Nutritional and Physical Rehabilitation in Post-Critical Coronavirus Disease 2019 (COVID-19) Ambulatory Patients: The NutriEcoMuscle Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Intervention
2.3. Variables
2.4. Sample Size
2.5. Statistical Analysis
3. Results
3.1. Nutritional Evaluation
3.2. Functional Assessment
3.3. Body Composition Assessment
3.4. Correlations Between Changes in BIA and Nutritional Ultrasound
3.5. Correlations Between Changes in BIA and Nutritional Ultrasound, and Changes in Handgrip Strength and TUG
3.6. Supplement Adherence and Tolerance
3.7. Exercise
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARDS | Acute Respiratory Distress Syndrome |
| ASMM | Appendicular skeletal muscle mass |
| ASMMI | Appendicular skeletal muscle mass index |
| BI | Barthel index |
| BIA | Bioelectrical impedance analysis |
| BIVA | Bioelectrical impedance vector analysis |
| BMI | Body mass index |
| CHF | Congestive heart failure |
| CI | Confidence interval |
| CKD | Chronic kidney disease |
| COPD | Chronic obstructive pulmonary disease |
| COVID-19 | Coronavirus disease 2019 |
| CRP | C-reactive protein |
| DM | Diabetes mellitus |
| DXA | Dual-energy X-ray absorptiometry |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| EWGSOP2 | European Working Group on Sarcopenia in Older People 2 |
| FFM | Fat-free mass |
| FFMI | Fat-free mass index |
| GLIM | Global Leadership Initiative on Malnutrition criteria |
| HBP | High blood pressure |
| HFNC | High flow nasal cannula |
| ICU | Intensive care unit |
| IQR | Interquartile range |
| NIMV | Non-invasive mechanical ventilation |
| NS | Not statistically significant |
| ONS | Oral nutritional supplement |
| PhA | Phase angle |
| RFCSA | Rectus femoris cross-sectional area |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| SD | Standard deviation |
| SGA | Subjective Global Assessment |
| SMMI | Skeletal muscle mass index |
| SOFA | Sequential Organ Failure Assessment Score |
| SPA | Standardized phase angle |
| TUG | Timed Up and Go test |
References
- Abate, S.M.; Ahmed Ali, S.; Mantfardo, B.; Basu, B. Rate of Intensive Care Unit admission and outcomes among patients with coronavirus: A systematic review and Meta-analysis. PLoS ONE 2020, 15, e0235653. [Google Scholar] [CrossRef] [PubMed]
- Singer, P. Preserving the quality of life: Nutrition in the ICU. Crit. Care 2019, 23, 139. [Google Scholar] [CrossRef] [PubMed]
- Rees, E.M.; Nightingale, E.S.; Jafari, Y.; Waterlow, N.R.; Clifford, S.; Pearson, C.A.B.; Group, C.W.; Jombart, T.; Procter, S.R.; Knight, G.M. COVID-19 length of hospital stay: A systematic review and data synthesis. BMC Med. 2020, 18, 270. [Google Scholar] [CrossRef]
- Joaquín, C.; Bretón, I.; Ocón Bretón, M.J.; Burgos, R.; Bellido, D.; Matía-Martín, P.; Martínez Olmos, M.Á.; Zugasti, A.; Riestra, M.; Botella, F.; et al. Nutritional and Morphofunctional Assessment of Post-ICU Patients with COVID-19 at Hospital Discharge: NutriEcoMuscle Study. Nutrients 2024, 16, 886. [Google Scholar] [CrossRef] [PubMed]
- Rives-Lange, C.; Zimmer, A.; Merazka, A.; Carette, C.; Martins-Bexinga, A.; Hauw-Berlemont, C.; Guerot, E.; Jannot, A.S.; Diehl, J.L.; Czernichow, S.; et al. Evolution of the nutritional status of COVID-19 critically-ill patients: A prospective observational study from ICU admission to three months after ICU discharge. Clin. Nutr. 2022, 41, 3026–3031. [Google Scholar] [CrossRef]
- Bedock, D.; Couffignal, J.; Bel Lassen, P.; Soares, L.; Mathian, A.; Fadlallah, J.P.; Amoura, Z.; Oppert, J.-M.; Faucher, P. Evolution of Nutritional Status after Early Nutritional Management in COVID-19 Hospitalized Patients. Nutrients 2021, 13, 2276. [Google Scholar] [CrossRef]
- Lakenman, P.L.; Joosten, K.F.; Bommel, J.V.; Bek, L.M.; Berg-Emons, R.J.V.D.; Olieman, J.F. Nutritional status of patients with COVID-19 1-y post-ICU stay: A prospective observational study. Nutrition 2023, 111, 112025. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Shadmand Foumani Moghadam, M.R.; Vaezi, A.; Jandari, S.; Araste, A.; Rezvani, R. Navigating sarcopenia in COVID-19 patients and survivors: Understanding the long-term consequences, transitioning from hospital to community with mechanisms and interventions for future preparedness. Aging Med. 2024, 7, 103–114. [Google Scholar] [CrossRef]
- Cornejo-Pareja, I.; Vegas-Aguilar, I.M.; García-Almeida, J.M.; Bellido-Guerrero, D.; Talluri, A.; Lukaski, H.; Tinahones, F.J. Phase angle and standardized phase angle from bioelectrical impedance measurements as a prognostic factor for mortality at 90 days in patients with COVID-19: A longitudinal cohort study. Clin. Nutr. 2022, 41, 3106–3114. [Google Scholar] [CrossRef]
- Hinkelmann, J.V.; De Oliveira, N.A.; Marcato, D.F.; Costa, A.R.R.O.; Ferreira, A.M.; Tomaz, M.; Rodrigues, T.J.; Mendes, A.P. Nutritional support protocol for patients with COVID-19. Clin. Nutr. ESPEN 2022, 49, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Besutti, G.; Pellegrini, M.; Ottone, M.; Bonelli, E.; Monelli, F.; Farì, R.; Milic, J.; Dolci, G.; Fasano, T.; Canovi, S.; et al. Modifications of Chest CT Body Composition Parameters at Three and Six Months after Severe COVID-19 Pneumonia: A Retrospective Cohort Study. Nutrients 2022, 14, 3764. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.S.; Mourtzakis, M.; Aronson Friedman, L.; Dinglas, V.D.; Hough, C.L.; Ely, E.W.; Morris, P.E.; Hopkins, R.O.; Needham, D.M. Evaluating Muscle Mass in Survivors of Acute Respiratory Distress Syndrome: A 1-Year Multicenter Longitudinal Study. Crit. Care Med. 2018, 46, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Pareja, I.; Soler-Beunza, A.G.; Vegas-Aguilar, I.M.; Fernández-Jiménez, R.; Tinahones, F.J.; García-Almeida, J.M. Predictors of Sarcopenia in Outpatients with Post-Critical SARS-CoV2 Disease. Nutritional Ultrasound of Rectus Femoris Muscle, a Potential Tool. Nutrients 2022, 14, 4988. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, I.M.; Ballesteros Pomar, M.D.; Cornejo-Pareja, I.M.; Fernández Medina, B.; de Luis Román, D.A.; Bellido Guerrero, D.; Bretón Lesmes, I.; Tinahones Madueño, F.J. Nutritional ultrasound®: Conceptualisation, technical considerations and standardisation. Endocrinol. Diabetes Nutr. 2023, 70 (Suppl. S1), 74–84. [Google Scholar] [CrossRef]
- De Luis Roman, D.; García Almeida, J.M.; Bellido Guerrero, D.; Guzmán Rolo, G.; Martín, A.; Primo Martín, D.; García-Delgado, Y.; Guirado-Peláez, P.; Palmas, F.; Tejera Pérez, C.; et al. Ultrasound Cut-Off Values for Rectus Femoris for Detecting Sarcopenia in Patients with Nutritional Risk. Nutrients 2024, 16, 1552. [Google Scholar] [CrossRef]
- Fortimel_Advanced—Nutricia. Available online: https://www.nutricia.es/productos/fortimel_advanced/ (accessed on 20 May 2024).
- Fortimel Advanced with ActiSynTM. Available online: https://www.nutricia.com/products/frailty-and-drm/fortimel-advanced.html (accessed on 20 May 2024).
- González, D.A.G.; Gómez González, A.; García Almeida, J.M. Programa de Ejercicio Físico y Rehabilitación Muscular. Available online: https://www.sspa.juntadeandalucia.es/servicioandaluzdesalud/hospital/virgen-victoria/sites/default/files/2024-11/%C2%B7Programa%20de%20ejercicio%20f%C3%ADsico%20y%20recuperaci%C3%B3n%20muscular%20.pdf (accessed on 10 April 2025).
- da Silva Fink, J.; Daniel de Mello, P.; Daniel de Mello, E. Subjective global assessment of nutritional status—A systematic review of the literature. Clin. Nutr. 2015, 34, 785–792. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- American Society of Hand Therapists. Clinical Assessment Recommendations; American Society of Hand Therapists: Mount Laurel, NJ, USA, 2015. [Google Scholar]
- Bahat, G.; Kilic, C.; Altinkaynak, M.; Akif Karan, M. Comparison of standard versus population-specific handgrip strength cut-off points in the detection of probable sarcopenia after launch of EWGSOP2. Aging Male 2020, 23, 1564–1569. [Google Scholar] [CrossRef]
- Kear, B.M.; Guck, T.P.; McGaha, A.L. Timed Up and Go (TUG) Test: Normative Reference Values for Ages 20 to 59 Years and Relationships with Physical and Mental Health Risk Factors. J. Prim. Care Community Health 2017, 8, 9–13. [Google Scholar] [CrossRef] [PubMed]
- García Almeida, J.M.; García García, C.; Bellido Castañeda, V.; Bellido Guerrero, D. Nuevo enfoque de la nutrición. Valoración del estado nutricional del paciente: Función y composición corporal. Nutr. Hosp. 2018, 35, 1–14. [Google Scholar] [CrossRef]
- Barichella, M.; Cereda, E.; Pinelli, G.; Iorio, L.; Caroli, D.; Masiero, I.; Ferri, V.; Cassani, E.; Bolliri, C.; Caronni, S.; et al. Muscle-targeted nutritional support for rehabilitation in patients with parkinsonian syndrome. Neurology 2019, 93, e485–e496. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Liu, K.; Katsukawa, H.; Nydahl, P.; Ely, E.W.; Kudchadkar, S.R.; Inoue, S.; Lefor, A.K.; Nishida, O. Nutrition therapy in the intensive care unit during the COVID-19 pandemic: Findings from the ISIIC point prevalence study. Clin. Nutr. 2022, 41, 2947–2954. [Google Scholar] [CrossRef]
- Eden, T.; McAuliffe, S.; Crocombe, D.; Neville, J.; Ray, S. Nutritional parameters and outcomes in patients admitted to intensive care with COVID-19: A retrospective single-centre service evaluation. BMJ Nutr. Prev. Health 2021, 4, 416–424. [Google Scholar] [CrossRef]
- Cuerda, C.; Sánchez López, I.; Gil Martínez, C.; Merino Viveros, M.; Velasco, C.; Cevallos Peñafiel, V.; Maíz Jiménez, M.; Gonzalo, I.; González-Sánchez, V.; Ramos Carrasco, A.; et al. Impact of COVID-19 in nutritional and functional status of survivors admitted in intensive care units during the first outbreak. Preliminary results of the NUTRICOVID study. Clin. Nutr. 2022, 41, 2934–2939. [Google Scholar] [CrossRef]
- Costa, A.; Gonçalves, A.F.; Rodrigues, M.; Santos, R.; Almeida, M.P.; Lima, A. Post-intensive Care Unit COVID-19 Survivors: Functional Status and Respiratory Function Three Months After an Inpatient Rehabilitation Program. Cureus 2022, 14, e31281. [Google Scholar] [CrossRef]
- Cavalleri, J.; Treguier, D.; Deliège, T.; Gurdebeke, C.; Ernst, M.; Lambermont, B.; Misset, B.; Rousseau, A.-F. One-Year Functional Decline in COVID-19 and Non-COVID-19 Critically Ill Survivors: A Prospective Study Incorporating a Pre-ICU Status Assessment. Healthcare 2022, 10, 2023. [Google Scholar] [CrossRef]
- Leite, L.C.; Carvalho, L.; de Queiroz, D.M.; Farias, M.S.Q.; Cavalheri, V.; Edgar, D.W.; Nery, B.R.A.; Vasconcelos Barros, N.; Maldaner, V.; Campos, N.G.; et al. Can the post-COVID-19 functional status scale discriminate between patients with different levels of fatigue, quality of life and functional performance? Pulmonology 2022, 28, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Boon, G.J.A.M.; Barco, S.; Endres, M.; Geelhoed, J.J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef]
- Kizilarslanoglu, M.C.; Kuyumcu, M.E.; Yesil, Y.; Halil, M. Sarcopenia in critically ill patients. J. Anesth. 2016, 30, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Hao, Q.; Ge, M.; Dong, B. Sarcopenia and hospital-related outcomes in the old people: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2019, 31, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Bein, T.; Weber-Carstens, S.; Apfelbacher, C. Long-term outcome after the acute respiratory distress syndrome: Different from general critical illness? Curr. Opin. Crit. Care 2018, 24, 35–40. [Google Scholar] [CrossRef]
- Ramos, A.; Joaquin, C.; Ros, M.; Martin, M.; Cachero, M.; Sospedra, M.; Martínez, E.; Sánchez Migallón, J.M.; Sendrós, M.-J.; Soldevila, B.; et al. Impact of COVID-19 on nutritional status during the first wave of the pandemic. Clin. Nutr. 2022, 41, 3032–3037. [Google Scholar] [CrossRef]
- Jones, C.; Eddleston, J.; McCairn, A.; Dowling, S.; McWilliams, D.; Coughlan, E.; Griffiths, R.D. Improving rehabilitation after critical illness through outpatient physiotherapy classes and essential amino acid supplement: A randomized controlled trial. J. Crit. Care 2015, 30, 901–907. [Google Scholar] [CrossRef]
- Bear, D.E.; Langan, A.; Dimidi, E.; Wandrag, L.; Harridge, S.D.R.; Hart, N.; Connolly, B.; Whelan, K. β-Hydroxy-β-methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 109, 1119–1132. [Google Scholar] [CrossRef]
- Li, F.; Yin, Y.; Tan, B.; Kong, X.; Wu, G. Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids 2011, 41, 1185–1193. [Google Scholar] [CrossRef]
- Salles, J.; Chanet, A.; Giraudet, C.; Patrac, V.; Pierre, P.; Jourdan, M.; Luiking, Y.C.; Verlaan, S.; Migné, C.; Boirie, Y.; et al. 1,25(OH)2-vitamin D3 enhances the stimulating effect of leucine and insulin on protein synthesis rate through Akt/PKB and m TOR mediated pathways in murine C2C12 skeletal myotubes. Mol. Nutr. Food Res. 2013, 57, 2137–2146. [Google Scholar] [CrossRef]
- Chanet, A.; Verlaan, S.; Salles, J.; Giraudet, C.; Patrac, V.; Pidou, V.; Pouyet, C.; Hafnaoui, N.; Blot, A.; Cano, N.; et al. Supplementing Breakfast with a Vitamin D and Leucine–Enriched Whey Protein Medical Nutrition Drink Enhances Postprandial Muscle Protein Synthesis and Muscle Mass in Healthy Older Men. J. Nutr. 2017, 147, 2262–2271. [Google Scholar] [CrossRef]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef]
- Rennie, M.J.; Bohé, J.; Smith, K.; Wackerhage, H.; Greenhaff, P. Branched-chain amino acids as fuels and anabolic signals in human muscle. J. Nutr. 2006, 136, 264S–268S. [Google Scholar] [CrossRef] [PubMed]
- Bohé, J.; Low, A.; Wolfe, R.R.; Rennie, M.J. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: A dose-response study. J. Physiol. 2003, 552, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Olezene, C.S.; Hansen, E.; Steere, H.K.; Giacino, J.T.; Polich, G.R.; Borg-Stein, J.; Zafonte, R.D.; Schneider, J.C. Functional outcomes in the inpatient rehabilitation setting following severe COVID-19 infection. PLoS ONE 2021, 16, e0248824. [Google Scholar] [CrossRef] [PubMed]
- Halabchi, F.; Selk-Ghaffari, M.; Tazesh, B.; Mahdaviani, B. The effect of exercise rehabilitation on COVID-19 outcomes: A systematic review of observational and intervention studies. Sport Sci. Health 2022, 18, 1201–1219. [Google Scholar] [CrossRef]
- Alfaro-Martínez, J.J.; Solís García Del Pozo, J.; Quílez Toboso, R.P.; García Blasco, L.; Rosa Felipe, C. Study of incidence of COVID-19 in Spain and its relationship to geographical province distribution. J. Healthc. Qual. Res. 2023, 38, 299–303. [Google Scholar] [CrossRef]


| Total | Men | Women | p-Value | |
|---|---|---|---|---|
| n (%) | 96 (100.0) | 69 (71.9) | 27 (28.1) | - |
| Age, mean (SD), years | 58.8 (8.5) | 58.5 (8.8) | 59.7 (7.7) | NS |
| Age ≥ 65 years, n (%) | 29 (30.2) | 20 (29.0) | 9 (33.3) | NS |
| Co-morbidities, n (%) | ||||
| Obesity | 40 (41.7) | 24 (34.8) | 16 (59.3) | 0.0386 * |
| HBP | 34 (35.4) | 22 (31.9) | 12 (44.4) | NS |
| Diabetes mellitus | 19 (19.8) | 13 (18.8) | 6 (22.2) | NS |
| COPD | 6 (6.3) | 5 (7.2) | 1 (3.7) | NS |
| CKD | 2 (2.1) | 2 (2.9) | 0 (0.0) | NS |
| CHF | 3 (3.1) | 3 (4.3) | 0 (0.0) | NS |
| Active oncologic pathology | 2 (2.1) | 2 (2.9) | 0 (0.0) | NS |
| Length of hospital stay, mean (SD), days | 48.2 (37.6) | 49.7 (40.9) | 44.6 (27.6) | NS |
| Pre-ICU hospital stay, mean (SD), days | 2.3 (3.2) | 2.0 (2.4) | 3.1 (4.7) | NS |
| ICU stay, mean (SD), days | 28.7 (7.5) | 30.3 (29.7) | 24.4 (20.4) | NS |
| SOFA score, mean (SD) | 4.1 (2.4) | 4.2 (2.7) | 4.0 (2.3) | NS |
| Mechanical ventilation, n (%) | 58 (60.4) | 40 (58.0) | 18 (66.7) | NS |
| NIMV | 5 (5.2) | 4 (5.8) | 1 (3.7) | NS |
| HFNC | 33 (34.4) | 25 (36.2) | 8 (29.6) | NS |
| CRP, median (IQR), mg/dL | 2.9 (0.5–9.0) | 2.9 (0.4–11.3) | 2.9 (0.7–8.4) | NS |
| Vitamin D, median (IQR), mg/dL | 13.1 (10–17) | 15.5 (9.8–18) | 11.7 (10–13.1) | NS |
| BI score at hospital discharge, median (IQR) | 90 (65–100) | 90 (65–100) | 85 (70–95) | NS |
| BI < 100, n (%) | 64 (66.7) | 42 (60.9) | 22 (81.5) | NS |
| Handgrip strength | ||||
| Mean (SD), kg | 21.6 (11.0) | 25.0 (10.9) | 13.0 (4.9) | <0.0001 |
| <27 men and <16 women; n (%) | 60 (62.5) | 39 (56.5) | 21 (77.8) | NS |
| TUG test | ||||
| Mean (SD), seconds | 19.9 (17.2) | 16.7 (14.2) | 28.0 (21.5) | 0.0004 |
| >20 s, n (%) | 26 (27.1) | 14 (20.3) | 12 (44.4) | 0.0224 |
| Day 0 * | Day 90 ** | p-Value | Change | ||
|---|---|---|---|---|---|
| Δ | % | ||||
| Weight, mean (SD), kg | 81.0 (16.8) | 87.8 (17.5) | <0.00001 | 6.8 (5.2) | 8.9% (6.6) |
| BMI, mean (SD), kg/m2 | 28.6 (6.0) | 30.7 (6.7) | <0.00001 | 1.8 (4.8) | 7.6% (15.0) |
| Waist circumference, mean (SD), cm | 103.7 (15.0) | 107.5 (14.3) | <0.00001 | 4.0 (7.5) | 4.3% (6.9) |
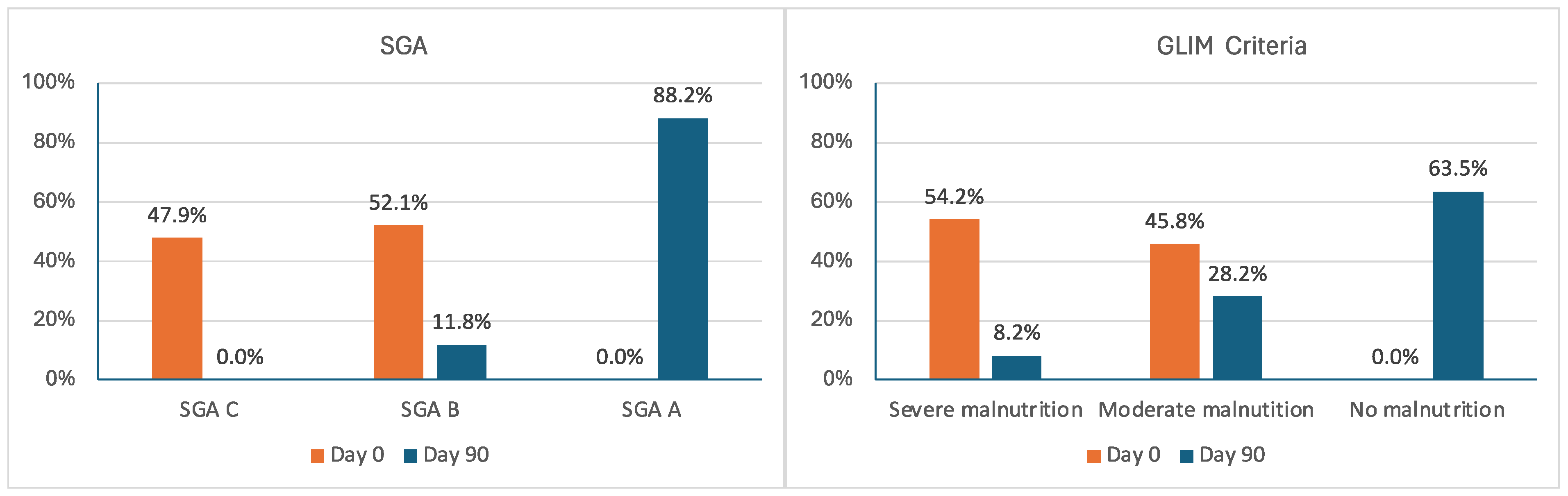
| Malnutrition by SGA, n (%) | |||||
| SGA A | 0 (0) | 75 (88.2) | <0.00001 | 88.2% | |
| SGA B | 50 (52.1) | 10 (11.8) | −40.3% | ||
| SGA C | 46 (47.9) | 0 (0) | −47.9% | ||
| Malnutrition by GLIM criteria, n (%) | |||||
| No malnutrition | 0 (0) | 54 (63.5) | <0.00001 | 63.5% | |
| Moderate | 44 (45.8) | 24 (28.2) | −17.6% | ||
| Severe | 52 (54.2) | 7 (8.2) | −46.0% | ||
| Day 0 * | Day 90 ** | p-Value | Change | ||
|---|---|---|---|---|---|
| Δ | % | ||||
| BI < 100, n (%) | 64 (66.7) | 23 (27.0) | <0.0001 | −39.7% | |
| Handgrip strength | |||||
| Mean (SD), kg | 21.6 (11.0) | 28.9 (11.7) | <0.00001 | 7.3 (6.7) | 48.2% (55.6) |
| <27 men and <16 women; n (%) | 60 (62.5) | 26 (31.3) | <0.0001 | −31.2% | |
| TUG test | |||||
| Mean (SD), seconds | 19.9 (17.2) | 8.9 (4.2) | <0.0001 | −10.5 (15.5) | −41.4% (24.7) |
| > 20 s, n (%) | 26 (27.1) | 4 (4.7) | <0.0001 | −22.4% | |
| Day 0 | Day 90 | p-Value | Change | ||||
|---|---|---|---|---|---|---|---|
| n | Result | n | Result | Δ | % | ||
| Weight, mean (SD), kg | 92 | 80.8 (16.7) | 83 | 87.4 (17.5) | <0.00001 | 6.6 (4.8) | 8.8% (6.4) |
| Fat mass, mean (SD), kg | 92 | 28.9 (11.6) | 83 | 29.7 (11.3) | 0.281 | 0.4 (6.9) | 5.5% (25.4) |
| Fat-free mass, mean (SD), kg | 90 | 49.6 (13.3) | 76 | 55.2 (14.5) | <0.00001 | 6.4 (9.5) | 19.7% (55.7) |
| FFMI, mean (SD), kg/m2 | 90 | 17.3 (3.9) | 76 | 19.3 (4.2) | <0.00001 | 2.2 (3.4) | 19.6% (55.0) |
| FFMI < 17 men and <15 kg/m2 women; n (%) | 90 | 30 (33.3) | 76 | 13 (17.1) | 0.0087 | −16.2% | |
| Body cell mass, mean (SD), kg | 78 | 28.2 (7.6) | 63 | 34.4 (8.7) | <0.00001 | 5.2 (4.4) | 21.1% (20.75) |
| SMMI, mean (SD), kg/m2 | 67 | 8.5 (2.4) | 59 | 9.6 (3.8) | <0.00001 | 1.4 (3.5) | 19.1% (48.5) |
| Total body water, mean (SD), L | 86 | 39.3 (8.4) | 79 | 43.4 (9.0) | <0.00001 | 4.3 (4.4) | 11.5% (11.0) |
| ASMM index, mean (SD), kg/m2 | 49 | 7.2 (2.1) | 41 | 7.7 (1.8) | 0.0002 | 0.6 (1.8) | 11.4% (20.0) |
| ASMM index < 7 men and <5.7 kg/m2 women, n (%) | 49 | 20 (40.8) | 41 | 12 (29.2) | 0.127 | −11.6% | |
| PhA, mean (SD), degrees | 87 | 4.5 (1.0) | 81 | 5.4 (0.9) | <0.00001 | 0.9 (0.7) | 24.3% (22.2) |
| PhA < 3.95°, n (%) | 87 | 26 (29.8) | 81 | 3 (3.7) | <0.0001 | −26.1% | |
| Standardized PhA, median (IQR) | 31 | −2.0 (−2.7 to −1.0) | 28 | −1.2 (−1.8 to −0.7) | 0.0071 | 0.5 (−0.2 to 1.7) | 34.8% (−11 to 66) |
| RZ, mean (SD), ohmios | 55 | 529.3 (108.0) | 49 | 465.2 (90.3) | <0.00001 | −74.3 (77.7) | −12.8% (13.7) |
| XC, mean (SD), ohmios | 55 | 43.1 (11.3) | 49 | 44.6 (9.2) | 0.267 | 1.3 (8.0) | 5.5% (19.5) |
| Day 0 | Day 90 | Change | |||||
|---|---|---|---|---|---|---|---|
| n | Result | n | Result | p-Value | Δ | % | |
| Rectus femoris | |||||||
| RFCSA, mean (SD), cm2 | 96 | 3.3 (1.3) | 85 | 4.6 (1.7) | <0.00001 | 1.2 (1.4) | 47.5% (61.0) |
| Muscle circumference, mean (SD), cm | 96 | 8.7 (1.3) | 85 | 9.3 (1.5) | <0.0001 | 0.57 (1.6) | 8.2% (20.8) |
| X-axis, mean (SD), cm | 96 | 3.6 (0.6) | 85 | 3.8 (0.6) | 0.0260 | 0.15 (0.7) | 7.8% (32.7) |
| Y-axis, mean (SD), cm | 96 | 1.0 (0.3) | 85 | 1.3 (0.4) | <0.00001 | 0.35 (0.3) | 41.4% (43.3) |
| Subcutaneous adipose tissue (SD), cm | 96 | 0.9 (0.5) | 85 | 0.9 (0.5) | 0.570 | −0.0004 (0.2) | 7.1% (31) |
| Abdominal wall | |||||||
| Total adipose tissue, mean (SD), cm | 95 | 2.1 (1.0) | 84 | 2.0 (0.9) | 0.207 | −0.105 (0.6) | −0.6% (27.3) |
| Superficial adipose tissue, mean (SD), cm | 95 | 1.0 (0.6) | 84 | 0.9 (0.5) | 0.338 | −0.05 (0.4) | 5.1% (49.4) |
| Preperitoneal adipose tissue mean (SD), cm | 95 | 0.8 (0.4) | 84 | 0.8 (0.5) | 0.799 | −0.005 (0.5) | 15.2% (101) |
| Baseline Characteristics | Beta | 95%CI | p |
|---|---|---|---|
| Male/female | −9.1026 | −20.9 to 2.7 | 0.130 |
| Age, years | −0.640 | −1.216 to −0.064 | 0.030 |
| Diabetes, yes/no | −16.942 | −33.097 to −0.787 | 0.040 |
| Length of hospital stay, days | 0.237 | 0.117 to 0.357 | <0.0001 |
| ICU stay, days | 0.398 | 0.239 to 0.556 | <0.0001 |
| SOFA score | 2.461 | −3.513 to 8.435 | 0.387 |
| CRP, mg/dL | −0.011 | −0.068 to 0.045 | 0.690 |
| Vitamin D, mg/dL | −0.209 | −1.403 to 0.983 | 0.720 |
| BI score | −0.607 | −1.539 to 0.325 | 0.199 |
| Handgrip strength, kg | −0.666 | −1.115 to −0.218 | 0.004 |
| TUG, seconds | 0.682 | 0.366 to 0.998 | <0.0001 |
| SMMI, kg/m2 | −1.444 | −3.893 to 1.003 | 0.242 |
| PhA, grades | −13.495 | −17.242 to −9.749 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joaquín, C.; Bretón, I.; Ocón-Bretón, M.J.; Zabalegui, A.; Bellido, D.; Matía Martín, P.; Martínez-Olmos, M.Á.; Zugasti, A.; Riestra, M.; Botella, F.; et al. Nutritional and Physical Rehabilitation in Post-Critical Coronavirus Disease 2019 (COVID-19) Ambulatory Patients: The NutriEcoMuscle Study. Nutrients 2025, 17, 1722. https://doi.org/10.3390/nu17101722
Joaquín C, Bretón I, Ocón-Bretón MJ, Zabalegui A, Bellido D, Matía Martín P, Martínez-Olmos MÁ, Zugasti A, Riestra M, Botella F, et al. Nutritional and Physical Rehabilitation in Post-Critical Coronavirus Disease 2019 (COVID-19) Ambulatory Patients: The NutriEcoMuscle Study. Nutrients. 2025; 17(10):1722. https://doi.org/10.3390/nu17101722
Chicago/Turabian StyleJoaquín, Clara, Irene Bretón, María Julia Ocón-Bretón, Alba Zabalegui, Diego Bellido, Pilar Matía Martín, Miguel Ángel Martínez-Olmos, Ana Zugasti, María Riestra, Francisco Botella, and et al. 2025. "Nutritional and Physical Rehabilitation in Post-Critical Coronavirus Disease 2019 (COVID-19) Ambulatory Patients: The NutriEcoMuscle Study" Nutrients 17, no. 10: 1722. https://doi.org/10.3390/nu17101722
APA StyleJoaquín, C., Bretón, I., Ocón-Bretón, M. J., Zabalegui, A., Bellido, D., Matía Martín, P., Martínez-Olmos, M. Á., Zugasti, A., Riestra, M., Botella, F., & García-Almeida, J. M., on behalf of the NutriEcoMuscle Study Team. (2025). Nutritional and Physical Rehabilitation in Post-Critical Coronavirus Disease 2019 (COVID-19) Ambulatory Patients: The NutriEcoMuscle Study. Nutrients, 17(10), 1722. https://doi.org/10.3390/nu17101722







