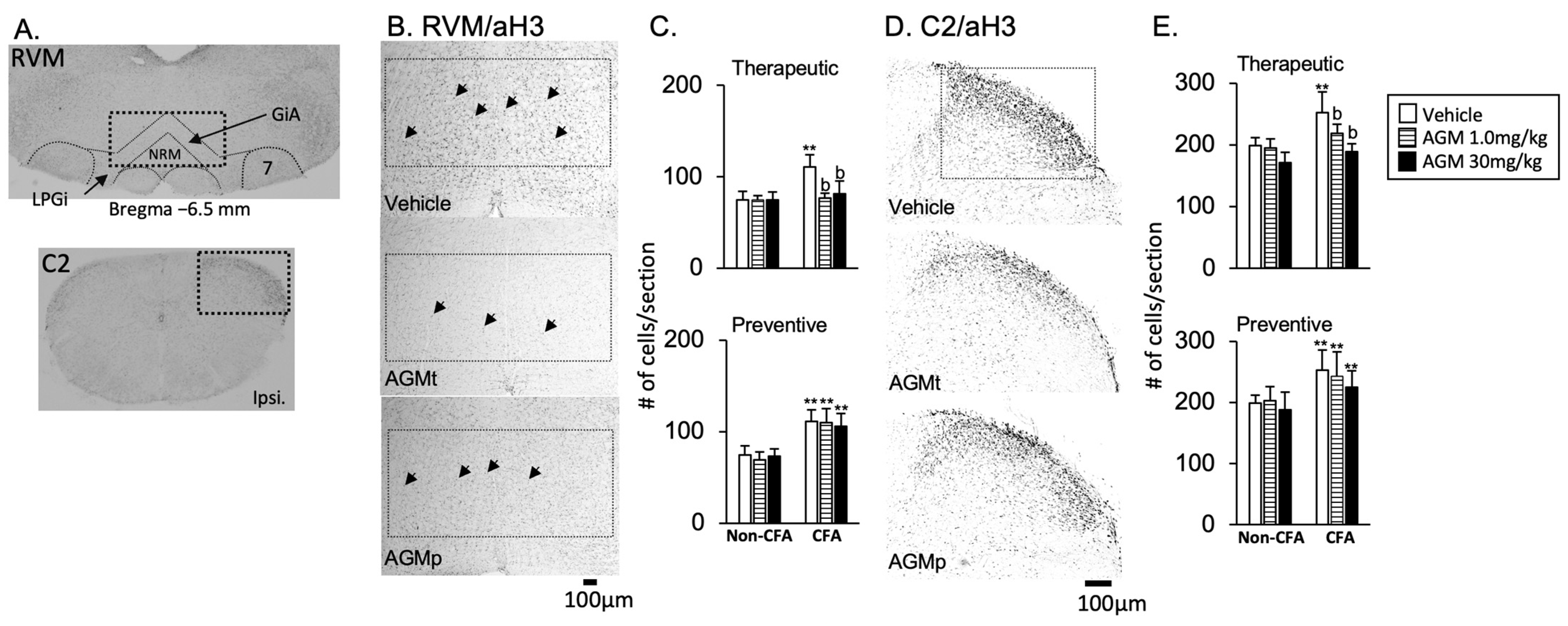
3.2.2. RVM and C2
- 1
- Acetylation histone H3 immunoreactivity
- AGMt (Figure 8C,E, upper panels)
- b.
- AGMp (Figure 8C,E, lower panels)
- 2
- FosB and c-Fos immunoreactivities
- FosB (Figure 9A–D)
- b.
- c-Fos (Figure 9E–H)
3.3. Determination of Agmatine in Sake Lees
4. Discussion
4.1. Effects of Craniofacial Inflammation on Anxiety-like Responses
4.2. Therapeutic Effects of Agmatine on Anxiety-like Behaviors
4.3. Preventive Effects of Agmatine on Anxiety-like Behaviors
4.4. Limitation
4.5. Future Perspective: Dietary Agmatine from Sake Lees Extracts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rafi, H.; Rafiq, H.; Farhan, M. Pharmacological profile of agmatine: An in-depth overview. Neuropeptides 2024, 105, 102429. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.O.; Goracke-Postle, C.J.; Kaminski, L.L.; Overland, A.C.; Morgan, A.D.; Fairbanks, C.A. Neuropharmacokinetic and dynamic studies of agmatine (decarboxylated arginine). Ann. N. Y. Acad. Sci. 2003, 1009, 82–105. [Google Scholar] [CrossRef] [PubMed]
- Piletz, J.E.; May, P.J.; Wang, G.; Zhu, H. Agmatine crosses the blood-brain barrier. Ann. N. Y. Acad. Sci. 2003, 1009, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.P.; Camargo, A.; Rodrigues, A.L.S. Agmatine as a novel candidate for rapid-onset antidepressant response. World J. Psychiatry 2021, 11, 981–996. [Google Scholar] [CrossRef]
- Kouba, B.R.; de Araujo Borba, L.; Borges de Souza, P.; Gil-Mohapel, J.; Rodrigues, A.L.S. Role of inflammatory mechanisms in major depressive disorder: From etiology to potential pharmacological targets. Cells 2024, 13, 423. [Google Scholar] [CrossRef]
- Xu, W.; Gao, L.; Li, T.; Shao, A.; Zhang, J. Neuroprotective role of agmatine in neurological diseases. Curr. Neuropharmacol. 2018, 16, 1296–1305. [Google Scholar] [CrossRef]
- Barua, S.; Kim, J.Y.; Kim, J.Y.; Kim, J.H.; Lee, J.E. Therapeutic effect of agmatine on neurological disease: Focus on ion channels and receptors. Neurochem. Res. 2019, 44, 735–750. [Google Scholar] [CrossRef]
- Chen, Z.D.; Chen, W.Q.; Wang, Z.Y.; Cao, D.N.; Wu, N.; Li, J. Antidepressant-like action of agmatine in the acute and sub-acute mouse models of depression: A receptor mechanism study. Metab. Brain Dis. 2018, 33, 1721–1731. [Google Scholar] [CrossRef]
- Akasaka, N.; Fujiwara, S. The therapeutic and nutraceutical potential of agmatine, and its enhanced production using Aspergillus oryzae. Amino Acids 2020, 52, 181–197. [Google Scholar] [CrossRef]
- Galgano, F.; Caruso, M.; Condelli, N.; Favati, F. Focused review: Agmatine in fermented foods. Front. Microbiol. 2012, 3, 199. [Google Scholar] [CrossRef]
- Nakatani, Y.; Kakihara, Y.; Shimizu, S.; Kurose, M.; Sato, T.; Kaneoke, M.; Saeki, M.; Takagi, R.; Yamamura, K.; Okamoto, K. Japanese Rice Wine can reduce psychophysical stress-induced depression-like behaviors and Fos expression in the trigeminal subnucleus caudalis evoked by masseter muscle injury in the rats. Biosci. Biotechnol. Biochem. 2019, 83, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.Y.; Lee, J.; Li, K.Y.; Leung, Y.Y.; Li, D.T.S. Psychological outcomes on anxiety and depression after interventions for temporomandibular disorders: A systematic review and meta-analysis. Diagnostics 2023, 13, 653. [Google Scholar] [CrossRef] [PubMed]
- Suvinen, T.I.; Reade, P.C.; Kemppainen, P.; Könönen, M.; Dworkin, S.F. Review of aetiological concepts of temporomandibular pain disorders: Towards a biopsychosocial model for integration of physical disorder factors with psychological and psychosocial illness impact factors. Eur. J. Pain 2005, 9, 613–633. [Google Scholar] [CrossRef] [PubMed]
- Del Val, B.M.; Shukla, S.R.; Oduoye, M.O.; Nsengiyumva, M.; Tesfaye, T.; Glinkowski, W.M. Prevalence of mental health disorders in knee osteoarthritis patients: A systematic review and meta-analysis. Ann. Med. Surg. 2024, 86, 4705–4713. [Google Scholar] [CrossRef]
- Alqarni, A.; Hosmani, J.; Alassiri, S.; AM, A.A.; Alfaifi, A.; Al Jazea, S.A. Association between psychosocial stressors and temporomandibular disorders in clinical dental students: A cross-sectional study. PeerJ 2025, 13, e19066. [Google Scholar] [CrossRef]
- Patel, R. The circuit basis for chronic pain and its comorbidities. Curr. Opin. Support. Palliat. Care 2023, 17, 156–160. [Google Scholar] [CrossRef]
- Crombez, G.; Vlaeyen, J.W.; Heuts, P.H.; Lysens, R. Pain-related fear is more disabling than pain itself: Evidence on the role of pain-related fear in chronic back pain disability. Pain 1999, 80, 329–339. [Google Scholar] [CrossRef]
- Piriyaprasath, K.; Kakihara, Y.; Hasegawa, M.; Iwamoto, Y.; Hasegawa, Y.; Fujii, N.; Yamamura, K.; Okamoto, K. Nutritional strategies for chronic craniofacial pain and temporomandibular disorders: Current clinical and preclinical insights. Nutrients 2024, 16, 2868. [Google Scholar] [CrossRef]
- Elma, Ö.; Yilmaz, S.T.; Deliens, T.; Clarys, P.; Nijs, J.; Coppieters, I.; Polli, A.; Malfliet, A. Chronic musculoskeletal pain and nutrition: Where are we and where are we heading? PM&R 2020, 12, 1268–1278. [Google Scholar] [CrossRef]
- Malau, I.A.; Chang, J.P.; Lin, Y.W.; Chang, C.C.; Chiu, W.C.; Su, K.P. Omega-3 fatty acids and neuroinflammation in depression: Targeting damage-associated molecular patterns and neural biomarkers. Cells 2024, 13, 1791. [Google Scholar] [CrossRef]
- Menéndez-Torre, Á.; Martin-Pintado-Zugasti, A.; Paris-Alemany, A.; Bocos-Corredor, E.; Molina-Álvarez, M.; Arribas-Romano, A.; Fernández-Carnero, J. Pain sensitization and pain-related psychological factors in patients with temporomandibular disorders: An observational cross-sectional study. Clin. Oral Investig. 2024, 28, 594. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.C.; Lucas, G.; Leite-Panissi, C.R.A. Emerging role of microglia and astrocyte in the affective-motivational response induced by a rat model of persistent orofacial pain. Brain Res. Bull. 2023, 195, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Hasegawa, M.; Piriyaprasath, K.; Kakihara, Y.; Saeki, M.; Yamamura, K. Preclinical models of deep craniofacial nociception and temporomandibular disorder pain. Jpn. Dent. Sci. Rev. 2021, 57, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Takahashi, Y.; Sugimura, Y.K.; Tokunaga, R.; Yajima, M.; Kato, F. Active role of the central amygdala in widespread mechanical sensitization in rats with facial inflammatory pain. Pain 2021, 162, 2273–2286. [Google Scholar] [CrossRef]
- Piriyaprasath, K.; Hasegawa, M.; Iwamoto, Y.; Kamimura, R.; Yusuf, A.S.H.; Fujii, N.; Yamamura, K.; Okamoto, K. Effects of treadmill running on anxiety- and craniofacial pain-like behaviors with histone H3 acetylation in the brain of mice subjected to social defeat stress. PLoS ONE 2025, 20, e0318292. [Google Scholar] [CrossRef]
- Piriyaprasath, K.; Kakihara, Y.; Kurahashi, A.; Taiyoji, M.; Kodaira, K.; Aihara, K.; Hasegawa, M.; Yamamura, K.; Okamoto, K. Preventive roles of rice-koji extracts and ergothioneine on anxiety- and pain-like responses under psychophysical stress conditions in Male Mice. Nutrients 2023, 15, 3989. [Google Scholar] [CrossRef]
- Hasegawa, M.; Piriyaprasath, K.; Otake, M.; Kamimura, R.; Saito, I.; Fujii, N.; Yamamura, K.; Okamoto, K. Effect of daily treadmill running exercise on masseter muscle nociception associated with social defeat stress in mice. Eur. J. Oral Sci. 2022, 130, e12882. [Google Scholar] [CrossRef]
- Chao, O.Y.; Nikolaus, S.; Yang, Y.M.; Huston, J.P. Neuronal circuitry for recognition memory of object and place in rodent models. Neurosci. Biobehav. Rev. 2022, 141, 104855. [Google Scholar] [CrossRef]
- Joaquim, A.F.; Appenzeller, S. Neuropsychiatric manifestations in rheumatoid arthritis. Autoimmun. Rev. 2015, 14, 1116–1122. [Google Scholar] [CrossRef]
- Viero, F.T.; Felix Morais, R.I.; Rodrigues, P.; Kudsi, S.Q.; Pereira, L.G.; Trevisan, G. Orofacial pain models induce impairment in spatial learning and working memory in rodents: A systematic review and meta-analysis. Eur. J. Pharmacol. 2025, 988, 177225. [Google Scholar] [CrossRef]
- Sánchez-Lafuente, C.L.; Reive, B.S.; Kalynchuk, L.E.; Caruncho, H.J. A scoping review of rodent studies investigating the epigenetic mechanisms in the brain underlying the effects of diet on depressive-like behaviour. Biomedicines 2022, 10, 3213. [Google Scholar] [CrossRef] [PubMed]
- López-Muñoz, E.; Mejía-Terrazas, G.E. Epigenetics and postsurgical pain: A scoping review. Pain Med. 2022, 23, 246–262. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, M.; Ye, L. Neuroimaging and artificial intelligence for assessment of chronic painful temporomandibular disorders-a comprehensive review. Int. J. Oral Sci. 2023, 15, 58. [Google Scholar] [CrossRef]
- Gonzalez-Hermosillo, D.C.; Gonzalez-Hermosillo, L.M.; Villaseñor-Almaraz, M.; Ballesteros-Herrera, D.; Moreno-Jimenez, S.; Corona-Cedillo, R.; Velasco-Campos, F.; Carrillo-Ruiz, J.D.; Roldan-Valadez, E. Current concepts of pain pathways: A brief review of anatomy, physiology, and medical imaging. Curr. Med. Imaging 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, Y.; Yamamoto, K.; Kobayashi, M. Descending projections from the insular cortex to the trigeminal spinal subnucleus caudalis facilitate excitatory outputs to the parabrachial nucleus in rats. Pain 2023, 164, e157–e173. [Google Scholar] [CrossRef]
- Mai, L.; Jia, S.; Liu, Q.; Chu, Y.; Liu, J.; Yang, S.; Huang, F.; Fan, W. Sympathectomy ameliorates CFA-induced mechanical allodynia via modulating phenotype of macrophages in sensory ganglion in mice. J. Inflamm. Res. 2022, 15, 6263–6274. [Google Scholar] [CrossRef]
- Gawali, N.B.; Bulani, V.D.; Gursahani, M.S.; Deshpande, P.S.; Kothavade, P.S.; Juvekar, A.R. Agmatine attenuates chronic unpredictable mild stress-induced anxiety, depression-like behaviours and cognitive impairment by modulating nitrergic signalling pathway. Brain Res. 2017, 1663, 66–77. [Google Scholar] [CrossRef]
- Rushaidhi, M.; Zhang, H.; Liu, P. Effects of prolonged agmatine treatment in aged male Sprague-Dawley rats. Neuroscience 2013, 234, 116–124. [Google Scholar] [CrossRef]
- Ghafarimoghadam, M.; Mashayekh, R.; Gholami, M.; Fereydani, P.; Shelley-Tremblay, J.; Kandezi, N.; Sabouri, E.; Motaghinejad, M. A review of behavioral methods for the evaluation of cognitive performance in animal models: Current techniques and links to human cognition. Physiol. Behav. 2022, 244, 113652. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, Compact, 3rd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2008; ISBN 978-0-12-374244-5. [Google Scholar]
- Gogolla, N. The insular cortex. Curr. Biol. 2017, 27, R580–R586. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, T.; Iwasaki, S.; Okada, M.; Okamoto, K.; Ikegaya, Y. Ethanol facilitates socially evoked memory recall in mice by recruiting pain-sensitive anterior cingulate cortical neurons. Nat. Commun. 2018, 9, 3526. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.B.; Leite-Morris, K.A.; Fan, W.; Young, A.J.; Guy, M.D. Opiate sensitization induces FosB/ΔFosB expression in prefrontal cortical, striatal and amygdala brain regions. PLoS ONE 2011, 6, e23574. [Google Scholar] [CrossRef] [PubMed]
- Doenni, V.M.; Song, C.M.; Hill, M.N.; Pittman, Q.J. Early-life inflammation with LPS delays fear extinction in adult rodents. Brain Behav. Immun. 2017, 63, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Hirayama, A.; Ishikawa, T.; Nakamura, S.; Shimizu, K.; Ueno, Y.; Tomita, M.; Soga, T. Depiction of metabolome changes in histidine-starved Escherichia coli by CE-TOFMS. Mol. Biosyst. 2008, 4, 135–147. [Google Scholar] [CrossRef]
- Ooga, T.; Sato, H.; Nagashima, A.; Sasaki, K.; Tomita, M.; Soga, T.; Ohashi, Y. Metabolomic anatomy of an animal model revealing homeostatic imbalances in dyslipidaemia. Mol. Biosyst. 2011, 7, 1217–1223. [Google Scholar] [CrossRef]
- Ou, F.; Su, K.; Sun, J.; Zhang, Z.; Peng, Y.; Liao, G. Temporomandibular joint disorders contribute to anxiety in BalB/C mice. Biochem. Biophys. Res. Commun. 2019, 516, 339–343. [Google Scholar] [CrossRef]
- do Nascimento, G.C.; Leite-Panissi, C.R. Time-dependent analysis of nociception and anxiety-like behavior in rats submitted to persistent inflammation of the temporomandibular joint. Physiol. Behav. 2014, 125, 1–7. [Google Scholar] [CrossRef]
- Parent, A.J.; Beaudet, N.; Beaudry, H.; Bergeron, J.; Bérubé, P.; Drolet, G.; Sarret, P.; Gendron, L. Increased anxiety-like behaviors in rats experiencing chronic inflammatory pain. Behav. Brain Res. 2012, 229, 160–167. [Google Scholar] [CrossRef]
- Chen, J.; Yan, Y.; Yuan, F.; Cao, J.; Li, S.; Eickhoff, S.B.; Zhang, J. Brain grey matter volume reduction and anxiety-like behavior in lipopolysaccharide-induced chronic pulmonary inflammation rats: A structural MRI study with histological validation. Brain Behav. Immun. 2019, 76, 182–197. [Google Scholar] [CrossRef]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef] [PubMed]
- Imbe, H.; Kimura, A. Repeated forced swim stress prior to complete Freund’s adjuvant injection enhances mechanical hyperalgesia and attenuates the expression of pCREB and ΔFosB and the acetylation of histone H3 in the insular cortex of rat. Neuroscience 2015, 301, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Imbe, H.; Iwai-Liao, Y. TMJ inflammation increases Fos expression in the nucleus raphe magnus induced by subsequent formalin injection of the masseter or hindpaw of rats. Okajimas Folia Anat. Jpn. 2006, 83, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Labrakakis, C. The role of the insular cortex in pain. Int. J. Mol. Sci. 2023, 24, 5736. [Google Scholar] [CrossRef]
- Kobayashi, M.; Nakaya, Y. Anatomical aspects of corticotrigeminal projections to the medullary dorsal horn. J. Oral Sci. 2020, 62, 144–146. [Google Scholar] [CrossRef]
- Iwata, K.; Miyachi, S.; Imanishi, M.; Tsuboi, Y.; Kitagawa, J.; Teramoto, K.; Hitomi, S.; Shinoda, M.; Kondo, M.; Takada, M. Ascending multisynaptic pathways from the trigeminal ganglion to the anterior cingulate cortex. Exp. Neurol. 2011, 227, 69–78. [Google Scholar] [CrossRef]
- Peterson, C.D.; Kitto, K.F.; Verma, H.; Pflepsen, K.; Delpire, E.; Wilcox, G.L.; Fairbanks, C.A. Agmatine requires GluN2B-containing NMDA receptors to inhibit the development of neuropathic pain. Mol. Pain 2021, 17, 17448069211029171. [Google Scholar] [CrossRef]
- Li, X.H.; Miao, H.H.; Zhuo, M. NMDA receptor dependent long-term potentiation in chronic pain. Neurochem. Res. 2019, 44, 531–538. [Google Scholar] [CrossRef]
- Fiori, L.M.; Turecki, G. Implication of the polyamine system in mental disorders. J. Psychiatry Neurosci. 2008, 33, 102–110. [Google Scholar]
- Chia, T.Y.; Zolp, A.; Miska, J. Polyamine immunometabolism: Central regulators of inflammation, cancer and autoimmunity. Cells 2022, 11, 896. [Google Scholar] [CrossRef]
- Caron, P.C.; Kremzner, L.T.; Cote, L.J. GABA and its relationship to putrescine metabolism in the rat brain and pancreas. Neurochem. Int. 1987, 10, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Inta, D.; Trusel, M.; Riva, M.A.; Sprengel, R.; Gass, P. Differential c-Fos induction by different NMDA receptor antagonists with antidepressant efficacy: Potential clinical implications. Int. J. Neuropsychopharmacol. 2009, 12, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Tasan, R.O.; Bukovac, A.; Peterschmitt, Y.N.; Sartori, S.B.; Landgraf, R.; Singewald, N.; Sperk, G. Altered GABA transmission in a mouse model of increased trait anxiety. Neuroscience 2011, 183, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Hu, X.-Z.; Wu, X.; Jiang, H.; Pan, H.; Marini, A.M.; Lipsky, R.H. Dynamic chromatin remodeling events in hippocampal neurons are associated with NMDA receptor-mediated activation of Bdnf gene promoter 1. J. Neurochem. 2009, 109, 1375–1388. [Google Scholar] [CrossRef]
- Kotagale, N.R.; Taksande, B.G.; Inamdar, N.N. Neuroprotective offerings by agmatine. NeuroToxicology 2019, 73, 228–245. [Google Scholar] [CrossRef]
- Rafi, H.; Rafiq, H.; Farhan, M. Inhibition of NMDA receptors by agmatine is followed by GABA/glutamate balance in benzodiazepine withdrawal syndrome. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 43. [Google Scholar] [CrossRef]
- Rushaidhi, M.; Collie, N.D.; Zhang, H.; Liu, P. Agmatine selectively improves behavioural function in aged male Sprague-Dawley rats. Neuroscience 2012, 218, 206–215. [Google Scholar] [CrossRef]
- Monarca, R.I.; Silva, R.F.B.; Gabriel, S.I.; Cerveira, A.M.; von Merten, S. The presence of a shelter in an open field test has differential effects on the behavior and stress response of two mouse species. J. Exp. Zool. A Ecol. Integr. Physiol. 2025, 343, 480–492. [Google Scholar] [CrossRef]
- Rosso, M.; Wirz, R.; Loretan, A.V.; Sutter, N.A.; Pereira da Cunha, C.T.; Jaric, I.; Würbel, H.; Voelkl, B. Reliability of common mouse behavioural tests of anxiety: A systematic review and meta-analysis on the effects of anxiolytics. Neurosci. Biobehav. Rev. 2022, 143, 104928. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, Z.; Li, Y.; Du, X.; Qian, K.; Yang, L.; Jia, G.; Yin, J.; Liao, S.; Zhou, Z. Agmatine attenuates the severity of immunometabolic disorders by suppressing macrophage polarization: An in vivo study using an ulcerative colitis mouse model. Biomed. Pharmacother. 2024, 180, 117549. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Jin, H.; Fan, X.; Yang, X.; Tang, W.; Yan, J.; Liang, H. Agmatine protects against zymosan-induced acute lung injury in mice by inhibiting NF-κB-mediated inflammatory response. Biomed. Res. Int. 2014, 2014, 583736. [Google Scholar] [CrossRef]
- Wei, X.L.; Su, R.B.; Lu, X.Q.; Liu, Y.; Yu, S.Z.; Yuan, B.L.; Li, J. Inhibition by agmatine on morphine-induced conditioned place preference in rats. Eur. J. Pharmacol. 2005, 515, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Neis, V.B.; Moretti, M.; Manosso, L.M.; Lopes, M.W.; Leal, R.B.; Rodrigues, A.L. Agmatine enhances antidepressant potency of MK-801 and conventional antidepressants in mice. Pharmacol. Biochem. Behav. 2015, 130, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, K.; Milosevic, A.; Stevanovic, I.; Zivkovic, A.; Laketa, D.; Janjic, M.M.; Bjelobaba, I.; Lavrnja, I.; Savic, D. Agmatine suppresses glycolysis via the PI3K/Akt/mTOR/HIF-1α signaling pathway and improves mitochondrial function in microglia exposed to lipopolysaccharide. Biofactors 2025, 51, e2149. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.L.; Tohidi, V.; Sherwood, K.; Gayen, S.; Medel, R.; Gilad, G.M. Evidence for Dietary Agmatine Sulfate Effectiveness in Neuropathies Associated with Painful Small Fiber Neuropathy. A Pilot Open-Label Consecutive Case Series Study. Nutrients 2020, 12, 576. [Google Scholar] [CrossRef]
- Shimizu, S.; Nakatani, Y.; Kakihara, Y.; Taiyoji, M.; Saeki, M.; Takagi, R.; Yamamura, K.; Okamoto, K. Daily administration of Sake Lees (Sake Kasu) reduced psychophysical stress-induced hyperalgesia and Fos responses in the lumbar spinal dorsal horn evoked by noxious stimulation to the hindpaw in the rats. Biosci. Biotechnol. Biochem. 2020, 84, 159–170. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwamoto, Y.; Piriyaprasath, K.; Yusuf, A.S.H.; Hasegawa, M.; Kakihara, Y.; Sato, T.; Fujii, N.; Yamamura, K.; Okamoto, K. Daily Administration of Agmatine Reduced Anxiety-like Behaviors and Neural Responses in the Brains of Male Mice with Persistent Inflammation in the Craniofacial Region. Nutrients 2025, 17, 1848. https://doi.org/10.3390/nu17111848
Iwamoto Y, Piriyaprasath K, Yusuf ASH, Hasegawa M, Kakihara Y, Sato T, Fujii N, Yamamura K, Okamoto K. Daily Administration of Agmatine Reduced Anxiety-like Behaviors and Neural Responses in the Brains of Male Mice with Persistent Inflammation in the Craniofacial Region. Nutrients. 2025; 17(11):1848. https://doi.org/10.3390/nu17111848
Chicago/Turabian StyleIwamoto, Yuya, Kajita Piriyaprasath, Andi Sitti Hajrah Yusuf, Mana Hasegawa, Yoshito Kakihara, Tsutomu Sato, Noritaka Fujii, Kensuke Yamamura, and Keiichiro Okamoto. 2025. "Daily Administration of Agmatine Reduced Anxiety-like Behaviors and Neural Responses in the Brains of Male Mice with Persistent Inflammation in the Craniofacial Region" Nutrients 17, no. 11: 1848. https://doi.org/10.3390/nu17111848
APA StyleIwamoto, Y., Piriyaprasath, K., Yusuf, A. S. H., Hasegawa, M., Kakihara, Y., Sato, T., Fujii, N., Yamamura, K., & Okamoto, K. (2025). Daily Administration of Agmatine Reduced Anxiety-like Behaviors and Neural Responses in the Brains of Male Mice with Persistent Inflammation in the Craniofacial Region. Nutrients, 17(11), 1848. https://doi.org/10.3390/nu17111848





