In Preclinical Epilepsy, GLUT1 and GFAP Dysregulation in Cells Surrounding the Third Ventricle, Including Tanycytes, Is Differentially Restored with Ketogenic Diet Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ketogenic Diet
2.3. Tissue Preparation
2.4. Immunohistochemistry
2.5. Study Design
2.6. Inclusion/Exclusion Criteria
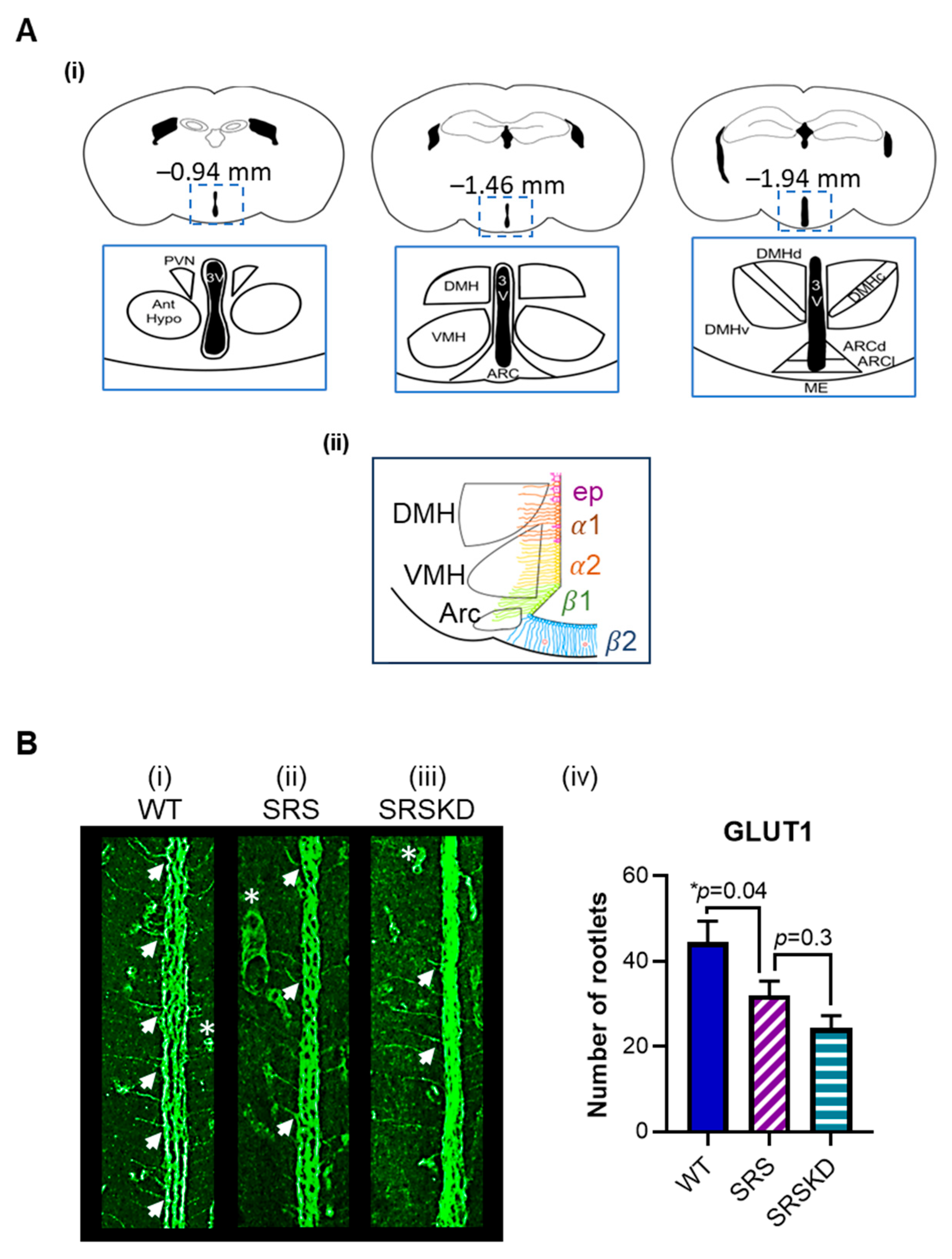
2.7. Image Acquisition and Analyses
2.8. Statistical Analysis
3. Results
3.1. The Number of Glut1-IR Rootlets Was Reduced in SRS Mice
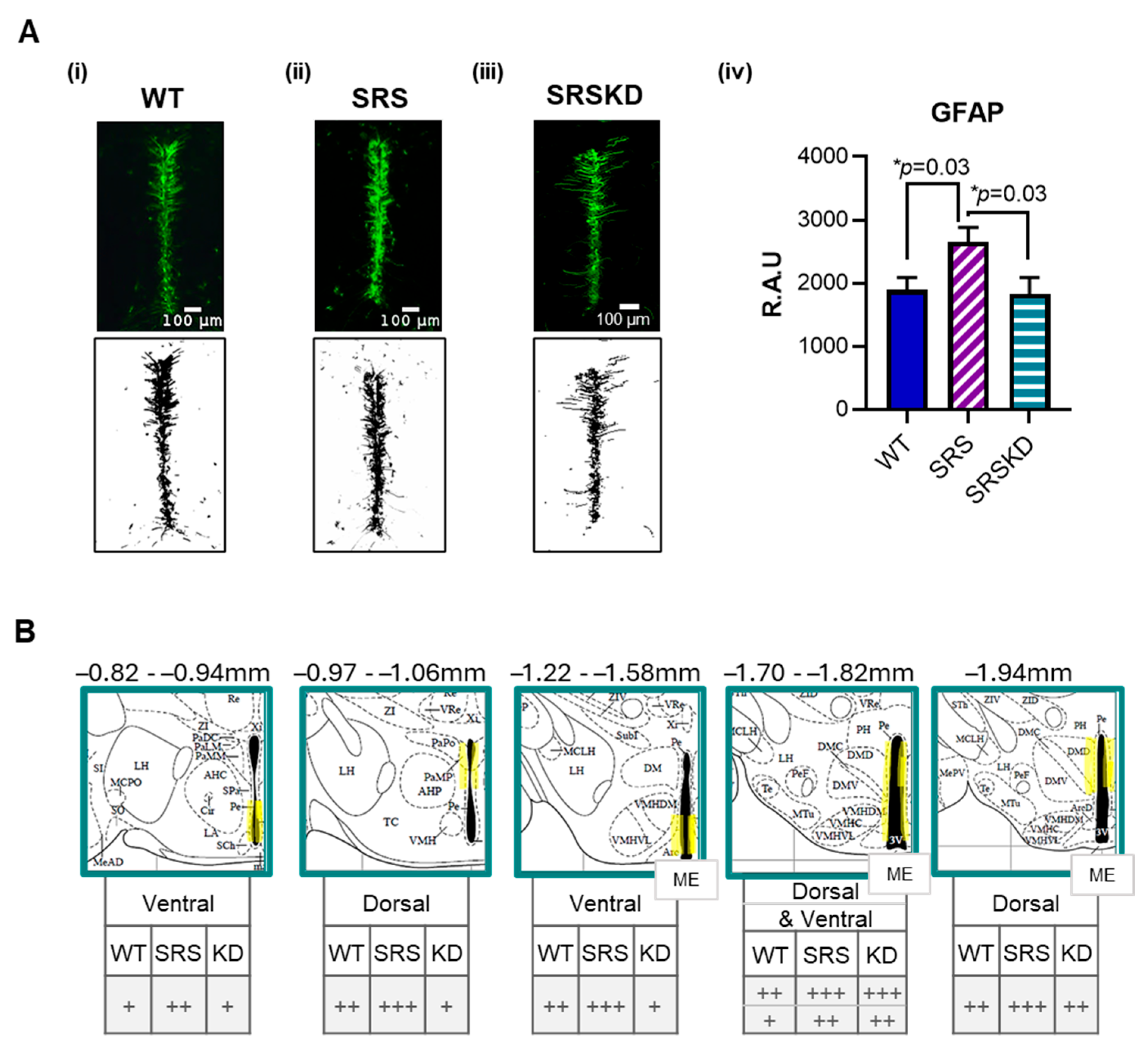
3.2. GFAP Was Increased in SRS Mice
3.3. KD Treatment Did Not Influence GLUT1-IR but Did Restore GFAP Levels in SRS Mice
3.4. The Number of DCX-IR Cells Did Not Differ Between WT and SRS Groups
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3V | Third Ventricle |
| DCX | Doublecortin |
| GFAP | Glial Fibrillary Acidic Protein |
| GLUT1 | Glucose Transporter 1 |
| IR | Immunoreactive |
| KD | Ketogenic Diet |
| NGS | Normal Goat Serum |
| PB | Phosphate Buffer |
| PBS | Phosphate Buffer Saline |
| PBST | Phosphate Buffer with Triton X-100 |
| PFA | Paraformaldehyde |
| SRS | Spontaneous Recurrent Seizure |
| WT | Wildtype |
References
- Elizondo-Vega, R.J.; Recabal, A.; Oyarce, K. Nutrient Sensing by Hypothalamic Tanycytes. Front. Endocrinol. 2019, 10, 244. [Google Scholar] [CrossRef]
- Dali, R.; Estrada-Meza, J.; Langlet, F. Tanycyte, the neuron whisperer. Physiol. Behav. 2023, 263, 114108. [Google Scholar] [CrossRef]
- Lee, D.A.; Bedont, J.L.; Pak, T.; Wang, H.; Song, J.; Miranda-Angulo, A.; Takiar, V.; Charubhumi, V.; Balordi, F.; Takebayashi, H.; et al. Tanycytes of the Hypothalamic Median Eminence Form a Diet-Responsive Neurogenic Niche. Nat. Neurosci. 2012, 15, 700–702. [Google Scholar] [CrossRef]
- Langlet, F. Tanycyte Gene Expression Dynamics in the Regulation of Energy Homeostasis. Front. Endocrinol. 2019, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.T.; Chou, I.C.; Wang, H.S. Role of vitamins in epilepsy. Epilepsy Behav. 2023, 139, 109062. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.; Puchowicz, M.; Borges, K. Impairments in Oxidative Glucose Metabolism in Epilepsy and Metabolic Treatments Thereof. Front. Cell Neurosci. 2018, 31, 274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elizondo-Vega, R.; Cortes-Campos, C.; Barahona, M.J.; Oyarce, K.A.; Carril, C.A.; García-Robles, M.A. The role of tanycytes in hypothalamic glucosensing. J. Cell Mol. Med. 2015, 19, 1471–1482. [Google Scholar] [CrossRef]
- Deng, S.; Gan, L.; Liu, C.; Xu, T.; Zhou, S.; Guo, Y.; Zhang, Z.; Yang, G.-Y.; Tian, H.; Tang, Y. Roles of Ependymal Cells in the Physiology and Pathology of the Central Nervous System. Aging Dis. 2023, 14, 468–483. [Google Scholar] [CrossRef]
- Furube, E.; Ishii, H.; Nambu, Y.; Kurganov, E.; Nagaoka, S.; Morita, M.; Miyata, S. Neural stem cell phenotype of tanycyte-like ependymal cells in the circumventricular organs and central canal of adult mouse brain. Sci. Rep. 2020, 10, 2826. [Google Scholar] [CrossRef]
- Mirzadeh, Z.; Kusne, Y.; Duran-Moreno, M.; Cabrales, E.; Gil-Perotin, S.; Ortiz, C.; Chen, B.; Garcia-Verdugo, J.M.; Sanai, N.; Alvarez-Buylla, A. Bi- and uniciliated ependymal cells define continuous floor-plate-derived tanycytic territories. Nat. Commun. 2017, 8, 13759. [Google Scholar] [CrossRef] [PubMed]
- Butruille, L.; Batailler, M.; Cateau, M.-L.; Sharif, A.; Leysen, V.; Prévot, V.; Vaudin, P.; Pillon, D.; Migaud, M. Selective Depletion of Adult GFAP-Expressing Tanycytes Leads to Hypogonadotropic Hypogonadism in Males. Front. Endocrinol. 2022, 13, 869019. [Google Scholar] [CrossRef] [PubMed]
- Batailler, M.; Droguerre, M.; Baroncini, M.; Fontaine, C.; Prevot, V.; Migaud, M. DCX-expressing cells in the vicinity of the hypothalamic neurogenic niche: A comparative study between mouse, sheep, and human tissues. J. Comp. Neurol. 2014, 522, 1966–1985. [Google Scholar] [CrossRef] [PubMed]
- Barahona, M.J.; Ferrada, L.; Vera, M.; Nualart, F. Tanycytes release glucose using the glucose-6-phosphatase system during hypoglycemia to control hypothalamic energy balance. Mol. Metab. 2024, 84, 101940. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, H.J.; Vacher, H.; Clark, E.; Trimmer, J.S.; Lee, A.L.; Sapolsky, R.M.; Tempel, B.L.; Schwartzkroin, P.A. Structural consequences of Kcna1 gene deletion and transfer in the mouse hippocampus. Epilepsia 2007, 48, 2023–2046. [Google Scholar] [CrossRef]
- Schwantje, M.; Verhagen, L.M.; van Hasselt, P.M.; Fuchs, S.A. Glucose transporter type 1 deficiency syndrome and the ketogenic diet. J. Inherit. Metab. Dis. 2020, 43, 216–222. [Google Scholar] [CrossRef]
- Tang, M.; Park, S.H.; Petri, S.; Yu, H.; Rueda, C.B.; Abel, E.D.; Kim, C.Y.; Hillman, E.M.; Li, F.; Lee, Y.; et al. An early endothelial cell–specific requirement for Glut1 is revealed in Glut1 deficiency syndrome model mice. JCI Insight 2021, 6, e145789. [Google Scholar] [CrossRef]
- Jessberger, S.; Römer, B.; Babu, H.; Kempermann, G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp. Neurol. 2005, 196, 342–351. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, L.; Konopka, H.; López, E.M.; Seoane, E.; Consalvo, D.; Oddo, S.; Kochen, S.; López-Costa, J.J. Doublecortin (DCX) immunoreactivity in hippocampus of chronic refractory temporal lobe epilepsy patients with hippocampal sclerosis. Seizure 2010, 19, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Simeone, K.A.; Matthews, S.A.; Rho, J.M.; Simeone, T.A. Ketogenic diet treatment increases longevity in Kcna1-null mice, a model of sudden unexpected death in epilepsy. Epilepsia 2016, 57, e178–e182. [Google Scholar] [CrossRef]
- Simeone, T.A.; Matthews, S.A.; Samson, K.K.; Simeone, K.A. Regulation of brain PPARgamma2 contributes to ketogenic diet anti-seizure efficacy. Exp. Neurol. 2017, 287 Pt 1, 54–64. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, D.Y.; Simeone, K.A.; Simeone, T.A.; Pandya, J.D.; Wilke, J.C.; Ahn, Y.; Geddes, J.W.; Sullivan, P.G.; Rho, J.M. Ketone bodies mediate antiseizure effects through mitochondrial permeability transition. Ann. Neurol. 2015, 78, 77–87. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Simeone, K.A.; Wilke, J.C.; Matthews, S.A.; Simeone, T.A.; Rho, J.M. Ketogenic diet-mediated seizure reduction preserves CA1 cell numbers in epileptic Kcna1-null mice: An unbiased stereological assessment. Epilepsia 2021, 62, e123–e128. [Google Scholar] [CrossRef] [PubMed]
- Borowicz-Reutt, K.; Krawczyk, M.; Czernia, J. Ketogenic Diet in the Treatment of Epilepsy. Nutrients 2024, 16, 1258. [Google Scholar] [CrossRef] [PubMed]
- Roundtree, H.M.; Simeone, T.A.; Johnson, C.; Matthews, S.A.; Samson, K.K.; Simeone, K.A. Orexin Receptor Antagonism Improves Sleep and Reduces Seizures in Kcna1-null Mice. Sleep 2016, 39, 357–368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Robins, S.C.; Stewart, I.; McNay, D.E.; Taylor, V.; Giachino, C.; Goetz, M.; Ninkovic, J.; Briancon, N.; Maratos-Flier, E.; Flier, J.S.; et al. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat. Commun. 2013, 4, 2049. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Paolone, N.A.; Bleichfeld, M.; Hayes, S.H.; Salvi, R.J.; Baizer, J.S. Expression of doublecortin, a neuronal migration protein, in unipolar brush cells of the vestibulocerebellum and dorsal cochlear nucleus of the adult rat. Neuroscience 2012, 202, 169–183. [Google Scholar] [CrossRef]
- Gleeson, J.G.; Lin, P.T.; Flanagan, L.A.; Walsh, C.A. Doublecortin Is a Microtubule-Associated Protein and Is Expressed Widely by Migrating Neurons. Neuron 1999, 23, 257–271. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose transporters in brain in health and disease. Pflugers. Arch. 2020, 472, 1299–1343. [Google Scholar] [CrossRef]
- Klepper, J.; Akman, C.; Armeno, M.; Auvin, S.; Cervenka, M.; Cross, H.J.; De Giorgis, V.; Della Marina, A.; Engelstad, K.; Heussinger, N.; et al. Glut1 Deficiency Syndrome (Glut1DS): State of the art in 2020 and recommendations of the international Glut1DS study group. Epilepsia Open 2020, 5, 354–365. [Google Scholar] [CrossRef]
- De Vivo, D.C.; Trifiletti, R.R.; Jacobson, R.I.; Ronen, G.M.; Behmand, R.A.; Harik, S.I. Defective Glucose Transport across the Blood-Brain Barrier as a Cause of Persistent Hypoglycorrhachia, Seizures, and Developmental Delay. N. Engl. J. Med. 1991, 325, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Zovi, A.; Cifani, C.; Confalonieri, C.; Lasala, R.; Sabbatucci, M.; Vitiello, A.; Vittori, S. Dietary management and access to treatment for patients with glucose deficiency syndrome type 1: An overview review with focus on the European regulatory framework. Eur. J. Clin. Nutr. 2024, 78, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.; Zhang, W.; McCourt, A.C.; Enerbäck, S. ATGL activity regulates GLUT1-mediated glucose uptake and lactate production via TXNIP stability in adipocytes. J. Biol. Chem. 2021, 296, 100332. [Google Scholar] [CrossRef]
- Liao, W.; Nguyen, M.T.; Yoshizaki, T.; Favelyukis, S.; Patsouris, D.; Imamura, T.; Verma, I.M.; Olefsky, J.M. Suppression of PPAR-gamma attenuates insulin-stimulated glucose uptake by affecting both GLUT1 and GLUT4 in 3T3-L1 adipocytes. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E219–E227. [Google Scholar] [CrossRef] [PubMed]
- Osman, I.; Wang, L.; Hu, G.; Zheng, Z.; Zhou, J. GFAP (Glial Fibrillary Acidic Protein)-Positive Progenitor Cells Contribute to the Development of Vascular Smooth Muscle Cells and Endothelial Cells—Brief Report. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1231–1238. [Google Scholar] [CrossRef]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an emerging biomarker in brain and spinal cord disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Dobson, H.; Al Maawali, S.; Malpas, C.; Santillo, A.F.; Kang, M.; Todaro, M.; Watson, R.; Yassi, N.; Blennow, K.; Zetterberg, H.; et al. Elevated plasma neurofilament light and glial fibrillary acidic protein in epilepsy versus nonepileptic seizures and nonepileptic disorders. Epilepsia 2024, 65, 2751–2763. [Google Scholar] [CrossRef]
- Gzielo, K.; Soltys, Z.; Rajfur, Z.; Setkowicz, Z.K. The Impact of the Ketogenic Diet on Glial Cells Morphology. A Quantitative Morphological Analysis. Neuroscience 2019, 413, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Saper, C.B.; Lowell, B.B. The hypothalamus. Curr. Biol. 2014, 24, R1111–R1116. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.H.; Aggarwal, A.; Warren, T.J.; Hallgren, J.; Abel, P.W.; Simeone, T.A.; Simeone, K.A. Progressive cardiorespiratory dysfunction in Kv1.1 knockout mice may provide temporal biomarkers of pending sudden unexpected death in epilepsy (SUDEP): The contribution of orexin. Epilepsia 2020, 61, 572–588. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Parent, J.M.; Yu, T.W.; Leibowitz, R.T.; Geschwind, D.H.; Sloviter, R.S.; Lowenstein, D.H. Dentate Granule Cell Neurogenesis Is Increased by Seizures and Contributes to Aberrant Network Reorganization in the Adult Rat Hippocampus. J. Neurosci. 1997, 17, 3727–3738. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.E.; Gray, W.P. Relevance of Seizure-Induced Neurogenesis in Animal Models of Epilepsy to the Etiology of Temporal Lobe Epilepsy. Epilepsia 2007, 48 (Suppl. 2), 33–41. [Google Scholar] [CrossRef] [PubMed]
- Goodman, T.; Hajihosseini, M.K. Hypothalamic tanycytes—Masters and servants of metabolic, neuroendocrine, and neurogenic functions. Front. Neurosci. 2015, 9, 387. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafiei, P.; Mian, H.S.; Iyer, S.H.; Draves, S.B.; Matthews, S.A.; Rendon, D.E.; Neesen, E.J.; Dunlay, M.; Revis, M.; Glisan, A.L.; et al. In Preclinical Epilepsy, GLUT1 and GFAP Dysregulation in Cells Surrounding the Third Ventricle, Including Tanycytes, Is Differentially Restored with Ketogenic Diet Treatment. Nutrients 2025, 17, 1824. https://doi.org/10.3390/nu17111824
Rafiei P, Mian HS, Iyer SH, Draves SB, Matthews SA, Rendon DE, Neesen EJ, Dunlay M, Revis M, Glisan AL, et al. In Preclinical Epilepsy, GLUT1 and GFAP Dysregulation in Cells Surrounding the Third Ventricle, Including Tanycytes, Is Differentially Restored with Ketogenic Diet Treatment. Nutrients. 2025; 17(11):1824. https://doi.org/10.3390/nu17111824
Chicago/Turabian StyleRafiei, Parisa, Huda S. Mian, Shruthi H. Iyer, Samantha B. Draves, Stephanie A. Matthews, Daniel E. Rendon, Emma J. Neesen, Madeline Dunlay, McKenna Revis, Adrianna L. Glisan, and et al. 2025. "In Preclinical Epilepsy, GLUT1 and GFAP Dysregulation in Cells Surrounding the Third Ventricle, Including Tanycytes, Is Differentially Restored with Ketogenic Diet Treatment" Nutrients 17, no. 11: 1824. https://doi.org/10.3390/nu17111824
APA StyleRafiei, P., Mian, H. S., Iyer, S. H., Draves, S. B., Matthews, S. A., Rendon, D. E., Neesen, E. J., Dunlay, M., Revis, M., Glisan, A. L., Simeone, T. A., & Simeone, K. A. (2025). In Preclinical Epilepsy, GLUT1 and GFAP Dysregulation in Cells Surrounding the Third Ventricle, Including Tanycytes, Is Differentially Restored with Ketogenic Diet Treatment. Nutrients, 17(11), 1824. https://doi.org/10.3390/nu17111824







