Dietary Omega-3 PUFAs in Metabolic Disease Research: A Decade of Omics-Enabled Insights (2014–2024)
Abstract
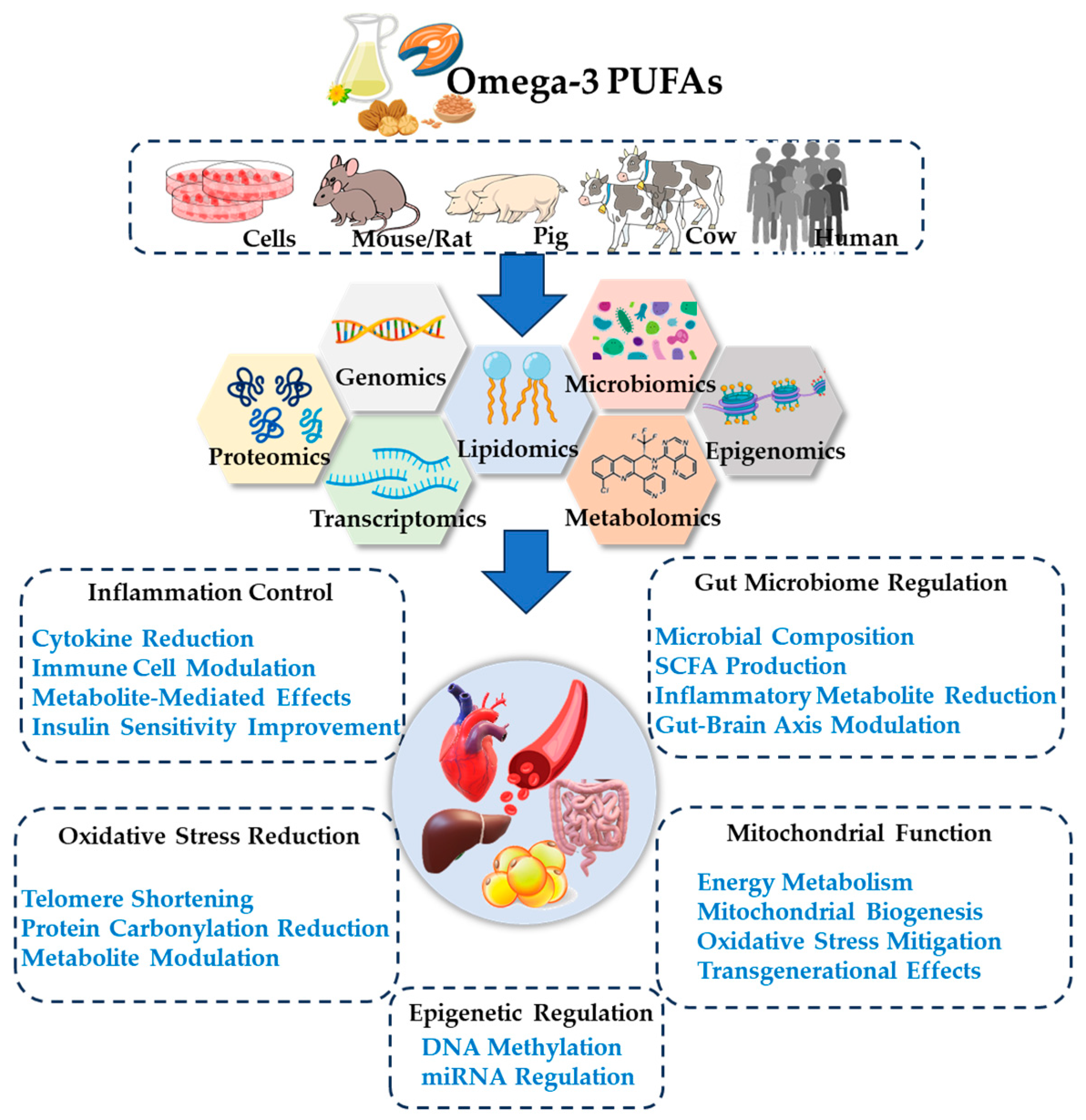
1. Introduction
2. Materials and Methods
2.1. Data Retrieval
2.2. Screening and Exclusion Criteria
2.3. Annotated Bibliography
3. Search Results and Study Characteristics
4. Omics Approaches in Animal Studies
4.1. Animal Transcriptomics: Gene Regulatory Mechanisms Mediated by Omega-3 PUFAs
4.2. Animal Proteomics: Protein Modifications and Pathways Induced by Omega-3 PUFAs
4.3. Animal Lipidomics: Effects of Omega-3 PUFAs on Lipid Profiles and Signaling Pathways
4.4. Animal Metabolomics: Effects of Omega-3 PUFAs on Metabolic Pathways and Biomarkers
4.5. Animal Microbiomics: Modulation of Gut Microbiota Composition and Function by Omega-3 PUFAs
5. Omics Approaches in Human Studies
5.1. Human Genomics and Epigenomics: Effects of Omega-3 PUFAs on Gene Expression and Epigenetics
5.2. Human Transcriptomics: Effects of Omega-3 PUFAs on Gene Expression in Metabolic Health
5.3. Human Proteomics: Protein Biomarkers and Metabolic Effects of Omega-3 PUFAs
5.4. Human Metabolomics: Metabolic Impact and Therapeutic Potential of Omega-3 PUFAs in Various Health Conditions
5.5. Human Lipidomics: Modulation of Lipid Metabolism by Omega-3 PUFAs in Health and Disease
5.6. Human Microbiomics: Modulation of the Gut Microbiome and Metabolic Outcomes by Omega-3 PUFAs
6. Mechanistic Insights into Omega-3 PUFAs and Omics
6.1. Epigenetic Regulation
6.2. Oxidative Stress Reduction
6.3. Modulation of Gut Microbiota and Metabolites
6.4. Mitochondrial Function Improvement
6.5. Inflammation Control
7. Challenges and Future Directions
7.1. Interindividual and Disease-Stage Variability
7.2. Challenges in Multi-Omics Technologies
7.3. Future Research Avenues: Direct Target Investigation
7.4. Future Research Avenues: Computational Biology Methods and Omega-3 Multi-Omics Integration
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABPP | Activity-based protein profiling |
| ALA | Alpha-linolenic acid |
| AMPK | Adenosine monophosphate-activated protein kinase |
| APC | Adipocyte precursor cell |
| CLA | Conjugated linoleic acid |
| CR | Calorie restriction |
| CVDs | Cardiovascular diseases |
| DALYs | Disability-adjusted life years |
| DHA | Docosahexaenoic acid |
| DHEA | Dehydroepiandrosterone |
| ECS | Endocannabinoid system |
| EEQs | Epoxyeicosatetraenoic acids |
| EPA | Eicosapentaenoic acid |
| Evs | Extracellular vesicles |
| FADS | Fatty acid desaturase |
| GBD | Global Burden of Disease |
| GDM | Gestational diabetes mellitus |
| GGT | Gamma-glutamyl transferase |
| GPD1 | Glycerol-3-phosphate dehydrogenase-1 |
| GPR120 | G protein-coupled receptor 120 |
| GPs | Glycerophospholipids |
| GSE | Grape seed polyphenols |
| HDL | High-density lipoprotein |
| HEPEs | Hydroxyeicosapentaenoic acids |
| HFD | High fat diet |
| HFHS | High-fat high-sucrose |
| Hhcy | Hyperhomocysteinemia |
| HUVECs | Human umbilical vein endothelial cells |
| LiP-MS | Limited proteolysis-mass spectrometry |
| lncRNAs | Long non-coding rnas |
| LPC | Lysophosphatidylcholine |
| LXR | Liver X receptor |
| LYCRPLs | Phospholipids from large yellow croaker roe |
| MASLD | Metabolic dysfunction-associated steatotic liver disease |
| MUFA | Monounsaturated fatty acids |
| NAFLD | Non-alcoholic fatty liver disease |
| NCDs | Non-communicable diseases |
| NF-κB | Nuclear factor kappa B |
| PBMCs | Peripheral blood mononuclear cells |
| PCOS | Polycystic ovary syndrome |
| PI3K | Phosphatidylinositol 3-kinase |
| PPARs | Peroxisome proliferator-activated receptors |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PUFAs | Polyunsaturated fatty acids |
| RNA-seq | RNA sequencing |
| SCFA | Short-chain fatty acids |
| scRNA-seq | Single-cell RNA sequencing |
| SIRT1 | Silent information regulator T1 |
| SPs | Sphingolipids |
| T2DM | Type 2 diabetes mellitus |
| TC | Total cholesterol |
| TCA cycle | Citric acid cycle |
| TPP | Thermal proteome profiling |
| UPLC-Q-TOF-MSE | Ultra-performance liquid chromatography-quadrupole/time-of-flight mass spectrometry |
| VAT | Visceral adipose tissue |
| WAT | White adipose tissue |
| WD | Western diet |
| WOS | Web of Science |
| 12-OH-17,18-EpETE | 12-hydroxy-17,18-epoxyeicosatetraenoic acid |
| 17,18-EpETE | 17,18-epoxyeicosatetraenoic acid |
References
- Zhang, H.; Zhou, X.D.; Shapiro, M.D.; Lip, G.Y.H.; Tilg, H.; Valenti, L.; Somers, V.K.; Byrne, C.D.; Targher, G.; Yang, W.; et al. Global burden of metabolic diseases, 1990-2021. Metabolism 2024, 160, 155999. [Google Scholar] [CrossRef] [PubMed]
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.X.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef] [PubMed]
- Kazibwe, J.; Tran, P.B.; Annerstedt, K.S. The household financial burden of non-communicable diseases in low- and middle-income countries: A systematic review. Health Res. Policy Syst. 2021, 19, 96. [Google Scholar] [CrossRef]
- Hoffman, D.J.; Powell, T.L.; Barrett, E.S.; Hardy, D.B. Developmental Origins of Metabolic Diseases. Physiol. Rev. 2021, 101, 739–795. [Google Scholar] [CrossRef]
- Cousin, E.; Duncan, B.B.; Stein, C.; Ong, K.L.; Vos, T.; Abbafati, C.; Abbasi-Kangevari, M.; Abdelmasseh, M.; Abdoli, A.; Abd-Rabu, R.; et al. Diabetes mortality and trends before 25 years of age: An analysis of the Global Burden of Disease Study 2019. Lancet Diabetes Endocrinol. 2022, 10, 177–192. [Google Scholar] [CrossRef]
- Qi, Y.; Fan, L.; Ran, D.; Xu, J.; Wang, Y.; Wu, J.; Zhang, Z. Main risk factors of type 2 diabetes mellitus with nonalcoholic fatty liver disease and hepatocellular carcinoma. J. Oncol. 2021, 2021, 7764817. [Google Scholar] [CrossRef] [PubMed]
- Samuel, P.O.; Edo, G.I.; Emakpor, O.L.; Oloni, G.O.; Ezekiel, G.O.; Essaghah, A.E.A.; Agoh, E.; Agbo, J.J. Lifestyle modifications for preventing and managing cardiovascular diseases. Sport Sci. Health 2024, 20, 23–36. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; López-Mora, C.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. New Insights and Potential Therapeutic Interventions in Metabolic Diseases. Int. J. Mol. Sci. 2023, 24, 10672. [Google Scholar] [CrossRef]
- Paulino, P.J.I.V.; Cuthrell, K.M.; Tzenios, N. Non Alcoholic Fatty Liver Disease; Disease Burden, Management, and Future Perspectives. Int. Res. J. Gastroenterol. Hepatol. 2024, 7, 1–13. [Google Scholar]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Shehzad, Q.; Su, Y.; Xu, L.; Yu, L.; Zeng, W.; Fang, Z.; Wu, G.; Wei, W.; et al. Does omega-3 PUFAs supplementation improve metabolic syndrome and related cardiovascular diseases? A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2024, 64, 9455–9482. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Park, K. Omega-3 and omega-6 polyunsaturated fatty acids and metabolic syndrome: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 765–773. [Google Scholar] [CrossRef]
- West, A.L.; Miles, E.A.; Lillycrop, K.A.; Napier, J.A.; Calder, P.C.; Burdge, G.C. Genetically modified plants are an alternative to oily fish for providing-3 polyunsaturated fatty acids in the human diet: A summary of the findings of a Biotechnology and Biological Sciences Research Council funded project. Nutr. Bull. 2020, 46, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Xu, Z.X.; Chen, W.C.; Huang, F.H.; Chen, S.W.; Wang, X.; Yang, C. Algal oil alleviates antibiotic-induced intestinal inflammation by regulating gut microbiota and repairing intestinal barrier. Front. Nutr. 2023, 9, 1081717. [Google Scholar] [CrossRef] [PubMed]
- Han, L.H.; Usher, S.; Sandgrind, S.; Hassall, K.; Sayanova, O.; Michaelson, L.V.; Haslam, R.P.; Napier, J.A. High level accumulation of EPA and DHA in field-grown transgenic Camelina—A multi-territory evaluation of TAG accumulation and heterogeneity. Plant Biotechnol. J. 2020, 18, 2280–2291. [Google Scholar] [CrossRef]
- Lin, X.L.; Baisley, J.; Bier, A.; Vora, D.; Holub, B. Transgenic Canola Oil Improved Blood Omega-3 Profiles: A Randomized, Placebo-Controlled Trial in Healthy Adults. Front. Nutr. 2022, 9, 847114. [Google Scholar] [CrossRef]
- Hong, J.; Bledsoe, J.W.; Overturf, K.E.; Lee, S.; Iassonova, D.; Small, B.C. LatitudeTM oil as a sustainable alternative to dietary fish oil in rainbow trout (Oncorhynchus mykiss): Effects on filet fatty acid profiles, intestinal histology, and plasma biochemistry. Front. Sustain. Food Syst. 2022, 6, 837628. [Google Scholar] [CrossRef]
- Weldon, A.; Davis, D.A.; Rhodes, M.; Morey, A.; Iassonova, D.; Roy, L.A.; Chen, L.Q. Use of Genetically Modified Canola Oil as a Replacement for Fish Oil in Practical Diets for Whiteleg Shrimp Litopeneaus vannamei Reared in Green Water Conditions. Aquac. Res. 2023, 2023, 2999827. [Google Scholar] [CrossRef]
- Crupi, R.; Cuzzocrea, S. Role of EPA in Inflammation: Mechanisms, Effects, and Clinical Relevance. Biomolecules 2022, 12, 242. [Google Scholar] [CrossRef]
- Borja-Magno, A.I.; Furuzawa-Carballeda, J.; Guevara-Cruz, M.; Arias, C.; Granados, J.; Bourges, H.; Tovar, A.R.; Sears, B.; Noriega, L.G.; Gómez, F.E. Supplementation with EPA and DHA omega-3 fatty acids improves peripheral immune cell mitochondrial dysfunction and inflammation in subjects with obesity. J. Nutr. Biochem. 2023, 120, 109415. [Google Scholar] [CrossRef]
- Abbott, K.A.; Burrows, T.L.; Acharya, S.; Thota, R.N.; Garg, M.L. DHA-enriched fish oil reduces insulin resistance in overweight and obese adults. Prostaglandins Leukot. Essent. Fat. Acids 2020, 159, 102154. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Xie, Q.Y.; Tan, W.F.; Hu, M.J.; Xu, G.L.; Zhang, X.; Xie, G.H.; Mao, L.M. Different ratios of DHA/EPA reverses insulin resistance by improving adipocyte dysfunction and lipid disorders in HFD-induced IR mice. Food Funct. 2023, 14, 1179–1197. [Google Scholar] [CrossRef]
- Aldhafiri, F.K. Investigating the role of EPA and DHA on cellular oxidative stress; profiling antidiabetic and antihypertensive potential. J. Pharm. Bioallied Sci. 2022, 14, 178–185. [Google Scholar] [CrossRef]
- Kelsey, M.D.; Pagidipati, N.J. Should we “RESPECT EPA” more now? EPA and DHA for cardiovascular risk reduction. Curr. Cardiol. Rep. 2023, 25, 1601–1609. [Google Scholar] [CrossRef]
- Khan, S.U.; Lone, A.N.; Khan, M.S.; Virani, S.S.; Blumenthal, R.S.; Nasir, K.; Miller, M.; Michos, E.D.; Ballantyne, C.M.; Boden, W.E. Effect of omega-3 fatty acids on cardiovascular outcomes: A systematic review and meta-analysis. EClinicalMedicine 2021, 38, 100997. [Google Scholar] [CrossRef] [PubMed]
- Sherratt, S.C.R.; Libby, P.; Budoff, M.J.; Bhatt, D.L.; Mason, R.P. Role of Omega-3 Fatty Acids in Cardiovascular Disease: The Debate Continues. Curr. Atheroscler. Rep. 2023, 25, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.P.; Chapman, M.J.; Parhofer, K.G.; Nelson, J.R. Differentiating EPA from EPA/DHA in cardiovascular risk reduction. Am. Heart J. Plus Cardiol. Res. Pract. 2022, 17, 100148. [Google Scholar] [CrossRef]
- Jones, B.D.M.; Farooqui, S.; Kloiber, S.; Husain, M.O.; Mulsant, B.H.; Husain, M.I. Targeting Metabolic Dysfunction for the Treatment of Mood Disorders: Review of the Evidence. Life 2021, 11, 819. [Google Scholar] [CrossRef]
- Yuan, Q.H.; Xie, F.; Huang, W.; Hu, M.; Yan, Q.L.; Chen, Z.M.; Zheng, Y.; Liu, L. The review of alpha-linolenic acid: Sources, metabolism, and pharmacology. Phytother. Res. 2022, 36, 164–188. [Google Scholar] [CrossRef]
- Yan, S.; Lu, J.J.; Chen, B.Q.; Yuan, L.X.; Chen, L.; Ju, L.L.; Cai, W.H.; Wu, J.Z. The Multifaceted Role of Alpha-Lipoic Acid in Cancer Prevention, Occurrence, and Treatment. Antioxidants 2024, 13, 897. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K.; Bishen, S.M.; Mishra, H.; Khurana, A. Molecular and Therapeutic Insights of Alpha-Lipoic Acid as a Potential Molecule for Disease Prevention. Rev. Bras. Farmacogn. 2023, 33, 272–287. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Xu, H.; Xia, J.; Lu, Y.; Xu, D.; Sun, J.; Wang, Y.; Liao, W.; Sun, G. Effect of alpha-linolenic acid supplementation on cardiovascular disease risk profile in individuals with obesity or overweight: A systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2023, 14, 1644–1655. [Google Scholar] [CrossRef]
- Najafi, N.; Mehri, S.; Rahbardar, M.G.; Hosseinzadeh, H. Effects of alpha lipoic acid on metabolic syndrome: A comprehensive review. Phytother. Res. 2022, 36, 2300–2323. [Google Scholar] [CrossRef]
- Bordoni, L.; Petracci, I.; Zhao, F.R.; Min, W.H.; Pierella, E.; Assmann, T.S.; Martinez, J.A.; Gabbianelli, R. Nutrigenomics of Dietary Lipids. Antioxidants 2021, 10, 994. [Google Scholar] [CrossRef]
- Brennan, L.; de Roos, B. Nutrigenomics: Lessons learned and future perspectives. Am. J. Clin. Nutr. 2021, 113, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Zuo, H.-L.; Huang, H.-Y.; Lin, Y.-C.-D.; Cai, X.-X.; Kong, X.-J.; Luo, D.-L.; Zhou, Y.-H.; Huang, H.-D. Enzyme Activity of Natural Products on Cytochrome P450. Molecules 2022, 27, 515. [Google Scholar] [CrossRef]
- Zuo, H.-L.; Huang, H.-Y.; Lin, Y.-C.-D.; Liu, K.-M.; Lin, T.-S.; Wang, Y.-B.; Huang, H.-D. Effects of Natural Products on Enzymes Involved in Ferroptosis: Regulation and Implications. Molecules 2023, 28, 7929. [Google Scholar] [CrossRef]
- Cai, X.-X.; Huang, Y.-H.; Lin, Y.-C.-D.; Huang, H.-Y.; Chen, Y.-G.; Zhang, D.-P.; Zhang, T.; Liu, Y.; Zuo, H.-L.; Huang, H.-D. A comprehensive review of small molecules, targets, and pathways in ulcerative colitis treatment. Eur. J. Med. Chem. 2025, 291, 117645. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.; Das, S.; Trahan, G.D.; Farriester, J.W.; Mullen, G.P.; Kyere-Davies, G.; Presby, D.M.; Houck, J.A.; Webb, P.G.; Dzieciatkowska, M.; et al. Neonatal intake of Omega-3 fatty acids enhances lipid oxidation in adipocyte precursors. iScience 2023, 26, 105750. [Google Scholar] [CrossRef]
- Ballester, M.; Quintanilla, R.; Ortega, F.J.; Serrano, J.C.E.; Cassanye, A.; Rodriguez-Palmero, M.; Moreno-Munoz, J.A.; Portero-Otin, M.; Tibau, J. Dietary intake of bioactive ingredients impacts liver and adipose tissue transcriptomes in a porcine model of prepubertal early obesity. Sci. Rep. 2020, 10, 5375. [Google Scholar] [CrossRef]
- Pinel, A.; Rigaudiere, J.P.; Morio, B.; Capel, F. Adipose Tissue Dysfunctions in Response to an Obesogenic Diet Are Reduced in Mice after Transgenerational Supplementation with Omega 3 Fatty Acids. Metabolites 2021, 11, 838. [Google Scholar] [CrossRef] [PubMed]
- Kunz, H.E.; Dasari, S.; Lanza, I.R. EPA and DHA elicit distinct transcriptional responses to high-fat feeding in skeletal muscle and liver. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E460–E472. [Google Scholar] [CrossRef]
- Manaig, Y.J.Y.; Criado-Mesas, L.; Esteve-Codina, A.; Marmol-Sanchez, E.; Castello, A.; Sanchez, A.; Folch, J.M. Identifying miRNA-mRNA regulatory networks on extreme n-6/n-3 polyunsaturated fatty acid ratio expression profiles in porcine skeletal muscle. PLoS ONE 2023, 18, e0283231. [Google Scholar] [CrossRef]
- Meng, Q.; Ying, Z.; Noble, E.; Zhao, Y.; Agrawal, R.; Mikhail, A.; Zhuang, Y.; Tyagi, E.; Zhang, Q.; Lee, J.H.; et al. Systems Nutrigenomics Reveals Brain Gene Networks Linking Metabolic and Brain Disorders. EBioMedicine 2016, 7, 157–166. [Google Scholar] [CrossRef]
- Soni, N.K.; Nookaew, I.; Sandberg, A.S.; Gabrielsson, B.G. Eicosapentaenoic and docosahexaenoic acid-enriched high fat diet delays the development of fatty liver in mice. Lipids Health Dis. 2015, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Dominguez, J.A.; Canovas, A.; Medrano, J.F.; Islas-Trejo, A.; Kim, K.; Taylor, S.L.; Villalba, J.M.; Lopez-Lluch, G.; Navas, P.; Ramsey, J.J. Omega-3 fatty acids partially revert the metabolic gene expression profile induced by long-term calorie restriction. Exp. Gerontol. 2016, 77, 29–37. [Google Scholar] [CrossRef]
- Soni, N.; Ross, A.B.; Scheers, N.; Nookaew, I.; Gabrielsson, B.G.; Sandberg, A.S. The Omega-3 Fatty Acids EPA and DHA, as a Part of a Murine High-Fat Diet, Reduced Lipid Accumulation in Brown and White Adipose Tissues. Int. J. Mol. Sci. 2019, 20, 5895. [Google Scholar] [CrossRef] [PubMed]
- Corral-Jara, K.F.; Cantini, L.; Poupin, N.; Ye, T.; Rigaudiere, J.P.; De Saint Vincent, S.; Pinel, A.; Morio, B.; Capel, F. An Integrated Analysis of miRNA and Gene Expression Changes in Response to an Obesogenic Diet to Explore the Impact of Transgenerational Supplementation with Omega 3 Fatty Acids. Nutrients 2020, 12, 3864. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Ni, Y.; Yi, C.; Fang, Y.; Ning, Q.; Shen, B.; Zhang, K.; Liu, Y.; Yang, L.; et al. Global Prevalence of Overweight and Obesity in Children and Adolescents: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2024, 178, 800–813. [Google Scholar] [CrossRef]
- Kra, G.; Daddam, J.R.; Moallem, U.; Kamer, H.; Mualem, B.; Levin, Y.; Kocvarova, R.; Nemirovski, A.; Contreras, A.G.; Tam, J.; et al. Alpha-linolenic acid modulates systemic and adipose tissue-specific insulin sensitivity, inflammation, and the endocannabinoid system in dairy cows. Sci. Rep. 2023, 13, 5280. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Balogun, K.A.; Bykova, N.V.; Cheema, S.K. Novel regulatory roles of omega-3 fatty acids in metabolic pathways: A proteomics approach. Nutr. Metab. 2014, 11, 6. [Google Scholar] [CrossRef]
- Munoz, S.; Mendez, L.; Dasilva, G.; Torres, J.L.; Ramos-Romero, S.; Romeu, M.; Nogues, M.R.; Medina, I. Targeting Hepatic Protein Carbonylation and Oxidative Stress Occurring on Diet-Induced Metabolic Diseases through the Supplementation with Fish Oils. Mar. Drugs 2018, 16, 353. [Google Scholar] [CrossRef] [PubMed]
- Mendez, L.; Munoz, S.; Miralles-Perez, B.; Nogues, M.R.; Ramos-Romero, S.; Torres, J.L.; Medina, I. Modulation of the Liver Protein Carbonylome by the Combined Effect of Marine Omega-3 PUFAs and Grape Polyphenols Supplementation in Rats Fed an Obesogenic High Fat and High Sucrose Diet. Mar. Drugs 2019, 18, 34. [Google Scholar] [CrossRef]
- Naoe, S.; Tsugawa, H.; Takahashi, M.; Ikeda, K.; Arita, M. Characterization of Lipid Profiles after Dietary Intake of Polyunsaturated Fatty Acids Using Integrated Untargeted and Targeted Lipidomics. Metabolites 2019, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Tognocchi, M.; Conte, M.; Testai, L.; Martucci, M.; Serra, A.; Salvioli, S.; Calderone, V.; Mele, M.; Conte, G. Supplementation of Enriched Polyunsaturated Fatty Acids and CLA Cheese on High Fat Diet: Effects on Lipid Metabolism and Fat Profile. Foods 2022, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- Nakandakari, S.; Gaspar, R.C.; Kuga, G.K.; Ramos, C.O.; Vieira, R.F.; Rios, T.D.S.; Munoz, V.R.; Sant’ana, M.R.; Simabuco, F.M.; da Silva, A.S.R.; et al. Short-term flaxseed oil, rich in omega 3, protects mice against metabolic damage caused by high-fat diet, but not inflammation. J. Nutr. Biochem. 2023, 114, 109270. [Google Scholar] [CrossRef]
- Pinel, A.; Rigaudière, J.P.; Jouve, C.; Montaurier, C.; Jousse, C.; Lhomme, M.; Morio, B.; Capel, F. Transgenerational supplementation with eicosapentaenoic acid reduced the metabolic consequences on the whole body and skeletal muscle in mice receiving an obesogenic diet. Eur. J. Nutr. 2021, 60, 3143–3157. [Google Scholar] [CrossRef]
- Moura-Assis, A.; Afonso, M.S.; de Oliveira, V.; Morari, J.; Dos Santos, G.A.; Koike, M.; Lottenberg, A.M.; Ramos Catharino, R.; Velloso, L.A.; Sanchez Ramos da Silva, A.; et al. Flaxseed oil rich in omega-3 protects aorta against inflammation and endoplasmic reticulum stress partially mediated by GPR120 receptor in obese, diabetic and dyslipidemic mice models. J. Nutr. Biochem. 2018, 53, 9–19. [Google Scholar] [CrossRef]
- Cao, H.; Chen, S.F.; Wang, Z.C.; Dong, X.J.; Wang, R.R.; Lin, H.; Wang, Q.; Zhao, X.J. Intervention of 4% salmon phospholipid on metabolic syndrome in mice based on colonic lipidomics analysis. J. Sci. Food Agric. 2022, 102, 3088–3098. [Google Scholar] [CrossRef]
- Rossmeisl, M.; Medrikova, D.; van Schothorst, E.M.; Pavlisova, J.; Kuda, O.; Hensler, M.; Bardova, K.; Flachs, P.; Stankova, B.; Vecka, M.; et al. Omega-3 phospholipids from fish suppress hepatic steatosis by integrated inhibition of biosynthetic pathways in dietary obese mice. Biochim. Biophys. Acta 2014, 1841, 267–278. [Google Scholar] [CrossRef]
- Miller, J.L.; Blaszkiewicz, M.; Beaton, C.; Johnson, C.P.; Waible, S., 2nd; Dubois, A.L.; Klemmer, A.; Kiebish, M.; Townsend, K.L. A peroxidized omega-3-enriched polyunsaturated diet leads to adipose and metabolic dysfunction. J. Nutr. Biochem. 2019, 64, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Skorve, J.; Hilvo, M.; Vihervaara, T.; Burri, L.; Bohov, P.; Tillander, V.; Bjorndal, B.; Suoniemi, M.; Laaksonen, R.; Ekroos, K.; et al. Fish oil and krill oil differentially modify the liver and brain lipidome when fed to mice. Lipids Health Dis. 2015, 14, 88. [Google Scholar] [CrossRef]
- Fan, R.; Kim, J.; You, M.; Giraud, D.; Toney, A.M.; Shin, S.H.; Kim, S.Y.; Borkowski, K.; Newman, J.W.; Chung, S. alpha-Linolenic acid-enriched butter attenuated high fat diet-induced insulin resistance and inflammation by promoting bioconversion of n-3 PUFA and subsequent oxylipin formation. J. Nutr. Biochem. 2020, 76, 108285. [Google Scholar] [CrossRef]
- Kubota, T.; Arita, M.; Isobe, Y.; Iwamoto, R.; Goto, T.; Yoshioka, T.; Urabe, D.; Inoue, M.; Arai, H. Eicosapentaenoic acid is converted via omega-3 epoxygenation to the anti-inflammatory metabolite 12-hydroxy-17,18-epoxyeicosatetraenoic acid. FASEB J. 2014, 28, 586–593. [Google Scholar] [CrossRef]
- Dong, Y.Q.; Zhang, X.Z.; Sun, L.L.; Zhang, S.Y.; Liu, B.; Liu, H.Y.; Wang, X.; Jiang, C.T. Omega-3 PUFA ameliorates hyperhomocysteinemia-induced hepatic steatosis in mice by inhibiting hepatic ceramide synthesis. Acta Pharmacol. Sin. 2017, 38, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- LeMieux, M.J.; Kalupahana, N.S.; Scoggin, S.; Moustaid-Moussa, N. Eicosapentaenoic acid reduces adipocyte hypertrophy and inflammation in diet-induced obese mice in an adiposity-independent manner. J. Nutr. 2015, 145, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Huang, L.; Chen, Y.; Hu, L.; Zhong, R.; Chen, L.; Cheng, W.; Zheng, B.; Liang, P. Effect of DHA-Enriched Phospholipids from Fish Roe on Rat Fecal Metabolites: Untargeted Metabolomic Analysis. Foods 2023, 12, 1687. [Google Scholar] [CrossRef]
- Liu, R.; Chen, L.; Wang, Y.; Zhang, G.; Cheng, Y.; Feng, Z.; Bai, X.; Liu, J. High ratio of omega-3/omega-6 polyunsaturated fatty acids targets mTORC1 to prevent high-fat diet-induced metabolic syndrome and mitochondrial dysfunction in mice. J. Nutr. Biochem. 2020, 79, 108330. [Google Scholar] [CrossRef]
- Gao, J.; Xiao, H.; Li, J.; Guo, X.; Cai, W.; Li, D. N-3 Polyunsaturated Fatty Acids Decrease Long-Term Diabetic Risk of Offspring of Gestational Diabetes Rats by Postponing Shortening of Hepatic Telomeres and Modulating Liver Metabolism. Nutrients 2019, 11, 1699. [Google Scholar] [CrossRef]
- Fang, X.; Cai, W.; Cheng, Q.; Ai, D.; Wang, X.; Hammock, B.D.; Zhu, Y.; Zhang, X. Omega-3 PUFA attenuate mice myocardial infarction injury by emerging a protective eicosanoid pattern. Prostaglandins Other Lipid Mediat. 2018, 139, 1–9. [Google Scholar] [CrossRef]
- Wang, C.; Liu, W.; Yao, L.; Zhang, X.; Zhang, X.; Ye, C.; Jiang, H.; He, J.; Zhu, Y.; Ai, D. Hydroxyeicosapentaenoic acids and epoxyeicosatetraenoic acids attenuate early occurrence of nonalcoholic fatty liver disease. Br. J. Pharmacol. 2017, 174, 2358–2372. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Xuan, B.; Jeong, Y.; Han, G.; Kim, E.B. Omega-3-Rich Fish-Oil-Influenced Mouse Gut Microbiome Shaped by Intermittent Consumption of Beef. Curr. Microbiol. 2023, 80, 119. [Google Scholar] [CrossRef]
- Salsinha, A.S.; Cima, A.; Araujo-Rodrigues, H.; Viana, S.; Reis, F.; Coscueta, E.R.; Rodriguez-Alcala, L.M.; Relvas, J.B.; Pintado, M. The use of an in vitro fecal fermentation model to uncover the beneficial role of omega-3 and punicic acid in gut microbiota alterations induced by a Western diet. Food Funct. 2024, 15, 6095–6117. [Google Scholar] [CrossRef] [PubMed]
- Newman, T.M.; Clear, K.Y.J.; Wilson, A.S.; Soto-Pantoja, D.R.; Ochs-Balcom, H.M.; Cook, K.L. Early-life dietary exposures mediate persistent shifts in the gut microbiome and visceral fat metabolism. Am. J. Physiol. Cell Physiol. 2023, 324, C644–C657. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, L.; Li, C.; Jing, J.; Li, Z.; Sun, S.; Xue, T.; Zhang, K.; Xue, M.; Cao, C.; et al. Effects of gut microbiota on omega-3-mediated ovary and metabolic benefits in polycystic ovary syndrome mice. J. Ovarian Res. 2023, 16, 138. [Google Scholar] [CrossRef]
- Lu, X.; Zhong, R.; Hu, L.; Huang, L.; Chen, L.; Cheng, W.; Zheng, B.; Liang, P. DHA-enriched phospholipids from large yellow croaker roe regulate lipid metabolic disorders and gut microbiota imbalance in SD rats with a high-fat diet. Food Funct. 2021, 12, 4825–4841. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Sha, L.; Li, K.; Wang, Z.; Wang, T.; Li, Y.; Liu, P.; Dong, X.; Dong, Y.; Zhang, X.; et al. Dietary flaxseed oil rich in omega-3 suppresses severity of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in rats. Lipids Health Dis. 2020, 19, 20. [Google Scholar] [CrossRef]
- Reemst, K.; Tims, S.; Yam, K.Y.; Mischke, M.; Knol, J.; Brul, S.; Schipper, L.; Korosi, A. The Role of the Gut Microbiota in the Effects of Early-Life Stress and Dietary Fatty Acids on Later-Life Central and Metabolic Outcomes in Mice. mSystems 2022, 7, e0018022. [Google Scholar] [CrossRef]
- Pal, A.; Sun, S.; Armstrong, M.; Manke, J.; Reisdorph, N.; Adams, V.R.; Kennedy, A.; Zu, Y.; Moustaid-Moussa, N.; Carroll, I.; et al. Beneficial effects of eicosapentaenoic acid on the metabolic profile of obese female mice entails upregulation of HEPEs and increased abundance of enteric Akkermansia muciniphila. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159059. [Google Scholar] [CrossRef]
- Lam, Y.Y.; Ha, C.W.; Hoffmann, J.M.; Oscarsson, J.; Dinudom, A.; Mather, T.J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J.; et al. Effects of dietary fat profile on gut permeability and microbiota and their relationships with metabolic changes in mice. Obesity 2015, 23, 1429–1439. [Google Scholar] [CrossRef]
- Monk, J.M.; Liddle, D.M.; Hutchinson, A.L.; Wu, W.; Lepp, D.; Ma, D.W.L.; Robinson, L.E.; Power, K.A. Fish oil supplementation to a high-fat diet improves both intestinal health and the systemic obese phenotype. J. Nutr. Biochem. 2019, 72, 108216. [Google Scholar] [CrossRef]
- Fumagalli, M.; Moltke, I.; Grarup, N.; Racimo, F.; Bjerregaard, P.; Jorgensen, M.E.; Korneliussen, T.S.; Gerbault, P.; Skotte, L.; Linneberg, A.; et al. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science 2015, 349, 1343–1347. [Google Scholar] [CrossRef]
- Tremblay, B.L.; Guenard, F.; Rudkowska, I.; Lemieux, S.; Couture, P.; Vohl, M.C. Epigenetic changes in blood leukocytes following an omega-3 fatty acid supplementation. Clin. Epigenet. 2017, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Alisi, A.; Fabrizi, M.; Vallone, C.; Rava, L.; Giannico, R.; Vernocchi, P.; Signore, F.; Manco, M. Maternal Intake of n-3 Polyunsaturated Fatty Acids During Pregnancy Is Associated With Differential Methylation Profiles in Cord Blood White Cells. Front. Genet. 2019, 10, 1050. [Google Scholar] [CrossRef]
- Massaro, M.; Martinelli, R.; Gatta, V.; Scoditti, E.; Pellegrino, M.; Carluccio, M.A.; Calabriso, N.; Buonomo, T.; Stuppia, L.; Storelli, C.; et al. Transcriptome-based identification of new anti-inflammatory and vasodilating properties of the n-3 fatty acid docosahexaenoic acid in vascular endothelial cell under proinflammatory conditions. PLoS ONE 2015, 10, e0129652, Erratum in PLoS ONE 2015, 11, e0154069. [Google Scholar] [CrossRef] [PubMed]
- Bottero, V.; Potashkin, J.A. A Comparison of Gene Expression Changes in the Blood of Individuals Consuming Diets Supplemented with Olives, Nuts or Long-Chain Omega-3 Fatty Acids. Nutrients 2020, 12, 3765. [Google Scholar] [CrossRef] [PubMed]
- Polus, A.; Zapala, B.; Razny, U.; Gielicz, A.; Kiec-Wilk, B.; Malczewska-Malec, M.; Sanak, M.; Childs, C.E.; Calder, P.C.; Dembinska-Kiec, A. Omega-3 fatty acid supplementation influences the whole blood transcriptome in women with obesity, associated with pro-resolving lipid mediator production. Biochim. Biophys. Acta 2016, 1861, 1746–1755. [Google Scholar] [CrossRef]
- Tait, S.; Calura, E.; Baldassarre, A.; Masotti, A.; Varano, B.; Gessani, S.; Conti, L.; Del Corno, M. Gene and lncRNA Profiling of omega3/omega6 Polyunsaturated Fatty Acid-Exposed Human Visceral Adipocytes Uncovers Different Responses in Healthy Lean, Obese and Colorectal Cancer-Affected Individuals. Int. J. Mol. Sci. 2024, 25, 3357. [Google Scholar] [CrossRef]
- Jimenez-Gomez, Y.; Cruz-Teno, C.; Rangel-Zuniga, O.A.; Peinado, J.R.; Perez-Martinez, P.; Delgado-Lista, J.; Garcia-Rios, A.; Camargo, A.; Vazquez-Martinez, R.; Ortega-Bellido, M.; et al. Effect of dietary fat modification on subcutaneous white adipose tissue insulin sensitivity in patients with metabolic syndrome. Mol. Nutr. Food Res. 2014, 58, 2177–2188. [Google Scholar] [CrossRef]
- Rangel-Zuniga, O.A.; Camargo, A.; Marin, C.; Pena-Orihuela, P.; Perez-Martinez, P.; Delgado-Lista, J.; Gonzalez-Guardia, L.; Yubero-Serrano, E.M.; Tinahones, F.J.; Malagon, M.M.; et al. Proteome from patients with metabolic syndrome is regulated by quantity and quality of dietary lipids. BMC Genom. 2015, 16, 509. [Google Scholar] [CrossRef]
- Manousopoulou, A.; Scorletti, E.; Smith, D.E.; Teng, J.; Fotopoulos, M.; Roumeliotis, T.I.; Clough, G.F.; Calder, P.C.; Byrne, C.D.; Garbis, S.D. Marine omega-3 fatty acid supplementation in non-alcoholic fatty liver disease: Plasma proteomics in the randomized WELCOME* trial. Clin. Nutr. 2019, 38, 1952–1955. [Google Scholar] [CrossRef]
- Yang, Z.H.; Amar, M.; Sampson, M.; Courville, A.B.; Sorokin, A.V.; Gordon, S.M.; Aponte, A.M.; Stagliano, M.; Playford, M.P.; Fu, Y.P.; et al. Comparison of Omega-3 Eicosapentaenoic Acid Versus Docosahexaenoic Acid-Rich Fish Oil Supplementation on Plasma Lipids and Lipoproteins in Normolipidemic Adults. Nutrients 2020, 12, 749. [Google Scholar] [CrossRef] [PubMed]
- Okada, L.; Oliveira, C.P.; Stefano, J.T.; Nogueira, M.A.; Silva, I.; Cordeiro, F.B.; Alves, V.A.F.; Torrinhas, R.S.; Carrilho, F.J.; Puri, P.; et al. Omega-3 PUFA modulate lipogenesis, ER stress, and mitochondrial dysfunction markers in NASH—Proteomic and lipidomic insight. Clin. Nutr. 2018, 37, 1474–1484. [Google Scholar] [CrossRef] [PubMed]
- Lambert, C.; Cubedo, J.; Padro, T.; Sanchez-Hernandez, J.; Antonijoan, R.M.; Perez, A.; Badimon, L. Phytosterols and Omega 3 Supplementation Exert Novel Regulatory Effects on Metabolic and Inflammatory Pathways: A Proteomic Study. Nutrients 2017, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Bozbas, E.; Zhou, R.; Soyama, S.; Allen-Redpath, K.; Mitchell, J.L.; Fisk, H.L.; Calder, P.C.; Jones, C.; Gibbins, J.M.; Fischer, R.; et al. Dietary n-3 polyunsaturated fatty acids alter the number, fatty acid profile and coagulatory activity of circulating and platelet-derived extracellular vesicles: A randomized, controlled crossover trial. Am. J. Clin. Nutr. 2024, 119, 1175–1186. [Google Scholar] [CrossRef]
- Michielsen, C.; Hangelbroek, R.W.J.; Bragt, M.C.E.; Verheij, E.R.; Wopereis, S.; Mensink, R.P.; Afman, L.A. Comparative Analysis of the Effects of Fish Oil and Fenofibrate on Plasma Metabolomic Profiles in Overweight and Obese Individuals. Mol. Nutr. Food Res. 2022, 66, e2100192. [Google Scholar] [CrossRef]
- Ding, X.; Xu, Y.; Nie, P.; Zhong, L.; Feng, L.; Guan, Q.; Song, L. Changes in the serum metabolomic profiles of subjects with NAFLD in response to n-3 PUFAs and phytosterol ester: A double-blind randomized controlled trial. Food Funct. 2022, 13, 5189–5201. [Google Scholar] [CrossRef]
- Chang, W.C.; So, J.; Lamon-Fava, S. Differential and shared effects of eicosapentaenoic acid and docosahexaenoic acid on serum metabolome in subjects with chronic inflammation. Sci. Rep. 2021, 11, 16324. [Google Scholar] [CrossRef]
- Duran, A.M.; Salto, L.M.; Camara, J.; Basu, A.; Paquien, I.; Beeson, W.L.; Firek, A.; Cordero-MacIntyre, Z.; De Leon, M. Effects of omega-3 polyunsaturated fatty-acid supplementation on neuropathic pain symptoms and sphingosine levels in Mexican-Americans with type 2 diabetes. Diabetes Metab. Syndr. Obes. 2019, 12, 109–120. [Google Scholar] [CrossRef]
- Xyda, S.E.; Vuckovic, I.; Petterson, X.M.; Dasari, S.; Lalia, A.Z.; Parvizi, M.; Macura, S.I.; Lanza, I.R. Distinct Influence of Omega-3 Fatty Acids on the Plasma Metabolome of Healthy Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 875–884. [Google Scholar] [CrossRef]
- Zio, S.; Tarnagda, B.; Tapsoba, F.; Zongo, C.; Savadogo, A. Health interest of cholesterol and phytosterols and their contribution to one health approach: Review. Heliyon 2024, 10, e40132. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Karlsson, P.; Gylfadottir, S.S.; Andersen, S.T.; Bennett, D.L.; Tankisi, H.; Finnerup, N.B.; Terkelsen, A.J.; Khan, K.; Themistocleous, A.C.; et al. Painful and non-painful diabetic neuropathy, diagnostic challenges and implications for future management. Brain 2021, 144, 1632–1645. [Google Scholar] [CrossRef]
- Padro, T.; Lopez-Yerena, A.; Perez, A.; Vilahur, G.; Badimon, L. Dietary omega3 Fatty Acids and Phytosterols in the Modulation of the HDL Lipidome: A Longitudinal Crossover Clinical Study. Nutrients 2023, 15, 3637. [Google Scholar] [CrossRef] [PubMed]
- Smid, V.; Dvorak, K.; Sedivy, P.; Kosek, V.; Lenicek, M.; Dezortova, M.; Hajslova, J.; Hajek, M.; Vitek, L.; Bechynska, K.; et al. Effect of Omega-3 Polyunsaturated Fatty Acids on Lipid Metabolism in Patients With Metabolic Syndrome and NAFLD. Hepatol. Commun. 2022, 6, 1336–1349. [Google Scholar] [CrossRef]
- Yan, M.; Cai, W.B.; Hua, T.; Cheng, Q.; Ai, D.; Jiang, H.F.; Zhang, X. Lipidomics reveals the dynamics of lipid profile altered by omega-3 polyunsaturated fatty acid supplementation in healthy people. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Shabrina, A.; Tung, T.H.; Nguyen, N.T.K.; Lee, H.C.; Wu, H.T.; Wang, W.; Huang, S.Y. n-3 PUFA and caloric restriction diet alters lipidomic profiles in obese men with metabolic syndrome: A preliminary open study. Eur. J. Nutr. 2020, 59, 3103–3112. [Google Scholar] [CrossRef]
- Bondia-Pons, I.; Poho, P.; Bozzetto, L.; Vetrani, C.; Patti, L.; Aura, A.M.; Annuzzi, G.; Hyotylainen, T.; Rivellese, A.A.; Oresic, M. Isoenergetic diets differing in their n-3 fatty acid and polyphenol content reflect different plasma and HDL-fraction lipidomic profiles in subjects at high cardiovascular risk. Mol. Nutr. Food Res. 2014, 58, 1873–1882. [Google Scholar] [CrossRef]
- Ortega, F.J.; Cardona-Alvarado, M.I.; Mercader, J.M.; Moreno-Navarrete, J.M.; Moreno, M.; Sabater, M.; Fuentes-Batllevell, N.; Ramirez-Chavez, E.; Ricart, W.; Molina-Torres, J.; et al. Circulating profiling reveals the effect of a polyunsaturated fatty acid-enriched diet on common microRNAs. J. Nutr. Biochem. 2015, 26, 1095–1101. [Google Scholar] [CrossRef]
- Veleba, J.; Kopecky, J., Jr.; Janovska, P.; Kuda, O.; Horakova, O.; Malinska, H.; Kazdova, L.; Oliyarnyk, O.; Skop, V.; Trnovska, J.; et al. Combined intervention with pioglitazone and n-3 fatty acids in metformin-treated type 2 diabetic patients: Improvement of lipid metabolism. Nutr. Metab. 2015, 12, 52. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Zhu, Y.; Huang, Y.; Zhang, Q.; Lin, S.; Fang, C.; Li, L.; Lv, Y.; Mei, W.; et al. Effect of Plant-Derived n-3 Polyunsaturated Fatty Acids on Blood Lipids and Gut Microbiota: A Double-Blind Randomized Controlled Trial. Front. Nutr. 2022, 9, 830960. [Google Scholar] [CrossRef]
- Pu, S.; Khazanehei, H.; Jones, P.J.; Khafipour, E. Interactions between Obesity Status and Dietary Intake of Monounsaturated and Polyunsaturated Oils on Human Gut Microbiome Profiles in the Canola Oil Multicenter Intervention Trial (COMIT). Front. Microbiol. 2016, 7, 1612. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, Y.; Zhou, X. The roles of microRNAs in epigenetic regulation. Curr. Opin. Chem. Biol. 2019, 51, 11–17. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, J.; Luo, Z.; Li, Y.; Huang, Y. Emerging mechanisms of lipid peroxidation in regulated cell death and its physiological implications. Cell Death Dis. 2024, 15, 859. [Google Scholar] [CrossRef]
- Daniel, N.; Le Barz, M.; Mitchell, P.L.; Varin, T.V.; Julien, I.B.; Farabos, D.; Pilon, G.; Gauthier, J.; Garofalo, C.; Kang, J.X.; et al. Comparing Transgenic Production to Supplementation of omega-3 PUFA Reveals Distinct But Overlapping Mechanisms Underlying Protection Against Metabolic and Hepatic Disorders. Function 2023, 4, zqac069. [Google Scholar] [CrossRef]
- Michaeloudes, C.; Christodoulides, S.; Christodoulou, P.; Kyriakou, T.C.; Patrikios, I.; Stephanou, A. Variability in the Clinical Effects of the Omega-3 Polyunsaturated Fatty Acids DHA and EPA in Cardiovascular Disease-Possible Causes and Future Considerations. Nutrients 2023, 15, 4830. [Google Scholar] [CrossRef]
- Murphy, C.H.; Connolly, C.; Flanagan, E.M.; Mitchelson, K.A.J.; de Marco Castro, E.; Egan, B.; Brennan, L.; Roche, H.M. Interindividual variability in response to protein and fish oil supplementation in older adults: A randomized controlled trial. J. Cachexia Sarcopenia Muscle 2022, 13, 872–883. [Google Scholar] [CrossRef]
- Coltell, O.; Sorlí, J.V.; Asensio, E.M.; Barragán, R.; González, J.I.; Giménez-Alba, I.M.; Zanón-Moreno, V.; Estruch, R.; Ramírez-Sabio, J.B.; Pascual, E.C.; et al. Genome-Wide Association Study for Serum Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Exploratory Analysis of the Sex-Specific Effects and Dietary Modulation in Mediterranean Subjects with Metabolic Syndrome. Nutrients 2020, 12, 310. [Google Scholar] [CrossRef]
- Carvajal, F.; Sánchez-Gil, A.; Cardona, D.; Rincón-Cervera, M.A.; Lerma-Cabrera, J.M. The Effect of Very-Long-Chain n-3 Polyunsaturated Fatty Acids in the Central Nervous System and Their Potential Benefits for Treating Alcohol Use Disorder: Reviewing Pre-Clinical and Clinical Data. Nutrients 2023, 15, 2993. [Google Scholar] [CrossRef]
- Scaglia, N.; Chatkin, J.; Chapman, K.R.; Ferreira, I.; Wagner, M.; Selby, P.; Allard, J.; Zamel, N. The relationship between omega-3 and smoking habit: A cross-sectional study. Lipids Health Dis. 2016, 15, 61. [Google Scholar] [CrossRef]
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef]
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahluwalia, T.S. Editorial: The Role of Genetic and Lifestyle Factors in Metabolic Diseases. Front. Endocrinol. 2019, 10, 475. [Google Scholar] [CrossRef]
- Ning, M.; Lo, E.H. Opportunities and challenges in omics. Transl. Stroke Res. 2010, 1, 233–237. [Google Scholar] [CrossRef]
- Hayes, C.N.; Nakahara, H.; Ono, A.; Tsuge, M.; Oka, S. From Omics to Multi-Omics: A Review of Advantages and Tradeoffs. Genes. 2024, 15, 1551. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Zhang, R.; He, J.; Chen, Y.; Bi, N.; Song, Y.; Wang, L.; Zhan, Q.; Abliz, Z. Development of a metabolic pathway-based pseudo-targeted metabolomics method using liquid chromatography coupled with mass spectrometry. Talanta 2019, 192, 160–168. [Google Scholar] [CrossRef]
- Tezel, G. A proteomics view of the molecular mechanisms and biomarkers of glaucomatous neurodegeneration. Prog. Retin. Eye Res. 2013, 35, 18–43. [Google Scholar] [CrossRef]
- Dai, X.; Shen, L. Advances and Trends in Omics Technology Development. Front. Med. 2022, 9, 911861. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Yang, J.; Dong, L.; Zhang, R.; Tian, S.; Yu, Y.; Ren, L.; Hou, W.; Zhu, F.; et al. Multi-omics data integration using ratio-based quantitative profiling with Quartet reference materials. Nat. Biotechnol. 2024, 42, 1133–1149. [Google Scholar] [CrossRef]
- Holmes, C.; McDonald, F.; Jones, M.; Ozdemir, V.; Graham, J.E. Standardization and omics science: Technical and social dimensions are inseparable and demand symmetrical study. Omics 2010, 14, 327–332. [Google Scholar] [CrossRef]
- Kaliannan, K.; Li, X.-Y.; Wang, B.; Pan, Q.; Chen, C.-Y.; Hao, L.; Xie, S.; Kang, J.X. Multi-omic analysis in transgenic mice implicates omega-6/omega-3 fatty acid imbalance as a risk factor for chronic disease. Commun. Biol. 2019, 2, 276. [Google Scholar] [CrossRef]
- Wörheide, M.A.; Krumsiek, J.; Kastenmüller, G.; Arnold, M. Multi-omics integration in biomedical research—A metabolomics-centric review. Anal. Chim. Acta 2021, 1141, 144–162. [Google Scholar] [CrossRef]
- Savitski, M.M.; Reinhard, F.B.; Franken, H.; Werner, T.; Savitski, M.F.; Eberhard, D.; Martinez Molina, D.; Jafari, R.; Dovega, R.B.; Klaeger, S.; et al. Tracking cancer drugs in living cells by thermal profiling of the proteome. Science 2014, 346, 1255784. [Google Scholar] [CrossRef]
- Malinovska, L.; Cappelletti, V.; Kohler, D.; Piazza, I.; Tsai, T.H.; Pepelnjak, M.; Stalder, P.; Dörig, C.; Sesterhenn, F.; Elsässer, F.; et al. Proteome-wide structural changes measured with limited proteolysis-mass spectrometry: An advanced protocol for high-throughput applications. Nat. Protoc. 2023, 18, 659–682. [Google Scholar] [CrossRef]
- Wang, S.; Tian, Y.; Wang, M.; Sun, G.-B.; Sun, X.-B. Advanced Activity-Based Protein Profiling Application Strategies for Drug Development. Front. Pharmacol. 2018, 9, 353. [Google Scholar] [CrossRef]
- Porta, E.O.J.; Steel, P.G. Activity-based protein profiling: A graphical review. Curr. Res. Pharmacol. Drug Discov. 2023, 5, 100164. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Ou, J.; Tu, H.; Shan, B.; Luk, A.; DeBose-Boyd, R.A.; Bashmakov, Y.; Goldstein, J.L.; Brown, M.S. Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. Proc. Natl. Acad. Sci. USA 2001, 98, 6027–6032. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Sundseth, S.S.; Jones, S.A.; Brown, P.J.; Wisely, G.B.; Koble, C.S.; Devchand, P.; Wahli, W.; Willson, T.M.; Lenhard, J.M.; et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc. Natl. Acad. Sci. USA 1997, 94, 4318–4323. [Google Scholar] [CrossRef]
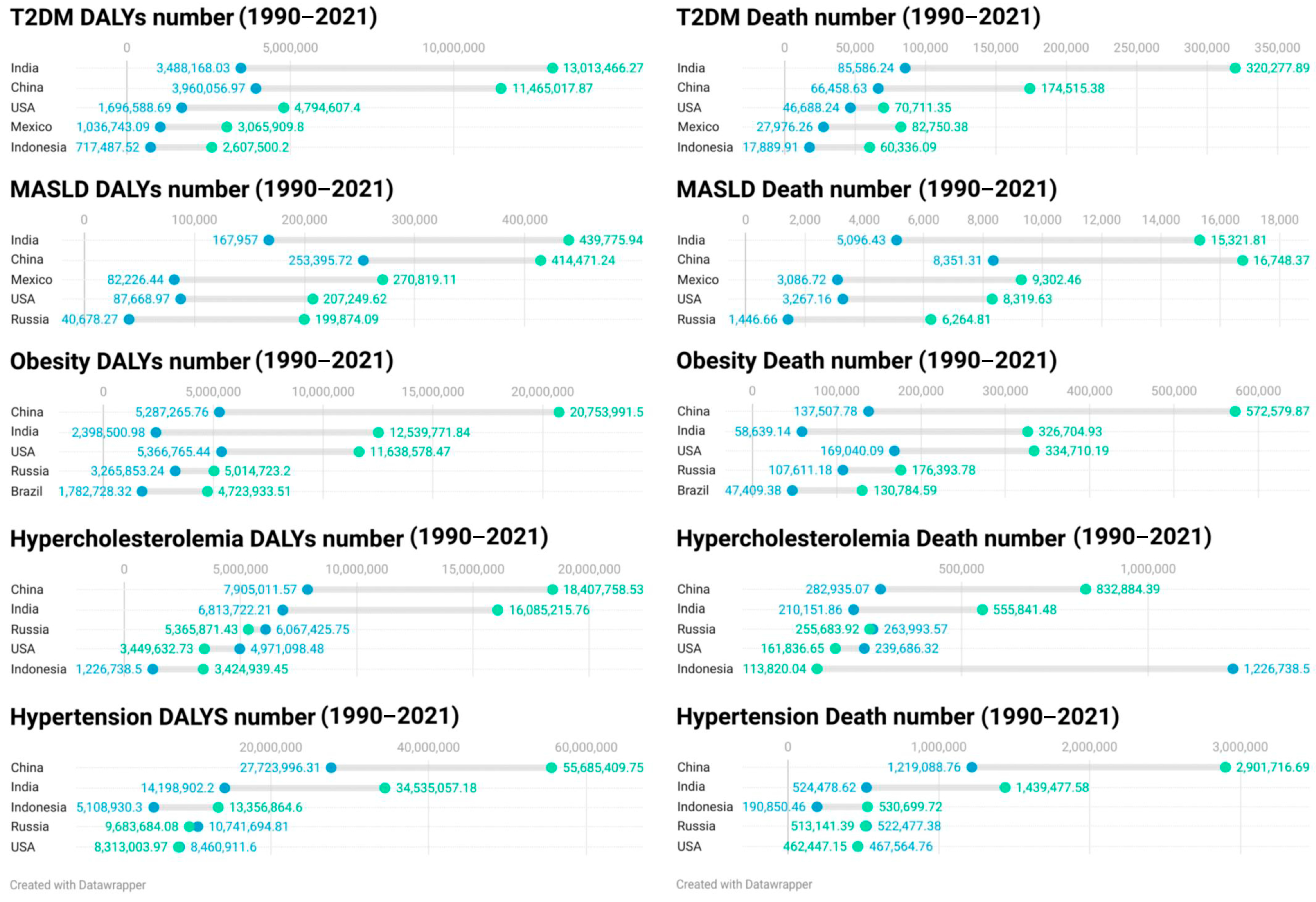
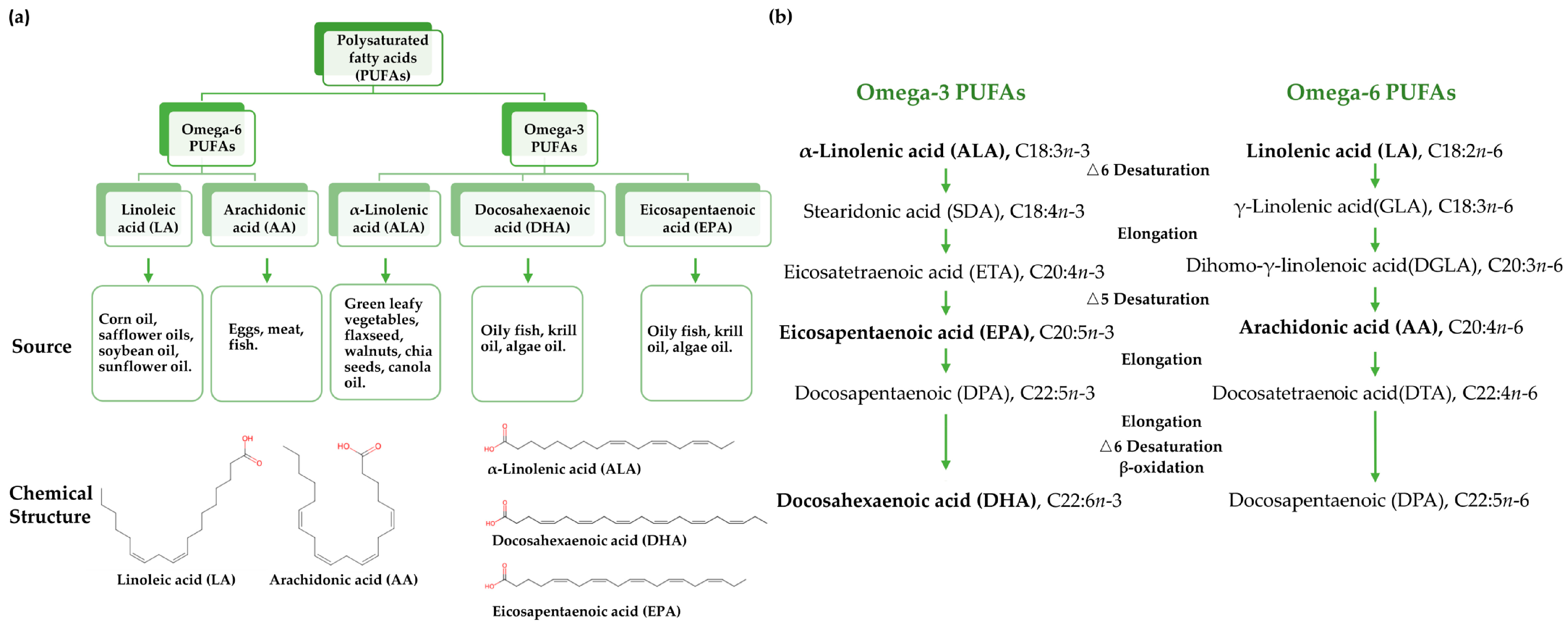
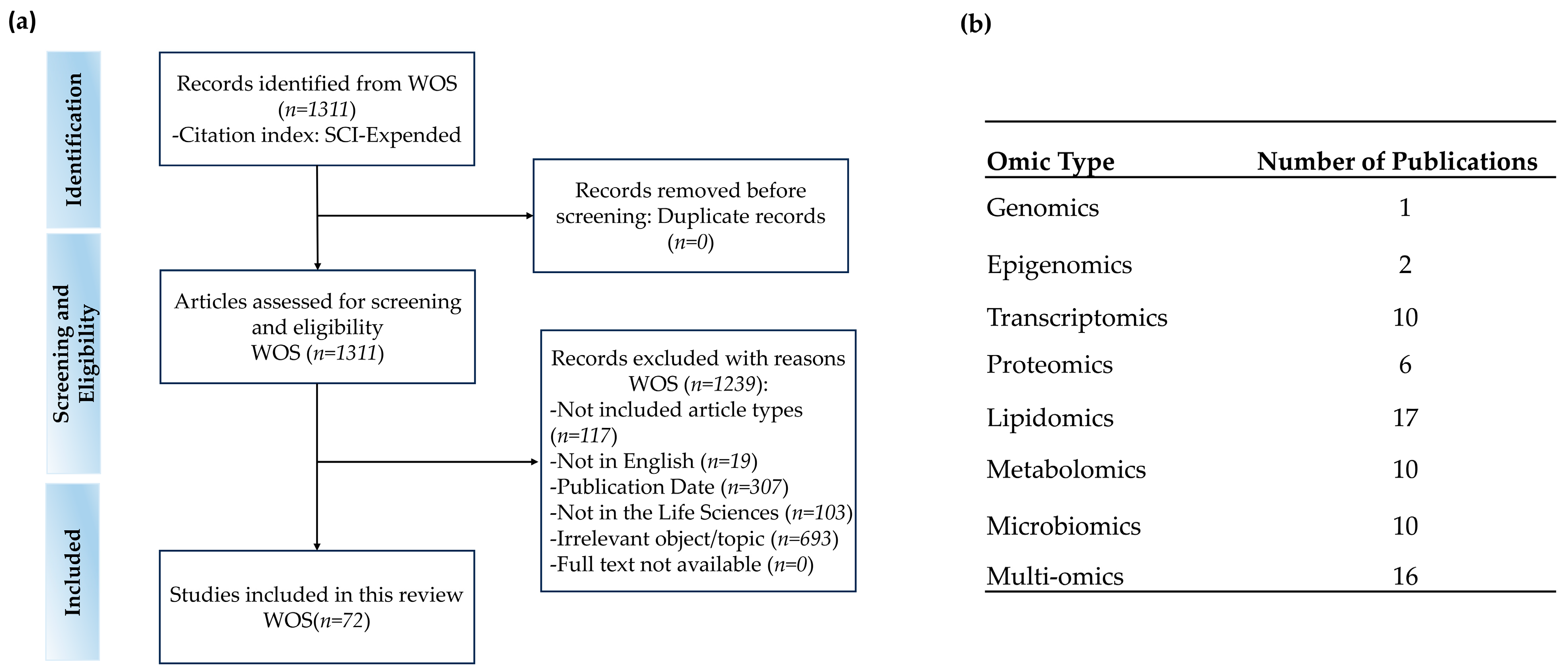
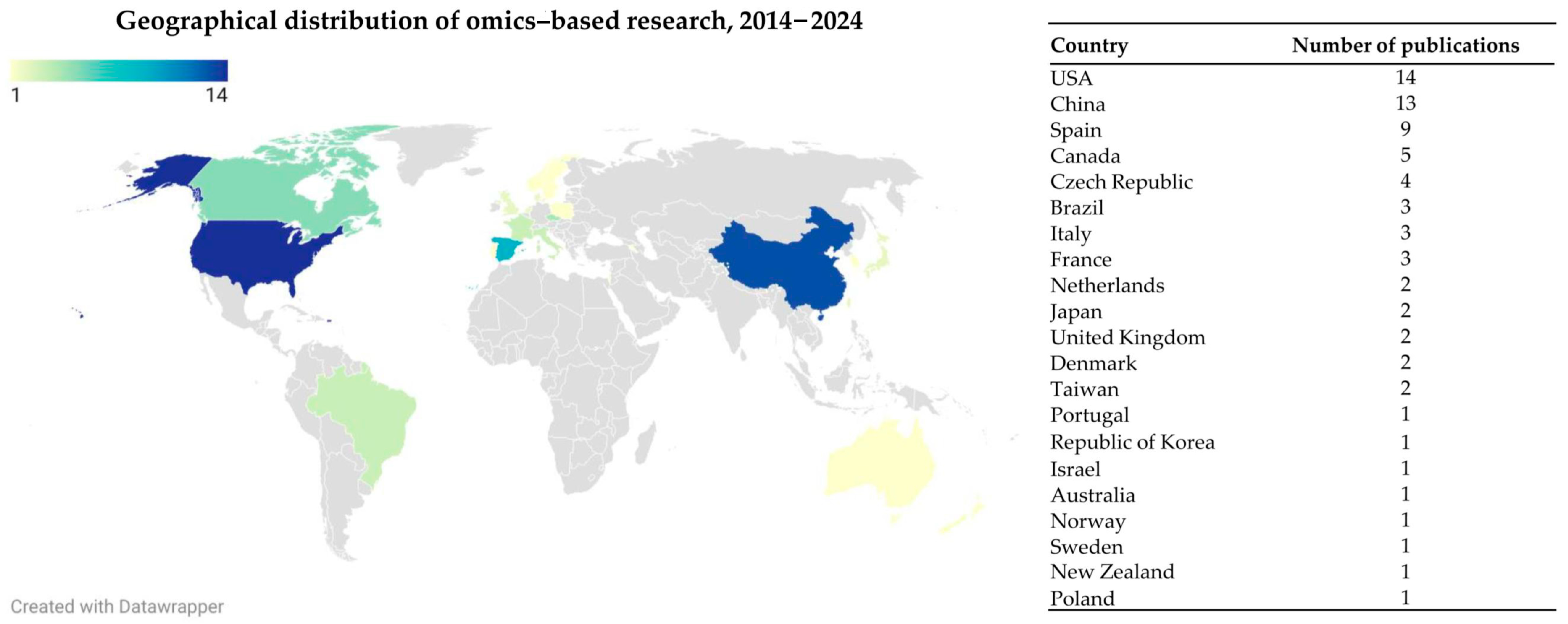
| No. | Exclusion Criteria |
|---|---|
| 1 | Excluded article types, e.g., review, proceedings, feature, editorial material |
| 2 | Not written in English |
| 3 | Publication date outside 2014–2024 |
| 4 | Not in the field of life sciences |
| 5 | Irrelevant objects or topics |
| 6 | Without available full-text |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Lin, Y.-C.-D.; Zuo, H.-L.; Huang, H.-Y.; Zhang, T.; Bai, J.-W.; Huang, H.-D. Dietary Omega-3 PUFAs in Metabolic Disease Research: A Decade of Omics-Enabled Insights (2014–2024). Nutrients 2025, 17, 1836. https://doi.org/10.3390/nu17111836
Li J, Lin Y-C-D, Zuo H-L, Huang H-Y, Zhang T, Bai J-W, Huang H-D. Dietary Omega-3 PUFAs in Metabolic Disease Research: A Decade of Omics-Enabled Insights (2014–2024). Nutrients. 2025; 17(11):1836. https://doi.org/10.3390/nu17111836
Chicago/Turabian StyleLi, Jing, Yang-Chi-Dung Lin, Hua-Li Zuo, Hsi-Yuan Huang, Tao Zhang, Jin-Wei Bai, and Hsien-Da Huang. 2025. "Dietary Omega-3 PUFAs in Metabolic Disease Research: A Decade of Omics-Enabled Insights (2014–2024)" Nutrients 17, no. 11: 1836. https://doi.org/10.3390/nu17111836
APA StyleLi, J., Lin, Y.-C.-D., Zuo, H.-L., Huang, H.-Y., Zhang, T., Bai, J.-W., & Huang, H.-D. (2025). Dietary Omega-3 PUFAs in Metabolic Disease Research: A Decade of Omics-Enabled Insights (2014–2024). Nutrients, 17(11), 1836. https://doi.org/10.3390/nu17111836







