Exogenous Ketone Supplementation Enhances the Anti-Epileptic Effect of Levetiracetam in Wistar Albino Glaxo/Rijswijk Rats
Abstract
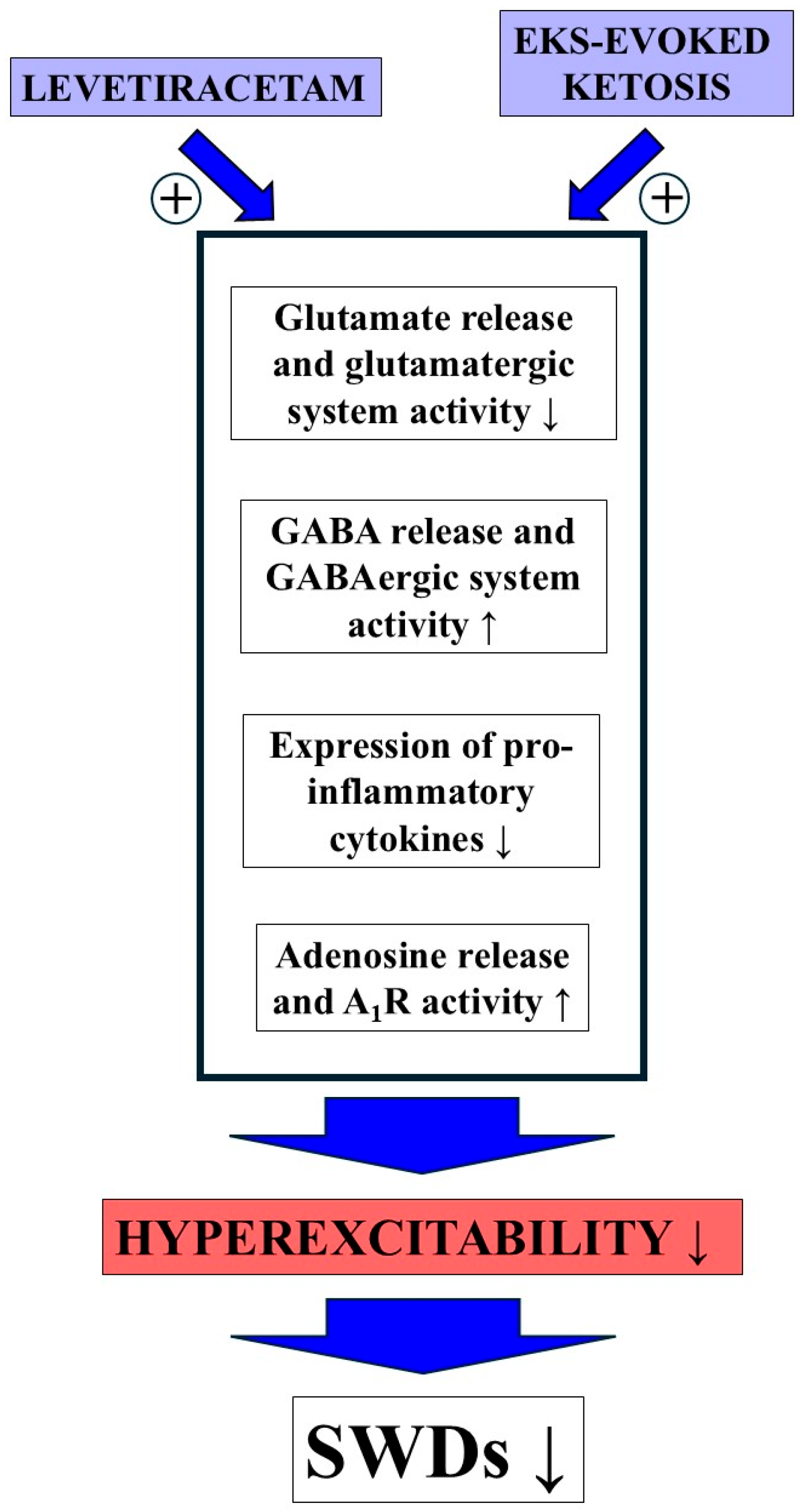
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. EEG Electrode Implantation
2.3. EEG Recording
2.4. Measuring of Blood Glucose and R-βHB Levels
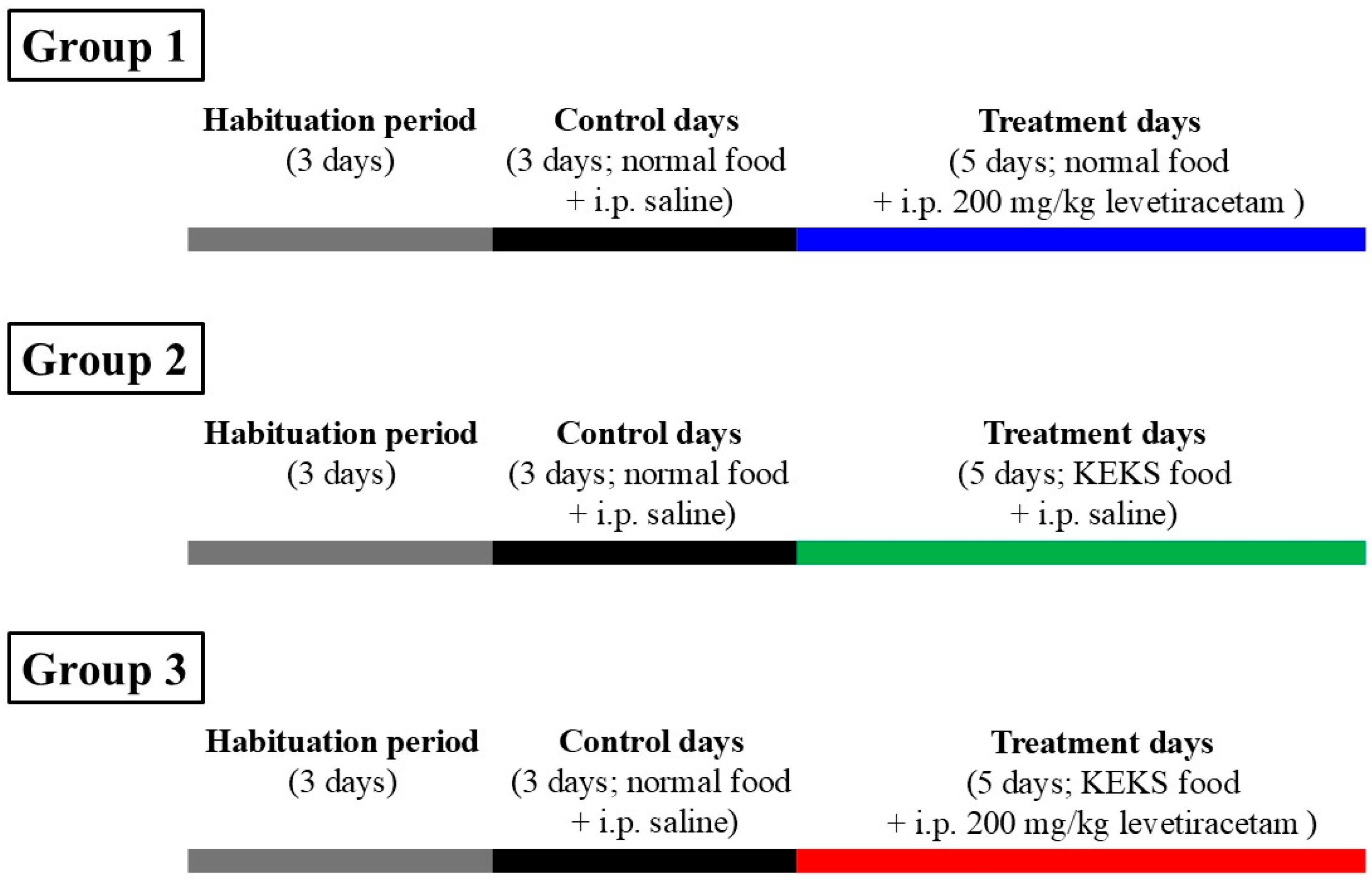
2.5. Treatment of Rats
2.6. Statistical Analysis
3. Results
3.1. Effect of Levetiracetam and KEKS Food Alone on SWDs
3.2. Effect of Combined Administration of Levetiracetam with KEKS Food on SWDs
3.3. Changes in Blood R-βHB and Glucose Levels and Body Weight
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Contreras-García, I.J.; Cárdenas-Rodríguez, N.; Romo-Mancillas, A.; Bandala, C.; Zamudio, S.R.; Gómez-Manzo, S.; Hernández-Ochoa, B.; Mendoza-Torreblanca, J.G.; Pichardo-Macías, L.A. Levetiracetam Mechanisms of Action: From Molecules to Systems. Pharmaceuticals 2022, 15, 475. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Z.; Skatchkov, S.N.; Veh, R.W.; Szabó, Z.; Németh, K.; Szabó, P.T.; Kardos, J.; Héja, L. Critical Role of Astrocytic Polyamine and GABA Metabolism in Epileptogenesis. Front. Cell. Neurosci. 2022, 15, 787319. [Google Scholar] [CrossRef] [PubMed]
- Lynch, B.A.; Lambeng, N.; Nocka, K.; Kensel-Hammes, P.; Bajjalieh, S.M.; Matagne, A.; Fuks, B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc. Natl. Acad. Sci. USA 2004, 101, 9861–9866. [Google Scholar] [CrossRef] [PubMed]
- Pichardo Macías, L.A.; Ramírez Mendiola, B.A.; Contreras García, I.J.; Zamudio Hernández, S.R.; Chávez Pacheco, J.L.; Sánchez Huerta, K.B.; Mendoza Torreblanca, J.G. Effect of levetiracetam on extracellular amino acid levels in the dorsal hippocampus of rats with temporal lobe epilepsy. Epilepsy Res. 2018, 140, 111–119. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.A.; Shaikh, I.A.; Khateeb, M.M.; Habeeb, S.M. Omega 3 polyunsaturated fatty acids enhance the protective effect of levetiracetam against seizures, cognitive impairment and hippocampal oxidative DNA damage in young kindled rats. Pharmacol. Biochem. Behav. 2015, 135, 105–113. [Google Scholar] [CrossRef]
- Ueda, Y.; Doi, T.; Nagatomo, K.; Tokumaru, J.; Takaki, M.; Willmore, L.J. Effect of levetiracetam on molecular regulation of hippocampal glutamate and GABA transporters in rats with chronic seizures induced by amygdalar FeCl3 injection. Brain Res. 2007, 1151, 55–61. [Google Scholar] [CrossRef]
- Ueda, Y.; Doi, T.; Takaki, M.; Nagatomo, K.; Nakajima, A.; Willmore, L.J. Levetiracetam enhances endogenous antioxidant in the hippocampus of rats: In vivo evaluation by brain microdialysis combined with ESR spectroscopy. Brain Res. 2009, 1266, 1–7. [Google Scholar] [CrossRef]
- Ari, C.; Kovács, Z.; Juhasz, G.; Murdun, C.; Goldhagen, C.R.; Koutnik, A.P.; Poff, A.M.; Kesl, S.L.; D’Agostino, D.P. Exogenous Ketone Supplements Reduce Anxiety-Related Behavior in Sprague-Dawley and Wistar Albino Glaxo/Rijswijk Rats. Front. Mol. Neurosci. 2016, 9, 137. [Google Scholar] [CrossRef]
- Kovács, Z.; D’Agostino, D.P.; Dobolyi, A.; Ari, C. Adenosine A1 Receptor Antagonism Abolished the Anti-seizure Effects of Exogenous Ketone Supplementation in Wistar Albino Glaxo Rijswijk Rats. Front. Mol. Neurosci. 2017, 10, 235. [Google Scholar] [CrossRef]
- Achanta, L.B.; Rae, C.D. β-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem. Res. 2017, 42, 35–49. [Google Scholar] [CrossRef]
- Poff, A.M.; Rho, J.M.; D’Agostino, D.P. Ketone Administration for Seizure Disorders: History and Rationale for Ketone Esters and Metabolic Alternatives. Front. Neurosci. 2019, 13, 1041. [Google Scholar] [CrossRef]
- McNally, M.A.; Hartman, A.L. Ketone bodies in epilepsy. J. Neurochem. 2012, 121, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Rani, E.; Waheed, A.; Rajput, S.K. Pharmacoresistant Epilepsy: A Current Update on Non-Conventional Pharmacological and Non-Pharmacological Interventions. J. Epilepsy Res. 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bolyard, M.L.; Graziano, C.M.; Fontaine, K.R.; Sayer, R.D.; Fisher, G.; Plaisance, E.P. Tolerability and Acceptability of an Exogenous Ketone Monoester and Ketone Monoester/Salt Formulation in Humans. Nutrients 2023, 15, 4876. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Cerminara, C.; Domizio, S.; Mohn, A.; Franzoni, E.; Coppola, G.; Zamponi, N.; Parisi, P.; Iannetti, P.; Curatolo, P. Levetiracetam in absence epilepsy. Dev. Med. Child Neurol. 2008, 50, 850–853. [Google Scholar] [CrossRef]
- Bouwman, B.M.; van Rijn, C.M. Effects of levetiracetam on spike and wave discharges in WAG/Rij rats. Seizure 2004, 13, 591–594. [Google Scholar] [CrossRef][Green Version]
- Leo, A.; Caro, C.; Nesci, V.; Palma, E.; Tallarico, M.; Iannone, M.; Constanti, A.; Sarro, G.; Russo, E.; Citraro, R. Antiepileptogenic effects of Ethosuximide and Levetiracetam in WAG/Rij rats are only temporary. Pharmacol. Rep. 2019, 71, 833–838. [Google Scholar] [CrossRef]
- Russo, E.; Citraro, R.; Scicchitano, F.; De Fazio, S.; Di Paola, E.D.; Constanti, A.; De Sarro, G. Comparison of the antiepileptogenic effects of an early long-term treatment with ethosuximide or levetiracetam in a genetic animal model of absence epilepsy. Epilepsia 2010, 51, 1560–1569. [Google Scholar] [CrossRef]
- Kovács, Z.; D’Agostino, D.P.; Diamond, D.M.; Ari, C. Exogenous Ketone Supplementation Decreased the Lipopolysaccharide-Induced Increase in Absence Epileptic Activity in Wistar Albino Glaxo Rijswijk Rats. Front. Mol. Neurosci. 2019, 12, 45. [Google Scholar] [CrossRef]
- Brunner, B.; Rauch, E.; Ari, C.; D’Agostino, D.P.; Kovács, Z. Enhancement of Ketone Supplements-Evoked Effect on Absence Epileptic Activity by Co-Administration of Uridine in Wistar Albino Glaxo Rijswijk Rats. Nutrients 2021, 13, 234. [Google Scholar] [CrossRef]
- Kaminski, R.M.; Matagne, A.; Patsalos, P.N.; Klitgaard, H. Benefit of combination therapy in epilepsy: A review of the preclinical evidence with levetiracetam. Epilepsia 2009, 50, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Azam, M.; Khan, S.A.; Ul-Haq, Z.; Simjee, S.U. Levetiracetam ameliorates epileptogenesis by modulating the adenosinergic pathway in a kindling model of epilepsy in mice. Turk. J. Med. Sci. 2023, 53, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Coenen, A.M.; Van Luijtelaar, E.L. Genetic animal models for absence epilepsy: A review of the WAG/Rij strain of rats. Behav. Genet. 2003, 33, 635–655. [Google Scholar] [CrossRef]
- Brunner, B.; Ari, C.; D’Agostino, D.P.; Kovács, Z. Adenosine Receptors Modulate the Exogenous Ketogenic Supplement-Evoked Alleviating Effect on Lipopolysaccharide-Generated Increase in Absence Epileptic Activity in WAG/Rij Rats. Nutrients 2021, 13, 4082. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain Stereotaxic Coordinates; Academic Press: Orlando, FL, USA, 1998; ISBN 978-0125476171. [Google Scholar]
- Nicolas, J.M.; Hannestad, J.; Holden, D.; Kervyn, S.; Nabulsi, N.; Tytgat, D.; Huang, Y.; Chanteux, H.; Staelens, L.; Matagne, A.; et al. Brivaracetam, a selective high-affinity synaptic vesicle protein 2A (SV2A) ligand with preclinical evidence of high brain permeability and fast onset of action. Epilepsia 2016, 57, 201–209. [Google Scholar] [CrossRef]
- Tong, X.; Patsalos, P.N. A microdialysis study of the novel antiepileptic drug levetiracetam: Extracellular pharmacokinetics and effect on taurine in rat brain. Br. J. Pharmacol. 2001, 133, 867–874. [Google Scholar] [CrossRef]
- Meehan, A.L.; Yang, X.; McAdams, B.D.; Yuan, L.; Rothman, S.M. A new mechanism for antiepileptic drug action: Vesicular entry may mediate the effects of levetiracetam. J. Neurophysiol. 2011, 106, 1227–1239. [Google Scholar] [CrossRef]
- Lukyanetz, E.A.; Shkryl, V.M.; Kostyuk, P.G. Selective blockade of N-type calcium channels by levetiracetam. Epilepsia 2002, 43, 9–18. [Google Scholar] [CrossRef]
- Niespodziany, I.; Klitgaard, H.; Margineanu, D.G. Levetiracetam inhibits the high-voltage-activated Ca2+ current in pyramidal neurones of rat hippocampal slices. Neurosci. Lett. 2001, 306, 5–8. [Google Scholar] [CrossRef]
- Lee, C.Y.; Chen, C.C.; Liou, H.H. Levetiracetam inhibits glutamate transmission through presynaptic P/Q-type calcium channels on the granule cells of the dentate gyrus. Br. J. Pharmacol. 2009, 158, 1753–1762. [Google Scholar] [CrossRef]
- Cortes-Altamirano, J.L.; Olmos-Hernández, A.; Bonilla-Jaime, H.; Bandala, C.; González-Maciel, A.; Alfaro-Rodríguez, A. Levetiracetam as an antiepileptic, neuroprotective, and hyperalgesic drug. Neurol. India 2016, 64, 1266–1275. [Google Scholar] [CrossRef]
- Madeja, M.; Margineanu, D.G.; Gorji, A.; Siep, E.; Boerrigter, P.; Klitgaard, H.; Speckmann, E.J. Reduction of voltage-operated potassium currents by levetiracetam: A novel antiepileptic mechanism of action? Neuropharmacology 2003, 45, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Hönack, D.; Bloms-Funke, P. The novel antiepileptic drug levetiracetam (ucb L059) induces alterations in GABA metabolism and turnover in discrete areas of rat brain and reduces neuronal activity in substantia nigra pars reticulata. Brain Res. 1996, 735, 208–216. [Google Scholar] [CrossRef]
- Li, Q.; Chen, C.; Gong, T. High-field MRS study of GABA+ in patients with migraine: Response to levetiracetam treatment. Neuroreport 2018, 29, 1007–1010. [Google Scholar] [CrossRef]
- Fukuyama, K.; Tanahashi, S.; Nakagawa, M.; Yamamura, S.; Motomura, E.; Shiroyama, T.; Tanii, H.; Okada, M. Levetiracetam inhibits neurotransmitter release associated with CICR. Neurosci. Lett. 2012, 518, 69–74. [Google Scholar] [CrossRef]
- Carunchio, I.; Pieri, M.; Ciotti, M.T.; Albo, F.; Zona, C. Modulation of AMPA receptors in cultured cortical neurons induced by the antiepileptic drug levetiracetam. Epilepsia 2007, 48, 654–662. [Google Scholar] [CrossRef]
- Stepanović-Petrović, R.M.; Micov, A.M.; Tomić, M.A.; Ugrešić, N.D. The local peripheral antihyperalgesic effect of levetiracetam and its mechanism of action in an inflammatory pain model. Anesth. Analg. 2012, 115, 1457–1466. [Google Scholar] [CrossRef]
- Christensen, K.V.; Leffers, H.; Watson, W.P.; Sánchez, C.; Kallunki, P.; Egebjerg, J. Levetiracetam attenuates hippocampal expression of synaptic plasticity-related immediate early and late response genes in amygdala-kindled rats. BMC Neurosci. 2010, 11, 9. [Google Scholar] [CrossRef]
- Itoh, K.; Taniguchi, R.; Matsuo, T.; Oguro, A.; Vogel, C.F.A.; Yamazaki, T.; Ishihara, Y. Suppressive effects of levetiracetam on neuroinflammation and phagocytic microglia: A comparative study of levetiracetam, valproate and carbamazepine. Neurosci. Lett. 2019, 708, 134363. [Google Scholar] [CrossRef]
- Kim, J.E.; Choi, H.C.; Song, H.K.; Jo, S.M.; Kim, D.S.; Choi, S.Y.; Kim, Y.I.; Kang, T.C. Levetiracetam inhibits interleukin-1 beta inflammatory responses in the hippocampus and piriform cortex of epileptic rats. Neurosci. Lett. 2010, 471, 94–99. [Google Scholar] [CrossRef]
- Itoh, K.; Ishihara, Y.; Komori, R.; Nochi, H.; Taniguchi, R.; Chiba, Y.; Ueno, M.; Takata-Tsuji, F.; Dohgu, S.; Kataoka, Y. Levetiracetam treatment influences blood-brain barrier failure associated with angiogenesis and inflammatory responses in the acute phase of epileptogenesis in post-status epilepticus mice. Brain Res. 2016, 1652, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gulcebi, M.I.; Kendirli, T.; Turgan, Z.A.; Patsalos, P.N.; Onat Yilmaz, F. The effect of serum levetiracetam concentrations on therapeutic response and IL1-beta concentration in patients with epilepsy. Epilepsy Res. 2018, 148, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Himmerich, H.; Bartsch, S.; Hamer, H.; Mergl, R.; Schönherr, J.; Petersein, C.; Munzer, A.; Kirkby, K.C.; Bauer, K.; Sack, U. Impact of mood stabilizers and antiepileptic drugs on cytokine production in-vitro. J. Psychiatr. Res. 2013, 47, 1751–1759. [Google Scholar] [CrossRef]
- Młodzikowska-Albrecht, J.; Steinborn, B.; Zarowski, M. Cytokines, epilepsy and epileptic drugs--is there a mutual influence? Pharmacol. Rep. 2007, 59, 129–138. [Google Scholar]
- Vezzani, A.; Granata, T. Brain inflammation in epilepsy: Experimental and clinical evidence. Epilepsia 2005, 46, 1724–1743. [Google Scholar] [CrossRef]
- Stellwagen, D.; Beattie, E.C.; Seo, J.Y.; Malenka, R.C. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J. Neurosci. 2005, 25, 3219–3228. [Google Scholar] [CrossRef]
- Vezzani, A.; Maroso, M.; Balosso, S.; Sanchez, M.A.; Bartfai, T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav. Immun. 2011, 25, 1281–1289. [Google Scholar] [CrossRef]
- Casamenti, F.; Prosperi, C.; Scali, C.; Giovannelli, L.; Colivicchi, M.A.; Faussone-Pellegrini, M.S.; Pepeu, G. Interleukin-1beta activates forebrain glial cells and increases nitric oxide production and cortical glutamate and GABA release in vivo: Implications for Alzheimer’s disease. Neuroscience 1999, 91, 831–842. [Google Scholar] [CrossRef]
- Hu, S.; Sheng, W.S.; Ehrlich, L.C.; Peterson, P.K.; Chao, C.C. Cytokine effects on glutamate uptake by human astrocytes. Neuroimmunomodulation 2000, 7, 153–159. [Google Scholar] [CrossRef]
- Wang, Y.S.; White, T.D. The bacterial endotoxin lipopolysaccharide causes rapid inappropriate excitation in rat cortex. J. Neurochem. 1999, 72, 652–660. [Google Scholar] [CrossRef]
- Eyal, S.; Yagen, B.; Sobol, E.; Altschuler, Y.; Shmuel, M.; Bialer, M. The activity of antiepileptic drugs as histone deacetylase inhibitors. Epilepsia 2004, 45, 737–744. [Google Scholar] [CrossRef]
- Stettner, M.; Dehmel, T.; Mausberg, A.K.; Köhne, A.; Rose, C.R.; Kieseier, B.C. Levetiracetam exhibits protective properties on rat Schwann cells in vitro. J. Peripher. Nerv. Syst. 2011, 16, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.E.; Walker, M.C.; Cock, H.R. Levetiracetam: Antiepileptic properties and protective effects on mitochondrial dysfunction in experimental status epilepticus. Epilepsia 2006, 47, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Van de Bovenkamp-Janssen, M.C.; Scheenen, W.J.; Kuijpers-Kwant, F.J.; Kozicz, T.; Veening, J.G.; van Luijtelaar, E.L.; McEnery, M.W.; Roubos, E.W. Differential expression of high voltage-activated Ca2+ channel types in the rostral reticular thalamic nucleus of the absence epileptic WAG/Rij rat. J. Neurobiol. 2004, 58, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Z.; Kékesi, K.A.; Dobolyi, Á.; Lakatos, R.; Juhász, G. Absence epileptic activity changing effects of non-adenosine nucleoside inosine, guanosine and uridine in Wistar Albino Glaxo Rijswijk rats. Neuroscience 2015, 300, 593–608. [Google Scholar] [CrossRef]
- Kovács, Z.; Skatchkov, S.N.; Szabó, Z.; Qahtan, S.; Méndez-González, M.P.; Malpica-Nieves, C.J.; Eaton, M.J.; Kardos, J.; Héja, L. Putrescine Intensifies Glu/GABA Exchange Mechanism and Promotes Early Termination of Seizures. Int. J. Mol. Sci. 2022, 23, 8191. [Google Scholar] [CrossRef]
- Kovács, Z.; Kékesi, K.A.; Szilágyi, N.; Abrahám, I.; Székács, D.; Király, N.; Papp, E.; Császár, I.; Szego, E.; Barabás, K.; et al. Facilitation of spike-wave discharge activity by lipopolysaccharides in Wistar Albino Glaxo/Rijswijk rats. Neuroscience 2006, 140, 731–742. [Google Scholar] [CrossRef]
- Kovács, Z.; Czurkó, A.; Kékesi, K.A.; Juhász, G. Intracerebroventricularly administered lipopolysaccharide enhances spike-wave discharges in freely moving WAG/Rij rats. Brain Res. Bull. 2011, 85, 410–416. [Google Scholar] [CrossRef]
- Russo, E.; Andreozzi, F.; Iuliano, R.; Dattilo, V.; Procopio, T.; Fiume, G.; Mimmi, S.; Perrotti, N.; Citraro, R.; Sesti, G.; et al. Early molecular and behavioral response to lipopolysaccharide in the WAG/Rij rat model of absence epilepsy and depressive-like behavior, involves interplay between AMPK, AKT/mTOR pathways and neuroinflammatory cytokine release. Brain Behav. Immun. 2014, 42, 157–168. [Google Scholar] [CrossRef]
- Van Luijtelaar, G.; Lyashenko, S.; Vastyanov, R.; Verbeek, G.; Oleinik, A.; van Rijn, C.; Volokhova, G.; Shandra, A.; Coenen, A.; Godlevsky, L. Cytokines and Absence Seizures in a Genetic Rat Model. Neurophysiology 2012, 43, 478–486. [Google Scholar] [CrossRef]
- Gessi, S.; Merighi, S.; Stefanelli, A.; Fazzi, D.; Varani, K.; Borea, P.A. A1 and A3 adenosine receptors inhibit LPS-induced hypoxia-inducible factor-1 accumulation in murine astrocytes. Pharmacol. Res. 2013, 76, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M., Jr.; Ruskin, D.N.; Masino, S.A. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J. Neurosci. 2010, 30, 3886–3895. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Z.; Juhász, G.; Palkovits, M.; Dobolyi, A.; Kékesi, K.A. Area, age and gender dependence of the nucleoside system in the brain: A review of current literature. Curr. Top. Med. Chem. 2011, 11, 1012–1033. [Google Scholar] [CrossRef]
- Martí Navia, A.; Dal Ben, D.; Lambertucci, C.; Spinaci, A.; Volpini, R.; Marques-Morgado, I.; Coelho, J.E.; Lopes, L.V.; Marucci, G.; Buccioni, M. Adenosine Receptors as Neuroinflammation Modulators: Role of A1 Agonists and A2A Antagonists. Cells 2020, 9, 1739. [Google Scholar] [CrossRef]
- Juge, N.; Gray, J.A.; Omote, H.; Miyaji, T.; Inoue, T.; Hara, C.; Uneyama, H.; Edwards, R.H.; Nicoll, R.A.; Moriyama, Y. Metabolic control of vesicular glutamate transport and release. Neuron 2010, 68, 99–112. [Google Scholar] [CrossRef]
- Yudkoff, M.; Daikhin, Y.; Melø, T.M.; Nissim, I.; Sonnewald, U.; Nissim, I. The ketogenic diet and brain metabolism of amino acids: Relationship to the anticonvulsant effect. Annu. Rev. Nutr. 2007, 27, 415–430. [Google Scholar] [CrossRef]
- Kovács, Z.; D’Agostino, D.P.; Ari, C. Neuroprotective and Behavioral Benefits of Exogenous Ketone Supplementation-Evoked Ketosis. In Ketogenic Diet and Metabolic Therapies: Expanded Roles in Health and Disease, 2nd ed.; Masino, S.A., Ed.; Oxford Academic: New York, NY, USA, 2022; pp. 423–465. [Google Scholar] [CrossRef]
- Kovács, Z.; Rauch, E.; D’Agostino, D.P.; Ari, C. Putative Role of Adenosine A1 Receptors in Exogenous Ketone Supplements-Evoked Anti-Epileptic Effect. Int. J. Mol. Sci. 2024, 25, 9869. [Google Scholar] [CrossRef]
- Youm, Y.H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Depaulis, A.; David, O.; Charpier, S. The genetic absence epilepsy rat from Strasbourg as a model to decipher the neuronal and network mechanisms of generalized idiopathic epilepsies. J. Neurosci. Methods 2016, 260, 159–174. [Google Scholar] [CrossRef]


| Treatments | Group 1 (Figure 2) | |||||
|---|---|---|---|---|---|---|
| Mean ± S.E.M. (Significance Level/p Value) | ||||||
| SWD Number | SWD Duration (s) | Time Spent in SWD (s) | ||||
| 30–90 min | 90–150 min | 30–90 min | 90–150 min | 30–90 min | 90–150 min | |
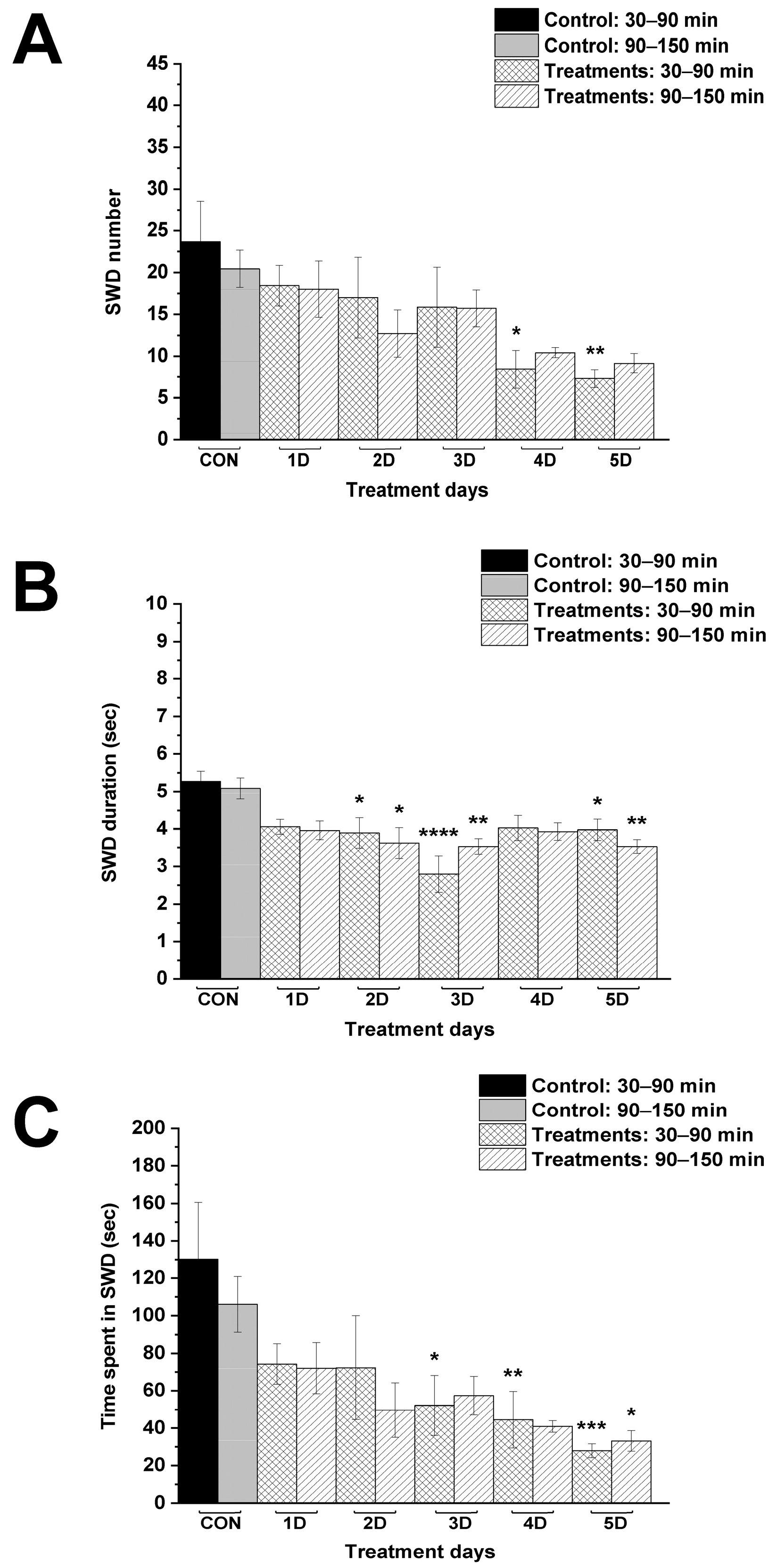
| Control (CON; i.p. saline + normal rat food) | 24.2 ± 2.99 | 20.1 ± 2.37 | 4.2 ± 0.30 | 4.2 ± 0.23 | 105.6 ± 18.75 | 82.5 ± 10.80 |
| 1st treatment day (1D; i.p. 200 mg/kg levetiracetam + normal rat food) | 6.1 ± 0.89 ****/<0.0001 | 5.1 ± 1.47 ****/<0.0001 | 3.1 ± 0.18 */0.0486 | 3.1 ± 0.24 ns/0.0586 | 19.4 ± 3.35 ****/<0.0001 | 15.6 ± 4.31 ****/<0.0001 |
| 2nd treatment day (2D; i.p. 200 mg/kg levetiracetam + normal rat food) | 10.0 ± 1.79 ****/<0.0001 | 4.7 ± 2.16 ****/<0.0001 | 3.00 ± 0.29 */0.016 | 3.1 ± 0.33 ns/0.1178 | 31.4 ± 7.82 ****/<0.0001 | 14.7 ± 6.68 ****/<0.0001 |
| 3rd treatment day (3D; i.p. 200 mg/kg levetiracetam + normal rat food) | 9.9 ± 2.13 ****/<0.0001 | 7.6 ± 3.17 ***/0.0007 | 3.1 ± 0.20 ns/0.0769 | 3.16 ± 0.33 ns/0.1497 | 31.5 ± 7.21 ****/<0.0001 | 22.5 ± 8.65 ****/<0.0001 |
| 4th treatment day (4D; i.p. 200 mg/kg levetiracetam + normal rat food) | 9.7 ± 1.81 ****/<0.0001 | 8.6 ± 2.00 **/0.0024 | 3.1 ± 0.28 ns/0.0586 | 2.9 ± 0.20 */0.033 | 33.2 ± 8.07 ****/<0.0001 | 25.7 ± 6.24 ***/0.0002 |
| 5th treatment day (5D; i.p. 200 mg/kg levetiracetam + normal rat food) | 8.1 ± 1.55 ****/<0.0001 | 8.1 ± 0.94 **/0.0015 | 3.4 ± 0.43 ns/0.2658 | 3.0 ± 0.16 ns/0.0534 | 30.0 ± 7.06 ****/<0.0001 | 24.8 ± 3.36 ***/0.0002 |
| Treatments | Group 2 (Figure 3) | |||||
|---|---|---|---|---|---|---|
| Mean ± S.E.M. (Significance Level/p Value) | ||||||
| SWD Number | SWD Duration (sec) | Time Spent in SWD (sec) | ||||
| 30–90 min | 90–150 min | 30–90 min | 90–150 min | 30–90 min | 90–150 min | |
| Control (CON; i.p. saline + normal rat food) | 23.7 ± 4.87 | 20.4 ± 2.22 | 5.3 ± 0.27 | 5.1 ± 0.28 | 130.3 ± 30.26 | 106.1 ± 14.84 |
| 1st treatment day (1D; i.p. saline + KEKS food) | 18.4 ± 2.44 ns/0.8451 | 18.0 ± 3.37 ns/0.9939 | 4.1 ± 0.20 ns/0.0715 | 4.0 ± 0.25 ns/0.1133 | 74.3 ± 10.88 ns/0.1454 | 72.1 ± 13.67 ns/0.664 |
| 2nd treatment day (2D; i.p. saline + KEKS food) | 17.0 ± 4.82 ns/0.6641 | 12.7 ± 2.82 ns/0.5102 | 3.9 ± 0.41 */0.0255 | 3.6 ± 0.41 */0.0146 | 72.3 ± 27.74 ns/0.1199 | 49.7 ± 14.58 ns/0.1398 |
| 3rd treatment day (3D; i.p. saline + KEKS food) | 15.9 ± 4.78 ns/0.4978 | 15.7 ± 2.19 ns/0.894 | 2.8 ± 0.49 ****/<0.0001 | 3.5 ± 0.21 **/0.0081 | 52.2 ± 16.05 */0.0115 | 57.4 ± 10.29 ns/0.274 |
| 4th treatment day (4D; i.p. saline + KEKS food) | 8.4 ± 2.28 */0.0396 | 10.4 ± 0.61 ns/0.2257 | 4.0 ± 0.33 ns/0.0608 | 3.9 ± 0.23 ns/0.0976 | 44.5 ± 15.11 **/0.004 | 41.0 ± 3.12 ns/0.0568 |
| 5th treatment day (5D; i.p. saline + KEKS food) | 7.3 ± 1.06 **/0.0055 | 9.1 ± 1.18 ns/0.1246 | 4.0 ± 0.29 */0.0435 | 3.5 ± 0.18 **/0.0081 | 27.9 ± 3.74 ***/0.0003 | 33.1 ± 5.54 */0.0225 |
| Treatments | Group 3 (Figure 4) | |||||
|---|---|---|---|---|---|---|
| Mean ± S.E.M. (Significance Level/p Value) | ||||||
| SWD Number | SWD Duration (sec) | Time Spent in SWD (sec) | ||||
| 30–90 min | 90–150 min | 30–90 min | 90–150 min | 30–90 min | 90–150 min | |
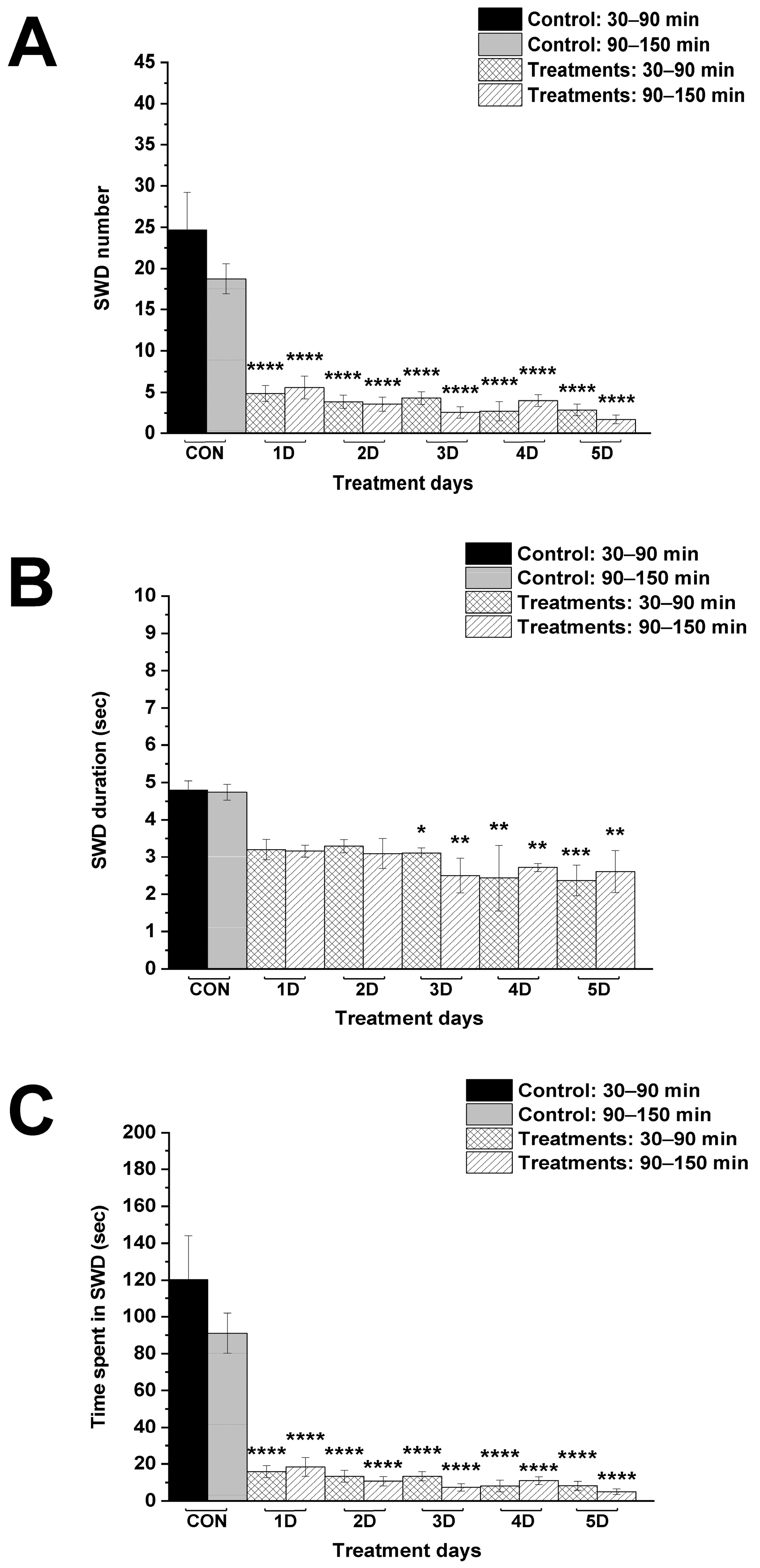
| Control (CON; i.p. saline + normal rat food) | 24.7 ± 4.55 | 18.8 ± 1.83 | 4.8 ± 0.25 | 4.7 ± 0.21 | 120.3 ± 23.80 | 91.0 ± 10.91 |
| 1st treatment day (1D; i.p. 200 mg/kg levetiracetam + KEKS food) | 4.9 ± 0.96 ****/<0.0001 | 5.6 ± 1.38 ****/<0.0001 | 3.2 ± 0.28 ns/0.0752 | 3.2 ± 0.16 ns/0.0707 | 15.9 ± 3.30 ****/<0.0001 | 18.5 ± 5.15 ****/<0.0001 |
| 2nd treatment day (2D; i.p. 200 mg/kg levetiracetam + KEKS food) | 3.9 ± 0.80 ****/<0.0001 | 3.6 ± 0.84 ****/<0.0001 | 3.3 ± 0.18 ns/0.1071 | 3.1 ± 0.41 ns/0.0516 | 13.4 ± 3.15 ****/<0.0001 | 10.7 ± 2.54 ****/<0.0001 |
| 3rd treatment day (3D; i.p. 200 mg/kg levetiracetam + KEKS food) | 4.3 ± 0.78 ****/<0.0001 | 2.6 ± 0.69 ****/<0.0001 | 3.1 ± 0.14 */0.0484 | 2.5 ± 0.46 **/0.0024 | 13.3 ± 2.49 ****/<0.0001 | 7.4 ± 2.03 ****/<0.0001 |
| 4th treatment day (4D; i.p. 200 mg/kg levetiracetam + KEKS food) | 2.7 ± 1.17 ****/<0.0001 | 4.0 ± 0.72 ****/<0.0001 | 2.4 ± 0.89 **/0.0013 | 2.7 ± 0.11 **/0.0075 | 8.1 ± 3.07 ****/<0.0001 | 11.0 ± 2.11 ****/<0.0001 |
| 5th treatment day (5D; i.p. 200 mg/kg levetiracetam + KEKS food) | 2.9 ± 0.71 ****/<0.0001 | 1.7 ± 0.52 ****/<0.0001 | 2.4 ± 0.41 ***/0.0009 | 2.6 ± 0.57 **/0.0047 | 8.2 ± 2.41 ****/<0.0001 | 5.1 ± 1.50 ****/<0.0001 |
| Treatments | Mean ± S.E.M. (Significance Level/p Value) | |
|---|---|---|
| R-βHB (mmol/L) | Glucose (mg/dL) | |
| Group 2 (Figure 6A,B) | ||
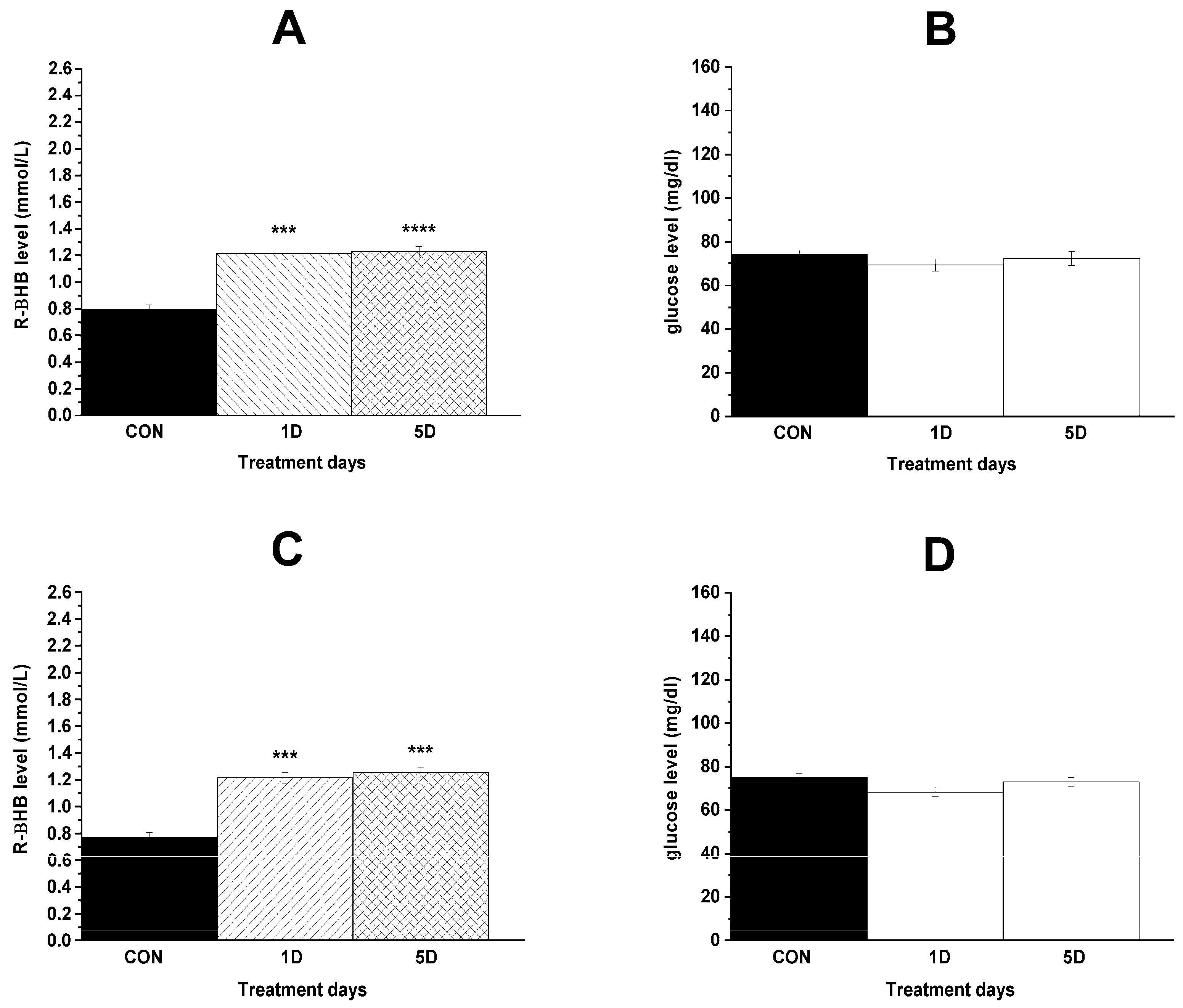
| Last control day (CON; i.p. saline + normal rat food) | 0.8 ± 0.03 | 74.0 ± 2.17 |
| 1st treatment day (1D; i.p. saline + KEKS food) | 1.2 ± 0.05 ***/0.0005 | 69.3 ± 2.76 ns/0.4284 |
| 5th treatment day (5D; i.p. saline + KEKS food) | 1.2 ± 0.04 ****/<0.0001 | 72.3 ± 3.21 ns/0.5219 |
| Group 3 (Figure 6C,D) | ||
| Last control day (CON; i.p. saline + normal rat food) | 0.8 ± 0.04 | 75.1 ± 1.89 |
| 1st treatment day (1D; i.p. 200 mg/kg levetiracetam + KEKS food) | 1.2 ± 0.04 ***/0.0002 | 68.3 ± 2.20 ns/0.0523 |
| 5th treatment day (5D; i.p. 200 mg/kg levetiracetam + KEKS food) | 1.3 ± 0.04 ***/0.0003 | 73.0 ± 2.15 ns/0.6092 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauch, E.; Ari, C.; D’Agostino, D.P.; Kovács, Z. Exogenous Ketone Supplementation Enhances the Anti-Epileptic Effect of Levetiracetam in Wistar Albino Glaxo/Rijswijk Rats. Nutrients 2025, 17, 1721. https://doi.org/10.3390/nu17101721
Rauch E, Ari C, D’Agostino DP, Kovács Z. Exogenous Ketone Supplementation Enhances the Anti-Epileptic Effect of Levetiracetam in Wistar Albino Glaxo/Rijswijk Rats. Nutrients. 2025; 17(10):1721. https://doi.org/10.3390/nu17101721
Chicago/Turabian StyleRauch, Enikő, Csilla Ari, Dominic P. D’Agostino, and Zsolt Kovács. 2025. "Exogenous Ketone Supplementation Enhances the Anti-Epileptic Effect of Levetiracetam in Wistar Albino Glaxo/Rijswijk Rats" Nutrients 17, no. 10: 1721. https://doi.org/10.3390/nu17101721
APA StyleRauch, E., Ari, C., D’Agostino, D. P., & Kovács, Z. (2025). Exogenous Ketone Supplementation Enhances the Anti-Epileptic Effect of Levetiracetam in Wistar Albino Glaxo/Rijswijk Rats. Nutrients, 17(10), 1721. https://doi.org/10.3390/nu17101721









