Short-Term Beetroot Juice Supplementation Enhances Strength, Reduces Fatigue, and Promotes Recovery in Physically Active Individuals: A Randomized, Double-Blind, Crossover Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Experimentation Protocol
2.3. Supplementation Protocol
2.4. Strength Exercise Protocol
2.5. Measurements
2.5.1. Incremental Strength Test and Performance
2.5.2. Heart Rate Variability (HRV) Analysis
2.5.3. Muscle Oxygenation
2.5.4. Lower-Limb Strength Tests
2.5.5. Blood Lactate Measurement
2.5.6. Delayed-Onset Muscle Soreness (DOMS)
2.6. Statistical Analysis
3. Results
3.1. Dietary Intake
3.2. Strenght Performance and Physiological Parameters
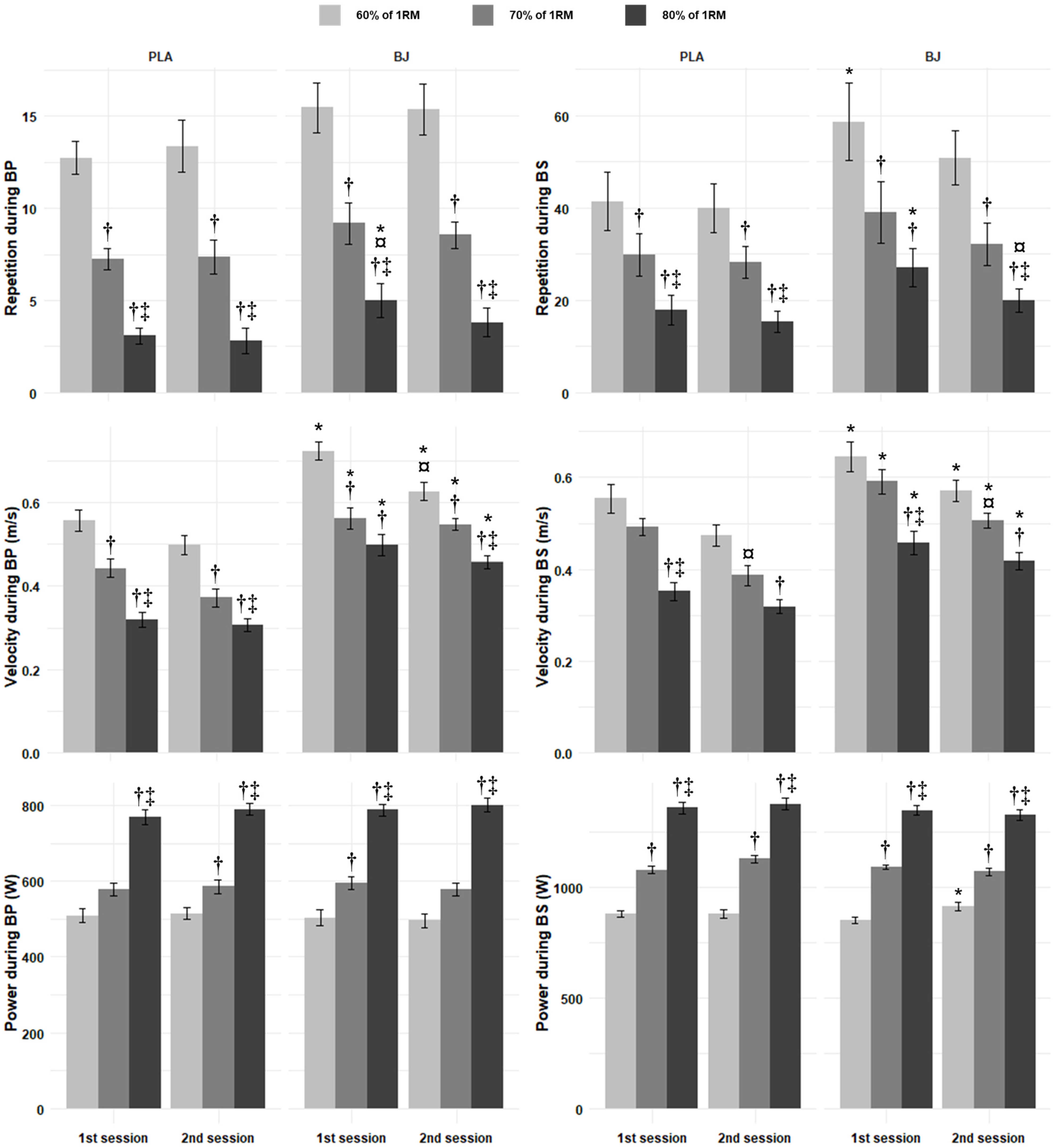
3.2.1. Reached Repetitions
3.2.2. Peak Velocity
3.2.3. Peak Power
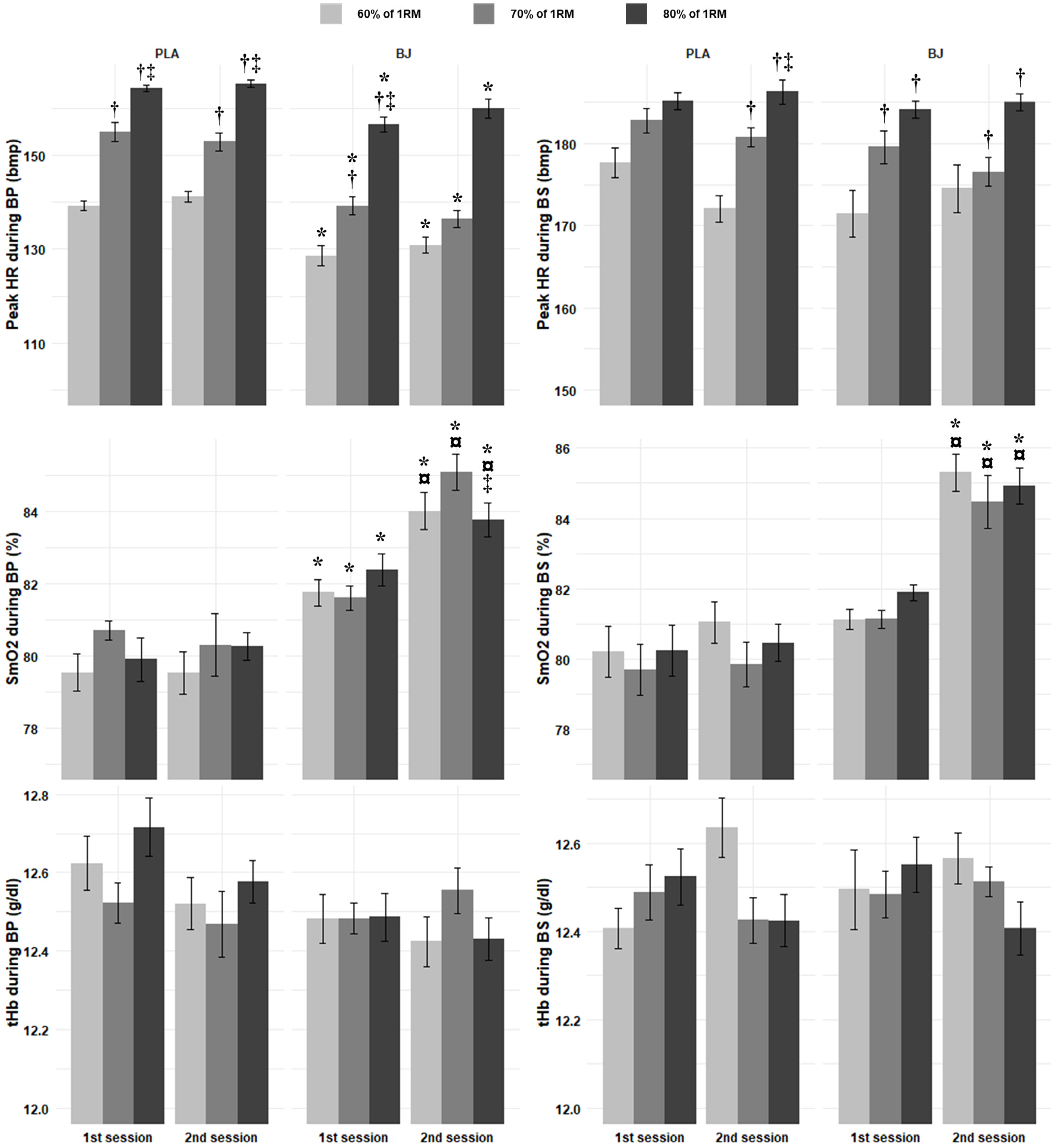
3.2.4. Peak HR
3.2.5. SmO2
3.2.6. Total Hemoglobin
3.3. ∆Pre-Post Change in HRV Indices and Lactate
3.4. Post-Session Recovery
3.4.1. CMJ
3.4.2. SJ
3.4.3. Upper-Limb DOMS
3.4.4. Lower-Limb DOMS
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BJ | Beetroot Juice |
| PLA | Placebo |
| 1RM | One-Repetition Maximum |
| BS | Back Squat |
| BP | Bench Press |
| HR | Heart Rate |
| HRV | Heart Rate Variability |
| RMSSD | Root Mean Square of Successive Differences |
| HF | High Frequency |
| CMJ | Countermovement Jump |
| SJ | Squat Jump |
| DOMS | Delayed Onset Muscle Soreness |
| RPE | Rate of Perceived Exertion |
| NO3− | Nitrate |
| NO2− | Nitrite |
| NO | Nitric Oxide |
| ATP | Adenosine Triphosphate |
| RT | Resistance Training |
| LF | Low Frequency |
| MeanRR | Mean RR Interval |
| SDNN | Standard Deviation of NN Intervals |
| SmO2 | Muscle Oxygen Saturation |
| THb | Total Hemoglobin |
| NIRS | Near-Infrared Spectroscopy |
| ∆Pre–Post | Delta Change from Pre- to Post-Intervention |
| η2p | Partial Eta-Squared |
| d | Cohen’s d (Effect Size) |
References
- Stoica, F.; Râpeanu, G.; Rațu, R.N.; Stănciuc, N.; Croitoru, C.; Țopa, D.; Jităreanu, G. Red Beetroot and Its By-Products: A Comprehensive Review of Phytochemicals, Extraction Methods, Health Benefits, and Applications. Agriculture 2025, 15, 270. [Google Scholar] [CrossRef]
- Domínguez, R.; Cuenca, E.; Maté-Muñoz, J.L.; García-Fernández, P.; Serra-Paya, N.; Estevan, M.C.L.; Herreros, P.V.; Garnacho-Castaño, M.V. Effects of beetroot juice supplementation on cardiorespiratory endurance in athletes. A systematic review. Nutrients 2017, 9, 43. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Bailey, S.J.; Fulford, J.; Vanhatalo, A.; Winyard, P.G.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol. 2010, 109, 135–148. [Google Scholar] [CrossRef]
- Ferguson, S.K.; Hirai, D.M.; Copp, S.W.; Holdsworth, C.T.; Allen, J.D.; Jones, A.M.; Musch, T.I.; Poole, D.C. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J. Physiol. 2013, 591, 547–557. [Google Scholar] [CrossRef]
- Tan, R.; Pennell, A.; Karl, S.T.; Cass, J.K.; Go, K.; Clifford, T.; Bailey, S.J.; Perkins Storm, C. Effects of dietary nitrate supplementation on back squat and bench press performance: A systematic review and meta-analysis. Nutrients 2023, 15, 2493. [Google Scholar] [CrossRef]
- Mosher, S.L.; Sparks, S.A.; Williams, E.L.; Bentley, D.J.; Mc Naughton, L.R. Ingestion of a nitric oxide enhancing supplement improves resistance exercise performance. J. Strength Cond. Res. 2016, 30, 3520–3524. [Google Scholar] [CrossRef]
- Flanagan, S.D.; Looney, D.P.; Miller, M.J.; DuPont, W.H.; Pryor, L.; Creighton, B.C.; Sterczala, A.J.; Szivak, T.K.; Hooper, D.R.; Maresh, C.M. The effects of nitrate-rich supplementation on neuromuscular efficiency during heavy resistance exercise. J. Am. Coll. Nutr. 2016, 35, 100–107. [Google Scholar] [CrossRef]
- Siervo, M.; Lara, J.; Ogbonmwan, I.; Mathers, J.C. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: A systematic review and meta-analysis. J. Nutr. 2013, 143, 818–826. [Google Scholar] [CrossRef]
- Lansley, K.E.; Winyard, P.G.; Bailey, S.J.; Vanhatalo, A.; Wilkerson, D.P.; Blackwell, J.R.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Acute dietary nitrate supplementation improves cycling time trial performance. Med. Sci. Sports Exerc. 2011, 43, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Ranchal-Sanchez, A.; Diaz-Bernier, V.M.; De La Florida-Villagran, C.A.; Llorente-Cantarero, F.J.; Campos-Perez, J.; Jurado-Castro, J.M. Acute effects of beetroot juice supplements on resistance training: A randomized double-blind crossover. Nutrients 2020, 12, 1912. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fernández, A.; Castillo, D.; Raya-González, J.; Domínguez, R.; Bailey, S.J. Beetroot juice supplementation increases concentric and eccentric muscle power output. J. Sci. Med. Sport 2021, 24, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Rael, B.; Alfaro-Magallanes, V.M.; Romero-Parra, N.; Castro, E.A.; Cupeiro, R.; Janse de Jonge, X.A.; Wehrwein, E.A.; Peinado, A.B.; Group, I.S. Menstrual cycle phases influence on cardiorespiratory response to exercise in endurance-trained females. Int. J. Environ. Res. Public Health 2021, 18, 860. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.D.; Martin, M.P.; Mintz, J.A.; Rogers, R.R.; Ballmann, C.G. Effect of acute beetroot juice supplementation on bench press power, velocity, and repetition volume. J. Strength Cond. Res. 2020, 34, 924–928. [Google Scholar] [CrossRef]
- Jurado-Castro, J.M.; Casanova-Rodriguez, D.; Campos-Perez, J.; Llorente-Cantarero, F.J.; De La Florida-Villagran, C.A.; Diaz-Bernier, V.M.; Ranchal-Sanchez, A. Beetroot Juice Produces Changes in Heart Rate Variability and Reduces Internal Load during Resistance Training in Men: A Randomized Double-Blind Crossover. Nutrients 2022, 14, 5119. [Google Scholar] [CrossRef]
- Dong, J.G. The role of heart rate variability in sports physiology. Exp. Ther. Med. 2016, 11, 1531–1536. [Google Scholar] [CrossRef]
- Tekin, R.T.; Kudas, S.; Buran, M.M.; Cabuk, S.; Akbasli, O.; Uludag, V.; Yosmaoglu, H.B. The relationship between resting heart rate variability and sportive performance, sleep and body awareness in soccer players. BMC Sports Sci. Med. Rehabil. 2025, 17, 58. [Google Scholar] [CrossRef]
- Olas, B. The cardioprotective role of nitrate-rich vegetables. Foods 2024, 13, 691. [Google Scholar] [CrossRef]
- Martinez, M.W.; Kim, J.H.; Shah, A.B.; Phelan, D.; Emery, M.S.; Wasfy, M.M.; Fernandez, A.B.; Bunch, T.J.; Dean, P.; Danielian, A. Exercise-induced cardiovascular adaptations and approach to exercise and cardiovascular disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2021, 78, 1453–1470. [Google Scholar] [CrossRef]
- Brown, L.E.; Weir, J.P. ASEP procedures recommendation I: Accurate assessment of muscular strength and power. J. Exerc. Physiol. Online 2001, 4, 1–21. [Google Scholar]
- Conceição, F.; Fernandes, J.; Lewis, M.; Gonzaléz-Badillo, J.J.; Jimenéz-Reyes, P. Movement velocity as a measure of exercise intensity in three lower limb exercises. J. Sports Sci. 2016, 34, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- González-Badillo, J.J.; Sánchez-Medina, L. Movement velocity as a measure of loading intensity in resistance training. Int. J. Sports Med. 2010, 31, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Morán-Navarro, R.; Pérez, C.E.; Mora-Rodríguez, R.; de la Cruz-Sánchez, E.; González-Badillo, J.J.; Sánchez-Medina, L.; Pallarés, J.G. Time course of recovery following resistance training leading or not to failure. Eur. J. Appl. Physiol. 2017, 117, 2387–2399. [Google Scholar] [CrossRef]
- Ammar, A.; Chtourou, H.; Trabelsi, K.; Padulo, J.; Turki, M.; El Abed, K.; Hoekelmann, A.; Hakim, A. Temporal specificity of training: Intra-day effects on biochemical responses and Olympic-Weightlifting performances. J. Sports Sci. 2015, 33, 358–368. [Google Scholar] [CrossRef]
- Ammar, A.; Turki, M.; Chtourou, H.; Hammouda, O.; Trabelsi, K.; Kallel, C.; Abdelkarim, O.; Hoekelmann, A.; Bouaziz, M.; Ayadi, F. Pomegranate supplementation accelerates recovery of muscle damage and soreness and inflammatory markers after a weightlifting training session. PLoS ONE 2016, 11, e0160305. [Google Scholar] [CrossRef]
- Ammar, A.; Bailey, S.J.; Chtourou, H.; Trabelsi, K.; Turki, M.; Hökelmann, A.; Souissi, N. Effects of pomegranate supplementation on exercise performance and post-exercise recovery in healthy adults: A systematic review. Br. J. Nutr. 2018, 120, 1201–1216. [Google Scholar] [CrossRef]
- Ammar, A.; Chtourou, H.; Souissi, N. Effect of time-of-day on biochemical markers in response to physical exercise. J. Strength Cond. Res. 2017, 31, 272–282. [Google Scholar] [CrossRef]
- Gallardo, E.J.; Coggan, A.R. What is in your beet juice? Nitrate and nitrite content of beet juice products marketed to athletes. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 345–349. [Google Scholar] [CrossRef]
- Govoni, M.; Jansson, E.Å.; Weitzberg, E.; Lundberg, J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 2008, 19, 333–337. [Google Scholar] [CrossRef]
- Sale, C.; Harris, R.C.; Florance, J.; Kumps, A.; Sanvura, R.; Poortmans, J.R. Urinary creatine and methylamine excretion following 4 × 5g·day−1 or 20 × 1g·day−1 of creatine monohydrate for 5 days. J. Sports Sci. 2009, 27, 759–766. [Google Scholar] [CrossRef]
- Sumaryanti, S.; Nugroho, S.; Visalim, A.; Ndayisenga, J. Development of physical fitness test for mild intellectual disabilities aged 13-15 years. J. Keolahragaan 2022, 10, 227–238. [Google Scholar] [CrossRef]
- Feuerbacher, J.F.; Jacobs, M.W.; Dragutinovic, B.; Goldmann, J.-P.; Cheng, S.; Schumann, M. Validity and test-retest reliability of the Vmaxpro sensor for evaluation of movement velocity in the deep squat. J. Strength Cond. Res. 2023, 37, 35–40. [Google Scholar] [CrossRef]
- Vondrasek, J.D.; Riemann, B.L.; Grosicki, G.J.; Flatt, A.A. Validity and efficacy of the elite HRV smartphone application during slow-paced breathing. Sensors 2024, 23, 9496. [Google Scholar] [CrossRef] [PubMed]
- Boushel, R.; Piantadosi, C. Near-infrared spectroscopy for monitoring muscle oxygenation. Acta Physiol. Scand. 2000, 168, 615–622. [Google Scholar] [CrossRef]
- Crum, E.; O’Connor, W.; Van Loo, L.; Valckx, M.; Stannard, S. Validity and reliability of the Moxy oxygen monitor during incremental cycling exercise. Eur. J. Sport Sci. 2017, 17, 1037–1043. [Google Scholar] [CrossRef]
- Coswig, V.; Silva, A.D.A.C.E.; Barbalho, M.; De Faria, F.R.; Nogueira, C.D.; Borges, M.; Buratti, J.R.; Vieira, I.B.; Román, F.J.L.; Gorla, J.I. Assessing the validity of the MyJUMP2 app for measuring different jumps in professional cerebral palsy football players: An experimental study. JMIR mHealth uHealth 2019, 7, e11099. [Google Scholar] [CrossRef]
- Forsyth, J.; Farrally, M. A comparison of lactate concentration in plasma collected from the toe, ear, and fingertip after a simulated rowing exercise. Br. J. Sports Med. 2000, 34, 35–38. [Google Scholar] [CrossRef]
- Goodwin, M.L.; Harris, J.E.; Hernández, A.; Gladden, L.B. Blood lactate measurements and analysis during exercise: A guide for clinicians. J. Diabetes Sci. Technol. 2007, 1, 558–569. [Google Scholar] [CrossRef]
- Newham, D.; Mills, K.; Quigley, B.; Edwards, R. Pain and fatigue after concentric and eccentric muscle contractions. Clin. Sci. 1983, 64, 55–62. [Google Scholar] [CrossRef]
- R Core Team, R. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Milton Park, UK, 2013. [Google Scholar]
- Hopkins, W.G. A scale of magnitudes for effect statistics. New View Stat. 2002, 502, 321. [Google Scholar]
- Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests; CRAN: Vienna, Austria, 2019. [Google Scholar]
- Singmann, H.; Bolker, B.; Westfall, J.; Aust, F. afex: Analysis of Factorial Experiments, version 0.16-1; CRAN: Vienna, Austria, 2016. [Google Scholar]
- Lenth, R.V. Least-squares means: The R package lsmeans. J. Stat. Softw. 2016, 69, 1–33. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Forbes, S.P.; Spriet, L.L. Potential effect of beetroot juice supplementation on exercise economy in well-trained females. Appl. Physiol. Nutr. Metab. 2022, 47, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Głowacz, J.; Popiel, M.; Dziekoński, K.; Rybowski, J.; Krzyśkowska, S.; Redner, A.; Kwiecień, I.; Zwierzchlewska, P.; Rusin, B.; Stanibuła, D. Beetroot Juice as a Natural Ergogenic Supplement: A Literature Review of Its Effects on Physical Performance and Training Adaptation. Qual. Sport 2025, 39, 59096. [Google Scholar] [CrossRef]
- Dumar, A.M.; Huntington, A.F.; Rogers, R.R.; Kopec, T.J.; Williams, T.D.; Ballmann, C.G. Acute beetroot juice supplementation attenuates morning-associated decrements in supramaximal exercise performance in trained sprinters. Int. J. Environ. Res. Public Health 2021, 18, 412. [Google Scholar] [CrossRef]
- Mueller, B.J.; Roberts, M.D.; Mobley, C.B.; Judd, R.L.; Kavazis, A.N. Nitric oxide in exercise physiology: Past and present perspectives. Front. Physiol. 2024, 15, 1504978. [Google Scholar] [CrossRef]
- Fulford, J.; Winyard, P.G.; Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; Jones, A.M. Influence of dietary nitrate supplementation on human skeletal muscle metabolism and force production during maximum voluntary contractions. Pflugers Arch. 2013, 465, 517–528. [Google Scholar] [CrossRef]
- Phillips, D.B.; Brotto, A.R.; Ross, B.A.; Bryan, T.L.; Wong, E.Y.; Meah, V.L.; Fuhr, D.P.; van Diepen, S.; Stickland, M.K.; Network, C.R.R. Inhaled nitric oxide improves ventilatory efficiency and exercise capacity in patients with mild COPD: A randomized-control cross-over trial. J. Physiol. 2021, 599, 1665–1683. [Google Scholar] [CrossRef]
- Garnacho-Castaño, M.V.; Sánchez-Nuño, S.; Molina-Raya, L.; Carbonell, T.; Maté-Muñoz, J.L.; Pleguezuelos-Cobo, E.; Serra-Payá, N. Circulating nitrate-nitrite reduces oxygen uptake for improving resistance exercise performance after rest time in well-trained CrossFit athletes. Sci. Rep. 2022, 12, 9671. [Google Scholar] [CrossRef]
- Crandall, C.G.; Wilson, T.E. Human cardiovascular responses to passive heat stress. Compr. Physiol. 2015, 5, 17–43. [Google Scholar] [CrossRef]
- Yuschen, X.; Choi, J.-H.; Seo, J.; Sun, Y.; Lee, E.; Kim, S.-W.; Park, H.-Y. Effects of acute beetroot juice supplementation and exercise on cardiovascular function in healthy men in preliminary study: A randomized, double-blinded, placebo-controlled, and crossover trial. Healthcare 2020, 8, 1240. [Google Scholar] [CrossRef]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Dietary nitrate reduces maximal oxygen consumption while maintaining work performance in maximal exercise. Free Radic. Biol. Med. 2010, 48, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Thompson, C.; Wylie, L.J.; Vanhatalo, A. Dietary nitrate and physical performance. Annu. Rev. Nutr. 2018, 38, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, Y.; Hu, Z.; Wu, S.; Jin, C. Beetroot as a functional food with huge health benefits: Antioxidant, antitumor, physical function, and chronic metabolomics activity. Food Sci. Nutr. 2021, 9, 6406–6420. [Google Scholar] [CrossRef]
- Jones, L.; Bailey, S.J.; Rowland, S.N.; Alsharif, N.; Shannon, O.M.; Clifford, T. The effect of nitrate-rich beetroot juice on markers of exercise-induced muscle damage: A systematic review and meta-analysis of human intervention trials. J. Diet. Suppl. 2022, 19, 749–771. [Google Scholar] [CrossRef]
- Ramanlal, R.; Gupta, V. Physiology, Vasodilation. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2025. [Google Scholar]
- Benjamim, C.J.R.; de Sousa Júnior, F.W.; Porto, A.A.; Andrade, C.V.G.; de Figueiredo, M.Í.L.S.; Benjamim, C.J.R.; da Silva Rodrigues, G.; Rocha, E.M.B.; Cavalcante, T.F.; Garner, D.M. Negligible Effects of Nutraceuticals from Beetroot Extract on Cardiovascular and Autonomic Recovery Response following Submaximal Aerobic Exercise in Physically Active Healthy Males: A Randomized Trial. Int. J. Environ. Res. Public Health 2023, 20, 4019. [Google Scholar] [CrossRef]
- Gobbo, H.R.; Barbosa, G.M.; de Oliveira, L.C.; de Oliveira, G.V. The Effect of Different Resistance Training Protocols on Cardiac Autonomic Modulation During Exercise Recovery: A Crossover, Randomized, and Controlled Pilot Study. J. Vasc. Dis. 2024, 3, 375–384. [Google Scholar] [CrossRef]
- Daab, W.; Bouzid, M.A.; Lajri, M.; Bouchiba, M.; Saafi, M.A.; Rebai, H. Chronic Beetroot Juice Supplementation Accelerates Recovery Kinetics following Simulated Match Play in Soccer Players. J. Am. Coll. Nutr. 2021, 40, 61–69. [Google Scholar] [CrossRef]
- Hemmatinafar, M.; Zaremoayedi, L.; Koushkie Jahromi, M.; Alvarez-Alvarado, S.; Wong, A.; Niknam, A.; Suzuki, K.; Imanian, B.; Bagheri, R. Effect of beetroot juice supplementation on muscle soreness and performance recovery after exercise-induced muscle damage in female volleyball players. Nutrients 2023, 15, 3763. [Google Scholar] [CrossRef]
- Varshney, K.; Mishra, K. An analysis of health benefits of beetroot. Int. J. Innov. Res. Eng. Manag. 2022, 9, 207–210. [Google Scholar] [CrossRef]
- Gao, C.; Gupta, S.; Adli, T.; Hou, W.; Coolsaet, R.; Hayes, A.; Kim, K.; Pandey, A.; Gordon, J.; Chahil, G. The effects of dietary nitrate supplementation on endurance exercise performance and cardiorespiratory measures in healthy adults: A systematic review and meta-analysis. J. Int. Soc. Sports Nutr. 2021, 18, 55. [Google Scholar] [CrossRef]
- Rojano-Ortega, D.; Peña Amaro, J.; Berral-Aguilar, A.J.; Berral-de la Rosa, F.J. Effects of Beetroot Supplementation on Recovery After Exercise-Induced Muscle Damage: A Systematic Review. Sports Health 2022, 14, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Dai, Z.; Heung-Sang Wong, S.; Zheng, C.; Tsz-Chun Poon, E. Acute effects of various doses of nitrate-rich beetroot juice on high-intensity interval exercise responses in women: A randomized, double-blinded, placebo-controlled, crossover trial. J. Int. Soc. Sports Nutr. 2024, 21, 2334680. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.M.; Melo, C.P.B.; Pinto, I.C.; Mendes-Pierotti, S.; Vignoli, J.A.; Verri, W.A.; Casagrande, R. Betalains: A Narrative Review on Pharmacological Mechanisms Supporting the Nutraceutical Potential Towards Health Benefits. Foods 2024, 13, 3909. [Google Scholar] [CrossRef]
- Esen, O.; Domínguez, R.; Karayigit, R. Acute Beetroot Juice Supplementation Enhances Intermittent Running Performance but Does Not Reduce Oxygen Cost of Exercise among Recreational Adults. Nutrients 2022, 14, 2839. [Google Scholar] [CrossRef]
- Vermeer, I.T.M.; Van Maanen, J. Nitrate exposure and endogenous formation of carcinogenic nitrosamines in humans. Rev. Environ. Health 2001, 16, 105–116. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2010; Volume 94, pp. v–vii, 1–412. [Google Scholar]
- Macuh, M.; Knap, B. Effects of Nitrate Supplementation on Exercise Performance in Humans: A Narrative Review. Nutrients 2021, 13, 3183. [Google Scholar] [CrossRef]
- Karwowska, M.; Kononiuk, A. Nitrates/Nitrites in Food-Risk for Nitrosative Stress and Benefits. Antioxidants 2020, 9, 241. [Google Scholar] [CrossRef]
- van Breda, S.G.; Mathijs, K.; Sági-Kiss, V.; Kuhnle, G.G.; Van der Veer, B.; Jones, R.R.; Sinha, R.; Ward, M.H.; de Kok, T.M. Impact of high drinking water nitrate levels on the endogenous formation of apparent N-nitroso compounds in combination with meat intake in healthy volunteers. Environ. Health 2019, 18, 87. [Google Scholar] [CrossRef]
- Berends, J.E.; van den Berg, L.M.; Guggeis, M.A.; Henckens, N.F.; Hossein, I.J.; de Joode, M.E.; Zamani, H.; van Pelt, K.A.; Beelen, N.A.; Kuhnle, G.G. Consumption of nitrate-rich beetroot juice with or without vitamin C supplementation increases the excretion of urinary nitrate, nitrite, and N-nitroso compounds in humans. Int. J. Mol. Sci. 2019, 20, 2277. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Kabir, A.; Azizi, F.; Ghasemi, A. The nitrate-independent blood pressure–lowering effect of beetroot juice: A systematic review and meta-analysis. Adv. Nutr. 2017, 8, 830–838. [Google Scholar] [CrossRef]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; De Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; Van Breda, S.G. Drinking water nitrate and human health: An updated review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [PubMed]
- Zamani, H.; De Joode, M.; Hossein, I.; Henckens, N.; Guggeis, M.; Berends, J.; De Kok, T.; Van Breda, S. The benefits and risks of beetroot juice consumption: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 61, 788–804. [Google Scholar] [CrossRef] [PubMed]


| Nutritional Value | 100 g | 15 g (Daily Dose) |
|---|---|---|
| Values provided by GymBeam | ||
| Energy value | 1305 kJ/310 kcal | 196 kJ/46.5 kcal |
| Fats | 0.7 g | 0.11 g |
| Saturated fats | 0 g | 0 g |
| Carbohydrates | 75 g | 11 g |
| Sugar | 53 g | 8 g |
| Protein | 12 g | 1.8 g |
| Salt | 0.36 g | 0.05 g |
| Vitamin B1 | 5.6 g | 0.84 g |
| Iron | 37 g | 0.55 g |
| Mangan | 2.7 g | 0.4 g |
| Values provided by the Centre of Biotechnology of Sfax | ||
| Sodium | 1510 mg | 226.5 mg |
| Potassium | 2690 mg | 403.5 mg |
| Magnesium | 210 mg | 31.5 mg |
| Chloride | 2260 mg | 339 mg |
| Nitrate | 3000 mg | 450 mg |
| Phosphate | 1060 mg | 159 mg |
| Sulfate | 360 mg | 54 mg |
| Variable | BJ | PLA |
|---|---|---|
| Energy (kcal) | 2450 ± 310 | 2380 ± 295 |
| Carbohydrates (g) | 312.4 ± 42.1 | 305.7 ± 39.8 |
| Protein (g) | 112.8 ± 18.6 | 110.5 ± 17.3 |
| Fat (g) | 87.1 ± 12.9 | 84.9 ± 11.7 |
| PLA | BJ | ANOVA Results | |||
|---|---|---|---|---|---|
| 1st Session | 2nd Session | 1st Session | 2nd Session | ||
| MeanRR | −26.48 ± 7.85 | −15.6 ± 11.18 ¤ | −25.6 ± 6.18 | −14.43 ± 9.74 ¤ | C: F(1, 10) = 0.13, p = 0.722, η2p = 0.013 S: F(1, 10) = 25.39, p < 0.001, η2p = 0.717 C × S: F(1, 10) = 0.00, p = 0.961, η2p < 0.001 |
| RMSSD | −40.78 ± 13.21 | −19.01 ± 20.2 ¤ | −32.74 ± 11.59 * | −19.66 ± 9.9 ¤ | C: F(1, 10) = 0.86, p = 0.377, η2p = 0.079 S: F(1, 10) = 17.10, p = 0.002, η2p = 0.631 C × S: F(1, 10) = 2.14, p = 0.174, η2p = 0.177 |
| SDNN | −22.87 ± 16.74 | −11.8 ± 16.94 | −25.37 ± 8.24 | −23.67 ± 12.92 | C: F(1, 10) = 2.01, p = 0.187, η2p = 0.167 S: F(1, 10) = 3.46, p = 0.093, η2p = 0.257 C × S: F(1, 10) = 2.05, p = 0.183, η2p = 0.170 |
| LF | −27.8 ± 19.6 | −11.5 ± 5.72 | −30.86 ± 15.11 | −22.19 ± 16.92 | C: F(1, 10) = 2.93, p = 0.118, η2p = 0.227 S: F(1, 10) = 6.94, p = 0.025, η2p = 0.410 C × S: F(1, 10) = 0.66, p = 0.435, η2p = 0.062 |
| HF | −34.56 ± 10.49 | −15.09 ± 16.06 ¤ | −23.08 ± 13.74 | −11.98 ± 10.78 ¤ | C: F(1, 10) = 1.49, p = 0.250, η2p = 0.130 S: F(1, 10) = 21.54, p < 0.001, η2p = 0.683 C × S: F(1, 10) = 3.16, p = 0.106, η2p = 0.240 |
| Lactate | 135.24 ± 116.13 | 150.96 ± 78.74 | 182.02 ± 142.74 | 280.66 ± 247.96 | C: F(1, 10) = 3.40, p = 0.095, η2p = 0.254 S: F(1, 10) = 1.17, p = 0.305, η2p = 0.105 C × S: F(1, 10) = 0.78, p = 0.397, η2p = 0.073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salem, A.; Ammar, A.; Kerkeni, M.; Boujelbane, M.A.; Uyar, A.M.; Köbel, L.M.; Selvaraj, S.; Zare, R.; Heinrich, K.M.; Jahrami, H.; et al. Short-Term Beetroot Juice Supplementation Enhances Strength, Reduces Fatigue, and Promotes Recovery in Physically Active Individuals: A Randomized, Double-Blind, Crossover Trial. Nutrients 2025, 17, 1720. https://doi.org/10.3390/nu17101720
Salem A, Ammar A, Kerkeni M, Boujelbane MA, Uyar AM, Köbel LM, Selvaraj S, Zare R, Heinrich KM, Jahrami H, et al. Short-Term Beetroot Juice Supplementation Enhances Strength, Reduces Fatigue, and Promotes Recovery in Physically Active Individuals: A Randomized, Double-Blind, Crossover Trial. Nutrients. 2025; 17(10):1720. https://doi.org/10.3390/nu17101720
Chicago/Turabian StyleSalem, Atef, Achraf Ammar, Mohamed Kerkeni, Mohamed Ali Boujelbane, Ayse Merve Uyar, Leonard Moritz Köbel, Saranya Selvaraj, Reza Zare, Katie M. Heinrich, Haitham Jahrami, and et al. 2025. "Short-Term Beetroot Juice Supplementation Enhances Strength, Reduces Fatigue, and Promotes Recovery in Physically Active Individuals: A Randomized, Double-Blind, Crossover Trial" Nutrients 17, no. 10: 1720. https://doi.org/10.3390/nu17101720
APA StyleSalem, A., Ammar, A., Kerkeni, M., Boujelbane, M. A., Uyar, A. M., Köbel, L. M., Selvaraj, S., Zare, R., Heinrich, K. M., Jahrami, H., Tounsi, S., Zmijewski, P., Schöllhorn, W. I., Trabelsi, K., & Chtourou, H. (2025). Short-Term Beetroot Juice Supplementation Enhances Strength, Reduces Fatigue, and Promotes Recovery in Physically Active Individuals: A Randomized, Double-Blind, Crossover Trial. Nutrients, 17(10), 1720. https://doi.org/10.3390/nu17101720











