Abstract
Background/Objectives: The preoperative improvement of patients’ functional capacity (prehabilitation) has gained attention in the surgical field, especially for colorectal cancer (CRC) patients. Despite the recognized benefits of prehabilitation programs, patients’ motivation to participate in and adhere to them remains a significant challenge. Several studies reported difficulties in recruiting participants and low adherence rates. This systematic review explored patients’ motivation for participation and adherence to prehabilitation programs for colorectal cancer surgery. Methods: A systematic search was conducted in the PubMed (MEDLINE), Embase, Cochrane, CINAHL, and Web of Science databases. Eligible studies included clinical trials published from inception until December 2024, written in English or Dutch, describing barriers and/or motivators affecting patient participation and adherence in prehabilitation programs. Results: A total of 89 studies, including 34 randomized controlled trials, were included. In total, 13,383 patients were included, with 7162 in the prehabilitation cohort. Participation rates ranged from 0 to 99.4%, and adherence rates ranged from 15% to 100%. Factors limiting participation included logistical issues and a busy schedule. Professional guidance, peer support, and regaining a sense of control improved adherence. Medical reasons, conflicting obligations, and intensive exercise limited adherence. Conclusions: This systematic review analyzed the current literature on participation and adherence in prehabilitation programs for colorectal cancer surgery patients. Overcoming logistical barriers and patient concerns through flexible, patient-centered approaches may improve participation and adherence. Future research should focus on large-scale randomized controlled trials, diverse healthcare settings, and strategies to enhance engagement with prehabilitation programs.
1. Introduction
In recent years, the number of studies on the effect of prehabilitation—the preoperative improvement of patients’ functional capacity—has expanded rapidly [,,,,]. However, patients’ motivation to participate in and their adherence to prehabilitation programs remain associated with significant challenges. Multiple studies reported difficulties in recruiting participants, highlighting these barriers [,].
Despite major improvements in perioperative care, such as minimally invasive surgery and enhanced recovery protocols, morbidity rates after colorectal surgery remain as high as 40% [,,]. Functional capacity has been identified as a key determinant for a favorable outcomes after surgery []. As a result, there is a strong impetus for clinicians to improve patients’ functional capacity []. Prehabilitation allows patients to improve their functional capacity before surgery, improving their ability to withstand the upcoming stress of a surgical procedure [,]. Recent studies have demonstrated the potential of multimodal prehabilitation to improve patients’ physical status, reduce the length of hospital stay (LOS), and potentially lead to cost savings [,,,,]. Early assessment by a nutritionist during the preoperative period is increasingly recommended as part of standard prehabilitation pathways [].
In the Netherlands, several hospitals have implemented a prehabilitation protocol []. However, no uniform nationwide prehabilitation protocol has been established. For prehabilitation to become the standard of care in clinical practice, recruitment and adherence rates need to increase. This remains challenging, particularly since frail, elderly patients, often suffering from decreased physical reserves and adaptive capacity at baseline, represent the primary population of interest [,,,]. Notably, many colorectal cancer (CRC) patients (55%) are aged 70 years or older []. This demographic shift is partly attributable to the widespread implementation of CRC screening programs. These programs allow for the early detection and removal of tumors, which reduces the overall number of advanced cases. As a result, the remaining patient population tends to be older and often presents with more complex medical issues []. Furthermore, participation in prehabilitation programs requires discipline, flexibility, and intrinsic motivation, which presents both practical and motivational challenges for patients [,,,].
Given the significant increase in prehabilitation studies, updating the literature on factors improving participation and adherence in prehabilitation is essential for generating robust data and facilitating its implementation.
Objective
This study aims to explore how to improve patients’ participation in and adherence to prehabilitation programs for colorectal cancer. To achieve this, the available literature will be systematically reviewed.
2. Materials and Methods
This systematic review was prospectively registered in the PROSPERO database (CRD42023481740). The review was conducted following the guidelines described in the Cochrane Handbook for Systematic Reviews of Interventions, Version 6.3, 2022, and the Preferred Reporting Items for Systematic Reviews and Meta-analysis of Individual Participant Data (PRISMA) guidelines [,].
2.1. Eligibility Criteria
2.1.1. Types of Studies
All clinical trials describing barriers and/or motivators of patient participation or adherence in prehabilitation programs for colorectal cancer surgery were eligible for inclusion. To ensure the inclusion of all relevant articles, studies were not restricted on study type, blinding, or sample size. However, studies were required to be published in peer-reviewed indexed journals.
2.1.2. Participants
Studies involving patients aged ≥ 18 years undergoing elective surgery for colorectal cancer, with or without (neo)adjuvant therapy, were eligible for inclusion. Articles that focus on patients with cancers other than colorectal cancer were excluded.
2.1.3. Interventions
There is no consensus on the design or content of prehabilitation programs and interventions. For this review, we focused on trials with a preoperative prehabilitation program of at least 7 days, involving exercise and/or nutritional interventions. The length of prehabilitation program was chosen based on a recently published Cochrane systematic review [].
2.1.4. Outcome Measure
The primary outcome parameter in this study was the participation rate. Secondary outcome parameters included motivators and barriers for participation, adherence rates, and patients’ motivators and barriers for adherence. Despite the focus on the participation and adherence of patients in prehabilitation programs, we did not exclude relevant studies not describing both measures.
Baseline characteristics included:
- Characteristics of the prehabilitation program: interventions (e.g., exercise, nutritional or psychological guidance), length of the program, motivators, and barriers
- Characteristics of the patients: baseline characteristics and location of cancer
- Characteristics of surgical/oncological treatment: type of surgery, type of (neo)adjuvant treatment, postoperative outcomes such as length of hospital stay (LOS), and overall complications, these will be graded by the Clavien–Dindo classification [] and further divided into both major (e.g., abdominal sepsis), and minor complications (e.g., wound infection), readmissions within 90 days after surgery.
2.2. Search Method for Identification of Studies
Literature Search
A systematic literature search in five key healthcare databases was conducted. Pubmed (MEDLINE), Embase, Cochrane, CINAHL, and Web of Science were searched from inception until 31 December 2024. The following search terms were used: prehabilitation, digestive surgery, digestive cancer, gastrointestinal surgery, and gastrointestinal cancer. The search strategy was developed following the guidelines described in the Cochrane Handbook for Systematic Reviews of Interventions, Version 6.3, 2022 []. The full search strategy is provided as Supplementary Data. All references from the four search databases were imported into Rayyan Bibliographic software []. A comprehensive cross-reference analysis of identified articles was conducted to ensure all relevant studies and sources were identified and included.
2.3. Data Collection and Analysis
2.3.1. Selection of Studies
The titles, abstracts, and descriptor terms of all downloaded material from the electronic searches were read by one researcher (MS), all irrelevant reports were excluded. All citations identified were independently assessed by two researchers (MS and OM) to determine the relevance of each article based on the pre-specified criteria. In cases of uncertainty regarding the relevance of the study, the full article was obtained. Studies were evaluated for relevance based on the study design, types of participants, types of interventions, and outcome measures. After identifying relevant articles, MS and OM independently applied the inclusion criteria. Differences were resolved by discussion with a third reviewer, JS, and a consensus among all reviewers was reached. The selection process is outlined in a flowchart according to PRISMA guidelines []. All identified studies were listed in the “Characteristics of Included Studies” table including an evaluation of whether the studies fulfilled the inclusion criteria. Excluded studies and the reasons for exclusion were listed as well.
2.3.2. Data Extraction and Management
All data were extracted independently by three researchers (EG, OM, and MS). The three types of studies we focused on were randomized control trials, observational studies, and qualitative studies. In the case of randomized trials, the following data were extracted: sample size (randomized and non-randomized), reasons for non-randomization, exclusions after randomization, drop-outs, and ‘intention-to-treat’ analysis. For all studies, available information on inclusion and exclusion criteria, mono- or multicenter study design, primary and secondary outcome measures, and surgical characteristics such as type of surgery and complications were registered. Furthermore, general descriptive data (such as sex, age, body mass index (BMI), and American Society of Anesthesiology (ASA) classification) were evaluated and presented in an additional table. Extracted data were stored and managed using the review manager software Covidence [].
2.3.3. Assessment of Risk of Bias in Included Studies
The methodological quality of RCTs was assessed using the Cochrane risk-of-bias tool (original (2016) version), RoB 2 []. The quality of observational studies was assessed using the ROBINS-I tool (2019 version) []. Every study was assessed independently by EG, OM, and MS. Discrepancies were solved by consensus discussion with a fourth reviewer, JS.
2.3.4. Measures of Treatment Effect
A descriptive quantitative analysis of the included quantitative studies was performed for continuous primary outcomes (participation rate) and secondary outcomes. For the qualitative data, a thematic synthesis was applied by identifying common themes and subthemes across the studies. An inductive approach was used. Subsequently, data were organized and categorized in a thematic framework.
2.3.5. Dealing with Missing Data
When data were missing, we investigated whether this data were missing at random using the Little MCAR test [], where indicated, combined with multiple imputation []. Depending on the outcome, missing data were either considered to have an impact on the outcome or not.
2.3.6. Handling Duplicate and Companion Publications
In cases of duplicate publications, companion papers, or multiple reports of a primary trial, the information was consolidated by collecting and assessing the available data, using the most comprehensive data set across all known publications. Excluded overlapping articles were checked for relevant information regarding outcome measures. If there was minimal overlap, both studies were included. Multiple reports of primary trials were cited as secondary references under the study ID of the included trial.
2.3.7. Assessment of Heterogeneity
Heterogeneity across studies was anticipated due to variations in inclusion and exclusion criteria, prehabilitation program components (e.g., nutritional, physical, psychological), duration, and outcome measures. To assess heterogeneity, we compared the fixed-effect and random-effect estimates of the intervention effect. In case of no discrepancy and no heterogeneity, the fixed-effect models were presented. In case of discrepancy between the two models, both results were reported.
In case of considerable heterogeneity (>75%) [], descriptive quantitative analysis and qualitative analysis were performed, and the outcomes of included studies were described.
2.3.8. Assessment of the Body of Evidence
The body of evidence was assessed by using the GRADE approach [,]. Four descriptors (‘very low’, ‘low’ ‘moderate’, and ‘high’) were used to indicate the confidence level in the evidence.
3. Results
3.1. Results of the Search
In the initial search, performed in October 2023, the systematic search identified 35,819 articles. The literature search and selection processes are shown in Figure 1. After removing the duplicates, 19,646 references were screened and assessed for eligibility based on title and abstract. Of these, 99 full-text articles were assessed for eligibility, of which 85 studies met the inclusion criteria. To incorporate relevant articles, that were recently published, the search was repeated in December 2024. This second search identified 2455 articles. Based on the screening of the title and abstract, 31 articles were subsequently assessed by full-text reading. Ten extra studies were included. No additional studies were included based on the reference checks. In total, 95 articles met the inclusion criteria. After excluding six studies due to the full overlap of study samples [,,,,,], a number of 89 studies were included in the final analysis.
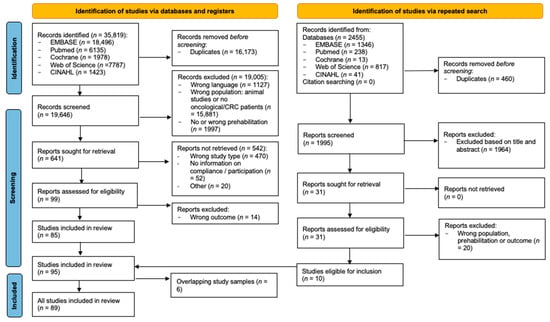
Figure 1.
PRISMA flowchart.
3.2. Included Studies
3.2.1. Study Design and Setting
This systematic review included 34 randomized controlled trials [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,] and 55 [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,] observational studies. Of the 55 observational studies, 6 articles had a qualitative study design [,,,,,]. Furthermore, most cohort studies were of a prospective nature.
The included studies involved a total number of 13,383 patients: 7162 patients in the prehabilitation cohort and 6272 in the control cohort. The total number of study participants ranged from 10 to 761 (median 75, IQR 29–191), with a range of 0–379 patients in the prehabilitation cohort (median 45, IQR 21–102) and 0–382 in the control cohort (median 24, IQR 0–80). Three studies (3.4%) reported a number of zero participants in the prehabilitation cohort [,,] after recruitment and enrollment.
The median length of follow-up after surgery was 56 days [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]. Studies were performed between 1995 and 2022. Most studies were conducted in Western countries such as Canada, the Netherlands, and the United Kingdom.
3.2.2. Participants
Regarding inclusion criteria, 69 studies included CRC patients only [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]. Other studies involved CRC patients and also patients undergoing prehabilitation for upper gastrointestinal (n = 7) [,,,,,,], hepato-pancreatic-biliary (n = 4) [,,,], lung (n = 3) [,,], urological (n = 3) [,,] or other surgeries (n = 10) [,,,,,,,,,]. The median number of patients in the prehabilitation cohort with colon cancer was 26 [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,], with a median percentage of 65.7% [29.9–72.1%]. The median number of patients with rectal cancer was 18 [,,,,,,,,,,,,,,,,,,,], with a median percentage of 32.0% [15.9–45.6%].
The control group included a median number of 24 participants [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,] with colon cancer, at a median percentage of 61.0% [39.5–76.1%], and 17 [,,,,,,,,,,,,,,,,,,,,] participants with rectal cancer, at a median percentage of 30.5% [18.0–47.8].
Sixty-eight studies included only patients undergoing oncological surgery [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,], and six studies included both benign and oncological surgery [,,,,,]. Nine studies included patients receiving neoadjuvant chemotherapy [,,,,,,,,]. In the prehabilitation cohort, the majority of patients underwent minimally invasive surgery, with a median of 80.7% [53.1–95.6]. In comparison, 11.9% [0.9–38.7] of patients underwent open surgery. Minimally invasive surgery was also most prevalent in the control group, with a median of 74.4% [40.0–93.0], versus 19.5% [1.6–47.1%] of patients receiving open surgery.
Regarding the exclusion criteria, emergency or non-elective surgery was an exclusion criterion for 22 studies [,,,,,,,,,,,,,,,,,,,,,], whereas 51 studies excluded patients based on limited physical or psychiatric functioning [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]. An overview of inclusion and exclusion criteria is provided in Supplementary Table S1, and patient characteristics are displayed in Supplementary Table S2.
3.3. Interventions and Comparisons
Prehabilitation Programs
Upon analyzing the prehabilitation programs, we noticed differences in prehabilitation modalities to improve patients’ condition before surgery. The majority of studies (n = 50, 56.2%) involved a multimodal prehabilitation program, with exercise or nutritional intervention. Twenty-seven studies (30.3%) described a unimodal exercise-based prehabilitation program, 10 studies (11.2%) described a unimodal nutrition-based prehabilitation program, and two studies (2.2%) involved a preoperative nutritional intervention and a (postoperative) rehabilitation program. An overview of the prehabilitation interventions is provided in Supplementary Tables S3 and S4.
Due to the large heterogeneity in prehabilitation programs, no meta-analysis could be performed. This heterogeneity arises from variations in inclusion and exclusion criteria, as well as differences in the length, content, and outcomes of the prehabilitation programs. Please refer to Tables S1, S3 and S4. The information was insufficient to conclude certainty of evidence.
3.4. Primary Outcome—Quantitative Data
Of the sixty-three studies describing participation, the participation rate ranged from 0 to 99.4%. The median rate of participation in the studies was 66.6% [55.6–83.6%]. Reasons for declining participation in the study were reported in 38 studies (42.7%). The most common reasons for declining participation were logistical factors, such as lack of transport to the training location and being too busy, as well as medical reasons, including contraindications for training due to arthrosis or cardiopulmonary failure. Other common reasons included a lack of interest in prehabilitation, the perceived burden of the prehabilitation program, concerns about a potential delay in surgery, or no need to prehabilitate (self-perceived), this is displayed in Figure 2.
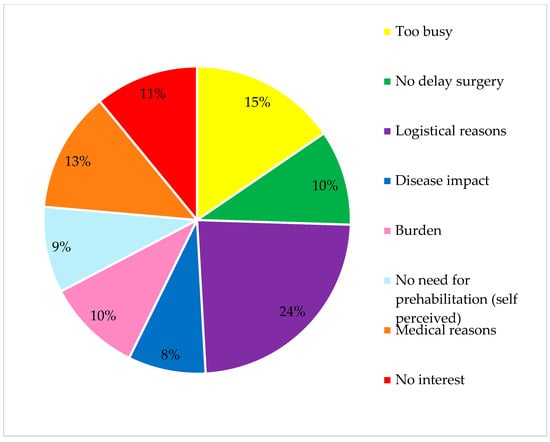
Figure 2.
Reasons for declining participation.
3.4.1. Participation—Unimodal Prehabilitation
In total, 27 studies (30.3%) involved a unimodal exercise prehabilitation program, including a total number of 1870 patients with 1058 patients in the prehabilitation cohort. The number of participants in the prehabilitation program ranged from 0 to 137, because two feasibility trials [,] (7.4%) could not include participants in their final data analysis. The median participation rate was 61.0% [41.5–73.6%]. Of these studies, 15 (55.6%) provided information about the participation rate or the reasons for agreeing/declining to participate. The most common reasons for declining participation were a busy schedule and logistical issues.
Ten studies (11.2%) involved a program with nutritional intervention, with 619 patients included in the prehabilitation cohort. The number of participants ranged from 20 to 132, with a median of 59 participants in the prehabilitation cohort [,,,,,,,,,,,,,,,,,,,,,,,]. Five studies (50%) described participation rates, with a median participation rate of 77.6% [IQR 53.9–90.6%]. In the four studies describing the reason for declining participation, the most common reason was the refusal of the nutritional intervention.
3.4.2. Participation—Multimodal Prehabilitation
Fifty studies (56.2%) described a multimodal prehabilitation program of which 36 (72%) provided information about participation. The range of participants was 5–641 with a total of 5413 participants in the prehabilitation cohort. The median participation rate was 70.0% [57.1–84.8]. Twenty-one studies reported reasons for not participating of which logistical reasons and the lack of interest were most common.
3.4.3. Participation—Exercise Intervention (n = 74)
When distinguishing between exercise and nutritional interventions, 57 studies involving exercise prehabilitation described participation rates (77.0%). The number of participants in the prehabilitation cohort varied from 0 to 641, with a total of 6258 patients included in the exercise prehabilitation cohort. The median participation rate was 67.1% [53.7–83.4%].
3.4.4. Participation—Nutritional Intervention (n = 57)
Thirty-seven nutritional prehabilitation studies described participation (64.9%). The number of participants varied from 0 to 641, with a total of 5073 patients included. The median participation rate was 75.0% [56.3–84.6%].
3.4.5. Adherence
The length of prehabilitation ranged from 7 to 115 days. Of studies describing mean prehabilitation duration, the mean was 28.2 days (95% CI 18,2–38.1, SD 22.4). Studies reporting a median prehabilitation duration had an overall median of 33 days [,,,,,,,,,,,,,,,,,,,]. Overall, 70 studies (78.7%) provided information on adherence to prehabilitation programs. The adherence rates ranged from 16 to 100%. Of studies reporting overall adherence (n = 14, 15.7%), the median was 72% [58.8–82.3%].
Reasons for non-adherence were reported by 38 studies, the most common reasons for non-adherence were medical reasons such as physical injuries and logistical issues (See Figure 3).
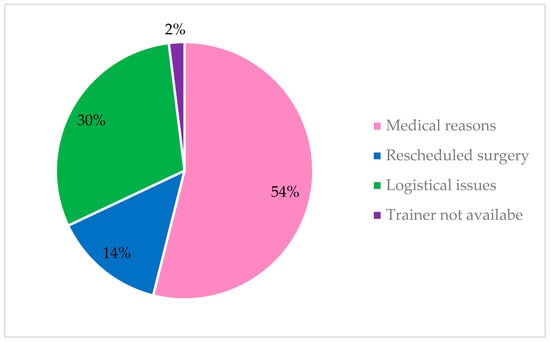
Figure 3.
Reasons for non-adherence.
3.4.6. Adherence—Exercise Intervention (n = 74)
Seventy-one studies involving exercise prehabilitation (95.9%) described the method of adherence measurement during exercise interventions. In most studies program adherence was reported by the supervisor (n = 23, 32.4%), or by both the patients and supervisors (n = 26, 36.6%) Less often patients reported their activity themselves (n = 20, 28.2%), for example by using an activity log.
Fifty-nine studies provided information on adherence to exercising (79.7%), with a median of 75.4% compliance rate [62.0–86.0%].
For unimodal exercise prehabilitation the median adherence rate was 78.0% [66.1–90.8%] in the 24 of 27 (88.9%) studies. Multimodal exercise prehabilitation had a median adherence rate of 72.0% [61.0–84.0%] in 35 of 47 studies describing multimodal exercise prehabilitation.
3.4.7. Adherence—Nutritional Intervention (n = 57)
The method of measuring adherence with nutritional interventions was described in 33 studies (57.9%) of 57 studies involving nutritional prehabilitation. Adherence was most often measured by self-reporting for most patients (75.8%), for example, in a food diary. Supervision was less common (12.1%), followed by 9.1% of a combination of self-reporting and supervision. Twenty-two studies provided information on the compliance rate to nutritional interventions (38.6%). The median compliance rate was 77.0% [65.4–96.3%].
The median adherence rate was 78.0% [66.1–90.8%] in the unimodal nutritional interventions (n = 24, 88.9%). Multimodal prehabilitation involving nutritional interventions was adhered to by 66.7% of patients (median, IQR [57.8–95.3%]).
3.5. Primary Outcome—Qualitative Data (n = 6)
Three studies involved a multimodal prehabilitation program [,,], one study a unimodal exercise program [], and two studies [,] interviewed CRC patients about their visions towards prehabilitation. The number of participants in the prehabilitation cohort ranged from 0 to 34, with a median participation rate of 80%.
3.5.1. Participation
The most common enablers and barriers to participation are displayed in Figure 4.
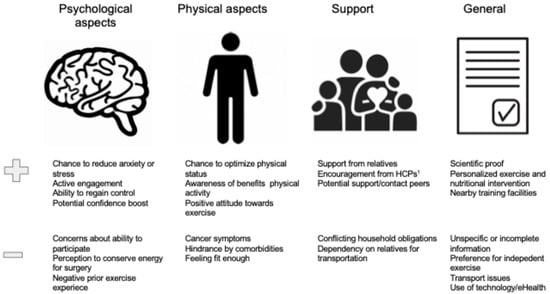
Figure 4.
Enablers and barriers to participation: qualitative studies. 1 HCP, Healthcare professional.
3.5.2. Adherence
In two studies, adherence to exercise interventions was measured by a supervisor [,]. The adherence to nutritional interventions was self-reported in two studies [,].
Figure 5 displays the most important reported enablers and barriers to adherence with prehabilitation.
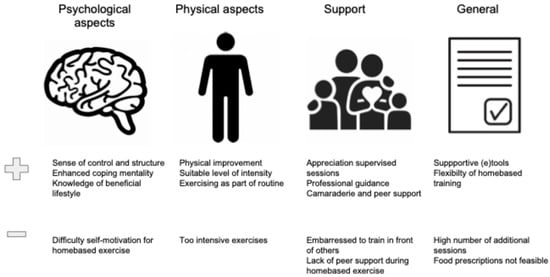
Figure 5.
Enablers and barriers to adherence: qualitative studies.
3.6. Risk of Bias
The methodological quality of the included quantitative studies (n = 83) is presented in risk of bias (RoB) summaries (Figure 6, Figure 7, Figure 8 and Figure 9). Fourteen studies [,,,,,,,,,,,,,] (16.8%) were assessed as having a serious or critical risk of bias, primarily due to confounding, selection bias, bias in measurement or bias in the selection of reported results. Sixty-one studies [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,] (73.5%) were deemed to have a moderate risk of bias. Eight studies [,,,,,,,] (9.6%) were considered to have a low risk of bias. Overall, the methodological quality of the included studies ranged from moderate to low.
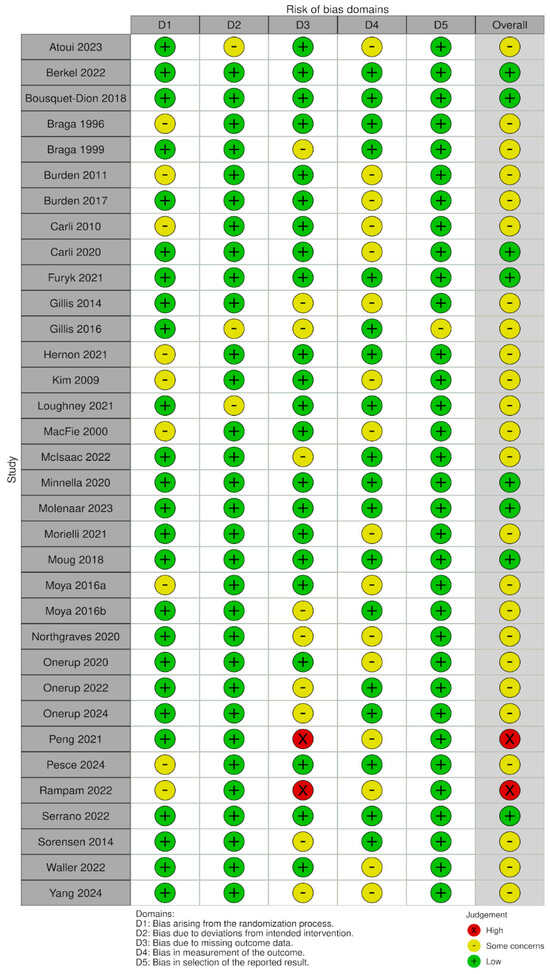
Figure 6.
RoB-2 plot risk of bias [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].
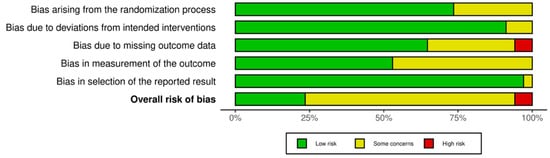
Figure 7.
RoB 2 Weighted summary plot.
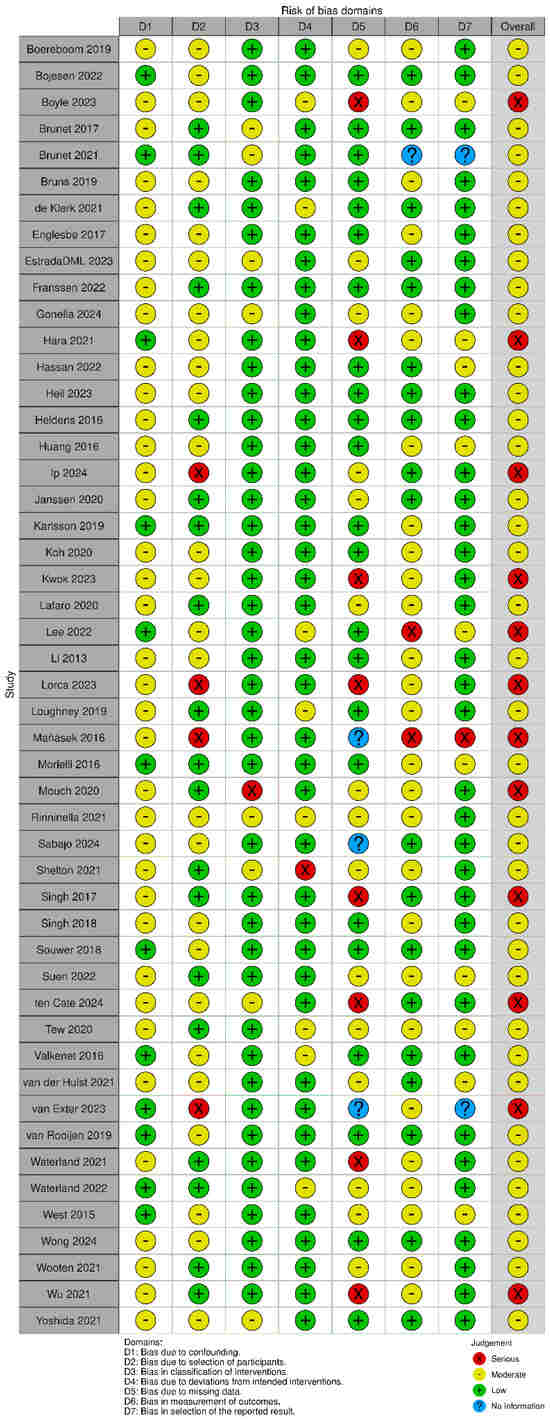
Figure 8.
ROBIN-I plots risk of bias [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,].
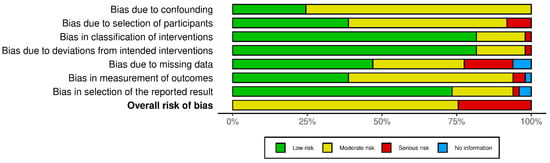
Figure 9.
ROBINS-I weighted summary plot.
An overview of the quality of the included qualitative studies is provided in Supplementary S1. One study [] had a low trustworthiness, the other five studies [,,,,] had a medium or high trustworthiness.
4. Discussion
This systematic review identified and analyzed 89 studies investigating prehabilitation programs for patients undergoing colorectal cancer surgery. It is the first review to collect qualitative and quantitative data to examine the current literature on participation and adherence to prehabilitation programs. Most of the included studies were prospective cohort studies. The results of the studied sample (n = 13,383) demonstrated a large variety in the participation rate (0–99.4%). Data on recruitment and declining participation was limited. The available data displayed that most patients eligible for inclusion were recruited. Participation was most often refused based on logistical or medical reasons. The highest participation rates were described for unimodal nutritional prehabilitation.
Adherence also varied widely among studies, with an adherence rate of 16–100%. A minority of studies described reasons for (non-) adherence. When reported, adherence was most often hindered due to medical reasons. The quantitative and qualitative results emphasize the importance of addressing accessibility for patients with medical limitations and providing help for logistical issues. Also, since high adherence is crucial for successful prehabilitation [,], more attention is paid to describing the measurement of adherence and the adherence rates to generate more information on how to improve adherence. The analyzed articles suggest that professional guidance, peer support, and supportive tools and content could improve adherence.
4.1. Participation in Prehabilitation Programs
The recruitment and enrollment of patients into clinical studies have long been recognized as challenging, with important implications for study outcomes, especially for prehabilitation programs, where the most motivated patients are also most likely to be adherent and achieve the best results [].
In general, approximately 50% of clinical trials are estimated to fail to meet their recruitment targets []. Our review reflects this difficulty, with a reported average participation rate of 66.6%. This finding is consistent with the meta-analysis of Xu et al. []. However, this figure is relatively high compared to some studies such as the pooled retrospective analysis of Lee et al. []. The difference in participation rate could be attributed to the difference in patient population, including patients undergoing surgery for lung, and esophageal cancer. In contrast, Cuijpers et al. [] reported a wide range in participation rates, which aligns with our findings underscoring the heterogeneity in patient recruitment and engagement across prehabilitation studies.
4.2. Participation—Barriers
Logistical barriers, such as transportation difficulties and scheduling conflicts, emerged as primary obstacles to participation in prehabilitation programs. These barriers align with the findings of the qualitative evidence synthesis of Houghton et al. [], who reported that additional appointments or travel requirements can be burdensome for patients. Medical contraindications, including comorbidities and perceived ineligibility, along with a perception of surgery urgency, were reported as reasons for non-participation in prehabilitation programs. Similarly, the systematic reviews of Alsuwaylihi et al. [] and Cuijpers et al. [] highlighted psychological barriers, such as feeling overwhelmed by the number of prehabilitation sessions or preferring immediate surgery, as significant deterrents. Furthermore, inadequate communication, lack of specific information, and an over-emphasis on benefits were reported as additional barriers. Although this study did not collect data on socio-economic status, qualitative findings suggest that financial burden could limit participation. This aligns with the findings of Lee et al. [] who found that individuals from lower socio-economic backgrounds were less likely to engage in prehabilitation programs, probably due to financial limitations and disparities in healthcare access.
4.3. Participation—Enablers
On the other hand, several factors were identified as enablers of higher participation rates in prehabilitation programs. The qualitative findings of this review highlight both psychological and physical benefits, such as creating a sense of regained control, a positive mindset, reduced stress, and the opportunity to optimize physical fitness to withstand surgical stress better.
Clear and direct communication from healthcare providers (HCPs) was a key facilitator for participation, as was the provision of detailed information about the prehabilitation program. Support from both relatives and HCPs played a crucial role in encouraging participation. These findings align with Houghton et al. [], who reported that clear, face-to-face communication from HCPs enhanced patient understanding of the prehabilitation process and improved participation in prehabilitation programs. Similarly, family support and encouragement from HCPs were found to be influential in motivating patients to participate in prehabilitation programs. These findings highlight the importance of effective communication and social support for improving patient engagement in prehabilitation programs.
Notably, this review showed the highest recruitment rate for unimodal nutritional intervention, suggesting that patients perceive this as less burdensome than multimodal prehabilitation programs.
4.4. Adherence to Prehabilitation Protocols
Adherence to prehabilitation programs is essential for an accurate evaluation of the effectiveness of these interventions. Adherence to long-term therapies has been reported to average around 50% [], and given that prehabilitation typically involves a multifaceted approach, adherence may be even more challenging. The average adherence rate of this review is consistent with findings by Alsuwaylihi et al. [] and O’Doherty et al. []. Similarly, Luther et al. [] emphasized the heterogeneity in adherence rates, particularly in studies involving exercise interventions, with adherence varying widely across different study populations.
4.5. Adherence—Barriers
This review reported medical reasons, rescheduled surgery, and logistical issues as the most important barriers to adherence. These findings are consistent with the review of O’Doherty et al. [] and Alsuwaylihi et al. [], describing reduced adherence when patients had the opportunity to undergo surgery earlier. Competing life responsibilities, such as work and family obligations, were also cited as reasons for non-adherence, as well as medical issues and fatigue. Additionally, logistical barriers, such as transportation difficulties and the need for frequent appointments, were common challenges that reduced adherence to prehabilitation interventions [,].
Cuijpers et al. [] reported that side effects from neoadjuvant chemoradiotherapy (NACRT) were also a barrier to adherence. Patients undergoing NACRT reported feeling unwell or fatigued, which negatively impacted their ability to participate in prehabilitation programs. Personal issues, such as stress or family responsibilities were also frequently reported as reasons for non-adherence.
4.6. Adherence—Enablers
Several factors were identified as contributing to higher adherence rates. This review highlights the positive impact of professional guidance, flexible and patient-centered programs, peer support, and supportive tools and content. These findings align with previous research, describing that a holistic, patient-centered approach that integrates multiple aspects of care contributed significantly to improved adherence [,]. Consistent with this review, the involvement of a multidisciplinary team also was a key enabler of adherence, as this approach provides comprehensive care and fosters a supportive environment for patients. Le Roy et al. [] noted that such teams contribute to higher patient adherence by addressing the diverse needs of patients, from exercise to psychological support. Furthermore, the early integration of prehabilitation into the treatment process was identified as an important factor, as it builds trust between patients and healthcare providers, which can enhance patient engagement and reduce dropouts [].
The highest adherence was reported for unimodal exercise or nutritional prehabilitation, suggesting that multimodality prehabilitation is more challenging for patients to adhere to.
In contrast, Alsuwaylihi et al. [] established that multimodal prehabilitation programs (which combined exercise, nutrition, and psychological support) were associated with higher adherence compared to unimodal interventions. Additionally, longer prehabilitation programs (≥3 weeks) and home-based training interventions were linked to better adherence, suggesting that the flexibility of the program and the opportunity to progress into prehabilitation may facilitate higher adherence.
4.7. Strengths and Limitations of the Review
The strengths of this systematic review are the large number of studies included and the diversity of study designs, which enhance the generalizability of the findings. By integrating qualitative and quantitative data, this review provides a comprehensive understanding of the factors influencing patient participation and adherence in prehabilitation programs.
However, several limitations must be acknowledged before the generalization of findings. We found the overall methodological quality of the studies about prehabilitation programs in colorectal cancer to be of medium to low quality. Nonetheless, all studies were included to provide a thorough overview of the current literature. Several studies lacked detailed information regarding participation rates. Also, both adherence measurement and adherence data are missing in various studies which hampers generalizability. Moreover, no meta-analysis could be conducted due to the large heterogeneity of study designs, intervention protocols, and outcome measures. This impairs direct comparisons between the studies. Additionally, the predominance of studies conducted in Western countries limits the applicability of these findings to non-Western healthcare settings, where cultural, socio-economic, and healthcare system differences may impact participation as well as the outcomes of prehabilitation programs. On the other hand, these countries have facilities to offer prehabilitation and are most likely to integrate it into their care pathway.
Moreover, the observational nature of many of the included studies introduces the potential for confounding, which could influence the reported outcomes. The low number of randomized controlled trials on the topic of prehabilitation in colorectal cancer underscores the need for further high-quality research to provide robust evidence for prehabilitation interventions. The limited number of studies reporting on participation and adherence rates further hinders the ability to draw definitive conclusions about the factors influencing these outcomes.
The lack of detailed information on patients who declined participation or did not adhere to the intervention introduces a risk of bias in outcome measurement and weakens the strength of evidence. Additionally, the absence of detailed descriptions of control group interventions in several studies may act as a potential confounder.
4.8. Recommendations for Future Research
Future research should focus on developing standardized protocols for measuring participation and adherence to enhance the comparability of results across studies. Furthermore, further studies should explore strategies to improve participation and adherence, particularly in home-based or digitally supported prehabilitation interventions. Large-scale randomized controlled trials or stepped-wedge design studies are necessary to identify the most effective and feasible prehabilitation approaches, especially in diverse healthcare settings. Additionally, research should examine the experiences of patients who decline participation in prehabilitation programs to better understand the barriers to engagement from a broader perspective.
5. Conclusions
This systematic review provides a comprehensive overview of the current literature on prehabilitation programs for patients undergoing colorectal cancer surgery. Despite the postulated beneficial impact of prehabilitation on physical and psychological status, significant variability in study designs, participation rates, and intervention strategies suggests that further research is needed to identify the most effective prehabilitation approaches. Addressing logistical barriers and patient concerns, particularly through flexible, patient-centered interventions, may enhance both participation and adherence, maximizing the benefits of prehabilitation in clinical practice. Future studies should focus on large-scale RCTs, explore diverse healthcare settings, and compare strategies to improve both participation and adherence to prehabilitation programs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17111792/s1, Table S1: Inclusion and exclusion criteria; Table S2: Patients’ characteristics; Table S3: Characteristics of exercise interventions and prehabilitation; Table S4: Characteristics of nutritional interventions and prehabilitation; Supplementary S1: EPPI Centre quality assessment.
Author Contributions
Conceptualization, M.A.T.S., M.P.P.J.C. and J.H.M.B.S.; methodology, M.A.T.S., M.P.P.J.C. and J.H.M.B.S.; software, M.A.T.S. and J.H.M.B.S.; validation, M.A.T.S., T.T.T.T. and J.H.M.B.S.; formal analysis, M.A.T.S., M.P.P.J.C., O.M. and E.G.; investigation, M.A.T.S., M.P.P.J.C., O.M., E.G. and J.H.M.B.S.; resources, J.H.M.B.S. and J.W.G.; data curation, J.H.M.B.S., T.T.T.T. and J.W.G.; writing—original draft preparation, M.A.T.S., M.P.P.J.C., O.M., E.G. and J.H.M.B.S.; writing—review and editing, M.A.T.S., M.P.P.J.C., O.M., E.G., T.T.T.T., J.W.G. and J.H.M.B.S.; visualization, M.A.T.S. and E.G.; supervision, J.H.M.B.S., T.T.T.T. and J.W.G.; project administration, M.A.T.S. and J.H.M.B.S.; funding acquisition, J.H.M.B.S. and J.W.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank Marion Heymans for taking the time and effort to provide guidance and advice for the literature search strategy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Molenaar, C.J.L.; Minnella, E.M.; Coca-Martinez, M.; ten Cate, D.W.G.; Regis, M.; Awasthi, R.; Martínez-Palli, G.; López-Baamonde, M.; Sebio-Garcia, R.; Feo, C.V.; et al. Effect of Multimodal Prehabilitation on Reducing Postoperative Complications and Enhancing Functional Capacity Following Colorectal Cancer Surgery: The PREHAB Randomized Clinical Trial. Arch. Surg. 2023, 158, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Barberan-Garcia, A.; Ubré, M.; Roca, J.; Lacy, A.; Burgos, F.; Risco, R.; Momblán, D.; Balust, J.; Blanco, I.; Martínez-Pallí, G. Personalised Prehabilitation in High-Risk Patients Undergoing Elective Major Abdominal Surgery: A Randomized Blinded Controlled Trial. Ann. Surg. 2018, 267, 50–56. [Google Scholar] [CrossRef]
- Gillis, C.; Buhler, K.; Bresee, L.; Carli, F.; Gramlich, L.; Culos-Reed, N.; Sajobi, T.T.; Fenton, T.R. Effects of Nutritional Prehabilitation, with and without Exercise, on Outcomes of Patients Who Undergo Colorectal Surgery: A Systematic Review and Meta-Analysis. Gastroenterology 2018, 155, 391–410.e4. [Google Scholar] [CrossRef]
- Minnella, E.M.; Bousquet-Dion, G.; Awasthi, R.; Scheede-Bergdahl, C.; Carli, F. Multimodal Prehabilitation Improves Functional Capacity before and After Colorectal Surgery for Cancer: A Five-Year Research Experience. Acta Oncol. 2017, 56, 295–300. [Google Scholar] [CrossRef]
- Molenaar, C.J.; Rooijen, S.J.; Fokkenrood, H.J.; Roumen, R.M.; Janssen, L.; Slooter, G.D. Prehabilitation Versus no Prehabilitation to Improve Functional Capacity, Reduce Postoperative Complications and Improve Quality of Life in Colorectal Cancer Surgery. Cochrane Database Syst. Rev. 2022, 2022, CD013259. [Google Scholar]
- Ferreira, V.; Agnihotram, R.V.; Bergdahl, A.; van Rooijen, S.J.; Awasthi, R.; Carli, F.; Scheede-Bergdahl, C. Maximizing Patient Adherence to Prehabilitation:: What do the Patients Say? Support. Care Cancer 2018, 26, 2717–2723. [Google Scholar] [CrossRef]
- Cooper, M.; Chmelo, J.; Sinclair, R.C.F.; Charman, S.; Hallsworth, K.; Welford, J.; Phillips, A.W.; Greystoke, A.; Avery, L. Exploring Factors Influencing Uptake and Adherence to a Home-Based Prehabilitation Physical Activity and Exercise Intervention for Patients Undergoing Chemotherapy before Major Surgery (ChemoFit): A Qualitative Study. BMJ Open 2022, 12, e062526. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, A.C.; Lee, R.M.; Turgeon, M.K.; Varlamos, C.; Regenbogen, S.E.; Hrebinko, K.A.; Holder-Murray, J.; Wiseman, J.T.; Ejaz, A.; Feng, M.P.; et al. Impact of Postoperative Complications on Oncologic Outcomes After Rectal Cancer Surgery: An Analysis of the US Rectal Cancer Consortium. Ann. Surg. Oncol. 2021, 28, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- van der Hulst, H.C.; van der Bol, J.M.; Bastiaannet, E.; Portielje, J.E.A.; Dekker, J.W.T. Surgical and Non-Surgical Complications After Colorectal Cancer Surgery in Older Patients; Time-Trends and Age-Specific Differences. Eur. J. Surg. Oncol. 2023, 49, 724–729. [Google Scholar] [CrossRef]
- Warps, A.K.; Tollenaar, R.A.E.M.; Tanis, P.J.; Dekker, J.W.T. Postoperative Complications After Colorectal Cancer Surgery and the Association with Long-Term Survival. Eur. J. Surg. Oncol. 2022, 48, 873–882. [Google Scholar] [CrossRef]
- Minnella, E.M.; Carli, F. Prehabilitation and Functional Recovery for Colorectal Cancer Patients. Eur. J. Surg. Oncol. 2018, 44, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Scheede-Bergdahl, C.; Minnella, E.M.; Carli, F. Multi-modal Prehabilitation: Addressing the Why, when, what, how, Who and Where Next? Anaesthesia 2019, 74, 20–26. [Google Scholar] [CrossRef]
- Cambriel, A.; Choisy, B.; Hedou, J.; Bonnet, M.; Fellous, S.; Lefevre, J.H.; Voron, T.; Gaudillière, D.; Kin, C.; Gaudillière, B.; et al. Impact of Preoperative Uni- Or Multimodal Prehabilitation on Postoperative Morbidity: Meta-Analysis. BJS Open 2023, 7, zrad129. [Google Scholar] [CrossRef]
- Groen, L.C.; van Gestel, T.; Daams, F.; van den Heuvel, B.; Taveirne, A.; Bruns, E.R.; Schreurs, H.W. Community-Based Prehabilitation in Older Patients and High-Risk Patients Undergoing Colorectal Cancer Surgery. Eur. J. Surg. Oncol. 2024, 50, 107293. [Google Scholar] [CrossRef]
- Sabajo, C.R.; Ten Cate, D.W.G.; Heijmans, M.H.M.; Koot, C.T.G.; van Leeuwen, L.V.L.; Slooter, G.D. Prehabilitation in Colorectal Cancer Surgery Improves Outcome and Reduces Hospital Costs. Eur. J. Surg. Oncol. 2024, 50, 107302. [Google Scholar] [CrossRef]
- De Felice, F.; Malerba, S.; Nardone, V.; Salvestrini, V.; Calomino, N.; Testini, M.; Boccardi, V.; Desideri, I.; Gentili, C.; De Luca, R.; et al. Progress and Challenges in Integrating Nutritional Care into Oncology Practice: Results from a National Survey on Behalf of the NutriOnc Research Group. Nutrients 2025, 17, 188. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, C.J.L.; Reudink, M.; Sabajo, C.R.; Janssen, L.; Roumen, R.M.H.; Klaase, J.M.; Slooter, G.D. Prehabilitation for Patients with Colorectal Cancer: A Snapshot of Current Daily Practice in Dutch Hospitals. Perioper. Med. 2023, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Berkel, A.E.M.; Bongers, B.C.; Kotte, H.; Weltevreden, P.; De Jongh, F.H.C.; Eijsvogel, M.M.M.; Wymenga, M.; Bigirwamungu-Bargeman, M.; Van Der Palen, J.; Van Det, M.J.; et al. Effects of Community-Based Exercise Prehabilitation for Patients Scheduled for Colorectal Surgery with High Risk for Postoperative Complications: Results of a Randomized Clinical Trial. Ann. Surg. 2022, 275, E299–E306. [Google Scholar] [CrossRef]
- Hoogeboom, T.J.; Dronkers, J.J.; Hulzebos, E.H.J.; van Meeteren, N.L.U. Merits of Exercise Therapy before and After Major Surgery. Curr. Opin. Anaesthesiol. 2014, 27, 161–166. [Google Scholar] [CrossRef]
- Minnella, E.M.; Awasthi, R.; Gillis, C.; Fiore, J.F., Jr.; Liberman, A.S.; Charlebois, P.; Stein, B.; Bousquet-Dion, G.; Feldman, L.S.; Carli, F. Patients with Poor Baseline Walking Capacity are most Likely to Improve their Functional Status with Multimodal Prehabilitation. Surgery 2016, 160, 1070–1079. [Google Scholar] [CrossRef]
- Integraal Kankercentrum Nederland. Cijfers Darmkanker; Integraal Kankercentrum Nederland: Utrecht, The Netherlands, 2022. [Google Scholar]
- Hu, F.Y.; Rowe, K.A.; O’Mara, L.M.; Bulger, A.; Bleday, R.; Groff, M.W.; Cooper, Z.; Bernacki, R.E. Evaluation of Interdisciplinary Care Pathway Implementation in Older Elective Surgery Patients. J. Am. Geriatr. Soc. 2023, 71, 1310–1322. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Price, J.; Delluc, C. An Exercise Trial for Adults Undergoing Neoadjuvant Chemoradiotherapy for Rectal Cancer Proves Not Feasible: Recommendations for Future Trials. Curr. Control Trials Cardiovasc. Med. 2021, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Sun, V.; Raz, D.J.; Kim, J.Y.; Melstrom, L.; Hite, S.; Varatkar, G.; Fong, Y. Barriers and Facilitators of Adherence to a Perioperative Physical Activity Intervention for Older Adults with Cancer and their Family Caregivers. J. Geriatr. Oncol. 2020, 11, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Vind Thaysen, H.; Hasselholt Soegaard, C.; Blaakaer, J.; Seibaek, L. What Matters to You? an Investigation of Patients’ Perspectives on and Acceptability of Prehabilitation in Major Cancer Surgery. Eur. J. Cancer Care 2021, 30, e13475. [Google Scholar] [CrossRef]
- West, M.A.; Loughney, L.; Lythgoe, D.; Barben, C.P.; Sripadam, R.; Kemp, G.J.; Grocott, M.P.W.; Jack, S. Effect of Prehabilitation on Objectively Measured Physical Fitness After Neoadjuvant Treatment in Preoperative Rectal Cancer Patients: A Blinded Interventional Pilot Study. Br. J. Anaesth. 2015, 114, 244–251. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2022; Volume 2023. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Veritas Health Innovation. Covidence Systematic Review Software; Veritas Health Innovation Ltd.: Oxford, UK, 2025. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Little, R.J.A. A Test of Missing Completely at Random for Multivariate Data with Missing Values. J. Am. Stat. Assoc. 1988, 83, 1198–1202. [Google Scholar] [CrossRef]
- Li, P.; Stuart, E.A.; Allison, D.B. Multiple Imputation: A Flexible Tool for Handling Missing Data. JAMA J. Am. Med. Assoc. 2015, 314, 1966–1967. [Google Scholar] [CrossRef] [PubMed]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE Guidelines: 3. Rating the Quality of Evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach; GRADE Working Group: Hamilton, ON, Canada, 2013; Volume 2023. [Google Scholar]
- Tweed, T.T.T.; Sier, M.A.T.; Van Bodegraven, A.A.; Van Nie, N.C.; Sipers, W.M.W.H.; Boerma, E.G.; Stoot, J.H.M.B. Feasibility and Efficiency of the BEFORE (Better Exercise and Food, Better Recovery) Prehabilitation Program. Nutrients 2021, 13, 3493. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.M.; Brunet, J.; Sabiston, C.M.; Jack, S.; Grocott, M.P.W.; West, M.A. Patients’ Perceptions of Quality of Life during Active Treatment for Locally Advanced Rectal Cancer: The Importance of Preoperative Exercise. Support. Care Cancer 2013, 21, 3345–3353. [Google Scholar] [CrossRef]
- Burke, S.M.; West, M.A.; Grocott, M.P.W.; Brunet, J.; Jack, S. Exploring the Experience of Adhering to a Prescribed Pre-Surgical Exercise Program for Patients with Advanced Rectal Cancer: A Phenomenological Study. Psychol. Sport Exerc. 2015, 16, 88–95. [Google Scholar] [CrossRef]
- Rinninella, E.; Persiani, R.; D’Ugo, D.; Pennestrì, F.; Cicchetti, A.; Di Brino, E.; Cintoni, M.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. NutriCatt Protocol in the Enhanced Recovery After Surgery (ERAS) Program for Colorectal Surgery: The Nutritional Support Improves Clinical and Cost-Effectiveness Outcomes. Nutrition 2018, 50, 74–81. [Google Scholar] [CrossRef]
- Janssen, T.L.; Steyerberg, E.W.; Langenberg, J.C.M.; van Hoof de Lepper, C.C.H.A.; Wielders, D.; Seerden, T.C.J.; de Lange, D.C.; Wijsman, J.H.; Ho, G.H.; Gobardhan, P.D.; et al. Multimodal Prehabilitation to Reduce the Incidence of Delirium and Other Adverse Events in Elderly Patients Undergoing Elective Major Abdominal Surgery: An Uncontrolled before-and-After Study. PLoS ONE 2019, 14, e0218152. [Google Scholar] [CrossRef]
- Hara, T.; Kogure, E.; Kubo, A.; Kakuda, W. Preoperative Improvement in Physical Function by Comprehensive Rehabilitation Leads to Decreased Postoperative Complications in Gastrointestinal Cancer Patients. Prog. Rehabil. Med. 2021, 6, 20210001. [Google Scholar] [CrossRef]
- Braga, M.; Gianotti, L.; Radaelli, G.; Vignali, A.; Mari, G.; Gentilini, O.; Di Carlo, V. Perioperative Immunonutrition in Patients Undergoing Cancer Surgery: Results of a Randomized Double-Blind Phase 3 Trial. Arch. Surg. 1999, 134, 428–433. [Google Scholar] [CrossRef]
- Braga, M.; Gianotti, L.; Cestari, A.; Vignali, A.; Pellegatta, F.; Dolci, A.; Carlo, V.D. Gut Function and Immune and Inflammatory Responses in Patients Perioperatively Fed with Supplemented Enteral Formulas. Arch. Surg. 1996, 131, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Carli, F.; Charlebois, P.; Stein, B.; Feldman, L.; Zavorsky, G.; Kim, D.J.; Scott, S.; Mayo, N.E. Randomized Clinical Trial of Prehabilitation in Colorectal Surgery. Br. J. Surg. 2010, 97, 1187–1197. [Google Scholar] [CrossRef]
- Gillis, C.; Loiselle, S.E.; Fiore, J.F., Jr.; Awasthi, R.; Wykes, L.; Liberman, A.S.; Stein, B.; Charlebois, P.; Carli, F. Prehabilitation with Whey Protein Supplementation on Perioperative Functional Exercise Capacity in Patients Undergoing Colorectal Resection for Cancer: A Pilot Double-Blinded Randomized Placebo-Controlled Trial. J. Acad. Nutr. Diet. 2016, 116, 802–812. [Google Scholar] [CrossRef]
- Moya, P.; Miranda, E.; Soriano-Irigaray, L.; Arroyo, A.; Aguilar, M.; Bellón, M.; Muñoz, J.; Candela, F.; Calpena, R.; Bellón, M.; et al. Perioperative Immunonutrition in Normo-Nourished Patients Undergoing Laparoscopic Colorectal Resection. Surg. Endosc. 2016, 30, 4946–4953. [Google Scholar] [CrossRef]
- Bousquet-Dion, G.; Awasthi, R.; Loiselle, S.; Minnella, E.M.; Agnihotram, R.V.; Bergdahl, A.; Carli, F.; Scheede-Bergdahl, C. Evaluation of Supervised Multimodal Prehabilitation Programme in Cancer Patients Undergoing Colorectal Resection: A Randomized Control Trial. Acta Oncol. 2018, 57, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Northgraves, M.J.; Arunachalam, L.; Madden, L.A.; Marshall, P.; Hartley, J.E.; MacFie, J.; Vince, R.V. Feasibility of a Novel Exercise Prehabilitation Programme in Patients Scheduled for Elective Colorectal Surgery: A Feasibility Randomised Controlled Trial. Support. Care Cancer 2020, 28, 3197–3206. [Google Scholar] [CrossRef]
- Carli, F.; Bousquet-Dion, G.; Awasthi, R.; Elsherbini, N.; Liberman, S.; Boutros, M.; Stein, B.; Charlebois, P.; Ghitulescu, G.; Morin, N.; et al. Effect of Multimodal Prehabilitation Vs Postoperative Rehabilitation on 30-Day Postoperative Complications for Frail Patients Undergoing Resection of Colorectal Cancer: A Randomized Clinical Trial. JAMA Surg. 2020, 155, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Onerup, A.; Thörn, S.; Angenete, E.; Bock, D.; Grybäck Gillheimer, E.; Haglind, E.; Nilsson, H. Effects of a Home-Based Exercise Program on the Insulin-Like Growth Factor Axis in Patients Operated for Colorectal Cancer in Sweden: Results from the Randomised Controlled Trial PHYSSURG-C. Growth Horm. IGF Res. 2020, 51, 27–33. [Google Scholar] [CrossRef]
- Minnella, E.; Ferreira, V.; Awasthi, R.; Charlebois, P.; Stein, B.; Liberman, A.; Scheede-Bergdahl, C.; Morais, J.; Carli, F. Effect of Two Different Pre-Operative Exercise Training Regimens before Colorectal Surgery on Functional Capacity: A Randomised Controlled Trial. Eur. J. Anaesthesiol. 2020, 37, 969–978. [Google Scholar] [CrossRef]
- Furyk, C.; Senthuran, S.; Nye, D.; Ho, Y.H.; Leicht, A.S. Prehabilitation for Frail Patients Undergoing Colorectal Surgery: Lessons Learnt from a Randomised Feasibility Study. Front. Rehabil. Sci. 2021, 2, 650835. [Google Scholar] [CrossRef]
- Peng, L.; Wang, W.; Chen, J.; Jin, J.; Min, S.; Qin, P. Implementation of the Pre-Operative Rehabilitation Recovery Protocol and its Effect on the Quality of Recovery After Colorectal Surgeries. Chin. Med. J. 2021, 134, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Atoui, S.; Carli, F.; Bernard, P.; Lee, L.; Stein, B.; Charlebois, P.; Liberman, A.S. Does a Multimodal Prehabilitation Program Improve Sleep Quality and Duration in Patients Undergoing Colorectal Resection for Cancer? Pilot Randomized Control Trial. J. Behav. Med. 2024, 47, 43–61. [Google Scholar] [CrossRef]
- Waller, E.; Sutton, P.; Rahman, S.; Allen, J.; Saxton, J.; Aziz, O. Prehabilitation with Wearables Versus Standard of Care before Major Abdominal Cancer Surgery: A Randomised Controlled Pilot Study (Trial Registration: NCT04047524). Surg. Endosc. 2022, 36, 1008–1017. [Google Scholar] [CrossRef]
- Kim, D.J.; Mayo, N.E.; Carli, F.; Montgomery, D.L.; Zavorsky, G.S. Responsive Measures to Prehabilitation in Patients Undergoing Bowel Resection Surgery. Tohoku J. Exp. Med. 2009, 217, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Li, C.; Lee, L.; Awasthi, R.; Augustin, B.; Gamsa, A.; Liberman, A.; Stein, B.; Charlebois, P.; Feldman, L.; et al. Prehabilitation Versus Rehabilitation: A Randomized Control Trial in Patients Undergoing Colorectal Resection for Cancer. Anesthesiology 2014, 121, 937–947. [Google Scholar] [CrossRef]
- MacFie, J.; Woodcock, N.P.; Palmer, M.D.; Walker, A.; Townsend, S.; Mitchell, C.J. Oral Dietary Supplements in Pre- and Postoperative Surgical Patients: A Prospective and Randomized Clinical Trial. Nutrition 2000, 16, 723–728. [Google Scholar] [CrossRef]
- Hernon, J.; Saxton, J.; Jones, M.; Howard, G.; Swart, A.M.; Clark, A.; Stirling, S.; Turner, D.; Murdoch, J.; Nortje, J.; et al. SupPoRtive Exercise Programmes for Accelerating REcovery After Major ABdominal Cancer Surgery Trial (PREPARE-ABC): Pilot Phase of a Multicentre Randomised Controlled Trial. Color. Dis. 2021, 23, 3008–3022. [Google Scholar]
- Morielli, A.R.; Usmani, N.; Boulé, N.G.; Severin, D.; Tankel, K.; Joseph, K.; Nijjar, T.; Fairchild, A.; Courneya, K.S. Feasibility, Safety, and Preliminary Efficacy of Exercise during and After Neoadjuvant Rectal Cancer Treatment: A Phase II Randomized Controlled Trial. Clin. Color. Cancer 2021, 20, 216–226. [Google Scholar] [CrossRef]
- Sorensen, L.S.; Rasmussen, H.H.; Aardestrup, I.V.; Thorlacius-Ussing, O.; Lindorff-Larsen, K.; Schmidt, E.B.; Calder, P.C. Rapid Incorporation of Ω-3 Fatty Acids into Colonic Tissue After Oral Supplementation in Patients with Colorectal Cancer. JPEN J. Parenter. Enter. Nutr. 2014, 38, 617–624. [Google Scholar] [CrossRef]
- Loughney, L.; West, M.A.; Moyses, H.; Bates, A.; Kemp, G.J.; Hawkins, L.; Varkonyi-Sepp, J.; Burke, S.; Barben, C.P.; Calverley, P.M.; et al. The Effects of Neoadjuvant Chemoradiotherapy and an in-Hospital Exercise Training Programme on Physical Fitness and Quality of Life in Locally Advanced Rectal Cancer Patients: A Randomised Controlled Trial (the EMPOWER Trial). Perioper. Med. 2021, 10, 23. [Google Scholar] [CrossRef]
- Moya, P.; Soriano-Irigaray, L.; Ramirez, J.M.; Garcea, A.; Blasco, O.; Blanco, F.J.; Brugiotti, C.; Miranda, E.; Arroyo, A. Perioperative Standard Oral Nutrition Supplements Versus Immunonutrition in Patients Undergoing Colorectal Resection in an Enhanced Recovery (ERAS) Protocol: A Multicenter Randomized Clinical Trial (SONVI Study). Medicine 2016, 95, e3704. [Google Scholar] [CrossRef] [PubMed]
- Rampam, S.; Sadiq, H.; Patel, J.; Meyer, D.; Uy, K.; Yates, J.; Schanzer, A.; Movahedi, B.; Lindberg, J.; Crawford, S.; et al. Supervised Preoperative Walking on Increasing Early Postoperative Stamina and Mobility in Older Adults with Frailty Traits: A Pilot and Feasibility Study. Health Sci. Rep. 2022, 5, e738. [Google Scholar] [CrossRef] [PubMed]
- McIsaac, D.I.; Hladkowicz, E.; Bryson, G.L.; Forster, A.J.; Gagne, S.; Huang, A.; Lalu, M.; Lavallée, L.T.; Moloo, H.; Nantel, J.; et al. Home-Based Prehabilitation with Exercise to Improve Postoperative Recovery for Older Adults with Frailty having Cancer Surgery: The PREHAB Randomised Clinical Trial. Br. J. Anaesth. 2022, 129, 41–48. [Google Scholar] [CrossRef]
- Burden, S.T.; Hill, J.; Shaffer, J.L.; Campbell, M.; Todd, C. An Unblinded Randomised Controlled Trial of Preoperative Oral Supplements in Colorectal Cancer Patients. J. Hum. Nutr. Diet. 2011, 24, 441–448. [Google Scholar] [CrossRef]
- Burden, S.T.; Gibson, D.J.; Lal, S.; Hill, J.; Pilling, M.; Soop, M.; Ramesh, A.; Todd, C. Pre-operative Oral Nutritional Supplementation with Dietary Advice Versus Dietary Advice Alone in Weight-losing Patients with Colorectal Cancer: Single-blind Randomized Controlled Trial. J. Cachexia Sarcopenia Muscle 2017, 8, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Moug, S.J.; Mutrie, N.; Barry, S.J.E.; Mackay, G.; Steele, R.J.C.; Boachie, C.; Buchan, C.; Anderson, A.S. Prehabilitation is Feasible in Patients with Rectal Cancer Undergoing Neoadjuvant Chemoradiotherapy and may Minimize Physical Deterioration: Results from the REx Trial. Color. Dis. 2019, 21, 548–562. [Google Scholar] [CrossRef]
- Onerup, A.; Andersson, J.; Angenete, E.; Bock, D.; Börjesson, M.; Ehrencrona, C.; Fagevik Olsén, M.; Larsson, P.; de la Croix, H.; Wedin, A.; et al. Effect of Short-Term Homebased Pre- and Postoperative Exercise on Recovery After Colorectal Cancer Surgery (PHYSSURG-C): A Randomized Clinical Trial. Ann. Surg. 2022, 275, 448–455. [Google Scholar] [CrossRef]
- Onerup, A.; Li, Y.; Afshari, K.; Angenete, E.; Croix, H.; Ehrencrona, C.; Wedin, A.; Haglind, E. Long-term Results of a Short-term Home-based Pre- and Postoperative Exercise Intervention on Physical Recovery After Colorectal Cancer Surgery (PHYSSURG-C): A Randomized Clinical Trial. Color. Dis. 2024, 26, 545–553. [Google Scholar] [CrossRef]
- Pesce, A.; Fabbri, N.; Colombari, S.; Uccellatori, L.; Grazzi, G.; Lordi, R.; Anania, G.; Feo, C.V. A Randomized Controlled Clinical Trial on Multimodal Prehabilitation in Colorectal Cancer Patients to Improve Functional Capacity: Preliminary Results. Surg. Endosc. 2024, 38, 7440–7450. [Google Scholar] [CrossRef]
- Serrano, P.E.; Parpia, S.; Simunovic, M.; Duceppe, E.; Pinto-Sanchez, M.I.; Bhandari, M.; Levine, M. Perioperative Optimization with Nutritional Supplements in Patients Undergoing Gastrointestinal Surgery for Cancer: A Randomized, Placebo-Controlled Feasibility Clinical Trial. Surgery 2022, 172, 670–676. [Google Scholar] [CrossRef]
- Yang, F.; Yuan, Y.; Liu, W.; Tang, C.; He, F.; Chen, D.; Xiong, J.; Huang, G.; Qian, K. Effect of Prehabilitation Exercises on Postoperative Frailty in Patients Undergoing Laparoscopic Colorectal Cancer Surgery. Front. Oncol. 2024, 14, 1411353. [Google Scholar] [CrossRef] [PubMed]
- Boereboom, C.L.; Blackwell, J.E.M.; Williams, J.P.; Phillips, B.E.; Lund, J.N. Short-term Pre-operative High-intensity Interval Training does Not Improve Fitness of Colorectal Cancer Patients. Scand. J. Med. Sci. Sports 2019, 29, 1383–1391. [Google Scholar] [CrossRef]
- Bojesen, R.D.; Jørgensen, L.B.; Grube, C.; Skou, S.T.; Johansen, C.; Dalton, S.O.; Gögenur, I. Fit for Surgery—Feasibility of Short-Course Multimodal Individualized Prehabilitation in High-Risk Frail Colon Cancer Patients Prior to Surgery. Pilot Feasibility Stud. 2022, 8, 11. [Google Scholar] [CrossRef]
- Boyle, H.; Fullbrook, A.; Wills, A.; Veal, I.; Peat, N.; Al-Noor, Z.; Bradshaw, R.; Raga, A.; Hegarty, A.; Hainsworth, A.; et al. Multimodal Prehabilitation Service for Patients with Colorectal Cancer: The Challenges of Implementation. BMJ Open Qual. 2023, 12, e002064. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Burke, S.; Grocott, M.P.W.; West, M.A.; Jack, S. The Effects of Exercise on Pain, Fatigue, Insomnia, and Health Perceptions in Patients with Operable Advanced Stage Rectal Cancer Prior to Surgery: A Pilot Trial. BMC Cancer 2017, 17, 153. [Google Scholar] [CrossRef] [PubMed]
- Bruns, E.R.J.; Argillander, T.E.; Schuijt, H.J.; van Duijvendijk, P.; van der Zaag, E.S.; Wassenaar, E.B.; Gerhards, M.F.; Consten, E.C.; Buskens, C.J.; van Munster, B.C.; et al. Fit4SurgeryTV at-Home Prehabilitation for Frail Older Patients Planned for Colorectal Cancer Surgery: A Pilot Study. Am. J. Phys. Med. Rehabil. 2019, 98, 399–406. [Google Scholar] [CrossRef]
- de Klerk, M.; van Dalen, D.H.; Nahar-van Venrooij, L.M.W.; Meijerink, W.J.H.J.; Verdaasdonk, E.G.G. A Multimodal Prehabilitation Program in High-Risk Patients Undergoing Elective Resection for Colorectal Cancer: A Retrospective Cohort Study. Eur. J. Surg. Oncol. 2021, 47, 2849–2856. [Google Scholar] [CrossRef]
- Englesbe, M.J.; Grenda, D.R.; Sullivan, J.A.; Derstine, B.A.; Kenney, B.N.; Sheetz, K.H.; Palazzolo, W.C.; Wang, N.C.; Goulson, R.L.; Lee, J.S.; et al. The Michigan Surgical Home and Optimization Program is a Scalable Model to Improve Care and Reduce Costs. Surgery 2017, 161, 1659–1666. [Google Scholar] [CrossRef]
- Estrada, D.M.L.; de Queiroz, F.L.; Guerra, L.I.; França-Neto, P.R.; Lacerda-Filho, A.; de Miranda Silvestre, S.C.; Coelho, J.M. Comparative Study using Propensity Score Matching Analysis in Patients Undergoing Surgery for Colorectal Cancer with or without Multimodal Prehabilitation. Int. J. Color. Dis. 2023, 38, 256. [Google Scholar] [CrossRef]
- Franssen, R.F.W.; Bongers, B.C.; Vogelaar, F.J.; Janssen-Heijnen, M.L.G. Feasibility of a Tele-Prehabilitation Program in High-Risk Patients with Colon or Rectal Cancer Undergoing Elective Surgery: A Feasibility Study. Perioper. Med. 2022, 11, 1–28. [Google Scholar] [CrossRef]
- Gonella, F.; Massucco, P.; Perotti, S.; Gianolio, S.; Vassallo, D.; Monasterolo, S.; Laezza, A.; De Zolt Ponte, B.; Ricotti, A.; Ferrero, A. Prehab, ERAS, Rehab: A Patient Care Continuum Around Colo-Rectal Surgery: Prehabilitation Combined with ERAS and Rehabilitation to Reduce Morbidity and Hospital Stay. Eur. J. Surg. Oncol. 2024, 50, 108688. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Kogure, E.; Kubo, A.; Kakuda, W. Does Pre-Operative Physical Rehabilitation Improve the Functional Outcomes of Patients Undergoing Gastrointestinal Cancer Surgery? J. Phys. Ther. Sci. 2021, 33, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Boyle, S.; Lai, W.; Barve, K.; Scanlon, K.; Shakeshaft, A.J.; Cox, M.R. Prehabilitation and Education in Major Abdominal and Thoracic Surgery Reduces Length of Stay and Ventilation Days. Physiother. Pract. Res. 2022, 43, 149–156. [Google Scholar] [CrossRef]
- Heil, T.C.; Verdaasdonk, E.G.G.; Maas, H.A.A.M.; van Munster, B.C.; Rikkert, M.G.M.O.; de Wilt, J.H.W.; Melis, R.J.F. Improved Postoperative Outcomes After Prehabilitation for Colorectal Cancer Surgery in Older Patients: An Emulated Target Trial. Ann. Surg. Oncol. 2023, 30, 244–254. [Google Scholar] [CrossRef]
- Heldens, A.F.J.M.; Bongers, B.C.; de Vos-Geelen, J.; van Meeteren, N.L.U.; Lenssen, A.F. Feasibility and Preliminary Effectiveness of a Physical Exercise Training Program during Neoadjuvant Chemoradiotherapy in Individual Patients with Rectal Cancer Prior to Major Elective Surgery. Eur. J. Surg. Oncol. 2016, 42, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.H.; Ismail, H.; Murnane, A.; Kim, P.; Riedel, B. Structured Exercise Program Prior to Major Cancer Surgery Improves Cardiopulmonary Fitness: A Retrospective Cohort Study. Support. Care Cancer 2016, 24, 2277–2285. [Google Scholar] [CrossRef]
- Ip, N.; Zhang, K.; Karimuddin, A.A.; Brown, C.J.; Campbell, K.L.; Puyat, J.H.; Sutherland, J.M.; Conklin, A.I. Preparing for Colorectal Surgery: A Feasibility Study of a Novel Web-based Multimodal Prehabilitation Programme in Western Canada. Color. Dis. 2024, 26, 534–544. [Google Scholar] [CrossRef]
- Janssen, T.L.; Steyerberg, E.W.; Lepper, C.C.H.A.v.H.-D.; Seerden, T.C.J.; de Lange, D.C.; Wijsman, J.H.; Ho, G.H.; Gobardhan, P.D.; van der Laan, L. Long-Term Outcomes of Major Abdominal Surgery and Postoperative Delirium After Multimodal Prehabilitation of Older Patients. Surg. Today 2020, 50, 1461–1470. [Google Scholar] [CrossRef]
- Karlsson, E.; Farahnak, P.; Franzén, E.; Nygren-Bonnier, M.; Dronkers, J.; van Meeteren, N.; Rydwik, E. Feasibility of Preoperative Supervised Home-Based Exercise in Older Adults Undergoing Colorectal Cancer Surgery—A Randomized Controlled Design. PLoS ONE 2019, 14, e0219158. [Google Scholar] [CrossRef]
- Koh, F.H.; Loh, C.H.; Tan, W.J.; Ho, L.M.L.; Yen, D.; Chua, J.M.W.; Kok, S.S.X.; Sivarajah, S.S.; Chew, M.; Foo, F. Structured Presurgery Prehabilitation for Aged Patients Undergoing Elective Surgery significantly Improves Surgical Outcomes and Reduces Cost: A Nonrandomized Sequential Comparative Prospective Cohort Study. Nutr. Clin. Pract. 2022, 37, 645–653. [Google Scholar] [CrossRef]
- Kwok, K.M.; Tay, S.S. Outcomes of a Multi-Modal Hospital-Associated Home-Based Cancer Prehabilitation Program. Ann. Rehabil. Med. 2023, 47, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Lafaro, K.J.; Raz, D.J.; Kim, J.Y.; Hite, S.; Ruel, N.; Varatkar, G.; Erhunmwunsee, L.; Melstrom, L.; Lee, B.; Singh, G.; et al. Pilot Study of a Telehealth Perioperative Physical Activity Intervention for Older Adults with Cancer and their Caregivers. Support. Care Cancer 2020, 28, 3867–3876. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Shelton, E.; Bidwell, S.; Shankar, K.; Ando, K.; Gaudilliere, B.; Shelton, A.; Kin, C. Association of Prehabilitation with Postoperative Opioid use in Colorectal Surgery: An Observational Cohort Study. J. Surg. Res. 2022, 273, 226–232. [Google Scholar] [CrossRef]
- Li, C.; Carli, F.; Lee, L.; Charlebois, P.; Stein, B.; Liberman, A.S.; Kaneva, P.; Augustin, B.; Wongyingsinn, M.; Gamsa, A.; et al. Impact of a Trimodal Prehabilitation Program on Functional Recovery After Colorectal Cancer Surgery: A Pilot Study. Surg. Endosc. 2013, 27, 1072–1082. [Google Scholar] [CrossRef]
- Lorca, L.A.; Ribeiro, I.L.; Pizarro, M.; Martínez, M.; Vivallos, J. Functional Results and Feasibility of a Teleprehabilitation Program in Patients Who are Candidates for Elective Colorectal Cancer Surgery during the COVID-19 Pandemic. Asia-Pac. J. Clin. Oncol. 2024, 20, 251–258. [Google Scholar] [CrossRef]
- Loughney, L.; Cahill, R.; O’Malley, K.; McCaffrey, N.; Furlong, B. Compliance, Adherence and Effectiveness of a Community-Based Pre-Operative Exercise Programme: A Pilot Study. Perioper. Med. 2019, 8, 17. [Google Scholar] [CrossRef]
- Maňásek, V.; Bezděk, K.; Foltys, A.; Klos, K.; Smitka, J.; Smehlik, D. The Impact of High Protein Nutritional Support on Clinical Outcomes and Treatment Costs of Patients with Colorectal Cancer. Klin. Onkol. 2016, 29, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Morielli, A.R.; Usmani, N.; Boulé, N.G.; Tankel, K.; Severin, D.; Nijjar, T.; Joseph, K.; Courneya, K.S. A Phase I Study Examining the Feasibility and Safety of an Aerobic Exercise Intervention in Patients with Rectal Cancer during and After Neoadjuvant Chemoradiotherapy. Oncol. Nurs. Forum 2016, 43, 352–362. [Google Scholar] [CrossRef]
- Mouch, C.A.; Kenney, B.C.; Lorch, S.; Montgomery, J.R.; Gonzalez-Walker, M.; Bishop, K.; Palazzolo, W.C.; Sullivan, J.A.; Wang, S.C.; Englesbe, M.J. Statewide Prehabilitation Program and Episode Payment in Medicare Beneficiaries. J. Am. Coll. Surg. 2020, 230, 306–313.e6. [Google Scholar] [CrossRef]
- Rinninella, E.; Biondi, A.; Cintoni, M.; Raoul, P.; Scialanga, F.; Persichetti, E.; Pulcini, G.; Pezzuto, R.; Persiani, R.; D’Ugo, D.; et al. NutriCatt Protocol Improves Body Composition and Clinical Outcomes in Elderly Patients Undergoing Colorectal Surgery in ERAS Program: A Retrospective Cohort Study. Nutrients 2021, 13, 1781. [Google Scholar] [CrossRef]
- Shelton, E.; Barreto, N.B.; Bidwell, S.; Folk-Tolbert, M.; Shelton, A.; Trickey, A.W.; Kin, C.J. Engagement and Adherence with a Web-Based Prehabilitation Program for Patients Awaiting Abdominal Colorectal Surgery. J. Gastrointest. Surg. 2021, 25, 3198–3207. [Google Scholar] [CrossRef] [PubMed]
- Singh, F.; Newton, R.U.; Baker, M.K.; Spry, N.A.; Taaffe, D.R.; Galvão, D.A. Feasibility and Efficacy of Presurgical Exercise in Survivors of Rectal Cancer Scheduled to Receive Curative Resection. Clin. Color. Cancer 2017, 16, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Singh, F.; Galvão, D.A.; Newton, R.U.; Spry, N.A.; Baker, M.K.; Taaffe, D.R. Feasibility and Preliminary Efficacy of a 10-Week Resistance and Aerobic Exercise Intervention during Neoadjuvant Chemoradiation Treatment in Rectal Cancer Patients. Integr. Cancer Ther. 2018, 17, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Souwer, E.T.D.; Bastiaannet, E.; de Bruijn, S.; Breugom, A.J.; van den Bos, F.; Portielje, J.E.A.; Dekker, J.W.T. Comprehensive Multidisciplinary Care Program for Elderly Colorectal Cancer Patients: “From Prehabilitation to Independence”. Eur. J. Surg. Oncol. 2018, 44, 1894–1900. [Google Scholar] [CrossRef]
- Suen, M.; Liew, A.; Turner, J.D.; Khatri, S.; Lin, Y.; Raso, K.L.; Vardy, J.L. Short-term Multimodal Prehabilitation Improves Functional Capacity for Colorectal Cancer Patients Prior to Surgery. Asia-Pac. J. Clin. Oncol. 2022, 18, e103–e110. [Google Scholar] [CrossRef]
- Ten Cate, D.W.G.; Sabajo, C.R.; Molenaar, C.J.L.; Janssen, L.; Bongers, B.C.; Slooter, G.D. Multimodal Prehabilitation in Elective Oncological Colorectal Surgery Enhances Preoperative Physical Fitness: A Single Center Prospective Real-World Data Analysis. Acta Oncol. 2024, 63, 35–43. [Google Scholar] [CrossRef]
- Tew, G.A.; Bedford, R.; Carr, E.; Durrand, J.W.; Gray, J.; Hackett, R.; Lloyd, S.; Peacock, S.; Taylor, S.; Yates, D.; et al. Community-Based Prehabilitation before Elective Major Surgery: The PREP-WELL Quality Improvement Project. BMJ Open Qual. 2020, 9, e000898. [Google Scholar] [CrossRef]
- Valkenet, K.; Trappenburg, J.C.A.; Schippers, C.C.; Wanders, L.; Lemmens, L.; Backx, F.J.G.; van Hillegersberg, R. Feasibility of Exercise Training in Cancer Patients Scheduled for Elective Gastrointestinal Surgery. Dig. Surg. 2016, 33, 439–447. [Google Scholar] [CrossRef]
- van Rooijen, S.J.; Molenaar, C.J.L.; Schep, G.; van Lieshout, R.H.M.A.; Beijer, S.; Dubbers, R.; Rademakers, N.; Papen-Botterhuis, N.E.; van Kempen, S.; Carli, F.; et al. Making Patients Fit for Surgery: Introducing a Four Pillar Multimodal Prehabilitation Program in Colorectal Cancer. Am. J. Phys. Med. Rehabil. 2019, 98, 888–896. [Google Scholar] [CrossRef]
- van der Hulst, H.C.; Bastiaannet, E.; Portielje, J.E.A.; van der Bol, J.M.; Dekker, J.W.T. Can Physical Prehabilitation Prevent Complications After Colorectal Cancer Surgery in Frail Older Patients? Eur. J. Surg. Oncol. 2021, 47, 2830–2840. [Google Scholar] [CrossRef]
- van Exter, S.H.; Drager, L.D.; van Asseldonk, M.J.M.D.; Strijker, D.; van der Schoot, N.D.; van den Heuvel, B.; Verlaan, S.; van den Berg, M.G.A. Adherence to and Efficacy of the Nutritional Intervention in Multimodal Prehabilitation in Colorectal and Esophageal Cancer Patients. Nutrients 2023, 15, 2133. [Google Scholar] [CrossRef] [PubMed]
- Waterland, J.L.; Ismail, H.; Amin, B.; Granger, C.L.; Denehy, L.; Riedel, B. Patient Acceptance of Prehabilitation for Major Surgery: An Exploratory Survey. Support. Care Cancer 2021, 29, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Waterland, J.L.; Ismail, H.; Granger, C.L.; Patrick, C.; Denehy, L.; Riedel, B.; Beaumont, A.; Bruns, E.; Burbury, K.; Carty, D.; et al. Prehabilitation in High-Risk Patients Scheduled for Major Abdominal Cancer Surgery: A Feasibility Study. Perioper. Med. 2022, 11, 1–32. [Google Scholar] [CrossRef]
- Wong, H.M.K.; Qi, D.; Ma, B.H.M.; Hou, P.Y.; Kwong, C.K.W.; Lee, A. Multidisciplinary Prehabilitation to Improve Frailty and Functional Capacity in High-Risk Elective Surgical Patients: A Retrospective Pilot Study. Perioper. Med. 2024, 13, 6–10. [Google Scholar] [CrossRef]
- Wooten, S.V.; Fleming, R.Y.D.; Wolf, J.S.; Stray-Gundersen, S.; Bartholomew, J.B.; Mendoza, D.; Stanforth, P.R.; Stanforth, D.; Hernandez, L.M.; Tanaka, H. Prehabilitation Program Composed of Blood Flow Restriction Training and Sports Nutrition Improves Physical Functions in Abdominal Cancer Patients Awaiting Surgery. Eur. J. Surg. Oncol. 2021, 47, 2952–2958. [Google Scholar] [CrossRef]
- Wu, F.; Rotimi, O.; Laza-Cagigas, R.; Rampal, T. The Feasibility and Effects of a Telehealth-Delivered Home-Based Prehabilitation Program for Cancer Patients during the Pandemic. Curr. Oncol. 2021, 28, 2248–2259. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Homma, S.; Ichikawa, N.; Iijima, H.; Taketomi, A. Preoperative Elemental Diet before Laparoscopic Anterior Resection in Patients with Advanced Stenotic Rectal Cancer. J. Anus Rectum Colon 2021, 5, 395–404. [Google Scholar] [CrossRef]
- Karlsson, E.; Dahl, O.; Rydwik, E.; Nygren-Bonnier, M.; Bergenmar, M. Older Patients’ Attitudes Towards, and Perceptions of, Preoperative Physical Activity and Exercise Prior to Colorectal Cancer Surgery—A Gap between Awareness and Action. Support. Care Cancer 2020, 28, 3945–3953. [Google Scholar] [CrossRef]
- Gillis, C.; Gill, M.; Gramlich, L.; Culos-Reed, S.; Nelson, G.; Ljungqvist, O.; Carli, F.; Fenton, T. Patients’ Perspectives of Prehabilitation as an Extension of Enhanced Recovery After Surgery Protocols. Can. J. Surg. 2021, 64, E578–E587. [Google Scholar] [CrossRef]
- Wang, R.; Yao, C.; Hung, S.H.; Meyers, L.; Sutherland, J.M.; Karimuddin, A.; Campbell, K.L.; Conklin, A.I. Preparing for Colorectal Surgery: A Qualitative Study of Experiences and Preferences of Patients in Western Canada. BMC Health Serv. Res. 2022, 22, 730. [Google Scholar] [CrossRef]
- Sier, M.A.T.; Cox, M.P.P.J.; Tweed, T.T.T.T.; Servaas, N.; Greve, J.W.M.; Stoot, J.H.M.B. Participation and Compliance in a Multimodal Prehabilitation Program for Colorectal Cancer (PACE): A Qualitative Study. Patient Prefer. Adherence 2024, 18, 2709–2720. [Google Scholar] [CrossRef] [PubMed]
- Talbot, A.; Jebb, S.A.; Foster, C.; Realpe, A.X.; Wheatstone, P.; Buczacki, S.; Koutoukidis, D.A. Participants’ Perspectives of being Recruited into a Randomised Trial of a Weight Loss Intervention before Colorectal Cancer Surgery: A Qualitative Interview Study. BMC Cancer 2024, 24, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Humeidan, M.L.; Otey, A.; Zuleta-Alarcon, A.; Mavarez-Martinez, A.; Stoicea, N.; Bergese, S. Perioperative Cognitive Protection—Cognitive Exercise and Cognitive Reserve (the Neurobics Trial): A Single-Blind Randomized Trial. Clin. Ther. 2015, 37, 2641–2650. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Halliday, L.J.; Doganay, E.; Wynter-Blyth, V.; Osborn, H.; Buckley, J.; Moorthy, K. Adherence to Pre-Operative Exercise and the Response to Prehabilitation in Oesophageal Cancer Patients. J. Gastrointest. Surg. 2021, 25, 890–899. [Google Scholar] [CrossRef]
- Houghton, C.; Dowling, M.; Meskell, P.; Hunter, A.; Gardner, H.; Conway, A.; Treweek, S.; Sutcliffe, K.; Noyes, J.; Devane, D.; et al. Factors that Impact on Recruitment to Randomised Trials in Health Care: A Qualitative Evidence Synthesis. Cochrane Database Syst. Rev. 2020, 2020, MR000045. [Google Scholar] [CrossRef]
- Xu, S.; Yin, R.; Zhu, H.; Gong, Y.; Zhu, J.; Li, C.; Xu, Q. The Role of Exercise-Based Prehabilitation in Enhancing Surgical Outcomes for Patients with Digestive System Cancers: A Meta-Analysis. BMC Gastroenterol. 2025, 25, 26. [Google Scholar] [CrossRef]
- Lee, D.; Wang, A.; Augustin, B.; Buajitti, E.; Tahasildar, B.; Carli, F.; Gillis, C. Socioeconomic Status Influences Participation in Cancer Prehabilitation and Preparation for Surgical Recovery: A Pooled Retrospective Analysis using a Validated Area-Level Socioeconomic Status Metric. Eur. J. Surg. Oncol. 2023, 49, 512–520. [Google Scholar] [CrossRef]
- Cuijpers, A.C.M.; Linskens, F.G.; Bongers, B.C.; Stassen, L.P.S.; Lubbers, T.; van Meeteren, N.L.U. Quality and Clinical Generalizability of Feasibility Outcomes in Exercise Prehabilitation before Colorectal Cancer Surgery—A Systematic Review. Eur. J. Surg. Oncol. 2022, 48, 1483–1497. [Google Scholar] [CrossRef]
- Alsuwaylihi, A.; Skořepa, P.; Prado, C.M.; Gomez, D.; Lobo, D.N.; O’Connor, D. Exploring the Acceptability of and Adherence to Prehabilitation and Rehabilitation in Patients Undergoing Major Abdominal Surgery: A Systematic Review and Meta-Analysis. Clin. Nutr. ESPEN 2024, 63, 709–726. [Google Scholar] [CrossRef]
- World Health Organization. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- O’Doherty, A.F.; West, M.; Jack, S.; Grocott, M.P.W. Preoperative Aerobic Exercise Training in Elective Intra-Cavity Surgery: A Systematic Review. Br. J. Anaesth. 2013, 110, 679–689. [Google Scholar] [CrossRef]
- Luther, A.; Gabriel, J.; Watson, R.P.; Francis, N.K. The Impact of Total Body Prehabilitation on Post-Operative Outcomes After Major Abdominal Surgery: A Systematic Review. World J. Surg. 2018, 42, 2781–2791. [Google Scholar] [CrossRef] [PubMed]
- Lotzke, H.; Brisby, H.; Gutke, A.; Hägg, O.; Jakobsson, M.; Smeets, R.; Lundberg, M. A Person-Centered Prehabilitation Program Based on Cognitive-Behavioral Physical Therapy for Patients Scheduled for Lumbar Fusion Surgery: A Randomized Controlled Trial. Phys. Ther. 2019, 99, 1069–1088. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Z.; Zhang, Y.; Cui, Y.; Pei, L.; Huang, Y. The Protocol for the Prehabilitation for Thoracic Surgery Study: A Randomized Pragmatic Trial Comparing a Short Home-Based Multimodal Program to Aerobic Training in Patients Undergoing Video-Assisted Thoracoscopic Surgery Lobectomy. Curr. Control Trials Cardiovasc. Med. 2023, 24, 194. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, B.; Selvy, M.; Slim, K. The Concept of Prehabilitation: What the Surgeon Needs to Know? J. Visc. Surg. 2016, 153, 109–112. [Google Scholar] [CrossRef]
- Daun, J.T.; Twomey, R.; Dort, J.C.; Capozzi, L.C.; Crump, T.; Francis, G.J.; Matthews, T.W.; Chandarana, S.P.; Hart, R.D.; Schrag, C.; et al. A Qualitative Study of Patient and Healthcare Provider Perspectives on Building Multiphasic Exercise Prehabilitation into the Surgical Care Pathway for Head and Neck Cancer. Curr. Oncol. 2022, 29, 5942–5954. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).