Fish Oil Supplementation Attenuates Offspring’s Neurodevelopmental Changes Induced by a Maternal High-Fat Diet in a Rat Model
Abstract
1. Introduction
2. Materials and Methods
3. Results
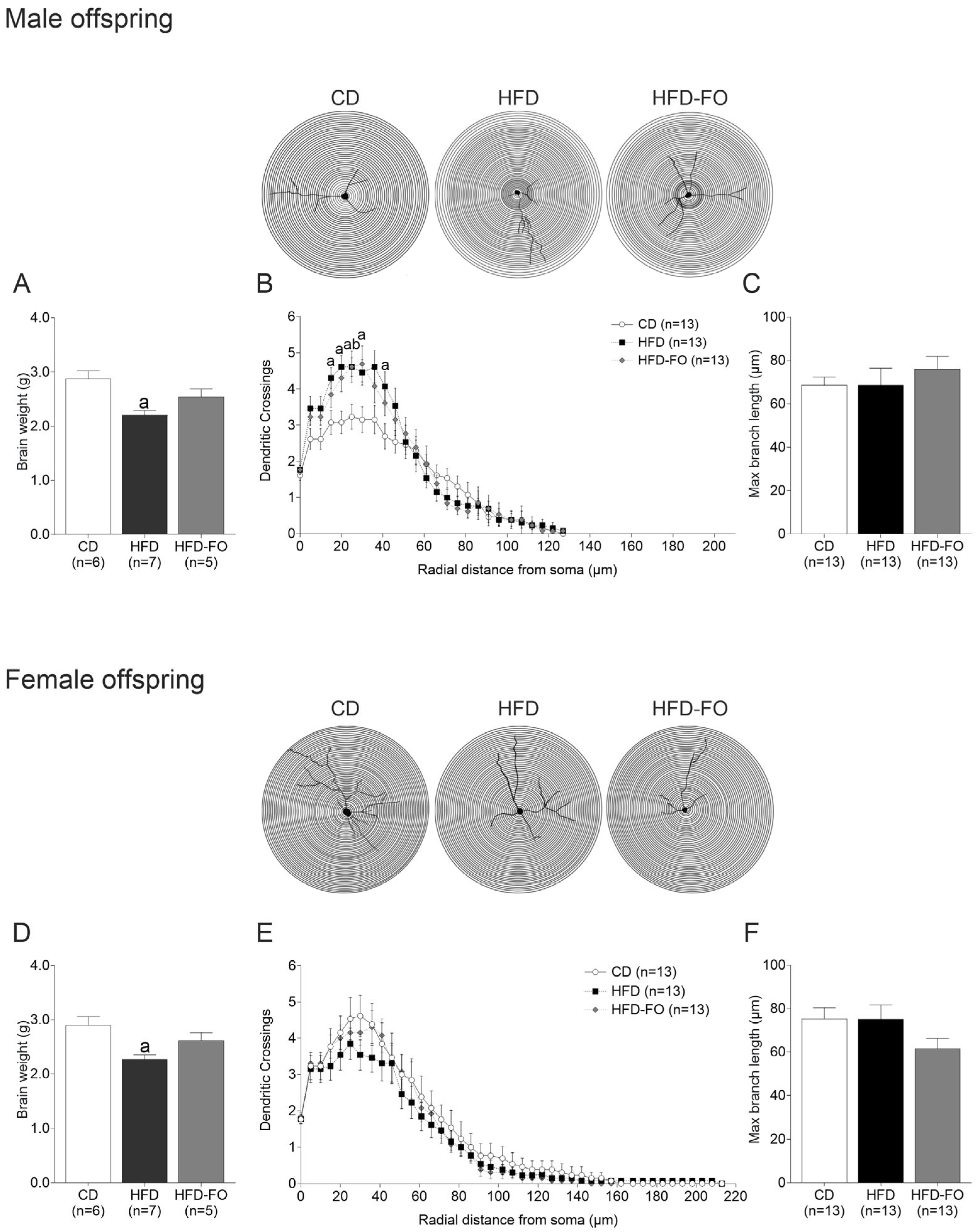
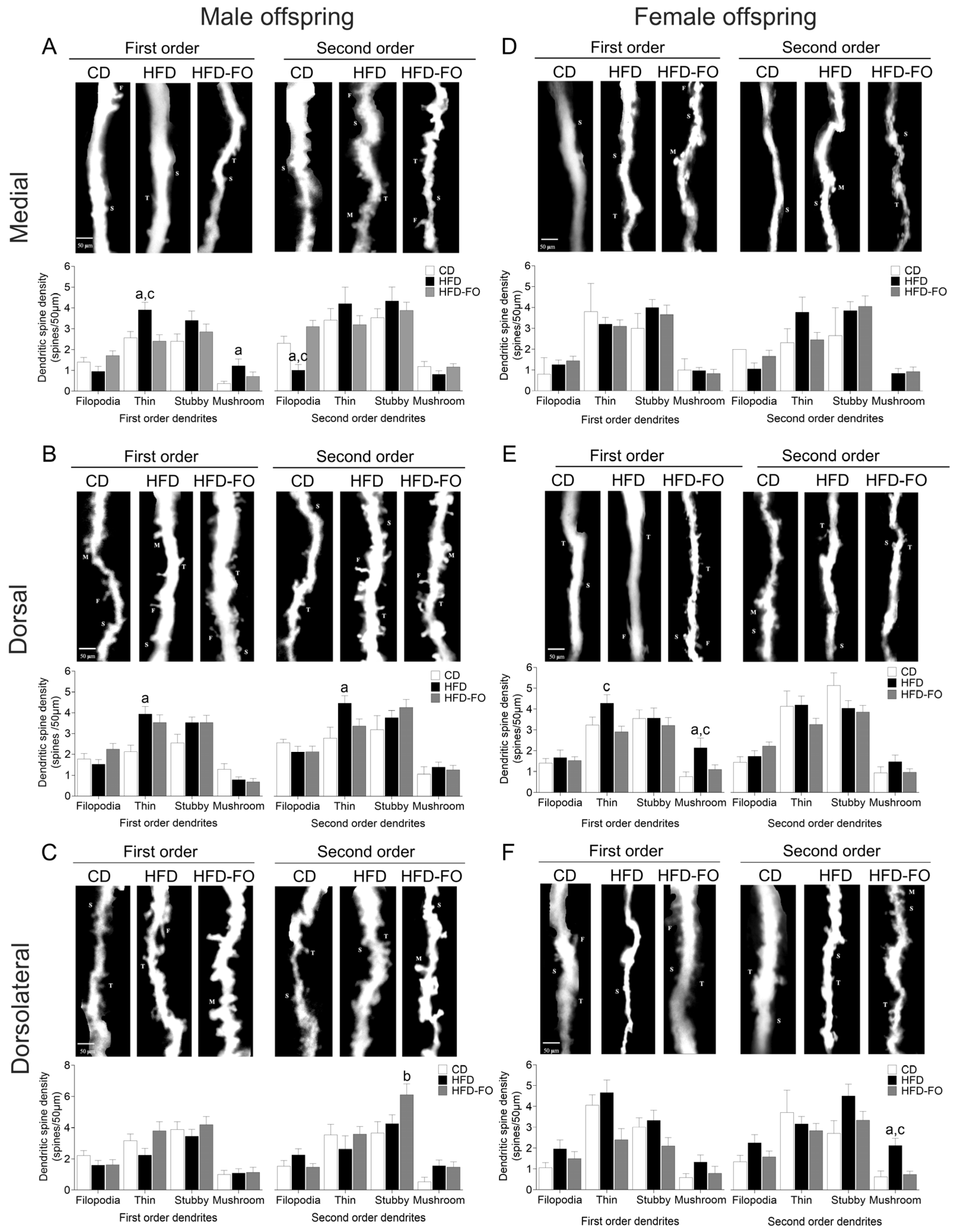
- Maternal FO supplementation counteracted HFD-induced effects on the offspring’s neurological reflexes and dendrite morphogenesis.
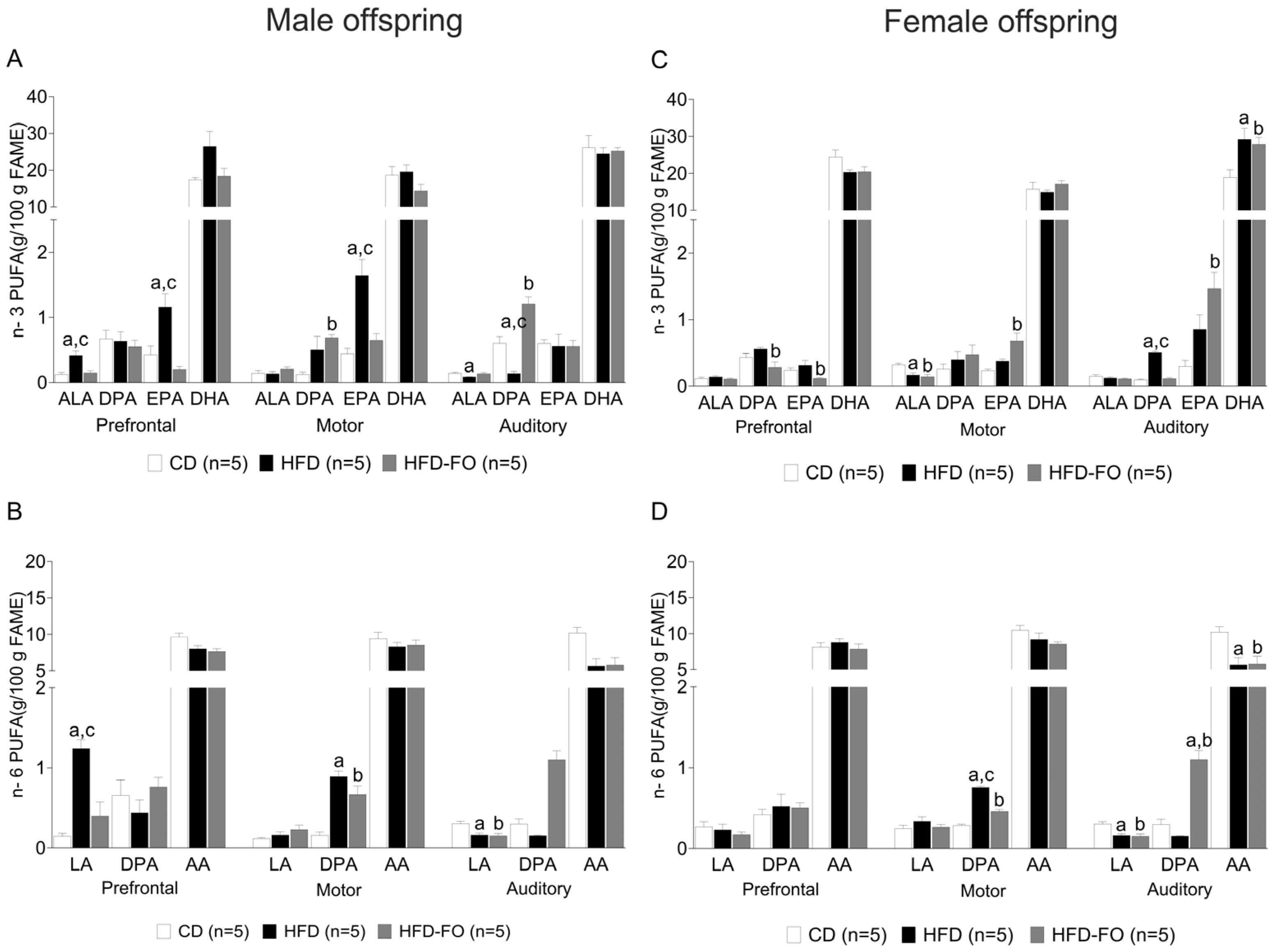
- Maternal FO supplementation restored the HFD-induced effects on the fatty acid profile in the cerebral cortex of the offspring.
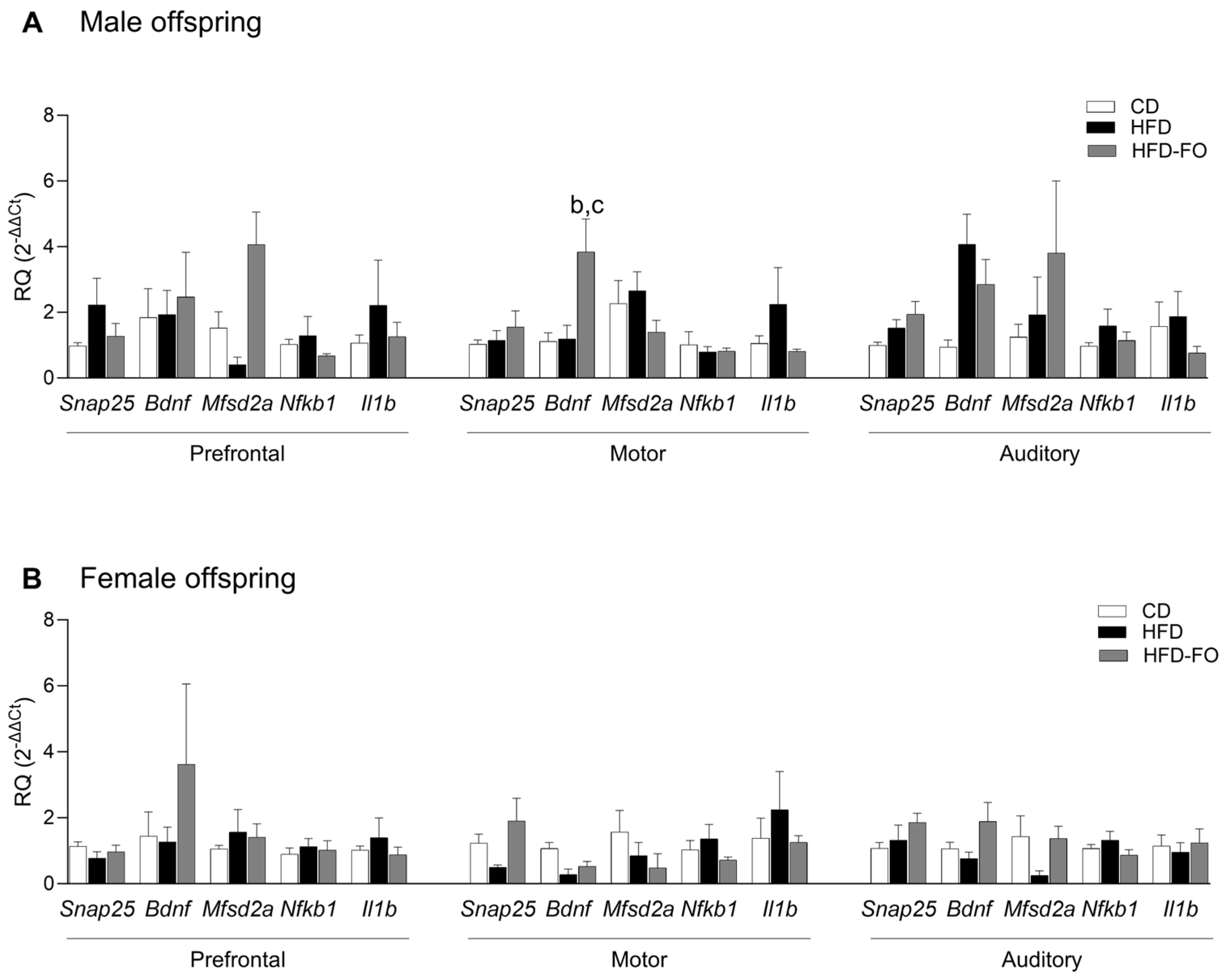
- Maternal FO supplementation increased transcript levels of Bdnf in the cerebral cortex of male offspring.
4. Discussion
Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid |
| ALA | α-linolenic acid |
| ANOVA | Analysis of variance |
| ASD | Autism spectrum disorders |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| BHT | Butylated hydroxytoluene |
| CaMKII | Ca2+/calmodulin-dependent protein kinase |
| CD | Control diet |
| DHA | Docosahexaenoic acid |
| DPA n-3 | Docosapentaenoic acid n-3 series |
| DPA n-6 | Docosapentaenoic acid n-6 series |
| ELOVL2 | Enzyme-specific elongation of very long chain 2 |
| EPA | Eicosapentaenoic acid |
| FAME | Fatty acid methyl esters |
| FFA | Free fatty acid |
| GABA | Gamma-aminobutyric acid |
| GC | Gas chromatography |
| GPR40 | G-protein coupled receptor 40 |
| HFD | High-fat diet |
| HFD-FO | High-fat diet—fish oil |
| IL-1β | Interleukin-1β |
| LA | Linoleic acid |
| MFSD2a | Major facilitator superfamily domain-containing protein 2 |
| mRNA | Messenger ribonucleic acid |
| MUFA | Monounsaturated fatty acid |
| NF-κB | Nuclear factor kappa-light-chain enhancer of activated B cells |
| PBS | Phosphate buffer saline |
| PND | Postnatal day |
| PUFA | Polyunsaturated fatty acid |
| RT-qPCR | Quantitative reverse transcription polymerase chain reaction |
| SCFA | Short-chain fatty acids |
| SFA | Saturated fatty acid |
| SNAP25 | Synaptosome-associated protein 25 |
| VPA | Valproic acid |
References
- Tong, L.; Kalish, B.T. The impact of maternal obesity on childhood neurodevelopment. J. Perinatol. 2021, 41, 928–939. [Google Scholar] [CrossRef]
- Edlow, A.G. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat. Diagn. 2017, 37, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, I.; Stull, N.D.; Tersey, S.A.; Mirmira, R.G. Phenotypic sexual dimorphism in response to dietary fat manipulation in C57BL/6J mice. J. Diabetes Complicat. 2021, 35, 107795. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Armstrong, E.A.; Yager, J.Y. Neurodevelopmental Reflex Testing in Neonatal Rat Pups. J. Vis. Exp. 2017, 122, 55261. [Google Scholar] [CrossRef]
- Horiquini Barbosa, E.; Vallim, J.H.; Lachat, J.J.; de Castro, V.L. Assessments of Motor Abnormalities on the Grid-Walking and Foot-Fault Tests From Undernutrition in Wistar Rats. J. Mot. Behav. 2016, 48, 5–12. [Google Scholar] [CrossRef]
- Fernandes, D.J.; Spring, S.; Roy, A.R.; Qiu, L.R.; Yee, Y.; Nieman, B.J.; Lerch, J.P.; Palmert, M.R. Exposure to maternal high-fat diet induces extensive changes in the brain of adult offspring. Transl. Psychiatry 2021, 11, 149. [Google Scholar] [CrossRef]
- Molteni, R.; Barnard, R.J.; Ying, Z.; Roberts, C.K.; Gómez-Pinilla, F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 2002, 112, 803–814. [Google Scholar] [CrossRef]
- Abidin, İ.; Aydin-Abidin, S.; Bodur, A.; İnce, İ.; Alver, A. Brain-derived neurotropic factor (BDNF) heterozygous mice are more susceptible to synaptic protein loss in cerebral cortex during high fat diet. Arch. Physiol. Biochem. 2018, 124, 442–447. [Google Scholar] [CrossRef]
- Basak, S.; Das, R.K.; Banerjee, A.; Paul, S.; Pathak, S.; Duttaroy, A.K. Maternal Obesity and Gut Microbiota Are Associated with Fetal Brain Development. Nutrients 2022, 14, 4515. [Google Scholar] [CrossRef]
- Cirulli, F.; De Simone, R.; Musillo, C.; Ajmone-Cat, M.A.; Berry, A. Inflammatory Signatures of Maternal Obesity as Risk Factors for Neurodevelopmental Disorders: Role of Maternal Microbiota and Nutritional Intervention Strategies. Nutrients 2022, 14, 3150. [Google Scholar] [CrossRef]
- Dresselhaus, E.C.; Meffert, M.K. Cellular Specificity of NF-κB Function in the Nervous System. Front. Immunol. 2019, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- Agustí, A.; García-Pardo, M.P.; López-Almela, I.; Campillo, I.; Maes, M.; Romaní-Pérez, M.; Sanz, Y. Interplay Between the Gut-Brain Axis, Obesity and Cognitive Function. Front. Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef]
- Di Miceli, M.; Bosch-Bouju, C.; Layé, S. PUFA and their derivatives in neurotransmission and synapses: A new hallmark of synaptopathies. Proc. Nutr. Soc. 2020, 79, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.J.; Mann, N.J.; Lewis, J.L.; Milligan, G.C.; Sinclair, A.J.; Howe, P.R. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 2003, 38, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, B.R.; Barrera, R.C.; González-Astorga, M.; Sanhueza, C.J.; Valenzuela, B.A. Alpha linolenic acid (ALA) from Rosa canina, sacha inchi and chia oils may increase ALA accretion and its conversion into n-3 LCPUFA in diverse tissues of the rat. Food Funct. 2014, 5, 1564–1572. [Google Scholar] [CrossRef]
- Echeverría, F.; Valenzuela, R.; Catalina Hernandez-Rodas, M.; Valenzuela, A. Docosahexaenoic acid (DHA), a fundamental fatty acid for the brain: New dietary sources. Prostaglandins Leukot. Essent. Fat. Acids 2017, 124, 1–10. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hossain, S.; Al Mamun, A.; Matsuzaki, K.; Arai, H. Docosahexaenoic acid: One molecule diverse functions. Crit. Rev. Biotechnol. 2017, 37, 579–597. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef]
- Sandoval, K.E.; Wooten, J.S.; Harris, M.P.; Schaller, M.L.; Umbaugh, D.S.; Witt, K.A. Mfsd2a and Glut1 Brain Nutrient Transporters Expression Increase with 32-Week Low and High Lard Compared with Fish-Oil Dietary Treatment in C57Bl/6 Mice. Curr. Dev. Nutr. 2018, 2, nzy065. [Google Scholar] [CrossRef]
- Haddad-Tóvolli, R.; Morari, J.; Barbizan, R.; Bóbbo, V.C.; Carraro, R.S.; Solon, C.; Dragano, N.R.; Torsoni, M.A.; Araujo, E.P.; Velloso, L.A. Maternal obesity damages the median eminence blood-brain barrier structure and function in the progeny: The beneficial impact of cross-fostering by lean mothers. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E154–E166. [Google Scholar] [CrossRef]
- Calderon, F.; Kim, H.Y. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J. Neurochem. 2004, 90, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Dagai, L.; Peri-Naor, R.; Birk, R.Z. Docosahexaenoic acid significantly stimulates immediate early response genes and neurite outgrowth. Neurochem. Res. 2009, 34, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Katakura, M.; Hashimoto, M.; Shahdat, H.M.; Gamoh, S.; Okui, T.; Matsuzaki, K.; Shido, O. Docosahexaenoic acid promotes neuronal differentiation by regulating basic helix-loop-helix transcription factors and cell cycle in neural stem cells. Neuroscience 2009, 160, 651–660. [Google Scholar] [CrossRef]
- Tanaka, K.; Farooqui, A.A.; Siddiqi, N.J.; Alhomida, A.S.; Ong, W.Y. Effects of docosahexaenoic Acid on neurotransmission. Biomol. Ther. 2012, 20, 152–157. [Google Scholar] [CrossRef]
- Zinkow, A.; Grodzicki, W.; Czerwińska, M.; Dziendzikowska, K. Molecular Mechanisms Linking Omega-3 Fatty Acids and the Gut-Brain Axis. Molecules 2024, 30, 71. [Google Scholar] [CrossRef] [PubMed]
- Helland, I.B.; Smith, L.; Saarem, K.; Saugstad, O.D.; Drevon, C.A. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics 2003, 111, e39–e44. [Google Scholar] [CrossRef]
- Dunstan, J.A.; Simmer, K.; Dixon, G.; Prescott, S.L. Cognitive assessment of children at age 2(1/2) years after maternal fish oil supplementation in pregnancy: A randomised controlled trial. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F45–F50. [Google Scholar] [CrossRef]
- Vollet, K.; Ghassabian, A.; Sundaram, R.; Chahal, N.; Yeung, E.H. Prenatal fish oil supplementation and early childhood development in the Upstate KIDS Study. J. Dev. Orig. Health Dis. 2017, 8, 465–473. [Google Scholar] [CrossRef]
- Mulder, K.A.; Elango, R.; Innis, S.M. Fetal DHA inadequacy and the impact on child neurodevelopment: A follow-up of a randomised trial of maternal DHA supplementation in pregnancy. Br. J. Nutr. 2018, 119, 271–279. [Google Scholar] [CrossRef]
- Ostadrahimi, A.; Salehi-Pourmehr, H.; Mohammad-Alizadeh-Charandabi, S.; Heidarabady, S.; Farshbaf-Khalili, A. The effect of perinatal fish oil supplementation on neurodevelopment and growth of infants: A randomized controlled trial. Eur. J. Nutr. 2018, 57, 2387–2397. [Google Scholar] [CrossRef]
- Colombo, J.; Shaddy, D.J.; Gustafson, K.; Gajewski, B.J.; Thodosoff, J.M.; Kerling, E.; Carlson, S.E. The Kansas University DHA Outcomes Study (KUDOS) clinical trial: Long-term behavioral follow-up of the effects of prenatal DHA supplementation. Am. J. Clin. Nutr. 2019, 109, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhao, T.; Kim, J. neuTube 1.0: A New Design for Efficient Neuron Reconstruction Software Based on the SWC Format. eNeuro 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Arshadi, C.; Günther, U.; Eddison, M.; Harrington, K.I.S.; Ferreira, T.A. SNT: A unifying toolbox for quantification of neuronal anatomy. Nat. Methods 2021, 18, 374–377. [Google Scholar] [CrossRef]
- Sholl, D.A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 1953, 87, 387–406. [Google Scholar]
- Berg, S.; Kutra, D.; Kroeger, T.; Straehle, C.N.; Kausler, B.X.; Haubold, C.; Schiegg, M.; Ales, J.; Beier, T.; Rudy, M.; et al. ilastik: Interactive machine learning for (bio)image analysis. Nat. Methods 2019, 16, 1226–1232. [Google Scholar] [CrossRef]
- Legland, D.; Arganda-Carreras, I.; Andrey, P. MorphoLibJ: Integrated library and plugins for mathematical morphology with ImageJ. Bioinformatics 2016, 32, 3532–3534. [Google Scholar] [CrossRef]
- Domander, R.; Felder, A.A.; Doube, M. BoneJ2—Refactoring established research software. Wellcome Open Res. 2021, 6, 37. [Google Scholar] [CrossRef]
- Risher, W.C.; Ustunkaya, T.; Singh Alvarado, J.; Eroglu, C. Rapid Golgi analysis method for efficient and unbiased classification of dendritic spines. PLoS ONE 2014, 9, e107591. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Morrison, W.R.; Smith, L.M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride—Methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.A.; Zylka, M.J. Controlling litter effects to enhance rigor and reproducibility with rodent models of neurodevelopmental disorders. J. Neurodev. Disord. 2021, 13, 2. [Google Scholar] [CrossRef]
- Joffre, C.; Grégoire, S.; De Smedt, V.; Acar, N.; Bretillon, L.; Nadjar, A.; Layé, S. Modulation of brain PUFA content in different experimental models of mice. Prostaglandins Leukot. Essent. Fatty Acids 2016, 114, 1–10. [Google Scholar] [CrossRef]
- Horman, T.; Fernandes, M.F.; Tache, M.C.; Hucik, B.; Mutch, D.M.; Leri, F. Dietary n-6/n-3 Ratio Influences Brain Fatty Acid Composition in Adult Rats. Nutrients 2020, 12, 1847. [Google Scholar] [CrossRef]
- Kiehn, O. Decoding the organization of spinal circuits that control locomotion. Nat. Rev. Neurosci. 2016, 17, 224–238. [Google Scholar] [CrossRef]
- Yeomans, J.S.; Frankland, P.W. The acoustic startle reflex: Neurons and connections. Brain Res. Rev. 1995, 21, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Jan, Y.N.; Jan, L.Y. Branching out: Mechanisms of dendritic arborization. Nat. Rev. Neurosci. 2010, 11, 316–328. [Google Scholar] [CrossRef]
- Gilbert, J.; Man, H.Y. Fundamental Elements in Autism: From Neurogenesis and Neurite Growth to Synaptic Plasticity. Front. Cell Neurosci. 2017, 11, 359. [Google Scholar] [CrossRef]
- Mahmood, U.; Ahn, S.; Yang, E.J.; Choi, M.; Kim, H.; Regan, P.; Cho, K.; Kim, H.S. Dendritic spine anomalies and PTEN alterations in a mouse model of VPA-induced autism spectrum disorder. Pharmacol. Res. 2018, 128, 110–121. [Google Scholar] [CrossRef]
- Choi, C.S.; Hong, M.; Kim, K.C.; Kim, J.W.; Yang, S.M.; Seung, H.; Ko, M.J.; Choi, D.H.; You, J.S.; Shin, C.Y.; et al. Effects of atomoxetine on hyper-locomotive activity of the prenatally valproate-exposed rat offspring. Biomol. Ther. 2014, 22, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Wang, Y.; Li, Y.; Chen, D.; Yang, F.; Wang, S. A Developmental Study of Abnormal Behaviors and Altered GABAergic Signaling in the VPA-Treated Rat Model of Autism. Front. Behav. Neurosci. 2018, 12, 182. [Google Scholar] [CrossRef]
- Courchesne, E.; Pierce, K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Curr. Opin. Neurobiol. 2005, 15, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Kurti, A.; Fair, D.A.; Fryer, J.D. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J. Neuroinflamm. 2014, 11, 156. [Google Scholar] [CrossRef]
- Gawlińska, K.; Gawliński, D.; Kowal-Wiśniewska, E.; Jarmuż-Szymczak, M.; Filip, M. Alteration of the Early Development Environment by Maternal Diet and the Occurrence of Autistic-like Phenotypes in Rat Offspring. Int. J. Mol. Sci. 2021, 22, 9662. [Google Scholar] [CrossRef]
- Gawlińska, K.; Gawliński, D.; Borczyk, M.; Korostyński, M.; Przegaliński, E.; Filip, M. A Maternal High-Fat Diet during Early Development Provokes Molecular Changes Related to Autism Spectrum Disorder in the Rat Offspring Brain. Nutrients 2021, 13, 3212. [Google Scholar] [CrossRef] [PubMed]
- Babikian, T.; Prins, M.L.; Cai, Y.; Barkhoudarian, G.; Hartonian, I.; Hovda, D.A.; Giza, C.C. Molecular and physiological responses to juvenile traumatic brain injury: Focus on growth and metabolism. Dev. Neurosci. 2010, 32, 431–441. [Google Scholar] [CrossRef]
- Stratton, H.J.; Khanna, R. Sculpting Dendritic Spines during Initiation and Maintenance of Neuropathic Pain. J. Neurosci. 2020, 40, 7578–7589. [Google Scholar] [CrossRef]
- Matsuzaki, M.; Honkura, N.; Ellis-Davies, G.C.; Kasai, H. Structural basis of long-term potentiation in single dendritic spines. Nature 2004, 429, 761–766. [Google Scholar] [CrossRef]
- Ziv, N.E.; Smith, S.J. Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron 1996, 17, 91–102. [Google Scholar] [CrossRef]
- Hamad, M.I.K.; Emerald, B.S.; Kumar, K.K.; Ibrahim, M.F.; Ali, B.R.; Bataineh, M.F. Extracellular molecular signals shaping dendrite architecture during brain development. Front. Cell Dev. Biol. 2023, 11, 1254589. [Google Scholar] [CrossRef] [PubMed]
- Sona, C.; Kumar, A.; Dogra, S.; Kumar, B.A.; Umrao, D.; Yadav, P.N. Docosahexaenoic acid modulates brain-derived neurotrophic factor via GPR40 in the brain and alleviates diabesity-associated learning and memory deficits in mice. Neurobiol. Dis. 2018, 118, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Engel, D.F.; Bobbo, V.C.D.; Solon, C.S.; Nogueira, G.A.; Moura-Assis, A.; Mendes, N.F.; Zanesco, A.M.; Papangelis, A.; Ulven, T.; Velloso, L.A. Activation of GPR40 induces hypothalamic neurogenesis through p38- and BDNF-dependent mechanisms. Sci. Rep. 2020, 10, 11047. [Google Scholar] [CrossRef] [PubMed]
- McAllister, A.K.; Katz, L.C.; Lo, D.C. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron 1996, 17, 1057–1064. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience 2008, 155, 751–759. [Google Scholar] [CrossRef]
- Horch, H.W.; Krüttgen, A.; Portbury, S.D.; Katz, L.C. Destabilization of cortical dendrites and spines by BDNF. Neuron 1999, 23, 353–364. [Google Scholar] [CrossRef]
- Vadisiute, A.; Meijer, E.; Szabó, F.; Hoerder-Suabedissen, A.; Kawashita, E.; Hayashi, S.; Molnár, Z. The role of snare proteins in cortical development. Dev. Neurobiol. 2022, 82, 457–475. [Google Scholar] [CrossRef]
- Cao, D.; Kevala, K.; Kim, J.; Moon, H.S.; Jun, S.B.; Lovinger, D.; Kim, H.Y. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J. Neurochem. 2009, 111, 510–521. [Google Scholar] [CrossRef]
- Yarmohammadi-Samani, P.; Vatanparast, J. Sex-specific dendritic morphology of hippocampal pyramidal neurons in the adolescent and young adult rats. Int. J. Dev. Neurosci. 2024, 84, 47–63. [Google Scholar] [CrossRef]
- Edlow, A.G.; Guedj, F.; Pennings, J.L.; Sverdlov, D.; Neri, C.; Bianchi, D.W. Males are from Mars, and females are from Venus: Sex-specific fetal brain gene expression signatures in a mouse model of maternal diet-induced obesity. Am. J. Obstet. Gynecol. 2016, 214, e621–e623. [Google Scholar] [CrossRef]
- Li, Y.M.; Ou, J.J.; Liu, L.; Zhang, D.; Zhao, J.P.; Tang, S.Y. Association Between Maternal Obesity and Autism Spectrum Disorder in Offspring: A Meta-analysis. J. Autism Dev. Disord. 2016, 46, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Iosif, A.M.; Hansen, R.L.; Schmidt, R.J. Maternal polyunsaturated fatty acids and risk for autism spectrum disorder in the. Autism 2020, 24, 1191–1200. [Google Scholar] [CrossRef]
- Perazzolo, S.; Hirschmugl, B.; Wadsack, C.; Desoye, G.; Lewis, R.M.; Sengers, B.G. The influence of placental metabolism on fatty acid transfer to the fetus. J. Lipid Res. 2017, 58, 443–454. [Google Scholar] [CrossRef]
- Larqué, E.; Demmelmair, H.; Gil-Sánchez, A.; Prieto-Sánchez, M.T.; Blanco, J.E.; Pagán, A.; Faber, F.L.; Zamora, S.; Parrilla, J.J.; Koletzko, B. Placental transfer of fatty acids and fetal implications. Am. J. Clin. Nutr. 2011, 94, 1908S–1913S. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sánchez, A.; Demmelmair, H.; Parrilla, J.J.; Koletzko, B.; Larqué, E. Mechanisms involved in the selective transfer of long chain polyunsaturated Fatty acids to the fetus. Front. Genet. 2011, 2, 57. [Google Scholar] [CrossRef]
- Chamorro, R.; Bascuñán, K.A.; Barrera, C.; Sandoval, J.; Puigrredon, C.; Valenzuela, R. Reduced n-3 and n-6 PUFA (DHA and AA) Concentrations in Breast Milk and Erythrocytes Phospholipids during Pregnancy and Lactation in Women with Obesity. Int. J. Environ. Res. Public Health 2022, 19, 1930. [Google Scholar] [CrossRef]
- Powell, T.L.; Uhlson, C.; Madi, L.; Berry, K.Z.; Chassen, S.S.; Jansson, T.; Ferchaud-Roucher, V. Fetal sex differences in placental LCPUFA ether and plasmalogen phosphatidylethanolamine and phosphatidylcholine contents in pregnancies complicated by obesity. Biol. Sex. Differ. 2023, 14, 66. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, D.; Ortiz, M.; Valdebenito, G.; Crisosto, N.; Echiburú, B.; Valenzuela, R.; Espinosa, A.; Maliqueo, M. Effects of a High-Fat Diet and Docosahexaenoic Acid during Pregnancy on Fatty Acid Composition in the Fetal Livers of Mice. Nutrients 2023, 15, 4696. [Google Scholar] [CrossRef]
- Metherel, A.H.; Valenzuela, R.; Klievik, B.J.; Cisbani, G.; Rotarescu, R.D.; Gonzalez-Soto, M.; Cruciani-Guglielmacci, C.; Layé, S.; Magnan, C.; Mutch, D.M.; et al. Dietary docosahexaenoic acid (DHA) downregulates liver DHA synthesis by inhibiting eicosapentaenoic acid elongation. J. Lipid Res. 2024, 65, 100548. [Google Scholar] [CrossRef]
- Valenzuela, R.; Metherel, A.H.; Cisbani, G.; Smith, M.E.; Chouinard-Watkins, R.; Klievik, B.J.; Videla, L.A.; Bazinet, R.P. Protein concentrations and activities of fatty acid desaturase and elongase enzymes in liver, brain, testicle, and kidney from mice: Substrate dependency. Biofactors 2024, 50, 89–100. [Google Scholar] [CrossRef]
- Domenichiello, A.F.; Chen, C.T.; Trepanier, M.O.; Stavro, P.M.; Bazinet, R.P. Whole body synthesis rates of DHA from α-linolenic acid are greater than brain DHA accretion and uptake rates in adult rats. J. Lipid Res. 2014, 55, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Metherel, A.H.; Rezaei, K.; Lacombe, R.J.S.; Bazinet, R.P. Plasma unesterified eicosapentaenoic acid is converted to docosahexaenoic acid (DHA) in the liver and supplies the brain with DHA in the presence or absence of dietary DHA. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158942. [Google Scholar] [CrossRef]
- Rodriguez-Navas, C.; Morselli, E.; Clegg, D.J. Sexually dimorphic brain fatty acid composition in low and high fat diet-fed mice. Mol. Metab. 2016, 5, 680–689. [Google Scholar] [CrossRef]
- Morselli, E.; Santos, R.S.; Gao, S.; Ávalos, Y.; Criollo, A.; Palmer, B.F.; Clegg, D.J. Impact of estrogens and estrogen receptor-α in brain lipid metabolism. Am. J. Physiol.-Endocrinol. Metab. 2018, 315, E7–E14. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Haven, S.; Lecaj, L.; Borgstrom, M.; Torabi, M.; SanGiovanni, J.P.; Hibbeln, J.R. Brain PUFA Concentrations Are Differentially Affected by Interactions of Diet, Sex, Brain Regions, and Phospholipid Pools in Mice. J. Nutr. 2020, 150, 3123–3132. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, A.; Ohishi, M.; Sato, Y.; Hata, N.; Misawa, Y.; Fujii, Y.; Okuyama, H. Reversibility of n-3 fatty acid deficiency-induced alterations of learning behavior in the rat: Level of n-6 fatty acids as another critical factor. J. Lipid Res. 2001, 42, 1655–1663. [Google Scholar] [CrossRef]
- Grygiel-Górniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13, 17. [Google Scholar] [CrossRef]
- Brown, R. Linoleic Acid and Alpha-Linolenic Acid Have Central Roles in Brain Energy Substrate Provision, Endogenous Lipid Production, Immune and Repair Function, via Peroxisomal Beta-Oxidation-Related Pathways? In Omega-3 Fatty Acids: Keys to Nutritional Health; Mahabaleshwar, V.H., Anand Arvind, Z., Sharad, P.A., Eds.; Springer: Cham, Switzerland, 2016; pp. 413–428. [Google Scholar]
- Azagra-Boronat, I.; Tres, A.; Massot-Cladera, M.; Franch, À.; Castell, M.; Guardiola, F.; Pérez-Cano, F.J.; Rodríguez-Lagunas, M.J. Associations of Breast Milk Microbiota, Immune Factors, and Fatty Acids in the Rat Mother-Offspring Pair. Nutrients 2020, 12, 319. [Google Scholar] [CrossRef]
- Venn-Watson, S.K.; Butterworth, C.N. Broader and safer clinically-relevant activities of pentadecanoic acid compared to omega-3: Evaluation of an emerging essential fatty acid across twelve primary human cell-based disease systems. PLoS ONE 2022, 17, e0268778. [Google Scholar] [CrossRef]
- Usui, N.; Iwata, K.; Miyachi, T.; Takagai, S.; Wakusawa, K.; Nara, T.; Tsuchiya, K.J.; Matsumoto, K.; Kurita, D.; Kameno, Y.; et al. VLDL-specific increases of fatty acids in autism spectrum disorder correlate with social interaction. EBioMedicine 2020, 58, 102917. [Google Scholar] [CrossRef]
- Gharami, K.; Das, M.; Das, S. Essential role of docosahexaenoic acid towards development of a smarter brain. Neurochem. Int. 2015, 89, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Sass, L.; Bjarnadóttir, E.; Stokholm, J.; Chawes, B.; Vinding, R.K.; Mora-Jensen, A.C.; Thorsen, J.; Noergaard, S.; Ebdrup, B.H.; Jepsen, J.R.M.; et al. Fish Oil Supplementation in Pregnancy and Neurodevelopment in Childhood-A Randomized Clinical Trial. Child Dev. 2021, 92, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- White, C.L.; Pistell, P.J.; Purpera, M.N.; Gupta, S.; Fernandez-Kim, S.O.; Hise, T.L.; Keller, J.N.; Ingram, D.K.; Morrison, C.D.; Bruce-Keller, A.J. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: Contributions of maternal diet. Neurobiol. Dis. 2009, 35, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Tuzun, F.; Kumral, A.; Dilek, M.; Ozbal, S.; Ergur, B.; Yesilirmak, D.C.; Duman, N.; Yilmaz, O.; Ozkan, H. Maternal omega-3 fatty acid supplementation protects against lipopolysaccharide-induced white matter injury in the neonatal rat brain. J. Matern.-Fetal Neonatal Med. 2012, 25, 849–854. [Google Scholar] [CrossRef]

| Nutritional Composition | CD (D12450H) | HFD (D12451) | HFD-FO (D21030416) |
|---|---|---|---|
| Calories per gram | 3.85 | 4.73 | 4.73 |
| Protein (%) | 20 | 20 | 20 |
| Carbohydrates (%) | 70 | 35 | 35 |
| Lipids (%) | 10 | 45 | 45 |
| Saturated fatty acids (%) | 23.50 | 31.59 | 29.27 |
| Stearic acid (g) | 3.08 | 19.78 | 11.98 |
| Palmitic acid (g) | 6.45 | 36.85 | 32.46 |
| Monounsaturated fatty acids (%) | 29.69 | 35.51 | 29.23 |
| Oleic acid (g) | 12.32 | 64.10 | 41.41 |
| Polyunsaturated fatty acids (%) | 46.81 | 32.91 | 41.50 |
| Linoleic acid (g) | 17.82 | 56.24 | 34.30 |
| Arachidonic acid (g) | 0.06 | 0.50 | 2.27 |
| Linolenic fatty acid (g) | 2.12 | 4.21 | 4.38 |
| Eicosapentaenoic acid (g) | 0.00 | 0.00 | 13.85 |
| Docosahexaenoic acid (g) | 0.00 | 0.00 | 10.00 |
| Omega-6 | 17.90 | 56.97 | 37.27 |
| Omega-3 | 2.13 | 4.36 | 34.04 |
| Ratio omega-6/omega-3 | 8.39 | 13.07 | 1.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, Y.; Kaune, H.; Dagnino-Subiabre, A.; Cruz, G.; Toledo, J.; Valenzuela, R.; Moraga, R.; Tabilo, L.; Flores, C.; Muñoz, A.; et al. Fish Oil Supplementation Attenuates Offspring’s Neurodevelopmental Changes Induced by a Maternal High-Fat Diet in a Rat Model. Nutrients 2025, 17, 1741. https://doi.org/10.3390/nu17101741
Muñoz Y, Kaune H, Dagnino-Subiabre A, Cruz G, Toledo J, Valenzuela R, Moraga R, Tabilo L, Flores C, Muñoz A, et al. Fish Oil Supplementation Attenuates Offspring’s Neurodevelopmental Changes Induced by a Maternal High-Fat Diet in a Rat Model. Nutrients. 2025; 17(10):1741. https://doi.org/10.3390/nu17101741
Chicago/Turabian StyleMuñoz, Yasna, Heidy Kaune, Alexies Dagnino-Subiabre, Gonzalo Cruz, Jorge Toledo, Rodrigo Valenzuela, Renato Moraga, Luis Tabilo, Cristian Flores, Alfredo Muñoz, and et al. 2025. "Fish Oil Supplementation Attenuates Offspring’s Neurodevelopmental Changes Induced by a Maternal High-Fat Diet in a Rat Model" Nutrients 17, no. 10: 1741. https://doi.org/10.3390/nu17101741
APA StyleMuñoz, Y., Kaune, H., Dagnino-Subiabre, A., Cruz, G., Toledo, J., Valenzuela, R., Moraga, R., Tabilo, L., Flores, C., Muñoz, A., Crisosto, N., Montiel, J. F., & Maliqueo, M. (2025). Fish Oil Supplementation Attenuates Offspring’s Neurodevelopmental Changes Induced by a Maternal High-Fat Diet in a Rat Model. Nutrients, 17(10), 1741. https://doi.org/10.3390/nu17101741








