A Comprehensive Scoping Review on Diet and Nutrition in Relation to Long COVID-19 Symptoms and Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Information Sources
2.3. Search Strategy
2.4. Terminology and Definitions of Long COVID-19
2.5. Eligibility Criteria
2.6. Selection of Sources of Evidence
2.7. Critical Appraisal of Sources of Evidence
2.8. Data Charting Process
2.9. Data Items
2.10. Synthesis of Results
2.11. Use of Large Language Models
3. Results
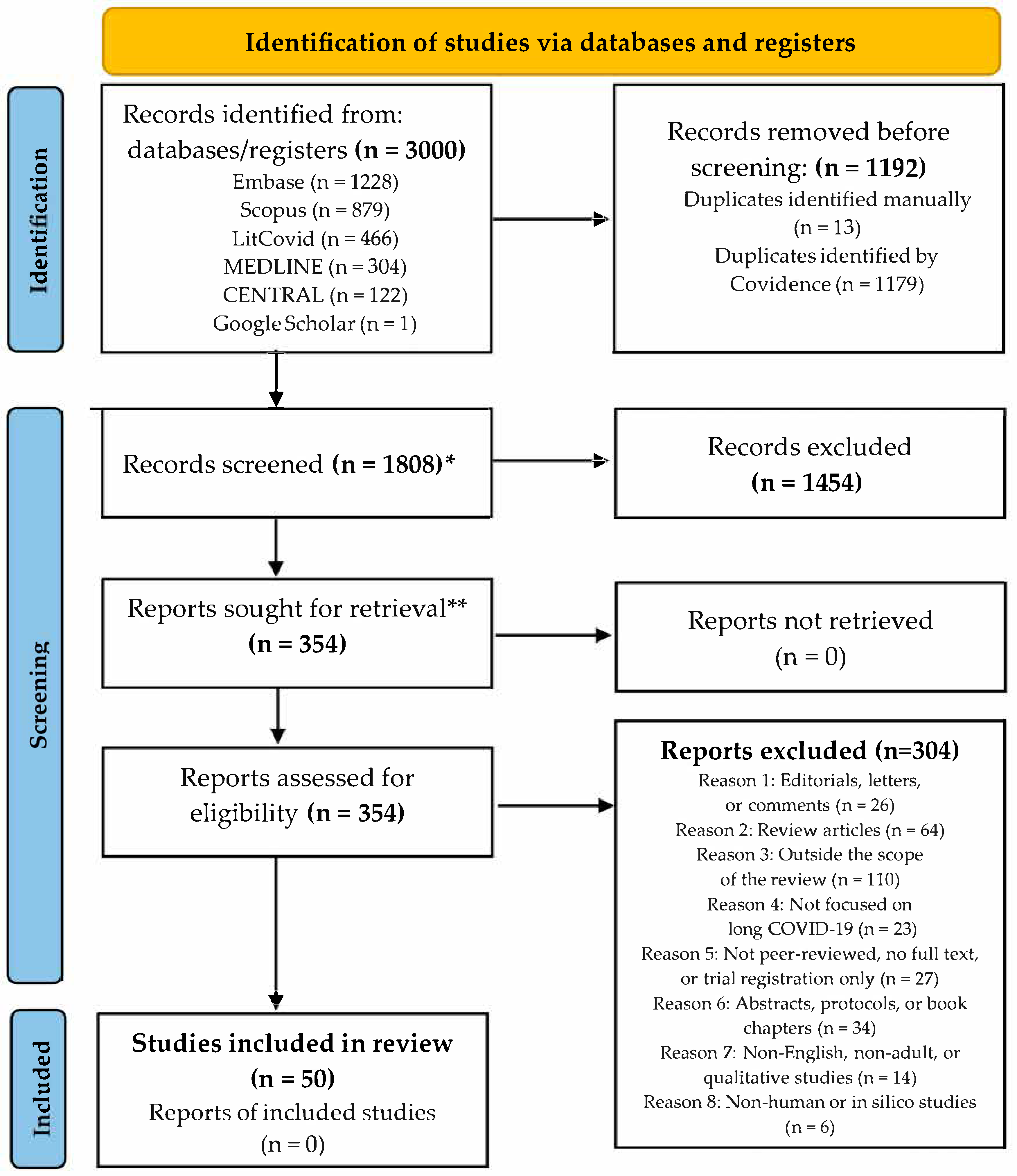
3.1. Selection of Sources of Evidence
3.2. Characteristics of Population, Design, Sample, and Study Country
3.3. Long COVID-19: Terminology and Definitions Across Studies
3.4. Strength of Evidence
3.5. Main Study Outcomes
3.6. Results of Individual Sources of Evidence
3.6.1. Vitamin D
3.6.2. Multinutrient, Nutraceutical, and Combined Formulations
3.6.3. Amino Acids and Metabolic Support
3.6.4. Gut Microbiota Modulation in Long COVID-19
3.6.5. Pre-Infection Lifestyle and Risk of Long COVID-19
3.6.6. Prolonged Nutritional Status After COVID-19
3.6.7. Multi-Professional Intervention and Diet Modification
3.7. Summary of Evidence on Key Nutritional Exposures in Long COVID-19
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 20-MFI | 20-item Multidimensional Fatigue Inventory |
| 25OHD | 25-hydroxyvitamin D |
| 6MWT | 6-Minute Walk Test |
| 7-day food diary | Seven-day dietary intake recording tool |
| ADMA | Asymmetric Dimethylarginine |
| AEO | Enol-Oxaloacetate |
| BDI | Beck Depression Inventory |
| BioICOPER | Biopsychosocial Integrated COVID Pathway for Evaluation and Rehabilitation |
| BMI | Body Mass Index |
| BTT | Butanol Threshold Test |
| CDC | Centers for Disease Control and Prevention |
| CES-D | Center for Epidemiologic Studies Depression Scale |
| CFS | Chalder Fatigue Score |
| COMPASS 31 | Composite Autonomic Symptom Score 31 |
| COVID-19 | Coronavirus Disease 2019 |
| CRP | C-reactive protein |
| CT | Controlled Trial |
| DQQ | Dietary Diversity Score |
| DSM | Diagnostic and Statistical Manual of Mental Disorders |
| ED | Emergency Department |
| EQ-5D | EuroQol 5-Dimension Questionnaire |
| EQ-5D-5L | EuroQol 5-Dimension 5-Level Questionnaire |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| FACIT | Functional Assessment of Chronic Illness Therapy |
| FAS | Fatigue Assessment Scale |
| FFMI | Fat-Free Mass Index |
| FMI | Fat Mass Index |
| GAD-7 | Generalized Anxiety Disorder—7 item scale |
| GLIM | Global Leadership Initiative on Malnutrition |
| HR | Hazard Ratio or Heart Rate (context-dependent) |
| HRQoL | Health-Related Quality of Life |
| ICD | International Classification of Diseases |
| ICU | Intensive Care Unit |
| IES-R | Impact of Event Scale—Revised |
| IL-6 | Interleukin-6 |
| IQR | Interquartile Range |
| IU | International Unit |
| LDH | Lactate Dehydrogenase |
| LDL | Low-Density Lipoprotein |
| MD | Mediterranean Diet |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MeSH | Medical Subject Headings |
| MEDAS | Mediterranean Diet Adherence Screener |
| ME/CFS | Myalgic Encephalomyelitis/Chronic Fatigue Syndrome |
| MetS | Metabolic Syndrome |
| MHC-SF | Mental Health Continuum—Short Form |
| MLR | Monocyte-to-lymphocyte ratio |
| MMS | Multiple Micronutrient Supplement |
| MoCA-BLIND | Montreal Cognitive Assessment—Blind Version |
| MNA | Mini Nutritional Assessment |
| NHS | National Health Service |
| NICE | National Institute for Health and Care Excellence |
| NLR | Neutrophil-to-lymphocyte ratio |
| NNLM | National Network of Libraries of Medicine |
| OFS | Oral Food Supplement |
| Omega-3/6 | Omega-3 and Omega-6 polyunsaturated fatty acids |
| ONS | Oral Nutritional Supplements |
| OR | Odds Ratio |
| PACS | Post-Acute COVID-19 Syndrome |
| PCC | Post-COVID-19 Condition |
| pCOVq | Post-COVID-19 Questionnaire |
| PCQ | Primary Care Questionnaire (or Patient Care Quality; context-dependent) |
| PHQ-9 | Patient Health Questionnaire-9 |
| PACSQ-14 | Post-Acute COVID-19 Syndrome Questionnaire |
| PREDIMED | Prevención con Dieta Mediterránea / Prevention with Mediterranean Diet |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews |
| PROMIS | Patient-Reported Outcomes Measurement Information System |
| PSQI | Pittsburgh Sleep Quality Index |
| QoL | Quality of life |
| RCT | randomized controlled trial |
| SARC-F | Strength, Assistance with walking, Rise from a chair, Climb stairs, and Falls |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SF-36 | 36-Item Short Form Survey |
| SIM01 | A specific probiotic strain/formulation (context-dependent) |
| TDR | Total Diet Replacement |
| UK | United Kingdom |
| VAS | Visual Analogue Scale |
| VDD | Vitamin D deficiency |
| WHO | World Health Organization |
| WHOQOL-BREF | World Health Organization Quality of Life—Brief Version |
References
- Nguyen, K.H.; Bao, Y.; Mortazavi, J.; Allen, J.D.; Chocano-Bedoya, P.O.; Corlin, L. Prevalence and factors associated with long COVID symptoms among U.S. adults, 2022. Vaccines 2024, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Bae, S.; Chang, H.H.; Kim, S.W. Long COVID prevalence and impact on quality of life 2 years after acute COVID-19. Sci. Rep. 2023, 13, 11960. [Google Scholar]
- Al-Aly, Z.; Topol, E. Solving the puzzle of Long Covid. Science 2024, 383, 830–832. [Google Scholar] [CrossRef] [PubMed]
- Krongthaeo, S.; Partiprajak, S.; Piaseu, N.; Ckumdee, S.; Taaon, C.; Kongsuwan, A. Prevalence and exploratory factor analysis of long COVID-19 symptoms among experienced infected population in Bangkok, Thailand. BMC Public Health 2024, 24, 2863. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Davis, H.; McCorkell, L.; Soares, L.; Wulf-Hanson, S.; Iwasaki, A.; Topol, E.J. Long COVID science, research and policy. Nat. Med. 2024, 30, 2148–2164. [Google Scholar] [CrossRef]
- Elbeltagi, R.; Al-Beltagi, M.; Saeed, N.K.; Bediwy, A.S. COVID-19-induced gastrointestinal autonomic dysfunction: A systematic review. World J. Clin. Cases 2023, 11, 5252–5272. [Google Scholar] [CrossRef]
- Anghel, L.; Manole, C.; Nechita, A.; Tatu, A.L.; Ștefănescu, B.I.; Nechita, L.; Bușilă, C.; Zainea, P.; Baroiu, L.; Mușat, C.L. Calcium, phosphorus and magnesium abnormalities associated with COVID-19 infection, and beyond. Biomedicines 2023, 11, 2362. [Google Scholar] [CrossRef]
- Hernández-Flores, T.d.J.; Pedraza-Brindis, E.J.; Cárdenas-Bedoya, J.; Ruíz-Carrillo, J.D.; Méndez-Clemente, A.S.; Martínez-Guzmán, M.A.; Iñiguez-Gutiérrez, L. Role of micronutrients and gut microbiota-derived metabolites in COVID-19 recovery. Int. J. Mol. Sci. 2022, 23, 12324. [Google Scholar] [CrossRef]
- Naidu, A.S.; Wang, C.K.; Rao, P.; Mancini, F.; Clemens, R.A.; Wirakartakusumah, A.; Chiu, H.F.; Yen, C.H.; Porretta, S.; Mathai, I.; et al. Precision nutrition to reset virus-induced human metabolic reprogramming and dysregulation (HMRD) in long COVID. NPJ Sci. Food 2024, 8, 19. [Google Scholar] [CrossRef]
- Ferrulli, A.; Senesi, P.; Terruzzi, I.; Luzi, L. Eating habits and body weight changes induced by variation in smell and taste in patients with previous SARS-CoV-2 infection. Nutrients 2022, 14, 5068. [Google Scholar] [CrossRef]
- Bradbury, J.; Wilkinson, S.; Schloss, J. Nutritional support during long COVID: A systematic scoping review. J. Integr. Complement. Med. 2023, 29, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Storz, M.A. Lifestyle adjustments in long COVID management: Potential benefits of plant-based diets. Curr. Nutr. Rep. 2021, 10, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Altooq, N.; Humood, A.; Alajaimi, A.; Alenezi, A.F.; Janahi, M.; AlHaj, O.; Jahrami, H. The role of micronutrients in the management of COVID-19 and optimizing vaccine efficacy. Hum. Nutr. Metab. 2022, 27, 200141. [Google Scholar] [CrossRef] [PubMed]
- Ben-Eltriki, M.; Hopefl, R.; Wright, J.M.; Deb, S. Association between vitamin D status and risk of developing severe COVID-19 infection: A meta-analysis of observational studies. J. Am. Nutr. Assoc. 2021, 41, 679–689. [Google Scholar] [CrossRef]
- Linneberg, A.; Kampmann, F.B.; Israelsen, S.B.; Andersen, L.R.; Jørgensen, H.L.; Sandholt, H.; Jørgensen, N.R.; Thysen, S.M.; Benfield, T. The association of low vitamin K status with mortality in a cohort of 138 hospitalized patients with COVID-19. Nutrients 2021, 13, 1985. [Google Scholar] [CrossRef]
- Azzolino, D.; Coelho-Junior, H.J.; Proietti, M.; Manzini, V.M.; Cesari, M. Fatigue in older persons: The role of nutrition. Proc. Nutr. Soc. 2023, 82, 39–46. [Google Scholar] [CrossRef]
- Bear, D.E.; Merriweather, J.L. Nutrition in postacute rehabilitation of COVID-19 survivors. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 154–158. [Google Scholar] [CrossRef]
- Schloss, J.V. Nutritional deficiencies that may predispose to long COVID. Inflammopharmacology 2023, 31, 573–583. [Google Scholar] [CrossRef]
- Suárez-Moreno, N.; Gómez-Sánchez, L.; Navarro-Caceres, A.; Arroyo-Romero, S.; Domínguez-Martín, A.; Lugones-Sánchez, C.; Tamayo-Morales, O.; González-Sánchez, S.; Castro-Rivero, A.B.; Rodríguez-Sánchez, E.; et al. Association of Mediterranean diet with cardiovascular risk factors and with metabolic syndrome in subjects with long COVID: BioICOPER study. Nutrients 2025, 17, 656. [Google Scholar] [CrossRef]
- Godos, J.; Guglielmetti, M.; Ferraris, C.; Frias-Toral, E.; Domínguez Azpíroz, I.; Lipari, V.; Di Mauro, A.; Furnari, F.; Castellano, S.; Galvano, F.; et al. Mediterranean diet and quality of life in adults: A systematic review. Nutrients 2025, 17, 577. [Google Scholar] [CrossRef]
- Bilal, M.; Ashraf, S.; Zhao, X. Dietary component-induced inflammation and its amelioration by prebiotics, probiotics, and synbiotics. Front. Nutr. 2022, 9, 931458. [Google Scholar] [CrossRef] [PubMed]
- Tandon, P.; Abrams, N.D.; Avula, L.R.; Carrick, D.M.; Chander, P.; Divi, R.L.; Dwyer, J.T.; Gannot, G.; Gordiyenko, N.; Liu, Q.; et al. Unraveling links between chronic inflammation and long COVID: Workshop report. J. Immunol. 2024, 212, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Prabhudesai, K.S.; Damle, A.; Ramakrishnan, S.; Durairaj, P.; Kalankariyan, S.; Vijayalakshmi, A.B.; Venkatesh, K.V. A systems biology-based mathematical model demonstrates the potential anti-stress effectiveness of a multi-nutrient botanical formulation. Sci. Rep. 2024, 14, 9582. [Google Scholar] [CrossRef] [PubMed]
- Dietz, T.K.; Brondstater, K.N. Long COVID management: A mini review of current recommendations and underutilized modalities. Front. Med. 2024, 11, 1430444. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef]
- Chen, Q.; Allot, A.; Leaman, R.; Wei, C.H.; Aghaarabi, E.; Guerrerio, J.J.; Xu, L.; Lu, Z. LitCovid in 2022: An information resource for the COVID-19 literature. Nucleic Acids Res. 2023, 51, D1512–D1518. [Google Scholar] [CrossRef]
- Veritas Health Innovation. Covidence Systematic Review Software. Melbourne, Australia. Available online: https://www.covidence.org (accessed on 9 May 2025).
- ChatGPT[Large Language Model], Version, 4.0; OpenAI: San Francisco, CA, USA, 2023. Available online: https://chat.openai.com (accessed on 9 May 2025).
- Pizzini, A.; Aichner, M.; Sahanic, S.; Böhm, A.; Egger, A.; Hoermann, G.; Kurz, K.; Widmann, G.; Bellmann-Weiler, R.; Weiss, G.; et al. Impact of vitamin D deficiency on COVID-19: A prospective analysis from the CovILD Registry. Nutrients 2020, 12, 2775. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; McCluskey, P.; O’Brien, K.; Dowds, J.; Laird, E.; Bannan, C.; Bourke, N.M.; Ní Cheallaigh, C.; Byrne, D.G.; et al. Investigating the relationship between vitamin D and persistent symptoms following SARS-CoV-2 infection. Nutrients 2021, 13, 2430. [Google Scholar] [CrossRef]
- Galluzzo, V.; Ciciarello, F.; Tosato, M.; Zazzara, M.B.; Pais, C.; Savera, G.; Calvani, R.; Picca, A.; Marzetti, E.; Landi, F. Association between vitamin D status and physical performance in COVID-19 survivors: Results from the Gemelli against COVID-19 post-acute care project. Mech. Ageing Dev. 2022, 205, 111684. [Google Scholar] [CrossRef]
- Fernandes, A.L.; Sales, L.P.; Santos, M.D.; Caparbo, V.F.; Murai, I.H.; Pereira, R.M.R. Persistent or new symptoms 1 year after a single high dose of vitamin D3 in patients with moderate to severe COVID-19. Front. Nutr. 2022, 9, 979667. [Google Scholar] [CrossRef] [PubMed]
- di Filippo, L.; Frara, S.; Nannipieri, F.; Cotellessa, A.; Locatelli, M.; Rovere Querini, P.; Giustina, A. Low vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J. Clin. Endocrinol. Metab. 2023, 108, e1106–e1116. [Google Scholar] [CrossRef] [PubMed]
- Hikmet, R.G.; Wejse, C.; Agergaard, J. Effect of vitamin D in long COVID patients. Int. J. Environ. Res. Public Health 2023, 20, 7058. [Google Scholar] [CrossRef]
- Chen, K.Y.; Lin, C.K.; Chen, N.H. Effects of vitamin D and zinc deficiency in acute and long COVID syndrome. J. Trace Elem. Med. Biol. 2023, 80, 127278. [Google Scholar] [CrossRef]
- Rodríguez-Morán, M.; Guerrero-Romero, F.; Barragán-Zuñiga, J.; Gamboa-Gómez, C.I.; Weyman-Vela, Y.; Arce-Quiñones, M.; Simmental-Mendía, L.E.; Martínez-Aguilar, G. Combined oral supplementation with magnesium plus vitamin D alleviates mild to moderate depressive symptoms related to long COVID: An open-label randomized, controlled clinical trial. Magnes Res. 2024, 37, 49–57. [Google Scholar] [CrossRef]
- Charoenporn, V.; Tungsukruthai, P.; Teacharushatakit, P.; Hanvivattanakul, S.; Sriyakul, K.; Sukprasert, S.; Kamalashiran, C.; Tungsukruthai, S.; Charernboon, T. Effects of an 8-week high-dose vitamin D supplementation on fatigue and neuropsychiatric manifestations in post-COVID syndrome: A randomized controlled trial. Psychiatry Clin. Neurosci. 2024, 78, 595–604. [Google Scholar] [CrossRef]
- Chadda, K.R.; Roberts, S.A.; Lugg, S.T.; Faniyi, A.A.; Faustini, S.E.; Webster, C.; Duffy, J.E.; Hewison, M.; Shields, A.; Richter, A.G.; et al. Vitamin D deficiency and duration of COVID-19 symptoms in UK healthcare workers. Front. Med. 2024, 11, 1494129. [Google Scholar] [CrossRef]
- Kaddah, S.Z.; Mousa, H.A.; Elhalafawy, M.Y.; Ashraf, H.; Osman, M.; Bayoumi, A.I. Investigating vitamin D status following severe acute respiratory syndrome coronavirus 2 infection and its relationship with long coronavirus disease. Egypt J. Chest Dis. Tuberc. 2024, 73, 126–131. [Google Scholar] [CrossRef]
- Wu, J.Y.; Liu, M.Y.; Hsu, W.H.; Tsai, Y.W.; Liu, T.H.; Huang, P.Y.; Chuang, M.H.; Chin, S.E.; Lai, C.C. Association between vitamin D deficiency and post-acute outcomes of SARS-CoV-2 infection. Eur. J. Nutr. 2024, 63, 613–622. [Google Scholar] [CrossRef]
- Atieh, O.; Daher, J.; Durieux, J.C.; Abboud, M.; Labbato, D.; Baissary, J.; Koberssy, Z.; Ailstock, K.; Cummings, M.; Funderburg, N.T.; et al. Vitamins K2 and D3 improve long COVID, fungal translocation, and inflammation: Randomized controlled trial. Nutrients 2025, 17, 304. [Google Scholar] [CrossRef]
- Chung, T.W.; Zhang, H.; Wong, F.K.; Sridhar, S.; Lee, T.M.; Leung, G.K.; Chan, K.H.; Lau, K.K.; Tam, A.R.; Ho, D.T.; et al. A pilot study of short-course oral vitamin A and aerosolised diffuser olfactory training for the treatment of smell loss in long COVID. Brain Sci. 2023, 13, 1014. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, S.; Mahmoudnejad, N.; Ahmadi Asour, A.; Keyvanfar, A.; Mahmoudi Chalmiani, E. Efficacy of thiamine (vitamin B1) on post-acute COVID-19 syndrome: An open-label, randomized, controlled trial. Arch. Clin. Infect. Dis. 2024, 19, e144280. [Google Scholar] [CrossRef]
- Rossato, M.S.; Brilli, E.; Ferri, N.; Giordano, G.; Tarantino, G. Observational study on the benefit of a nutritional supplement, supporting immune function and energy metabolism, on chronic fatigue associated with the SARS-CoV-2 post-infection progress. Clin. Nutr. ESPEN 2021, 46, 510–518. [Google Scholar] [CrossRef]
- Naureen, Z.; Dautaj, A.; Nodari, S.; Fioretti, F.; Dhuli, K.; Anpilogov, K.; Lorusso, L.; Paolacci, S.; Michelini, S.; Guda, T.; et al. Proposal of a food supplement for the management of post-COVID syndrome. Eur. Rev. Med. Pharmacol. Sci. 2021, 25 (Suppl. S1), 67–73. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Martone, A.M.; Ciciarello, F.; Galluzzo, V.; Savera, G.; Calvani, R.; Picca, A.; Marzetti, E.; Tosato, M.; Gemelli Against COVID-19 Post-Acute Care Team. Effects of a new multicomponent nutritional supplement on muscle mass and physical performance in adult and old patients recovered from COVID-19: A pilot observational case-control study. Nutrients 2022, 14, 2316. [Google Scholar] [CrossRef] [PubMed]
- Galluzzo, V.; Zazzara, M.B.; Ciciarello, F.; Savera, G.; Pais, C.; Calvani, R.; Picca, A.; Marzetti, E.; Landi, F.; Tosato, M.; et al. Gemelli Against COVID-19 Post-Acute Care Team Fatigue in COVID-19 survivors: The potential impact of a nutritional supplement on muscle strength function. Clin. Nutr. ESPEN 2022, 51, 215–221. [Google Scholar] [CrossRef]
- Kharaeva, Z.; Shokarova, A.; Shomakhova, Z.; Ibragimova, G.; Trakhtman, P.; Trakhtman, I.; Chung, J.; Mayer, W.; De Luca, C.; Korkina, L. Fermented Carica papaya and Morinda citrifolia as perspective food supplements for the treatment of post-COVID symptoms: Randomized placebo-controlled clinical laboratory study. Nutrients 2022, 14, 2203. [Google Scholar] [CrossRef]
- Gaylis, N.B.; Kreychman, I.; Sagliani, J.; Mograbi, J.; Gabet, Y. The results of a unique dietary supplement (nutraceutical formulation) used to treat the symptoms of long-haul COVID. Front. Nutr. 2022, 9, 1034169. [Google Scholar] [CrossRef]
- Tomasa-Irriguible, T.M.; Monfà, R.; Miranda-Jiménez, C.; Morros, R.; Robert, N.; Bordejé-Laguna, L.; Vidal, S.; Torán-Monserrat, P.; Barriocanal, A.M. Preventive intake of a multiple micronutrient supplement during mild, acute SARS-CoV-2 infection to reduce the post-acute COVID-19 condition: A double-blind, placebo-controlled, randomized clinical trial. Nutrients 2024, 16, 1631. [Google Scholar] [CrossRef]
- Noce, A.; Marrone, G.; Di Lauro, M.; Vita, C.; Montalto, G.; Giorgino, G.; Chiaramonte, C.; D’Agostini, C.; Bernardini, S.; Pieri, M. Potential anti-inflammatory and anti-fatigue effects of an oral food supplement in long COVID patients. Pharmaceuticals 2024, 17, 463. [Google Scholar] [CrossRef]
- Marra, A.M.; Giardino, F.; Anniballo, A.; Ferazzoli, S.; Salzano, A.; Arcopinto, M.; D’Assante, R.; De Mare, A.; Esposito, G.; Saldamarco, L.; et al. Beneficial effects on exercise capacity associated with a combination of lactoferrin, lysozyme, Lactobacillus, resveratrol, vitamins, and oligoelements in patients with post-COVID-19 syndrome: A single-center retrospective study. J. Clin. Med. 2024, 13, 4444. [Google Scholar] [CrossRef] [PubMed]
- Tosato, M.; Calvani, R.; Picca, A.; Ciciarello, F.; Galluzzo, V.; Coelho-Júnior, H.J.; Di Giorgio, A.; Di Mario, C.; Gervasoni, J.; Gremese, E.; et al. Effects of L-arginine plus vitamin C supplementation on physical performance, endothelial function, and persistent fatigue in adults with long COVID: A single-blind randomized controlled trial. Nutrients 2022, 14, 4984. [Google Scholar] [CrossRef] [PubMed]
- Cash, A.; Kaufman, D.L. Oxaloacetate treatment for mental and physical fatigue in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and long COVID fatigue patients: A non-randomized controlled clinical trial. J. Transl. Med. 2022, 20, 295. [Google Scholar] [CrossRef]
- Izzo, R.; Trimarco, V.; Mone, P.; Aloè, T.; Capra Marzani, M.; Diana, A.; Fazio, G.; Mallardo, M.; Maniscalco, M.; Marazzi, G.; et al. Combining L-arginine with vitamin C improves long COVID symptoms: The LINCOLN Survey. Pharmacol. Res. 2022, 183, 106360. [Google Scholar] [CrossRef]
- Turcu-Stiolica, A.; Ionele, C.M.; Ungureanu, B.S.; Subtirelu, M.S. The effects of arginine-based supplements on fatigue levels following COVID-19 infection: A prospective study in Romania. Healthcare 2023, 11, 1477. [Google Scholar] [CrossRef]
- Slankamenac, J.; Ranisavljev, M.; Todorovic, N.; Ostojic, J.; Stajer, V.; Ostojic, S.M. Effects of six-month creatine supplementation on patient- and clinician-reported outcomes, and tissue creatine levels in patients with post-COVID-19 fatigue syndrome. Food Sci. Nutr. 2023, 11, 6899–6906. [Google Scholar] [CrossRef]
- Slankamenac, J.; Ranisavljev, M.; Todorovic, N.; Ostojic, J.; Stajer, V.; Candow, D.G.; Ratgeber, L.; Betlehem, J.; Acs, P.; Ostojic, S.M. Eight-week creatine-glucose supplementation alleviates clinical features of long COVID. J. Nutr. Sci. Vitaminol. 2024, 70, 174–178. [Google Scholar] [CrossRef]
- Thomas, R.; Williams, M.; Aldous, J.; Yanagisawa, Y.; Kumar, R.; Forsyth, R.; Chater, A. A randomised, double-blind, placebo-controlled trial evaluating concentrated phytochemical-rich nutritional capsule in addition to a probiotic capsule on clinical outcomes among individuals with COVID-19—The UK Phyto-V Study. COVID 2022, 2, 433–449. [Google Scholar] [CrossRef]
- Docampo, M.J.; Batruch, M.; Oldrati, P.; Berenjeno-Correa, E.; Hilty, M.; Leventhal, G.; Lutterotti, A.; Martin, R.; Sospedra, M. Clinical and immunologic effects of paraprobiotics in long COVID patients: A pilot study. Neurol. Neuroimmunol. Neuroinflamm. 2024, 11, e200296. [Google Scholar] [CrossRef]
- Lau, R.I.; Su, Q.; Lau, I.S.F.; Ching, J.Y.L.; Wong, M.C.S.; Lau, L.H.S.; Tun, H.M.; Mok, C.K.P.; Chau, S.W.H.; Tse, Y.K.; et al. A synbiotic preparation (SIM01) for post-acute COVID-19 syndrome in Hong Kong (RECOVERY): A randomized, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2024, 24, 256–265. [Google Scholar] [CrossRef]
- Calvani, R.; Giampaoli, O.; Marini, F.; Del Chierico, F.; De Rosa, M.; Conta, G.; Sciubba, F.; Tosato, M.; Picca, A.; Ciciarello, F.; et al. Beetroot juice intake positively influenced gut microbiota and inflammation but failed to improve functional outcomes in adults with long COVID: A pilot randomized controlled trial. Clin. Nutr. 2024, 43, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Sierra, A.; de la, O.V.; Higuera-Gómez, A.; Chero-Sandoval, L.; de Cuevillas, B.; Martínez-Urbistondo, M.; Moreno-Torres, V.; Pintos-Pascual, I.; Castejón, R.; Martínez, J.A.; et al. Mediterranean diet and olive oil redox interactions on lactate dehydrogenase mediated by gut Oscillibacter in patients with long COVID-19 syndrome. Antioxidants 2024, 13, 1358. [Google Scholar] [CrossRef] [PubMed]
- Ranisavljev, M.; Stajer, V.; Todorovic, N.; Ostojic, J.; Cvejic, J.H.; Steinert, R.E.; Ostojic, S.M. The effects of 3-month supplementation with synbiotic on patient-reported outcomes, exercise tolerance, and brain and muscle metabolism in adult patients with post-COVID-19 chronic fatigue syndrome (STOP-FATIGUE): A randomized placebo-controlled clinical trial. Eur. J. Nutr. 2024, 64, 28. [Google Scholar] [PubMed]
- Wang, S.; Li, Y.; Yue, Y.; Yuan, C.; Kang, J.H.; Chavarro, J.E.; Bhupathiraju, S.N.; Roberts, A.L. Adherence to a healthy lifestyle prior to infection and risk of post-COVID-19 condition. JAMA Intern. Med. 2023, 183, 232–241. [Google Scholar] [CrossRef]
- Wang, Y.; Su, B.; Alcalde-Herraiz, M.; Barclay, N.L.; Tian, Y.; Li, C.; Wareham, N.J.; Paredes, R.; Xie, J.; Prieto-Alhambra, D. Modifiable lifestyle factors and the risk of post-COVID-19 multisystem sequelae, hospitalization, and death. Nat. Commun. 2024, 15, 6363. [Google Scholar] [CrossRef]
- Gérard, M.; Mahmutovic, M.; Malgras, A.; Michot, N.; Scheyer, N.; Jaussaud, R.; Nguyen-Thi, P.L.; Quilliot, D. Long-term evolution of malnutrition and loss of muscle strength after COVID-19: A major and neglected component of long COVID-19. Nutrients 2021, 13, 3964. [Google Scholar] [CrossRef]
- Álvarez-Hernández, J.; Matía-Martín, P.; Cáncer-Minchot, E.; Cuerda, C. Long-term outcomes in critically ill patients who survived COVID-19: The NUTRICOVID observational cohort study. Clin. Nutr. 2023, 42, 2029–2035. [Google Scholar] [CrossRef]
- Lakenman, P.L.; Joosten, K.F.; Bommel, J.V.; Bek, L.M.; van den Berg-Emons, R.J.; Olieman, J.F. Nutritional status of patients with COVID-19 one year post-ICU stay: A prospective observational study. Nutrition 2023, 111, 112025. [Google Scholar] [CrossRef]
- Muzaffar, F.A.S.; Tan, S.T. Comparison of sleep quality, diet quality, and weight change between COVID-19-recovered patients and healthy controls: A matched case-control study. Health Sci. Rep. 2024, 7, e70042. [Google Scholar] [CrossRef]
- Mejía Alonso, L.A.; Espinosa-Poblano, E.; de Regil López, S.; Lemus Eslava, V.; Serrano Sánchez, J.G.; Paredes-Manjarrez, C.; Balderas-Chairéz, A.T.; Anda-Garay, J.C.; Miguel-Puga, J.A.; Jáuregui-Renaud, K.; et al. Malnutrition contribution to the functional status and health related quality of life after COVID-19: A correlational follow-up study. Sci. Rep. 2024, 14, 15005. [Google Scholar] [CrossRef]
- Sousa-Catita, D.; Godinho, C.; Mascarenhas, P.; Quaresma, F.; Fonseca, J. The effects of an intensive rehabilitation program on the nutritional and functional status of post-COVID-19 pneumonia patients. Nutrients 2022, 14, 2501. [Google Scholar] [CrossRef] [PubMed]
- Nikolic Turnic, T.; Vasiljevic, I.; Stanic, M.; Jakovljevic, B.; Mikerova, M.; Ekkert, N.; Reshetnikov, V.; Jakovljevic, V. Post-COVID-19 status and its physical, nutritional, psychological, and social effects in working-age adults—A prospective questionnaire study. J. Clin. Med. 2022, 11, 6668. [Google Scholar] [CrossRef] [PubMed]
- Sordi, A.F.; Lemos, M.M.; de Souza Marques, D.C.; Ryal, J.J.; de Paula Silva Lalucci, P.; Marques, M.G.; Amaro Camilo, M.L.; De Paula Ramos, S.; Franzói De Moraes, S.M.; Valdés-Badilla, P.; et al. Effects of a multi-professional intervention on body composition, physical fitness and biochemical markers in overweight COVID-19 survivors: A clinical trial. Front. Physiol. 2023, 14, 1219252. [Google Scholar] [CrossRef]
- Ryal, J.J.; Perli, V.A.S.; Marques, D.C.S.; Sordi, A.F.; Marques, M.G.S.; Camilo, M.L.; Milani, R.G.; Mota, J.; Valdés-Badilla, P.; Magnani Branco, B.H. Effects of a multi-professional intervention on mental health of middle-aged overweight survivors of COVID-19: A clinical trial. Int. J. Environ. Res. Public Health 2023, 20, 4132. [Google Scholar] [CrossRef]
- Pink, I.; Wiestler, M.; Pueschel, L.; Ruwisch, J.; Drick, N.; Boblitz, L.; Scharbau, M.; Welte, T.; Haufe, S.; Tegtbur, U.; et al. Exploring physical activity, sleep, and nutrition’s role in fatigue among post-COVID-19 patients. Nutrients 2024, 16, 4056. [Google Scholar] [CrossRef]
- Combet, E.; Haag, L.; Richardson, J.; Haig, C.E.; Cunningham, Y.; Fraser, H.L.; Brosnahan, N.; Ibbotson, T.; Ormerod, J.; White, C.; et al. Remotely delivered weight management for people with long COVID and overweight: The randomized wait-list-controlled ReDIRECT trial. Nat. Med. 2025, 31, 258–266. [Google Scholar] [CrossRef]
- Rusu, M.E.; Bigman, G.; Ryan, A.S.; Popa, D.S. Investigating the effects and mechanisms of combined vitamin D and K supplementation in postmenopausal women: An up-to-date comprehensive review of clinical studies. Nutrients 2024, 16, 2356. [Google Scholar] [CrossRef]
- Tosato, M.; Ciciarello, F.; Zazzara, M.B.; Pais, C.; Savera, G.; Picca, A.; Galluzzo, V.; Coelho-Júnior, H.J.; Calvani, R.; Marzetti, E.; et al. Nutraceuticals and dietary supplements for older adults with long COVID-19. Clin. Geriatr. Med. 2022, 38, 565–591. [Google Scholar] [CrossRef] [PubMed]
- Mey, J.T.; Kirwan, J.P.; Axelrod, C.L. The role of nutrition in mitigating the effects of COVID-19 from infection through PASC. Nutrients 2023, 15, 866. [Google Scholar] [CrossRef]
- Reyes, Z.; Stovall, M.C.; Punyamurthula, S.; Longo, M.; Maraganore, D.; Solch-Ottaiano, R.J. The impact of gut microbiome and diet on post-acute sequelae of SARS-CoV-2 infection. J. Neurol. Sci. 2024, 467, 123295. [Google Scholar] [CrossRef]
- Barrea, L.; Vetrani, C.; Caprio, M.; Cataldi, M.; Ghoch, M.E.; Elce, A.; Camajani, E.; Verde, L.; Savastano, S.; Colao, A.; et al. From the ketogenic diet to the Mediterranean diet: The potential dietary therapy in patients with obesity after COVID-19 infection (post COVID syndrome). Curr. Obes. Rep. 2022, 11, 144–165. [Google Scholar] [CrossRef] [PubMed]
- Montes-Ibarra, M.; Godziuk, K.; Thompson, R.B.; Chan, C.B.; Pituskin, E.; Gross, D.P.; Lam, G.; Schlögl, M.; Felipe Mota, J.; Ian Paterson, D.; et al. Protocol for a pilot study: Feasibility of a web-based platform to improve nutrition, mindfulness, and physical function in people living with post COVID-19 condition (BLEND). Methods 2024, 231, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Haag, L.; Richardson, J.; Cunningham, Y.; Fraser, H.; Brosnahan, N.; Ibbotson, T.; Ormerod, J.; White, C.; McIntosh, E.; O’Donnell, K.; et al. The remote diet intervention to reduce Long COVID symptoms trial (ReDIRECT): Protocol for a randomised controlled trial to determine the effectiveness and cost-effectiveness of a remotely delivered supported weight management programme for people with Long COVID and excess weight, with personalised improvement goals. NIHR Open Res. 2023, 2, 57. [Google Scholar] [CrossRef]
- Bigman, G.; Rusu, M.E.; Kleckner, A.S.; Sorkin, J.D.; Jin, Y.; Talegawkar, S.A.; Tanaka, T.; Ferrucci, L.; Ryan, A.S. Plant-based diets and their associations with physical performance in the Baltimore Longitudinal Study of Aging. Nutrients 2024, 16, 4249. [Google Scholar] [CrossRef] [PubMed]
- Guerra, G.; Lucariello, A.; Komici, K. Editorial: Long COVID: Nutrition and lifestyle changes. Front. Nutr. 2024, 11, 1375449. [Google Scholar] [CrossRef]
- Gartlehner, G.; Affengruber, L.; Titscher, V.; Noel-Storr, A.; Dooley, G.; Ballarini, N.; König, F. Single-reviewer abstract screening missed 13 percent of relevant studies: A crowd-based, randomized controlled trial. J. Clin. Epidemiol. 2020, 121, 20–28. [Google Scholar] [CrossRef]

| Inclusion | Exclusion |
|---|---|
| Peer-reviewed journal articles. | Commentaries, case reports, letters, books, dissertations, editorials, conference proceedings. |
| Participants must have long COVID-19 symptoms or a history of post-COVID-19 recovery. | Long COVID-19 or post-COVID-19 symptoms were not under investigation. |
| Measurable outcomes related to post-COVID-19 recovery/symptoms. | |
| Human adults (≥18 years old). | Children |
| Published in English. | Not published in English. |
| Quantity study designs, such as RCTs, cohort, case–control, or observational studies. | Qualitative, non-quantitative, or review study. |
| Publication date from 2020 onward to ensure relevance to long COVID-19. | Studies published before 2020. |
| Observational or Intervention studies focused on nutrition, diet, or nutritional supplements. | Interventions involving rehabilitation programs without dietary components. |
| Study, Year, Ref. | Country | Study Design | Study Sample | Long COVID-19 Assessment | Type of Nutritional Exposure | Outcomes Assessed | Assessment Tools/Criteria | Main Results | Significance and Strength of Evidence |
|---|---|---|---|---|---|---|---|---|---|
| Pizzini et al., 2020 [30] | Austria | Prospective CovILD study | N = 109 Age: 58 ± 14 y 40%, female. | CovILD registry 8 weeks after hospitalization/ outpatients with COVID-19 symptoms | Biomarker assessment: serum 25(OH)D levels | Parathyroid hormone (PTH), inflammatory markers, lung function, and CT findings. | Standard pulmonary function tests, VDD (25(OH)D < 30 nmol/L) CRP, IL-6 | VDD is frequent among COVID-19 patients but not associated with disease outcomes after eight weeks | Non-sig; Moderate |
| Townsend et al., 2021 [31] | Ireland | Cross- sectional | N = 149 Age 48.0 ± 15.0 y 59.1% female. | Post-COVID-19 outpatient symptoms at a median of 79 days post-infection | Biomarker assessment: serum 25(OH)D levels | Fatigue; exercise tolerance; inflammation | CFS, 6MWT, modified Borg scale; CRP, IL-6 | No significant association between serum vitamin D levels and fatigue or exercise tolerance. | Non-sig; Moderate |
| Galluzzo et al., 2022 [32] | Italy | Cross- sectional | N = 681. Age 53.4 ± 15.2 y 49% female | COVID-19 survivors admitted to the post-COVID-19 outpatient setting | Biomarker assessment: serum 25(OH)D levels | Physical performance | 6MWT, One-minute chair stand, Handgrip strength | VDD was associated with reduced physical performance; participants with normal levels walked farther on the 6MWT (475.0 m vs. 421.9 m; p < 0.01). | Sig; Moderate |
| Fernandes et al., 2022 [33] | Brazil | Double-blind, placebo-controlled, RCT | N = 144 Age: 54.3 ± 13.1 y 47.9%, female | Hospitalized patients with moderate to severe COVID-19 that were followed-up for 1 year | Supplement intervention: a single high dose of vitamin D3 (200,000 IU) | Persistent or new symptoms (e.g., fatigue, joint pain, myalgia) | Symptoms at 6 and 12 months after discharge QoL (SF-36) | No significant differences between groups were observed for fever, cough, fatigue, fever, myalgia, joint pain, QoL, and new or persistent symptoms up to 1-year of follow-up | Non-sig; Moderate |
| Di Filippo et al., 2023 [34] | Italy | Cross- sectional Retrospective | N = 100 Age: 61 (51–70) y 44% female | National Institute for Health and Care Excellence (NICE) n = 50 long COVID-19 vs. n = 50 without | Biomarker assessment: serum 25(OH)D levels | Long COVID-19 diagnosis | NICE case definition | Lower vitamin D levels at follow-up were associated with long COVID-19 (20.1 vs. 23.2 ng/mL; p = 0.03; OR = 1.09, 95% CI: 1.01–1.16; p = 0.008). | Sig; Moderate |
| Hikmet et al., 2023 [35] | Denmark | Cohort study | N = 442 Age 47 ± 12.7 y 72% female | long COVID-19 at specialized post-COVID-19 clinic; symptoms > 12 weeks | Biomarker classification: serum 25(OH)D3 levels (cutoff: <50 nmol/L vs. normal) | Symptom burden | PCQ (31-symptom sum score) | No significant differences in symptom prevalence or severity between vitamin D-deficient and -sufficient groups. | Non-sig; Moderate |
| Chen et al., 2023 [36] | Taiwan | Retrospective case | N = 55 Age 49.3 ± 17.5 y 61.8% female | Symptoms persisted for more than 42 days | Biomarker assessment: serum vitamin D and zinc levels | Duration of long COVID-19 symptoms; inflammation | Self-report (symptom duration); inflammatory biomarkers | Vitamin D (29.1%) and zinc (27.3%) deficiencies were associated with long COVID-19 (p < 0.05); zinc deficiency linked to elevated fibrinogen, and VDD to delayed recovery. | Sig; Low |
| Rodríguez-Morán et al., 2024 [37] | Mexico | RCT Open-label | N = 60 Age 52.8 ± 12.6 y | Hypomagnesemia, VDD, and MMD related to long COVID-19 | Supplement intervention: Magnesium chloride (1300 mg/day) + vitamin D (4000 IU/day) vs. vitamin D alone for 4 months | Depressive symptoms MMD | MMD defined as BDI score 11–30; improvement defined as BDI <11 | Depressive symptoms improved more in the intervention group (BDI 28.8 → 9.2; p < 0.01) than in controls (28.4 → 21.6; p < 0.05); 73.2% vs. 34.5% achieved BDI <11 (p = 0.006). | Sig; Moderate |
| Charoenporn et al., 2024 [38] | Thailand | Double-blind RCT | N = 80 Age 34.1 ± 10.5 y 75%, female | at least one post-symptom within 3 months of COVID-19 onset and lasting for >1 month. | Supplement intervention: 60,000 IU/week of vitamin D2 vs. placebo for 8 weeks | Fatigue, anxiety, depression, sleep, cognition, inflammation | Questionnaires; IL-6, CRP | Vitamin D supplementation improved fatigue (p = 0.024), anxiety (p = 0.011), and cognition (p = 0.012); no significant effects were observed for sleep, depression, or inflammatory markers. | Sig; High |
| Chadda et al., 2024 [39] | UK | Cross- sectional | N = 392 Age 42 (30–50) y 73% female | From the COVID-19 convalescent immunity study | Biomarker classification: vitamin D deficiency defined as <30 nmol/L | Symptom presence, onset, duration | Self-report (8 symptoms including fatigue, breathlessness, etc.) | Vitamin D deficiency (15.6% prevalence) was associated with a greater number of symptoms (p = 0.003), prolonged body aches (OR = 3.07; p = 0.001), fatigue (OR = 2.09; p = 0.027). | Sig; Moderate |
| Kaddah et al., 2024 [40] | Egypt | Cross- sectional | N = 84 Age 40.5 ± 14.0 y 72.6% female | 3–6 months post-COVID-19 infection | Biomarker assessment: serum 25(OH)D levels | Post-COVID-19 symptoms | CFS, mMRC dyspnea scale, CRP | Vitamin D insufficiency (44%) and deficiency (36%) were not significantly associated with post-COVID-19 symptoms or outcomes. | Non-sig; Low |
| Wu et al., 2024 [41] | Taiwan | Retrospective | N = 16,600 Age 49.5 ± 17.6 y 66.4% female | ICD-10 code U09 TriNetX research network | Biomarker: vitamin D deficiency (<20 ng/mL) vs. non-deficient (≥20 ng/mL) | ED visits, hospitalization, mortality, post-COVID-19 diagnosis | TriNetX database; ICD-10 U09 | VDD was not associated with post-COVID-19 diagnosis (HR = 0.980; 95% CI: 0.630–1.523). | Non-sig; High |
| Atieh et al., 2025 [42] | USA | RCT | N = 151. Age 45 ± 12.9 y 70.4% female | ≥2 moderate symptoms >3 months | Supplement intervention: Vitamin K2 (240 µg/day) + vitamin D3 (2000 IU/day) vs. standard care for 24 weeks | Long COVID-19 symptom burden and diagnosis | RECOVER long COVID-19 Research Index (threshold ≥12); number and type of symptoms | Vitamin K2/D3 reduced RECOVER Index by 7.1% vs. a 7.2% increase in controls (p = 0.01); symptoms remained stable in the intervention group but worsened in controls (p = 0.03). | Sig; High |
| Chung et al., 2023 [43] | China (Hong Kong) | RCT Open-label | N = 24 Age 36 (26.0–43.0) y 56% female | olfactory dysfunction (OD) post-COVID-19. | Supplement intervention: Vitamin A (25,000 IU/day) with or without aromatherapy OT vs. clinical observation for 4 weeks | Olfactory function (primary), smell identification, olfactory bulb/tract volume, and olfactory cortical network connectivity | Butanol Threshold Test (≥2-point increase = clinical improvement); imaging and functional connectivity analysis | Oral vitamin A combined with olfactory training significantly improved olfactory function vs. training alone and control (BTT score, p < 0.001); also increased olfactory network activity. | Sig; Moderate |
| Tehrani et al., 2024 [44] | Iran | RCT Open-label | N = 66 Age 49.35 ± 13.83 y 47% female | Persistent symptoms ≥3 weeks after onset | Supplement intervention: Vitamin B1 (600 mg/day) + supportive therapy vs. supportive therapy alone for 8 weeks | Symptom severity (e.g., fatigue, anosmia, sleep disorders); sleep quality | 0–10 Visual Assessment Tool (weekly); PSQI | Vitamin B1 led to greater symptom resolution from week 6 onward; by week 8, 87% recovered vs. 40% in controls (p < 0.0001). | Sig; Moderate |
| Study, Year, Ref. | Country | Study Design | Study Sample | Long COVID-19 Assessment | Type of Nutritional Exposure | Outcomes Assessed | Assessment Tools/ Criteria | Main Results | Significance and Strength of Evidence |
|---|---|---|---|---|---|---|---|---|---|
| Rossato et al., 2021 [45] | Italy | Pre-post Intervention | N = 201 Age 48.11 ± 13.16 y 60.7%, female | Persistent fatigue following infection, with a median of 37 days since disease onset | Supplement intervention: Apportal® (daily sachet containing 19 nutrients including B vitamins, minerals, amino acids, and plant extracts) for 28 days | QoL, fatigue, mental fatigue | EQ-5D index, VAS, FACIT-Fatigue, modified Chalder Q (baseline, day 14 and 28) | Significant improvements in QoL and fatigue at days 14 and 28 (p < 0.0001); 76.6% improved FACIT-F by day 14 | Sig; Moderate |
| Naureen et al., 2021 [46] | Italy | Pilot Study | N = 40 Age 52.25 ± 12.0 y | Self-reported fatigue persisting after recovery from COVID-19 | Combination supplement: Acetyl L-carnitine, hydroxytyrosol, vitamins B1, B6, B9, B12, C, and D3 | Perceived fatigue, tension, energy, calmness | AD-ACL; 15-day pre-post | 123% ↑ in energy, ~50% ↓ in tiredness/tension in supplement users; no significant change in controls | Sig; Low |
| Landi et al., 2022 [47] | Italy | Intervention vs. Control | N = 66 Age 61.0 ± 11.8 y 44%, female | Experiencing fatigue, with a mean of 94.2 ± 22.3 days since onset | Supplement intervention: Amino-Ther Pro (10 amino acids + vitamins B1 and B6 + organic acids), 2 servings/day for 8 weeks vs. no supplement | Muscle index, handgrip, chair-stand, 6MWT, QoL | Handgrip dynamometry, 1-minute chair-stand, 6MWT, EuroQol, VAS | Significant improvements in physical function and muscle metrics; gains remained significant after adjustment | Sig; Moderate |
| Galluzzo et al., 2022 [48] | Italy | Pre-post Intervention | N = 30 Age 56.14 ± 13.9 y 70%, female | Fatigue and reduced exercise tolerance 30–90 days after COVID-19 diagnosis. | Supplement intervention: Apportal® (same formulation as above), daily for 28 days | Muscle strength, performance, body composition, inflammation | Handgrip, sit-to-stand test, BIA (phase angle, SMI, fat mass), CRP, VAS | Increased handgrip (26.3→28.9 kg), strength time, chair-stands (22→28.3), phase angle, and VAS (all p < 0.05) | Sig; Low |
| Kharaeva et al., 2022 [49] | Slovenia | RCT | N = 160 Age 38–69 y 60.6%, female | Post-COVID-19 following moderate or severe infection | Supplement intervention: Fermented Carica papaya and Morinda citrifolia extract vs. placebo for 20 days | Post-COVID-19 symptoms, inflammation | Self-reported symptoms, IL-6, TNF-α assays | Reduced fatigue, joint pain, cognitive symptoms; decreased IL-6, TNF-α | Sig; High |
| Gaylis et al., 2022 [50] | USA/ Israel | RCT Open-label | N = 51 Age 21–73 y 66.7%, female | symptoms ≥3 months | Supplement intervention: Nutraceutical blend (β-caryophyllene, pregnenolone, DHEA, quercetin, bromelain, zinc, vitamin D), twice daily for 4 weeks | Symptom severity, global well-being | Self-reported symptom checklist (12 items), global rating scale | 72–84% reported symptom improvement; 88% showed overall benefit | Sig; Low |
| Tomasa- Irriguible et al., 2024 [51] | Spain | Double-blind RCT | N = 246 Age 46.8 ± 16.3 y 68%, female | Outpatients with acute COVID-19 | Supplement intervention: MMS tablet (13 nutrients including vitamins A, B6, B12, C, D3, E, folic acid, zinc, selenium, iron, copper) vs. placebo for 14 days | Long COVID-19 incidence, cognition, QoL | ICD diagnosis tracking (6 months), MoCA-BLIND, EQ-5D-5L | No effect on long COVID-19 incidence; non-significant cognitive benefit; no QoL differences | Non-sig; High |
| Noce et al., 2024 [52] | Italy | Double-blind RCT | N = 33 Age 47.6 ± 16.y 57.6%, female | Long COVID-19 patients time since infection 73.7 ± 35.9 days | Supplement intervention: OFS (Echinacea angustifolia, rosehip, propolis, royal jelly, zinc, vitamin C, polyphenols) vs. placebo for 2 months | Inflammation, vitamin D, fatigue, QoL | CRP (lab), serum vitamin D, Fatigue Severity Scale, QoL scale | Reduced CRP (p = 0.0145), increased vitamin D (p = 0.0005), improved fatigue and QoL | Sig; Moderate |
| Marra et al., 2024 [53] | Italy | Retrospective | N = 44 Age 49.1 ± 18.1 y 56.8%, female | at least one persistent symptom consistent with post-COVID-19 syndrome | Supplement intervention: PIRV-F20® (lactoferrin, lysozyme, Lactobacillus, resveratrol, vitamins A, C, D3, E, K2, zinc, copper) vs. control for 6 weeks | 6MWT, handgrip, symptoms, cardiac function | 6MWT, handgrip dynamometry, symptom report, cardiac evaluation (not specified) | 6MWT improved more in intervention group (+40 m vs. +10 m, p = 0.01); no difference in strength or cardiac function | Sig; Low |
| Study, Year, Ref. | Country | Study Design | Study Sample | Long COVID-19 Assessment | Type of Nutritional Exposure | Main Study Outcome | Assessment Tools/Criteria | Main Results | Significance and Strength of Evidence |
|---|---|---|---|---|---|---|---|---|---|
| Tosato et al., 2022 [54] | Italy | Single-blind RCT | N = 46 Age 50.5 ± 14.0 y 65.2% female. | WHO criteria + persistent fatigue responding “most or all the time” to item seven of the CES-D (“I felt that everything I did was an effort”) | Supplement intervention: L-arginine (1.66 g) + liposomal vitamin C (500 mg), twice daily vs. placebo for 28 days | Physical function, endothelial function, fatigue | 6MWT, handgrip dynamometry, flow-mediated dilation, CES-D fatigue item | Intervention group showed greater gains in walking (+30 m), handgrip (+3.4 kg), and dilation (14.3% vs. 9.4%); fatigue reported by 8.7% vs. 80.1% in placebo (all p < 0.05) | Sig; Moderate |
| Cash et al., 2022 [55] | USA | Non-randomized CT | N = 43 Age 47 years; 73.7% female. | long COVID-19 experienced at least 6 months of fatigue with no prior fatigue | Supplement intervention: Anhydrous Enol-Oxaloacetate (500 or 1000 mg, twice daily) for 6 weeks | Fatigue | Chalder Fatigue Questionnaire, PROMIS Fatigue 7A, Fatigue Severity Scale | Fatigue scores decreased by up to 46.8% at 6 weeks | Sig; Low |
| Izzo et al., 2022 [56] | Italy | Observational (LINCOLN Survey) | N = 1390 Age 55.5 ± 15.7 y 49.5% female | Presence of COVID-19 sequelae that extend beyond four weeks after initial infection. | Supplement comparison: L-arginine (1.66 g) + liposomal vitamin C (500 mg) vs. multivitamins (B1, B2, B6, B12, folic acid, niacin, pantothenic acid) for 30 days | Long COVID-19 symptom burden, physical exertion | Symptom checklist; modified Borg scale (0–10) | L-arginine + vitamin C group had lower symptom scores (8.15 ± 1.3 vs. 13.9 ± 2.3; p < 0.001) and Borg effort scores (p < 0.0001) | Sig; Moderate |
| Turcu-Stiolica et al., 2023 [57] | Romania | Prospective | N = 505 Age 50(39–63) y 54.3% female | SARS-CoV-2 infection with mental and/or physical fatigue during or after illness. | Supplement intervention: Astenor Energy® or Astenor Forte® based on liver enzyme status, 10 days/month for 3 months | Fatigue | FAS | FAS scores improved in both groups (median 33→17 and 25→17; p < 0.0001); fatigue type varied by group | Sig; Moderate |
| Slankamenac et al., 2023 [58] | Serbia | Double-blind RCT | N = 12 Age 27.5 ± 6.8 y 50% female | COVID-19~3 months, fatigue 20-MFI score >43.5) +1 symptom | Supplement intervention: creatine monohydrate (4 g/day) vs. placebo for 6 months | Muscle creatine, fatigue, symptoms, endurance | 1.5T MRS, MFI-20, symptom VAS, treadmill time-to-exhaustion | Creatine increased brain/muscle creatine (p < 0.05), reduced fatigue (p = 0.04), and improved time to exhaustion (+65 s); large symptom effect sizes (d = 1.26–3.03) | Sig; Low |
| Slankamenac et al., 2024 [59] | Serbia | Double-blind RCT | N = 15 Age 39.7 ± 16.0 y 60%, females | long COVID-19 with fatigue and at least one other long COVID-19 symptom | Supplement comparison: creatine (8 g/day) ± glucose (3 g/day) vs. glucose alone, for 8 weeks | Brain/muscle creatine, fatigue, symptoms, endurance | MRS, MFIS-20, symptom VAS, treadmill test | Creatine (±glucose) increased brain creatine (p < 0.05), improved exhaustion time (+205 s; p = 0.03), and reduced fatigue (p = 0.008) | Sig; Low |
| Study, Year, Ref. | Country | Study Design | Study Sample | Long COVID-19 Assessment | Type of Nutritional Exposure | Main Study Outcome | Assessment Tools/Criteria | Main Results | Significance and Strength of Evidence |
|---|---|---|---|---|---|---|---|---|---|
| Thomas et al., 2022 [60] | UK | Double-blind RCT | N = 147 Age 53 y 44%, female | Long COVID-19 mean duration of symptoms: 108 days | Supplement intervention: phytochemical-rich capsule (curcumin, chamomile, citrus bioflavonoids, pomegranate, resveratrol) + probiotic (Lactobacillus + inulin) vs. placebo for 28 days | Fatigue, cough, well-being | CFS, Cough Symptom Score, Subjective Well-being Score | Intervention group had 2× greater fatigue reduction, 3× cough improvement, and >2× well-being gains vs. placebo (p = 0.02) | Sig; High |
| Docampo et al., 2024 [61] | Switzerland | Pre-post Intervention | N = 6 Age 31.7 ± 16 y 67% female | Long COVID-19 over 12 months with symptoms of fatigue, cognitive difficulties, and dizziness. | Supplement intervention: Paraprobiotic formula (Abiprol + Brexibiol) twice daily for 4 weeks | Fatigue, QoL, dysautonomia, depression, digital activity, immune markers | CFS, Bell Disability Scale, SF-36, COMPASS 31, PHQ-9, smartphone/wearable data, immune profiling | 30–80% symptom improvement across domains; reduced TLR2/CD40/HLA-DR immune activation; better sleep and fatigue in most participants | Sig; Low |
| Lau et al., 2024 [62] | China (Hong Kong) | Double-blind RCT | N = 463 Age 49·3 ±13 y 66%, female | CDC criteria+ at least one symptom from the PACSQ-14 persisting for ≥4 weeks after infection. | Supplement intervention: synbiotic (SIM01 with Bifidobacteria + prebiotics) vs. placebo | PACS symptoms (fatigue, cognitive, GI, general unwellness) | Symptom checklist; odds ratios for symptom alleviation | Significantly greater alleviation of fatigue (OR = 2.27), memory loss, concentration difficulty, GI symptoms, and general unwellness (all p < 0.01) | Sig; High |
| Calvani et al., 2024 [63] | Italy | Double-blind RCT | N = 31 Age 50.3 ± 12.9 y 45.5%, female | WHO criteria, with persistent fatigue defined as responding “most or all the time” to item 7 of the CES-D scale (“I felt that everything I did was an effort”). | Supplement intervention: beetroot juice (600 mg nitrate/day) vs. placebo for 14 days | Fatigue, physical function, vascular response, microbiota, inflammation | Fatigue resistance test, 6MWT, handgrip, FMD, gut microbiota, cytokine panels | Both groups improved from baseline in fatigue and 6MWT; beetroot group had gut microbiota shifts and increased IFN-γ, MIP-1β (no group differences in primary outcomes) | Non-sig; Moderate |
| Cuevas- Sierra et al., 2024 [64] | Spain | Cross- Sectional | N = 188 Age 49 ± 0.9 y 87%, female | Diagnosed with long COVID-19 by the internist doctor | Dietary pattern: high vs. low Mediterranean diet adherence (cutoff ≥7 points) | Inflammation, oxidative stress, gut microbiota | Blood biomarkers (LDL, glucose, LDH), redox indices, microbiota analysis | High MD adherence linked to better metabolic profile, redox balance, and lower Oscillibacter abundance; stronger effects with high olive oil intake | Sig; Moderate |
| Ranisavljev et al., 2025 [65] | Serbia | Double-blind RCT | N = 26 Age 42.5 ± 13.4 y 50%, female | COVID-19~3 months, fatigue 20-MFI score >43.5) +1 symptom | Supplement intervention: daily synbiotic (L. rhamnosus, L. plantarum, B. lactis, B. longum, FOS, zinc) for 3 months | Fatigue, post-exertional malaise, brain/muscle metabolism, endurance | 20-MFI, VAS, treadmill test, brain MRS (tCho, tCr, NAA) | Improved post-exertional malaise, increased brain choline/creatine, reduced fatigue, and extended time to exhaustion | Sig; Moderate |
| Study, Year, Ref. | Country | Study Design | Study Sample | Long COVID-19 Assessment | Type of Nutritional Exposure | Main Study Outcome | Assessment Tools/Criteria | Main Results | Significance and Strength of Evidence |
|---|---|---|---|---|---|---|---|---|---|
| Pre-Infection Lifestyle Factors and Risk of long COVID-19 | |||||||||
| Wang et al., 2023 [66] | USA | Prospective cohort (Nurses’ Health Study II) | N = 32,249 Age 64.7 ± 4.6 y 100%, female | 1981 tested positive for SARS-CoV-2 | Healthy lifestyle factors pre-COVID-19 (0–6 score) | Post-COVID-19 condition (PCC) risk | Self-reported PCC (≥4 weeks) | 5–6 healthy factors linked to 49% lower PCC risk (RR = 0.51); BMI and sleep showed independent associations | Sig; High |
| Wang et al., 2024 [67] | UK | Prospective cohort study (UK Biobank) | N = 68,896 Age 68.1 ± 8.1 y 44.7%, female | Confirmed SARS-CoV-2 infection from the UK Biobank cohort | Lifestyle factors pre-infection (diet, BMI, PA, etc.) | Post-COVID-19 sequelae, death, hospitalization | Health records and lifestyle surveys | Favorable lifestyle (6–10 factors) linked to lower risk of sequelae (HR = 0.64), mortality (HR = 0.59), and hospitalization (HR = 0.78) | Sig; High |
| Prolong Nutritional status after COVID-19 | |||||||||
| Gérard et al., 2021 [68] | France | Prospective cohort | N = 288 Age 59.8 ± 16.6 y 45.8%, female | Post-hospitalized COVID-19 patients at 6 months with sequelae | Nutrition support (ONS, diet, activity) in post-hospital patients | Malnutrition, performance, fatigue | GLIM, SES, VAS | 36% still malnourished at 6 months; ICU stay and obesity predicted worse outcomes | Sig; Moderate |
| Álvarez-Hernández et al., 2023 [69] | Spain | Prospective cohort | N = 199 Age 60.7 ± 10.1 y 29.6%, female | COVID-19 ICU survivors evaluated 3, 6, and 12 months after discharge. | Nutrition support (ONS, energy/protein) post-ICU | Malnutrition risk, physical function, sarcopenia | MUST, EQ-5D-3L, SARC-F | 35% at risk after 1 year; 25% had sarcopenia risk; low intake common; support linked to better outcomes | Sig; Moderate |
| Lakenman et al., 2023 [70] | Netherlands | Prospective | N = 48 Age 60 [52; 65] y 73% male | COVID-19 ICU survivors evaluated 1-year post-discharge. | Protein/energy intake at 1-year post-ICU | Nutrition status, strength, body comp. | MUST, FFMI, FMI, GLIM, handgrip | Weight regained but 50% had high fat mass; protein intake suboptimal despite no malnutrition | Non-sig (mixed); Low–Moderate |
| Muzaffar et al., 2024 [71] | Malaysia | Case– Control | N = 108 Age 21.06 ± 1.37 y 74.1%, female | n = 54 COVID-19-recovered vs. n = 54 healthy controls | Diet quality in COVID-19-recovered vs. controls | Diet diversity, sleep, weight | DQQ Malaysia, sleep self-report | COVID-19 group had lower diet diversity; no significant sleep–weight associations | Non-sig; Low |
| Mejía Alonso et al., 2024 [72] | Mexico | Correlational follow-up | N = 66 Age 51.3 y 25% female | Hospitalized for COVID-19 and referred to rehabilitation >139 days post-discharge. | Nutrition protocol and counseling in rehab patients | Nutritional recovery, muscle mass/function | Ultrasound (muscle), HRQoL, mobility tests | Improved muscle strength, mass, respiratory function; excess weight reduced 6MWT performance | Sig; Low–Moderate |
| Multi-professional intervention and diet modification in Post-COVID-19 Recovery | |||||||||
| Sousa-Catita et al., 2022 [73] | Portugal | Prospective intervention study | N = 118 Age 71.9 (41–90) y 52%, female | Post-COVID-19 patients admitted to rehabilitation care units > 30 days. | Interdisciplinary rehab (ESPEN-based nutrition, exercise, therapy) | Nutrition and functional status | BMI, MUAC, MNA®, HGS | BMI, HGS, and MNA® improved; largest gains in ICU group and final 15 days of rehab | Sig; Moderate |
| Nikolic Turnic et al., 2022 [74] | Serbia | Cross- Sectional | N = 80 Age 30.6± 1.5 y 75%, female | Working-age adults (mostly under 30, with confirmed COVID-19 prior 6 months | Self-reported diet and lifestyle 6 months post-COVID-19 | QoL, dietary change, activity | pCOVq, WHOQOL-BREF | Water intake ↑; no major nutrient changes; reduced physical/social activity; slight QoL decline | Non-sig (lifestyle only); Low |
| Sordi et al., 2023 [75] | Brazil | Non- randomized CT | N = 35 Age 42 ± 12 y 90% male | Overweight adults post-COVID-19 (BMI ≥25), self-reported post-COVID-19 symptoms, | 8-week group program: nutrition + exercise + education | Body comp, fitness, biomarkers | CRP, glucose, HDL-c, strength tests | Strength, flexibility, inflammation, and metabolic markers improved, esp. in moderate/severe cases | Sig; Low–Moderate |
| Ryal et al., 2023 [76] | Brazil | Controlled trial with parallel groups and repeated measures | N = 55 Age 49 ± 13 y 65.4% male | Middle-aged overweight or obese COVID-19 survivors with self-reported post-COVID-19 symptoms | 8-week intervention: nutrition + mental health + exercise | Mental health (anxiety, depression, PTSD, well-being) | GAD-7, PHQ-9, IES-R, MHC-SF | Mental health improved most in mild/control groups; less consistent gains in moderate/severe groups | Sig; Moderate |
| Pink et al., 2024 [77] | Germany | RCT (Secondary analysis) | N = 92 Age 47 (40, 53) y 75% female | Post-COVID-19 patients with fatigue FAS ≥22 matched to healthy controls ratio 1:1 | Diet behavior (7-day diary), omega-3/6, sleep | Fatigue, mental health, nutrient intake | FAS, SF-36, sleep/activity tracking | PASC group had healthier diet trends, longer sleep (+49 min); no intake/activity differences | Non-sig; Moderate |
| Combet et al., 2025 [78] | UK | RCT Open-label | N = 234 Age 46.4 ± 9.1 y 84.5%, female | long COVID-19 with symptoms >12 weeks) and BMI >27 kg/m2 | 12-week remote weight-loss program (diet replacement + reintro) | Long COVID-19 symptom change | VAS, symptom questionnaires | Intervention led to greater symptom improvement (mean diff = –0.34; 95% CI: –0.67 to –0.01) | Sig; High |
| Suárez-Moreno et al., 2025 [19] | Spain | Cross- Sectional | N = 305 Age 52.8 ± 11.9 y 68%, female | WHO’s definition of long COVID-19. History of different SARS-CoV-2 infection symptoms >3 months after onset. | Mediterranean diet adherence (assessed via MEDAS) | Metabolic syndrome components | BMI, HDL-c, waist, uric acid link to long COVID-19 | Higher MD adherence linked to lower BMI, waist, uric acid, and MetS risk; higher HDL | Sig; Moderate |
| Type of Nutritional Exposure | No. of Studies | % Reporting Significant Findings | General Strength of Evidence | Details in Table |
|---|---|---|---|---|
| Vitamin D (e.g., Biomarker, deficiency, supplement) | 10 | 40% | Mostly moderate | Table 2 |
| Vitamin D with Zinc/Magnesium/ Vitamin K2 | 3 | 100% | Low-to-high | |
| Vitamins A/ B1 | 2 | 100% | Moderate | |
| Multinutrient/Nutraceutical (e.g., Apportal®, MMS, OFS, fermented papaya extract, and blends with vitamins, amino acids, minerals, and polyphenols) | 9 | 89% | Low-to-Moderate | Table 3 |
| Amino Acids/Metabolic Support (e.g., L-arginine + vitamin C, oxaloacetate, creatine, and Astenor formulations with amino acids and cofactors) | 6 | 100% | Low-to-Moderate | Table 4 |
| Gut Microbiota-Targeted Therapies (e.g., SIM01 synbiotic, paraprobiotics, beetroot juice, phytochemical + probiotic blends, and Mediterranean diet adherence) | 6 | 83% | Moderate-to-high | Table 5 |
| Pre-Infection Lifestyle Factors (e.g., healthy diet, BMI and other modifiable risk factors) | 2 | 100% | High | Table 6 |
| Nutritional Status / Deficiency Studies (e.g., oral nutrition support, protein/energy intake, diet quality, and post-ICU malnutrition risk) | 5 | 60% | Low-to-Moderate | |
| Multicomponent Lifestyle Interventions (e.g., diet combined with physical activity, mental health support, weight loss, or rehabilitation programs) | 7 | 71% | Mostly moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bigman, G.; Rusu, M.E.; Shelawala, N.; Sorkin, J.D.; Beamer, B.A.; Ryan, A.S. A Comprehensive Scoping Review on Diet and Nutrition in Relation to Long COVID-19 Symptoms and Recovery. Nutrients 2025, 17, 1802. https://doi.org/10.3390/nu17111802
Bigman G, Rusu ME, Shelawala N, Sorkin JD, Beamer BA, Ryan AS. A Comprehensive Scoping Review on Diet and Nutrition in Relation to Long COVID-19 Symptoms and Recovery. Nutrients. 2025; 17(11):1802. https://doi.org/10.3390/nu17111802
Chicago/Turabian StyleBigman, Galya, Marius Emil Rusu, Nicole Shelawala, John D. Sorkin, Brock A. Beamer, and Alice S. Ryan. 2025. "A Comprehensive Scoping Review on Diet and Nutrition in Relation to Long COVID-19 Symptoms and Recovery" Nutrients 17, no. 11: 1802. https://doi.org/10.3390/nu17111802
APA StyleBigman, G., Rusu, M. E., Shelawala, N., Sorkin, J. D., Beamer, B. A., & Ryan, A. S. (2025). A Comprehensive Scoping Review on Diet and Nutrition in Relation to Long COVID-19 Symptoms and Recovery. Nutrients, 17(11), 1802. https://doi.org/10.3390/nu17111802








