CONUT Score as a Predictor of Mortality Risk in Acute and Chronic Heart Failure: A Meta-Analytic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria and Search Strategy
2.2. Selection Process
Data Extraction and Quality Assessment
2.3. Study Characteristics
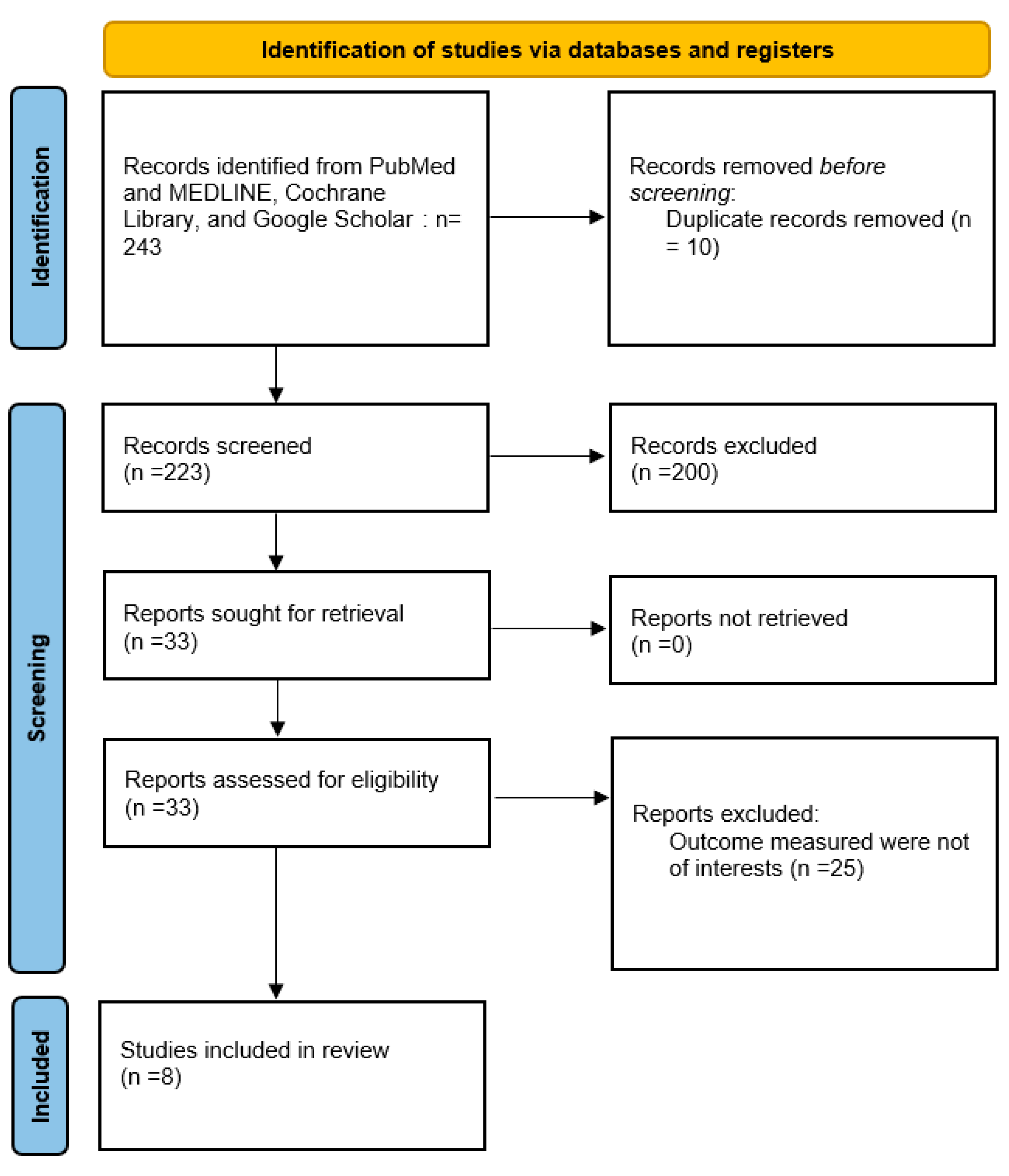
3. Results
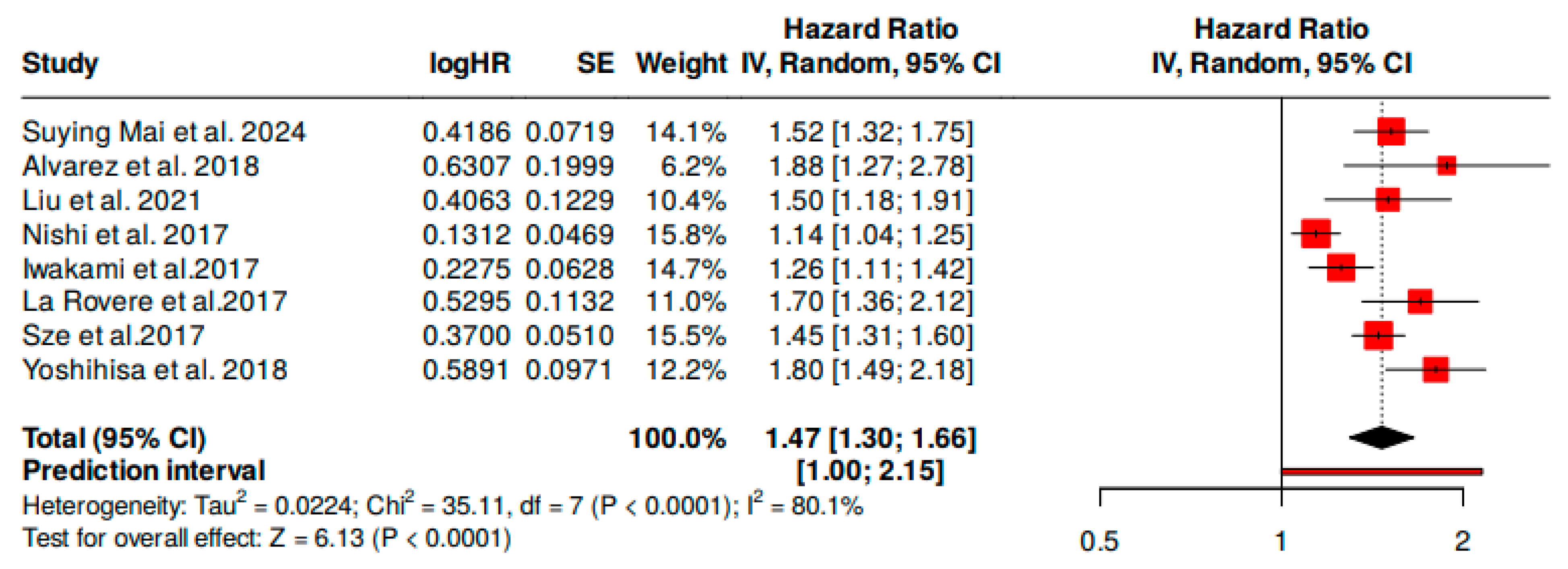
3.1. Overall Mortality Risk
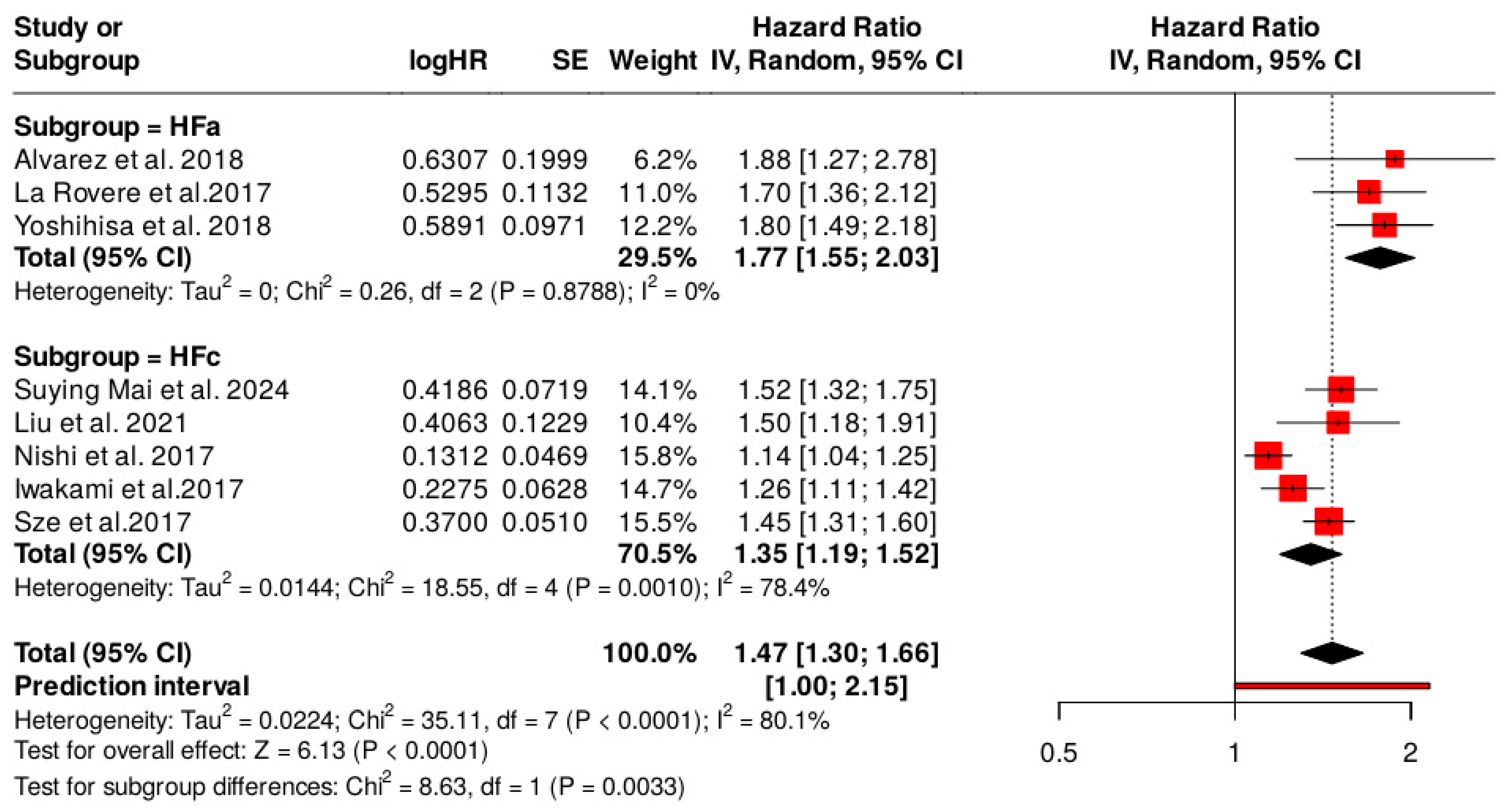
3.2. Subgroup Analyses for the Type of Heart Failure
3.3. Publication Bias
4. Discussion
4.1. Comparison with Other Prognostic Tools
4.2. Clinical Implications of CONUT in Heart Failure Management
4.3. Integration with Other Prognostic Markers
4.4. Heterogeinity
4.5. Strengths and Limitations of the Review
4.6. Directions for Future Research
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HF | Heart failure |
| ACE | Angiotensin-converting enzyme |
| CRT | Cardiac resynchronization therapy |
| 6MWT | 6-min walk test |
| CFS | Clinical frailty scale |
| BNP | B-type natriuretic peptide (a marker of heart failure) |
| LVEF | Left ventricular ejection fraction |
| BMI | Body mass index |
| EF | Ejection fraction |
| CRP | C-reactive protein |
| MAGGIC | Meta-Analysis Global Group in Chronic Heart Failure |
| CONUT | Controlling Nutritional Status |
| SGA | Subjective Global Assessment |
| GNRI | Geriatric Nutritional Risk Index |
| PNI | Prognostic Nutritional Index |
| MNA | Mini Nutritional Assessment |
| NYHA | New York Heart Association |
| CVD | Cardiovascular disease |
| COPD | Chronic obstructive pulmonary disease |
| ARB | Angiotensin receptor blocker |
| SGLT | Sodium–glucose cotransporter |
| ESC | European Society of Cardiology |
| HFA | Heart Failure Association |
| TNF | Tumor necrosis factor |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PICO | Patient, Intervention, Comparison, Outcome (framework for clinical questions) |
| HR | Hazard ratio |
| CI | Confidence interval |
| NOS | Newcastle–Ottawa Scale |
| OR | Odds ratio |
| NHANES | National Health and Nutrition Examination Survey |
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Severino, P.; D’Amato, A.; Prosperi, S.; Mariani, M.V.; Myftari, V.; Labbro Francia, A.; Cestiè, C.; Tomarelli, E.; Manzi, G.; Birtolo, L.I.; et al. Strategy for an early simultaneous introduction of four-pillars of heart failure therapy: Results from a single center experience. Am. J. Cardiovasc. Drugs. 2024, 24, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Baptista, R.; Silva Cardoso, J.; Canhão, H.; Maria Rodrigues, A.; Kislaya, I.; Franco, F.; Bernardo, F.; Pimenta, J.; Mendes, L.; Gonçalves, S.; et al. Portuguese heart failure prevalence: Observational study based on primary care records. Rev. Port. Cardiol. (Engl. Ed.) 2023, 42, 985–995. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Forse, R.A.; Shizgal, H.M. Serum albumin and nutritional status. J. Parenter. Enter. Nutr. 1980, 4, 450–454. [Google Scholar] [CrossRef]
- Bonilla-Palomas, J.L.; Gámez-López, A.L.; Castillo-Domínguez, J.C.; Moreno-Conde, M.; López Ibáñez, M.C.; Alhambra Expósito, R.; Ramiro Ortega, E.; Anguita-Sánchez, M.P.; Villar-Ráez, A. Nutritional Intervention in Malnourished Hospitalized Patients with Heart Failure. Arch. Med. Res. 2016, 47, 535–540. [Google Scholar] [CrossRef]
- Lv, S.; Ru, S. The prevalence of malnutrition and its effects on the all-cause mortality among patients with heart failure: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0259300. [Google Scholar] [CrossRef]
- Nishi, I.; Seo, Y.; Hamada-Harimura, Y.; Sato, K.; Sai, S.; Yamamoto, M.; Ishizu, T.; Sugano, A.; Obara, K.; Wu, L.; et al. Nutritional screening based on the controlling nutritional status (CONUT) score at the time of admission is useful for long-term prognostic prediction in patients with heart failure requiring hospitalization. Heart Vessels. 2017, 32, 1337–1349. [Google Scholar] [CrossRef]
- Ulíbarri, J.I.; González-Madroño, A.; de Villar, N.G.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Li, H.; Zhou, P.; Zhao, Y.; Ni, H.; Luo, X.; Li, J. Prediction of all-cause mortality with malnutrition assessed by controlling nutritional status score in patients with heart failure: A systematic review and meta-analysis. Public Health Nutr. 2022, 25, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.W.; Luo, J.J.; Baldinger, B. The controlling nutritional status score and clinical outcomes in patients with heart failure: Pool analysis of observational studies. Front. Cardiovasc. Med. 2022, 9, 961141. [Google Scholar] [CrossRef]
- Narumi, T.; Arimoto, T.; Funayama, A.; Kadowaki, S.; Otaki, Y.; Nishiyama, S.; Takahashi, H.; Shishido, T.; Miyashita, T.; Miyamoto, T.; et al. Prognostic importance of objective nutritional indexes in patients with chronic heart failure. J. Cardiol. 2013, 62, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Iwakami, N.; Nagai, T.; Furukawa, T.A.; Sugano, Y.; Honda, S.; Okada, A.; Asaumi, Y.; Aiba, T.; Noguchi, T.; Kusano, K.; et al. Prognostic value of malnutrition assessed by Controlling Nutritional Status score for long-term mortality in patients with acute heart failure. Int. J. Cardiol. 2017, 230, 529–536. [Google Scholar] [CrossRef]
- Kato, Y.; Yamada, S.; Suenaga, M.; Takami, H.; Niwa, Y.; Hayashi, M.; Iwata, N.; Kanda, M.; Tanaka, C.; Nakayama, G.; et al. Impact of the Controlling Nutritional Status Score on the Prognosis After Curative Resection of Pancreatic Ductal Adenocarcinoma. Pancreas 2018, 47, 823–829. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, X.; Qian, L.; Chen, Z.; Hua, X.; Zhang, D.; Wei, H. The association between nutritional-inflammatory status and chronic kidney disease prognosis: A population-based study. Ren Fail. 2025, 47, 2471016. [Google Scholar] [CrossRef]
- Zuo, J.; Huang, Y.; Huang, Z.; Zhang, J.; Hou, W.; Wang, C.; Wang, X.; Bu, X. Comparison of three objective nutritional screening tools for identifying GLIM-defined malnutrition in patients with gastric cancer. Eur. J. Clin. Nutr. 2025, 79, 64–70. [Google Scholar] [CrossRef]
- Chávez-Tostado, M.; Cervantes-Guevara, G.; López-Alvarado, S.E.; Cervantes-Pérez, G.; Barbosa-Camacho, F.J.; Fuentes-Orozco, C.; Hernández-Corona, D.M.; González-Heredia, T.; Cervantes-Cardona, G.A.; González-Ojeda, A. Comparison of nutritional screening tools to assess nutritional risk and predict clinical outcomes in Mexican patients with digestive diseases. BMC Gastroenterol. 2020, 20, 79. [Google Scholar] [CrossRef]
- Kyo, D.; Tokuoka, S.; Katano, S.; Hisamune, R.; Yoshimoto, H.; Murao, S.; Umemura, Y.; Takasu, A.; Yamakawa, K. Comparison of Nutrition Indices for Prognostic Utility in Patients with Sepsis: A Real-World Observational Study. Diagnostics 2023, 13, 1302. [Google Scholar] [CrossRef]
- Komorita, T.; Yamamoto, E.; Sueta, D.; Tokitsu, T.; Fujisue, K.; Usuku, H.; Nishihara, T.; Oike, F.; Takae, M.; Egashira, K.; et al. The controlling nutritional status score predicts outcomes of cardiovascular events in patients with heart failure with preserved ejection fraction. Int. J. Cardiol. Heart Vasc. 2020, 29, 100563. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing Non Randomised Studies; Ottawa Hospital Research Institute: 2011. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 12 May 2025).
- Toyokawa, G.; Kozuma, Y.; Matsubara, T.; Haratake, N.; Takamori, S.; Akamine, T.; Takada, K.; Katsura, M.; Shimokawa, M.; Shoji, F.; et al. Prognostic impact of controlling nutritional status score in resected lung squamous cell carcinoma. J. Thorac. Dis. 2017, 9, 2942–2951. [Google Scholar] [CrossRef] [PubMed]
- Mai, S.; Nan, Y.; Peng, L.; Chen, Q. Controlling nutritional status score in the prediction of cardiovascular disease prevalence, all-cause and cardiovascular mortality in chronic obstructive pulmonary disease population: NHANES 1999–2018. BMC Pulm. Med. 2024, 24, 175. [Google Scholar] [CrossRef]
- Alvarez-Alvarez, B.; García-Seara, J.; Rodríguez-Mañero, M.; Iglesias-Alvarez, D.; Martínez-Sande, J.L.; Agra-Bermejo, R.M.; López, X.A.F.; González-Melchor, L.; Sampedro, F.G.; Díaz-Louzao, C.; et al. Prognostic value of nutrition status in the response of cardiac resynchronization therapy. Indian Pacing Electrophysiol. J. 2018, 18, 133–139. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, M.; Yang, X.; Cui, H.; Li, Z.; Wei, J. Controlling Nutritional Status Score as a Predictive Marker of In-hospital Mortality in Older Adult Patients. Front. Nutr. 2021, 8, 738045. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Maestri, R.; Olmetti, F.; Paganini, V.; Riccardi, G.; Riccardi, R.; Pinna, G.D.; Traversi, E. Additional predictive value of nutritional status in the prognostic assessment of heart failure patients. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 274–280. [Google Scholar] [CrossRef]
- Sze, S.; Zhang, J.; Pellicori, P.; Morgan, D.; Hoye, A.; Clark, A.L. Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin. Res. Cardiol. 2017, 106, 533–541. [Google Scholar] [CrossRef]
- Yoshihisa, A.; Kanno, Y.; Watanabe, S.; Yokokawa, T.; Abe, S.; Miyata, M.; Sato, T.; Suzuki, S.; Oikawa, M.; Kobayashi, A.; et al. Impact of nutritional indices on mortality in patients with heart failure. Open Heart 2018, 5, e000730. [Google Scholar] [CrossRef]
- Huang, L.; He, R.; Sun, X.; Lv, J.; Chen, S. Controlling Nutritional Status Score With Adverse Outcomes in Patients With Coronary Artery Disease: A Systematic Review and Meta-Analysis. Angiology 2022, 74, 149–158. [Google Scholar] [CrossRef]
- Xu, H.; Tan, P.; Jin, X.; Ai, J.; Lin, T.; Lei, H.; Yang, L.; Wei, Q. Validation of the preoperative controlling nutritional status score as an independent predictor in a large Chinese cohort of patients with upper tract urothelial carcinoma. Cancer Med. 2018, 7, 6112–6123. [Google Scholar] [CrossRef]
- Agra Bermejo, R.M.; González Ferreiro, R.; Varela Román, A.; Gómez Otero, I.; Kreidieh, O.; Conde Sabarís, P.; Rodríguez-Mañero, M.; Moure González, M.; Seoane Blanco, A.; Virgós Lamela, A.; et al. Nutritional status is related to heart failure severity and hospital readmissions in acute heart failure. Int. J. Cardiol. 2017, 230, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Ommen, S.R.; Gibbons, R.J.; Hodge, D.O.; Thomson, S.P. Usefulness of the Lymphocyte Concentration as a Prognostic Marker in Coronary Artery Disease. Am. J. Cardiol. 1997, 79, 812–814. [Google Scholar] [CrossRef]
- Ommen, S.R.; Hodge, D.O.; Rodeheffer, R.J.; McGregor, C.G.; Thomson, S.P.; Gibbons, R.J. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation 1998, 97, 19–25. [Google Scholar] [CrossRef]
- Bonilla-Palomas, J.L.; Gámez-López, A.L.; Moreno-Conde, M.; López-Ibáñez, M.C.; Anguita-Sánchez, M.; de la Sacristana, Á.G.; García-Catalán, F.; Villar-Ráez, A. Hypoalbuminemia in Acute Heart Failure Patients: Causes and Its Impact on Hospital and Long-Term Mortality. J. Card. Fail. 2014, 20, 350–358. [Google Scholar] [CrossRef]
- Rauchhaus, M.; Clark, A.L.; Doehner, W.; Davos, C.; Bolger, A.; Sharma, R.; Coats, A.J.; Anker, S.D. The relationship between cholesterol and survival in patients with chronic heart failure. J. Am. Coll. Cardiol. 2003, 42, 1933–1940. [Google Scholar] [CrossRef]
- Huo, Q.; He, T.; Xiong, J.; Zhao, J. Controlling nutritional status score is associated with renal progression, cardiovascular events, and all-cause mortality in biopsy-proved diabetic kidney disease. Front. Physiol. 2023, 14, 1231448. [Google Scholar] [CrossRef]
- Takagi, K.; Yagi, T.; Umeda, Y.; Shinoura, S.; Yoshida, R.; Nobuoka, D.; Kuise, T.; Araki, H.; Fujiwara, T. Preoperative Controlling Nutritional Status (CONUT) Score for Assessment of Prognosis Following Hepatectomy for Hepatocellular Carcinoma. World J. Surg. 2017, 41, 2353–2360. [Google Scholar] [CrossRef]
- Yasui, K.; Shida, D.; Ahiko, Y.; Takamizawa, Y.; Moritani, K.; Tsukamoto, S.; Kanemitsu, Y. Risk of non-colorectal cancer-related death in elderly patients with the disease: A comparison of five preoperative risk assessment indices. Cancer Med. 2023, 12, 2290–2302. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Abraham, W.T.; Albert, N.M.; Stough, W.G.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; Pieper, K.; Sun, J.L.; Yancy, C.; et al. Association between performance measures and clinical outcomes for patients hospitalized with heart failure. JAMA 2007, 297, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yaku, H.; Morimoto, T.; Inuzuka, Y.; Tamaki, Y.; Yamamoto, E.; Yoshikawa, Y.; Kitai, T.; Taniguchi, R.; Iguchi, M.; et al. Association with Controlling Nutritional Status (CONUT) Score and In-hospital Mortality and Infection in Acute Heart Failure. Sci. Rep. 2020, 10, 3320. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, W.; Ye, S.; Zhang, S.; Shi, W.; Cui, M.; Wang, L. The clinical value of the Controlling Nutritional Status score for predicting prognosis in systolic heart failure cases in the vulnerable phase. Front. Nutr. 2023, 10, 1084107. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Tang, J.; Zhang, N.; Zhang, Z.; Wang, D.; He, Y. Association between controlling nutritional status score and the prognosis of patients with acute myocardial infarction: A systematic review and meta-analysis. Front. Nutr. 2025, 11, 1518822. [Google Scholar] [CrossRef] [PubMed]
- Arfsten, H.; Cho, A.; Prausmüller, S.; Spinka, G.; Novak, J.; Goliasch, G.; Bartko, P.E.; Raderer, M.; Gisslinger, H.; Kornek, G.; et al. Inflammation-Based Scores as a Common Tool for Prognostic Assessment in Heart Failure or Cancer. Front Cardiovasc. Med. 2021, 8, 725903. [Google Scholar] [CrossRef]
- Xu, D.; Shen, R.; Hu, M.; Fan, Q.; Wu, J. Prognostic impact of CONUT score in older patients with chronic heart failure. BMC Geriatr. 2024, 24, 738. [Google Scholar] [CrossRef]
- Singh, G.; Bamba, H.; Inban, P.; Chandrasekaran, S.H.; Priyatha, V.; John, J.; Prajjwal, P. The prognostic significance of pro-BNP and heart failure in acute pulmonary embolism: A systematic review. Dis.-A-Mon. 2024, 70, 101783. [Google Scholar] [CrossRef]
- Huang, X.; Qiu, J.; Kuang, M.; Wang, C.; He, S.; Yu, C.; Xie, G.; Sheng, G.; Zou, Y. Assessing the predictive value of the controlling nutritional status score on all-cause mortality during hospitalization in patients with acute decompensated heart failure: A retrospective cohort study from Jiangxi, China. Front Nutr. 2024, 11, 1392268. [Google Scholar] [CrossRef]
- Bittner, D.O.; Mayrhofer, T.; Budoff, M.; Szilveszter, B.; Foldyna, B.; Hallett, T.R.; Ivanov, A.; Janjua, S.; Meyersohn, N.M.; Staziaki, S.V.; et al. Prognostic Value of Coronary CTA in Stable Chest Pain: CAD-RADS, CAC, and Cardiovascular Events in PROMISE. JACC Cardiovasc Imaging. 2020, 13, 1534–1545. [Google Scholar] [CrossRef]
- Deng, H.; Bai, Y.; Shantsila, A.; Fauchier, L.; Potpara, T.S.; Lip, G.Y.H. Clinical scores for outcomes of rhythm control or arrhythmia progression in patients with atrial fibrillation: A systematic review. Clin. Res. Cardiol. 2017, 106, 813–823. [Google Scholar] [CrossRef]
- Sze, S.; Pellicori, P.; Zhang, J.; Weston, J.; Clark, A.L. Agreement and Classification Performance of Malnutrition Tools in Patients with Chronic Heart Failure. Curr. Dev. Nutr. 2020, 4, nzaa071. [Google Scholar] [CrossRef]
- Fan, X.; He, Q.; Zhang, K.; Lan, X.; Li, Y.; Zhang, H. Comparison of the value of four objective nutritional indices in assessing the long-term prognosis of elderly patients with heart failure with preserved ejection fraction. Rev. Cardiovasc. Med. 2024, 25, 201. [Google Scholar] [CrossRef]
- Buglio, A.; Bellanti, F.; Carmignano, D.F.P.; Serviddio, G.; Vendemiale, G. Association between Controlling Nutritional Status (CONUT) score and body composition, inflammation, and frailty in hospitalized elderly patients. Nutrients 2024, 16, 576. [Google Scholar] [CrossRef] [PubMed]
- Driggin, E.; Cohen, L.P.; Gallagher, D.; Karmally, W.; Maddox, T.; Hummel, S.L.; Carbone, S.; Maurer, M.S. Nutrition assessment and dietary interventions in heart failure: JACC review topic of the week. J. Am. Coll. Cardiol. 2022, 79, 1623–1635. [Google Scholar] [CrossRef]
- Anker, S.D.; Coats, A.J.S. Cardiac cachexia: A syndrome with impaired survival and immune and neuroendocrine activation. Chest 1999, 115, 836–847. [Google Scholar] [CrossRef]
- Sze, S.; Pellicori, P.; Zhang, J.; Weston, J.; Clark, A.L. The impact of malnutrition on short-term morbidity and mortality in ambulatory patients with heart failure. Am. J. Clin. Nutr. 2021, 113, 695–705. [Google Scholar] [CrossRef]
- Takagi, K.; Domagala, P.; Polak, W.G.; Buettner, S.; Wijnhoven, B.P.L.; Ijzermans, J.N.M. Prognostic significance of the controlling nutritional status (CONUT) score in patients undergoing gastrectomy for gastric cancer: A systematic review and meta-analysis. BMC Surg. 2019, 19, 129. [Google Scholar] [CrossRef]
- Januzzi, J.L., Jr. Natriuretic Peptides as Biomarkers in Heart Failure. J. Investig. Med. 2013, 61, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Akagi, S.; Ejiri, K.; Nakamura, K.; Ito, H. Impact of malnutrition on prognosis in patients with pulmonary arterial hypertension. Pulm Circ. 2023, 13, e12286. [Google Scholar] [CrossRef]
- Rich, J.D.; Burns, J.; Freed, B.H.; Maurer, M.S.; Burkhoff, D.; Shah, S.J. Meta-Analysis Global Group in Chronic (MAGGIC) Heart Failure Risk Score: Validation of a Simple Tool for the Prediction of Morbidity and Mortality in Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2018, 16, e009594. [Google Scholar] [CrossRef]

| Parameters | Normal | Mild | Moderate | Severe |
|---|---|---|---|---|
| Serum albumin (g/mL) | ≥3.5 | 3.0–3.4 | 2.5–2.9 | <2.50 |
| Score | 0 | 2 | 4 | 6 |
| Total lymphocyte count | ≥1600 | 1200–1599 | 800–1199 | <800 |
| Score | 0 | 1 | 2 | 3 |
| Total cholesterol | ≥180 | 140–179 | 100–139 | <100 |
| Score | 0 | 1 | 2 | 3 |
| Total score | 0–1 | 2–4 | 5–8 | 9–12 |
| Dysnutritional states | Normal | Mild | Moderate | Severe |
| Study | Mean Age (Years) | Male (%) | Female (%) |
|---|---|---|---|
| S1. Suying Mai et al. * [24] | NA | NA | NA |
| S2. Alvarez et al. [25] | 66 ± 10 | 77 | 23 |
| S3. Liu et al. [26] | 74.8 ± 7.0 | 50.2 | 49.8 |
| S4. Nishi et al. [9] | 2 ± 12 | 60 | 40 |
| S5. Iwakami et al. [14] | 78 | 55 | 45 |
| S6. La Rovere et al. [27] | 67 ± 11 | 70 | 30 |
| S7. Sze et al. [28] | 76 ± 11 | 60 | 40 |
| S8. Yoshihisa et al. [29] | 68 ± 13 | 65 | 35 |
| Study | Author/Year | Type of Study | Country | Quality Assessment Results (Newcastle–Ottawa *) | Sample Size | Effects on the Main Outcomes | Reference | |
|---|---|---|---|---|---|---|---|---|
| 1. | Controlling nutritional status score in the prediction of cardiovascular disease prevalence, all-cause and cardiovascular mortality in chronic obstructive pulmonary disease population: NHANES 1999–2018 | Suying Mai/2024 | Cohort study | USA | NOS = 9/9 | N = 309 people ≥ 35 years of age with COPD and HF from NHANES 1999–2018 | A count score > 2 is associated with a high prevalence of cardiovascular disease and overall mortality risk, and studies suggest that it is a good nutritional tool for patients’ risk stratification. | [24] |
| 2. | Prognostic value of nutrition status in the response of cardiac resynchronization therapy. | Alvarez/2018 | Retrospective Observational Study | Spain | NOS = 8/9 | N = 302 | Results showed that those with moderate-severe malnutrition had the highest risk of acute heart failure hospitalization and mortality risk, as well as an association with ventricular remodelling. | [25] |
| 3. | Controlling Nutritional Status Score as a Predictive Marker of In-hospital Mortality in Older Adult Patients | Cheng Liu/2021 | Retrospective Cohort Study | China | NOS = 8/9 | N = 11,795 | CONUT ≥ 6 was associated with a high prevalence of long-term adverse outcomes, proving that it is an independent predictor of mortality, especially among the elderly. | [26] |
| 4. | Nutritional screening based on the controlling nutritional status (CONUT) score at the time of admission is useful for long-term prognostic prediction in patients with heart failure requiring hospitalization | Isao Nishi/2017 | Retrospective Observational Study | Japan | NOS = 9/9 | N = 482 | Analyses revealed that a per-point increase in the CONUT score was associated with an increased risk of all-cause death | [9] |
| 5. | Prognostic value of malnutrition assessed by Controlling Nutritional Status score for long-term mortality in patients with acute heart failure | Naotsugu Iwakami/2017 | Retrospective Observational Study | Japan | NOS = 8/9 | N = 635 | Higher CONUT score at admission was significantly associated with increased long-term mortality. The CONUT score also improved the predictive accuracy of existing risk models. | [14] |
| 6. | Additional predictive value of nutritional status in the prognostic assessment of heart failure patients | M.T. La Rovere/2017 | Prospective Observational Study | Italy | NOS = 8/9 | N = 466 | The results showed that a higher CONUT score was associated with increased mortality over a 12-month period, demonstrating the importance of nutritional evaluation in risk stratification for patients with heart failure. | [27] |
| 7. | Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction | S.Sze/2017 | Prospective Observational study | United Kingdom | NOS = 9/9 | N = 265 | The findings indicated that frailty and malnutrition are strongly associated with adverse outcomes, improving mortality prediction. | [28] |
| 8. | Impact of nutritional indices on mortality in patients with heart failure | Akiomi Yoshihisa/2018 | Retrospective cohort study | Japan | NOS = 8/9 | N = 1307 | The results indicated that malnutrition was associated with increased all-cause mortality, with PNI and GNRI demonstrating superior predictive accuracy compared to CONUT. | [29] |
| Study | HR * (95% CI *) | Weight (%) |
|---|---|---|
| Suying Mai et al., 2024 [24] | 1.52 [1.32–1.75] | 11.1% |
| Alvarez et al., 2018 [25] | 1.88 [1.27–2.78] | 7.3% |
| Liu et al., 2021 [26] | 1.50 [1.18–1.91] | 12.4% |
| Nishi et al., 2017 [9] | 1.14 [1.04–1.25] | 15.3% |
| Iwakami et al., 2017 [14] | 1.26 [1.11–1.42] | 14.5% |
| La Rovere et al., 2017 [27] | 1.70 [1.36–2.12] | 11.7% |
| Sze et al., 2017 [28] | 1.45 [1.31–1.60] | 12.6% |
| Yoshihisa et al., 2018 [29] | 1.80 [1.49–2.18] | 15.1% |
| Study Name | Subgroup Within Study | Point | Lower Limit | Upper Limit | Z-Value | p-Value | Hazard Ratio 95%CI with Study Removed | ||
|---|---|---|---|---|---|---|---|---|---|
| 0.01 | 1.00 | 10.00 | |||||||
| Suying Mai et al., 2024 [24] | HFc | 1.462 | 1.273 | 1.679 | 5.384 | 0.000 |  | ||
| Alvarez et al., 2018 [25] | HFa | 1.442 | 1.274 | 1.633 | 5.772 | 0.000 |  | ||
| Liu et al., 2021 [26] | HFc | 1.465 | 1.283 | 1.674 | 5.633 | 0.000 |  | ||
| Nishi et al., 2017 [9] | HFc | 1.519 | 1.376 | 1.676 | 8.283 | 0.000 |  | ||
| Iwakami et al., 2017 [14] | HFc | 1.511 | 1.310 | 1.743 | 5.676 | 0.000 |  | ||
| La Rovere et al., 2017 [27] | HFa | 1.439 | 1.267 | 1.635 | 5.591 | 0.000 |  | ||
| Sze et al., 2017 [28] | HFc | 1.479 | 1.274 | 1.717 | 5.134 | 0.000 |  | ||
| Yoshihisa et al., 2018 [29] | HFa | 1.420 | 1.259 | 1.601 | 5.723 | 0.000 | 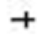 | ||
| Random | 1.467 | 1.298 | 1.657 | 6.152 | 0.000 | 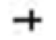 | |||
| Pred Int | 1.467 | 0.987 | 2.180 | 0.000 | 0.000 | ||||
| Groups | Effect Size and 95% Interval | Test of Null (2-Tail) | Prediction Interval | Between-Study | Other Heterogeneity Statistics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Number of Studies | Point Estimate | 95% CI (Lower) | 95% CI (Upper) | Z-Value | p-Value | Lower | Upper | Tau | TauSq | Q-Value | df (Q) | Q p-Value | I2 (%) |
| Fixed effect analysis | ||||||||||||||
| HFa | 3 | 1.771 | 1.547 | 2.028 | 8.267 | 0.000 | 0.248 | 2 | 0.883 | 0.0 | ||||
| HFc | 5 | 1.314 | 1.245 | 1.386 | 10.027 | 0.000 | 18.598 | 4 | 0.001 | 78.493 | ||||
| Total within | 18.846 | 6 | 0.004 | |||||||||||
| Total between | 16.178 | 1 | 0.000 | |||||||||||
| Overall | 8 | 1.367 | 1.301 | 1.437 | 12.358 | 0.000 | 35.024 | 7 | 0.000 | 80.014 | ||||
| Random effects analysis | ||||||||||||||
| HFa | 3 | 1.775 | 1.471 | 2.142 | 5.994 | 0.000 | 1.251 | 2.520 | 0.106 | 0.011 | ||||
| HFc | 5 | 1.347 | 1.205 | 1.506 | 5.240 | 0.000 | 1.003 | 1.809 | 0.106 | 0.011 | ||||
| Total between | 6.148 | 1 | 0.013 | |||||||||||
| Overall | 8 | 1.530 | 1.168 | 2.003 | 3.089 | 0.002 | 0.985 | 2.127 | 0.149 | 0.022 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fărcaș, D.A.; Cerghizan, A.; Maior, R.; Mîndrilă, A.-C.; Tarcea, M. CONUT Score as a Predictor of Mortality Risk in Acute and Chronic Heart Failure: A Meta-Analytic Review. Nutrients 2025, 17, 1736. https://doi.org/10.3390/nu17101736
Fărcaș DA, Cerghizan A, Maior R, Mîndrilă A-C, Tarcea M. CONUT Score as a Predictor of Mortality Risk in Acute and Chronic Heart Failure: A Meta-Analytic Review. Nutrients. 2025; 17(10):1736. https://doi.org/10.3390/nu17101736
Chicago/Turabian StyleFărcaș, Diana Andreea, Anda Cerghizan, Raluca Maior, Andreea-Cornelia Mîndrilă, and Monica Tarcea. 2025. "CONUT Score as a Predictor of Mortality Risk in Acute and Chronic Heart Failure: A Meta-Analytic Review" Nutrients 17, no. 10: 1736. https://doi.org/10.3390/nu17101736
APA StyleFărcaș, D. A., Cerghizan, A., Maior, R., Mîndrilă, A.-C., & Tarcea, M. (2025). CONUT Score as a Predictor of Mortality Risk in Acute and Chronic Heart Failure: A Meta-Analytic Review. Nutrients, 17(10), 1736. https://doi.org/10.3390/nu17101736







