Association of Comorbidity and Inflammatory and Nutritional Markers with Epilepsy and Seizure Frequency
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Definition of Epilepsy
2.3. Modified Charlson Comorbidity Index (mCCI)
2.4. Hematological Parameters
2.5. Statistical Analysis
3. Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beghi, E. The epidemiology of epilepsy. Neuroepidemiology 2020, 54, 185–191. [Google Scholar] [CrossRef] [PubMed]
- WHO. Epilepsy. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 4 January 2025).
- Zaitsev, A.V.; Khazipov, R. Molecular and Cellular Mechanisms of Epilepsy. Int. J. Mol. Sci. 2023, 24, 12415. [Google Scholar] [CrossRef] [PubMed]
- GBD Epilepsy Collaborators. Global, regional, and national burden of epilepsy, 1990-2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Public Health 2025, 10, e203–e227. [Google Scholar] [CrossRef]
- Kerr, M.P. The impact of epilepsy on patients’ lives. Acta Neurol. Scand. 2012, 126, 1–9. [Google Scholar] [CrossRef]
- McCormick, D.A.; Contreras, D. On the cellular and network bases of epileptic seizures. Annu. Rev. Physiol. 2001, 63, 815–846. [Google Scholar] [CrossRef]
- Badawy, R.A.; Harvey, A.S.; Macdonell, R.A. Cortical hyperexcitability and epileptogenesis: Understanding the mechanisms of epilepsy–part 1. J. Clin. Neurosci. 2009, 16, 355–365. [Google Scholar] [CrossRef]
- Da Silva, F.L.; Blanes, W.; Kalitzin, S.N.; Parra, J.; Suffczynski, P.; Velis, D.N. Epilepsies as dynamical diseases of brain systems: Basic models of the transition between normal and epileptic activity. Epilepsia 2003, 44, 72–83. [Google Scholar] [CrossRef]
- Engelborghs, S.; D’hooge, R.; De Deyn, P. Pathophysiology of epilepsy. Acta Neurol. Belg. 2000, 100, 201–213. [Google Scholar]
- Stafstrom, C.E. Pathophysiological mechanisms of seizures and epilepsy: A primer. In Epilepsy; CRC Press: Boca Raton, FL, USA, 2010; pp. 25–42. [Google Scholar]
- Cao, Z.; Li, Y.; Liu, S.; He, Z.; Li, J. Clinical characteristics and impact of comorbidities on the prognosis of senile epilepsy in Southwest China: A retrospective cohort study. Acta Epileptol. 2024, 6, 11. [Google Scholar] [CrossRef]
- Seidenberg, M.; Pulsipher, D.T.; Hermann, B. Association of epilepsy and comorbid conditions. Future Neurol. 2009, 4, 663–668. [Google Scholar] [CrossRef]
- Lee, J.; Choi, A.; Kim, S. Effects of Psychiatric Comorbidities on the Prognosis of New-Onset Pediatric Epilepsy: A Retrospective Nationwide Cohort Study. J. Clin. Med. 2024, 13, 4500. [Google Scholar] [CrossRef] [PubMed]
- Gaitatzis, A.; Carroll, K.; Majeed, A.; Sander, J.W. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia 2004, 45, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Hitiris, N.; Mohanraj, R.; Norrie, J.; Brodie, M.J. Mortality in epilepsy. Epilepsy Behav. 2007, 10, 363–376. [Google Scholar] [CrossRef]
- Belluzzo, M.; Furlanis, G.; Stragapede, L.; Monti, F. Role of comorbidities and in-hospital complications in short-term status epilepticus outcome. Clin. Neurol. Neurosurg. 2017, 154, 13–18. [Google Scholar] [CrossRef]
- Giussani, G.; Bianchi, E.; Beretta, S.; Carone, D.; DiFrancesco, J.C.; Stabile, A.; Zanchi, C.; Pirovano, M.; Trentini, C.; Padovano, G.; et al. Comorbidities in patients with epilepsy: Frequency, mechanisms and effects on long-term outcome. Epilepsia 2021, 62, 2395–2404. [Google Scholar] [CrossRef]
- Kabboord, A.D.; Godfrey, D.; Gordon, A.L.; Gladman, J.R.F.; Van Eijk, M.; van Balen, R.; Achterberg, W.P. The modified functional comorbidity index performed better than the Charlson index and original functional comorbidity index in predicting functional outcome in geriatric rehabilitation: A prospective observational study. BMC Geriatr. 2020, 20, 114. [Google Scholar] [CrossRef]
- Denti, L.; Artoni, A.; Casella, M.; Giambanco, F.; Scoditti, U.; Ceda, G.P. Validity of the modified Charlson comorbidity index as predictor of short-term outcome in older stroke patients. J. Stroke Cerebrovasc. Dis. 2015, 24, 330–336. [Google Scholar] [CrossRef]
- Maldonado-Cabrera, A.; Angulo-Molina, A.; Haque, U.; Velazquez, C.; Álvarez-Villaseñor, A.S.; Santacruz-Gómez, K.J.; Gallego-Hernández, A.L. Acute inflammatory mediators in young adult patients with COVID-19 in Mexico. Pathogens 2021, 10, 1056. [Google Scholar] [CrossRef]
- Çinar, Z.; Yiğit, Ö.; Ertugay, Ç.K. Evaluation of inflammation parameters in complete blood count in patients diagnosed with vestibular migraine. KBB-Forum 2021, 20, 37–44. [Google Scholar]
- Qin, B.; Ma, N.; Tang, Q.; Wei, T.; Yang, M.; Fu, H.; Hu, Z.; Liang, Y.; Yang, Z.; Zhong, R. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Mod. Rheumatol. 2016, 26, 372–376. [Google Scholar] [CrossRef]
- Tefferi, A.; Nicolosi, M.; Penna, D.; Mudireddy, M.; Szuber, N.; Lasho, T.L.; Hanson, C.A.; Ketterling, R.P.; Gangat, N.; Pardanani, A.D. Development of a prognostically relevant cachexia index in primary myelofibrosis using serum albumin and cholesterol levels. Blood Adv. 2018, 2, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- Lucijanic, M.; Veletic, I.; Rahelic, D.; Pejsa, V.; Cicic, D.; Skelin, M.; Livun, A.; Tupek, K.M.; Stoos-Veic, T.; Lucijanic, T.; et al. Assessing serum albumin concentration, lymphocyte count and prognostic nutritional index might improve prognostication in patients with myelofibrosis. Wien. Klin. Wochenschr. 2018, 130, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Allen Hauser, W.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984, 85, 1001–1005. [Google Scholar]
- Keezer, M.R.; Bell, G.S.; Jetté, N.; Sander, J.W. The performance of three mortality risk-adjustment comorbidity indices in a community epilepsy cohort. Epilepsia 2015, 56, e68–e72. [Google Scholar] [CrossRef]
- Hosseini, S.; Mofrad, A.M.E.; Mokarian, P.; Nourigheimasi, S.; Azarhomayoun, A.; Khanzadeh, S.; Habibzadeh, S.; Ghaedi, A. Neutrophil to lymphocyte ratio in epilepsy: A systematic review. Mediat. Inflamm. 2022, 2022, 4973996. [Google Scholar] [CrossRef]
- Ozdemir, A.F.; Kemerdere, R.; Orhan, B.; Emre, H.O.; Inal, B.B.; Kayhan, A.; Yeni, S.N.; Tanriverdi, T. Serum endocan and preoperative systemic inflammatory markers in patients with epilepsy. Neurochirurgie 2020, 66, 29–35. [Google Scholar] [CrossRef]
- Güneş, M.; Büyükgöl, H. Relationship between generalized epileptic seizure and neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and neutrophil mediated inflammation. Int. J. Neurosci. 2020, 130, 1095–1100. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Song, S.; Pan, H.; Huang, C.; Sun, T.; Wang, S.; Xu, J. Correlation between inflammatory markers over time and disease severity in status epilepticus: A preliminary study. Front. Neurol. 2024, 15, 1334415. [Google Scholar] [CrossRef] [PubMed]
- Olivo, S.; Stella, A.B.; Pavan, S.; Cegalin, M.; Furlanis, G.; Cheli, M.; Tomaselli, M.; Stokelj, D.; Manganotti, P. Admission neutrophil-to-lymphocyte ratio predicts length of hospitalization and need for ICU admission in adults with Status Epilepticus. Seizure 2023, 106, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, Z.; Fan, R.; Liu, S.; Zheng, W.; Xiao, F. Association of blood count-derived immunoinflammatory makers and risk of epilepsy: A prospective cohort of 497,291 participants. Seizure 2024, 123, 9–16. [Google Scholar] [CrossRef]
- Ates, I.; Ozkayar, N.; Ates, H.; Karakulak, U.N.; Kursun, O.; Topcuoglu, C.; Inan, B.; Yilmaz, N. Elevated circulating sST2 associated with subclinical atherosclerosis in newly diagnosed primary hypertension. Hypertens. Res. 2016, 39, 513–518. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Maida, C.; Pinto, A. Inflammation and inflammatory cell recruitment in acute cerebrovascular diseases. Curr. Immunol. Rev. 2015, 11, 24–32. [Google Scholar] [CrossRef]
- Sarejloo, S.; Dehesh, M.; Fathi, M.; Khanzadeh, M.; Lucke-Wold, B.; Ghaedi, A.; Khanzadeh, S. Meta-analysis of differences in neutrophil to lymphocyte ratio between hypertensive and non-hypertensive individuals. BMC Cardiovasc. Disord. 2023, 23, 283. [Google Scholar] [CrossRef]
- Larmann, J.; Handke, J.; Scholz, A.S.; Dehne, S.; Arens, C.; Gillmann, H.-J.; Uhle, F.; Motsch, J.; Weigand, M.A.; Janssen, H. Preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are associated with major adverse cardiovascular and cerebrovascular events in coronary heart disease patients undergoing non-cardiac surgery. BMC Cardiovasc. Disord. 2020, 20, 230. [Google Scholar] [CrossRef]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef]
- Rana, A.; Musto, A.E. The role of inflammation in the development of epilepsy. J. Neuroinflamm. 2018, 15, 144. [Google Scholar] [CrossRef]
- Ichiyama, T.; Okada, K.; Lipton, J.M.; Matsubara, T.; Hayashi, T.; Furukawa, S. Sodium valproate inhibits production of TNF-alpha and IL-6 and activation of NF-kappaB. Brain Res. 2000, 857, 246–251. [Google Scholar] [CrossRef]
- Labh, R.; Gupta, R.; Narang, M.; Halder, S.; Kar, R. Effect of valproate and add-on levetiracetam on inflammatory biomarkers in children with epilepsy. Epilepsy Behav. 2021, 125, 108358. [Google Scholar] [CrossRef] [PubMed]
- Guenther, S.; Bauer, S.; Hagge, M.; Knake, S.; Olmes, D.G.; Tackenberg, B.; Rosenow, F.; Hamer, H.M. Chronic valproate or levetiracetam treatment does not influence cytokine levels in humans. Seizure 2014, 23, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Tsukagoshi, M.; Araki, K.; Igarashi, T.; Ishii, N.; Kawai, S.; Hagiwara, K.; Hoshino, K.; Seki, T.; Okuyama, T.; Fukushima, R.; et al. Lower Geriatric Nutritional Risk Index and Prognostic Nutritional Index Predict Postoperative Prognosis in Patients with Hepatocellular Carcinoma. Nutrients 2024, 16, 940. [Google Scholar] [CrossRef]
- Misirlioglu, N.F.; Uzun, N.; Ozen, G.D.; Çalik, M.; Altinbilek, E.; Sutasir, N.; Baykara Sayili, S.; Uzun, H. The Relationship between Neutrophil-Lymphocyte Ratios with Nutritional Status, Risk of Nutritional Indices, Prognostic Nutritional Indices and Morbidity in Patients with Ischemic Stroke. Nutrients 2024, 16, 1225. [Google Scholar] [CrossRef]
- Jhang, S.-W.; Liu, Y.-T.; Kor, C.-T.; Wu, Y.-P.; Lai, C.-H. Low Prognostic Nutritional Index Predicts In-Hospital Complications and Case Fatality in Patients with Spontaneous Intracerebral Hemorrhage: A Retrospective Study. Nutrients 2024, 16, 1841. [Google Scholar] [CrossRef]
- Ismail, R.S.; Kishk, N.A.; Rizk, H.I.; El-Kholy, T.; El-Maoula, L.M.A.; El-Desoky, O.I.; Shaheen, S.; El-Sawy, E. Nutritional intake and its impact on patients with epilepsy: An analytical cross-sectional study. Nutr. Neurosci. 2022, 25, 1813–1822. [Google Scholar] [CrossRef]
- Mendonça, C.N.; Henriques-Souza, A.M.M.; Viana, L.A.; Souza, P.A.; Alves Neto, L.B.; Mello, M.J.G. Ketogenic diet in pharmacoresistant epilepsies: A clinical nutritional assessment. Dieta cetogênica na epilepsia fármaco-resistente: Avaliação clínica-nutricional. Arq. Neuropsiquiatr. 2024, 82, s00441779269. [Google Scholar]
- Bertoli, S.; Cardinali, S.; Veggiotti, P.; Trentani, C.; Testolin, G.; Tagliabue, A. Evaluation of nutritional status in children with refractory epilepsy. Nutr. J. 2006, 5, 14. [Google Scholar] [CrossRef]
- Çetin, I.D.; Çetin, O. A preliminary study on the association between prognostic nutritional index and neutrophil-to-lymphocyte ratio with nutritional status and inflammation in febrile children’s susceptibility to seizures. Rev. Assoc. Médica Bras. 2024, 70, e20240166. [Google Scholar] [CrossRef]
- He, F.; Gu, X.-S.; Chu, X.-L.; Song, X.-Z.; Li, Q.; Li, Y.-R.; Ming, D. Basic mechanisms of peripheral nerve injury and treatment via electrical stimulation. Neural Regen. Res. 2022, 17, 2185–2193. [Google Scholar] [CrossRef]


| Variables | Control n = 53 | Epilepsy | p-Value | |
|---|---|---|---|---|
| Without Comorbidity n = 53 | With Comorbidity n = 53 | |||
| Age, years | 44.0 ± 14.2 | 33.0 ± 12.5 | 56.2 ± 13.8 | <0.001 * |
| Sex, n (%) | ||||
| Female | 21 (39.6) | 28 (52.8) | 22 (41.5) | 0.369 |
| Male | 32 (60.4) | 25 (47.2) | 31 (58.5) | |
| Epilepsy type, n (%) | ||||
| Focal | - | 34 (64.2) | 40 (75.5) | 0.441 |
| Generalized | - | 14 (26.4) | 9 (17.0) | |
| Combined | - | 5 (9.4) | 4 (17.5) | |
| MRI findings, n (%) | ||||
| Normal | - | 41 (77.4) | 31 (58.5) | 0.060 |
| Abnormal | - | 12 (22.6) | 22 (41.5) | |
| EEG, n (%) | ||||
| Normal | - | 4 (7.5) | 5 (9.4) | 0.999 |
| Epileptiform | - | 45 (84.9) | 44 (83.0) | |
| Nonepileptiform | - | 4 (7.5) | 4 (7.5) | |
| Duration of disease, years | - | 3 (2–9) | 4 (2–7) | |
| mCCI | - | - | 5 (3–6) | - |
| Low comorbidity | - | - | 9 (17.0) | - |
| Moderate comorbidity | - | - | 15 (28.3) | |
| High comorbidity | - | - | 29 (54.7) | |
| Laboratory findings | ||||
| Hemoglobin, g/dL | 14.1 ± 1.5 | 13.3 ± 1.3 | 13.6 ± 1.5 | 0.007 * |
| RBC, ×106 µL | 4.8 ± 0.6 | 4.5 ± 0.4 | 4.7 ± 0.7 | 0.031 * |
| Hematocrit, % | 42.0 ± 3.5 | 39.2 ± 3.4 | 40.8 ± 3.5 | <0.001 * |
| MCV, fL | 86.6 ± 3.3 | 85.9 ± 5.0 | 85.3 ± 5.7 | 0.352 |
| MCH, pg | 29.8 ± 1.5 | 29.3 ± 1.9 | 28.7 ± 2.0 | 0.008 * |
| MCHC, g/dL | 34.2 ± 1.6 | 33.8 ± 1.2 | 33.7 ± 1.3 | 0.183 |
| Leukocytes, ×103 µL | 6.0 ± 1.5 | 6.8 ± 1.8 | 8.0 ± 2.0 | <0.001 * |
| Lymphocytes, ×103 µL | 2.5 (1.9–2.7) | 2.3 (1.8–2.6) | 2.1 (1.7–2.5) | 0.030 * |
| Neutrophils, ×103 µL | 2.8 (2.3–3.1) | 3.5 (3.0–4.6) | 4.7 (4–5.8) | <0.001 * |
| Monocytes, ×103 µL | 0.5 ± 0.1 | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.222 |
| Platelets, ×103 µL | 241.5 ± 70 | 262.6 ± 76.7 | 249.7 ± 65.9 | 0.306 |
| MPV, fL | 9.4 ± 1.1 | 9.6 ± 0.9 | 9.4 ± 1.2 | 0.424 |
| PCT, % | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.265 |
| PDW, % | 10.2 ± 1.8 | 10.4 ± 1.9 | 11.7 ± 2.4 | 0.005 * |
| CRP, mg/dL | 0.5 (0.1–1.0) | 0.5 (0.1–1.0) | 0.9 (0.5–2.3) | 0.045 * |
| Albumin, g/dL | 4.2 ± 0.5 | 3.7 ± 0.7 | 3.2 ± 0.6 | <0.001 * |
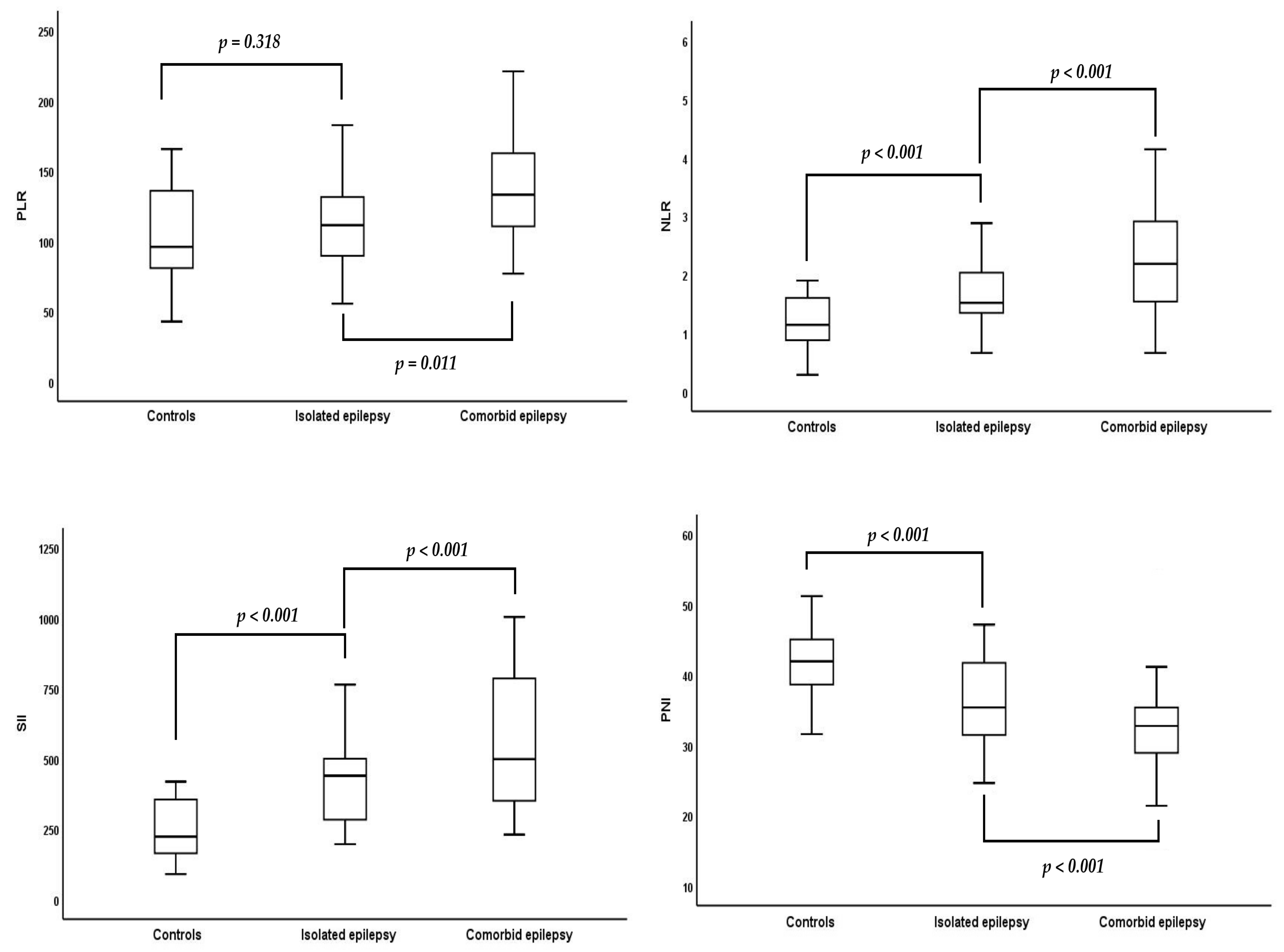
| PLR | 95.9 (80.9–135.8) | 111.4 (89.6–131.4) | 133.7 (114.2–168.1) | 0.044 * |
| NLR | 1.1 (0.9–1.6) | 1.5 (1.3–2.0) | 2.2 (1.5–2.9) | <0.001 * |
| SII | 244.3 (185.0–376.3) | 449.4 (283.4–504.1) | 500 (371.5–787.7) | <0.001 * |
| PNI | 42.2 ± 4.7 | 36.5 ± 7.6 | 32.2 ± 6.7 | <0.001 * |
| ≥35 (normal) | 35 (66.0) | 23 (43.4) | 16 (30.2) | <0.001 * |
| <35 (malnutrition) | 18 (34.0) | 30 (56.6) | 37 (69.8) | |
| Seizure frequency, n (%) | ||||
| Seizure-freedom | - | 24 (45.3) | 13 (24.5) | 0.020 * |
| Once a month | - | 5 (9.4) | 9 (17.0) | |
| More than once a month | - | 5 (9.4) | 8 (15.1) | |
| Low comorbidity | - | 13 (24.5) | 7 (13.2) | |
| Once a year | - | 6 (11.3) | 16 (30.2) | |
| More than once a year | ||||
| Variables | Seizure Freedom | p-Value | |
|---|---|---|---|
| Yes n = 37 | No n = 69 | ||
| Age, years | 38.8 ± 15.9 | 48.6 ± 16.8 | 0.004 * |
| Sex, n (%) | |||
| Female | 16 (43.2) | 34 (49.3) | 0.684 |
| Male | 21 (56.8) | 35 (50.7) | |
| Epilepsy type, n (%) | |||
| Focal | 23 (62.2) | 51 (73.9) | 0.296 |
| Generalized | 9 (24.3) | 14 (20.3) | |
| Combined | 5 (13.5) | 4 (5.8) | |
| MRI findings, n (%) | |||
| Normal | 28 (75.7) | 44 (63.8) | 0.276 |
| Abnormal | 9 (24.3) | 25 (36.2) | |
| EEG, n (%) | |||
| Normal | 5 (13.5) | 4 (5.8) | 0.426 |
| Epileptiform | 29 (78.4) | 60 (87.0) | |
| Nonepileptiform | 3 (8.1) | 5 (7.2) | |
| Duration of disease, years | 3 (2–9) | 4 (2–8) | 0.883 |
| mCCI | 3 (2–5) | 5 (3.5–6) | 0.008 * |
| No comorbid conditions | 24 (64.9) | 29 (42.0) | 0.022 * |
| Low comorbidity | 4 (10.8) | 5 (7.2) | |
| Moderate comorbidity | 5 (13.5) | 10 (14.5) | |
| High comorbidity | 4 (10.8) | 25 (36.2) | |
| Laboratory findings | |||
| Hemoglobin, g/dL | 13.1 ± 1.0 | 13.6 ± 1.6 | 0.053 |
| RBC, ×106 µL | 4.6 ± 0.6 | 4.7 ± 0.5 | 0.290 |
| Hematocrit, % | 39.4 ± 3.4 | 40.2 ± 3.5 | 0.114 |
| MCV, fL | 85.4 ± 5.4 | 85.7 ± 5.4 | 0.788 |
| MCH, pg | 28.7 ± 1.8 | 29.2 ± 2.0 | 0.228 |
| MCHC, g/dL | 33.7 ± 1.3 | 33.8 ± 1.2 | 0.716 |
| Leukocytes, ×103 µL | 7.1 ± 2.0 | 7.5 ± 1.9 | 0.291 |
| Lymphocytes, ×103 µL | 2.6 (2.2–2.8) | 2.1 (1.8–2.7) | 0.014 * |
| Neutrophils, ×103 µL | 3.6 (3–4.7) | 4.6 (3.5–5.7) | 0.005 * |
| Monocytes, ×103 µL | 0.6 ± 0.2 | 0.6 ± 0.2 | 0.363 |
| Platelets, ×103 µL | 239.3 ± 74.8 | 265.2 ± 68.4 | 0.075 |
| MPV, fL | 9.5 ± 1.1 | 9.5 ± 1.0 | 0.926 |
| PCT, % | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.223 |
| PDW, % | 10.7 ± 3.0 | 11.3 ± 2.7 | 0.336 |
| CRP, mg/dL | 0.1 (0.1–1.2) | 0.2 (0.1–1.5) | 0.620 |
| Albumin, g/dL | 3.7 ± 0.7 | 3.2 ± 0.6 | <0.001 * |
| PLR | 91.0 (77.9–112.6) | 118.6 (95.0–153.1) | <0.001 * |
| NLR | 1.5 (1.3–1.7) | 2.2 (1.5–2.8) | <0.001 * |
| SII | 328.6 (274.4–449.4) | 534 (439.6–733.8) | <0.001 * |
| PNI | 37.6 ± 7.6 | 32.0 ± 6.5 | <0.001 * |
| 35≥ (normal) | 21 (56.8) | 18 (26.1) | <0.001 * |
| <35 (malnutrition) | 16 (43.2) | 51 (73.9) | |
| Variables | Univariable Regression | Multivariable Regression | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age, years | 1.04 (1.01–1.07) | 0.006 * | - | - |
| mCCI | 1.68 (1.10–2.56) | 0.016 * | - | - |
| No comorbid conditions | ref | ref | ||
| Low comorbidity | 1.03 (0.25–4.28) | 0.963 | 0.46 (0.09–2.33) | 0.650 |
| Moderate comorbidity | 1.66 (0.50–5.51) | 0.411 | 1.52 (0.41–5.65) | 0.528 |
| High comorbidity | 5.17 (1.58–16.93) | 0.007 * | 4.56 (1.30–16.01) | 0.018 * |
| Laboratory findings | ||||
| Lymphocytes | 0.48 (0.26–0.85) | 0.013 * | - | - |
| Neutrophils | 1.47 (1.10–1.98) | 0.010 * | - | - |
| Albumin | 0.32 (0.17–0.61) | 0.001 * | - | - |
| PLR | 1.03 (1.02–1.05) | <0.001 * | - | - |
| NLR | 4.35 (1.96–9.63) | <0.001 * | - | - |
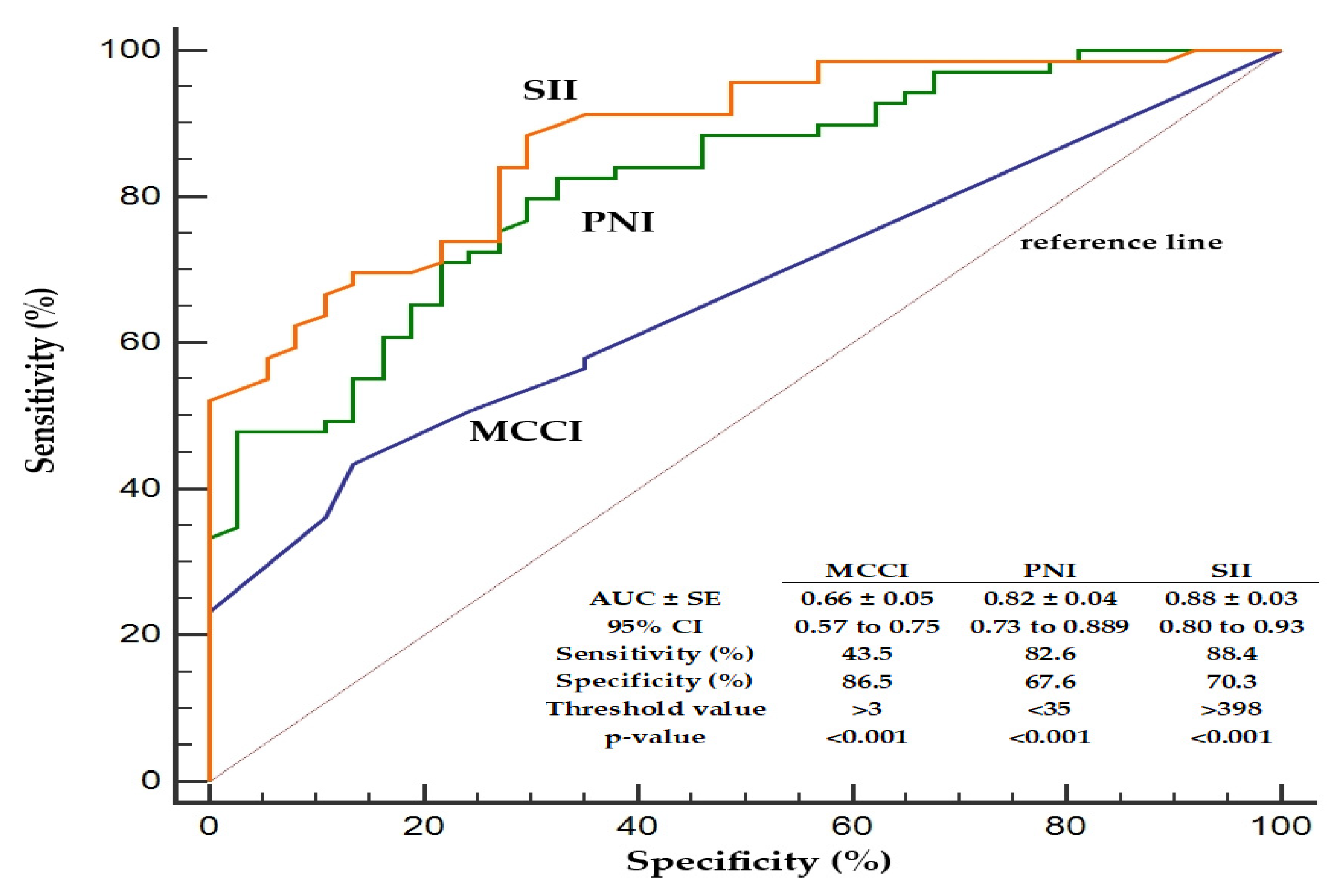
| SII | 1.13 (1.08–1.19) | <0.001 * | 1.13 (1.08–1.19) | <0.001 * |
| PNI | 0.89 (0.84–0.95) | <0.001 * | 0.88 (0.81–0.96) | 0.004 * |
| Nagelkerke R2 = 0.59 | ||||
| Variables | Crude Regression | Adjusted Regression | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Hypertension | 1.70 (0.36–8.05) | 0.504 | 1.92 (0.38–9.76) | 0.432 |
| Cerebrovascular disease | 4.5 (1.08–22.97) | 0.021 * | 4.15 (1.10–20.30) | 0.045 * |
| Hemiplegia | 5.14 (1.06–24.12) | 0.025 * | 4.48 (1.05–21.6) | 0.048 * |
| Dementia | 0.40 (0.10–1.55) | 0.187 | 0.25 (0.04–1.07) | 0.106 |
| Coronary artery disease | 2.08 (0.39–10.59) | 0.385 | 1.03 (0.09–12.17) | 0.981 |
| Peptic ulcer disease | 0.48 (0.11–2.00) | 0.312 | 0.42 (0.09–1.95) | 0.271 |
| Congestive heart failure | 1.11 (0.25–4.86) | 0.889 | 1.17 (0.25–5.44) | 0.841 |
| Hyperlipidemia | 3.48 (0.40–30.54) | 0.26 | 3.22 (0.36–29.14) | 0.299 |
| Diabetes mellitus | 1.71 (0.18–16.18) | 0.638 | 1.22 (0.11–13.45) | 0.872 |
| Peripheral vascular disease | 1.33 (0.14–13.12) | 0.805 | 1.36 (0.13–14.80) | 0.799 |
| Connective tissue disease | 0.63 (0.05–7.59) | 0.717 | 0.98 (0.07–13.75) | 0.989 |
| Chronic pulmonary disease | 0.31 (0.02–5.30) | 0.417 | 0.19 (0.01–3.72) | 0.275 |
| Variables | mCCI | |
|---|---|---|
| r | p | |
| Age | 0.479 | <0.001 * |
| Sex | ||
| Female | 5 (3–6) | 0.355 |
| Male | 5 (3–6) | |
| Epilepsy type | ||
| Focal | 5 (3–6) | 0.806 |
| Generalized | 5 (4–6) | |
| Combined | 5 (4–6) | |
| MRI findings | ||
| Normal | 4 (3–5) | 0.280 |
| Abnormal | 5 (3–6) | |
| EEG | ||
| Normal | 4 (3–5) | 0.648 |
| Epileptiform | 5 (3–6) | |
| Nonepileptiform | 4 (3–5) | |
| Duration of disease | −0.058 | 0.683 |
| Laboratory findings | ||
| Hemoglobin | 0.098 | 0.484 |
| RBC | 0.168 | 0.230 |
| Hematocrit | −0.059 | 0.673 |
| MCV | 0.126 | 0.370 |
| MCH | 0.192 | 0.168 |
| MCHC | −0.039 | 0.784 |
| Leukocytes | 0.169 | 0.225 |
| Lymphocytes | −0.069 | 0.622 |
| Neutrophils | 0.066 | 0.637 |
| Monocytes | 0.154 | 0.271 |
| Platelets | 0.189 | 0.174 |
| MPV | 0.092 | 0.513 |
| PCT | 0.025 | 0.858 |
| PDW | 0.068 | 0.633 |
| CRP | 0.075 | 0.594 |
| Albumin | −0.292 | 0.089 |
| PLR | 0.226 | 0.104 |
| NLR | 0.126 | 0.369 |
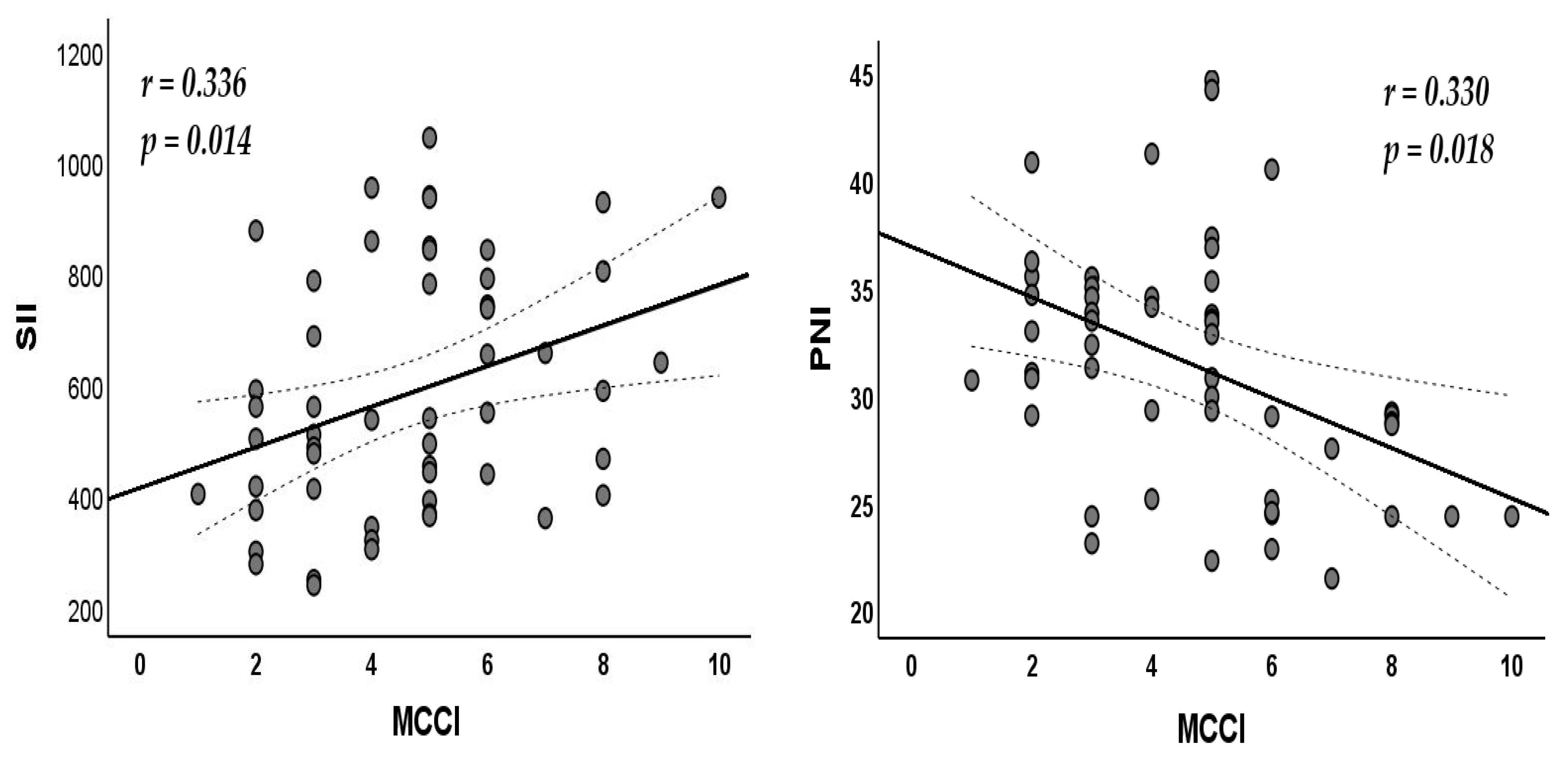
| SII | 0.336 | 0.014 * |
| PNI | −0.330 | 0.018 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aygun, D.; Uzun, H. Association of Comorbidity and Inflammatory and Nutritional Markers with Epilepsy and Seizure Frequency. Nutrients 2025, 17, 1847. https://doi.org/10.3390/nu17111847
Aygun D, Uzun H. Association of Comorbidity and Inflammatory and Nutritional Markers with Epilepsy and Seizure Frequency. Nutrients. 2025; 17(11):1847. https://doi.org/10.3390/nu17111847
Chicago/Turabian StyleAygun, Demet, and Hafize Uzun. 2025. "Association of Comorbidity and Inflammatory and Nutritional Markers with Epilepsy and Seizure Frequency" Nutrients 17, no. 11: 1847. https://doi.org/10.3390/nu17111847
APA StyleAygun, D., & Uzun, H. (2025). Association of Comorbidity and Inflammatory and Nutritional Markers with Epilepsy and Seizure Frequency. Nutrients, 17(11), 1847. https://doi.org/10.3390/nu17111847







