Low-Calorie, High-Protein Ketogenic Diet Versus Low-Calorie, Low-Sodium, and High-Potassium Mediterranean Diet in Overweight Patients and Patients with Obesity with High-Normal Blood Pressure or Grade I Hypertension: The Keto–Salt Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Office and Ambulatory Blood Pressure Measurement
2.3. Dietary Intervention
2.4. Baseline and Follow-Up Assessments
2.5. Statistical Analysis
3. Results
3.1. Study Population
3.2. Changes Following Dietary Intervention in the Overall Population
3.3. Comparative Effects of the KD and MD
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OW | Overweight |
| OB | Obesity |
| BP | Blood Pressure |
| KD | Ketogenic Diet |
| MD | Mediterranean Diet |
| ABPM | Ambulatory Blood Pressure Monitoring |
| BIA | Bioelectrical Impedance Analysis |
| BMI | Body Mass Index |
| SCORE2 | Systematic Coronary Risk Estimation 2 |
| FFM | Free Fat Mass |
| FM | Fat Mass |
| CVD | Cardiovascular Disease |
| SBP | Systolic Blood Pressure |
| DBP | Diastolic Blood Pressure |
| ESH | European Society of Hypertension |
| WC | Waist Circumference |
| DM | Diabetes Mellitus |
| CKD | Chronic Kidney Disease |
| eGFR | Estimated Glomerular Filtration Rate |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| TG | Triglycerides |
| GCP | Good Clinical Practice |
| TBW | Total Body Water |
| ECW | Extra-Cellular Water |
| ICW | Intra-Cellular Water |
| TC | Total Cholesterol |
| HDL-C | High-Density Lipoprotein Cholesterol |
| ApoB | Apolipoprotein B |
| UA | Uric Acid |
| HbA1c | Glycosylated Haemoglobin |
| HS-CRP | High-Sensitivity C-Reactive Protein |
| IR | Insulin Resistance |
| HOMA-IR | Homeostasis Model Assessment of Insulin Resistance |
| SD | Standard Deviation |
| IQR | Interquartile Range |
| SPSS | Statistical Package for Social Sciences |
| NCC | Sodium-Chloride Cotransporter |
| DASH | Dietary Approach to Stop Hypertension |
| VLCKD | Very-Low-Calorie Ketogenic Diet |
| RCT | Randomized Controlled Trial |
References
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the Management of Arterial Hypertension The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA): Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the Management of Elevated Blood Pressure and Hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar]
- Prinelli, F.; Yannakoulia, M.; Anastasiou, C.A.; Adorni, F.; Di Santo, S.G.; Musicco, M.; Scarmeas, N.; Correa Leite, M.L. Mediterranean Diet and Other Lifestyle Factors in Relation to 20-Year All-Cause Mortality: A Cohort Study in an Italian Population. Br. J. Nutr. 2015, 113, 1003–1011. [Google Scholar] [CrossRef]
- Barrea, L.; Boschetti, M.; Gangitano, E.; Guglielmi, V.; Verde, L.; Muscogiuri, G. Long-Term Efficacy and Safety of Nutritional and Pharmacological Strategies for Obesity. Curr. Obes. Rep. 2025, 14, 1. [Google Scholar] [CrossRef]
- Brouns, F. Overweight and Diabetes Prevention: Is a Low-Carbohydrate–High-Fat Diet Recommendable? Eur. J. Nutr. 2018, 57, 1301–1312. [Google Scholar] [CrossRef]
- Landolfo, M.; Spannella, F.; Gezzi, A.; Giulietti, F.; Sabbatini, L.; Bari, I.; Alessandroni, R.; Di Agostini, A.; Turri, P.; Alborino, F.; et al. Validation of the Novel Web-Based Application HUMTELEMED for a Comprehensive Assessment of Cardiovascular Risk Based on the 2021 European Society of Cardiology Guidelines. J. Clin. Med. 2024, 13, 2295. [Google Scholar] [CrossRef]
- Spannella, F.; Giulietti, F.; Fedecostante, M.; Ricci, M.; Balietti, P.; Cocci, G.; Landi, L.; Bonfigli, A.R.; Boemi, M.; Espinosa, E.; et al. Interarm Blood Pressure Differences Predict Target Organ Damage in Type 2 Diabetes. J. Clin. Hypertens. 2017, 19, 472–478. [Google Scholar] [CrossRef]
- Omboni, S.; Palatini, P.; Parati, G. Working Group on Blood Pressure Monitoring of the Italian Society of Hypertension Standards for Ambulatory Blood Pressure Monitoring Clinical Reporting in Daily Practice: Recommendations from the Italian Society of Hypertension. Blood Press. Monit. 2015, 20, 241–244. [Google Scholar] [CrossRef]
- Gregório, M.J.; Rodrigues, A.M.; Salvador, C.; Dias, S.S.; de Sousa, R.D.; Mendes, J.M.; Coelho, P.S.; Branco, J.C.; Lopes, C.; Martínez-González, M.A.; et al. Validation of the telephone-administered version of the Mediterranean diet adherence screener (MEDAS) questionnaire. Nutrients 2020, 12, 1511. [Google Scholar] [CrossRef]
- Mannocci, A.; Di Thiene, D.; Del Cimmuto, A.; Masala, D.; Boccia, A.; De Vito, E. International Physical Activity Questionnaire: Validation and assessment in an Italian sample. Ital. J. Public Health 2010, 7, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Giugliano, R.P.; Murphy, S.A.; Wasserman, S.M.; Stein, E.A.; Ceška, R.; López-Miranda, J.; Georgiev, B.; Lorenzatti, A.J.; Tikkanen, M.J.; et al. Comparison of Low-Density Lipoprotein Cholesterol Assessment by Martin/Hopkins Estimation, Friedewald Estimation, and Preparative Ultracentrifugation: Insights from the FOURIER Trial. JAMA Cardiol. 2018, 3, 749–753. [Google Scholar] [CrossRef]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity-Induced Hypertension: Interaction of Neurohumoral and Renal Mechanisms. Circ. Res. 2015, 116, 991–1006. [Google Scholar] [CrossRef]
- Landolfo, M.; Spannella, F.; Poliseno, C.; Massacesi, A.; Giulietti, F.; Festa, R.; Cavazzin, E.; Sasso, G.; Mazza, A.; Sarzani, R. The Effects of ESC/ESH-Based Written Generic Lifestyle Advice and a Nutraceutical on 24-Hour Blood Pressure in Patients with High-Normal Office Blood Pressure and Low-Moderate Cardiovascular Risk. Nutrients 2023, 15, 5099. [Google Scholar] [CrossRef]
- Leslie, W.S.; Ali, E.; Harris, L.; Messow, C.M.; Brosnahan, N.T.; Thom, G.; McCombie, E.L.; Barnes, A.C.; Sattar, N.; Taylor, R.; et al. Antihypertensive Medication Needs and Blood Pressure Control with Weight Loss in the Diabetes Remission Clinical Trial (DiRECT). Diabetologia 2021, 64, 1927–1938. [Google Scholar] [CrossRef]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Efficacy and Safety of Once-Weekly Subcutaneous Semaglutide 2.4 MG in Adults with Overweight or Obesity (STEP 1). J. Endocr. Soc. 2021, 5, A10–A10. [Google Scholar] [CrossRef]
- de Lemos, J.A.; Linetzky, B.; le Roux, C.W.; Laffin, L.J.; Vongpatanasin, W.; Fan, L.; Hemmingway, A.; Ahmad, N.N.; Bunck, M.C.; Stefanski, A. Tirzepatide Reduces 24-Hour Ambulatory Blood Pressure in Adults with Body Mass Index ≥27 Kg/M2: SURMOUNT-1 Ambulatory Blood Pressure Monitoring Substudy. Hypertension 2024, 81, e41–e43. [Google Scholar] [CrossRef]
- Krumholz, H.M.; de Lemos, J.A.; Sattar, N.; Linetzky, B.; Sharma, P.; Mast, C.J.; Ahmad, N.N.; Bunck, M.C.; Stefanski, A. Tirzepatide and Blood Pressure Reduction: Stratified Analyses of the SURMOUNT-1 Randomised Controlled Trial. Heart 2024, 110, 1165–1171. [Google Scholar] [CrossRef]
- Covassin, N.; Sert-Kuniyoshi, F.H.; Singh, P.; Romero-Corral, A.; Davison, D.E.; Lopez-Jimenez, F.; Jensen, M.D.; Somers, V.K. Experimental Weight Gain Increases Ambulatory Blood Pressure in Healthy Subjects: Implications of Visceral Fat Accumulation. Mayo Clin. Proc. 2018, 93, 618–626. [Google Scholar] [CrossRef]
- Filippini, T.; Malavolti, M.; Whelton, P.K.; Naska, A.; Orsini, N.; Vinceti, M. Blood Pressure Effects of Sodium Reduction: Dose-Response Meta-Analysis of Experimental Studies. Circulation 2021, 143, 1542–1567. [Google Scholar] [CrossRef]
- Bailey, M.A.; Dhaun, N. Salt Sensitivity: Causes, Consequences, and Recent Advances. Hypertension 2024, 81, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., 3rd; Simons-Morton, D.G.; et al. Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef] [PubMed]
- La Dieta Mediterranea. Available online: https://www.iss.it/web/guest/-/dieta-mediterranea (accessed on 15 April 2025).
- Di Raimondo, D.; Buscemi, S.; Musiari, G.; Rizzo, G.; Pirera, E.; Corleo, D.; Pinto, A.; Tuttolomondo, A. Ketogenic Diet, Physical Activity, and Hypertension-A Narrative Review. Nutrients 2021, 13, 2567. [Google Scholar] [CrossRef]
- Caprio, M.; Infante, M.; Moriconi, E.; Armani, A.; Fabbri, A.; Mantovani, G.; Mariani, S.; Lubrano, C.; Poggiogalle, E.; Migliaccio, S.; et al. Very-Low-Calorie Ketogenic Diet (VLCKD) in the Management of Metabolic Diseases: Systematic Review and Consensus Statement from the Italian Society of Endocrinology (SIE). J. Endocrinol. Invest. 2019, 42, 1365–1386. [Google Scholar] [CrossRef]
- O’Hearn, L.A. The Therapeutic Properties of Ketogenic Diets, Slow-Wave Sleep, and Circadian Synchrony. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 503–508. [Google Scholar] [CrossRef]
- Barrea, L.; Pugliese, G.; Frias-Toral, E.; Napolitano, B.; Laudisio, D.; Aprano, S.; Ceriani, F.; Savastano, S.; Colao, A.; Muscogiuri, G. Is there a relationship between the ketogenic diet and sleep disorders? Int. J. Food Sci. Nutr. 2022, 73, 285–295. [Google Scholar] [CrossRef]
- Katsuya, S.; Kawata, Y.; Kawamura, Y.; Kawamura, J.; Tsubota, J. Effect of d-β-hydroxybutyrate on sleep quality in healthy participants: A randomized, double-blind, placebo-controlled study. Biosci. Biotechnol. Biochem. 2025, 89, 769–775. [Google Scholar] [CrossRef]
- Gonzalez, J.E.; Cooke, W.H. Influence of an acute fast on ambulatory blood pressure and autonomic cardiovascular control. Am J. Physiol. Regul. Integr. Comp. Physiol. 2022, 322, R542–R550. [Google Scholar] [CrossRef]
- Polito, R.; Valenzano, A.; Monda, V.; Cibelli, G.; Monda, M.; Messina, G.; Villano, I.; Messina, A. Heart rate variability and sympathetic activity is modulated by very low-calorie ketogenic diet. Int. J. Environ. Res. Public Health 2022, 19, 2253. [Google Scholar] [CrossRef]
- Perticone, M.; Maio, R.; Sciacqua, A.; Suraci, E.; Pinto, A.; Pujia, R.; Zito, R.; Gigliotti, S.; Sesti, G.; Perticone, F. Ketogenic Diet-Induced Weight Loss Is Associated with an Increase in Vitamin D Levels in Obese Adults. Molecules 2019, 24, 2499. [Google Scholar] [CrossRef]
- Leung, L.Y.-L.; Tam, H.-L.; Ho, J.K.-M. Effects of Ketogenic and Low-Carbohydrate Diets on the Body Composition of Adults with Overweight or Obesity: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Clin. Nutr. 2025, 46, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Mancini, J.G.; Filion, K.B.; Atallah, R.; Eisenberg, M.J. Systematic Review of the Mediterranean Diet for Long-Term Weight Loss. Am. J. Med. 2016, 129, 407–415.e4. [Google Scholar] [CrossRef] [PubMed]
- Moreno, B. Comparison of a Very Low-Calorie Ketogenic Diet with a Standard Low-Calorie Diet in the Treatment of Obesity. Endocrine 2014, 47, 793–805. [Google Scholar] [CrossRef]
- Landry, M.J.; Crimarco, A.; Perelman, D.; Durand, L.R.; Petlura, C.; Aronica, L.; Robinson, J.L.; Kim, S.H.; Gardner, C.D. Adherence to Ketogenic and Mediterranean Study Diets in a Crossover Trial: The Keto-Med Randomized Trial. Nutrients 2021, 13, 967. [Google Scholar] [CrossRef]
- Balietti, P.; Spannella, F.; Giulietti, F.; Rosettani, G.; Bernardi, B.; Cocci, G.; Bonfigli, A.R.; Sarzani, R. Ten-year Changes in Ambulatory Blood Pressure: The Prognostic Value of Ambulatory Pulse Pressure. J. Clin. Hypertens. 2018, 20, 1230–1237. [Google Scholar] [CrossRef]

| Parameters | KD (n = 15) | MD (n = 11) | p-Value * | ||
|---|---|---|---|---|---|
| T0 | T3 | T0 | T3 | ||
| Age (years) | 41.7 ± 11.4 | - | 52.0 ± 10.2 | - | - |
| Sex (%, male) | 73.3% | - | 54.5% | - | - |
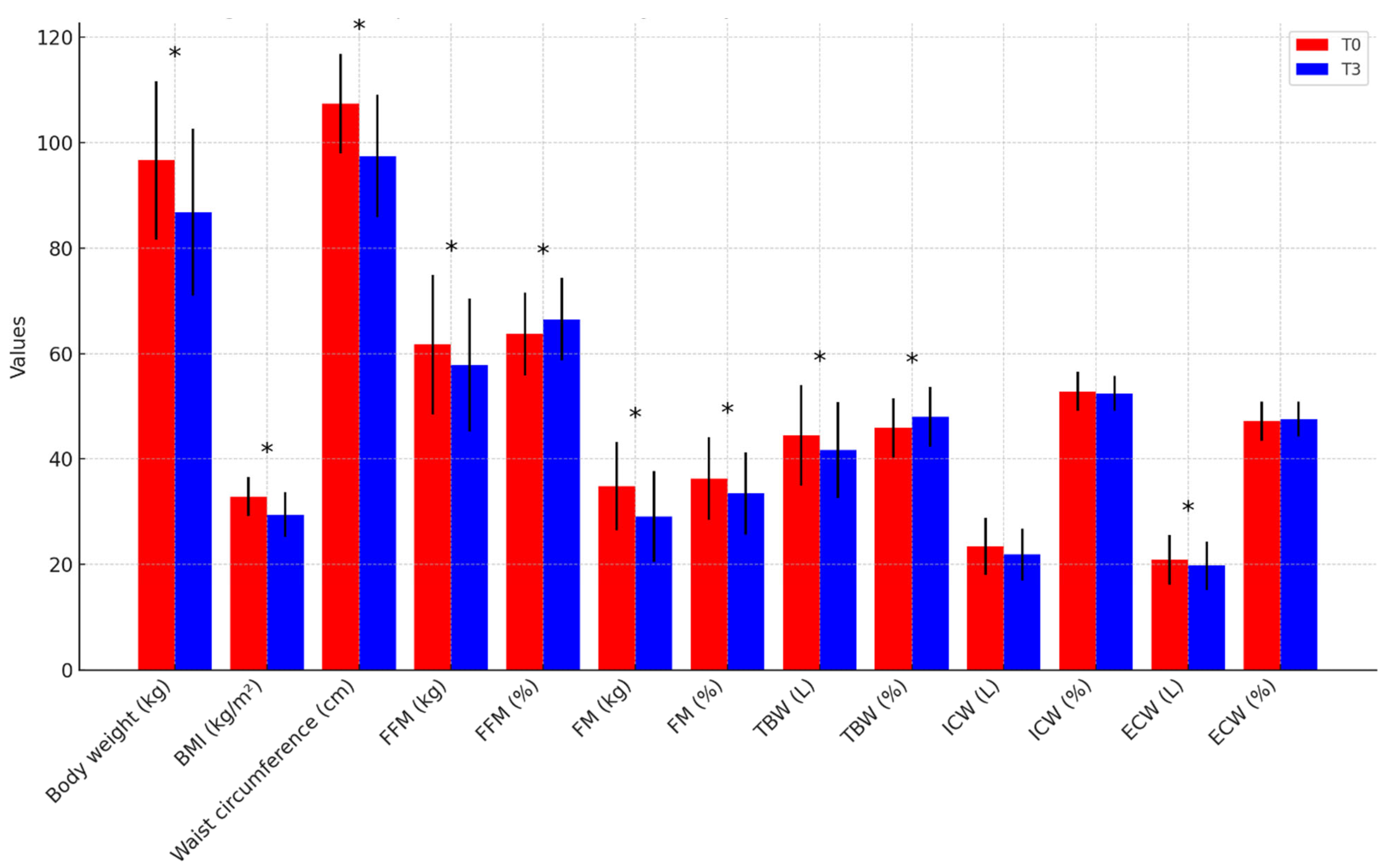
| Body weight (kg) | 98.6 ± 13.0 | 87.3 ± 13.4 | 93.8 ± 17.7 | 86.1 ± 19.3 | p = 0.849 |
| BMI (kg/m2) | 33.5 ± 4.0 | 29.6 ± 4.2 | 32.0 ± 3.4 | 29.4 ± 4.2 | p = 0.874 |
| Waist circumference (cm) | 107.2 ± 7.2 | 95.4 ± 9.4 | 107.8 ± 12.1 | 100.0 ± 13.4 | p = 0.313 |
| FFM (kg) | 63.6 ± 11.3 | 59.1 ± 10.4 | 59.0 ± 15.6 | 56.1 ± 15.5 | p = 0.557 |
| FFM (%) | 64.6 ± 8.0 | 67.8 ± 8.2 | 62.4 ± 7.7 | 64.8 ± 7.2 | p = 0.340 |
| FM (kg) | 34.9 ± 9.3 | 28.4 ± 9.2 | 34.7 ± 7.4 | 30.0 ± 8.0 | p = 0.638 |
| FM (%) | 35.4 ± 8.0 | 32.2 ± 8.2 | 37.6 ± 7.7 | 35.2 ± 7.2 | p = 0.340 |
| TBW (L) | 45.9 ± 8.1 | 42.6 ± 7.5 | 42.5 ± 11.2 | 40.5 ± 11.1 | p = 0.557 |
| TBW (%) | 46.5 ± 5.7 | 48.9 ± 5.9 | 45.0 ± 5.6 | 46.7 ± 5.2 | p = 0.341 |
| ICW (L) | 24.8 ± 4.9 | 22.8 ± 4.3 | 21.7 ± 5.8 | 20.5 ± 5.5 | p = 0.236 |
| ICW (%) | 54.0 ± 3.8 | 53.6 ± 3.1 | 51.2 ± 2.8 | 50.8 ± 3.1 | p = 0.029 |
| ECW (L) | 21.1 ± 4.1 | 19.8 ± 3.6 | 20.8 ± 5.7 | 19.9 ± 5.9 | p = 0.926 |
| ECW (%) | 46.0 ± 3.8 | 46.4 ± 3.1 | 48.8 ± 2.8 | 49.2 ± 3.1 | p = 0.029 |
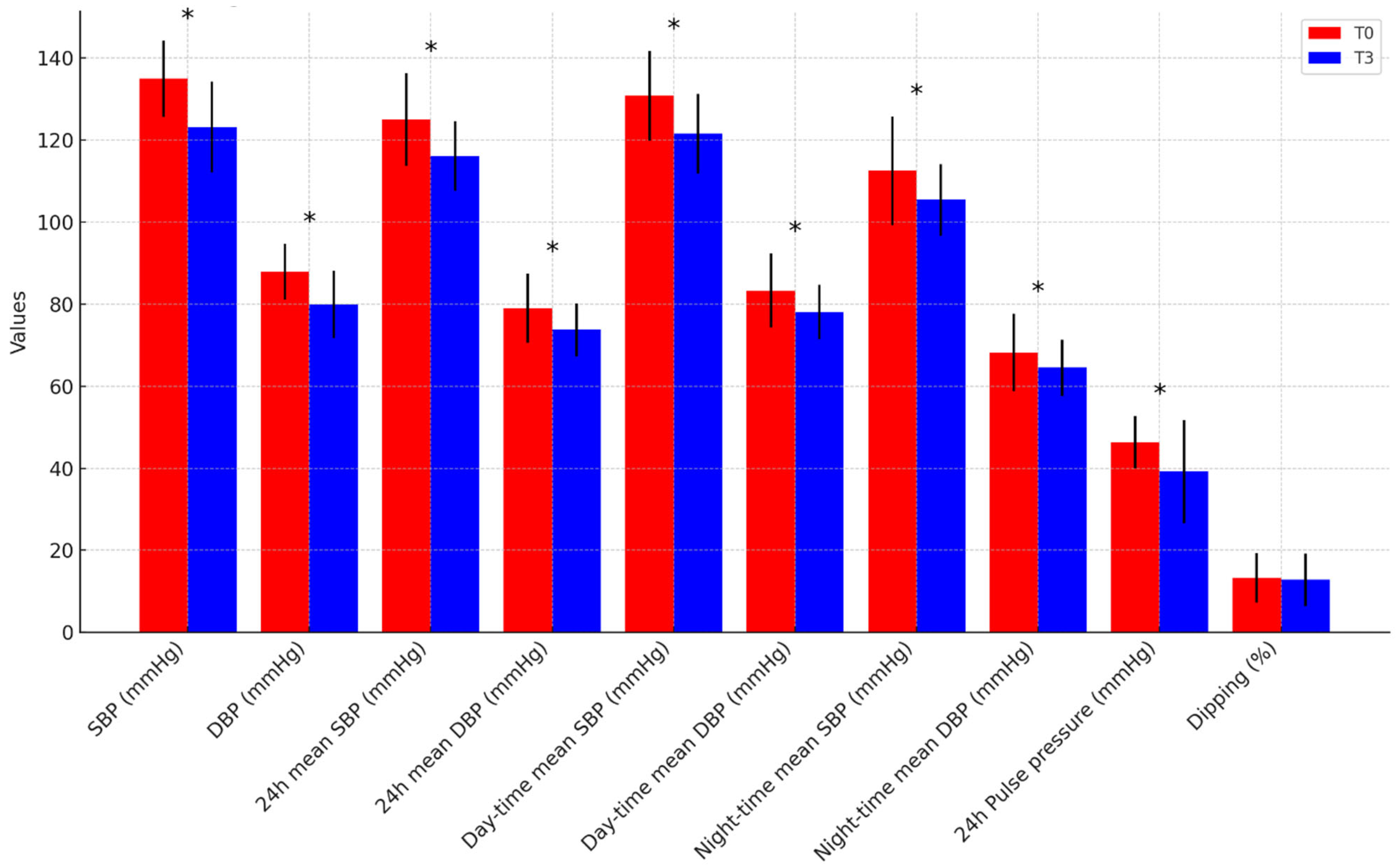
| SBP (mmHg) | 134.2 ± 8.4 | 122.7 ± 7.2 | 135.9 ± 10.8 | 123.7 ± 15.4 | p = 0.824 |
| DBP (mmHg) | 88.6 ± 6.6 | 79.5 ± 6.8 | 86.9 ± 7.3 | 80.6 ± 10.1 | p = 0.741 |
| 24 h mean SBP (mmHg) | 127.3 ± 9.9 | 118.3 ± 7.4 | 123.9 ± 13.2 | 113.1 ± 9.5 | p = 0.146 |
| 24 h mean DBP (mmHg) | 81.2 ± 8.0 | 75.6 ± 5.8 | 77.2 ± 8.4 | 71.0 ± 6.6 | p = 0.085 |
| Daytime mean SBP (mmHg) | 131.8 ± 9.4 | 124.6 ± 7.0 | 131.3 ± 13.2 | 117.3 ± 11.6 | p = 0.069 |
| Daytime mean DBP (mmHg) | 84.3 ± 8.6 | 80.3 ± 5.0 | 83.2 ± 9.6 | 75.0 ± 7.5 | p = 0.050 |
| Night-time mean SBP (mmHg) | 116.2 ± 12.8 | 105.6 ± 9.0 | 110.1 ± 13.9 | 104.7 ± 8.6 | p = 0.756 |
| Night-time mean DBP (mmHg) | 71.2 ± 10.4 | 65.6 ± 7.4 | 65.5 ± 6.7 | 63.1 ± 5.8 | p = 0.391 |
| 24 h pulse pressure (mmHg) | 46.1 ± 5.3 | 39.9 ± 11.8 | 46.7 ± 8.0 | 38.3 ± 14.2 | p = 0.758 |
| Dipping (%) | 12.4 ± 6.3 | 15 ± 4.9 | 16.3 ± 5.8 | 10.3 ±7.3 | p = 0.074 |
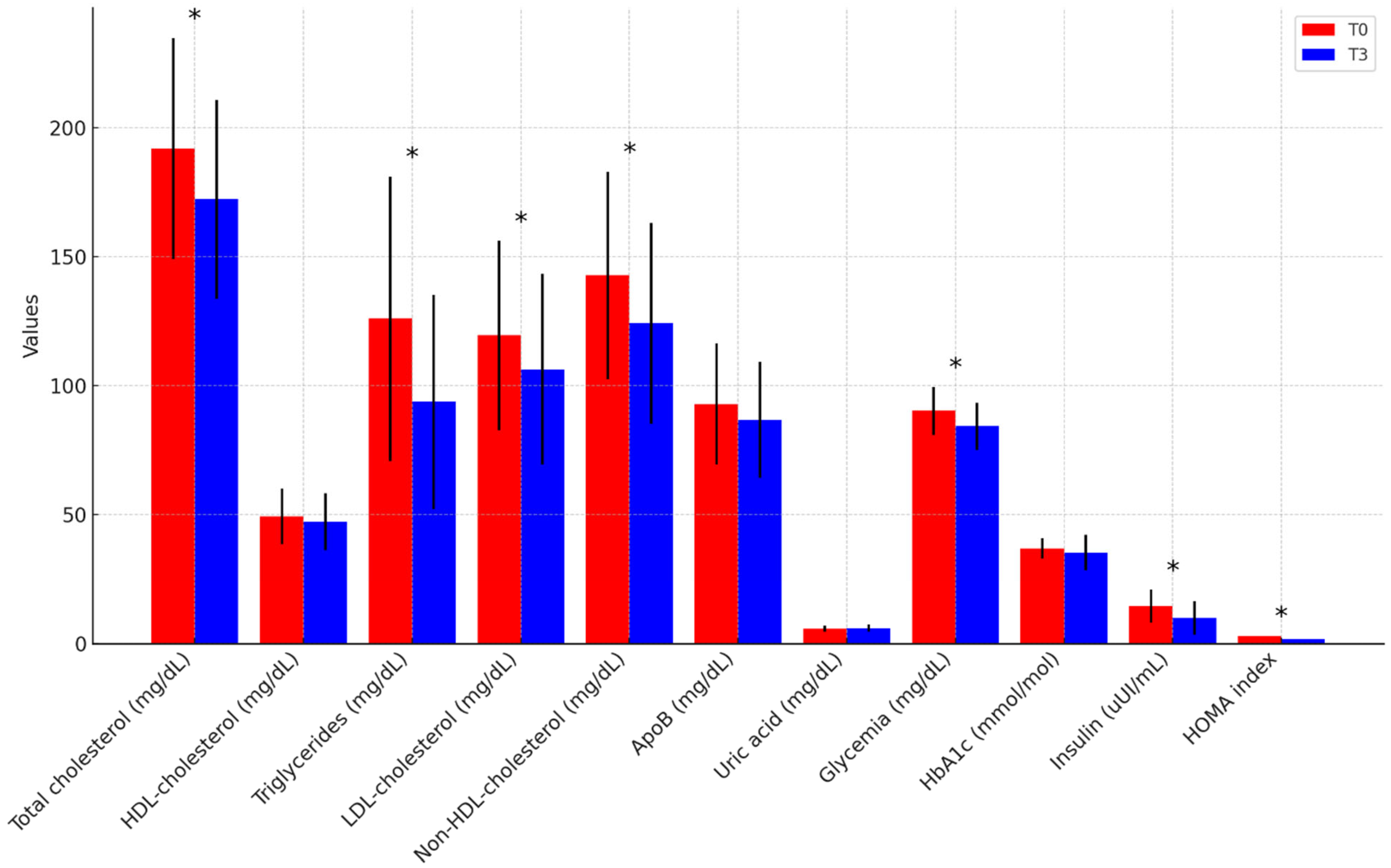
| Total cholesterol (mg/dL) | 192.2 ± 44.1 | 170.6 ± 40.8 | 191.9 ± 43.5 | 174.7 ± 37.4 | p = 0.794 |
| HDL-cholesterol (mg/dL) | 47.9 ± 10.0 | 47.2 ± 12.1 | 51.2 ± 12.1 | 47.2 ± 10.2 | p = 0.997 |
| Triglycerides (mg/dL) | 129.7 ± 66.3 | 86.1 ± 47.3 | 120.8 ± 37.3 | 104.0 ± 31.6 | p = 0.289 |
| LDL-cholesterol (mg/dL) | 120.5 ± 36.8 | 105.7 ± 38.7 | 118.1 ± 38.4 | 107.2 ± 36.2 | p = 0.925 |
| Non-HDL-cholesterol (mg/dL) | 144.3 ± 41.8 | 122.3 ± 40.1 | 140.7 ± 40.1 | 126.7 ± 39.1 | p = 0.783 |
| ApoB (mg/dL) | 92.9 ± 21.4 | 85.9 ± 19.9 | 92.6 ± 27.0 | 89.2 ± 25.7 | p = 0.714 |
| Uric acid (mg/dL) | 5.8 ± 0.8 | 6.2 ± 1.3 | 5.2 ± 1.4 | 5.2 ± 1.3 | p = 0.063 |
| Glycemia (mg/dL) | 87.7 ± 8.9 | 83.3 ± 10.3 | 93.5 ± 9.3 | 85.3 ± 7.8 | p = 0.605 |
| HbA1c (mmol/mol) | 35.4 ± 3.8 | 32.7 ± 8.1 | 38.8 ± 3.2 | 38.6 ± 2.4 | p = 0.028 |
| Insulin (uUI/mL) | 14.8 ± 7.4 | 9.8 ± 7.4 | 14.0 ± 5.0 | 9.7 ± 5.1 | p = 0.971 |
| Creatinine (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | p = 0.402 |
| Urea (mg/dL) | 34.1 ± 7.3 | 35.6 ± 10.6 | 37.2 ± 7.5 | 33.4 ± 8.9 | p = 0.576 |
| Sodium (mEq/L) | 139.9 ± 1.9 | 140.5 ± 2.2 | 140.5 ± 1.9 | 142.1 ± 3.1 | p = 0.145 |
| Potassium (mEq/L) | 4.2 ± 0.6 | 4.4 ± 0.5 | 4.5 ± 0.3 | 4.4 ± 0.3 | p = 0.699 |
| HS-CRP (mg/dL) | 0.82 (0.52–3.31) | 0.79 (0.32–2.11) | 3.29 (1.71–5.49) | 2.76 (1.43–6.83) | p = 0.081 |
| 24 h Na excretion (mEq/24 h) | 198.1 ± 89.6 | 149.4 ± 84.0 | 169.0 ± 61.3 | 140.0 ± 72.0 | p = 0.790 |
| 24 h K excretion (mEq/24 h) | 58.3 ± 26.0 | 70.5 ± 36.9 | 67.7 ± 29.6 | 61.8 ± 24.1 | p = 0.548 |
| HOMA index | 2.86 (1.69–3.75) | 1.79 (1.01–2.88) | 2.90 (2.09–4.36) | 1.71 (1.38–3.00) | p = 0.967 |
| Albuminuria/Creatininuria (mg/g) | 7.00 (2.35–12) | - | 5.6 (4.10–14.70) | - | p = 0.849 |
| 24 h cortisol excretion (mcg/24 h) | 164.6 (97.4–345) | - | 120 (41.4–272.1) | - | p = 0.874 |
| Parameter | Δ T3-T0 KD | Δ T3-T0 MD | p-Value |
|---|---|---|---|
| Body weight (kg) | −11.3 ± 4.2 | −7.7 ± 5.1 | p = 0.058 |
| BMI (kg/m2) | −3.9 ± 1.4 | −2.7 ± 1.8 | p = 0.064 |
| Waist circumference (cm) | −11.8 ± 4.1 | −7.7 ± 7.0 | p = 0.077 |
| FFM (kg) | −4.5 ± 2.3 | −2.9 ± 2.3 | p = 0.083 |
| FFM (%) | 3.2 ± 1.7 | 2.4 ± 2.2 | p = 0.250 |
| FM (kg) | −6.6 ± 2.3 | −4.7 ± 3.4 | p = 0.107 |
| FM (%) | −3.2 ± 1.6 | −2.3 ± 2.1 | p = 0.250 |
| TBW (L) | −3.2 ± 1.7 | −2.1 ± 1.7 | p = 0.090 |
| TBW (%) | 2.3 ± 1.2 | 1.7 ± 1.6 | p = 0.258 |
| ICW (L) | −1.9 ± 1.7 | −1.2 ± 0.9 | p = 0.223 |
| ICW (%) | −0.4 ± 3.2 | −0.4 ± 1.1 | p = 0.955 |
| ECW (L) | −1.3 ± 1.8 | −0.8 ± 1.1 | p = 0.420 |
| ECW (%) | 0.4 ± 3.2 | 0.4 ± 1.1 | p = 0.955 |
| SBP (mmHg) | −11.5 ± 6.6 | −12.2 ± 12.0 | p = 0.852 |
| DBP (mmHg) | −9.1 ± 6.4 | −6.2 ± 7.0 | p = 0.294 |
| 24 h mean SBP (mmHg) | −8.6 ± 6.2 | −9.2 ± 19.0 | p = 0.909 |
| 24 h mean DBP (mmHg) | −5.4 ± 4.5 | −5.2 ± 8.6 | p = 0.933 |
| 24 h daytime mean SBP (mmHg) | −7.1 ± 6.0 | −12.4 ± 19.9 | p = 0.352 |
| 24 h daytime mean DBP (mmHg) | −3.9 ± 4.9 | −7.1 ± 9.0 | p = 0.267 |
| 24 h night-time mean SBP (mmHg) | −9.5 ± 7.9 | −3.8 ± 17.3 | p = 0.287 |
| 24 h night-time mean DBP (mmHg) | −5.1 ± 6.2 | −1.6 ± 7.6 | p = 0.231 |
| 24 h differential pressure (mmHg) | −3.1 ± 5.1 | −8.5 ± 18.0 | p = 0.302 |
| Dipping (%) | 2.6 ± 3.8 | −6.0 ± 5.7 | p < 0.001 |
| Total cholesterol (mg/dL) | −21.6 ± 45.5 | −17.2 ± 22.3 | p = 0.770 |
| HDL-cholesterol (mg/dL) | −0.7 ± 8.0 | −4.0 ± 6.8 | p = 0.277 |
| Triglycerides (mg/dL) | −43.6 ± 75.5 | −16.8 ± 40.7 | p = 0.298 |
| LDL-cholesterol (mg/dL) | −14.9 ± 39.9 | −11.0 ± 18.4 | p = 0.769 |
| Non-HDL-cholesterol (mg/dL) | −22.0 ± 43.4 | −14.0 ± 22.8 | p = 0.584 |
| ApoB (mg/dL) | −8.1 ± 24.6 | −3.5 ± 17.4 | p = 0.604 |
| Uric acid (mg/dL) | 0.4 ± 0.9 | 0.0 ± 1.0 | p = 0.319 |
| Glycemia (mg/dL) | −4.3 ± 8.2 | −8.3 ± 9.9 | p = 0.277 |
| HbA1c (%) | −0.3 ± 0.7 | −0.0 ± 0.2 | p = 0.338 |
| Insulin (µUI/mL) | −4.9 ± 7.4 | −4.3 ± 5.9 | p = 0.813 |
| Creatinine (mg/dL) | −0.0 ± 0.1 | −0.0 ± 0.1 | p = 0.810 |
| Urea (mg/dL) | 1.5 ± 6.8 | −3.8 ± 6.0 | p = 0.048 |
| Sodium (mEq/L) | 0.6 ± 2.2 | 1.6 ± 2.8 | p = 0.301 |
| Potassium (mEq/L) | 0.2 ± 0.5 | −0.1 ± 0.3 | p = 0.081 |
| HS-CRP (mg/L) | −1.7 ± 4.7 | 4.9 ± 13.6 | p = 0.195 |
| 24 h Na excretion (mEq/24 h) | −42.6 ± 127.4 | −34.3 ± 70.3 | p = 0.863 |
| 24 h K excretion (mEq/24 h) | 15.2 ± 49.4 | −5.5 ± 28.0 | p = 0.276 |
| HOMA index | −1.1 ± 1.3 | −1.1 ± 1.5 | p = 0.993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landolfo, M.; Stella, L.; Gezzi, A.; Spannella, F.; Turri, P.; Sabbatini, L.; Cecchi, S.; Lucchetti, B.; Petrelli, M.; Sarzani, R. Low-Calorie, High-Protein Ketogenic Diet Versus Low-Calorie, Low-Sodium, and High-Potassium Mediterranean Diet in Overweight Patients and Patients with Obesity with High-Normal Blood Pressure or Grade I Hypertension: The Keto–Salt Pilot Study. Nutrients 2025, 17, 1739. https://doi.org/10.3390/nu17101739
Landolfo M, Stella L, Gezzi A, Spannella F, Turri P, Sabbatini L, Cecchi S, Lucchetti B, Petrelli M, Sarzani R. Low-Calorie, High-Protein Ketogenic Diet Versus Low-Calorie, Low-Sodium, and High-Potassium Mediterranean Diet in Overweight Patients and Patients with Obesity with High-Normal Blood Pressure or Grade I Hypertension: The Keto–Salt Pilot Study. Nutrients. 2025; 17(10):1739. https://doi.org/10.3390/nu17101739
Chicago/Turabian StyleLandolfo, Matteo, Lucia Stella, Alessandro Gezzi, Francesco Spannella, Paolo Turri, Lucia Sabbatini, Sofia Cecchi, Beatrice Lucchetti, Massimiliano Petrelli, and Riccardo Sarzani. 2025. "Low-Calorie, High-Protein Ketogenic Diet Versus Low-Calorie, Low-Sodium, and High-Potassium Mediterranean Diet in Overweight Patients and Patients with Obesity with High-Normal Blood Pressure or Grade I Hypertension: The Keto–Salt Pilot Study" Nutrients 17, no. 10: 1739. https://doi.org/10.3390/nu17101739
APA StyleLandolfo, M., Stella, L., Gezzi, A., Spannella, F., Turri, P., Sabbatini, L., Cecchi, S., Lucchetti, B., Petrelli, M., & Sarzani, R. (2025). Low-Calorie, High-Protein Ketogenic Diet Versus Low-Calorie, Low-Sodium, and High-Potassium Mediterranean Diet in Overweight Patients and Patients with Obesity with High-Normal Blood Pressure or Grade I Hypertension: The Keto–Salt Pilot Study. Nutrients, 17(10), 1739. https://doi.org/10.3390/nu17101739







