Cultural and Molecular Factors Predisposed to Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. History of NAFLD
3. Methods
4. Signs and Symptoms of NAFLD
5. Prevalence of NAFLD
5.1. Global Perspectives
5.2. Prevalence of NAFLD Amongst Different Ethnicities and Cultures in the USA
6. Cultural Factors That Lead to the Development of NAFLD and Type 2 Diabetes in Different Ethnicities
6.1. Diet
6.1.1. Diet and Overnutrition
6.1.2. Dietary Patterns in Asians
6.1.3. Dietary Patterns in the Hispanic Population
6.1.4. Dietary Patterns in Caucasian Americans
6.1.5. Dietary Patterns in African Americans
6.2. Assimilation of Culture
6.3. The Perception of an Individual Body
6.4. Emotional Distress
6.5. The Role of Adverse Childhood Experiences and Obesity
6.6. Literacy Regarding Health Issues
6.7. Judgement and Beliefs Concerning Diseases
6.8. The Role of Leisure Time and Physical Activities
6.9. Religion
6.10. Financial Status
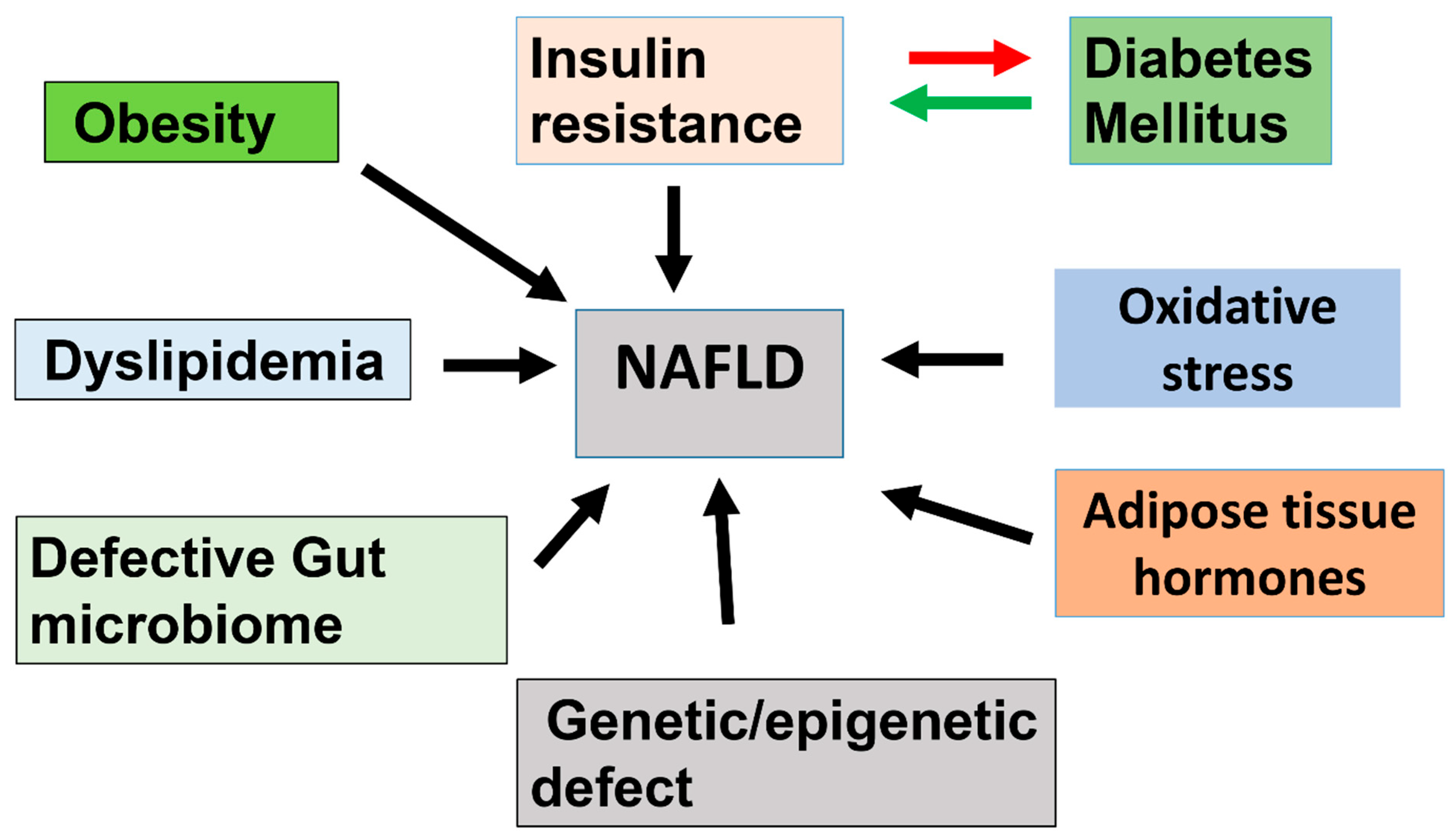
7. Molecular Mechanisms Leading to NAFLD
7.1. Pathogenesis of NAFLD
7.2. Autophagy and NAFLD
7.3. Increased Stress in Endoplasmic Reticulum
7.4. The Role of Oxidative Stress
7.5. Role of Lipo-Oxygenase Enzymes
7.6. Association of Genetics and NAFLD
8. NAFLD’S Association with Type 2 Diabetes Mellitus and Obesity
8.1. Genetic Association of NAFLD and T2DM
8.2. Inflammation Pathway in the Development of Insulin Resistance
9. Future Options and Treatment of NAFLD
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khoonsari, M.; Azar, M.M.H.; Ghavam, R.; Hatami, K.; Asobar, M.; Gholami, A.; Rajabi, A.; Tameshkel, F.S.; Amirkalali, B.; Sohrabi, M. Clinical Manifestations and Diagnosis of Nonalcoholic Fatty Liver Disease. Iran. J. Pathol. 2017, 12, 99–105. [Google Scholar] [CrossRef]
- Adeghate, E.A. GLP-1 receptor agonists in the treatment of diabetic non-alcoholic steatohepatitis patients. Expert Opin. Pharmacother. 2024, 25, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Leoni, S.; Alswat, K.A.; Fouad, Y. History of Non-alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2020, 21, 5888. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, J.; Viggiano, T.R.; McGILL, D.B.; Ott, B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980, 55, 434–438. [Google Scholar] [CrossRef]
- Ayonrinde, O.T. Historical narrative from fatty liver in the nineteenth century to contemporary NAFLD—Reconciling the present with the past. JHEP Rep. 2021, 3, 100261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schaffner, F.; Thaler, H. Nonalcoholic fatty liver disease. Prog. Liver Dis. 1986, 8, 283–298. [Google Scholar]
- Shinde, S.; Taylor, N.; Chinthammit, C.; Wilson, R.; Burgess, S.M.; Poon, J.L. Understanding the impact of non-alcoholic steatohepatitis with metabolic comorbidities on adults: A real-world qualitative study. Curr. Med. Res. Opin. 2024, 40, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Murag, S.; Ahmed, A.; Kim, D. Recent Epidemiology of Non-alcoholic Fatty Liver Disease. Gut Liver 2021, 15, 206–216. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.; Owrangi, S.; Yilmaz, Y.; El-Kassas, M.; Alswat, K.; Alqahtani, S.A. Prevalence of metabolic dysfunction-associated steatotic liver disease in the Middle East and North Africa. Liver Int. 2024, 44, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, R.W.; Kim, H.J.; Therneau, T.M. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013, 57, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Bonacini, M.; Kassamali, F.; Kari, S.; Barrera, N.L.; Kohla, M. Racial differences in prevalence and severity of non-alcoholic fatty liver disease. World J. Hepatol. 2021, 13, 763–773. [Google Scholar] [CrossRef]
- Rojas, Y.A.O.; Cuellar, C.L.V.; Barrón, K.M.A.; Arab, J.P.; Miranda, A.L. Nonalcoholic fatty liver disease prevalence in Latin America: A systematic review and meta-analysis. Ann. Hepatol. 2022, 27, 100706. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zou, B.; Yeo, Y.H.; Feng, Y.; Xie, X.; Lee, D.H.; Fujii, H.; Wu, Y.; Kam, L.Y.; Ji, F.; et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2019, 4, 389–398. [Google Scholar] [CrossRef]
- Spearman, C.W.; Afihene, M.; Betiku, O.; Bobat, B.; Cunha, L.; Kassianides, C.; Katsidzira, L.; Mekonnen, H.D.; Ocama, P.; Ojo, O.; et al. Gastroenterology and Hepatology Association of sub-Saharan Africa (GHASSA). Epidemiology, risk factors, social determinants of health, and current management for non-alcoholic fatty liver disease in sub-Saharan Africa. Lancet Gastroenterol. Hepatol. 2021, 6, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Abeysekera, K.W.M.; Fernandes, G.S.; Hammerton, G.; Portal, A.J.; Gordon, F.H.; Heron, J.; Hickman, M. Prevalence of steatosis and fibrosis in young adults in the UK: A population-based study. Lancet Gastroenterol. Hepatol. 2020, 5, 295–305. [Google Scholar] [CrossRef]
- Ye, D.; Wang, J.; Shi, J.; Ma, Y.; Li, Y.; Li, Q.; Hu, X.; Chen, J.; Bao, Z. Prevalence of MAFLD in the U.S. based on NHANES 2009-2018: Differences in demographic characteristics, physical indices and lifestyle conditions. BMC Gastroenterol. 2025, 25, 329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bates, D.G.; Plog, F. Cultural Anthropology; McGraw-Hill: New York, NY, USA, 1990. [Google Scholar]
- Mulholland, J. The Language of Negotiation; Routledge: London, UK, 1991. [Google Scholar]
- Tsitsou, S.; Bali, T.; Adamantou, M.; Saridaki, A.; Poulia, K.; Karagiannakis, D.S.; Papakonstantinou, E.; Cholongitas, E. Effects of a 12-Week Mediterranean-Type Time-Restricted Feeding Protocol in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: A Randomised Controlled Trial-The ‘CHRONO-NAFLD Project’. Aliment. Pharmacol. Ther. 2025, 61, 1290–1309. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nikparast, A.; Sohouli, M.H.; Forouzan, K.; Farani, M.A.; Dehghan, P.; Rohani, P.; Asghari, G. The association between total, animal, and plant protein intake and metabolic dysfunction-associated fatty liver disease in overweight and obese children and adolescents. Nutr. J. 2025, 24, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sharif, R.; Shahar, S.; Rajab, N.F.; Fenech, M. Dietary Pattern, Genomic Stability and Relative Cancer Risk in Asian Food Landscape. Nutr. Cancer 2021, 74, 1171–1187. [Google Scholar] [CrossRef]
- Cuy-Castellanos, D. Dietary Acculturation in Latinos/Hispanics in the United States. Am. J. Lifestyle Med. 2014, 9, 31–36. [Google Scholar] [CrossRef]
- Grotto, D.; Zied, E. The Standard American Diet and Its Relationship to the Health Status of Americans. Nutr. Clin. Pract. 2010, 25, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, M.O.; Ali, I.I.; Adeghate, J.O.; Tekes, K.; Kalász, H.; Adeghate, E.A. An Update on the Molecular and Cellular Basis of Pharmacotherapy in Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 9328. [Google Scholar] [CrossRef] [PubMed]
- Baptiste-Roberts, K.; Gary, T.L.; Beckles, G.L.; Gregg, E.W.; Owens, M.; Porterfield, D.; Engelgau, M.M. Family history of diabetes, awareness of risk factors, and health behaviors among African Americans. American J. Public Health 2007, 97, 907–912. [Google Scholar] [CrossRef]
- Horowitz, C.R.; Colson, K.A.; Herbert, P.L.; Lancaster, K. Barriers to buying healthy foods for people with diabetes: Evidence of environmental disparities. Am. J. Public Health 2004, 94, 1549–1554. [Google Scholar] [CrossRef]
- Caballero, A.E. Diabetes in Culturally Diverse Populations: From Biology to Culture. In Principles of Diabetes Mellitus; Springer: Berlin/Heidelberg, Germany, 2017; pp. 159–177. [Google Scholar] [CrossRef]
- Fallon, A. Culture in the mirror: Sociocultural determinants of body image. In Body Images: Development, Deviance, and Change; Guilford Press: New York, NY, USA, 1990; pp. 80–109. [Google Scholar]
- Wiseman, C.V.; Gray, J.J.; Mosimann, J.E.; Ahrens, A.H. Cultural expectations of thinness in women: An update. Int. J. Eat. Disord. 1992, 11, 85–89. [Google Scholar] [CrossRef]
- Halliwell, E.; Dittmar, H. Does size matter? The impact of model’s body size on women’s body-focused anxiety and advertising effectiveness. J. Soc. Clin. Psychol. 2004, 23, 104–122. [Google Scholar] [CrossRef]
- Schrider, E. Available online: https://www2.census.gov/library/publications/2024/demo/p60-283.pdf (accessed on 14 May 2025).
- Bellehumeur-Béchamp, L.; Legendre, M.; Bégin, C. From Childhood Interpersonal Trauma to Binge Eating in Adults: Unraveling the Role of Personality and Maladaptive Regulation. Nutrients 2024, 16, 4427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rienecke, R.D.; Johnson, C.; Le Grange, D.; Manwaring, J.; Mehler, P.S.; Duffy, A.; McClanahan, S.; Blalock, D.V. Adverse childhood experiences among adults with eating disorders: Comparison to a nationally representative sample and identification of trauma. J. Eat. Disord. 2022, 10, 72, Erratum in J. Eat. Disord. 2022, 10, 115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paasche-Orlow, M.K.; Parker, R.M.; Gazmararian, J.A.; Nielsen-Bohlman, L.T.; Rudd, R.R. The prevalence of limited health literacy. J. Gen. Intern. Med. 2005, 20, 175–184. [Google Scholar] [CrossRef]
- Berkman, N.D.; Sheridan, S.L.; Donahue, K.E.; Halpern, D.J.; Crotty, K. Low Health Literacy and Health Outcomes: An Updated Systematic Review. Ann. Intern. Med. 2011, 155, 97. [Google Scholar] [CrossRef]
- Devlin, H.; Roberts, M.; Okaya, A.; Xiong, Y.M. Our Lives Were Healthier Before: Focus Groups with African American, American Indian, Hispanic/Latino, and Hmong People with Diabetes. Health Promot. Pract. 2006, 7, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Al Jaberi, S.; Cohen, A.; Saeed, Z.; Ojha, S.; Singh, J.; Adeghate, E. Obesity: Molecular Mechanisms, Epidemiology, Complications and Pharmacotherapy. In Cellular and Biochemical Mechanisms of Obesity; Tappia, P.S., Ramjiawan, B., Dhalla, N.S., Eds.; Advances in Biochemistry in Health and Disease; Springer: Cham, Switzerland, 2021; Volume 23. [Google Scholar] [CrossRef]
- Stefan, N.; Häring, H.U.; Cusi, K. Non-alcoholic fatty liver disease: Causes, diagnosis, cardio metabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Kruger, J.; Ham, S.A.; Kohl, H.I. Trends in leisure-time physical inactivity by age, sex, and race/ethnicity-United States, 1994–2004. Morb. Mortal. Wkly. Rep. 2005, 54, 331–994. [Google Scholar]
- Wood, F.G. Leisure time activity of Mexican Americans with diabetes. J. Adv. Nurs. 2004, 45, 190–196. [Google Scholar] [CrossRef]
- Sorkin, D.H.; Biegler, K.A.; Billimek, J. Differences in Self-Reported Physical Activity and Body Mass Index among Older Hispanic and Non-Hispanic White Men and Women: Findings from the 2009 California Health Interview Survey. J. Am. Geriatr. Soc. 2015, 63, 2158–2163. [Google Scholar] [CrossRef]
- Clark, D.O. Physical activity efficacy and effectiveness among older adults and minorities. Diabetes Care 1997, 20, 1176–1182. [Google Scholar] [CrossRef]
- Han, B.H.; Sadarangani, T.; Wyatt, L.C.; Zanowiak, J.M.; Kwon, S.C.; Trinh-Shevrin, C.; Lee, L.; Islam, N.S. Correlates of Physical Activity among Middle-Aged and Older Korean Americans at Risk for Diabetes. J. Nurs. Scholarsh. Off. Publ. Sigma Theta Tau Int. Honor. Soc. Nurs. 2016, 48, 48–57. [Google Scholar] [CrossRef]
- Al-Arouj, M.; Bouguerra, R.; Buse, J.; Hafez, S.; Hassanein, M.; Ibrahim, M.A.; Ismail-Beigi, F.; El-Kebbi, I.; Khatib, O.; Kishawi, S.; et al. Recommendations for Management of Diabetes during Ramadan. Diabetes Care 2005, 28, 2305–2311. [Google Scholar] [CrossRef]
- Watchel, M.S. Family poverty accounts for differences in lower-extremity amputation rates of minorities 50 years old or more with diabetes. J. Natl. Med. Assoc. 2005, 97, 334–338. [Google Scholar]
- Carr, R.M.; Oranu, A.; Khungar, V. Nonalcoholic Fatty Liver Disease. Gastroenterol. Clin. North Am. 2016, 45, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Caldwell, S.H. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology 2003, 37, 1202–1219, Erratum in Hepatology 2003, 38, 536. [Google Scholar] [CrossRef] [PubMed]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Matteoni, C.; Younossi, Z.; Gramlich, T.; Boparai, N.; Liu, Y.; Mccullough, A. Nonalcoholic fatty liver disease: A spectrum of clinical and pathological severity. Gastroenterology 1999, 116, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Khare, T.; Liu, K.; Chilambe, L.O.; Khare, S. NAFLD and NAFLD Related HCC: Emerging Treatments and Clinical Trials. Int. J. Mol. Sci. 2025, 26, 306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ludwig, J.; McGILL, D.B.; Lindor, K.D. Review: Nonalcoholic Steatohepatitis. J. Gastroenterol. Hepatol. 1997, 12, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Valencia, O.; López, C.; Vanegas-Duarte, E.; Fillizola, C.; Ramírez, D.F.B.; Mejía, N.A.C.; Torres, A.V. Risk Factors Related to the Development of Nonalcoholic Fatty Liver: A Systematic Review. Can. J. Gastroenterol. Hepatol. 2025, 2025, 9964486. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres, D.M.; Shaw, J.; Contreras, M.; Landt, C.L.; Harrison, S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011, 140, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Argo, C.K.; Northup, P.G.; Al-Osaimi, A.M.; Caldwell, S.H. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J. Hepatol. 2009, 51, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Henry, L.; Bush, H.; Mishra, A. Clinical and Economic Burden of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Clin. Liver Dis. 2018, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.M.; Golabi, P.; Younossi, Y.; Mishra, A.; Younossi, Z.M. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of NAFLD. Hepatology 2020, 72, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, M.L.; Abenavoli, L. Metabolic Dysfunction-Associated Steatotic Liver Disease vs. Metabolic Dysfunction-Associated Fatty Liver Disease: Which Option is the Better Choice? Br. J. Hosp. Med. 2025, 86, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J. NASH: A global health problem. Hepatol. Res. 2011, 41, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, L.; Trainer, T.; Janiec, D. Prevalence of non-alcoholic steatohepatitis in morbidly obese subjects undergoing gastric bypass. Obes. Surg. 2003, 13, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Luo, Y.; Liu, Y.; Liu, J.; Chen, Y.; Zeng, B.; Liao, X.; Liu, Y.; Wang, X. DNA hypermethylation-induced suppression of ALKBH5 is required for folic acid to alleviate hepatic lipid deposition by enhancing autophagy in an ATG12-dependent manner. J. Nutr. Biochem. 2025, 140, 109870. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; He, S.; Chen, J.; Deng, C.; Duan, J. Ellagic Acid Modulates Necroptosis, Autophagy, Inflammations, and Stress to Ameliorate Non-alcoholic Liver Fatty Disease in a Rat Model. Food Sci. Nutr. 2025, 13, e4694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samala, N.; Tersey, S.A.; Chalasani, N.; Anderson, R.M.; Mirmira, R.G. Molecular mechanisms of nonalcoholic fatty liver disease: Potential role for 12-lipoxygenase. J. Diabetes Complicat. 2017, 31, 1630–1637. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Komatsu, M.; Waguri, S.; Ueno, T.; Iwata, J.; Murata, S.; Tanida, I.; Ezaki, J.; Mizushima, N.; Ohsumi, Y.; Uchiyama, Y.; et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 2005, 169, 425–434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kiffin, R.; Christian, C.; Knecht, E.; Cuervo, A.M. Activation of chaperone-mediated autophagy during oxidative stress. Mol. Biol. Cell 2004, 15, 4829–4840. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, S.; Allen, A.M.; Wang, Z.; Prokop, L.J.; Murad, M.H.; Loomba, R. Fibrosis Progression in Nonalcoholic Fatty Liver vs Nonalcoholic Steatohepatitis: A Systematic Review and Meta-analysis of Paired-Biopsy Studies. Clin. Gastroenterol. Hepatol. 2015, 13, 643–654.e9. [Google Scholar] [CrossRef]
- Singh, A.; Dhaliwal, A.S.; Singh, S.; Kumar, A.; Lopez, R.; Gupta, M.; Noureddin, M.; Carey, W.; McCullough, A.; Alkhouri, N. Awareness of Nonalcoholic Fatty Liver Disease Is Increasing but Remains Very Low in a Representative US Cohort. Dig. Dis. Sci. 2020, 65, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Sriramdasu, S.; Sharma, S.; Ansari, A.R.; Phatak, N.V.; Tikoo, K. Borneol Ameliorates Non-Alcoholic Fatty Liver Disease via Promoting AMPK-Mediated Lipophagy. J. Biochem. Mol. Toxicol. 2025, 39, e70182. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Ansari, A.; Gupta, J.; Singh, H.; Jagavelu, K.; Sashidhara, K.V. Androsin alleviates non-alcoholic fatty liver disease by activating autophagy and attenuating de novo lipogenesis. Phytomedicine 2024, 129, 155702. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V. Autophagy of intracellular microbes and mitochondria: Two sides of the same coin? F1000 Biol. Rep. 2010, 2, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, Z.; Wang, Y.; Long, Z.; He, G. Interaction between autophagy and the NLRP3 inflammasome. Acta Biochim. Biophys. Sin. 2019, 51, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Sluimer, J.C.; Wang, Y.; Subramanian, M.; Brown, K.; Pattison, J.S.; Robbins, J.; Martinez, J.; Tabas, I. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012, 15, 545–553. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borradaile, N.M.; Han, X.; Harp, J.D.; Gale, S.E.; Ory, D.S.; Schaffer, J.E. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res. 2006, 47, 2726–2737. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; He, W.; Meng, H.; Li, P.; Qu, J. Endoplasmic reticulum stress in acute lung injury and pulmonary fibrosis. FASEB J. 2024, 38, e70232. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, L.M. A BiP-centric view of endoplasmic reticulum functions and of my career. J. Mol. Biol. 2025, 437, 169052. [Google Scholar] [CrossRef] [PubMed]
- Lebeaupin, C.; Proics, E.; de Bieville, C.H.; Rousseau, D.; Bonnafous, S.; Patouraux, S.; Adam, G.; Lavallard, V.J.; Rovere, C.; Le Thuc, O.; et al. ER stress induces NLRP3 inflammasome activation and hepatocyte death. Cell Death Dis. 2015, 6, e1879. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mooli, R.G.R.; Mukhi, D.; Ramakrishnan, S.K. Oxidative Stress and Redox Signaling in the Pathophysiology of Liver Diseases. Compr. Physiol. 2022, 12, 3167–3192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iozzo, P.; Bucci, M.; Roivainen, A.; Någren, K.; Järvisalo, M.J.; Kiss, J.; Guiducci, L.; Fielding, B.; Naum, A.G.; Borra, R.; et al. Fatty acid metabolism in the liver, measured by positron emission tomography, is increased in obese individuals. Gastroenterology 2010, 139, 846–856.e6. [Google Scholar] [CrossRef] [PubMed]
- Hall, Z.; Bond, N.J.; Ashmore, T.; Sanders, F.; Ament, Z.; Wang, X.; Murray, A.J.; Bellafante, E.; Virtue, S.; Vidal-Puig, A.; et al. Lipid zonation and phospholipid remodeling in nonalcoholic fatty liver disease. Hepatology 2017, 65, 1165–1180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Powell, W.S.; Rokach, J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim. Biophys. Acta 2015, 1851, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Tersey, S.A.; Bolanis, E.; Holman, T.R.; Maloney, D.J.; Nadler, J.L.; Mirmira, R.G. Minireview: 12-Lipoxygenase and Islet β-Cell Dysfunction in Diabetes. Mol. Endocrinol. 2015, 29, 791–800. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, S.; McPhaul, C.; Li, J.Z.; Garuti, R.; Kinch, L.; Grishin, N.V.; Cohen, J.C.; Hobbs, H.H. A Sequence Variation (I148M) in PNPLA3 Associated with Nonalcoholic Fatty Liver Disease Disrupts Triglyceride Hydrolysis. J. Biol. Chem. 2010, 285, 6706–6715. [Google Scholar] [CrossRef]
- Donati, B.; Motta, B.M.; Pingitore, P.; Meroni, M.; Pietrelli, A.; Alisi, A.; Petta, S.; Xing, C.; Dongiovanni, P.; del Menico, B.; et al. The rs2294918 E434K variant modulates patatin-like phospholipase domain-containing 3 expression and liver damage. Hepatology 2016, 63, 787–798. [Google Scholar] [CrossRef]
- Kozlitina, J.; Smagris, E.; Stender, S.; Nordestgaard, B.G.; Zhou, H.H.; Tybjærg-Hansen, A.; Vogt, T.F.; Hobbs, H.H.; Cohen, J.C. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2014, 46, 352–356. [Google Scholar] [CrossRef]
- Zhou, Y.; Llauradó, G.; Orešič, M.; Hyötyläinen, T.; Orho-Melander, M.; Yki-Järvinen, H. Circulating triacylglycerol signatures and insulin sensitivity in NAFLD associated with the E167K variant in TM6SF2. J. Hepatol. 2015, 62, 657–663. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Roden, M. NAFLD and diabetes mellitus. Nat. Rev. Gastroenterol. Hepatol. 2016, 14, 32–42. [Google Scholar] [CrossRef]
- Adeghate, E.A.; Kalász, H.; Al Jaberi, S.; Adeghate, J.; Tekes, K. Tackling type 2 diabetes-associated cardiovascular and renal comorbidities: A key challenge for drug development. Expert Opin. Investig. Drugs 2021, 30, 85–93. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Konstantopoulos, N.; Lee, J.; Hansen, L.; Li, Z.-W.; Karin, M.; Shoelson, S.E. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science 2001, 293, 1673–1677. [Google Scholar] [CrossRef]
- White, M.F. Insulin signalling in health and disease. Science 2003, 302, 1710–1711. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. Nuclear factor-κB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef]
- Arkan, M.C.; Hevener, A.L.; Greten, F.R.; Maeda, S.; Li, Z.-W.; Long, J.M.; Wynshaw-Boris, A.; Poli, G.; Olefsky, J.; Karin, M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 2005, 11, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Carreras, M.; Del Hoyo, P.; Martín, M.A.; Rubio, J.C.; Martín, A.; Castellano, G.; Colina, F.; Arenas, J.; Solis-Herruzo, J.A. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 2003, 38, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, N.; Deeg, M.A.; Crabb, D.W. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2004, 99, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Sjøgaard-Frich, L.M.; Henriksen, M.S.; Lam, S.M.; Birkbak, F.J.; Czaplinska, D.; Flinck, M.; Pedersen, S.F. NHE1 regulation in NAFLD in vitro contributes to hepatocyte injury and HSC crosstalk. J. Endocrinol. 2024, 263, e240099. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Niki, E.; Naito, Y.; Yoshikawa, T. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free. Radic. Res. 2013, 7, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, M. Current Options and Future Directions for NAFLD and NASH Treatment. Int. J. Mol. Sci. 2021, 22, 7571. [Google Scholar] [CrossRef] [PubMed]
- Howarth, F.; Jacobson, M.; Shafiullah, M.; Adeghate, E. Effects of insulin treatment on heart rhythm, body temperature and physical activity in streptozotocin-induced diabetic rat. Clin. Exp. Pharmacol. Physiol. 2006, 33, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Albitar, O.; D’souza, C.M.; Adeghate, E.A. Effects of Lipoproteins on Metabolic Health. Nutrients 2024, 16, 2156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, R.B.; Saboo, B.; Mahashwari, A.; Bharatdwaj, K.; Verma, N.; Hristova, K.; Ghosh, S.; Niaz, M.A.; Singh, J.; Earrnst, A.; et al. Effects of Indo-Mediterranean style diet and low fat diet on incidence of diabetes in acute coronary syndromes. World Heart J. 2017, 9, 25–36. [Google Scholar]



| # | Region/Country | Prevalence (%) | Reference |
|---|---|---|---|
| 1 | Worldwide | 25.2 | [2,8] |
| 2 | Middle East and North Africa | 40.0 | [9,10] |
| 2 | USA | 34.0 | [8,11,12,17] |
| 3 | Asia | 29.0 | [14] |
| 4 | South America | 59.0 | [8,9,10,13] |
| 5 | Africa | 13.5−25.2 | [8,10] |
| 6 | United Kingdom | 20.7 | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
George, H.; Permata, F.S.; D'Souza, C.M.; Adeghate, E.A. Cultural and Molecular Factors Predisposed to Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. Nutrients 2025, 17, 1797. https://doi.org/10.3390/nu17111797
George H, Permata FS, D'Souza CM, Adeghate EA. Cultural and Molecular Factors Predisposed to Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. Nutrients. 2025; 17(11):1797. https://doi.org/10.3390/nu17111797
Chicago/Turabian StyleGeorge, Hanna, Fajar Shodiq Permata, Crystal M. D'Souza, and Ernest A. Adeghate. 2025. "Cultural and Molecular Factors Predisposed to Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus" Nutrients 17, no. 11: 1797. https://doi.org/10.3390/nu17111797
APA StyleGeorge, H., Permata, F. S., D'Souza, C. M., & Adeghate, E. A. (2025). Cultural and Molecular Factors Predisposed to Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. Nutrients, 17(11), 1797. https://doi.org/10.3390/nu17111797







