A Cross-Sectional Study on Protein Substitutes for Paediatric Phenylketonuria Diet: Time to Pay Attention
Abstract
1. Introduction
2. Materials and Methods
2.1. Protein Substitutes
2.2. Estimation of Micronutrient Daily Requirement
2.3. Micronutrient Content Calculation and Statistical Analysis
3. Results
3.1. Comparison of Micronutrient Intake by Age Range with RDA
3.2. Variability of Micronutrient Content Among Different PSs
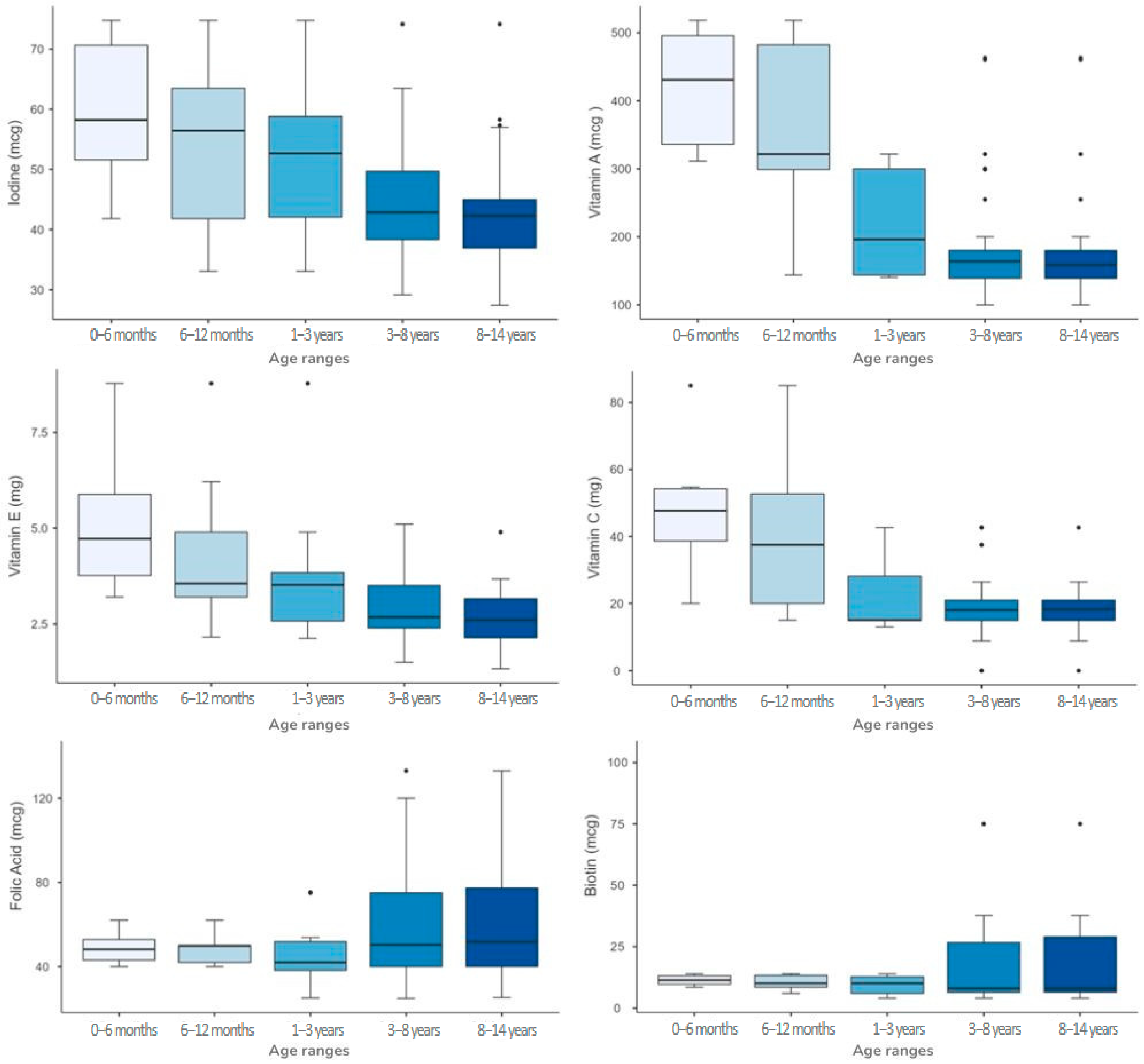
3.3. Variability of Single Micronutrient Content Among Different Age Ranges
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PS | Protein substitute |
| PHE | Phenylalanine |
| PKU | Phenylketonuria |
| RDA | Recommended dietary allowance |
| WHO | World Health Organization |
| IEM | Inborn error of metabolism |
| RNI | Reference nutrient intakes |
References
- Blau, N.; van Spronsen, F.J.; Levy, H.L. Phenylketonuria. Lancet 2010, 376, 1417–1427. [Google Scholar] [CrossRef] [PubMed]
- Boyer, S.W.; Barclay, L.J.; Burrage, L.C. Inherited Metabolic Disorders: Aspects of Chronic Nutrition Management. Nutr. Clin. Pract. 2015, 30, 502–510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ahring, K.; Bélanger-Quintana, A.; Dokoupil, K.; Gokmen Ozel, H.; Lammardo, A.M.; MacDonald, A.; Motzfeldt, K.; Nowacka, M.; Robert, M.; van Rijn, M. Dietary management practices in phenylketonuria across European centres. Clin. Nutr. 2009, 28, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MacDonald, A.; van Wegberg, A.M.J.; Ahring, K.; Beblo, S.; Bélanger-Quintana, A.; Burlina, A.; Campistol, J.; Coşkun, T.; Feillet, F.; Giżewska, M.; et al. PKU dietary handbook to accompany PKU guidelines. Orphanet J. Rare Dis. 2020, 15, 171, Erratum in Orphanet J Rare Dis. 2020, 15, 230. https://doi.org/10.1186/s13023-020-01486-6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ney, D.M. Nutritional Management of Phenylketonuria. Ann. Nestle Eng. 2010, 68, 58–69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Protein and Amino acid Requirements in Human Nutrition; Report of a Joint WHO/FAO/UNU Expert Consultation WHO Technical Report Series; WHO: Geneva, Switzerland, 2007; Volume 935.
- MacDonald, A.; Singh, R.H.; Rocha, J.C.; van Spronsen, F.J. Optimising amino acid absorption: Essential to improve nitrogen balance and metabolic control in phenylketonuria. Nutr. Res. Rev. 2019, 32, 70–78. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brestenský, M.; Nitrayová, S.; Patráš, P.; Nitray, J. Dietary Requirements for Proteins and Amino Acids in Human Nutrition. Curr. Nutr. Food Sci. 2019, 15, 638–645. [Google Scholar] [CrossRef]
- Richter, M.; Baerlocher, K.; Bauer, J.M.; Elmadfa, I.; Heseker, H.; Leschik-Bonnet, E.; Stangl, G.; Volkert, D.; Stehle, P.; on behalf of the German Nutrition Society (DGE). Revised Reference Values for the Intake of Protein. Ann. Nutr. Metab. 2019, 74, 242–250. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- MacDonald, A.; Chakrapani, A.; Hendriksz, C.; Daly, A.; Davies, P.; Asplin, D.; Hall, K.; Booth, I.W. Protein substitute dosage in PKU: How much do young patients need? Arch. Dis. Child. 2006, 91, 588–593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Crujeiras, V.; Aldámiz-Echevarría, L.; Dalmau, J.; Vitoria, I.; Andrade, F.; Roca, I.; Leis, R.; Fernandez-Marmiesse, A.; Couce, M.L. Vitamin and Mineral Status in Patients with Hyperphenylalaninemia. Mol. Genet. Metab. 2015, 115, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.; Rocha, J.C.; van Rijn, M.; Ahring, K.; Bélanger-Quintana, A.; MacDonald, A.; Dokoupil, K.; Gokmen Ozel, H.; Lammardo, A.M.; Goyens, P.; et al. Micronutrient status in phenylketonuria. Mol Genet Metab. 2013, 110, S6–S17. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, K.; Rodenburg, I.L.; van Ginkel, W.G.; Lubout, C.M.A.; Wolffenbuttel, B.H.R.; van der Klauw, M.M.; Heiner-Fokkema, M.R.; van Spronsen, F.J. Biomarkers of Micronutrients in Regular Follow-Up for Tyrosinemia Type 1 and Phenylketonuria Patients. Nutrients 2019, 11, 2011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stølen, L.H.; Lilje, R.; Jørgensen, J.V.; Bliksrud, Y.T.; Almaas, R. High dietary folic Acid and high plasma folate in children and adults with phenylketonuria. JIMD Rep. 2014, 13, 83–90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lammardo, A.M.; Robert, M.; Rocha, J.C.; van Rijn, M.; Ahring, K.; Bélanger-Quintana, A.; MacDonald, A.; Dokoupil, K.; Ozel, H.G.; Goyens, P.; et al. Main issues in micronutrient supplementation in phenylketonuria. Mol. Genet. Metab. 2013, 110, S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Tummolo, A.; Carella, R.; De Giovanni, D.; Paterno, G.; Simonetti, S.; Tolomeo, M.; Leone, P.; Barile, M. Micronutrient Deficiency in Inherited Metabolic Disorders Requiring Diet Regimen: A Brief Critical Review. Int. J. Mol. Sci. 2023, 24, 17024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiig, I.; Motzfeldt, K.; Løken, E.B.; Kase, B.F. Nutritional Consequences of Adhering to a Low Phenylalanine Diet for Late-Treated Adults with PKU: Low Phe Diet for Adults with PKU. JIMD Rep. 2013, 7, 109–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stroup, B.M.; Ney, D.M.; Murali, S.G.; Rohr, F.J.; Gleason, S.T.; van Calcar, S.C.; Levy, H.L. Metabolomic Insights into the Nutritional Status of Adults and Adolescents with Phenylketonuria Consuming a Low-Phenylalanine Diet in Combination with Amino Acid and Glycomacropeptide Medical Foods. J. Nutr. Metab. 2017, 2017, 6859820. [Google Scholar] [CrossRef]
- Barretto, J.R.; Silva, L.R.; Leite, M.E.; Boa-Sorte, N.; Pimentel, H.; Purificacao, A.C.; Carvalho, G.; Fontes, M.I.; Amorim, T. Poor zinc and selenium status in phenylketonuric children and adolescents in Brazil. Nutr. Res. 2008, 28, 208–211. [Google Scholar] [CrossRef]
- Prochazkova, D.; Jarkovsky, J.; Vinohradska, H.; Konecna, P.; Machacova, L.; Dolezel, Z. Controlled diet in phenylketonuria and hyperphenylalaninemia may cause serum selenium deficiency in adult patients: The Czech experience. Biol. Trace Elem. Res. 2013, 154, 178–184. [Google Scholar] [CrossRef]
- National Institutes of Health. Nutrients Reccomandations and Databases. Available online: https://ods.od.nih.gov/HealthInformation/nutrientrecommendations.aspx#dri (accessed on 24 November 2023).
- Gropper, S.S.; Acosta, P.B.; Clarke-Sheehan, N.; Wenz, E.; Cheng, M.; Koch, R. Trace element status of children with PKU and normal children. J. Am. Diet. Assoc. 1988, 88, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Schulz, B.; Bremer, H.J. Nutrient intake and food consumption of adolescents and young adults with phenylketonuria. Acta Paediatr. 1995, 84, 743–748. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A. Diet and compliance in phenylketonuria. Eur. J. Pediatr. 2000, 159, S136–S141. [Google Scholar] [CrossRef] [PubMed]
- Tosi, M.; Fiori, L.; Tagi, V.M.; Gambino, M.; Montanari, C.; Bosetti, A.; Zuccotti, G.; Verduci, E. Glycomacropeptide-Based Protein Substitutes for Children with Phenylketonuria in Italy: A Nutritional Comparison. Nutrients 2024, 16, 956. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Habibzadeh, F. Data Distribution: Normal or Abnormal? J. Korean Med. Sci. 2024, 39, e35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, Y.; Walmsley, R.P. Learning and understanding the Kruskal-Wallis one-way analysis-of-variance-by-ranks test for differences among three or more independent groups. Phys. Ther. 1997, 77, 1755–1762, Erratum in Phys. Ther. 1998, 78, 322. [Google Scholar] [CrossRef] [PubMed]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 10: Further nonparametric methods. Crit. Care. 2004, 8, 196–199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Vaz, M.A.B.; Pacheco, P.S.; Seidel, E.J.; Ansuj, A.P. Classification of the coefficient of variation to variables in beef cattle experiments. Ciência Rural 2017, 47, e20160946. [Google Scholar] [CrossRef]
- O’Neill, M.E.; Mathews, K.L. Levene tests of homogeneity of variance for general block and treatment designs. Biometrics 2002, 58, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.; Evans, S.; Pinto, A.; Ashmore, C.; MacDonald, A. Protein Substitutes in PKU; Their Historical Evolution. Nutrients 2021, 13, 484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zeisel, S.H.; da Costa, K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Derbyshire, E.; Maes, M. The Role of Choline in Neurodevelopmental Disorders-A Narrative Review Focusing on ASC, ADHD and Dyslexia. Nutrients 2023, 15, 2876. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conly, J.M.; Stein, K.; Worobetz, L.; Rutledge-Harding, S. The contribution of vitamin K2 (menaquinones) produced by the intestinal microflora to human nutritional requirements for vitamin K. Am. J. Gastroenterol. 1994, 89, 915–923. [Google Scholar] [PubMed]
- Shearer, M.J. Vitamin K in parenteral nutrition. Gastroenterology 2009, 137, S105–S118. [Google Scholar] [CrossRef] [PubMed]
- Bargellini, A.; Venturelli, F.; Casali, E.; Ferrari, A.; Marchesi, I.; Borella, P. Trace elements in starter infant formula: Dietary intake and safety assessment. Environ. Sci. Pollut. Res. Int. 2018, 25, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- East, P.L.; Reid, B.; Blanco, E.; Burrows, R.; Lozoff, B.; Gahagan, S. Iron supplementation given to nonanemic infants: Neurocognitive functioning at 16 years. Nutr. Neurosci. 2023, 26, 40–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lozoff, B.; Castillo, M.; Clark, K.M.; Smith, J.B. Iron-fortified vs low-iron infant formula: Developmental outcome at 10 years. Arch. Pediatr. Adolesc. Med. 2012, 166, 208–215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Björmsjö, M.; Hernell, O.; Lönnerdal, B.; Berglund, S.K. Reducing Iron Content in Infant Formula from 8 to 2 mg/L Does Not Increase the Risk of Iron Deficiency at 4 or 6 Months of Age: A Randomized Controlled Trial. Nutrients 2020, 13, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pike, V.; Zlotkin, S. Excess micronutrient intake: Defining toxic effects and upper limits in vulnerable populations. Ann. N. Y. Acad. Sci. 2019, 1446, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.J.; Pedersen, K.B.; Watt, J. Adverse Effects of Nutraceuticals and Dietary Supplements. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 583–601. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guidance for establishing and applying tolerable upper intake levels for vitamins and essential minerals EFSA. EFSA J. 2024, 22, e9052. [CrossRef]
- Commission Delegated Regulation (EU) 2016/128; Commission Delegated Regulation (EU) 2016/128-of 25 September 2015-Supplementing Regulation (EU) No 609/2013 of the European Parliament and of the Council as Regards the Specific Compositional and Information Requirements for Food for Special Medical Purposes. European Commission: Brussels, Belgium, 2015.
- Verduci, E.; Tosi, M.; Montanari, C.; Gambino, M.; Eletti, F.; Bosetti, A.; Di Costanzo, M.; Carbone, M.T.; Biasucci, G.; Fiori, L.; et al. Are Phe-Free Protein Substitutes Available in Italy for Infants with PKU All the Same? Nutrients 2023, 16, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evans, S.; Daly, A.; MacDonald, J.; Preece, M.A.; Santra, S.; Vijay, S.; Chakrapani, A.; MacDonald, A. The micronutrient status of patients with phenylketonuria on dietary treatment: An ongoing challenge. Ann. Nutr. Metab. 2014, 65, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Arslan, E.; Gokcay, G.F. Special low protein foods for phenylketonuria in Turkey: An examination of their nutritional composition compared to regular food. Nutr. Health 2024, 30, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Pena, M.J.; de Almeida, M.F.; van Dam, E.; Ahring, K.; Bélanger-Quintana, A.; Dokoupil, K.; Gokmen-Ozel, H.; Lammardo, A.M.; MacDonald, A.; Robert, M.; et al. Protein substitutes for phenylketonuria in Europe: Access and nutritional composition. Eur. J. Clin. Nutr. 2016, 70, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Ilgaz, F.; Marsaux, C.; Pinto, A.; Singh, R.; Rohde, C.; Karabulut, E.; Gökmen-Özel, H.; Kuhn, M.; MacDonald, A. Protein Substitute Requirements of Patients with Phenylketonuria on BH4 Treatment: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 1040. [Google Scholar] [CrossRef] [PubMed]
- Brantley, K.D.; Douglas, T.D.; Singh, R.H. One-year follow-up of B vitamin and Iron status in patients with phenylketonuria provided tetrahydrobiopterin (BH4). Orphanet J. Rare Dis. 2018, 13, 192. [Google Scholar] [CrossRef]
- Hennermann, J.B.; Roloff, S.; Gebauer, C.; Vetter, B.; von Arnim-Baas, A.; Mönch, E. Long-term treatment with tetrahydrobiopterin in phenylketonuria: Treatment strategies and prediction of long-term responders. Mol. Genet. Metab. 2012, 107, 294–301. [Google Scholar] [CrossRef] [PubMed]
- McWhorter, N.; Ndugga-Kabuye, M.K.; Puurunen, M.; Ernst, S.L. Complications of the Low Phenylalanine Diet for Patients with Phenylketonuria and the Benefits of Increased Natural Protein. Nutrients 2022, 14, 4960. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kose, E.; Arslan, N. Vitamin/mineral and micronutrient status in patients with classical phenylketonuria. Clin. Nutr. 2019, 38, 197–203. [Google Scholar] [CrossRef] [PubMed]


| Age Range | |||||
|---|---|---|---|---|---|
| 0–6 Months | 6–12 Months | 1–3 Years | 3–8 Years | 8–14 Years | |
| Total number of products | 7 | 10 | 14 | 55 | 50 |
| Powders | 6 | 9 | 13 | 32 | 27 |
| Liquids | 1 | 1 | 1 | 15 | 15 |
| Micro-tablets | 0 | 0 | 0 | 2 | 2 |
| Bars | 0 | 0 | 0 | 4 | 4 |
| Tablets | 0 | 0 | 0 | 1 | 1 |
| Puddings | 0 | 0 | 0 | 1 | 1 |
| Age Range | |||||
|---|---|---|---|---|---|
| Prescribability | 0–6 Months | 6–12 Months | 1–3 Years | 3–8 Years | 8–14 Years |
| 0–12 months | 4 | 4 | |||
| 0–3 years | 1 | 1 | 1 | ||
| from birth | 2 | 2 | 2 | 2 | 2 |
| 6 months–5 years | 1 | 1 | 1 | ||
| 6 months–10 years | 1 | 1 | 1 | 1 | |
| from 6 months | 1 | 1 | 1 | 1 | |
| 1–6 years | 2 | 2 | |||
| 1–8 years | 2 | 2 | |||
| 1–10 years | 2 | 2 | 2 | ||
| from 1 years | 2 | 2 | 2 | ||
| 3–6 years | 3 | ||||
| from 3 years | 31 | 31 | |||
| from 4 years | 4 | 4 | |||
| 7–14 years | 3 | 3 | |||
| from 7 years | 1 | 1 | |||
| 8–14 years | 1 | ||||
| from 8 years | 1 | ||||
| from 8 years and pregnancy | 1 | ||||
| Age Range | |||||
|---|---|---|---|---|---|
| 0–6 Months | 6–12 Months | 1–3 Years | 3–8 Years | 8–14 Years | |
| Infant formulas (from birth) | 7 | 7 | 3 | 2 | 2 |
| Second stage infant formulas (from 6 months) | 0 | 3 | 3 | 3 | 2 |
| Third stage formulas (from 3 years) | 0 | 0 | 0 | 34 | 31 |
| Other | 0 | 0 | 8 | 16 | 15 |
| Protein Requirement in PKU (g/day) = Protein Requirement UNU/FAO (g/day) × 140% | ||||||
|---|---|---|---|---|---|---|
| Age Range | Boys | Girls | Average of Total Protein Requirements in the 2 Genders | Average of Protein Intake from PSs in the 2 Genders (66%) | ||
| 0–6 months | 14.28 | 16.24 | 13.16 | 15.12 | 14.70 | 9.70 |
| 6–12 months | 14.28 | 16.24 | 13.16 | 15.12 | 14.70 | 9.70 |
| 1–3 years | 16.24 | 18.34 | 15.12 | 17.78 | 16.87 | 11.13 |
| 3–8 years | 23.94 | 36.26 | 22.68 | 36.68 | 29.89 | 19.73 |
| 8–14 years | 36.26 | 56.7 | 36.68 | 57.4 | 46.76 | 30.86 |
| Micronutrients | Age Range | ||||
|---|---|---|---|---|---|
| 0–6 Months | 6–12 Months | 1–3 Years | 3–8 Years | 8–14 Years | |
| Biotin | 20% | 31% | 40% | 134% | 128% |
| Choline (mg) | 60% | 42% | 51% | 46% | 40% |
| Folic Acid (mcg) | 17% | 14% | 33% | 53% | 52% |
| Niacin (mg) | 53% | 57% | 32% | 47% | 49% |
| Pantothenic Acid (mg) | 29% | 37% | 25% | 43% | 45% |
| Riboflavin (mg) | 19% | 22% | 14% | 24% | 24% |
| Thiamine (mg) | 22% | 30% | 24% | 24% | 21% |
| Vitamin A (mcg) | 22% | 41% | 37% | 42% | 42% |
| Vitamin B12 (mcg) | 76% | 81% | 25% | 48% | 52% |
| Vitamin B6 (mg) | 30% | 26% | 15% | 31% | 32% |
| Vitamin C (mg) | 45% | 56% | 51% | 37% | 35% |
| Vitamin D (mcg) | 10% | 30% | 31% | 41% | 45% |
| Vitamin E (mg) | 40% | 48% | 50% | 30% | 31% |
| Vitamin K (mcg) | 37% | 45% | 64% | 42% | 42% |
| Calcium (mg) | 20% | 21% | 15% | 39% | 41% |
| Chlorine (mg) | 21% | 34% | 28% | 69% | 79% |
| Chromium (mcg) | 37% | 32% | 38% | 42% | 44% |
| Copper (mg) | 16% | 24% | 27% | 26% | 595% |
| Iodine (mcg) | 22% | 29% | 28% | 21% | 22% |
| Iron (mg) | 22% | 27% | 20% | 29% | 31% |
| Magnesium (mg) | 20% | 16% | 16% | 34% | 34% |
| Manganese (mg) | 70% | 53% | 59% | 54% | 56% |
| Molybdenum (mcg) | 36% | 40% | 29% | 33% | 35% |
| Phosphorus (mg) | 14% | 14% | 17% | 38% | 37% |
| Potassium (mg) | 19% | 31% | 43% | 49% | 50% |
| Selenio (mcg) | 18% | 23% | 31% | 23% | 22% |
| Sodium (mg) | 28% | 33% | 53% | 75% | 83% |
| Zinc (mg) | 22% | 25% | 24% | 23% | 23% |
| Micronutrient | Age Range | Group Differences (*) | Homogeneity of Variability Across Formulations (^) | ||||
|---|---|---|---|---|---|---|---|
| 0–6 Months | 6–12 Months | 1–3 Years | 4–8 Years | 8–14 Years | |||
| Biotin (mcg) | 11.4 [9.6–13.3] | 10 [7.9–13.5] | 10 [6–13] | 8 [6.4–26.8] | 8 [6.4–30.1] | 0.788 | 0.013 |
| Choline (mg) | 70 [37–97.2] | 66.9 [56.7–79.1] | 66.9 [36.5–76.8] | 100 [72.7–105] | 100 [90–113.1] | 0.232 | 0.872 |
| Folic Acid (mcg) | 48.2 [42–53.8] | 49.9 [42–51] | 42 [38.2–52.8] | 50.4 [40–75.2] | 51.8 [40–78.5] | 0.483 | 0.003 |
| Niacin (mg) | 5 [2.3–7.7] | 3.4 [2.2–6.1] | 3.4 [2.5–4.1] | 4.1 [3.3–5.1] | 4 [3.3–5.4] | 0.662 | 0.180 |
| Pantothenic Acid (mg) | 2.3 [2–3] | 2.1 [1.6–2.6] | 1.7 [1.3–2] | 1.3 [1.2–1.7] | 1.3 [1.1–1.5] | <0.001 | 0.771 |
| Riboflavin (mg) | 0.5 [0.4–0.5] | 0.4 [0.3–0.5] | 0.4 [0.3–0.4] | 0.4 [0.3–0.4] | 0.4 [0.3–0.4] | 0.263 | 0.813 |
| Thiamine (mg) | 0.4 [0.3–0.4] | 0.3 [0.3–0.4] | 0.3 [0.3–0.4] | 0.3 [0.3–0.4] | 0.3 [0.3–0.4] | 0.747 | 0.425 |
| Vitamin A (mcg) | 431 [322–500] | 322 [260–487] | 196 [144–300] | 164 [139–180] | 159 [139–180] | <0.001 | 0.013 |
| Vitamin B12 (mcg) | 1 [0.9–1.2] | 0.9 [0.7–1.1] | 0.8 [0.5–0.9] | 0.8 [0.7–0.9] | 0.8 [0.6–0.8] | 0.035 | 0.018 |
| Vitamin B6 (mg) | 0.4 [0.3–0.4] | 0.3 [0.3–0.4] | 0.4 [0.3–0.4] | 0.3 [0.3–0.4] | 0.3 [0.3–0.4] | 0.325 | 0.618 |
| Vitamin C (mg) | 47.7 [37.3–54.7] | 37.5 [18.8–53.2] | 15.1 [15–32.7] | 18 [15–21] | 18.3 [15–21] | <0.001 | <0.001 |
| Vitamin D (mcg) | 8.4 [8–8.5] | 8 [5.7–8.5] | 6.6 [4.2–7.4] | 4.2 [3.9–5.3] | 4 [2.5–5] | <0.001 | 0.382 |
| Vitamin E (mg) | 4.7 [3.5–6.2] | 3.6 [3–5.2] | 3.5 [2.4–3.9] | 2.7 [2.4–3.5] | 2.6 [2.1–3.2] | <0.001 | <0.001 |
| Vitamin K (mcg) | 24.9 [21.8–28.5] | 21.8 [9.8–28.5] | 9.8 [8.7–24.3] | 13 [12.2–18.4] | 13 [12.1–17.9] | 0.100 | 0.136 |
| Calcium (mg) | 349 [300–412] | 313 [275–392] | 313 [285–369] | 262 [200–312] | 235 [199–280] | <0.001 | 0.588 |
| Chlorine (mg) | 276 [255–295] | 255 [155–284] | 160 [148–200] | 110 [14–162] | 108 [11.3–160] | <0.001 | 0.178 |
| Chromium (mcg) | 13.4 [10.1–16.5] | 16 [10.4–17] | 10.7 [10–16.5] | 10 [8.6–16.7] | 10 [7.4–16.8] | 0.259 | 0.793 |
| Copper (mg) | 0.3 [0.3–0.4] | 0.3 [0.3–0.3] | 0.3 [0.2–0.4] | 0.4 [0.3–0.4] | 0.4 [0.3–0.4] | 0.668 | 0.133 |
| Iodine (mcg) | 58.2 [50–74.1] | 56.4 [39.6–66.2] | 52.7 [38.3–61.2] | 42.9 [38.2–49.8] | 42.3 [36.3–45] | 0.007 | 0.028 |
| Iron (mg) | 5.8 [4.1–6.3] | 5.5 [3.9–6.2] | 4.5 [4–4.8] | 3.6 [3.4–4.2] | 3.6 [3.3–3.8] | <0.001 | 0.336 |
| Magnesium (mg) | 42.3 [31.5–47.3] | 40 [37.9–45.2] | 44 [40.1–51.9] | 54 [48.4–62.8] | 54.8 [49.1–62.8] | <0.001 | 0.116 |
| Manganese (mg) | 0.3 [0.1–0.4] | 0.4 [0.2–0.4] | 0.4 [0.3–0.6] | 0.5 [0.3–0.8] | 0.5 [0.3–0.8] | 0.006 | 0.125 |
| Molybdenum (mcg) | 20.5 [11.2–23.6] | 12 [10.7–21.6] | 12 [10.3–15.9] | 14.2 [11.3–17] | 12.9 [11.3–17] | 0.637 | 0.266 |
| Phosphorus (mg) | 229 [225–242] | 229 [207–232] | 210 [179–230] | 183 [138–213] | 178 [139–211] | 0.008 | 0.071 |
| Potassium (mg) | 418 [353–455] | 353 [253–451] | 213 [195–300] | 175 [122–226] | 166 [120–225] | <0.001 | 0.814 |
| Selenio (mcg) | 12.8 [11.8–14.7] | 11.8 [9.6–13.8] | 11 [8.6–13.1] | 13.4 [11.9–14.8] | 13.9 [12.9–14.8] | 0.142 | 0.927 |
| Sodium (mg) | 139 [133–146] | 133 [101–142] | 99.4 [90.9–133] | 104 [76.4–160] | 101 [52.2–145] | 0.297 | 0.108 |
| Zinc (mg) | 4.1 [3.3–4.5] | 3.8 [2.6–4.4] | 3.3 [2.6–3.7] | 3.3 [2.4–3.6] | 3.2 [2.3–3.6] | 0.058 | 0.832 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tummolo, A.; Carella, R.; De Giovanni, D.; Di Tullio, V.; Lorusso, L.; Bartolomeo, N. A Cross-Sectional Study on Protein Substitutes for Paediatric Phenylketonuria Diet: Time to Pay Attention. Nutrients 2025, 17, 1767. https://doi.org/10.3390/nu17111767
Tummolo A, Carella R, De Giovanni D, Di Tullio V, Lorusso L, Bartolomeo N. A Cross-Sectional Study on Protein Substitutes for Paediatric Phenylketonuria Diet: Time to Pay Attention. Nutrients. 2025; 17(11):1767. https://doi.org/10.3390/nu17111767
Chicago/Turabian StyleTummolo, Albina, Rosa Carella, Donatella De Giovanni, Vito Di Tullio, Letizia Lorusso, and Nicola Bartolomeo. 2025. "A Cross-Sectional Study on Protein Substitutes for Paediatric Phenylketonuria Diet: Time to Pay Attention" Nutrients 17, no. 11: 1767. https://doi.org/10.3390/nu17111767
APA StyleTummolo, A., Carella, R., De Giovanni, D., Di Tullio, V., Lorusso, L., & Bartolomeo, N. (2025). A Cross-Sectional Study on Protein Substitutes for Paediatric Phenylketonuria Diet: Time to Pay Attention. Nutrients, 17(11), 1767. https://doi.org/10.3390/nu17111767






