Medium-Chain Triglyceride Dietary Supplements Reduce Glucose Metabolism of Gait-Related Skeletal Muscle in Older Adults: A Longitudinal 18F-FDG PET/CT Analysis
Highlights
- MCT supplementation suppressed glucose metabolism in gait-related skeletal muscles and increased their muscle densities.
- The metabolic changes in MCT were negatively correlated with blood β-hydroxybutyrate.
- The main findings highlighted the positive effect of MCT on skeletal muscle metabolism from glycolysis to ketone body utilization and related changes in muscle quality.
- The main findings demonstrated the potential role of MCT as a novel dietary supplement for the stabilization of walking ability in elderly individuals.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics Approval
2.2. Participants
2.3. Image Acquisition
2.4. Measurements
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Participants
3.2. Effects of MCT on Blood Ketone Bodies and Glucose Metabolism
3.3. Effects of MCT on Glucose Metabolism and Muscle Density
4. Discussion
4.1. Mechanism by Which MCTs Modulate Energy Metabolism
4.2. Effects of MCTs on Skeletal Muscle Mass and Density
4.3. Practical Application of MCTs
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CT | Computed Tomography |
| FDG | 18F-2-fluoro-2-deoxy-D-glucose |
| HU | Hounsfield units |
| LCT | Long-chain triglyceride |
| MCT | Medium-chain triglyceride |
| PET | Positron emission tomography |
| RCT | Randomized clinical trial |
| ROI | Region of interest |
| SUV | Standardized uptake value |
| 3D | Three-dimensional. |
References
- Balachandran, A.; de Beer, J.; James, K.S.; van Wissen, L.; Janssen, F. Comparison of Population Aging in Europe and Asia Using a Time-Consistent and Comparative Aging Measure. J. Aging Health 2020, 32, 340–351. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Wu, D. Risk factors for frailty in older adults. Medicine 2022, 101, e30169. [Google Scholar] [CrossRef]
- Takei, S.; Okada, Y.; Ochi, M.; Miura, S.; Shiraoka, A.; Ochi, H.; Matsumoto, S.; Igase, M.; Fujishita, S.; Ohyagi, Y. Frailty and aging are associated with cognitive decline and dermal advanced glycation end-product accumulation in older Japanese men. Arch. Gerontol. Geriat. 2024, 1, 100071. [Google Scholar] [CrossRef]
- Arosio, B.; Calvani, R.; Ferri, E.; Coelho-Junior, H.J.; Carandina, A.; Campanelli, F.; Ghiglieri, V.; Marzetti, E.; Picca, A. Sarcopenia and Cognitive Decline in Older Adults: Targeting the Muscle-Brain Axis. Nutrients 2023, 15, 1853. [Google Scholar] [CrossRef]
- Kapogiannis, D.; Avgerinos, K.I. Brain glucose and ketone utilization in brain aging and neurodegenerative diseases. Int. Rev. Neurobiol. 2020, 154, 79–110. [Google Scholar] [PubMed]
- Wang, Y.; Liu, Z.; Han, Y.; Xu, J.; Huang, W.; Li, Z. Medium Chain Triglycerides enhances exercise endurance through the increased mitochondrial biogenesis and metabolism. PLoS ONE 2018, 13, e0191182. [Google Scholar] [CrossRef]
- Ashton, J.S.; Roberts, J.W.; Wakefield, C.J.; MacLaren, D.P.M.; Marwood, S.; Malone, J.J. Medium chain triglycerides with a C8:C10 ratio of 30:70 enhances cognitive performance and mitigates the cognitive decline associated with prolonged exercise in young and healthy adults. Physiol. Behav. 2023, 269, 114284. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Ishikawa, H.; Watanabe, S.; Nosaka, N.; Mutoh, T. A Randomized, Double-Blind, Controlled Trial Assessing If Medium-Chain Triglycerides in Combination with Moderate-Intensity Exercise Increase Muscle Strength in Healthy Middle-Aged and Older Adults. Nutrients 2023, 15, 3275. [Google Scholar] [CrossRef]
- Ishikawa, H.; Kojima, K.; Watanabe, S.; Nosaka, N.; Mutoh, T. Effect of medium-chain triglycerides supplements and walking on health-related quality of life in sedentary, healthy middle-aged, and older adults with low BMIs: A randomized, double-blind, placebo-controlled, parallel-group trial. Front. Nutr. 2023, 10, 1296896. [Google Scholar] [CrossRef]
- Mutoh, T.; Kunitoki, K.; Tatewaki, Y.; Yamamoto, S.; Thyreau, B.; Matsudaira, I.; Kawashima, R.; Taki, Y. Impact of medium-chain triglycerides on gait performance and brain metabolic network in healthy older adults: A double-blind, randomized controlled study. Geroscience 2022, 44, 1325–1338. [Google Scholar] [CrossRef]
- Watanabe, S.; Tsujino, S. Applications of Medium-Chain Triglycerides in Foods. Front. Nutr. 2022, 9, 802805. [Google Scholar] [CrossRef] [PubMed]
- Ezaki, O.; Abe, S. Medium-chain triglycerides (8:0 and 10:0) increase muscle mass and function in frail older adults: A combined data analysis of clinical trials. Front. Nutr. 2023, 10, 1284497. [Google Scholar] [CrossRef]
- Zurlo, F.; Larson, K.; Bogardus, C.; Ravussin, E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Investig. 1990, 86, 1423–1427. [Google Scholar] [CrossRef]
- Goncalves, M.D.; Green-McKenzie, J.; Alavi, A.; Torigian, D.A. Regional Variation in Skeletal Muscle and Adipose Tissue FDG Uptake Using PET/CT and Their Relation to BMI. Acad. Radiol. 2017, 24, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Looijaard, W.G.; Dekker, I.M.; Stapel, S.N.; Girbes, A.R.; Twisk, J.W.; Oudemans-van Straaten, H.M.; Weijs, P.J. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit. Care 2016, 20, 386. [Google Scholar] [CrossRef] [PubMed]
- Engelke, K.; Museyko, O.; Wang, L.; Laredo, J.D. Quantitative analysis of skeletal muscle by computed tomography imaging-State of the art. J. Orthop. Transl. 2018, 15, 91–103. [Google Scholar] [CrossRef]
- Zwezerijnen, G.J.C.; Eertink, J.J.; Ferrández, M.C.; Wiegers, S.E.; Burggraaff, C.N.; Lugtenburg, P.J.; Heymans, M.W.; de Vet, H.C.W.; Zijlstra, J.M.; Boellaard, R. Reproducibility of [18F]FDG PET/CT liver SUV as reference or normalisation factor. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 486–493. [Google Scholar] [CrossRef]
- Kimoto, A.; Ohnuma, T.; Toda, A.; Takebayashi, Y.; Higashiyama, R.; Tagata, Y.; Ito, M.; Ota, T.; Shibata, N.; Arai, H. Medium-chain triglycerides given in the early stage of mild-to-moderate Alzheimer’s disease enhance memory function. Psychogeriatrics 2017, 17, 520–521. [Google Scholar] [CrossRef]
- Yakupova, E.I.; Bocharnikov, A.D.; Plotnikov, E.Y. Effects of Ketogenic Diet on Muscle Metabolism in Health and Disease. Nutrients 2022, 14, 3842. [Google Scholar] [CrossRef]
- Moya-Garzon, M.D.; Wang, M.; Li, V.L.; Lyu, X.; Wei, W.; Tung, A.S.; Raun, S.H.; Zhao, M.; Coassolo, L.; Islam, H.; et al. A β-hydroxybutyrate shunt pathway generates anti-obesity ketone metabolites. Cell 2025, 188, 175–186.e120. [Google Scholar] [CrossRef]
- Clarke, K.; Tchabanenko, K.; Pawlosky, R.; Carter, E.; Todd King, M.; Musa-Veloso, K.; Ho, M.; Roberts, A.; Robertson, J.; Vanitallie, T.B.; et al. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul. Toxicol. Pharmacol. 2012, 63, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Courchesne-Loyer, A.; St-Pierre, V.; Vandenberghe, C.; Pierotti, T.; Fortier, M.; Croteau, E.; Castellano, C.A. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2016, 1367, 12–20. [Google Scholar] [CrossRef]
- Courchesne-Loyer, A.; Croteau, E.; Castellano, C.A.; St-Pierre, V.; Hennebelle, M.; Cunnane, S.C. Inverse relationship between brain glucose and ketone metabolism in adults during short-term moderate dietary ketosis: A dual tracer quantitative positron emission tomography study. J. Cereb. Blood Flow Metab. 2017, 37, 2485–2493. [Google Scholar] [CrossRef] [PubMed]
- De Marco, D.; Mamane, S.; Choo, W.; Mullie, L.; Xue, X.; Afilalo, M.; Afilalo, J. Muscle Area and Density Assessed by Abdominal Computed Tomography in Healthy Adults: Effect of Normal Aging and Derivation of Reference Values. J. Nutr. Health Aging 2022, 26, 243–246. [Google Scholar] [CrossRef]
- Amini, B.; Boyle, S.P.; Boutin, R.D.; Lenchik, L. Approaches to Assessment of Muscle Mass and Myosteatosis on Computed Tomography: A Systematic Review. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1671–1678. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, D.W.; Ko, Y.; Ha, J.; Shin, Y.B.; Lee, J.; Sung, Y.S.; Kim, K.W. Updated systematic review and meta-analysis on diagnostic issues and the prognostic impact of myosteatosis: A new paradigm beyond sarcopenia. Ageing Res. Rev. 2021, 70, 101398. [Google Scholar] [CrossRef]
- Song, G.; Zhou, J.; Wang, K.; Yao, D.; Chen, S.; Shi, Y. Segmentation of multi-regional skeletal muscle in abdominal CT image for cirrhotic sarcopenia diagnosis. Front. Neurosci. 2023, 17, 1203823. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.; Sayer, A.A. Sarcopenia and frailty: New challenges for clinical practice. Clin. Med. 2016, 16, 455–458. [Google Scholar] [CrossRef]
- Giannos, P.; Prokopidis, K.; Lidoriki, I.; Triantafyllidis, K.K.; Kechagias, K.S.; Celoch, K.; Candow, D.G.; Ostojic, S.M.; Forbes, S.C. Medium-chain triglycerides may improve memory in non-demented older adults: A systematic review of randomized controlled trials. BMC Geriatr. 2022, 22, 817. [Google Scholar] [CrossRef]
- Kjaerulff, M.L.G.; Nielsen, E.N.; Gormsen, L.C.; Dias, A.H. First-in-human whole-body PET/CT imaging with the optimized ketone tracer [(11)C]β-hydroxybutyrate using a long axial field-of-view scanner. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 3802–3804. [Google Scholar] [CrossRef]

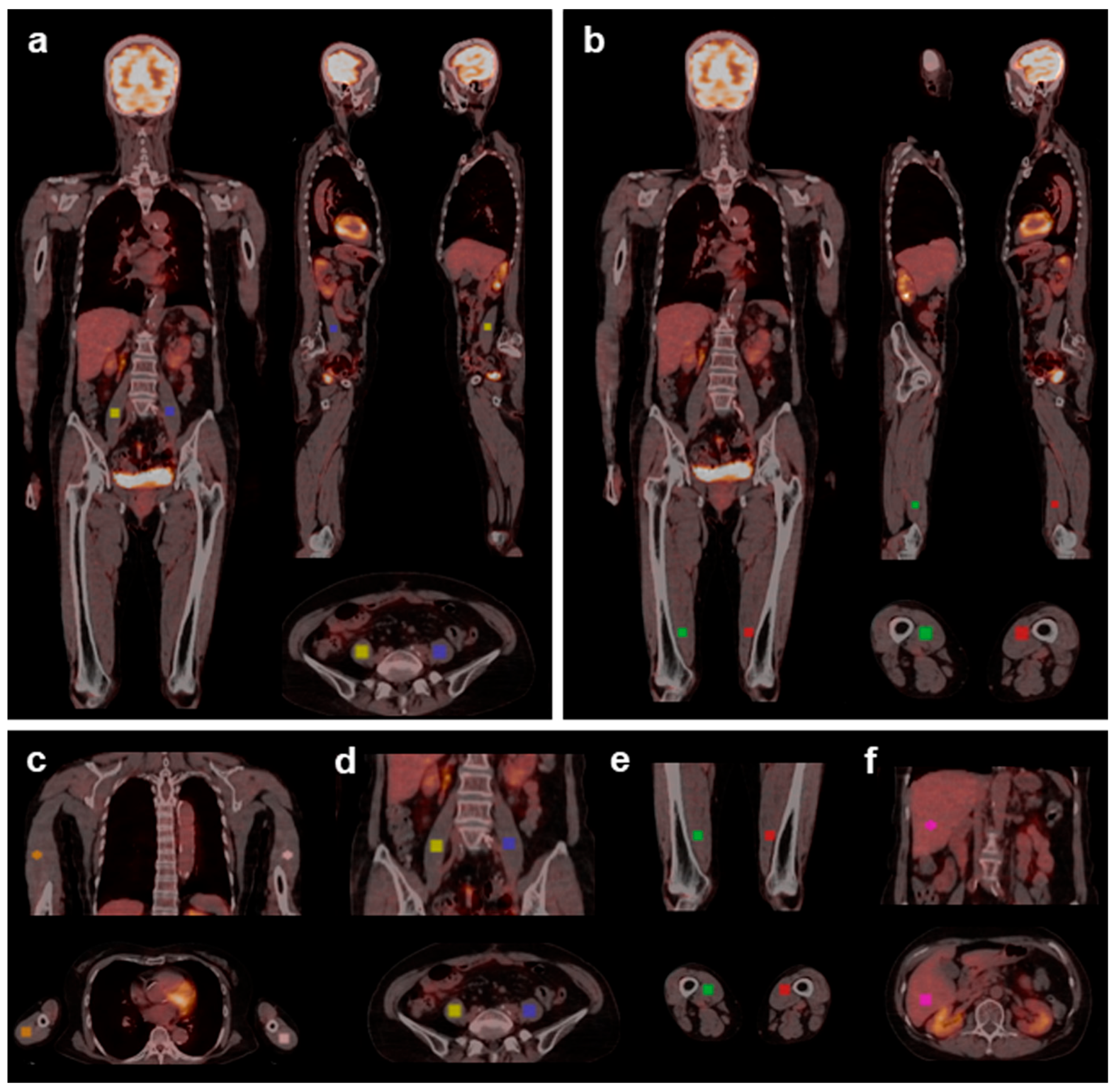
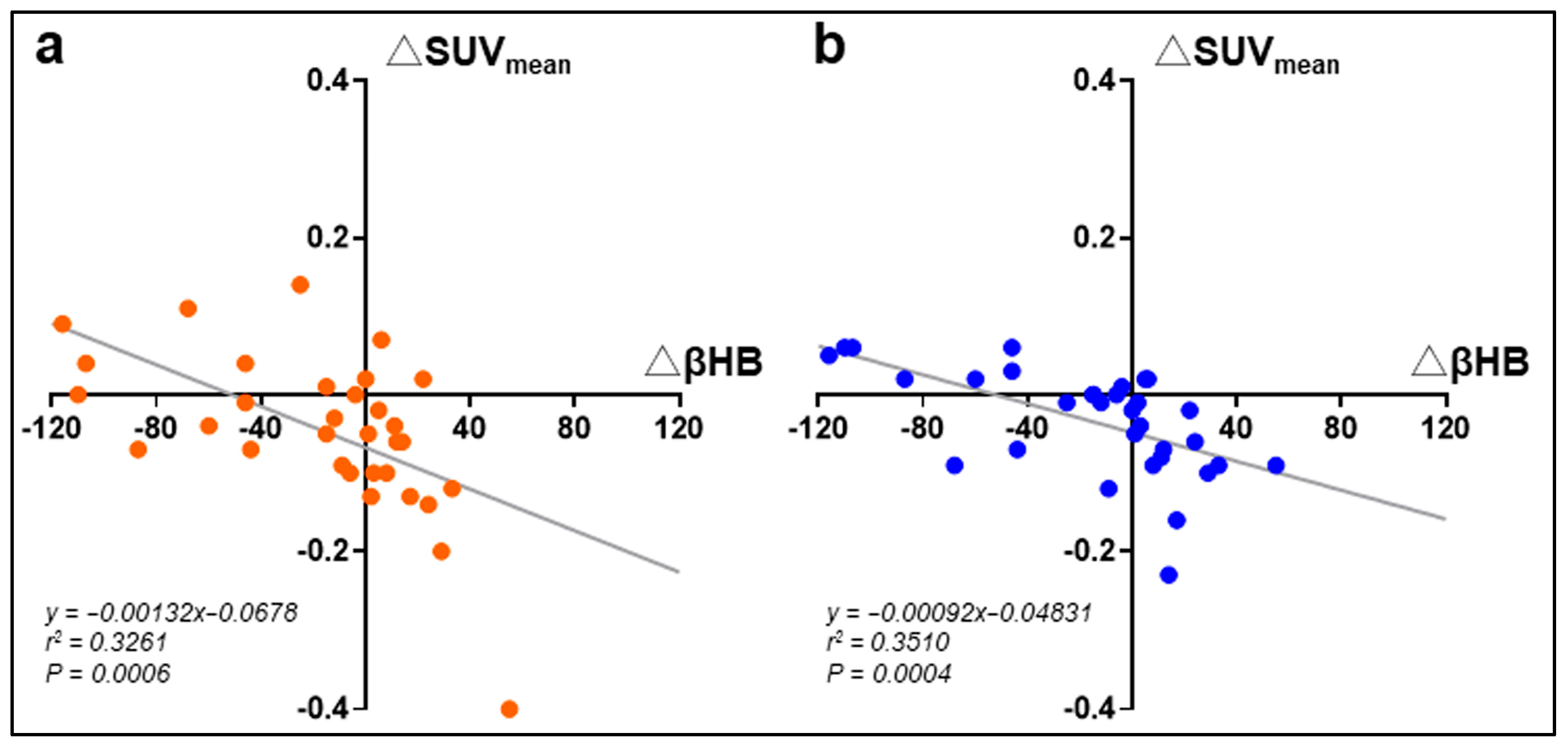
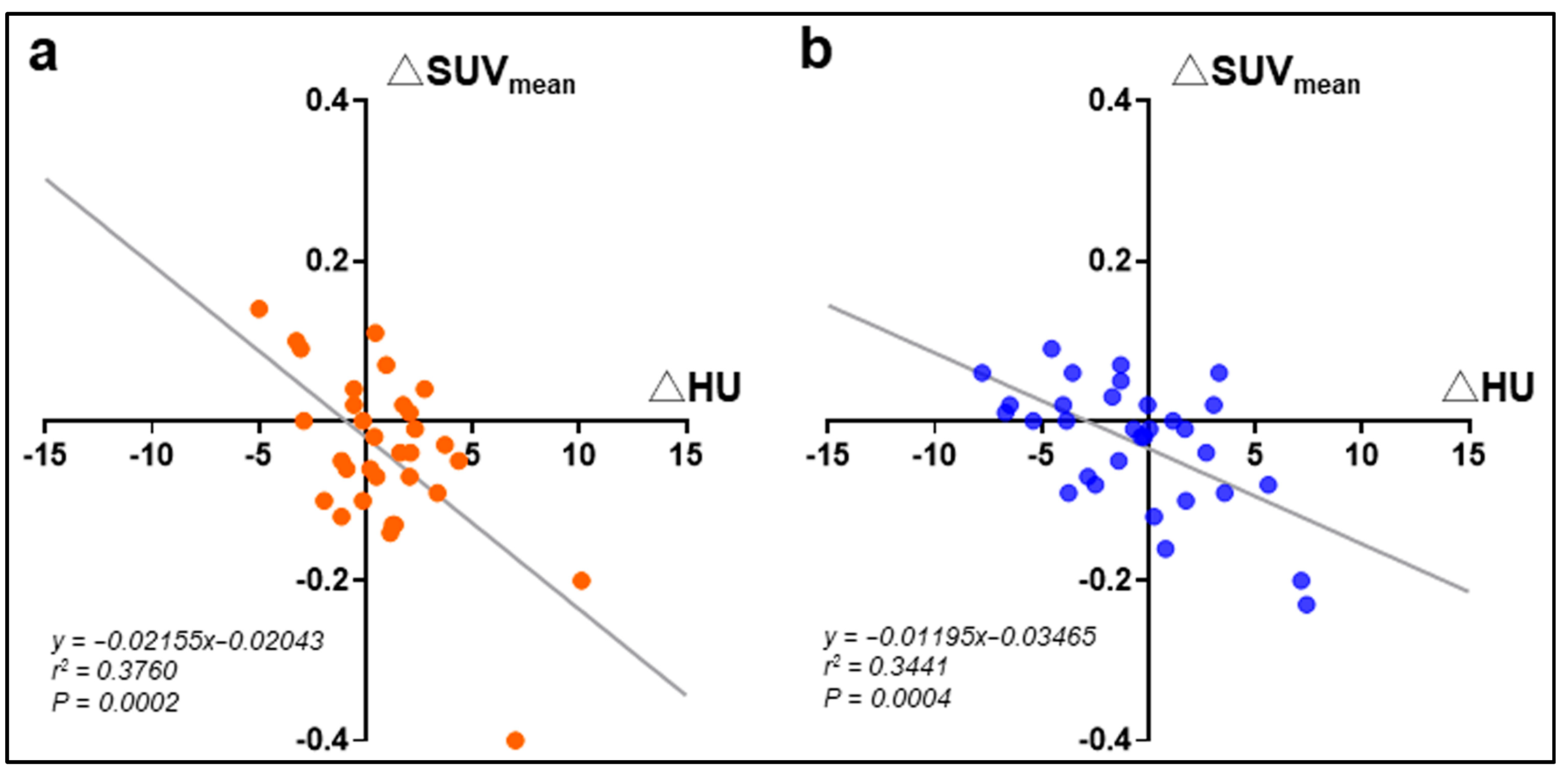
| Placebo | Medium-Chain Triglycerides | Intergroup p Value | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | p | Pre | Post | p | ||
| Total ketone bodies (μmol/L) | 155 ± 96 | 112 ± 78 | 0.01 | 110 ± 87 | 90 ± 62 | 0.17 | 0.09 |
| β-hydroxybutyrate (μmol/L) | 105 ± 65 | 74 ± 53 | 0.03 | 74 ± 61 | 58 ± 46 | 0.46 | 0.05 |
| Acetoacetate (μmol/L) | 50 ± 33 | 38 ± 26 | 0.006 | 36 ± 28 | 32 ± 17 | 0.10 | 0.08 |
| Glucose (mmol/L) | 5.3 ± 0.3 | 5.3 ± 0.3 | 0.57 | 5.4 ± 0.5 | 5.5 ± 0.4 | 0.06 | 0.06 |
| Placebo | Medium-Chain Triglycerides | |||||
|---|---|---|---|---|---|---|
| r | 95%CI | p | r | 95%CI | p | |
| Triceps | −0.102 | −0.446 0.268 | 0.59 | −0.341 | −0.617 0.008 | 0.056 |
| Psoas | −0.029 | −0.385 0.335 | 0.88 | −0.571 | −0.767 −0.278 | 0.0006 |
| Vastus medialis | 0.026 | −0.337 0.383 | 0.89 | −0.593 | −0.780 −0.307 | 0.0004 |
| Mean Standardized Uptake Value | Intergroup p Value | Hounsfield Units | Intergroup p Value | |||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | p | Pre | Post | p | |||
| Triceps | ||||||||
| Placebo | 0.64 ± 0.11 | 0.62 ± 0.08 | 0.07 | 0.98 | 60.5 ± 7.3 | 59.6 ± 7.9 | 0.36 | 0.06 |
| MCT | 0.63 ± 0.10 | 0.63 ± 0.09 | 0.80 | 63.4 ± 6.4 | 63.1 ± 7.8 | 0.73 | ||
| Psoas | ||||||||
| Placebo | 0.60 ± 0.12 | 0.59 ± 0.10 | 0.51 | 0.43 | 54.2 ± 4.1 | 53.1 ± 2.9 | 0.30 | <0.001 |
| MCT | 0.63 ± 0.08 | 0.59 ± 0.10 | 0.002 | 49.8 ± 3.8 | 53.0 ± 3.9 | 0.002 | ||
| Vastus medialis | ||||||||
| Placebo | 0.60 ± 0.10 | 0.61 ± 0.10 | 0.67 | 0.69 | 46.5 ± 6.0 | 44.3 ± 5.1 | 0.15 | 0.11 |
| MCT | 0.63 ± 0.09 | 0.60 ± 0.08 | 0.03 | 42.4 ± 6.5 | 45.4 ± 4.8 | 0.04 | ||
| Liver | ||||||||
| Placebo | 2.28 ± 0.31 | 2.29 ± 0.37 | 0.64 | 0.97 | 62.6 ± 6.4 | 64.3 ± 6.8 | 0.05 | 0.95 |
| MCT | 2.23 ± 0.39 | 2.35 ± 0.34 | 0.003 | 63.3 ± 5.9 | 63.4 ± 7.0 | 0.89 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutoh, T.; Kataoka, H.; Tatewaki, Y.; Taki, Y. Medium-Chain Triglyceride Dietary Supplements Reduce Glucose Metabolism of Gait-Related Skeletal Muscle in Older Adults: A Longitudinal 18F-FDG PET/CT Analysis. Nutrients 2025, 17, 1707. https://doi.org/10.3390/nu17101707
Mutoh T, Kataoka H, Tatewaki Y, Taki Y. Medium-Chain Triglyceride Dietary Supplements Reduce Glucose Metabolism of Gait-Related Skeletal Muscle in Older Adults: A Longitudinal 18F-FDG PET/CT Analysis. Nutrients. 2025; 17(10):1707. https://doi.org/10.3390/nu17101707
Chicago/Turabian StyleMutoh, Tatsushi, Hiroki Kataoka, Yasuko Tatewaki, and Yasuyuki Taki. 2025. "Medium-Chain Triglyceride Dietary Supplements Reduce Glucose Metabolism of Gait-Related Skeletal Muscle in Older Adults: A Longitudinal 18F-FDG PET/CT Analysis" Nutrients 17, no. 10: 1707. https://doi.org/10.3390/nu17101707
APA StyleMutoh, T., Kataoka, H., Tatewaki, Y., & Taki, Y. (2025). Medium-Chain Triglyceride Dietary Supplements Reduce Glucose Metabolism of Gait-Related Skeletal Muscle in Older Adults: A Longitudinal 18F-FDG PET/CT Analysis. Nutrients, 17(10), 1707. https://doi.org/10.3390/nu17101707





