Association of Nutritional Status and Possible Sarcopenia Among Formerly Older Homeless Adults in Supportive Housing, Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
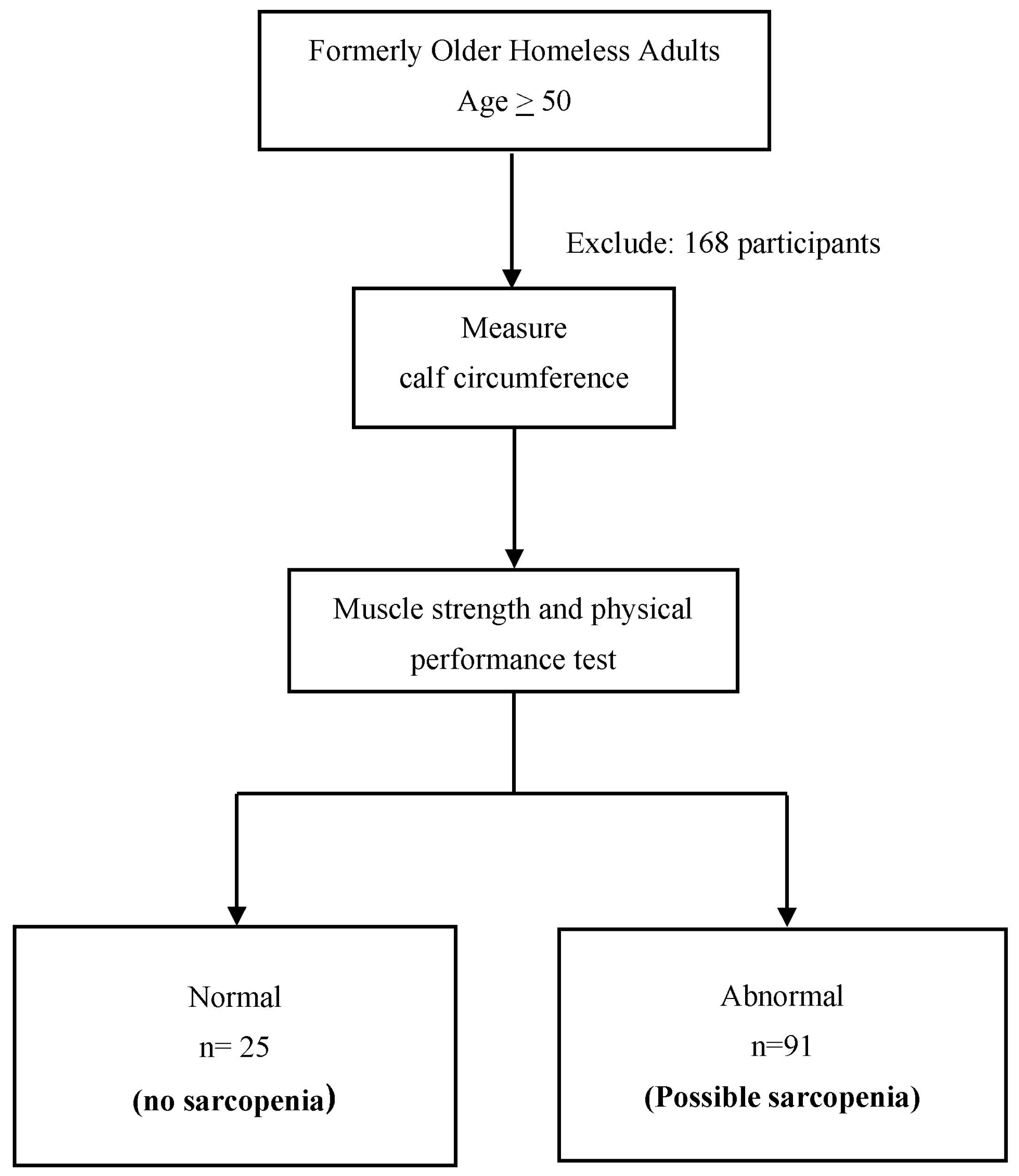
2.2. Participants
2.3. Data Collection
2.3.1. Data Collection Tools
- 1.
- Screening for possible Sarcopenia
- Assessment of Calf circumference
- Assessment of Muscle Strength
- Assessment of a 6 m gait speed test was used to assess physical performance
- 2.
- Nutritional Status
- 3.
- Sociodemographic and health-related information
2.3.2. Data Collection Process
2.3.3. Statistical Analysis
3. Results
4. Discussion
4.1. Prevalence of Possible Sarcopenia and Nutrition Status
4.2. Association Between Nutritional Status and Possible Sarcopenia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Gandham, A.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Kanis, J.A.; Karlsson, M.K.; von Schoultz, A.; Ohlsson, C.; Vandenput, L. Sarcopenia definitions and their association with injurious falls in older Swedish women from the Sahlgrenska University Hospital Prospective Evaluation of Risk of Bone Fractures (SUPERB) study. Osteoporos. Int. 2024, 35, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Gandham, A.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Harvey, N.C.; Kanis, J.A.; Karlsson, M.K.; von Schoultz, A.; Ohlsson, C.; Vandenput, L. Sarcopenia definitions and their association with fracture risk in older Swedish women. J. Bone Miner. Res. 2024, 39, 453–461. [Google Scholar] [CrossRef]
- Beaudart, C.; Tilquin, N.; Abramowicz, P.; Baptista, F.; Peng, D.J.; de Souza Orlandi, F.; Bruyère, O. Quality of life in sarcopenia measured with the SarQoL questionnaire: A meta-analysis of individual patient data. Maturitas 2024, 180, 107902. [Google Scholar] [CrossRef]
- Xu, J.; Wan, C.S.; Ktoris, K.; Reijnierse, E.M.; Maier, A.B. Sarcopenia is associated with mortality in adults: A systematic review and meta-analysis. Gerontology 2022, 68, 361–376. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Chen, Z.; Ho, M.; Chau, P.H. Prevalence, incidence, and associated factors of possible sarcopenia in community-dwelling Chinese older adults: A population-based longitudinal study. Front. Med. 2022, 8, 769708. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, Y.A. Prevalence and risk factors of possible sarcopenia in patients with subacute stroke. PLoS ONE 2023, 18, e0291452. [Google Scholar] [CrossRef] [PubMed]
- Fazel, S.; Geddes, J.R.; Kushel, M. The health of homeless people in high-income countries: Descriptive epidemiology, health consequences, and clinical and policy recommendations. Lancet 2014, 384, 1529–1540. [Google Scholar] [CrossRef]
- Petersen, C.L.; Brooks, J.M.; Titus, A.J.; Vasquez, E.; Batsis, J.A. Relationship between food insecurity and functional limitations in older adults from 2005–2014 NHANES. J. Nutr. Gerontol. Geriatr. 2019, 38, 231–246. [Google Scholar] [CrossRef]
- Bowen, E.A.; Lahey, J.; Rhoades, H.; Henwood, B.F. Food insecurity among formerly homeless individuals living in permanent supportive housing. Am. J. Public Health 2019, 109, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Loopstra, R.; Lambie-Mumford, H. Food banks: Understanding their role in the food insecure population in the UK. Proc. Nutr. Soc. 2023, 82, 253–263. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Tey, S.L.; Huynh, D.T.T.; Berde, Y.; Baggs, G.; How, C.H.; Low, Y.L.; Cheong, M.; Chow, W.L.; Tan, N.C.; Chew, S.T.H. Prevalence of low muscle mass and associated factors in community-dwelling older adults in Singapore. Sci. Rep. 2021, 11, 23071. [Google Scholar] [CrossRef]
- Xie, L.; Jiang, J.; Fu, H.; Zhang, W.; Yang, L.; Yang, M. Malnutrition in relation to muscle mass, muscle quality, and muscle strength in hospitalized older adults. J. Am. Med. Dir. Assoc. 2022, 23, 722–728. [Google Scholar] [CrossRef]
- Sanchez-Garcia, E.; Cruz-Jentoft, A.J.; Ravasco, P.; Suominen, M.; Pitkälä, P.K. Nutritional care in older adults: Are we doing everything? An expert opinion review. Curr. Med. Res. Opin. 2024, 40, 1563–1576. [Google Scholar] [CrossRef] [PubMed]
- Jitapunkul, S. (Ed.) Principles of geriatric medicine. In Analysis of Geriatric Medicine; Chulalongkorn University: Bangkok, Thailand, 1998; pp. 88–89. [Google Scholar]
- Jitapunkul, S.; Kamolratanakul, P.; Ebrahim, S. The meaning of activities of daily living in a Thai elderly population: Development of a new index. Age Ageing 1994, 23, 97–101. [Google Scholar] [CrossRef]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef]
- Veronese, N.; De Rui, M.; Toffanello, E.D.; De Ronch, I.; Perissinotto, E.; Bolzetta, F.; D’Avanzo, B.; Cardin, F.; Coin, A.; Manzato, E.; et al. Body mass index as a predictor of all-cause mortality in nursing home residents during a 5-year follow-up. J. Am. Med. Dir. Assoc. 2013, 14, 53–57. [Google Scholar] [CrossRef]
- Shuremu, M.; Belachew, T.; Hassen, K. Nutritional status and its associated factors among elderly people in Ilu Aba Bor Zone, Southwest Ethiopia: A community-based cross-sectional study. BMJ Open 2023, 13, e067787. [Google Scholar] [CrossRef]
- Sharma, S.; Yadav, D.K.; Karmacharya, I.; Pandey, R. Quality of life and nutritional status of the geriatric population of the south-central part of Nepal. J. Nutr. Metab. 2021, 2021, 6621278. [Google Scholar] [CrossRef] [PubMed]
- Whaikid, P.; Aurmaor, T.; Suvankereekhun, K. Factors influencing low muscle strength among community-dwelling older adults with non-communicable diseases. J. Thail. Nurs. Midwifery Counc. 2024, 39, 191–204. [Google Scholar] [CrossRef]
- Iida, H.; Seki, T.; Sakai, Y.; Watanabe, T.; Wakao, N.; Matsui, H.; Imagama, S. Low muscle mass affect hip fracture treatment outcomes in older individuals: A single-institution case-control study. BMC Musculoskelet. Disord. 2021, 22, 259. [Google Scholar] [CrossRef]
- Melsæter, K.N.; Tangen, G.G.; Skjellegrind, H.K.; Bergland, A.; Steindal, S.A.; Aas, B. Physical performance in older age by sex and educational level: The HUNT Study. BMC Geriatr. 2022, 22, 821. [Google Scholar] [CrossRef] [PubMed]
- Tangen, G.G.; Robinson, H.S. Measuring physical performance in highly active older adults: Associations with age and gender. Aging Clin. Exp. Res. 2020, 32, 229–237. [Google Scholar] [CrossRef]
- Champaiboon, J.; Petchlorlian, A.; Manasvanich, B.A.; Ubonsutvanich, N.; Jitpugdee, W.; Kittiskulnam, P.; Wongwatthananart, S.; Menorngwa, Y.; Pornsalnuwat, S.; Praditpornsilpa, K. Calf circumference as a screening tool for low skeletal muscle mass: Cut-off values in independent Thai older adults. BMC Geriatr. 2023, 23, 826. [Google Scholar] [CrossRef]
- Rubenstein, L.Z. Falls in older people: Epidemiology, risk factors and strategies for prevention. Age Ageing 2006, 35 (Suppl. S2), ii37–ii41. [Google Scholar] [CrossRef] [PubMed]
- Kiss, C.M.; Bertschi, D.; Beerli, N.; Berres, M.; Kressig, R.W.; Fischer, A.M. Calf circumference as a surrogate indicator for detecting low muscle mass in hospitalized geriatric patients. Aging Clin. Exp. Res. 2024, 36, 25. [Google Scholar] [CrossRef]
- Ryu, M.; Jo, J.; Lee, Y.; Chung, Y.S.; Kim, K.M.; Baek, W.C. Association of physical activity with sarcopenia and sarcopenic obesity in community-dwelling older adults: The Fourth Korea National Health and Nutrition Examination Survey. Age Ageing 2013, 42, 734–740. [Google Scholar] [CrossRef]
- Lee, G.; Choi, S.; Park, S.M. Association of waist circumference with muscle and fat mass in adults with a normal body mass index. Nutr. Res. Pract. 2021, 15, 604–612. [Google Scholar] [CrossRef]
- de Carvalho, D.H.T.; Scholes, S.; Santos, J.L.F.; de Oliveira, C.; Alexandre, T.D.S. Does abdominal obesity accelerate muscle strength decline in older adults? Evidence from the English Longitudinal Study of Ageing. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cheng, K.Y.; Tong, X.; Cheung, W.H.; Chow, S.K.; Law, S.W.; Wong, R.M.Y. The role of obesity in sarcopenia and the optimal body composition to prevent against sarcopenia and obesity. Front. Endocrinol. 2023, 14, 1077255. [Google Scholar] [CrossRef]
- Crovetto Mattassi, M.; Henríquez Mella, C.; Pérez Bocaz, L. Association between sarcopenia and nutritional status in Chilean older people aged 65 years and older. Nutrients 2022, 14, 5228. [Google Scholar] [CrossRef]
- Tostes, N.F.; da Cunha Antunes Saraiva, D.; Martucci, R.B. Association between nutritional status and muscle strength in pediatric cancer patients. Clin. Nutr. ESPEN 2021, 43, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.A.; Meskers, C.G.M.; Trappenburg, M.C.; Verlaan, S.; Reijnierse, E.M.; Whittaker, A.C.; Maier, A.B. Malnutrition is associated with dynamic physical performance. Aging Clin. Exp. Res. 2020, 32, 1085–1092. [Google Scholar] [CrossRef]
- Hai, S.; Wang, H.; Liu, Y.; Liu, P.; Yang, Y.; Zhu, X.; Cao, L.; Liu, Y.; Dong, B. Association between sarcopenia and nutritional status and physical activity among community-dwelling Chinese adults aged 60 years and older. Geriatr. Gerontol. Int. 2017, 17, 1959–1966. [Google Scholar] [CrossRef]
- Ganapathy, A.; Nieves, J.W. Nutrition and sarcopenia—What do we know? Nutrients 2020, 12, 1755. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Suzuki, H.; Mimura, M.; Inoue, Y.; Sugita, M.; Suzuki, K.; Kobayashi, H. Leucine-enriched essential amino acids attenuate muscle soreness and improve muscle protein synthesis after eccentric contractions in rats. Amino Acids 2015, 47, 1193–1201. [Google Scholar] [CrossRef]
- Dickinson, J.M.; Volpi, E.; Rasmussen, B.B. Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exerc. Sport Sci. Rev. 2013, 41, 216–223. [Google Scholar] [CrossRef]
- Holmes, C.J.; Racette, S.B. The Utility of body composition assessment in nutrition and clinical practice: An overview of current methodology. Nutrients 2021, 13, 2493. [Google Scholar] [CrossRef]
- Schneider, D.A.; Trence, D.L. Possible Role of Nutrition in Prevention of Sarcopenia and Falls. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2019, 25, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Brau, F.; Galluzzo, V.; Santagada, D.A.; Loreti, C.; Biscotti, L.; Laudisio, A.; Zuccalà, G.; Bernabei, R. Falls among older adults: Screening, identification, rehabilitation, and management. Appl. Sci. 2022, 12, 7934. [Google Scholar] [CrossRef]
- Zuo, X.; Li, X.; Tang, K.; Zhao, R.; Wu, M.; Wang, Y.; Li, T. Sarcopenia and cardiovascular diseases: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 1183–1198. [Google Scholar] [CrossRef]
- Chin, S.O.; Rhee, S.Y.; Chon, S.; Hwang, Y.C.; Jeong, I.K.; Oh, S.; Ahn, K.J.; Chung, H.Y.; Woo, J.T.; Kim, S.W.; et al. Sarcopenia is independently associated with cardiovascular disease in older Korean adults: The Korea National Health and Nutrition Examination Survey (KNHANES) from 2009. PLoS ONE 2013, 8, e60119. [Google Scholar] [CrossRef]
| Sociodemographic Characteristics | Total (n) | Non-Sarcopenia | Possible Sarcopenia | p-Value | |
|---|---|---|---|---|---|
| All participants, n (%) | 116 | 25 (21.6%) | 91 (78.4%) | ||
| Age (years), mean ± SD | 116 | 54.76 ± 5.59 | 60.34 ± 7.90 | 0.001 | |
| Age, n (%) | |||||
| 50–59 | 70 | 19 (76.0%) | 51 (56.0%) | ||
| ≥60 | 46 | 6 (24.0%) | 40 (44.0%) | 0.071 | |
| Gender, n (%) | |||||
| Female | 39 | 10 (25.6%) | 29 (74.4%) | 0.446 | |
| Male | 77 | 15 (19.5%) | 62 (80.5%) | ||
| Body mass index (kg/m2), mean ± SD | 116 | 26.44 ± 4.84 | 19.73 ± 2.69 | <0.001 | |
| Body mass index, n (%) | |||||
| <20 kg/m2 | 56 | 2 (3.6%) | 54 (96.4%) | <0.001 | |
| ≥20 kg/m2 | 60 | 23 (38.3%) | 37 (61.7%) | ||
| MNA-SF, n (%) | |||||
| <12 | 60 | 5 (8.3%) | 55 (91.7%) | <0.001 | |
| ≥12 | 56 | 20 (35.7%) | 36 (64.3%) | ||
| Comorbidity, n (%) | |||||
| No | 15 | 2 (13.3%) | 13 (86.7%) | 0.407 | |
| Yes | 101 | 23 (22.8%) | 78 (77.2%) | ||
| Waist circumference (cm), mean ± SD | 116 | 91.76 ± 12.33 | 76.57 ± 8.67 | <0.001 | |
| Waist circumference, n (%) | |||||
| High | 33 | 16 (48.5%) | 17 (51.5%) | ||
| Normal | 83 | 9 (10.8%) | 74 (89.2%) | <0.001 | |
| Frailty, n (%) | |||||
| Low | 6 | 2 (33.3%) | 4 (66.7%) | ||
| Normal | 110 | 23 (20.9%) | 87 (79.1%) | 0.608 | |
| Sociodemographic Characteristics | Total (n) | Non-Sarcopenia | Possible Sarcopenia | p-Value | |
|---|---|---|---|---|---|
| Calf circumference (cm), | 116 | 35.88 ± 2.74 | 30.32 ± 2.00 | <0.001 | |
| mean ± SD | |||||
| Calf circumference, n (%) | |||||
| Low | 93 | 2 (2.2%) | 91 (97.8%) | ||
| Normal | 23 | 23 (100%) | - | <0.001 | |
| Handgrip strength (kg), | 116 | 22.30 ± 5.70 | 19.14 ± 7.48 | 0.027 | |
| mean ± SD | |||||
| Handgrip strength, n (%) | |||||
| Low | 90 | 15 (16.7%) | 75 (83.3%) | ||
| Normal | 26 | 10 (38.5%) | 16 (61.5%) | 0.017 | |
| Gait speed (m/s), mean ± SD | 116 | 0.92 ± 0.31 | 0.89 ± 0.29 | 0.485 | |
| Gait speed, n (%) | |||||
| Low | 43 | 9 (20.9%) | 34 (79.1%) | ||
| Normal | 73 | 16 (21.9%) | 57 (78.1%) | 0.901 | |
| Variables | Crude OR | 95% CI | p-Value | Adjusted OR | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|
| Age | 1.154 | 1.050–1.268 | 0.003 | 1.152 | 1.023–1.299 | 0.020 | |
| Gender | |||||||
| Female * | |||||||
| Male | 0.702 | 0.281–1.749 | 0.447 | ||||
| Body mass index | |||||||
| <20 kg/m2 | 16.784 | 3.729–75.535 | <0.001 | 5.315 | 1.000–28.266 | 0.050 | |
| ≥20 kg/m2 * | |||||||
| MNA-SF | |||||||
| Normal nutritional status * | |||||||
| At risk of malnutrition or malnourished | 6.111 | 2.104–17.750 | 0.001 | 4.757 | 1.170–19.346 | 0.029 | |
| Waist circumference | |||||||
| High | |||||||
| Normal * | 0.129 | 0.049–0.342 | <0.001 | 0.181 | 0.044–0.741 | 0.017 | |
| Frail | |||||||
| Low * | 0.529 | 0.091–3.069 | 0.478 | ||||
| Normal | |||||||
| Comorbidity | |||||||
| No * | |||||||
| Yes | 0.522 | 0.110–2.482 | 0.414 | ||||
| Variables | Nagelkerke R-Square | Adjusted OR | 95% CI | p-Value | |
|---|---|---|---|---|---|
| Possible Sarcopenia | |||||
| MNA < 12 | 0.338 | 3.429 | 1.093–10.763 | 0.035 | |
| Low BMI | 11.732 | 2.523–54.567 | 0.002 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whaikid, P.; Piaseu, N. Association of Nutritional Status and Possible Sarcopenia Among Formerly Older Homeless Adults in Supportive Housing, Thailand. Nutrients 2025, 17, 1776. https://doi.org/10.3390/nu17111776
Whaikid P, Piaseu N. Association of Nutritional Status and Possible Sarcopenia Among Formerly Older Homeless Adults in Supportive Housing, Thailand. Nutrients. 2025; 17(11):1776. https://doi.org/10.3390/nu17111776
Chicago/Turabian StyleWhaikid, Phatcharaphon, and Noppawan Piaseu. 2025. "Association of Nutritional Status and Possible Sarcopenia Among Formerly Older Homeless Adults in Supportive Housing, Thailand" Nutrients 17, no. 11: 1776. https://doi.org/10.3390/nu17111776
APA StyleWhaikid, P., & Piaseu, N. (2025). Association of Nutritional Status and Possible Sarcopenia Among Formerly Older Homeless Adults in Supportive Housing, Thailand. Nutrients, 17(11), 1776. https://doi.org/10.3390/nu17111776







