Low Vitamin D Status Attenuates Hypolipidemic and Pleiotropic Effects of Atorvastatin in Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Laboratory Assays
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blais, J.E.; Wei, Y.; Yap, K.K.; Alwafi, H.; Ma, T.T.; Brauer, R.; Lau, W.C.; Man, K.K.; Siu, C.W.; Tan, K.C.; et al. Trends in lipid-modifying agent use in 83 countries. Atherosclerosis 2021, 328, 44–51. [Google Scholar] [CrossRef]
- Barter, P.J.; Rye, K.A. New era of lipid-lowering drugs. Pharmacol. Rev. 2016, 68, 458–475. [Google Scholar] [CrossRef]
- Goldsborough, E., 3rd; Osuji, N.; Blaha, M.J. Assessment of cardiovascular disease risk: A 2022 update. Endocrinol. Metab. Clin. 2022, 51, 483–509. [Google Scholar] [CrossRef]
- Yu, D.; Liao, J.K. Emerging views of statin pleiotropy and cholesterol lowering. Cardiovasc. Res. 2022, 118, 413–423. [Google Scholar] [CrossRef]
- Satny, M.; Hubacek, J.A.; Vrablik, M. Statins and inflammation. Curr. Atheroscler. Rep. 2021, 23, 80. [Google Scholar] [CrossRef]
- Davignon, J.; Jacob, R.F.; Mason, R.P. The antioxidant effects of statins. Coron. Artery Dis. 2004, 15, 251–258. [Google Scholar] [CrossRef]
- Blum, A.; Shamburek, R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis 2009, 203, 325–330. [Google Scholar] [CrossRef]
- Siniscalchi, C.; Basaglia, M.; Riva, M.; Meschi, M.; Meschi, T.; Castaldo, G.; Di Micco, P. Statins effects on blood clotting: A review. Cells 2023, 12, 2719. [Google Scholar] [CrossRef]
- Ng, D.S. The role of statins in oxidative stress and cardiovascular disease. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005, 5, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, A.; Indolfi, C.; Sorrentino, S.; Esposito, G.; Spaccarotella, C.A. The Effects of statins, ezetimibe, PCSK9-Inhibitors, inclisiran, and icosapent ethyl on platelet function. Int. J. Mol. Sci. 2023, 24, 11739. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M., Jr.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef] [PubMed]
- Heart Protection Study Collaborative Group. Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: A randomised controlled trial. Lancet 2011, 378, 2013–2020. [Google Scholar] [CrossRef]
- Schwartz, G.G.; Olsson, A.G.; Ezekowitz, M.D.; Ganz, P.; Oliver, M.F.; Waters, D.; Zeiher, A.; Chaitman, B.R.; Leslie, S.; Stern, T.; et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: The MIRACL study: A randomized controlled trial. JAMA 2001, 285, 1711. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar]
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; de Craen, A.J.; Seshasai, S.R.; McMurray, J.J.; Freeman, D.J.; Jukema, J.W.; et al. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010, 375, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Schooling, C.M. Statins, type 2 diabetes, and body mass index: A univariable and multivariable mendelian randomization study. J. Clin. Endocrinol. Metab. 2023, 108, 385–396. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Verheyen, N.; Grübler, M.R.; Tomaschitz, A.; März, W. Vitamin D and cardiovascular disease prevention. Nat. Rev. Cardiol. 2016, 13, 404–417. [Google Scholar] [CrossRef]
- Podzolkov, V.I.; Pokrovskaya, A.E.; Panasenko, O.I. Vitamin D deficiency and cardiovascular pathology. Ter. Arkh. 2018, 90, 144–150. [Google Scholar] [CrossRef]
- Daraghmeh, A.H.; Bertoia, M.L.; Al-Qadi, M.O.; Abdulbaki, A.M.; Roberts, M.B.; Eaton, C.B. Evidence for the vitamin D hypothesis: The NHANES III extended mortality follow-up. Atherosclerosis 2016, 25, 96–101. [Google Scholar] [CrossRef]
- Milazzo, V.; De Metrio, M.; Cosentino, N.; Marenzi, G.; Tremoli, E. Vitamin D and acute myocardial infarction. World J. Cardiol. 2017, 9, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Perrelli, A.; Ragni, A.; Retta, F.; De Silva, T.M.; Sobey, C.G.; Retta, S.F. Vitamin D deficiency and the risk of cerebrovascular disease. Antioxidants 2020, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Melamed, M.L.; Muntner, P.; Michos, E.D.; Uribarri, J.; Weber, C.; Sharma, J.; Raggi, P. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease results from NHANES 2001 to 2004. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1179–1185. [Google Scholar] [CrossRef]
- Marquina, C.; Mousa, A.; Scragg, R.; de Courten, B. Vitamin D and cardiometabolic disorders: A review of current evidence, genetic determinants and pathomechanisms. Obes. Rev. 2019, 20, 262–277. [Google Scholar] [CrossRef]
- Song, Y.; Wang, L.; Pittas, A.G.; Del Gobbo, L.C.; Zhang, C.; Manson, J.E.; Hu, F.B. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: A meta-analysis of prospective studies. Diabetes Care 2013, 36, 1422–1428. [Google Scholar] [CrossRef]
- Brewer, L.C.; Michos, E.D.; Reis, J.P. Vitamin D in atherosclerosis, vascular disease, and endothelial function. Curr. Drug Targets 2011, 12, 54–60. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; García-Recio, E.; De Luna-Bertos, E.; Ruiz, C.; Illescas-Montes, R. Role of vitamin D in the metabolic syndrome. Nutrients 2021, 13, 830. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Zheng, Y.; Wang, P.; Zhang, Y. The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: A systematic review and meta-analysis. Nutrients 2018, 10, 375. [Google Scholar] [CrossRef]
- Thompson, B.; Waterhouse, M.; English, D.R.; McLeod, D.S.; Armstrong, B.K.; Baxter, C.; Duarte Romero, B.; Ebeling, P.R.; Hartel, G.; Kimlin, M.G.; et al. Vitamin D supplementation and major cardiovascular events: D-Health randomised controlled trial. BMJ 2023, 381, e075230. [Google Scholar] [CrossRef]
- Krysiak, R.; Basiak, M.; Machnik, G.; Szkróbka, W.; Okopień, B. Vitamin D status determines cardiometabolic effects of cabergoline in women with elevated prolactin levels: A pilot study. Nutrients 2023, 15, 2303. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. The impact of vitamin D status on cardiometabolic effects of fenofibrate in women with atherogenic dyslipidemia. Clin. Exp. Pharmacol. Physiol. 2021, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Gilowska, M.; Okopień, B. Different cardiometabolic effects of atorvastatin in men with normal vitamin D status and vitamin D insufficiency. Clin. Cardiol. 2016, 39, 715–720. [Google Scholar]
- Clark, D., 3rd; Virani, S.S. 2018 cholesterol guidelines: Key topics in primary prevention. Curr. Atheroscler. Rep. 2019, 21, 17. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The impact of atorvastatin on cardiometabolic risk factors in brothers of women with polycystic ovary syndrome. Pharmacol. Rep. 2021, 73, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Guidance for Clinical Investigators, Sponsors, and IRBs Adverse Effect Reporting to IRBs—Improving Human Subject Protection; Food and Drug Administration: Washington, DC, USA, 2009.
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 7, 412–419. [Google Scholar] [CrossRef]
- Anderson, T.S.; Wilson, L.M.; Sussman, J.B. Atherosclerotic cardiovascular disease risk estimates using the predicting risk of cardiovascular disease events equations. JAMA Intern. Med. 2024, 184, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Sethwala, A.M.; Gohmm, I.; Amerena, J.V. Combating inflammation in cardiovascular disease. Heart Lung Circ. 2021, 30, 197–206. [Google Scholar] [CrossRef]
- Guieu, R.; Ruf, J.; Mottola, G. Hyperhomocysteinemia and cardiovascular diseases. Ann. Biol. Clin. 2022, 80, 7–14. [Google Scholar] [CrossRef]
- Rossing, P.; Epstein, M. Microalbuminuria constitutes a clinical action item for clinicians in 2021. Am. J. Med. 2022, 135, 576–580. [Google Scholar] [CrossRef]
- Santos, R.D.; Waters, D.D.; Tarasenko, L.; Messig, M.; Jukema, J.W.; Ferrières, J.; Verdejo, J.; Chiang, C.W. L-TAP 2 Investigators. Low- and high-density lipoprotein cholesterol goal attainment in dyslipidemic women: The Lipid Treatment Assessment Project (L-TAP) 2. Am. Heart J. 2009, 158, 860–866. [Google Scholar] [CrossRef]
- Victor, B.M.; Teal, V.; Ahedor, L.; Karalis, D.G. Gender differences in achieving optimal lipid goals in patients with coronary artery disease. Am. J. Cardiol. 2014, 113, 1611–1615. [Google Scholar] [CrossRef]
- Ashraf, H.; Maghbouli, N.; Abolhasani, M.; Zandi, N.; Nematizadeh, M.; Omidi, N.; Davoodi, G.; Boroumand, M.A.; Ali, J.H. Serum vitamin D concentration and anthropometric indicators of adiposity in adults without or with low dose statin users: A cross-sectional study. J. Health Popul. Nutr. 2024, 43, 206. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Pergolini, P.; Rolla, R.; Nardin, M.; Schaffer, A.; Barbieri, L.; Daffara, V.; Marino, P.; Bellomo, G.; Suryapranata, H.; et al. Impact of high-dose statins on vitamin D levels and platelet function in patients with coronary artery disease. Thromb. Res. 2017, 150, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Sygitowicz, G.; Sitkiewicz, D.; Wrzosek, K.; Dłuźniewski, M. The impact of atorvastatin treatment on the distribution of low-density lipoprotein subfractions and the level of vitamin D in patients after acute myocardial infarction: Preliminary findings. Int. J. Mol. Sci. 2024, 25, 11264. [Google Scholar] [CrossRef]
- Anagnostis, P.; Adamidou, F.; Slavakis, A.; Polyzos, S.A.; Selalmatzidou, D.; Panagiotou, A.; Athyros, V.G.; Karagiannis, A.; Kita, M. Comparative effect of atorvastatin and rosuvastatin on 25-hydroxy-vitamin D levels in non-diabetic patients with dyslipidaemia: A prospective randomized open-label pilot study. Open Cardiovasc. Med. J. 2014, 8, 55–60. [Google Scholar] [CrossRef]
- Chihaoui, M.; Terzi, A.; Hammami, B.; Oueslati, I.; Khessairi, N.; Chaker, F.; Yazidi, M.; Feki, M. Effects of high-intensity statin therapy on steroid hormones and vitamin D in type 2 diabetic men: A prospective self-controlled study. Lipids 2024, 59, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Rezaie, P.; Vatanparast, H.; Kengne, A.P. Effect of statins on serum vitamin D concentrations: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2017, 47, 93–101. [Google Scholar] [CrossRef]
- Delrue, C.; Speeckaert, M.M. Vitamin D and vitamin D-binding protein in health and disease. Int. J. Mol. Sci. 2023, 24, 4642. [Google Scholar] [CrossRef]
- Hirota, T.; Fujita, Y.; Ieiri, I. An updated review of pharmacokinetic drug interactions and pharmacogenetics of statins. Expert Opin. Drug Metab. Toxicol. 2020, 16, 809–822. [Google Scholar] [CrossRef]
- Pérez-Castrillón, J.L.; Abad Manteca, L.; Vega, G.; Del Pino Montes, J.; de Luis, D.; Duenas Laita, A. Vitamin D levels and lipid response to atorvastatin. Int. J. Endocrinol. 2010, 2010, 320721. [Google Scholar] [CrossRef]
- Catalano, A.; Morabito, N.; Basile, G.; Cucinotta, D.; Lasco, A. Calcifediol improves lipid profile in osteopenic atorvastatin-treated postmenopausal women. Eur. J. Clin. Investig. 2015, 45, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Sexton, R.C.; Rudney, H. Effect of vitamin D3 derivatives on cholesterol synthesis and HMG-CoA reductase activity in cultured cells. J. Lipid Res. 1989, 30, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Krhač, M.; Lovrenčić, M.V. Update on biomarkers of glycemic control. World J. Diabetes 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Pang, C.; Chen, Y. Association between vitamin D and statin-related myopathy: A meta-analysis. Am. J. Cardiovasc. Drugs 2022, 22, 183–193. [Google Scholar] [CrossRef]
- Teo, C.B.; Tan, P.Y.; Tay, R.Y.; Khoo, J.; Watts, G.F.; Loh, W.J. Association Between Vitamin D Supplementation and statin-associated muscle symptoms: A systematic review. High Blood Press. Cardiovasc. Prev. 2022, 29, 337–351. [Google Scholar] [CrossRef]
- Peyrel, P.; Mauriège, P.; Frenette, J.; Laflamme, N.; Greffard, K.; Dufresne, S.S.; Huth, C.; Bergeron, J.; Joanisse, D.R. No benefit of vitamin D supplementation on muscle function and health-related quality of life in primary cardiovascular prevention patients with statin-associated muscle symptoms: A randomized controlled trial. J. Clin. Lipidol. 2024, 18, e269–e284. [Google Scholar] [CrossRef]

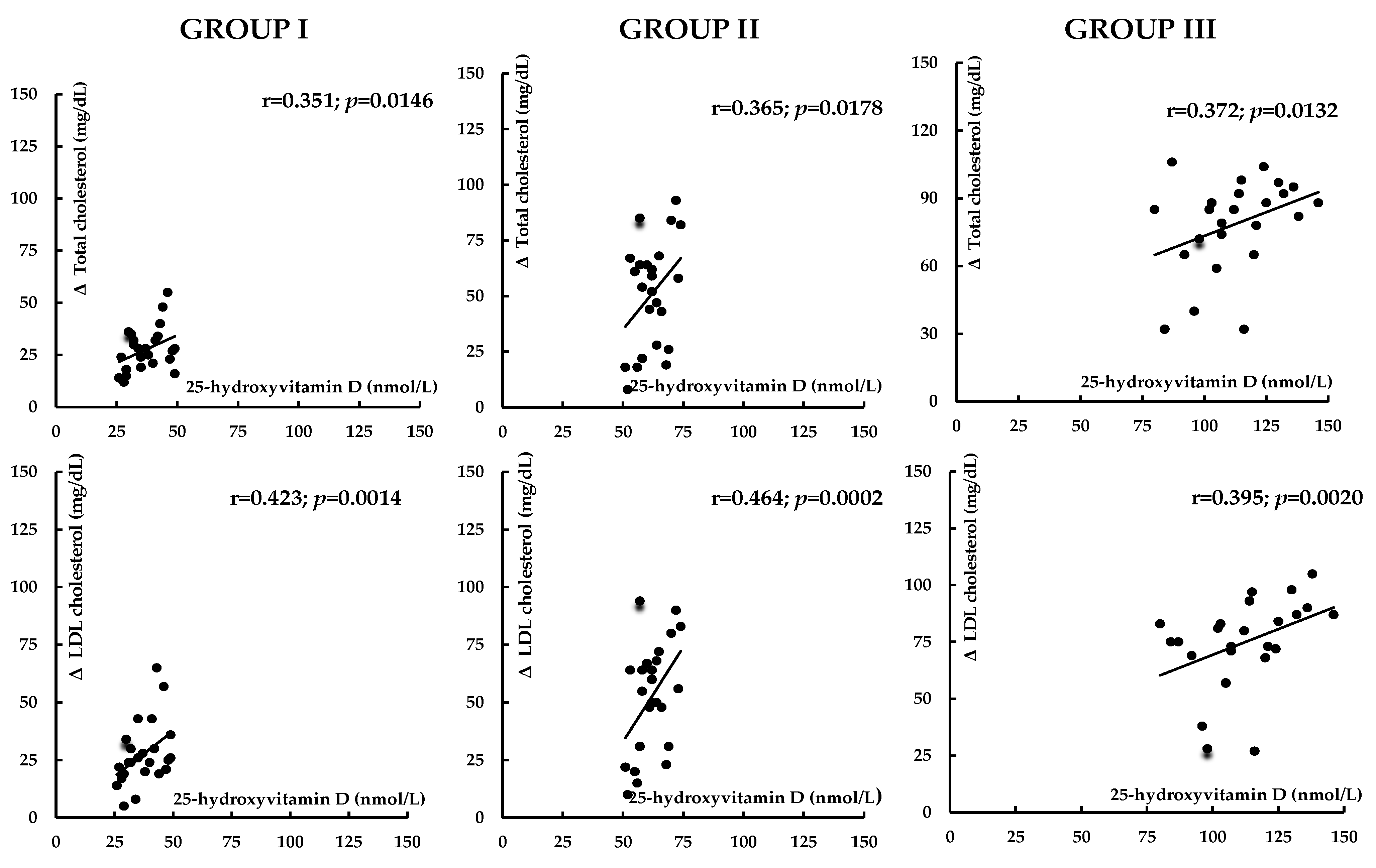
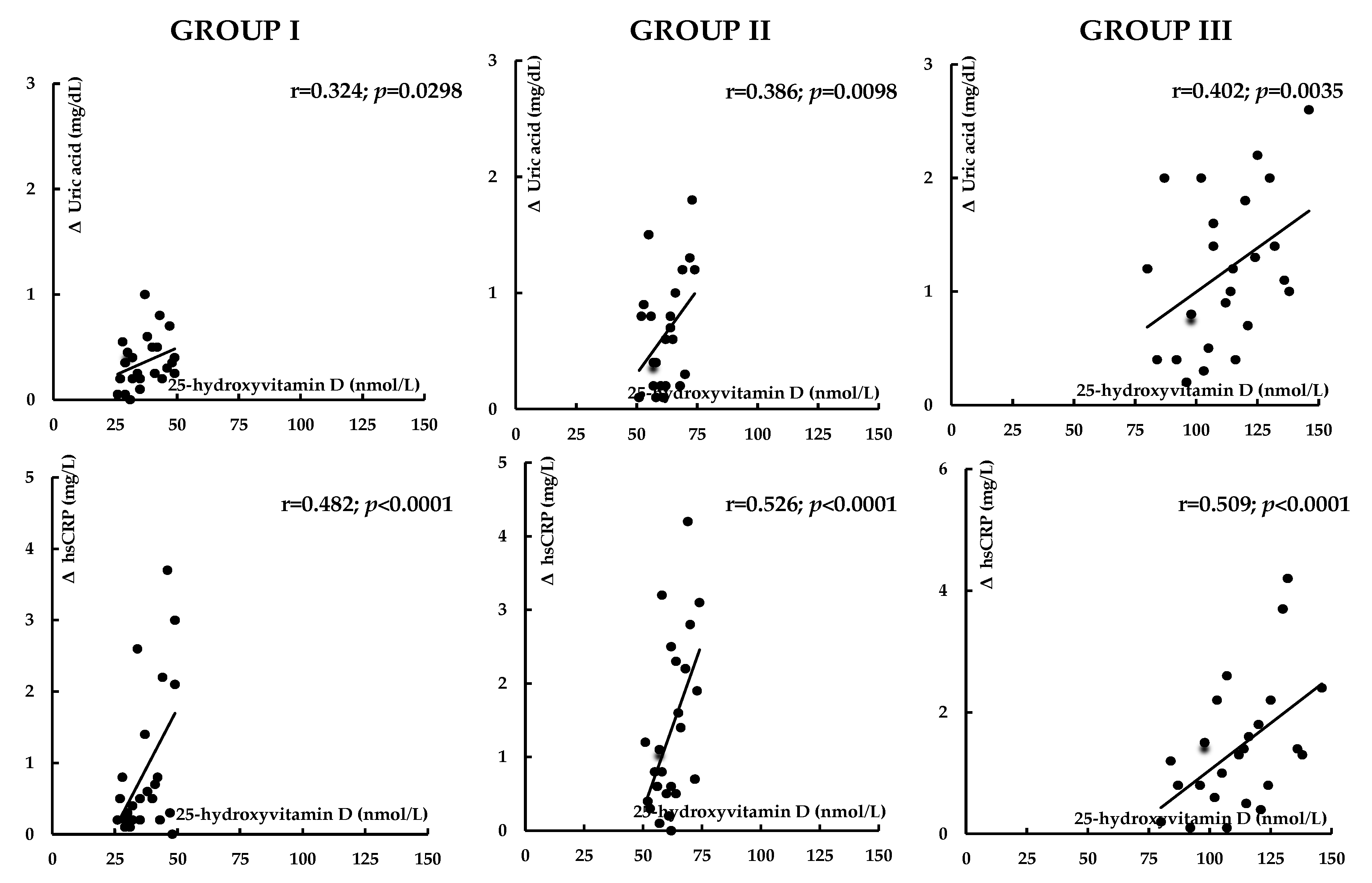
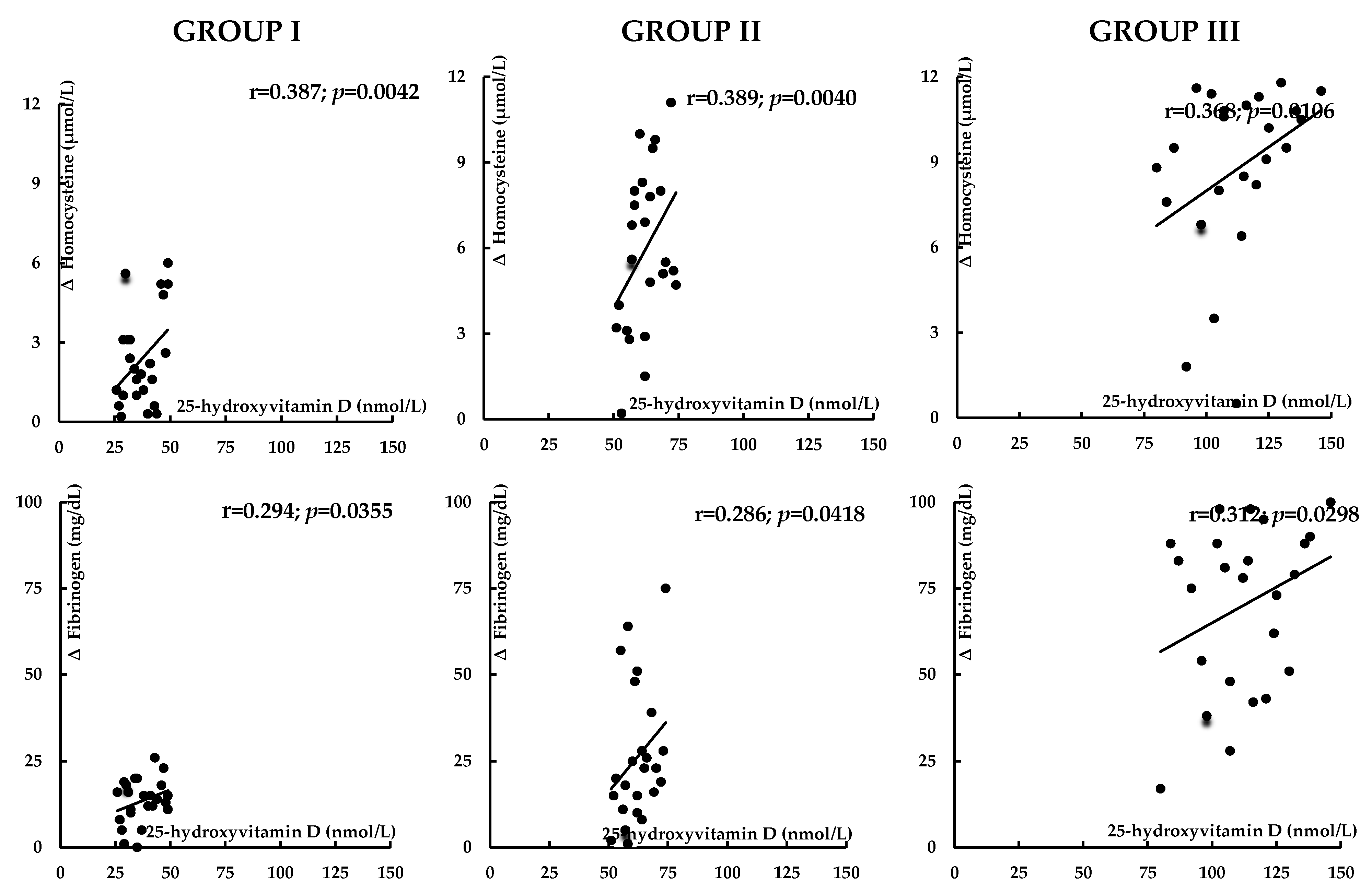
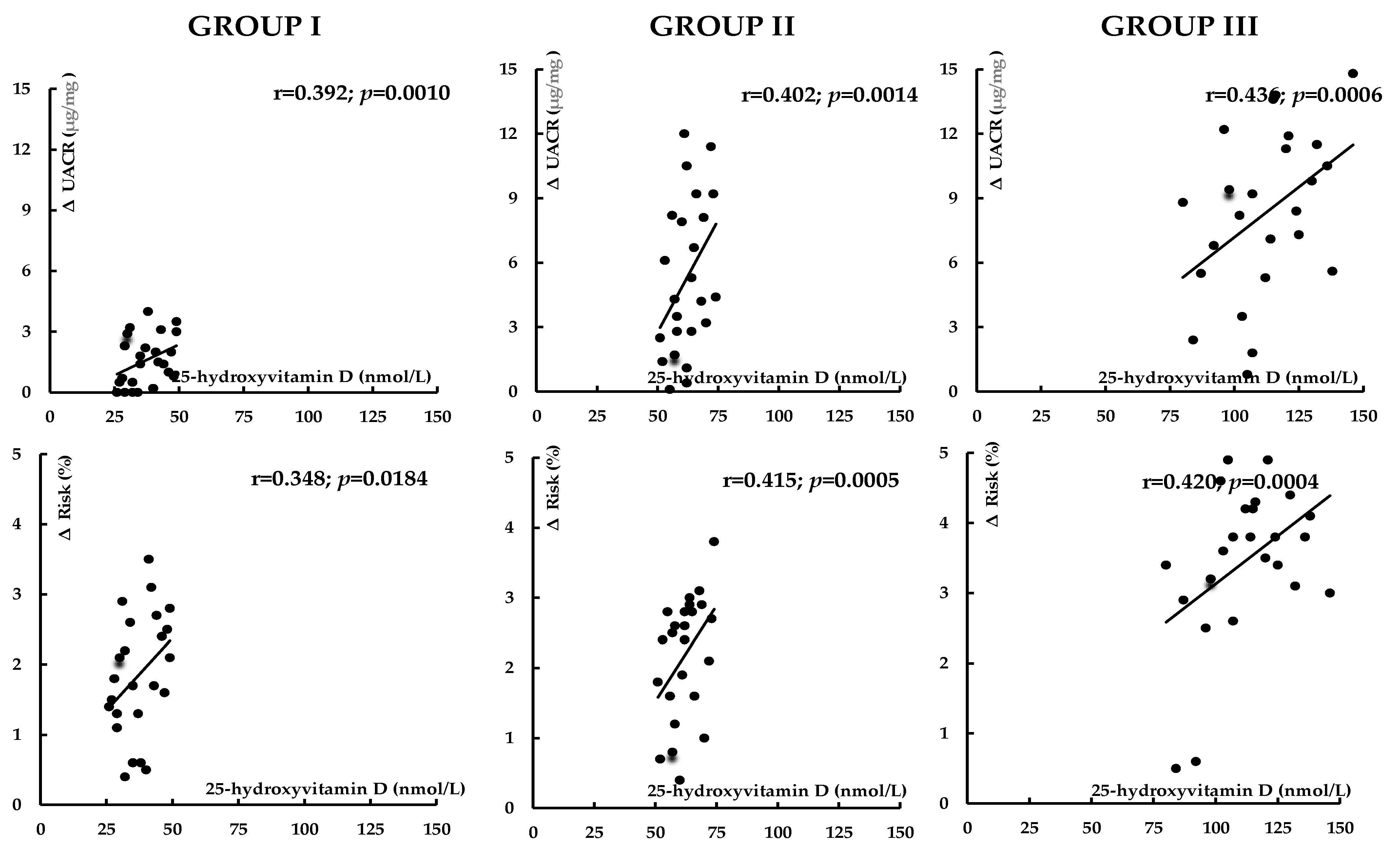
| Variable | Group I | Group II | Group III |
|---|---|---|---|
| Number (n) | 24 | 24 | 24 |
| Age (years) | 60 ± 8 | 59 ± 8 | 58 ± 9 |
| Smokers (%)/Number of cigarettes a day (n)/Duration of smoking (years) | 58/10 ± 6/29 ± 8 | 54/9 ± 5/27 ± 8 | 50/11 ± 7/30 ± 9 |
| BMI (kg/m2) | 27.4 ± 5.0 | 26.8 ± 5.2 | 26.4 ± 4.9 |
| Systolic blood pressure (mmHg) | 132 ± 16 | 128 ± 17 | 126 ± 19 |
| Diastolic blood pressure (mmHg) | 86 ± 7 | 85 ± 7 | 84 ± 7 |
| Total daily calciferol intake (µg) | 8 ± 3 &$ | 13 ± 4 $ | 29 ± 8 |
| Users of calciferol supplements (%) | 4 &$ | 29 $ | 50 |
| Variable | Group I | Group II | Group III |
|---|---|---|---|
| 25-hydroxyvitamin D (nmol/L) | |||
| Before atorvastatin treatment | 37.2 ± 6.5 &$ | 62.0 ± 6.7 $ | 112.1 ± 18.7 |
| After atorvastatin treatment | 38.5 ± 6.4 &$ | 63.8 ± 6.2 $ | 118.1 ± 14.6 |
| Percentage change from baseline | 3 ± 8 | 2 ± 8 | 5 ± 10 |
| Total cholesterol (mg/dL) | |||
| Before atorvastatin treatment | 232 ± 41 | 236 ± 46 | 241 ± 50 |
| After atorvastatin treatment | 206 ± 38 *&$ | 184 ± 35 *$ | 164 ± 29 * |
| Percentage change from baseline | −11 ± 14 | −22 ± 12 # | −32 ± 16 #& |
| HDL cholesterol (mg/dL) | |||
| Before atorvastatin treatment | 48 ± 10 | 50 ± 10 | 51 ± 9 |
| After atorvastatin treatment | 49 ± 12 | 50 ± 11 | 53 ± 11 |
| Percentage change from baseline | 2 ± 5 | 0 ± 8 | 4 ± 10 |
| LDL cholesterol (mg/dL) | |||
| Before atorvastatin treatment | 146 ± 23 | 150 ± 20 | 153 ± 25 |
| After atorvastatin treatment | 120 ± 28 *&$ | 98 ± 23 *$ | 78 ± 19 * |
| Percentage change from baseline | −18 ± 10 | −35 ± 17 # | −49 ± 20 #& |
| Triglycerides (mg/dL) | |||
| Before atorvastatin treatment | 176 ± 69 | 171 ± 78 | 168 ± 74 |
| After atorvastatin treatment | 168 ± 74 | 161 ± 73 | 153 ± 64 |
| Percentage change from baseline | −3 ± 14 | −6 ± 15 | −9 ± 18 |
| Glucose (mg/dL) | |||
| Before atorvastatin treatment | 93 ± 12 | 92 ± 13 | 90 ± 11 |
| After atorvastatin treatment | 95 ± 14 | 93 ± 14 | 90 ± 13 |
| Percentage change from baseline | 2 ± 6 | 1 ± 5 | 0 ± 5 |
| HOMA-IR | |||
| Before atorvastatin treatment | 2.6 ± 0.9 $ | 2.3 ± 0.9 | 1.9 ± 0.8 |
| After atorvastatin treatment | 3.2 ± 1.0 *&$ | 2.5 ± 1.0 | 2.0 ± 0.8 |
| Percentage change from baseline | 23 ± 20 &$ | 9 ± 15 | 5 ± 10 |
| HbA1c (%) | |||
| Before atorvastatin treatment | 5.3 ± 0.5 $ | 5.1 ± 0.5 | 5.0 ± 0.4 |
| After atorvastatin treatment | 5.6 ± 0.4 *&$ | 5.2 ± 0.5 | 5.0 ± 0.4 |
| Percentage change from baseline | 6 ± 7 &$ | 2 ± 5 | 0 ± 5 |
| Uric acid (mg/dL) | |||
| Before atorvastatin treatment | 5.0 ± 1.7 $ | 4.6 ± 1.8 | 4.1 ± 1.3 |
| After atorvastatin treatment | 4.7 ± 1.6 $ | 4.0 ± 1.4 $ | 3.0 ± 1.1 * |
| Percentage change from baseline | −6 ± 16 | −15 ± 20 | −27 ± 19 #& |
| hsCRP (mg/L) | |||
| Before atorvastatin treatment | 3.8 ± 1.4 &$ | 3.0 ± 1.1 $ | 2.3 ± 0.8 |
| After atorvastatin treatment | 3.0 ± 0.8 *&$ | 1.7 ± 1.0 *$ | 1.0 ± 0.6 * |
| Percentage change from baseline | −21 ± 23 | −43 ± 25 # | −57 ± 20 #& |
| Homocysteine (μmol/L) | |||
| Before atorvastatin treatment | 34.1 ± 11.9 &$ | 27.5 ± 9.8 $ | 20.4 ± 8.8 |
| After atorvastatin treatment | 31.8 ± 10.2 &$ | 21.6 ± 9.4 *$ | 11.7 ± 8.4 * |
| Percentage change from baseline | −7 ± 14 | −21 ± 22 # | −43 ± 26 #& |
| Fibrinogen (mg/dL) | |||
| Before atorvastatin treatment | 443 ± 93 &$ | 380 ± 102 | 346 ± 88 |
| After atorvastatin treatment | 429 ± 87 &$ | 358 ± 90 $ | 275 ± 76 * |
| Percentage change from baseline | −3 ± 10 | −6 ± 12 | −21 ± 18 #& |
| UACR (μg/mg) | |||
| Before atorvastatin treatment | 35.0 ±11.3 &$ | 27.1 ± 11.0 $ | 20.4 ± 8.2 |
| After atorvastatin treatment | 34.0 ± 12.4 &$ | 22.1 ± 8.7 $ | 11.8 ± 5.8 * |
| Percentage change from baseline | −3 ± 20 | −18 ± 35 | −42 ± 40 #& |
| 10-year risk of atherosclerotic events (%) | |||
| Before atorvastatin treatment | 10.3 ± 2.6 $ | 9.2 ± 2.8 | 8.9 ± 2.0 |
| After atorvastatin treatment | 8.4 ± 2.5 *&$ | 7.0 ± 2.2 *$ | 5.3 ± 2.0 * |
| Percentage change from baseline | −18 ± 9 | −24 ± 11 # | −40 ± 14 #& |
| CK (U/L) | |||
| Before atorvastatin treatment | 70 ± 40 | 62 ± 30 | 58 ± 28 |
| After atorvastatin treatment | 110 ± 48 *&$ | 80 ± 42 | 68 ± 35 |
| Percentage change from baseline | 57 ± 46 &$ | 29 ± 42 | 17 ± 40 |
| Correlated Variables | Group I | Group II | Group III | |
|---|---|---|---|---|
| HOMA-IR | 25-hydroxyvitamin D | −0.386 * | −0.223 | −0.202 |
| HbA1c | 25-hydroxyvitamin D | −0.345 * | −0.207 | −0.195 |
| Uric acid | 25-hydroxyvitamin D | −0.285 * | −0.205 | −0.186 |
| hsCRP | 25-hydroxyvitamin D | −0.523 * | −0.490 * | −0.465 * |
| Homocysteine | 25-hydroxyvitamin D | −0.486 * | −0.423 * | −0.402 * |
| Fibrinogen | 25-hydroxyvitamin D | −0.351 * | −0.258 | −0.224 |
| UACR | 25-hydroxyvitamin D | −0.398 * | −0.370 * | −0.311 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krysiak, R.; Kowalcze, K.; Szkróbka, W.; Okopień, B. Low Vitamin D Status Attenuates Hypolipidemic and Pleiotropic Effects of Atorvastatin in Women. Nutrients 2025, 17, 1674. https://doi.org/10.3390/nu17101674
Krysiak R, Kowalcze K, Szkróbka W, Okopień B. Low Vitamin D Status Attenuates Hypolipidemic and Pleiotropic Effects of Atorvastatin in Women. Nutrients. 2025; 17(10):1674. https://doi.org/10.3390/nu17101674
Chicago/Turabian StyleKrysiak, Robert, Karolina Kowalcze, Witold Szkróbka, and Bogusław Okopień. 2025. "Low Vitamin D Status Attenuates Hypolipidemic and Pleiotropic Effects of Atorvastatin in Women" Nutrients 17, no. 10: 1674. https://doi.org/10.3390/nu17101674
APA StyleKrysiak, R., Kowalcze, K., Szkróbka, W., & Okopień, B. (2025). Low Vitamin D Status Attenuates Hypolipidemic and Pleiotropic Effects of Atorvastatin in Women. Nutrients, 17(10), 1674. https://doi.org/10.3390/nu17101674






