Gender-Specific Dietary and Lifestyle Patterns Associated with Cardiometabolic Risk: A Cross-Sectional Analysis
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Investigation
2.3. Body Composition
2.4. Dietary Pattern Scores and Calculation of ABSI
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.-C.; Zhou, Z.; Wu, T.; Zhong, K.; Hu, H.; Zhang, H.; Sun, R.; Liu, W. Association between composite lifestyle factors and cardiometabolic multimorbidity in Chongqing, China: A cross-sectional exploratory study in people over 45 years and older. Front. Public Health 2023, 11, 1118628. [Google Scholar] [CrossRef] [PubMed]
- Venditti, E.; Emery, R.L.; Kolko, R. Biobehavioral Factors Related to the Development and Course of Type 2 Diabetes and Cardiometabolic Impairment in Adults: The Critical Role of Weight, Diet, Physical Activity, and Other Lifestyle Behaviors. In Handbook of Life Course Health Development; Springer: Cham, Switzerland, 2020; pp. 279–301. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Appel, L.J.; Van Horn, L.V. Components of a Cardioprotective Diet: New Insights. Circulation 2011, 123, 2870–2891. [Google Scholar] [CrossRef]
- Gupta, C.; Vincent, G.; Coates, A.; Khalesi, S.; Irwin, C.; Dorrian, J.; Ferguson, S. A Time to Rest, a Time to Dine: Sleep, Time-Restricted Eating, and Cardiometabolic Health. Nutrients 2022, 14, 420. [Google Scholar] [CrossRef] [PubMed]
- Rozenbajgier, M.; Wójcik-Grudzień, J.; Pawłowska, P.; Ozga-Stachurska, A. Plant Based Diets and the Risk of Type 2 Diabetes. J. Educ. Health Sport 2022, 12, 9. [Google Scholar] [CrossRef]
- Said, T.; Khalid, A.; Takhar, K.; Srinivasan, S.; Kaelber, K.K.; Werner, J. An Update on the Effects of Plant-Based Diets on Cardiometabolic Factors in Adults with Type 2 Diabetes Mellitus. Curr. Cardiovasc. Risk Rep. 2022, 16, 25–30. [Google Scholar] [CrossRef]
- Austin, G.; Ferguson, J.; Eslick, S.; Oldmeadow, C.; Wood, L.; Garg, M. Cardiovascular Disease Risk in Individuals Following Plant-Based Dietary Patterns Compared to Regular Meat-Eaters. Nutrients 2024, 16, 1063. [Google Scholar] [CrossRef]
- Jia, M.; Li, M. Association of cardiometabolic index with sleep quality in adults: A population-based study. Sci. Rep. 2024, 14, 26019. [Google Scholar] [CrossRef]
- Lonnie, M.; Wądołowska, L. Empirically derived dietary-lifestyle patterns and cardiometabolic health in young men: A review. Proc. Nutr. Soc. 2020, 79, 324–330. [Google Scholar] [CrossRef]
- Barstad, L.H.; Juliusson, P.; Johnson, L.K.; Hertel, J.; Lekhal, S.; Hjelmesæth, J. Gender-related differences in cardiometabolic risk factors and lifestyle behaviors in treatment-seeking adolescents with severe obesity. BMC Pediatr. 2018, 18, 61. [Google Scholar] [CrossRef]
- Masella, R.; Malorni, W. Lifestyle, nutrition, and gender. Ital. J. Gend.-Specif. Med. 2020, 6, 152. [Google Scholar] [CrossRef]
- Storz, M.A.; Beckschulte, K.; Brommer, M.; Lombardo, M. Current Sex Distribution of Cooking and Food Shopping Responsibilities in the United States: A Cross-Sectional Study. Foods 2022, 11, 2840. [Google Scholar] [CrossRef]
- Saldarriaga-Giraldo, C.; Ramírez-Ramos, C.F.; López-Santi, R.; Lanas, F.; Martín, A.V.; Perales, J.L.S.; Juárez-Lloclla, J.P.; Ruise, M.; Arcela, J.P.C.; de Espinal, E.H.F.; et al. Gender-related Differences in the Impact of COVID-19 Pandemic in Cardiometabolic Patients in Latin America: The CorCOVID LATAM Gender Sub-study. Curr. Probl. Cardiol. 2021, 47, 101075. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.L.; Rubín-García, M.; Álvarez-Álvarez, L.; Toledo, E.; Corella, D.; Salas-Salvadó, J.; Pérez-Vega, K.A.; Martínez, J.A.; Alonso-Gómez, Á.M.; Wärnberg, J.; et al. Sex-specific dietary patterns and their association with metabolic syndrome: Insights from a cross-sectional analysis. Diabetes Metab. Syndr. 2024, 18, 103123. [Google Scholar] [CrossRef]
- Andrews, R.R.; Anderson, K.R.; Fry, J.L. Sex-Specific Variation in Metabolic Responses to Diet. Nutrients 2024, 16, 2921. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, N.Y.; Krakauer, J.C. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE 2012, 7, e39504. [Google Scholar] [CrossRef]
- Krakauer, N.Y.; Krakauer, J.C. Diet Composition, Anthropometrics, and Mortality Risk. Int. J. Environ. Res. Public Health 2022, 19, 12885. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E.; Stallone, D.; Juneja, M.; Bingham, S.; Marmot, M. Dietary assessment in Whitehall II: Comparison of 7 d diet diary and food-frequency questionnaire and validity against biomarkers. Br. J. Nutr. 2001, 86, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Callmer, E.; Riboli, E.; Saracci, R.; Akesson, B.; Lindgärde, F. Dietary assessment methods evaluated in the Malmö food study. J. Intern. Med. 1993, 233, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.A.; Oluwagbemigun, K.; Nöthlings, U. Advances in dietary pattern analysis in nutritional epidemiology. Eur. J. Nutr. 2021, 60, 4115–4130. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hooson, J.; Hutchinson, J.; Warthon-Medina, M.; Hancock, N.; Greathead, K.; Knowles, B.; Vargas-Garcia, E.; Gibson, L.E.; Bush, L.A.; Margetts, B.; et al. A systematic review of reviews identifying UK validated dietary assessment tools for inclusion on an interactive guided website for researchers: www.nutritools.org. Crit. Rev. Food Sci. Nutr. 2020, 60, 1265–1289. [Google Scholar] [CrossRef]
- Vasold, K.L.; Parks, A.C.; Phelan, D.M.L.; Pontifex, M.B.; Pivarnik, J.M. Reliability and Validity of Commercially Available Low-Cost Bioelectrical Impedance Analysis. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 406–410. [Google Scholar] [CrossRef]
- Berryman, C.E.; Agarwal, S.; Lieberman, H.R.; Fulgoni, V.L.; Pasiakos, S.M. Diets higher in animal and plant protein are associated with lower adiposity and do not impair kidney function in US adults. Am. J. Clin. Nutr. 2016, 104, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, F. Plant Protein, Animal Protein, and Cardiometabolic Health. In Vegetarian and Plant-Based Diets in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2017; pp. 643–665. [Google Scholar] [CrossRef]
- Veilleux, A.; Tchernof, A. Sex Differences in Body Fat Distribution. In Sex and Gender Factors Affecting Metabolic Homeostasis, Diabetes and Obesity; Springer: Cham, Switzerland, 2017; pp. 123–166. [Google Scholar] [CrossRef]
- Azizi, Z.; Gupte, T.; Kho, P.F.; Nzenkue, K.; Zhou, J.; Guarischi-Sousa, R.; Panyard, D.; Chen, M.-L.; Abbasi, F.; Clarke, S.L.; et al. Sex-Stratified Machine Learning Analysis of Proteomics, Phenotypic, and Genomic Influences on Visceral Adipose Tissue Volume in the UK Biobank. Circulation 2024, 149 (Suppl. S1), AP273. [Google Scholar] [CrossRef]
- Fontana, L.; Sieri, S.; Ricceri, F.; Agnoli, C.; Pala, V.; Masala, G.; Saieva, C.; Catalano, A.; Macciotta, A.; Tumino, R.; et al. Dietary intake of animal and plant proteins and risk of all-cause and cause-specific mortality: The EPIC-Italy cohort. Nutr. Healthy Aging 2022, 6, 257–268. [Google Scholar] [CrossRef]
- Gagnon, É.; Paulin, A.; Mitchell, P.L.; Arsenault, B.J. Disentangling the impact of gluteofemoral versus visceral fat accumulation on cardiometabolic health using sex-stratified Mendelian randomization. Atherosclerosis 2023, 386, 117371. [Google Scholar] [CrossRef]
- Namazi, N.; Anjom-Shoae, J.; Darbandi, M.; Rezaeian, S.; Pasdar, Y. Dietary intake of total, animal, and vegetable protein and cardiometabolic risk factors in patients with type 2 diabetes: Using iso-energetic substitution models. J. Diabetes Metab. Disord. 2025, 24, 60. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef]
- Kuryłowicz, A.; Cąkała-Jakimowicz, M.; Puzianowska-Kuźnicka, M. Targeting Abdominal Obesity and Its Complications with Dietary Phytoestrogens. Nutrients 2020, 12, 582. [Google Scholar] [CrossRef]
- Gavin, K.M.; Bessesen, D.H. Sex Differences in Adipose Tissue Function. Endocrinol. Metab. Clin. N. Am. 2020, 49, 215–228. [Google Scholar] [CrossRef]
- Anderson, W.D.; Guertin, M.J.; Civelek, M. Integrative analysis of sex differences in adipose tissue gene expression. FASEB J. 2018, 32, 803.7. [Google Scholar] [CrossRef]
- Vučić Lovrenčić, M.; Gerić, M.; Košuta, I.; Dragičević, M.; Garaj-Vrhovac, V.; Gajski, G. Sex-specific effects of vegetarian diet on adiponectin levels and insulin sensitivity in healthy non-obese individuals. Nutrition 2020, 79–80, 110862. [Google Scholar] [CrossRef]
- Lederer, A.-K.; Storz, M.A.; Huber, R.; Hannibal, L.; Neumann, E. Plasma Leptin and Adiponectin after a 4-Week Vegan Diet: A Randomized-Controlled Pilot Trial in Healthy Participants. Int. J. Environ. Res. Public Health 2022, 19, 11370. [Google Scholar] [CrossRef] [PubMed]
- Tsaban, G.; Yaskolka Meir, A.; Rinott, E.; Zelicha, H.; Kaplan, A.; Shalev, A.; Katz, A.; Rudich, A.; Tirosh, A.; Shelef, I.; et al. The effect of green Mediterranean diet on cardiometabolic risk: A randomised controlled trial. Heart 2021, 107, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Gepner, Y.; Shelef, I.; Komy, O.; Cohen, N.; Schwarzfuchs, D.; Bril, N.; Rein, M.; Serfaty, D.; Kenigsbuch, S.; Zelicha, H.; et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J. Hepatol. 2019, 71, 379–388. [Google Scholar] [CrossRef]
- Kiortsis, D.; Simos, Y. Mediterranean Diet for the Prevention and Treatment of Metabolic Syndrome. Angiology 2014, 65, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Koyama, T.; Kuriyama, N.; Ozaki, E.; Uehara, R. The Association of Daily Physical Activity Behaviors with Visceral Fat. Obes. Res. Clin. Pract. 2020, 14, 531–535. [Google Scholar] [CrossRef]
- Plinta, R.; Olszanecka-Glinianowicz, M.; Chudek, J.; Skrzypulec-Plinta, V. Sports training and circulating adipokine levels. Postep. Hig. Med. Dosw. 2013, 67, 35–42. [Google Scholar] [CrossRef]
- Mongraw-Chaffin, M.; Anderson, C.; Allison, M.; Ouyang, P.; Szklo, M.; Vaidya, D.; Woodward, M.; Golden, S. Association between sex hormones and adiposity: Qualitative differences in women and men in the Multi-Ethnic Study of Atherosclerosis. J. Clin. Endocrinol. Metab. 2015, 100, E596–E600. [Google Scholar] [CrossRef]
- Shaw, B.; Shaw, I.; Mamen, A. Contrasting effects in anthropometric measures of total fatness and abdominal fat mass following endurance and concurrent endurance and resistance training. J. Sports Med. Phys. Fit. 2010, 50, 207–213. [Google Scholar]
- Lehmann, S.; Retschlag, U.; Oberbach, A.; Morgenroth, R.; Linder, N.; Schaudinn, A.; Garnov, N.; Busse, H.; Solty, K.; Prettin, C.; et al. Visceral fat mass dynamics in a 2-year randomized strength versus endurance training trial in people with obesity. Diabetes Obes. Metab. 2024, 26, 4087–4099. [Google Scholar] [CrossRef]
- Green, J.; Stanforth, P.; Rankinen, T.; Leon, A.; Rao, D.C.; Skinner, J.; Bouchard, C.; Wilmore, J. The effects of exercise training on abdominal visceral fat, body composition, and indicators of the metabolic syndrome in postmenopausal women with and without estrogen replacement therapy: The HERITAGE family study. Metabolism 2004, 53, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Cirella, S.M., Jr.; Hartman, M.E.; Webster, K.; Harlow, L.L. Examining Gender Norms of Eating Behavior and Body Checking in NCAA Student-Athletes. J. Athl. Train. 2025. [Google Scholar] [CrossRef] [PubMed]
- Bracht, J.; Vieira-Potter, V.; De Souza Santos, R.; Öz, O.K.; Palmer, B.; Clegg, D. The role of estrogens in the adipose tissue milieu. Ann. N. Y. Acad. Sci. 2019, 1461, 127–143. [Google Scholar] [CrossRef]
- Lizcano, F.; Guzmán, G. Estrogen deficiency and the origin of obesity during menopause. Biomed Res. Int. 2014, 2014, 757461. [Google Scholar] [CrossRef]
- Steiner, B.M.; Berry, D.C. The Regulation of Adipose Tissue Health by Estrogens. Front. Endocrinol. 2022, 13, 889923. [Google Scholar] [CrossRef] [PubMed]
- Blouin, K.; Boivin, A.; Tchernof, A. Androgens and body fat distribution. J. Steroid Biochem. Mol. Biol. 2008, 108, 272–280. [Google Scholar] [CrossRef]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- Tao, Z.; Zheng, L.D.; Smith, C.; Luo, J.; Robinson, A.; Almeida, F.A.; Wang, Z.; Olumi, A.; Liu, D.; Cheng, Z. Estradiol signaling mediates gender difference in visceral adiposity via autophagy. Cell Death Dis. 2018, 9, 309. [Google Scholar] [CrossRef]
- Jovanovic, N.; Zach, V.; Crocini, C.; Bahr, L.S.; Forslund-Startceva, S.K.; Franz, K. A gender perspective on diet, microbiome, and sex hormone interplay in cardiovascular disease. Acta Physiol. 2024, 240, e14228. [Google Scholar] [CrossRef]
- Vella, S.; Cliff, D. Organised sports participation and adiposity among a cohort of adolescents over a two year period. PLoS ONE 2018, 13, e0206500. [Google Scholar] [CrossRef]
- Clark, E.; Drignei, D.; Brown, E.C. Sex Differences in the Association Between Muscular Strength and Adiposity in Healthy Adults. J. Health Sports Kinesiol. 2021, 2, 29. [Google Scholar] [CrossRef]
- Joyner, M.; Hunter, S.K.; Senefeld, J.W. Evidence on sex differences in sports performance. J. Appl. Physiol. 2024, 138, 274–281. [Google Scholar] [CrossRef] [PubMed]




| Variable | Total | Male Mean (SD) | Female Mean (SD) | p-Value |
|---|---|---|---|---|
| n | 1631 | 690 | 941 | |
| Age (y) | 41.5 (13.5) | 40.42 (13.37) | 42.30 (13.55) | 0.0054 |
| Weight (kg) | 80.31 (17.76) | 89.85 (17.29) | 73.31 (14.55) | <0.001 |
| Height (m) | 1.68 (0.09) | 1.76 (0.07) | 1.63 (0.06) | <0.001 |
| BMI (kg/m2) | 28.24 (5.23) | 28.93 (5.11) | 27.73 (5.26) | <0.001 |
| ABSI | 0.08 (0.00) | 0.08 (0.00) | 0.08 (0.01) | 0.0007 |
| zABSI | −0.01 (1.0) | 0.09 (0.86) | −0.08 (1.08) | 0.0007 |
| fat mass (kg) | 25.25 (10.72) | 23.48 (10.85) | 26.55 (10.44) | <0.001 |
| fat mass (%) | 30.79 (9.05) | 25.03 (7.54) | 35.01 (7.62) | <0.001 |
| FFM (kg) | 52.36 (11.4) | 63.11 (8.27) | 44.48 (5.29) | <0.001 |
| FFM (%) | 65.82 (8.67) | 71.28 (7.26) | 61.81 (7.32) | <0.001 |
| BMR | 1660.24 (348.12) | 1966.33 (277.96) | 1434.20 (182.57) | <0.001 |
| Plant-Based Protein Score | 1.93 (1.96) | 2.00 (2.14) | 1.87 (1.81) | 0.2198 |
| Mediterranean Model Score | 2.07 (1.01) | 2.02 (1.00) | 2.11 (1.01) | 0.0724 |
| Interaction Term | Beta (β) | 95% CI Lower | 95% CI Upper | p-Value |
|---|---|---|---|---|
| Sex × Physical Activity | −0.013 | −0.173 | 0.147 | 0.8728 |
| Sex × Plant-Based Protein Intake | 0.094 | −0.077 | 0.266 | 0.2790 |
| Sex × Smoker | −0.012 | −0.241 | 0.216 | 0.9158 |
| Sex × Disturbed Sleep | 0.048 | −0.133 | 0.229 | 0.6031 |
| Key Message | Details |
|---|---|
| zABSI is a useful marker of central adiposity | The standardised ABSI (zABSI) effectively captures abdominal fat distribution beyond BMI. |
| Sex differences in abdominal fat are evident | Women had significantly lower zABSI values than men, indicating less central adiposity. |
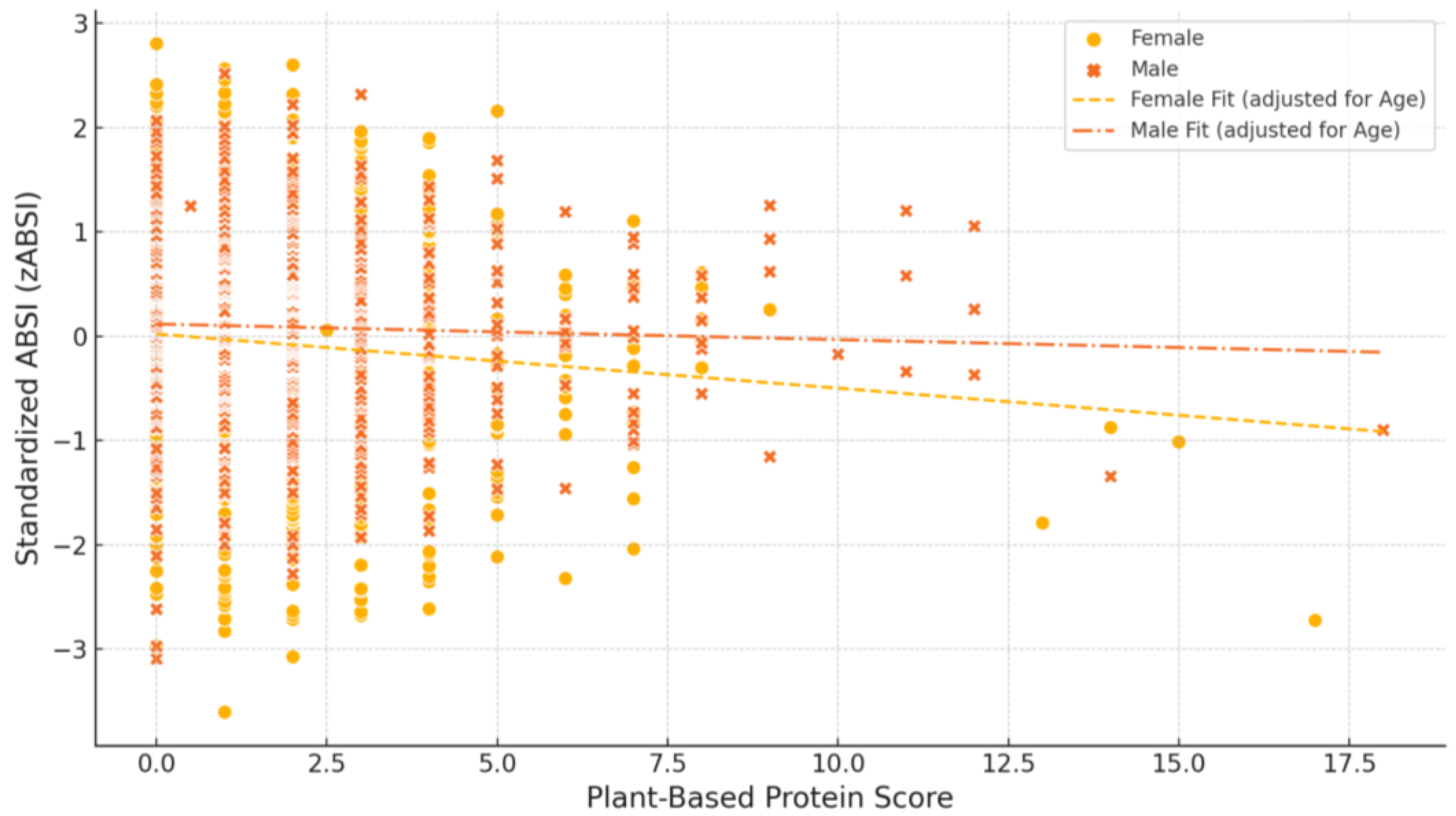
| Plant-based diets may benefit women more than men | Higher plant protein intake was associated with lower zABSI in women but not in men. |
| Physical activity may be linked to lower central adiposity | Active individuals, particularly women, had more favourable zABSI profiles. |
| Gender-tailored strategies may enhance cardiometabolic prevention | Findings support the design of sex-specific dietary and lifestyle interventions. |
| Limitations include use of non-validated tools for behavioural assessment | Lifestyle data were collected via structured but non-validated questionnaires, potentially affecting accuracy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, M.; Krakauer, J.C.; Krakauer, N.Y.; Caprio, M.; Armani, A.; Feraco, A. Gender-Specific Dietary and Lifestyle Patterns Associated with Cardiometabolic Risk: A Cross-Sectional Analysis. Nutrients 2025, 17, 1705. https://doi.org/10.3390/nu17101705
Lombardo M, Krakauer JC, Krakauer NY, Caprio M, Armani A, Feraco A. Gender-Specific Dietary and Lifestyle Patterns Associated with Cardiometabolic Risk: A Cross-Sectional Analysis. Nutrients. 2025; 17(10):1705. https://doi.org/10.3390/nu17101705
Chicago/Turabian StyleLombardo, Mauro, Jesse C. Krakauer, Nir Y. Krakauer, Massimiliano Caprio, Andrea Armani, and Alessandra Feraco. 2025. "Gender-Specific Dietary and Lifestyle Patterns Associated with Cardiometabolic Risk: A Cross-Sectional Analysis" Nutrients 17, no. 10: 1705. https://doi.org/10.3390/nu17101705
APA StyleLombardo, M., Krakauer, J. C., Krakauer, N. Y., Caprio, M., Armani, A., & Feraco, A. (2025). Gender-Specific Dietary and Lifestyle Patterns Associated with Cardiometabolic Risk: A Cross-Sectional Analysis. Nutrients, 17(10), 1705. https://doi.org/10.3390/nu17101705











