The Antiaging Potential of Dietary Plant-Based Polyphenols: A Review on Their Role in Cellular Senescence Modulation
Abstract
1. Introduction
1.1. Cell Senescence
1.2. Senolytics and Senomorphics
2. Methods
3. Polyphenols
3.1. Phenolic Acids
3.2. Stilbenes
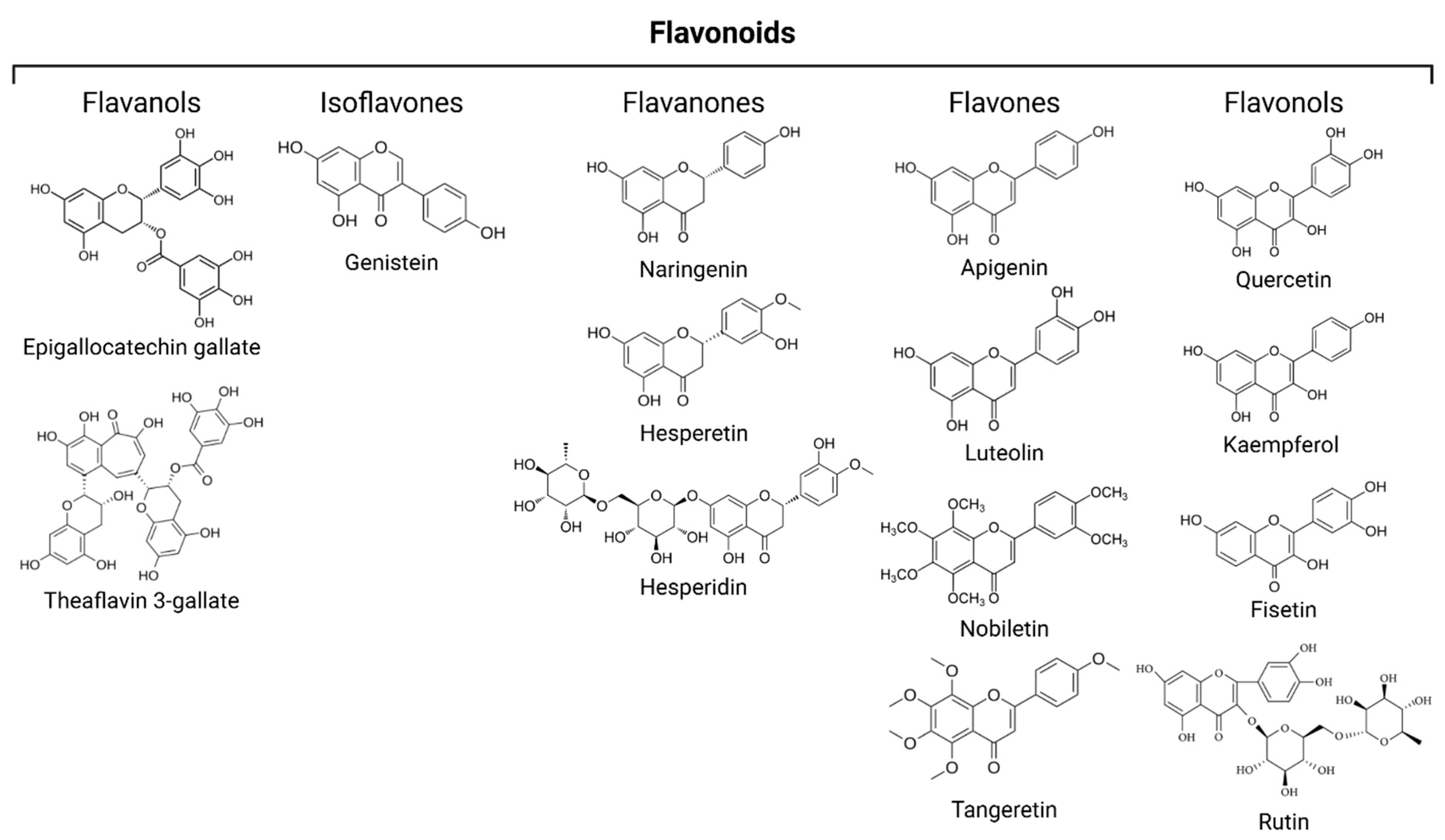
3.3. Flavonoids
3.3.1. Flavanols
3.3.2. Isoflavones
3.3.3. Flavanones
3.3.4. Flavones
3.3.5. Flavonols
3.4. Tannins
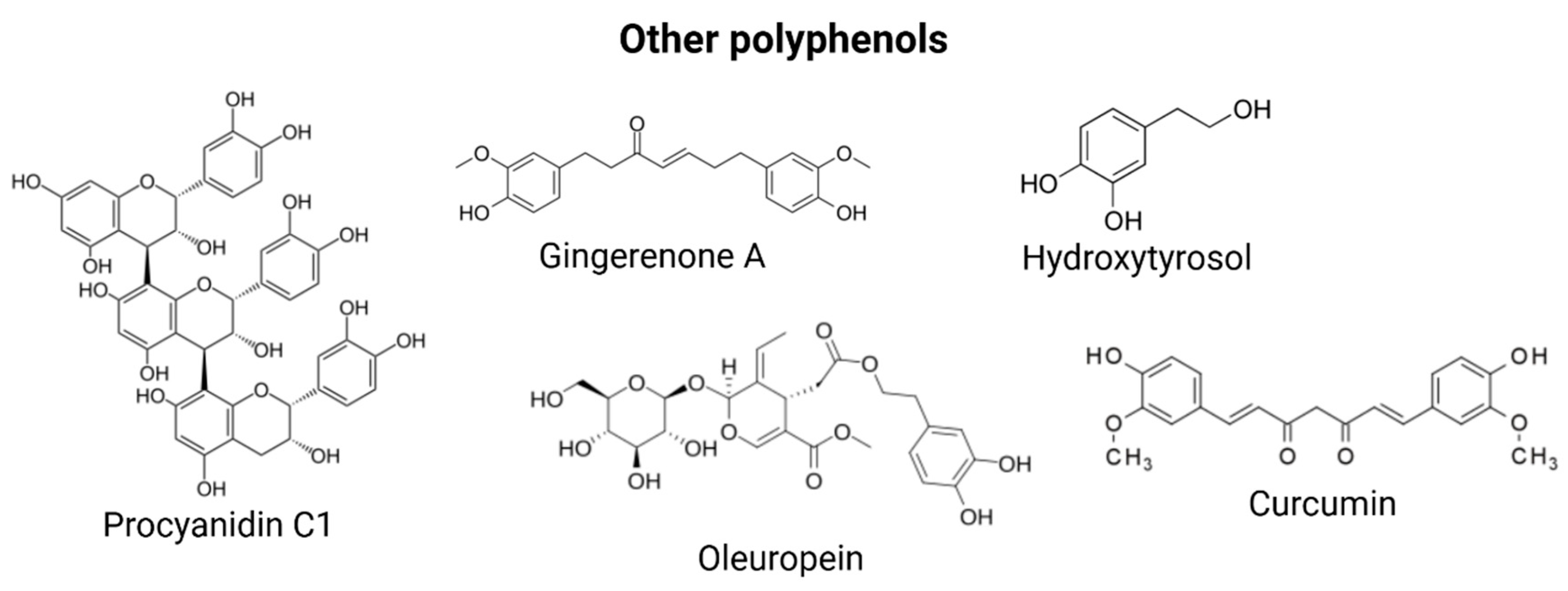
3.5. Other Polyphenols
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ADSCs | adipose-derived stem cells |
| AMPK | AMP-activated protein kinase |
| ASAP | acute stress-associated phenotype |
| ATM | ataxia-telangiectasia mutated |
| Bax | Bcl-2 associated X protein |
| Bcl | B Cell Lymphoma |
| BMSCs | bone-marrow-derived mesenchymal stem cells |
| CCL2 | C-C motif chemokine ligand 2 |
| COX-2 | Cyclooxygenase-2 |
| DNA-SCARS | DNA segments with chromatin alterations reinforcing senescence |
| ECs | endothelial cells |
| EGCG | Epigallocatechin gallate |
| ERRα | estrogen-related receptor alpha |
| EVOO | extra virgin olive oil |
| EVs | extracellular vesicles |
| GA | gallic acid |
| GSE | grape seed extract |
| HaCaT | human keratinocytes |
| HDF | human dermal fibroblasts |
| HEI-OC1 | House Ear Institute-Organ of Corti 1 cells |
| hHBMCs | human bone-marrow-derived mesenchymal stem cells |
| HIF-1α | hypoxia-inducible factor 1-alpha |
| HIIT | high-intensity interval training |
| hMSCs | human mesenchymal stem cells |
| htNSCs | hypothalamic neural stem cells |
| hUC-MSC | human umbilical cord mesenchymal stem cell |
| HUVECs | human umbilical vein endothelial cells |
| IFN-γ | Interferon Gamma |
| IP-10 | interferon-γ-induced protein 10 |
| JNK | c-Jun N-terminal kinase |
| KAE | Kaempferol (3,4′,5,7-tetrahydroxyflavone) |
| Ki-67 | Kiel 67 |
| LKB1 | liver kinase B1 |
| lncRNAs | long non-coding RNAs |
| LPS | lipopolysaccharide |
| MMP | Matrix Metalloproteinase |
| MSCs | mesenchymal stromal cells |
| mTOR | mammalian Target Of Rapamycin |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric Oxide |
| ROS | Reactive Oxygen Species |
| Nrf2 | nuclear factor erythroid 2–related factor 2 |
| OA | osteoarthritis |
| OLE | oleuropein |
| OVX-BMMSCs | Bone marrow mesenchymal stem cells from ovariectomized rats |
| ox-LDL | oxidized low-density lipoprotein |
| p38 MAPK | p38 mitogen-activated protein kinases |
| PCA | protocatechuic acid |
| PCC1 | Procyanidin C1 |
| PD | Parkinson’s disease |
| PDK1 | pyruvate dehydrogenase kinase 1 |
| PGC-1α | proliferator-activated receptor gamma coactivator 1-alpha |
| PIC | Piceatannol |
| PM2.5 | Particular matter 2.5 |
| RES | resveratrol |
| SAHF | senescence-associated heterochromatin foci |
| SAMP8 | senescence-accelerated mouse prone 8 |
| SASP | senescence-associated secretory phenotype |
| SA-β-gal | senescence-associated beta-galactosidase |
| SCs | senescent cells |
| SDF | senescence-associated DNA damage foci |
| SIRT | sirtuin |
| TF2A | theaflavin-3-gallate |
| TGF-β | Transforming Growth Factor beta |
| TNF-α | Tumor Necrosis Factor Alpha |
| TRAF6 | TNF receptor-associated factor 6 |
| UV | ultraviolet |
| VSMCs | vascular smooth muscle cells |
| WS | Werner syndrome |
References
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef] [PubMed]
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Campisi, J.; d’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Wiley, C.D.; Campisi, J. From Ancient Pathways to Aging Cells—Connecting Metabolism and Cellular Senescence. Cell Metab. 2016, 23, 1013–1021. [Google Scholar] [CrossRef]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Narita, M.; Nuñez, S.; Heard, E.; Lin, A.W.; Hearn, S.A.; Spector, D.L.; Hannon, G.J.; Lowe, S.W. Rb-Mediated Heterochromatin Formation and Silencing of E2F Target Genes during Cellular Senescence. Cell 2003, 113, 703–716. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular Senescence: Defining a Path Forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.-M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef]
- Muñoz-Espín, D.; Serrano, M. Cellular senescence: From physiology to pathology. Nat. Rev. Mol. Cell Biol. 2014, 15, 482–496. [Google Scholar] [CrossRef]
- Campisi, J. Senescent Cells, Tumor Suppression, and Organismal Aging: Good Citizens, Bad Neighbors. Cell 2005, 120, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, D.; Mandal, M. Senescence in polyploid giant cancer cells: A road that leads to chemoresistance. Cytokine Growth Factor Rev. 2020, 52, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Tyutyunyk-Massey, L.; Gewirtz, D.A. Tumor Cell Escape from Therapy-Induced Senescence as a Model of Disease Recurrence after Dormancy. Cancer Res. 2019, 79, 1044–1046. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.J.; Wijshake, T.; Tchkonia, T.; Lebrasseur, N.K.; Childs, B.G.; Van De Sluis, B.; Kirkland, J.L.; Van Deursen, J.M. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 2011, 479, 232–236. [Google Scholar] [CrossRef]
- Zhu, Y.; Doornebal, E.J.; Pirtskhalava, T.; Giorgadze, N.; Wentworth, M.; Fuhrmann-Stroissnigg, H.; Niedernhofer, L.J.; Robbins, P.D.; Tchkonia, T.; Kirkland, J.L. New agents that target senescent cells: The flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging 2017, 9, 955–963. [Google Scholar] [CrossRef]
- Yosef, R.; Pilpel, N.; Tokarsky-Amiel, R.; Biran, A.; Ovadya, Y.; Cohen, S.; Vadai, E.; Dassa, L.; Shahar, E.; Condiotti, R.; et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 2016, 7, 11190. [Google Scholar] [CrossRef]
- Luís, C.; Maduro, A.T.; Pereira, P.; Mendes, J.J.; Soares, R.; Ramalho, R. Nutritional senolytics and senomorphics: Implications to immune cells metabolism and aging—From theory to practice. Front. Nutr. 2022, 9, 958563. [Google Scholar] [CrossRef]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Wan, M.L.Y.; Co, V.A.; El-Nezami, H. Dietary polyphenol impact on gut health and microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 690–711. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.; Xu, B.; Wang, Y.; Zhang, C.; Cao, Y.; Xing, X. Research progress on classification, sources and functions of dietary polyphenols for prevention and treatment of chronic diseases. J. Future Foods 2023, 3, 289–305. [Google Scholar] [CrossRef]
- Virgili, F.; Marino, M. Regulation of cellular signals from nutritional molecules: A specific role for phytochemicals, beyond antioxidant activity. Free Radic. Biol. Med. 2008, 45, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Ojcius, D.M.; Ko, Y.-F.; Ke, P.-Y.; Wu, C.-Y.; Peng, H.-H.; Young, J.D. Hormetic Effects of Phytochemicals on Health and Longevity. Trends Endocrinol. Metab. 2019, 30, 335–346. [Google Scholar] [CrossRef] [PubMed]
- de Córdova, M.F.; Medina, A.R. Analytical Methods for Determination of Polyphenols in Beer. In Processing and Impact on Antioxidants in Beverages; Elsevier: Amsterdam, The Netherlands, 2014; pp. 289–299. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Islam, M.S. Phenolics: Therapeutic applications against oxidative injury in obesity and type 2 diabetes pathology. In Pathology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 297–307. [Google Scholar] [CrossRef]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant Properties of Hydroxycinnamic Acids: A Review of Structure- Activity Relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef] [PubMed]
- Rahimifard, M.; Baeeri, M.; Bahadar, H.; Moini-Nodeh, S.; Khalid, M.; Haghi-Aminjan, H.; Mohammadian, H.; Abdollahi, M. Therapeutic Effects of Gallic Acid in Regulating Senescence and Diabetes; an In Vitro Study. Molecules 2020, 25, 5875. [Google Scholar] [CrossRef]
- Shan, H.; Geng, L.; Jiang, X.; Song, M.; Wang, J.; Liu, Z.; Zhuo, X.; Wu, Z.; Hu, J.; Ji, Z.; et al. Large-scale chemical screen identifies Gallic acid as a geroprotector for human stem cells. Protein Cell 2022, 13, 532–539. [Google Scholar] [CrossRef]
- Kim, Y.S.; Seo, H.W.; Lee, M.-H.; Kim, D.K.; Jeon, H.; Cha, D.S. Protocatechuic acid extends lifespan and increases stress resistance in Caenorhabditis elegans. Arch. Pharmacal Res. 2014, 37, 245–252. [Google Scholar] [CrossRef]
- Son, J.H.; Kim, S.-Y.; Jang, H.H.; Lee, S.N.; Ahn, K.J. Protective effect of protocatechuic acid against inflammatory stress induced in human dermal fibroblasts. Biomed. Dermatol. 2018, 2, 9. [Google Scholar] [CrossRef]
- Osorio-Paz, I.; Valle-Jiménez, X.; Brunauer, R.; Alavez, S. Vanillic Acid Improves Stress Resistance and Substantially Extends Life Span in Caenorhabditis elegans. J. Gerontol. Ser. A 2023, 78, 1100–1107. [Google Scholar] [CrossRef]
- Saenno, R.; Suwannakot, K.; Prajit, R.; Sirichoat, A.; Aranarochana, A.; Sritawan, N.; Pannangrong, W.; Wigmore, P.; Welbat, J.U. Caffeic Acid Attenuates Neuronal Apoptosis, Oxidative Stress, and Memory Deficits via Antioxidant Properties in Aging Rats Induced by D-Galactose. Mol. Neurobiol. 2025, 62, 5143–5155. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Khongkow, M.; Iempridee, T.; Lourith, N. Food hydroxycinnamic acids alleviate ageing in dermal cells. Food Prod. Process. Nutr. 2024, 6, 86. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Pratsinis, H.; Kletsas, D.; Haroutounian, S.A. Resveratrol and related stilbenes: Their anti-aging and anti-angiogenic properties. Food Chem. Toxicol. 2013, 61, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Lin, C. Effect of resveratrol and pterostilbene on aging and longevity. BioFactors 2018, 44, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.N.; Lim, J.H.; Kim, M.Y.; Ban, T.H.; Jang, I.A.; Yoon, H.E.; Park, C.W.; Chang, Y.S.; Choi, B.S. Resveratrol, an Nrf2 activator, ameliorates aging-related progressive renal injury. Aging 2018, 10, 83–99. [Google Scholar] [CrossRef]
- Farhadnejad, H.; Emamat, H.; Zand, H. The Effect of Resveratrol on Cellular Senescence in Normal and Cancer Cells: Focusing on Cancer and Age-Related Diseases. Nutr. Cancer 2019, 71, 1175–1180. [Google Scholar] [CrossRef]
- Franco, F.N.; Peixoto, B.E.; de Araújo, G.R.; Chaves, M.M. Silencing of the Nrf2 pathway in aging promotes a decrease in the anti-inflammatory effect of resveratrol. Arch. Gerontol. Geriatr. 2025, 129, 105694. [Google Scholar] [CrossRef]
- Mehrabi, A.; Nuori, R.; Gaeini, A.; Amirazodi, M.; Mehrtash, M.; Esfahlani, M.A.; Bahrami, M.; Bejeshk, M.A.; Rajizadeh, M.A. The Antiaging and Antioxidative Effects of a Combination of Resveratrol and High-Intensity Interval Training on the Frontal Lobe in Aged Rats: The Role of SIRTS 4, SIRTS 5, SOD1, and SOD2. Oxidative Med. Cell. Longev. 2025, 2025, 8251896. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Z.; Ji, S.; Liu, T.; Hou, Y.; Li, S.; Li, G. Resveratrol reduces senescence-associated secretory phenotype by SIRT1/NF-κB pathway in gut of the annual fish Nothobranchius guentheri. Fish Shellfish Immunol. 2018, 80, 473–479. [Google Scholar] [CrossRef]
- Howitz, K.T.; Bitterman, K.J.; Cohen, H.Y.; Lamming, D.W.; Lavu, S.; Wood, J.G.; Zipkin, R.E.; Chung, P.; Kisielewski, A.; Zhang, L.-L.; et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 2003, 425, 191–196. [Google Scholar] [CrossRef]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef]
- Bauer, J.H.; Goupil, S.; Garber, G.B.; Helfand, S.L. An accelerated assay for the identification of lifespan-extending interventions in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2004, 101, 12980–12985. [Google Scholar] [CrossRef] [PubMed]
- Rascón, B.; Hubbard, B.P.; Sinclair, D.A.; Amdam, G.V. The lifespan extension effects of resveratrol are conserved in the honey bee and may be driven by a mechanism related to caloric restriction. Aging 2012, 4, 499–508. [Google Scholar] [CrossRef]
- Gocmez, S.S.; Gacar, N.; Utkan, T.; Gacar, G.; Scarpace, P.J.; Tumer, N. Protective effects of resveratrol on aging-induced cognitive impairment in rats. Neurobiol. Learn. Mem. 2016, 131, 131–136. [Google Scholar] [CrossRef]
- Zhang, L.-F.; Yu, X.-L.; Ji, M.; Liu, S.-Y.; Wu, X.-L.; Wang, Y.-J.; Liu, R.-T. Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T α-synuclein mouse model of Parkinson’s disease. Food Funct. 2018, 9, 6414–6426. [Google Scholar] [CrossRef]
- Pearson, K.J.; Baur, J.A.; Lewis, K.N.; Peshkin, L.; Price, N.L.; Labinskyy, N.; Swindell, W.R.; Kamara, D.; Minor, R.K.; Perez, E.; et al. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell Metab. 2008, 8, 157–168. [Google Scholar] [CrossRef]
- Wang, X.; Ma, S.; Yang, B.; Huang, T.; Meng, N.; Xu, L.; Xing, Q.; Zhang, Y.; Zhang, K.; Li, Q.; et al. Resveratrol promotes hUC-MSCs engraftment and neural repair in a mouse model of Alzheimer’s disease. Behav. Brain Res. 2018, 339, 297–304. [Google Scholar] [CrossRef]
- Ei, Z.Z.; Srithawirat, T.; Chunhacha, P.; Chaotham, C.; Arunmanee, W.; Phookphan, P.; Chanvorachote, P. Resveratrol Shows Potent Senescence Reversal in Experimental Cellular Models of Particular Matter 2.5-induced Cellular Senescence in Human Dermal Papilla Cells. In Vivo 2024, 38, 665–673. [Google Scholar] [CrossRef]
- Sawda, C.; Moussa, C.; Turner, R.S. Resveratrol for Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2017, 1403, 142–149. [Google Scholar] [CrossRef]
- Wang, X.; Ma, S.; Meng, N.; Yao, N.; Zhang, K.; Li, Q.; Zhang, Y.; Xing, Q.; Han, K.; Song, J.; et al. Resveratrol Exerts Dosage-Dependent Effects on the Self-Renewal and Neural Differentiation of hUC-MSCs. Mol. Cells 2016, 39, 418–425. [Google Scholar] [CrossRef]
- Witte, A.V.; Kerti, L.; Margulies, D.S.; Flöel, A. Effects of Resveratrol on Memory Performance, Hippocampal Functional Connectivity, and Glucose Metabolism in Healthy Older Adults. J. Neurosci. 2014, 34, 7862–7870. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Rahimi, R.; Nikfar, S.; Abdollahi, M. Effect of resveratrol on cognitive and memory performance and mood: A meta-analysis of 225 patients. Pharmacol. Res. 2018, 128, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Seyed, M.A.; Jantan, I.; Bukhari, S.N.A.; Vijayaraghavan, K. A Comprehensive Review on the Chemotherapeutic Potential of Piceatannol for Cancer Treatment, with Mechanistic Insights. J. Agric. Food Chem. 2016, 64, 725–737. [Google Scholar] [CrossRef]
- Alessio, N.; Squillaro, T.; Lettiero, I.; Galano, G.; De Rosa, R.; Peluso, G.; Galderisi, U.; Di Bernardo, G. Biomolecular Evaluation of Piceatannol’s Effects in Counteracting the Senescence of Mesenchymal Stromal Cells: A New Candidate for Senotherapeutics? Int. J. Mol. Sci. 2021, 22, 11619. [Google Scholar] [CrossRef]
- Suzuki, T.; Tetsuka, R.; Iwasaki, A.; Shimura, T.; Hirayama, R.; Nakamura, A.J. Piceatannol reduces radiation-induced DNA double-strand breaks by suppressing superoxide production and enhancing ATM-dependent repair efficiency. Adv. Redox Res. 2024, 13, 100114. [Google Scholar] [CrossRef]
- Yang, X.; Wu, Y.; Zhang, M.; Zhang, L.; Zhao, T.; Qian, W.; Zhu, M.; Wang, X.; Zhang, Q.; Sun, J.; et al. Piceatannol protects against age-related hearing loss by inhibiting cellular pyroptosis and inflammation through regulated Caspase11-GSDMD pathway. Biomed. Pharmacother. 2023, 163, 114704. [Google Scholar] [CrossRef]
- Kunsorn, P.; Payuhakrit, W.; Petit, V.; Larue, L.; Champakam, S.; Suwannalert, P. Skin anti-aging and wound healing effects of a passion fruit seed extract rich in piceatannol. Nutr. Health Aging 2024, 9, 101–112. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Sun, Q.; Kasireddy, N.; Kim, K.; Park, Y. Piceatannol extends the lifespan of Caenorhabditis elegans via DAF-16. BioFactors 2017, 43, 379–387. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.-H.; Chen, X.; Zhang, N.; Li, G. Piceatannol attenuates behavioral disorder and neurological deficits in aging mice via activating the Nrf2 pathway. Food Funct. 2018, 9, 371–378. [Google Scholar] [CrossRef]
- Mbara, K.C.; Devnarain, N.; Owira, P.M.O. Potential Role of Polyphenolic Flavonoids as Senotherapeutic Agents in Degenerative Diseases and Geroprotection. Pharm. Med. 2022, 36, 331–352. [Google Scholar] [CrossRef]
- Joshipura, K.J.; Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Rimm, E.B.; Speizer, F.E.; Colditz, G.; Ascherio, A.; Rosner, B.; Spiegelman, D.; et al. The Effect of Fruit and Vegetable Intake on Risk for Coronary Heart Disease. Ann. Intern. Med. 2001, 134, 1106–1114. [Google Scholar] [CrossRef]
- Cassidy, A.; Rimm, E.B.; O’Reilly, É.J.; Logroscino, G.; Kay, C.; Chiuve, S.E.; Rexrode, K.M. Dietary Flavonoids and Risk of Stroke in Women. Stroke 2012, 43, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Beecher, G.R. Overview of Dietary Flavonoids: Nomenclature, Occurrence and Intake. J. Nutr. 2003, 133, 3248S–3254S. [Google Scholar] [CrossRef] [PubMed]
- Lilja, S.; Oldenburg, J.; Pointner, A.; Dewald, L.; Lerch, M.; Hippe, B.; Switzeny, O.; Haslberger, A. Epigallocatechin Gallate Effectively Affects Senescence and Anti-SASP via SIRT3 in 3T3-L1 Preadipocytes in Comparison with Other Bioactive Substances. Oxidative Med. Cell. Longev. 2020, 2020, 4793125. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Kumari, A.; Gulati, A.; Padwad, Y.; Sharma, R. Epigallocatechin gallate suppresses premature senescence of preadipocytes by inhibition of PI3K/Akt/mTOR pathway and induces senescent cell death by regulation of Bax/Bcl-2 pathway. Biogerontology 2019, 20, 171–189. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A.; Padwad, Y.; Sharma, R. Preadipocyte secretory factors differentially modulate murine macrophage functions during aging which are reversed by the application of phytochemical EGCG. Biogerontology 2020, 21, 325–343. [Google Scholar] [CrossRef]
- Patel, S.; Ellis, K.; Scipione, C.A.; Fish, J.E.; Howe, K.L. Epigallocatechin gallate (EGCG) modulates senescent endothelial cell-monocyte communication in age-related vascular inflammation. Front. Cardiovasc. Med. 2025, 11, 1506360. [Google Scholar] [CrossRef]
- Han, D.-W.; Lee, M.H.; Kim, B.; Lee, J.J.; Hyon, S.-H.; Park, J.-C. Preventive Effects of Epigallocatechin-3-O-Gallate against Replicative Senescence Associated with p53 Acetylation in Human Dermal Fibroblasts. Oxidative Med. Cell. Longev. 2012, 2012, 850684. [Google Scholar] [CrossRef]
- Shin, J.-H.; Jeon, H.-J.; Park, J.; Chang, M.-S. Epigallocatechin-3-gallate prevents oxidative stress-induced cellular senescence in human mesenchymal stem cells via Nrf2. Int. J. Mol. Med. 2016, 38, 1075–1082. [Google Scholar] [CrossRef]
- Xiao, Y.-Z.; Yang, M.; Xiao, Y.; Guo, Q.; Huang, Y.; Li, C.-J.; Cai, D.; Luo, X.-H. Reducing Hypothalamic Stem Cell Senescence Protects against Aging-Associated Physiological Decline. Cell Metab. 2020, 31, 534–548.e5. [Google Scholar] [CrossRef]
- Cai, G.; Xiao, Y.; Yang, M.; Guo, Q.; Su, T.; Liu, Y.; Jiang, T.; Li, C. Long noncoding RNA Gm31629 promotes bone regeneration by maintaining bone marrow mesenchymal stem cells activity. PeerJ 2022, 10, e13475. [Google Scholar] [CrossRef]
- Jung, Y.S.; Rha, C.-S.; Baik, M.-Y.; Baek, N.-I.; Kim, D.-O. A brief history and spectroscopic analysis of soy isoflavones. Food Sci. Biotechnol. 2020, 29, 1605–1617. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Kim, J.-R.; Choi, H.C. Genistein-induced LKB1–AMPK activation inhibits senescence of VSMC through autophagy induction. Vasc. Pharmacol. 2016, 81, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, Y.; Xue, K.; Li, J.; Son, G.; Wang, J.; Qian, W.; Wang, S.; Zheng, J.; Yang, C.; et al. Genistein mitigates senescence of bone marrow mesenchymal stem cells via ERRα-mediated mitochondrial biogenesis and mitophagy in ovariectomized rats. Redox Biol. 2023, 61, 102649. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.; Pang, X.; Zhao, Z.; Yu, H.; Zhou, H. Genistein protects against ox-LDL-induced senescence through enhancing SIRT1/LKB1/AMPK-mediated autophagy flux in HUVECs. Mol. Cell. Biochem. 2019, 455, 127–134. [Google Scholar] [CrossRef]
- Han, D.; Gong, H.; Wei, Y.; Xu, Y.; Zhou, X.; Wang, Z.; Feng, F. Hesperidin inhibits lung fibroblast senescence via IL-6/STAT3 signaling pathway to suppress pulmonary fibrosis. Phytomedicine 2023, 112, 154680. [Google Scholar] [CrossRef]
- Tsai, Y.-F.; Chen, Y.-R.; Chen, J.-P.; Tang, Y.; Yang, K.-C. Effect of hesperidin on anti-inflammation and cellular antioxidant capacity in hydrogen peroxide-stimulated human articular chondrocytes. Process. Biochem. 2019, 85, 175–184. [Google Scholar] [CrossRef]
- Habauzit, V.; Sacco, S.M.; Gil-Izquierdo, A.; Trzeciakiewicz, A.; Morand, C.; Barron, D.; Pinaud, S.; Offord, E.; Horcajada, M.-N. Differential effects of two citrus flavanones on bone quality in senescent male rats in relation to their bioavailability and metabolism. Bone 2011, 49, 1108–1116. [Google Scholar] [CrossRef]
- Choi, E.M.; Lee, Y.S. Effects of hesperetin on the production of inflammatory mediators in IL-1β treated human synovial cells. Cell. Immunol. 2010, 264, 1–3. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Shen, Z.-Q.; Wang, T.-W.; Kao, C.-H.; Teng, Y.-C.; Yeh, T.-K.; Lu, C.-K.; Tsai, T.-F. Hesperetin promotes longevity and delays aging via activation of Cisd2 in naturally aged mice. J. Biomed. Sci. 2022, 29, 53. [Google Scholar] [CrossRef]
- Shen, Z.-Q.; Chang, C.-Y.; Yeh, C.-H.; Lu, C.-K.; Hung, H.-C.; Wang, T.-W.; Wu, K.-S.; Tung, C.-Y.; Tsai, T.-F. Hesperetin activates CISD2 to attenuate senescence in human keratinocytes from an older person and rejuvenates naturally aged skin in mice. J. Biomed. Sci. 2024, 31, 15. [Google Scholar] [CrossRef]
- Nyane, N.A.; Tlaila, T.B.; Malefane, T.G.; Ndwandwe, D.E.; Owira, P.M.O. Metformin-like antidiabetic, cardio-protective and non-glycemic effects of naringenin: Molecular and pharmacological insights. Eur. J. Pharmacol. 2017, 803, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wu, Y.; He, D.; Zhu, X.; Li, H.; Liu, H.; Liu, H. Anti-aging effects of Ribes meyeri anthocyanins on neural stem cells and aging mice. Aging 2020, 12, 17738–17753. [Google Scholar] [CrossRef] [PubMed]
- Da Pozzo, E.; Costa, B.; Cavallini, C.; Testai, L.; Martelli, A.; Calderone, V.; Martini, C. The Citrus Flavanone Naringenin Protects Myocardial Cells against Age-Associated Damage. Oxidative Med. Cell. Longev. 2017, 2017, 9536148. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Kim, G.R. Inhibitory effect of naringenin on LPS-induced skin senescence by SIRT1 regulation in HDFs. Biomed. Dermatol. 2018, 2, 26. [Google Scholar] [CrossRef]
- Tang, X.; Zhong, J.; Luo, H.; Zhou, F.; Wang, L.; Lin, S.; Xiong, J.; Lv, H.; Zhou, Z.; Yu, H.; et al. Efficacy of Naringenin against aging and degeneration of nucleus pulposus cells through IGFBP3 inhibition. Sci. Rep. 2025, 15, 6780. [Google Scholar] [CrossRef]
- Testai, L.; Piragine, E.; Piano, I.; Flori, L.; Da Pozzo, E.; Miragliotta, V.; Pirone, A.; Citi, V.; Mannelli, L.D.C.; Brogi, S.; et al. The Citrus Flavonoid Naringenin Protects the Myocardium from Ageing-Dependent Dysfunction: Potential Role of SIRT1. Oxidative Med. Cell. Longev. 2020, 2020, 4650207. [Google Scholar] [CrossRef]
- Piragine, E.; De Felice, M.; Germelli, L.; Brinkmann, V.; Flori, L.; Martini, C.; Calderone, V.; Ventura, N.; Da Pozzo, E.; Testai, L. The Citrus flavanone naringenin prolongs the lifespan in C. elegans and slows signs of brain aging in mice. Exp. Gerontol. 2024, 194, 112495. [Google Scholar] [CrossRef]
- Wang, J.; Wu, R.; Hua, Y.; Ling, S.; Xu, X. Naringenin ameliorates vascular senescence and atherosclerosis involving SIRT1 activation. J. Pharm. Pharmacol. 2023, 75, 1021–1033. [Google Scholar] [CrossRef]
- Hostetler, G.L.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Lim, H.; Park, H.; Kim, H.P. Effects of flavonoids on senescence-associated secretory phenotype formation from bleomycin-induced senescence in BJ fibroblasts. Biochem. Pharmacol. 2015, 96, 337–348. [Google Scholar] [CrossRef]
- Perrott, K.M.; Wiley, C.D.; Desprez, P.-Y.; Campisi, J. Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. GeroScience 2017, 39, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, J.-L.; Liu, R.; Li, X.-X.; Li, J.-F.; Zhang, L. Neuroprotective, Anti-Amyloidogenic and Neurotrophic Effects of Apigenin in an Alzheimer’s Disease Mouse Model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef] [PubMed]
- Cavalier, A.N.; Clayton, Z.S.; Wahl, D.; Hutton, D.A.; McEntee, C.M.; Seals, D.R.; LaRocca, T.J. Protective effects of apigenin on the brain transcriptome with aging. Mech. Ageing Dev. 2024, 217, 111889. [Google Scholar] [CrossRef]
- Ali, D.; Okla, M.; Abuelreich, S.; Vishnubalaji, R.; Ditzel, N.; Hamam, R.; Kowal, J.M.; Sayed, A.; Aldahmash, A.; Alajez, N.M.; et al. Apigenin and Rutaecarpine reduce the burden of cellular senescence in bone marrow stromal stem cells. Front. Endocrinol. 2024, 15, 1360054. [Google Scholar] [CrossRef]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. BioFactors 2021, 47, 170–180. [Google Scholar] [CrossRef]
- Zhu, R.Z.; Li, B.S.; Gao, S.S.; Seo, J.H.; Choi, B.-M. Luteolin inhibits H2O2-induced cellular senescence via modulation of SIRT1 and p53. Korean J. Physiol. Pharmacol. 2021, 25, 297–305. [Google Scholar] [CrossRef]
- Xie, T.; Yuan, J.; Mei, L.; Li, P.; Pan, R. Luteolin suppresses TNF-α-induced inflammatory injury and senescence of nucleus pulposus cells via the Sirt6/NF-κB pathway. Exp. Ther. Med. 2022, 24, 469. [Google Scholar] [CrossRef]
- Yan, Y.; Huang, H.; Su, T.; Huang, W.; Wu, X.; Chen, X.; Ye, S.; Zhong, J.; Li, C.; Li, Y. Luteolin Mitigates Photoaging Caused by UVA-Induced Fibroblast Senescence by Modulating Oxidative Stress Pathways. Int. J. Mol. Sci. 2025, 26, 1809. [Google Scholar] [CrossRef]
- Younis, R.L.; El-Gohary, R.M.; Ghalwash, A.A.; Hegab, I.I.; Ghabrial, M.M.; Aboshanady, A.M.; Mostafa, R.A.; El-Azeem, A.H.A.; Farghal, E.E.; Belal, A.A.; et al. Luteolin Mitigates D-Galactose-Induced Brain Ageing in Rats: SIRT1-Mediated Neuroprotection. Neurochem. Res. 2024, 49, 2803–2820. [Google Scholar] [CrossRef]
- Keshtkar, S.; Kaviani, M.; Jabbarpour, Z.; Geramizadeh, B.; Motevaseli, E.; Nikeghbalian, S.; Shamsaeefar, A.; Motazedian, N.; Al-Abdullah, I.H.; Ghahremani, M.H.; et al. Protective effect of nobiletin on isolated human islets survival and function against hypoxia and oxidative stress-induced apoptosis. Sci. Rep. 2019, 9, 11701. [Google Scholar] [CrossRef]
- Nakajima, A.; Aoyama, Y.; Nguyen, T.-T.L.; Shin, E.-J.; Kim, H.-C.; Yamada, S.; Nakai, T.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; et al. Nobiletin, a citrus flavonoid, ameliorates cognitive impairment, oxidative burden, and hyperphosphorylation of tau in senescence-accelerated mouse. Behav. Brain Res. 2013, 250, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Dusabimana, T.; Kim, S.R.; Kim, H.J.; Park, S.W.; Kim, H. Nobiletin ameliorates hepatic ischemia and reperfusion injury through the activation of SIRT-1/FOXO3a-mediated autophagy and mitochondrial biogenesis. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef]
- Wang, H.-H.; Sun, Y.-N.; Qu, T.-Q.; Sang, X.-Q.; Zhou, L.-M.; Li, Y.-X.; Ren, F.-Z. Nobiletin Prevents D-Galactose-Induced C2C12 Cell Aging by Improving Mitochondrial Function. Int. J. Mol. Sci. 2022, 23, 11963. [Google Scholar] [CrossRef]
- Xie, L.; Xie, H.; Chen, C.; Tao, Z.; Zhang, C.; Cai, L. Inhibiting the PI3K/AKT/NF-κB signal pathway with nobiletin for attenuating the development of osteoarthritis: In vitro and in vivo studies. Food Funct. 2019, 10, 2161–2175. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Yin, L.; Zhu, M.; Wang, F.; Zhang, L.; Wang, H.; Zhou, Z.; Zhu, H.; Huang, C.; et al. Tangeretin promotes lifespan associated with insulin/insulin-like growth factor-1 signaling pathway and heat resistance in Caenorhabditis elegans. BioFactors 2022, 48, 442–453. [Google Scholar] [CrossRef]
- Yang, T.; Feng, C.; Wang, D.; Qu, Y.; Yang, Y.; Wang, Y.; Sun, Z. Neuroprotective and Anti-inflammatory Effect of Tangeretin Against Cerebral Ischemia-Reperfusion Injury in Rats. Inflammation 2020, 43, 2332–2343. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Lee, E.-J.; Park, J.-S.; Jang, S.-E.; Kim, D.-H.; Kim, H.-S. Anti-Inflammatory and Antioxidant Mechanism of Tangeretin in Activated Microglia. J. Neuroimmune Pharmacol. 2016, 11, 294–305. [Google Scholar] [CrossRef]
- Mahmud, A.R.; Ema, T.I.; Siddiquee, M.F.-R.; Shahriar, A.; Ahmed, H.; Hasan, M.U.; Rahman, N.; Islam, R.; Uddin, M.R.; Mizan, F.R. Natural flavonols: Actions, mechanisms, and potential therapeutic utility for various diseases. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 47. [Google Scholar] [CrossRef]
- Geng, L.; Liu, Z.; Zhang, W.; Li, W.; Wu, Z.; Wang, W.; Ren, R.; Su, Y.; Wang, P.; Sun, L.; et al. Chemical screen identifies a geroprotective role of quercetin in premature aging. Protein Cell 2019, 10, 417–435. [Google Scholar] [CrossRef]
- Chondrogianni, N.; Kapeta, S.; Chinou, I.; Vassilatou, K.; Papassideri, I.; Gonos, E.S. Anti-ageing and rejuvenating effects of quercetin. Exp. Gerontol. 2010, 45, 763–771. [Google Scholar] [CrossRef]
- Bientinesi, E.; Ristori, S.; Lulli, M.; Monti, D. Quercetin induces senolysis of doxorubicin-induced senescent fibroblasts by reducing autophagy, preventing their pro-tumour effect on osteosarcoma cells. Mech. Ageing Dev. 2024, 220, 111957. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Jiang, K.; Ogrodnik, M.; Chen, X.; Zhu, X.-Y.; Lohmeier, H.; Ahmed, L.; Tang, H.; Tchkonia, T.; Hickson, L.J.; et al. Increased renal cellular senescence in murine high-fat diet: Effect of the senolytic drug quercetin. Transl. Res. 2019, 213, 112–123. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, B.; Shi, Y.; Xie, C.; Huang, C.; Chen, B.; Zhang, H.; Zeng, G.; Liang, H.; Wu, Y.; et al. Senolytic agent Quercetin ameliorates intervertebral disc degeneration via the Nrf2/NF-κB axis. Osteoarthr. Cartil. 2021, 29, 413–422. [Google Scholar] [CrossRef]
- Belinha, I.; Amorim, M.A.; Rodrigues, P.; de Freitas, V.; Moradas-Ferreira, P.; Mateus, N.; Costa, V. Quercetin Increases Oxidative Stress Resistance and Longevity in Saccharomyces cerevisiae. J. Agric. Food Chem. 2007, 55, 2446–2451. [Google Scholar] [CrossRef]
- Kampkötter, A.; Timpel, C.; Zurawski, R.F.; Ruhl, S.; Chovolou, Y.; Proksch, P.; Wätjen, W. Increase of stress resistance and lifespan of Caenorhabditis elegans by quercetin. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 149, 314–323. [Google Scholar] [CrossRef]
- Zhu, Y.I.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef]
- Fan, T.; Du, Y.; Zhang, M.; Zhu, A.R.; Zhang, J. Senolytics Cocktail Dasatinib and Quercetin Alleviate Human Umbilical Vein Endothelial Cell Senescence via the TRAF6-MAPK-NF-κB Axis in a YTHDF2-Dependent Manner. Gerontology 2022, 68, 920–934. [Google Scholar] [CrossRef]
- Wang, X.; Tan, Y.; Liu, F.; Wang, J.; Liu, F.; Zhang, Q.; Li, J. Pharmacological network analysis of the functions and mechanism of kaempferol from Du Zhong in intervertebral disc degeneration (IDD). J. Orthop. Transl. 2023, 39, 135–146. [Google Scholar] [CrossRef]
- Yao, X.; Jiang, H.; Li, Y.; Gao, Q.; Xu, Y.N.; Kim, N. Kaempferol alleviates the reduction of developmental competence during aging of porcine oocytes. Anim. Sci. J. 2019, 90, 1417–1425. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.-S.; Choi, D.-H.; Choi, J.; Cho, S.Y.; Kim, S.-H.; Baek, H.-S.; Yoon, K.D.; Son, S.W.; Son, E.D.; et al. Kaempferol tetrasaccharides restore skin atrophy via PDK1 inhibition in human skin cells and tissues: Bench and clinical studies. Biomed. Pharmacother. 2022, 156, 113864. [Google Scholar] [CrossRef]
- Kampkötter, A.; Nkwonkam, C.G.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Wätjen, W.; Kahl, R. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and FoxO transcription factor DAF-16 in the model organism Caenorhabditis elegans. Arch. Toxicol. 2007, 81, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Wang, T.; Li, Z.; Gao, Y.; Cui, S.W.; Qiu, J. Comparison of quercetin and rutin inhibitory influence on Tartary buckwheat starch digestion in vitro and their differences in binding sites with the digestive enzyme. Food Chem. 2022, 367, 130762. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Q.; Wufuer, H.; Li, Z.; Sun, R.; Jiang, Z.; Dou, X.; Fu, Q.; Campisi, J.; Sun, Y. Rutin is a potent senomorphic agent to target senescent cells and can improve chemotherapeutic efficacy. Aging Cell 2024, 23, e13921. [Google Scholar] [CrossRef]
- Li, Y.; Qin, R.; Yan, H.; Wang, F.; Huang, S.; Zhang, Y.; Zhong, M.; Zhang, W.; Wang, Z. Inhibition of vascular smooth muscle cells premature senescence with rutin attenuates and stabilizes diabetic atherosclerosis. J. Nutr. Biochem. 2018, 51, 91–98. [Google Scholar] [CrossRef]
- Xinghua, L.; Yingying, H.; Shuai, W.; Guangping, L. Anti-aging Effect of Rutin in Caenorhabditis elegans and D-Gal-Induced Aging Mouse Model. Dokl. Biochem. Biophys. 2023, 513, 350–354. [Google Scholar] [CrossRef]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A Dietary Antioxidant for Health Promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef]
- Mahoney, S.A.; Venkatasubramanian, R.; Darrah, M.A.; Ludwig, K.R.; VanDongen, N.S.; Greenberg, N.T.; Longtine, A.G.; Hutton, D.A.; Brunt, V.E.; Campisi, J.; et al. Intermittent supplementation with fisetin improves arterial function in old mice by decreasing cellular senescence. Aging Cell 2024, 23, e14060. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Huard, C.A.; Gao, X.; Hazra, M.E.D.; Hazra, R.-O.D.; Lebsock, K.; Easley, J.T.; Millett, P.J.; Huard, J. Effects of Fisetin Treatment on Cellular Senescence of Various Tissues and Organs of Old Sheep. Antioxidants 2023, 12, 1646. [Google Scholar] [CrossRef]
- Mullen, M.; Nelson, A.L.; Goff, A.; Billings, J.; Kloser, H.; Huard, C.; Mitchell, J.; Hambright, W.S.; Ravuri, S.; Huard, J. Fisetin Attenuates Cellular Senescence Accumulation During Culture Expansion of Human Adipose-Derived Stem Cells. Stem Cells 2023, 41, 698–710. [Google Scholar] [CrossRef]
- Xu, Q.; Fu, Q.; Li, Z.; Liu, H.; Wang, Y.; Lin, X.; He, R.; Zhang, X.; Ju, Z.; Campisi, J.; et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat. Metab. 2021, 3, 1706–1726. [Google Scholar] [CrossRef] [PubMed]
- Moaddel, R.; Rossi, M.; Rodriguez, S.; Munk, R.; Khadeer, M.; Abdelmohsen, K.; Gorospe, M.; Ferrucci, L. Identification of gingerenone A as a novel senolytic compound. PLoS ONE 2022, 17, e0266135. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Choi, M. Anti-inflammatory and anti-aging effects of hydroxytyrosol on human dermal fibroblasts (HDFs). Biomed. Dermatol. 2018, 2, 21. [Google Scholar] [CrossRef]
- Menicacci, B.; Cipriani, C.; Margheri, F.; Mocali, A.; Giovannelli, L. Modulation of the Senescence-Associated Inflammatory Phenotype in Human Fibroblasts by Olive Phenols. Int. J. Mol. Sci. 2017, 18, 2275. [Google Scholar] [CrossRef]
- Giovannelli, L. Beneficial effects of olive oil phenols on the aging process: Experimental evidence and possible mechanisms of action. Nutr. Aging 2012, 1, 207–223. [Google Scholar] [CrossRef]
- Lee, K.-S.; Lee, B.-S.; Semnani, S.; Avanesian, A.; Um, C.-Y.; Jeon, H.-J.; Seong, K.-M.; Yu, K.; Min, K.-J.; Jafari, M. Curcumin Extends Life Span, Improves Health Span, and Modulates the Expression of Age-Associated Aging Genes in Drosophila melanogaster. Rejuvenation Res. 2010, 13, 561–570. [Google Scholar] [CrossRef]
- Liao, V.H.-C.; Yu, C.-W.; Chu, Y.-J.; Li, W.-H.; Hsieh, Y.-C.; Wang, T.-T. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech. Ageing Dev. 2011, 132, 480–487. [Google Scholar] [CrossRef]
- Ray Hamidie, R.D.; Yamada, T.; Ishizawa, R.; Saito, Y.; Masuda, K. Curcumin treatment enhances the effect of exercise on mitochondrial biogenesis in skeletal muscle by increasing cAMP levels. Metabolism 2015, 64, 1334–1347. [Google Scholar] [CrossRef]
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology 2017, 18, 447–476. [Google Scholar] [CrossRef]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, Inflammation, and Chronic Diseases: How Are They Linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef]
- Mazzanti, G.; Di Giacomo, S. Curcumin and Resveratrol in the Management of Cognitive Disorders: What is the Clinical Evidence? Molecules 2016, 21, 1243. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Pipingas, A.; Scholey, A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. 2015, 29, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef]
- Williamson, G.; Holst, B. Dietary reference intake (DRI) value for dietary polyphenols: Are we heading in the right direction? Br. J. Nutr. 2008, 99, S55–S58. [Google Scholar] [CrossRef]
- Khokhar, S.; Magnusdottir, S.G.M. Total Phenol, Catechin, and Caffeine Contents of Teas Commonly Consumed in the United Kingdom. J. Agric. Food Chem. 2002, 50, 565–570. [Google Scholar] [CrossRef]
- Arts, I.C.W.; van de Putte, B.; Hollman, P.C.H. Catechin Contents of Foods Commonly Consumed in The Netherlands. 2. Tea, Wine, Fruit Juices, and Chocolate Milk. J. Agric. Food Chem. 2000, 48, 1752–1757. [Google Scholar] [CrossRef]
- Long, H.; Zhu, Y.; Huang, T.; Coury, L.A.; Kissinger, P.T. Identification and determination of polyphenols in tea by liquid chromatography with multi-channel electrochemical detection. J. Liq. Chromatogr. Relat. Technol. 2001, 24, 1105–1114. [Google Scholar] [CrossRef]
- Lee, B.-L.; Ong, C.-N. Comparative analysis of tea catechins and theaflavins by high-performance liquid chromatography and capillary electrophoresis. J. Chromatogr. A 2000, 881, 439–447. [Google Scholar] [CrossRef]
- Kiehne, A.; Engelhardt, U.H. Thermospray-LC-MS analysis of various groups of polyphenols in tea. Eur. Food Res. Technol. 1996, 202, 48–54. [Google Scholar] [CrossRef]
- Natsume, M.; Osakabe, N.; Yamagishi, M.; Takizawa, T.; Nakamura, T.; Miyatake, H.; Hatano, T.; Yoshida, T. Analyses of Polyphenols in Cacao Liquor, Cocoa, and Chocolate by Normal-Phase and Reversed-Phase HPLC. Biosci. Biotechnol. Biochem. 2000, 64, 2581–2587. [Google Scholar] [CrossRef]
- Gökmen, V.; Acar, J.; Kahraman, N. Influence of conventional clarification and ultrafiltration on the phenolic composition of golden delicious apple juice. J. Food Qual. 2003, 26, 257–266. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A. Effects of various clarification treatments on phenolic compounds and color of apple juice. Eur. Food Res. Technol. 2007, 224, 755–762. [Google Scholar] [CrossRef]
- de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Quantitative Analysis of Flavan-3-ols in Spanish Foodstuffs and Beverages. J. Agric. Food Chem. 2000, 48, 5331–5337. [Google Scholar] [CrossRef] [PubMed]
- Marín, F.; Martinez, M.; Uribesalgo, T.; Castillo, S.; Frutos, M. Changes in nutraceutical composition of lemon juices according to different industrial extraction systems. Food Chem. 2002, 78, 319–324. [Google Scholar] [CrossRef]
- Caristi, C.; Bellocco, E.; Panzera, V.; Toscano, G.; Vadalà, R.; Leuzzi, U. Flavonoids Detection by HPLC-DAD-MS-MS in Lemon Juices from Sicilian Cultivars. J. Agric. Food Chem. 2003, 51, 3528–3534. [Google Scholar] [CrossRef]
- Pupin, A.; Dennis, M.; Toledo, M. Flavanone glycosides in Brazilian orange juice. Food Chem. 1998, 61, 275–280. [Google Scholar] [CrossRef]
- Rouseff, R.L.; Martin, S.F.; Youtsey, C.O. Quantitative survey of narirutin, naringin, hesperidin, and neohesperidin in citrus. J. Agric. Food Chem. 1987, 35, 1027–1030. [Google Scholar] [CrossRef]
- Mouly, P.P.; Gaydou, E.M.; Faure, R.; Estienne, J.M. Blood Orange Juice Authentication Using Cinnamic Acid Derivatives. Variety Differentiations Associated with Flavanone Glycoside Content. J. Agric. Food Chem. 1997, 45, 373–377. [Google Scholar] [CrossRef]
- Guedon, D.J.; Pasquier, B.P. Analysis and Distribution of Flavonoid Glycosides and Rosmarinic Acid in 40 Mentha x piperita Clones. J. Agric. Food Chem. 1994, 42, 679–684. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Mukhopadhyay, S.; Robbins, R.J.; Harnly, J.M. Identification and quantification of flavonoids of Mexican oregano (Lippia graveolens) by LC-DAD-ESI/MS analysis. J. Food Compos. Anal. 2007, 20, 361–369. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant Activity and Phenolic Compounds in Selected Herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Proestos, C.; Komaitis, M. Ultrasonically assisted extraction of phenolic compounds from aromatic plants: Comparison with conventional extraction technics. J. Food Qual. 2006, 29, 567–582. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Cantini, C.; Cimato, A.; Heimler, D. Characterization of Violetto di Toscana, a typical Italian variety of artichoke (Cynara scolymus L.). Food Chem. 2006, 95, 221–225. [Google Scholar] [CrossRef]
- Leuzzi, U.; Caristi, C.; Panzera, V.; Licandro, G. Flavonoids in Pigmented Orange Juice and Second-Pressure Extracts. J. Agric. Food Chem. 2000, 48, 5501–5506. [Google Scholar] [CrossRef]
- Inocencio, C.; Rivera, D.; Alcaraz, F.; Tomás-Barberán, F.A. Flavonoid content of commercial capers (Capparis spinosa, C. sicula and C. orientalis) produced in mediterranean countries. Eur. Food Res. Technol. 2000, 212, 70–74. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- Counet, C.; Callemien, D.; Collin, S. Chocolate and cocoa: New sources of trans-resveratrol and trans-piceid. Food Chem. 2006, 98, 649–657. [Google Scholar] [CrossRef]
- Kaack, K.; Austed, T. Interaction of vitamin C and flavonoids in elderberry (Sambucus nigra L.) during juice processing. Plant Foods Hum. Nutr. 1998, 52, 187–198. [Google Scholar] [CrossRef]
- Franke, A.A.; Hankin, J.H.; Yu, M.C.; Maskarinec, G.; Low, S.-H.; Custer, L.J. Isoflavone Levels in Soy Foods Consumed by Multiethnic Populations in Singapore and Hawaii. J. Agric. Food Chem. 1999, 47, 977–986. [Google Scholar] [CrossRef]
- López-Gutiérrez, N.; Romero-González, R.; Frenich, A.G.; Vidal, J.L.M. Identification and quantification of the main isoflavones and other phytochemicals in soy based nutraceutical products by liquid chromatography–orbitrap high resolution mass spectrometry. J. Chromatogr. A 2014, 1348, 125–136. [Google Scholar] [CrossRef]
- Chan, S.G.; Murphy, P.A.; Ho, S.C.; Kreiger, N.; Darlington, G.; So, E.K.F.; Chong, P.Y.Y. Isoflavonoid Content of Hong Kong Soy Foods. J. Agric. Food Chem. 2009, 57, 5386–5390. [Google Scholar] [CrossRef] [PubMed]
- Johns, P.; Dowlati, L.; Wargo, W. Determination of Isoflavones in Ready-to-Feed Soy-Based Infant Formula. J. AOAC Int. 2003, 86, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.A.; Song, T.; Buseman, G.; Barua, K.; Beecher, G.R.; Trainer, D.; Holden, J. Isoflavones in Retail and Institutional Soy Foods. J. Agric. Food Chem. 1999, 47, 2697–2704. [Google Scholar] [CrossRef]
- Song, T.; Barua, K.; Buseman, G.; Murphy, P.A. Soy isoflavone analysis: Quality control and a new internal standard. Am. J. Clin. Nutr. 1998, 68, 1474S–1479S. [Google Scholar] [CrossRef]
- Tayyem, R.F.; Heath, D.D.; Al-Delaimy, W.K.; Rock, C.L. Curcumin Content of Turmeric and Curry Powders. Nutr. Cancer 2006, 55, 126–131. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Rao, L.J.M.; Sakariah, K.K. Improved HPLC Method for the Determination of Curcumin, Demethoxycurcumin, and Bisdemethoxycurcumin. J. Agric. Food Chem. 2002, 50, 3668–3672. [Google Scholar] [CrossRef]
- Bianco, A.; Uccella, N. Biophenolic components of olives. Food Res. Int. 2000, 33, 475–485. [Google Scholar] [CrossRef]
- Bianco, A.; Buiarelli, F.; Cartoni, G.; Coccioli, F.; Jasionowska, R.; Margherita, P. Analysis by liquid chromatography-tandem mass spectrometry of biophenolic compounds in olives and vegetation waters, Part I. J. Sep. Sci. 2003, 26, 409–416. [Google Scholar] [CrossRef]
- Boskou, G.; Salta, F.N.; Chrysostomou, S.; Mylona, A.; Chiou, A.; Andrikopoulos, N.K. Antioxidant capacity and phenolic profile of table olives from the Greek market. Food Chem. 2006, 94, 558–564. [Google Scholar] [CrossRef]
- Blekas, G.; Vassilakis, C.; Harizanis, C.; Tsimidou, M.; Boskou, D.G. Biophenols in Table Olives. J. Agric. Food Chem. 2002, 50, 3688–3692. [Google Scholar] [CrossRef]
- Medina, E.; de Castro, A.; Romero, C.; Brenes, M. Comparison of the Concentrations of Phenolic Compounds in Olive Oils and Other Plant Oils: Correlation with Antimicrobial Activity. J. Agric. Food Chem. 2006, 54, 4954–4961. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Mulinacci, N.; Pinelli, P.; Vincieri, F.F.; Cimato, A. Polyphenolic Content in Five Tuscany Cultivars of Olea europaea L. J. Agric. Food Chem. 1999, 47, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Liberatore, L.; Procida, G.; D’Alessandro, N.; Cichelli, A. Solid-phase extraction and gas chromatographic analysis of phenolic compounds in virgin olive oil. Food Chem. 2001, 73, 119–124. [Google Scholar] [CrossRef]
- Jakopic, J.; Colaric, M.; Veberic, R.; Hudina, M.; Solar, A.; Stampar, F. How much do cultivar and preparation time influence on phenolics content in walnut liqueur? Food Chem. 2007, 104, 100–105. [Google Scholar] [CrossRef]
- Kilinc, E.; Kalkan, H. High-performance Liquid Chromatographic Determination of Some Phenolic Acids of Turkish Commercial Wines: An Electrochemical Approach. J. Wine Res. 2003, 14, 17–23. [Google Scholar] [CrossRef]
- Gambelli, L.; Santaroni, G. Polyphenols content in some Italian red wines of different geographical origins. J. Food Compos. Anal. 2004, 17, 613–618. [Google Scholar] [CrossRef]
- Burns, J.; Gardner, P.T.; O’Neil, J.; Crawford, S.; Morecroft, I.; McPhail, D.B.; Lister, C.; Matthews, D.; MacLean, M.R.; Lean, M.E.J.; et al. Relationship among Antioxidant Activity, Vasodilation Capacity, and Phenolic Content of Red Wines. J. Agric. Food Chem. 2000, 48, 220–230. [Google Scholar] [CrossRef]
- Pathak, S.B.; Niranjan, K.; Padh, H.; Rajani, M. TLC Densitometric Method for the Quantification of Eugenol and Gallic Acid in Clove. Chromatographia 2004, 60, 241–244. [Google Scholar] [CrossRef]
- De Vasconcelos, M.D.C.B.M.; Bennett, R.N.; Rosa, E.A.S.; Cardoso, J.V.F. Primary and Secondary Metabolite Composition of Kernels from Three Cultivars of Portuguese Chestnut (Castanea sativa Mill.) at Different Stages of Industrial Transformation. J. Agric. Food Chem. 2007, 55, 3508–3516. [Google Scholar] [CrossRef]
- Rossetto, M.; Lante, A.; Vanzani, P.; Spettoli, P.; Scarpa, M.; Rigo, A. Red Chicories as Potent Scavengers of Highly Reactive Radicals: A Study on Their Phenolic Composition and Peroxyl Radical Trapping Capacity and Efficiency. J. Agric. Food Chem. 2005, 53, 8169–8175. [Google Scholar] [CrossRef]
- Beta, T.; Rooney, L.W.; Marovatsanga, L.T.; Taylor, J.R. Phenolic compounds and kernel characteristics of Zimbabwean sorghums. J. Sci. Food Agric. 1999, 79, 1003–1010. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of Antioxidant Activity, Anthocyanins, Carotenoids, and Phenolics of Three Native Fresh and Sun-Dried Date (Phoenix dactylifera L.) Varieties Grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef]
- Wang, H.; Provan, G.J.; Helliwell, K. Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem. 2004, 87, 307–311. [Google Scholar] [CrossRef]
- Lamikanra, O.; Grimm, C.C.; Ben Rodin, J.; Inyang. Hydroxylated Stilbenes in Selected American Wines. J. Agric. Food Chem. 1996, 44, 1111–1115. [Google Scholar] [CrossRef]
- Ehala, S.; Vaher, M.; Kaljurand, M. Characterization of Phenolic Profiles of Northern European Berries by Capillary Electrophoresis and Determination of their Antioxidant Activity. J. Agric. Food Chem. 2005, 53, 6484–6490. [Google Scholar] [CrossRef]
- Kallithraka, S.; Arvanitoyannis, I.; El-Zajouli, A.; Kefalas, P. The application of an improved method for trans-resveratrol to determine the origin of Greek red wines. Food Chem. 2001, 75, 355–363. [Google Scholar] [CrossRef]


| Polyphenol | Source | Mean Content | References |
|---|---|---|---|
| (−)-Epigallocatechin 3-O-Gallate | Tea [Black], infusion | 9.12 mg/100 mL | [147,148] |
| Tea [Green], infusion | 27.16 mg/100 mL | [147,149] | |
| Tea [Oolong], infusion | 17.89 mg/100 mL | [150,151] | |
| Procyanidin Trimer C1 | Chocolate, dark | 26.00 mg/100 g FW | [152] |
| Apple [Dessert], pure juice | 29.97 mg/100 mL | [153,154] | |
| Plum, fresh | 10.01 mg/100 g FW | [155] | |
| Hesperidin | Lemon, pure juice | 17.81 mg/100 mL | [156,157] |
| Orange [Blond], pure juice | 25.85 mg/100 mL | [158,159] | |
| Orange [Blood], pure juice | 43.61 mg/100 mL | [160] | |
| Peppermint, dried | 480.65 mg/100 g FW | [161] | |
| Naringenin | Mexican oregano, dried | 372.00 mg/100 g FW | [162] |
| Apigenin | Italian oregano, fresh | 3.50 mg/100 g FW | [163] |
| Marjoram, dried | 4.40 mg/100 g FW | [164] | |
| Common sage, fresh | 2.40 mg/100 g FW | [163] | |
| Luteolin | Common sage, fresh | 33.40 mg/100 g FW | [163] |
| Common thyme, fresh | 39.50 mg/100 g FW | [163] | |
| Mexican oregano, dried | 56.33 mg/100 g FW | [162] | |
| Globe artichoke, heads, raw | 42.10 mg/100 g FW | [165] | |
| Nobiletin | Orange [Blood], pure juice | 0.31 mg/100 mL | [166] |
| Tangeretin | Orange [Blood], pure juice | 0.04 mg/100 mL | [166] |
| Kaempferol | Capers | 104.29 mg/100 g FW | [167] |
| Cloves | 23.80 mg/100 g FW | [168] | |
| Cumin | 38.60 mg/100 g FW | [168] | |
| Quercetin | Chocolate, dark | 25.00 mg/100 g FW | [169] |
| Black elderberry | 42.00 mg/100 g FW | [170] | |
| Mexican oregano, dried | 42.00 mg/100 g FW | [162] | |
| Capers | 32.82 mg/100 g FW | [167] | |
| Cloves | 28.40 mg/100 g FW | [168] | |
| Genistein | Soy paste, miso | 7.25 mg/100 g FW | [171,172,173] |
| Soy, tempe | 10.00 mg/100 g FW | [174,175,176] | |
| Soy, tofu, fermented | 9.68 mg/100 g FW | [171,173] | |
| Curcumin | Turmeric, dried | 2213.57 mg/100 g FW | [177,178] |
| Curry, powder | 285.26 mg/100 g FW | [177] | |
| Hydroxytyrosol | Olive [Black], raw | 65.93 mg/100 g FW | [179,180,181] |
| Olive [Green], raw | 55.57 mg/100 g FW | [179,182] | |
| Oleuropein-Aglycone | Olive, oil, refined | 12.54 mg/100 g FW | [183] |
| Olive [Black], raw | 81.82 mg/100 g FW | [179,184] | |
| Olive [Green], raw | 58.50 mg/100 g FW | [179] | |
| Olive, oil, virgin | 12.06 mg/100 g FW | [183,185] | |
| Gallic Acid | Walnut, liquor | 15.15 mg/100 mL | [186] |
| Wine [Red] | 3.59 mg/100 mL | [187,188,189] | |
| Cloves | 458.19 mg/100 g FW | [190] | |
| Chestnut, raw | 479.78 mg/100 g FW | [191] | |
| Chicory [Green], raw | 25.84 mg/100 g FW | [192] | |
| Protocatechuic Acid | Sorghum, whole grain | 2.55 mg/100 g FW | [193] |
| Date, dried | 4.94 mg/100 g FW | [194] | |
| Star anise | 32.20 mg/100 g FW | [168] | |
| Chicory [Red], raw | 16.78 mg/100 g FW | [192] | |
| Caffeic Acid | Common sage, dried | 26.40 mg/100 g FW | [195] |
| Spearmint, dried | 25.00 mg/100 g FW | [195] | |
| Ceylan cinnamon | 24.20 mg/100 g FW | [168] | |
| Star anise | 20.20 mg/100 g FW | [168] | |
| Resveratrol | Muscadine grape, red wine | 3.02 mg/100 mL | [196] |
| European cranberry | 1.92 mg/100 g FW | [197] | |
| Lingonberry, raw | 3.00 mg/100 g FW | [197] | |
| Wine [Red] | 0.27 mg/100 mL | [188,189,198] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Centonze, M.; Aloisio Caruso, E.; De Nunzio, V.; Cofano, M.; Saponara, I.; Pinto, G.; Notarnicola, M. The Antiaging Potential of Dietary Plant-Based Polyphenols: A Review on Their Role in Cellular Senescence Modulation. Nutrients 2025, 17, 1716. https://doi.org/10.3390/nu17101716
Centonze M, Aloisio Caruso E, De Nunzio V, Cofano M, Saponara I, Pinto G, Notarnicola M. The Antiaging Potential of Dietary Plant-Based Polyphenols: A Review on Their Role in Cellular Senescence Modulation. Nutrients. 2025; 17(10):1716. https://doi.org/10.3390/nu17101716
Chicago/Turabian StyleCentonze, Matteo, Emanuela Aloisio Caruso, Valentina De Nunzio, Miriam Cofano, Ilenia Saponara, Giuliano Pinto, and Maria Notarnicola. 2025. "The Antiaging Potential of Dietary Plant-Based Polyphenols: A Review on Their Role in Cellular Senescence Modulation" Nutrients 17, no. 10: 1716. https://doi.org/10.3390/nu17101716
APA StyleCentonze, M., Aloisio Caruso, E., De Nunzio, V., Cofano, M., Saponara, I., Pinto, G., & Notarnicola, M. (2025). The Antiaging Potential of Dietary Plant-Based Polyphenols: A Review on Their Role in Cellular Senescence Modulation. Nutrients, 17(10), 1716. https://doi.org/10.3390/nu17101716






