Dietary Insulinogenic Amino Acid Restriction Improves Glucose Metabolism in a Neonatal Piglet Model
Abstract
1. Introduction
2. Materials and Methods
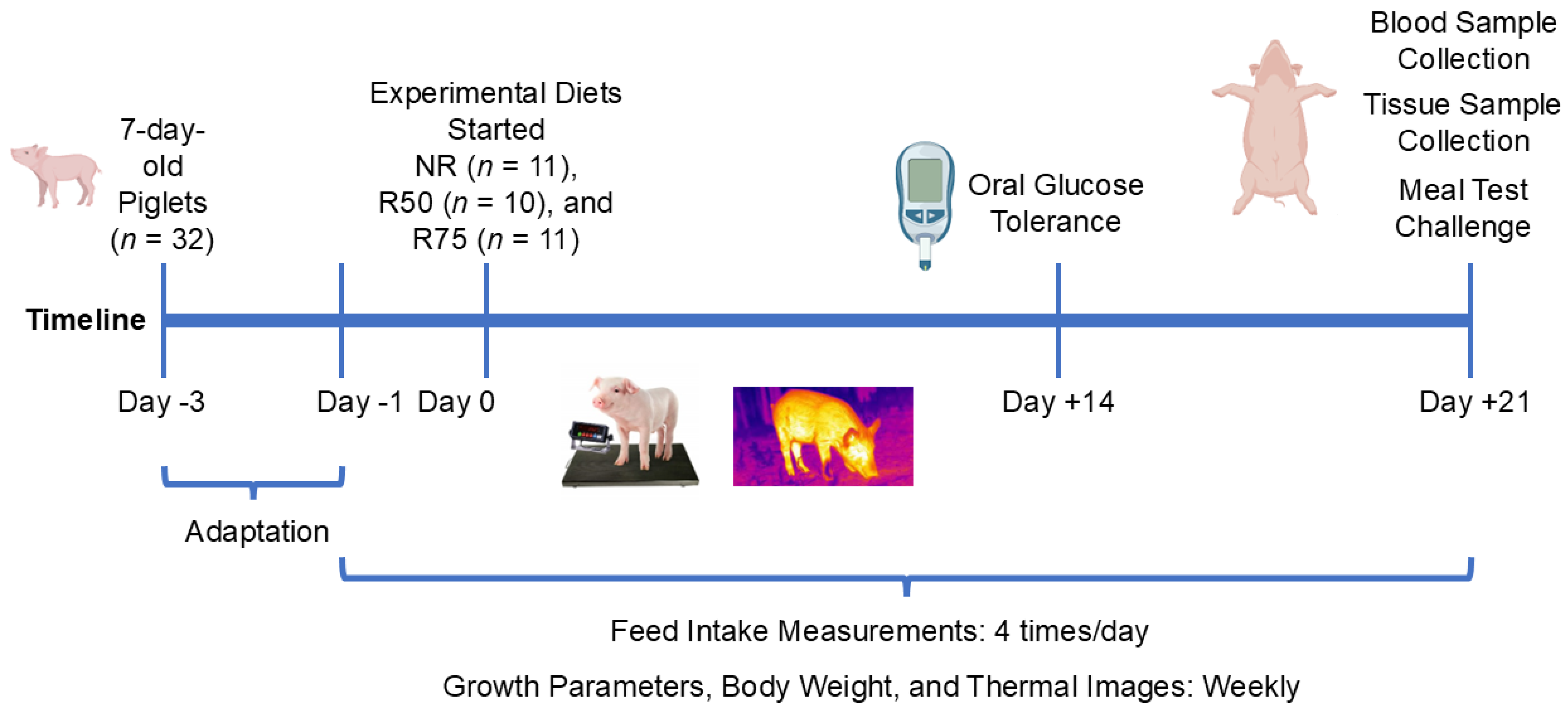
2.1. Animals and Housing
2.2. Experimental Design and Diets
2.3. Feed Intake and Growth Measurements
2.4. Thermal Images
2.5. Oral Glucose Tolerance Test
2.6. Feed Samples
2.7. Meal Test and Blood and Tissue Collection
2.8. Diets Proximate and Amino Acid Analysis
2.9. Thermal Radiation Analysis
2.10. Plasma Insulin
2.11. Insulin Sensitivity Calculations
2.12. H&E Staining and Adipocyte Measurements
2.13. RNA Isolation and RT-qPCR
2.14. Immunoblot
2.15. Statistical Analysis
3. Results
3.1. Body Weight, Growth Measurements, and Feed Intake
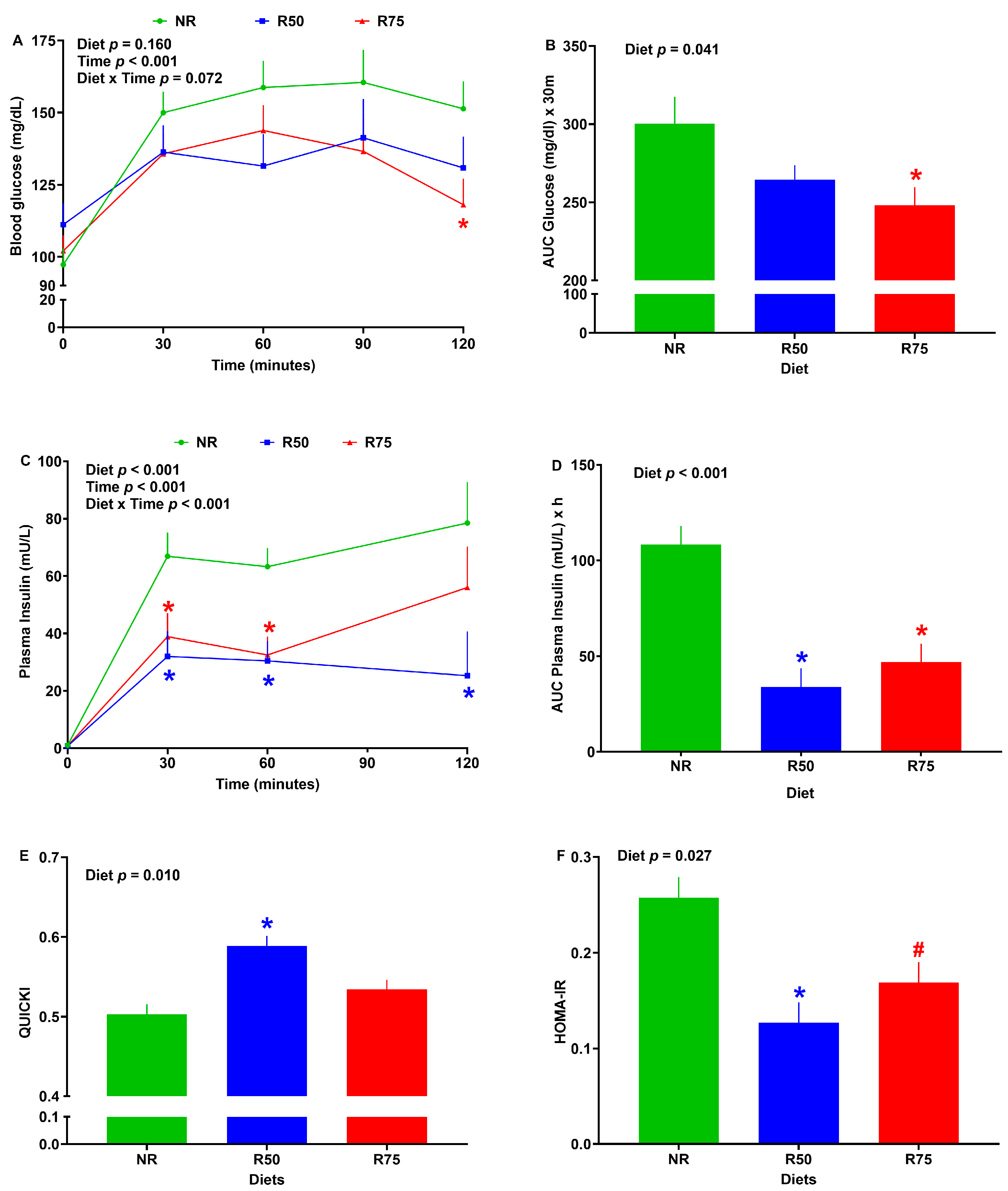
3.2. Glucose Tolerance, Plasma Insulin, and Insulin Sensitivity Indices
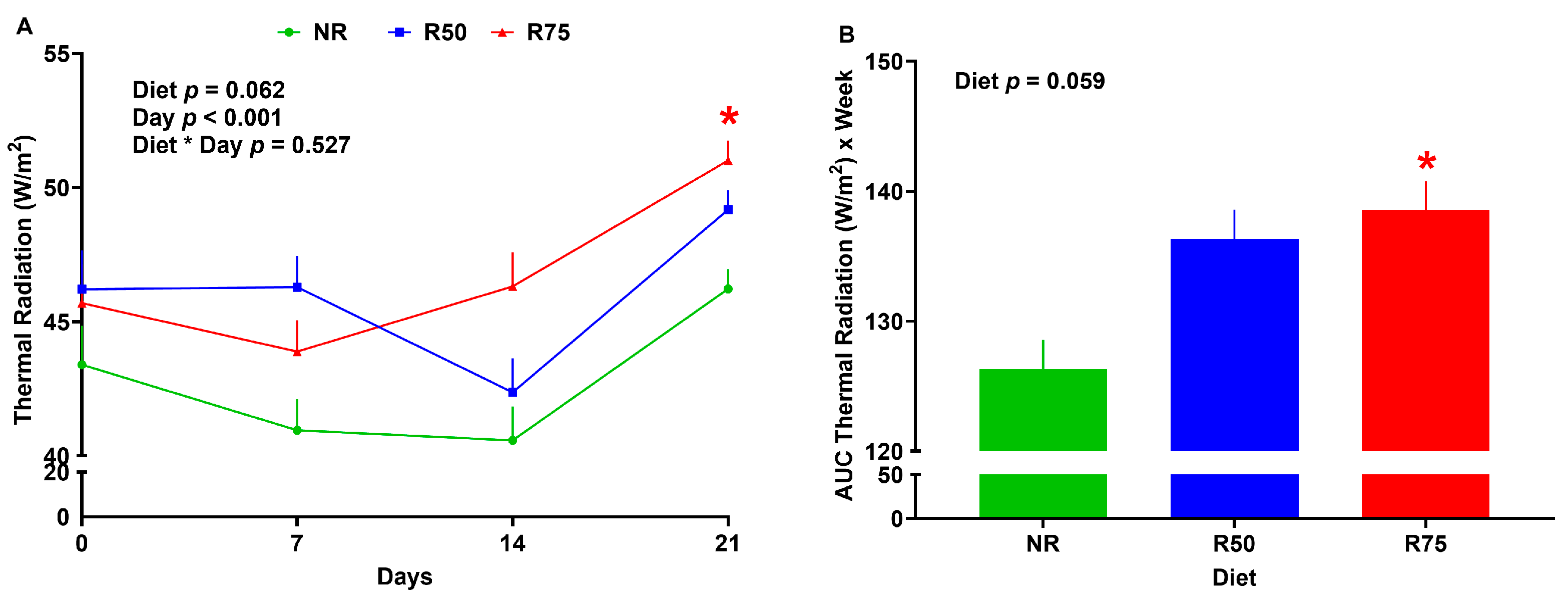
3.3. Thermal Radiation
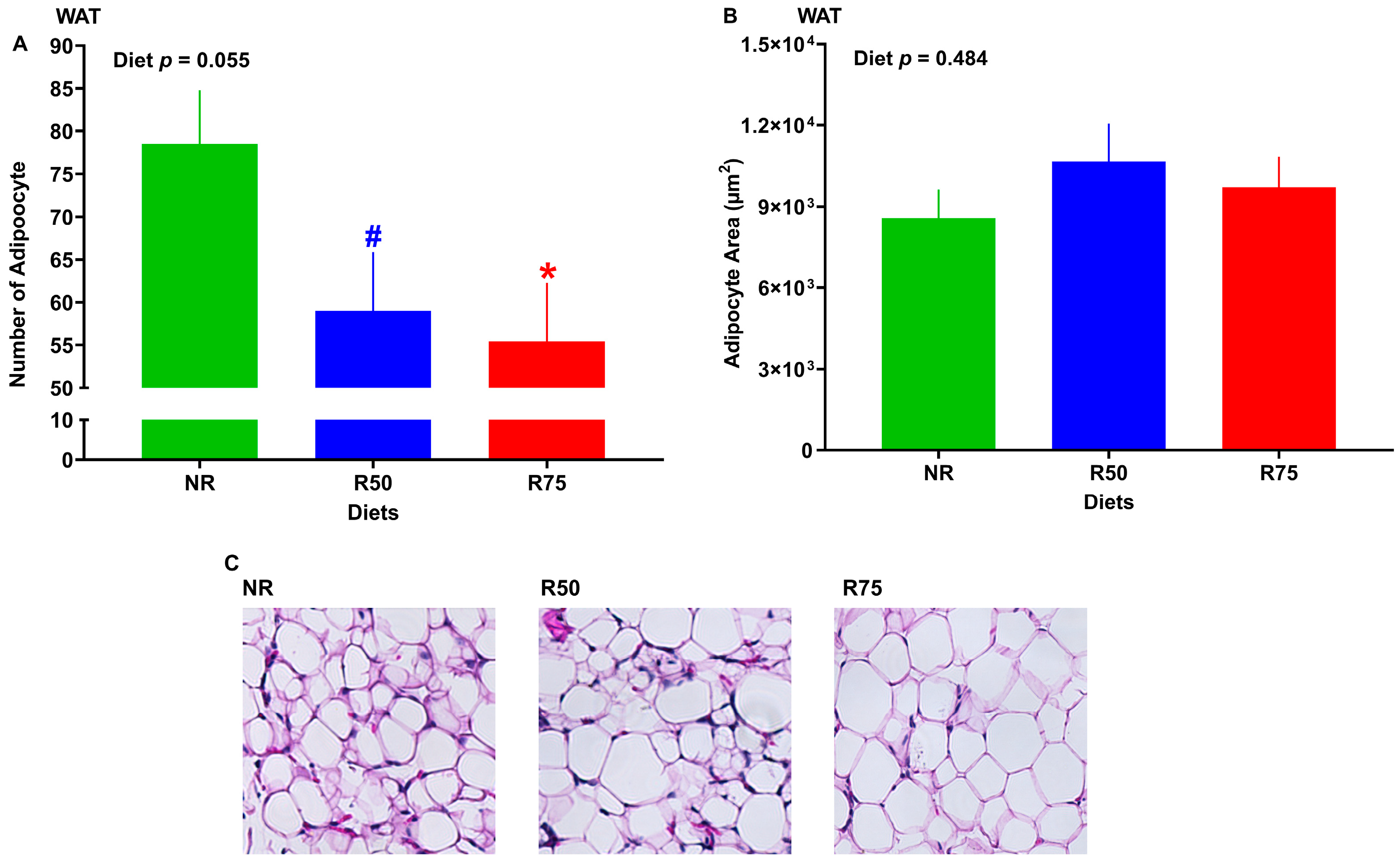
3.4. Size and Number of Adipocytes in White Adipose Tissue
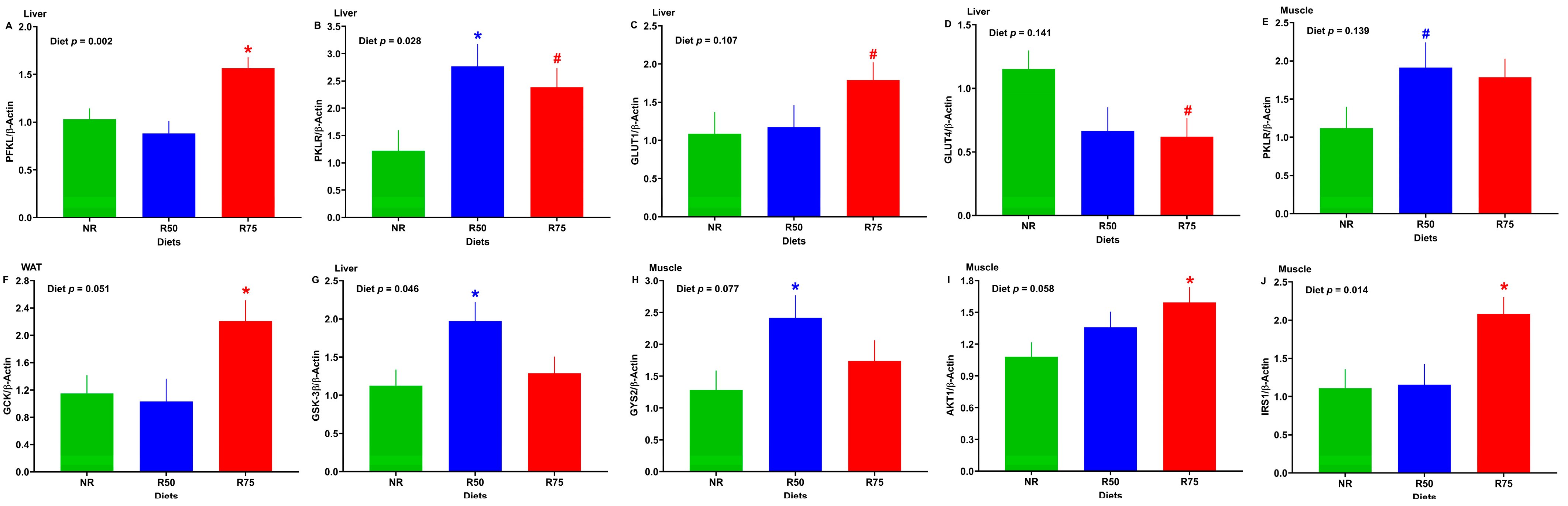
3.5. mRNA Abundance of Genes Involved in Glucose Transport and Metabolism, Glycogen Metabolism, and Insulin Signaling
3.6. mRNA Abundance of Genes Involved in Lipid Metabolism
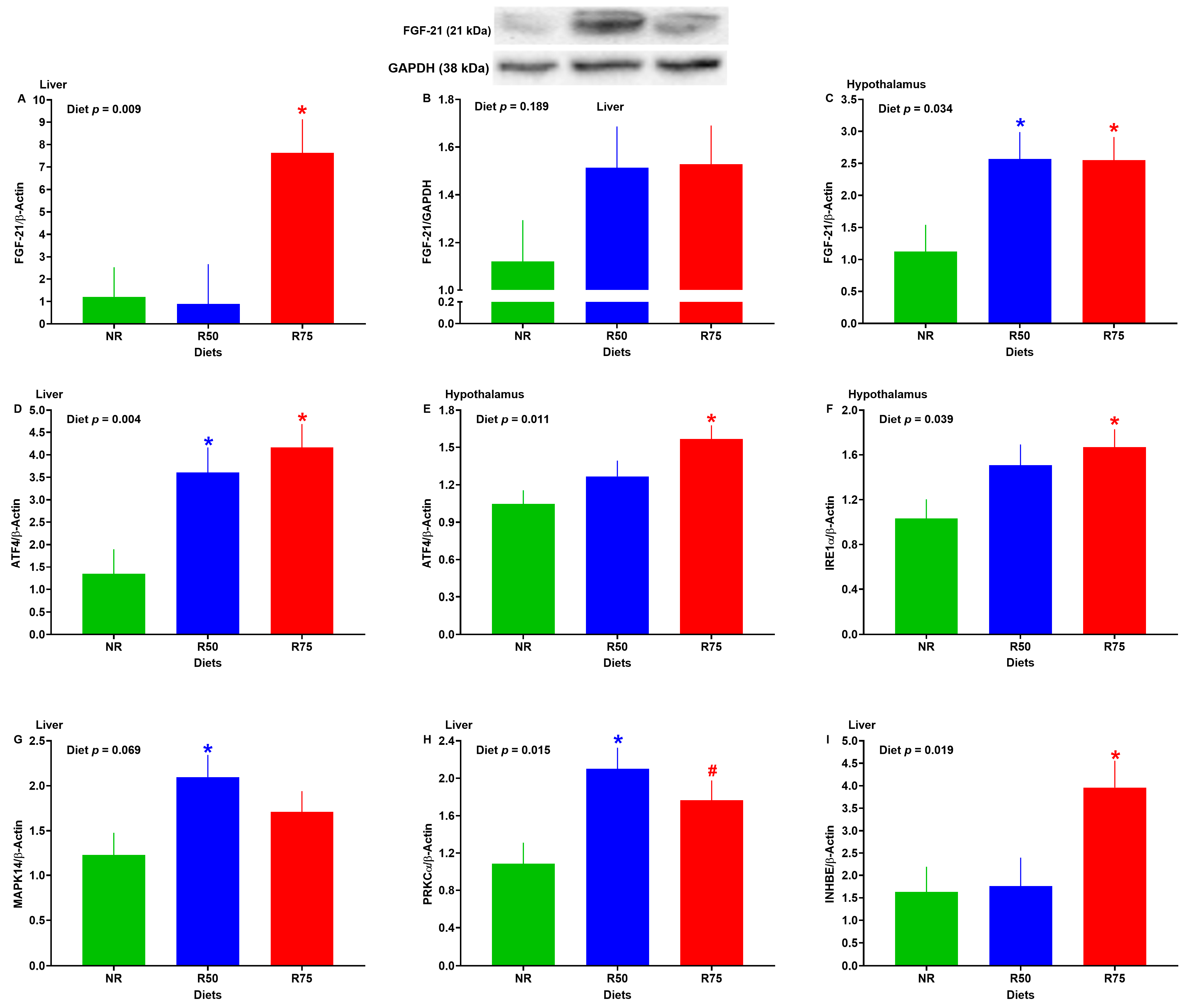
3.7. mRNA Abundance of Genes Associated with FGF-21 Pathway or FGF-21 Protein Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Amino Acids |
| ADG | Average Daily Gain |
| ADMI | Average Dry Matter Intake |
| CDMI | Cumulative Dry Matter Intake |
| ADPI | Average Daily Protein Intake |
| Ala | Alanine |
| Arg | Arginine |
| BCAA | Branched Chain Amino Acids |
| DM | Dry Matter |
| G:F | Gain-to-Feed Ratio |
| G:P | Gain-to-Protein Ratio |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
| IAA | Insulinogenic Amino Acids |
| Ile | Isoleucine |
| Leu | Leucine |
| Lys | lysine |
| NR | No Restriction |
| OGTT | Oral Glucose Tolerance Test |
| Phe | Phenylalanine |
| qPCR | Real-Time Quantitative PCR |
| QUICKI | Quantitative Insulin Sensitivity Check Index |
| T2D | Type 2 Diabetes |
| Thr | Threonine |
| Val | Valine |
References
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide Trends in Body-Mass Index, Underweight, Overweight, and Obesity from 1975 to 2016: A Pooled Analysis of 2416 Population-Based Measurement Studies in 128·9 Million Children, Adolescents, and Adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- de Onis, M.; Blössner, M.; Borghi, E. Global Prevalence and Trends of Overweight and Obesity among Preschool Children1234. Am. J. Clin. Nutr. 2010, 92, 1257–1264. [Google Scholar] [CrossRef]
- Mayer-Davis, E.J.; Lawrence, J.M.; Dabelea, D.; Divers, J.; Isom, S.; Dolan, L.; Imperatore, G.; Linder, B.; Marcovina, S.; Pettitt, D.J.; et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N. Engl. J. Med. 2017, 376, 1419–1429. [Google Scholar] [CrossRef]
- Dabelea, D.; Mayer-Davis, E.J.; Saydah, S.; Imperatore, G.; Linder, B.; Divers, J.; Bell, R.; Badaru, A.; Talton, J.W.; Crume, T.; et al. Prevalence of Type 1 and Type 2 Diabetes among Children and Adolescents from 2001 to 2009. JAMA 2014, 311, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Sanyaolu, A.; Okorie, C.; Qi, X.; Locke, J.; Rehman, S. Childhood and Adolescent Obesity in the United States: A Public Health Concern. Glob. Pediatr. Health 2019, 6, 2333794X19891305. [Google Scholar] [CrossRef]
- Andes, L.J.; Cheng, Y.J.; Rolka, D.B.; Gregg, E.W.; Imperatore, G. Prevalence of Prediabetes Among Adolescents and Young Adults in the United States, 2005–2016. JAMA Pediatr. 2020, 174, e194498. [Google Scholar] [CrossRef]
- Divers, J.; Mayer-Davis, E.J.; Lawrence, J.M.; Isom, S.; Dabelea, D.; Dolan, L.; Imperatore, G.; Marcovina, S.; Pettitt, D.J.; Pihoker, C.; et al. Trends in Incidence of Type 1 and Type 2 Diabetes Among Youths-Selected Counties and Indian Reservations, United States, 2002–2015. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control. National Diabetes Statistics Report 2020: Estimates of Diabetes and Its Burden in the United States. 2020. Available online: https://stacks.cdc.gov/view/cdc/85309 (accessed on 5 January 2024).
- Imperatore, G.; Boyle, J.P.; Thompson, T.J.; Case, D.; Dabelea, D.; Hamman, R.F.; Lawrence, J.M.; Liese, A.D.; Liu, L.L.; Mayer-Davis, E.J.; et al. Projections of Type 1 and Type 2 Diabetes Burden in the U.S. Population Aged <20 Years through 2050: Dynamic Modeling of Incidence, Mortality, and Population Growth. Diabetes Care 2012, 35, 2515–2520. [Google Scholar] [CrossRef] [PubMed]
- Rolland-Cachera, M.-F.; Deheeger, M.; Akrout, M.; Bellisle, F. Influence of Macronutrients on Adiposity Development: A Follow up Study of Nutrition and Growth from 10 Months to 8 Years of Age. Int. J. Obes. Relat. Metab. Disord. 1995, 19, 573–578. [Google Scholar]
- Koletzko, B.; von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Gruszfeld, D.; Dobrzanska, A.; et al. Lower Protein in Infant Formula Is Associated with Lower Weight up to Age 2 y: A Randomized Clinical Trial. Am. J. Clin. Nutr. 2009, 89, 1836–1845. [Google Scholar] [CrossRef]
- Koletzko, B.; Von Kries, R.; Monasterolo, R.C.; Subías, J.E.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Anton, B.; Gruszfeld, D.; et al. Can Infant Feeding Choices Modulate Later Obesity Risk? Am. J. Clin. Nutr. 2009, 89, 1502S–1508S. [Google Scholar] [CrossRef] [PubMed]
- Brands, B.; Demmelmair, H.; Koletzko, B.; EarlyNutrition, P. How Growth Due to Infant Nutrition Influences Obesity and Later Disease Risk. Acta Paediatr. 2014, 103, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, K.F.; Greer, F.R. Protein Needs Early in Life and Long-Term Health. Am. J. Clin. Nutr. 2014, 99, 718S–722S. [Google Scholar] [CrossRef]
- Camier, A.; Davisse-Paturet, C.; Scherdel, P.; Lioret, S.; Heude, B.; Charles, M.A.; de Lauzon-Guillain, B. Early Growth According to Protein Content of Infant Formula: Results from the EDEN and ELFE Birth Cohorts. Pediatr. Obes. 2021, 16, e12803. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Grote, V.; Closa-Monasterolo, R.; Escribano, J.; Langhendries, J.P.; Dain, E.; Giovannini, M.; Verduci, E.; Gruszfeld, D.; Socha, P.; et al. Lower Protein Content in Infant Formula Reduces BMI and Obesity Risk at School Age: Follow-up of a Randomized Trial. Am. J. Clin. Nutr. 2014, 99, 1041–1051. [Google Scholar] [CrossRef]
- Kouwenhoven, S.M.P.; Antl, N.; Finken, M.J.J.; Twisk, J.W.R.; van der Beek, E.M.; Abrahamse-Berkeveld, M.; van de Heijning, B.J.M.; van Goudoever, J.B.; Koletzko, B.V. Long-Term Effects of a Modified, Low-Protein Infant Formula on Growth and Body Composition: Follow-up of a Randomized, Double-Blind, Equivalence Trial. Clin. Nutr. 2021, 40, 3914–3921. [Google Scholar] [CrossRef]
- Kouwenhoven, S.M.P.; Muts, J.; Finken, M.J.J.; van Goudoever, J.B. Low-Protein Infant Formula and Obesity Risk. Nutrients 2022, 14, 2728. [Google Scholar] [CrossRef]
- Koletzko, B.; Demmelmair, H.; Grote, V.; Prell, C.; Weber, M. High Protein Intake in Young Children and Increased Weight Gain and Obesity Risk. Am. J. Clin. Nutr. 2016, 103, 303–304. [Google Scholar] [CrossRef]
- Kalhan, S.C. Optimal Protein Intake in Healthy Infants. Am. J. Clin. Nutr. 2009, 89, 1719–1720. [Google Scholar] [CrossRef]
- Newsholme, P.; Brennan, L.; Bender, K. Amino Acid Metabolism, β-Cell Function, and Diabetes. Diabetes 2006, 55, S39–S47. [Google Scholar] [CrossRef]
- Nilsson, M.; Holst, J.J.; Björck, I.M. Metabolic Effects of Amino Acid Mixtures and Whey Protein in Healthy Subjects: Studies Using Glucose-Equivalent Drinks2. Am. J. Clin. Nutr. 2007, 85, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulou, D.; LaFave, L.; Schweim, K.; Gannon, M.C.; Nuttall, F.Q. Lysine Ingestion Markedly Attenuates the Glucose Response to Ingested Glucose without a Change in Insulin Response. Am. J. Clin. Nutr. 2009, 90, 314–320. [Google Scholar] [CrossRef] [PubMed]
- van Sloun, B.; Goossens, G.H.; Erdos, B.; Lenz, M.; van Riel, N.; Arts, I.C.W. The Impact of Amino Acids on Postprandial Glucose and Insulin Kinetics in Humans: A Quantitative Overview. Nutrients 2020, 12, 3211. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, W.-W.; Chen, J.-C.; Zhang, H.-L.; Liu, J.; Lin, Y.; Lin, P.-C.; Wu, B.-X.; An, Y.-P.; Huang, L.; et al. Phenylalanine Impairs Insulin Signaling and Inhibits Glucose Uptake through Modification of IRβ. Nat. Commun. 2022, 13, 4291. [Google Scholar] [CrossRef]
- Anthony, T.G.; Morrison, C.D.; Gettys, T.W. Remodeling of Lipid Metabolism by Dietary Restriction of Essential Amino Acids. Diabetes 2013, 62, 2635–2644. [Google Scholar] [CrossRef]
- Yap, Y.W.; Rusu, P.M.; Chan, A.Y.; Fam, B.C.; Jungmann, A.; Solon-Biet, S.M.; Barlow, C.K.; Creek, D.J.; Huang, C.; Schittenhelm, R.B.; et al. Restriction of Essential Amino Acids Dictates the Systemic Metabolic Response to Dietary Protein Dilution. Nat. Commun. 2020, 11, 2894. [Google Scholar] [CrossRef]
- Kuhara, T.; Ikeda, S.; Ohneda, A.; Sasaki, Y. Effects of Intravenous Infusion of 17 Amino Acids on the Secretion of GH, Glucagon, and Insulin in Sheep. Am. J. Physiol. 1991, 260, E21–E26. [Google Scholar] [CrossRef]
- Xiao, F.; Huang, Z.; Li, H.; Yu, J.; Wang, C.; Chen, S.; Meng, Q.; Cheng, Y.; Gao, X.; Li, J.; et al. Leucine Deprivation Increases Hepatic Insulin Sensitivity via GCN2/mTOR/S6K1 and AMPK Pathways. Diabetes 2011, 60, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Yu, J.; Guo, Y.; Deng, J.; Li, K.; Du, Y.; Chen, S.; Zhu, J.; Sheng, H.; Guo, F. Effects of Individual Branched-Chain Amino Acids Deprivation on Insulin Sensitivity and Glucose Metabolism in Mice. Metabolism 2014, 63, 841–850. [Google Scholar] [CrossRef]
- Fontana, L.; Cummings, N.E.; Arriola Apelo, S.I.; Neuman, J.C.; Kasza, I.; Schmidt, B.A.; Cava, E.; Spelta, F.; Tosti, V.; Syed, F.A.; et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell. Rep. 2016, 16, 520–530. [Google Scholar] [CrossRef]
- Cummings, N.E.; Williams, E.M.; Kasza, I.; Konon, E.N.; Schaid, M.D.; Schmidt, B.A.; Poudel, C.; Sherman, D.S.; Yu, D.; Arriola Apelo, S.I.; et al. Restoration of Metabolic Health by Decreased Consumption of Branched-Chain Amino Acids. J. Physiol. 2018, 596, 623–645. [Google Scholar] [CrossRef] [PubMed]
- Karusheva, Y.; Koessler, T.; Strassburger, K.; Markgraf, D.; Mastrototaro, L.; Jelenik, T.; Simon, M.C.; Pesta, D.; Zaharia, O.P.; Bodis, K.; et al. Short-Term Dietary Reduction of Branched-Chain Amino Acids Reduces Meal-Induced Insulin Secretion and Modifies Microbiome Composition in Type 2 Diabetes: A Randomized Controlled Crossover Trial. Am. J. Clin. Nutr. 2019, 110, 1098–1107. [Google Scholar] [CrossRef]
- Yu, D.; Richardson, N.E.; Green, C.L.; Spicer, A.B.; Murphy, M.E.; Flores, V.; Jang, C.; Kasza, I.; Nikodemova, M.; Wakai, M.H.; et al. The Adverse Metabolic Effects of Branched-Chain Amino Acids Are Mediated by Isoleucine and Valine. Cell. Metab. 2021, 33, 905–922.e6. [Google Scholar] [CrossRef]
- Miller, E.R.; Ullrey, D.E. The Pig as a Model for Human Nutrition. Annu. Rev. Nutr. 1987, 7, 361–382. [Google Scholar] [CrossRef]
- Puiman, P.; Stoll, B. Animal Models to Study Neonatal Nutrition in Humans. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.A.; Merricks, E.P.; Nichols, T.C. Swine Models of Type 2 Diabetes Mellitus: Insulin Resistance, Glucose Tolerance, and Cardiovascular Complications. ILAR J. 2006, 47, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Harwood, H.J.; Listrani, P.; Wagner, J.D. Nonhuman Primates and Other Animal Models in Diabetes Research. J. Diabetes Sci. Technol. 2012, 6, 503–514. [Google Scholar] [CrossRef] [PubMed]
- McGlone, J. Guide for the Care and Use of Agricultural Animals in Research and Teaching; FASS: Champaign, IL, USA, 2010. [Google Scholar]
- Rafiee-Tari, N.; Fan, M.Z.; Archbold, T.; Arranz, E.; Corredig, M. Effect of Milk Protein Composition and Amount of Beta-Casein on Growth Performance, Gut Hormones, and Inflammatory Cytokines in an in Vivo Piglet Model. J. Dairy Sci. 2019, 102, 8604–8613. [Google Scholar] [CrossRef]
- Goodarzi, P.; Habibi, M.; Roberts, K.; Sutton, J.; Shili, C.N.; Lin, D.; Pezeshki, A. Dietary Tryptophan Supplementation Alters Fat and Glucose Metabolism in a Low-Birthweight Piglet Model. Nutrients 2021, 13, 2561. [Google Scholar] [CrossRef]
- Calbet, J.A.L. Plasma Glucagon and Insulin Responses Depend on the Rate of Appearance of Amino Acids after Ingestion of Different Protein Solutions in Humans. J. Nutr. 2002, 132, 2174–2182. [Google Scholar] [CrossRef]
- Nilsson, M. Glycemia and Insulinemia in Healthy Subjects after Lactose-Equivalent Meals of Milk and Other Food Proteins: The Role of Plasma Amino Acids and Incretins. Am. J. Clin. Nutr. 2004, 80, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Wanders, D.; Stone, K.P.; Dille, K.; Simon, J.; Pierse, A.; Gettys, T.W. Metabolic Responses to Dietary Leucine Restriction Involve Remodeling of Adipose Tissue and Enhanced Hepatic Insulin Signaling. Biofactors 2015, 41, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Kaya, A.; Ma, S.; Kim, G.; Gerashchenko, M.V.; Yim, S.H.; Hu, Z.; Harshman, L.G.; Gladyshev, V.N. Methionine Restriction Extends Lifespan of Drosophila Melanogaster under Conditions of Low Amino-Acid Status. Nat. Commun. 2014, 5, 3592. [Google Scholar] [CrossRef] [PubMed]
- Lees, E.K.; Banks, R.; Cook, C.; Hill, S.; Morrice, N.; Grant, L.; Mody, N.; Delibegovic, M. Direct Comparison of Methionine Restriction with Leucine Restriction on the Metabolic Health of C57BL/6J Mice. Sci. Rep. 2017, 7, 9977. [Google Scholar] [CrossRef]
- Zhou, Z.; Yin, H.; Guo, Y.; Fang, Y.; Yuan, F.; Chen, S.; Guo, F. A Fifty Percent Leucine-Restricted Diet Reduces Fat Mass and Improves Glucose Regulation. Nutr. Metab. 2021, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.R.; Berman, A.S.; Harrell, R.J.; Kessler, A.M.; Cornelius, S.G.; Odle, J. Vegetable Proteins Enhance the Growth of Milk-Fed Piglets, despite Lower Apparent Ileal Digestibility. J. Nutr. 2005, 135, 2137–2143. [Google Scholar] [CrossRef][Green Version]
- DeRouchey, J.M.; Goodband, R.D.; Tokach, M.D.; Nelssen, J.L.; Dritz, S.S. Nursery Swine Nutrient Recommendations and Feeding Management. In National Swine Nutrition Guide; Meisinger, D.J., Ed.; U.S. Pork Center of Excellence: Ames, IA, USA, 2010; pp. 65–79. [Google Scholar]
- Kim, S.W.; Wu, G. Dietary Arginine Supplementation Enhances the Growth of Milk-Fed Young Pigs. J. Nutr. 2004, 134, 625–630. [Google Scholar] [CrossRef]
- Madsen, J.G.; Mueller, S.; Kreuzer, M.; Bigler, M.B.; Silacci, P.; Bee, G. Milk Replacers Supplemented with Either L-Arginine or L-Carnitine Potentially Improve Muscle Maturation of Early Reared Low Birth Weight Piglets from Hyperprolific Sows. Animal 2018, 12, 43–53. [Google Scholar] [CrossRef]
- Montelius, C.; Szwiec, K.; Kardas, M.; Lozinska, L.; Erlanson-Albertsson, C.; Pierzynowski, S.; Rehfeld, J.F.; Weström, B. Dietary Thylakoids Suppress Blood Glucose and Modulate Appetite-Regulating Hormones in Pigs Exposed to Oral Glucose Tolerance Test. Clin. Nutr. 2014, 33, 1122–1126. [Google Scholar] [CrossRef]
- Habibi, M.; Shili, C.; Sutton, J.; Goodarzi, P.; Maylem, E.R.; Spicer, L.; Pezeshki, A. Branched-Chain Amino Acids Partially Recover the Reduced Growth of Pigs Fed with Protein-Restricted Diets through Both Central and Peripheral Factors. Anim. Nutr. 2021, 7, 868–882. [Google Scholar] [CrossRef]
- Spring, S.; Premathilake, H.; Bradway, C.; Shili, C.; Desilva, U.; Carter, S.; Pezeshki, A. Effect of Very Low-Protein Diets Supplemented with Branched-Chain Amino Acids on Energy Balance, Plasma Metabolomics and Fecal Microbiome of Pigs. Sci. Rep. 2020, 10, 15859. [Google Scholar] [CrossRef] [PubMed]
- Spring, S.; Premathilake, H.; Desilva, U.; Shili, C.; Carter, S.; Pezeshki, A. Low Protein-High Carbohydrate Diets Alter Energy Balance, Gut Microbiota Composition and Blood Metabolomics Profile in Young Pigs. Sci. Rep. 2020, 10, 3318. [Google Scholar] [CrossRef]
- Shili, C.N.; Broomhead, J.N.; Spring, S.C.; Lanahan, M.B.; Pezeshki, A. A Novel Corn-Expressed Phytase Improves Daily Weight Gain, Protein Efficiency Ratio and Nutrients Digestibility and Alters Fecal Microbiota in Pigs Fed with Very Low Protein Diets. Animals 2020, 10, 1926. [Google Scholar] [CrossRef]
- Goodarzi, P.; Wileman, C.M.; Habibi, M.; Walsh, K.; Sutton, J.; Shili, C.N.; Chai, J.; Zhao, J.; Pezeshki, A. Effect of Isoleucine and Added Valine on Performance, Nutrients Digestibility and Gut Microbiota Composition of Pigs Fed with Very Low Protein Diets. Int. J. Mol. Sci. 2022, 23, 14886. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.; Habibi, M.; Shili, C.N.; Beker, A.; Salak-Johnson, J.L.; Foote, A.; Pezeshki, A. Low-Protein Diets Differentially Regulate Energy Balance during Thermoneutral and Heat Stress in Cobb Broiler Chicken (Gallus Domesticus). Int. J. Mol. Sci. 2024, 25, 4369. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.; Goodarzi, P.; Shili, C.N.; Sutton, J.; Wileman, C.M.; Kim, D.M.; Lin, D.; Pezeshki, A. A Mixture of Valine and Isoleucine Restores the Growth of Protein-Restricted Pigs Likely through Improved Gut Development, Hepatic IGF-1 Pathway, and Plasma Metabolomic Profile. Int. J. Mol. Sci. 2022, 23, 3300. [Google Scholar] [CrossRef]
- Goodarzi, P.; Habibi, M.; Gorton, M.W.; Walsh, K.; Tarkesh, F.; Fuhrig, M.; Pezeshki, A. Dietary Isoleucine and Valine: Effects on Lipid Metabolism and Ureagenesis in Pigs Fed with Protein Restricted Diets. Metabolites 2023, 13, 89. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and β-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity In Humans. J. Clin. Endocr. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Parlee, S.D.; Lentz, S.I.; Mori, H.; MacDougald, O.A. Quantifying Size and Number of Adipocytes in Adipose Tissue. Methods Enzymol. 2014, 537, 93–122. [Google Scholar] [CrossRef]
- Shili, C.N.; Habibi, M.; Sutton, J.; Barnes, J.; Burch-Konda, J.; Pezeshki, A. Effect of a Phytogenic Water Additive on Growth Performance, Blood Metabolites and Gene Expression of Amino Acid Transporters in Nursery Pigs Fed with Low-Protein/High-Carbohydrate Diets. Animals 2021, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, A.; Muench, G.P.; Chelikani, P.K. Short Communication: Expression of Peptide YY, Proglucagon, Neuropeptide Y Receptor Y2, and Glucagon-like Peptide-1 Receptor in Bovine Peripheral Tissues. J. Dairy Sci. 2012, 95, 5089–5094. [Google Scholar] [CrossRef]
- Habibi, M.; Shili, C.N.; Sutton, J.; Goodarzi, P.; Pezeshki, A. Dietary Branched-Chain Amino Acids Modulate the Dynamics of Calcium Absorption and Reabsorption in Protein-Restricted Pigs. J. Anim. Sci. Biotechnol. 2022, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Vitali, M.; Dimauro, C.; Sirri, R.; Zappaterra, M.; Zambonelli, P.; Manca, E.; Sami, D.; Lo Fiego, D.P.; Davoli, R. Effect of Dietary Polyunsaturated Fatty Acid and Antioxidant Supplementation on the Transcriptional Level of Genes Involved in Lipid and Energy Metabolism in Swine. PLoS ONE 2018, 13, e0204869. [Google Scholar] [CrossRef]
- Kim, S.-C.; Jang, H.-C.; Lee, S.-D.; Jung, H.-J.; Park, J.-C.; Lee, S.-H.; Kim, T.-H.; Choi, B.-H. Changes in Expression of Insulin Signaling Pathway Genes by Dietary Fat Source in Growing-Finishing Pigs. J. Anim. Sci. Technol. 2014, 56, 12. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, H.; Sun, H. Effects of Low-Protein Diet Supplementation with Alpha-Ketoglutarate on Growth Performance, Nitrogen Metabolism and mTOR Signalling Pathway of Skeletal Muscle in Piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Chomwisarutkun, K.; Murani, E.; Ponsuksili, S.; Wimmers, K. Gene Expression Analysis of Mammary Tissue during Fetal Bud Formation and Growth in Two Pig Breeds--Indications of Prenatal Initiation of Postnatal Phenotypic Differences. BMC. Dev. Biol. 2012, 12, 13. [Google Scholar] [CrossRef]
- Xie, C.; Wang, Q.; Wang, J.; Tan, B.; Fan, Z.; Deng, Z.; Wu, X.; Yin, Y. Developmental Changes in Hepatic Glucose Metabolism in a Newborn Piglet Model: A Comparative Analysis for Suckling Period and Early Weaning Period. Biochem. Biophys. Res. Commun. 2016, 470, 824–830. [Google Scholar] [CrossRef]
- Cervantes, M.; Cota, M.; Arce, N.; Castillo, G.; Avelar, E.; Espinoza, S.; Morales, A. Effect of Heat Stress on Performance and Expression of Selected Amino Acid and Glucose Transporters, HSP90, Leptin and Ghrelin in Growing Pigs. J. Therm. Biol. 2016, 59, 69–76. [Google Scholar] [CrossRef]
- Fang, L.; Jiang, X.; Su, Y.; Zhu, W. Long-Term Intake of Raw Potato Starch Decreases Back Fat Thickness and Dressing Percentage but Has No Effect on the Longissimus Muscle Quality of Growing–Finishing Pigs. Livest. Sci. 2014, 170, 116–123. [Google Scholar] [CrossRef]
- Espinosa, C.D.; Fry, R.S.; Kocher, M.E.; Stein, H.H. Effects of Copper Hydroxychloride on Growth Performance and Abundance of Genes Involved in Lipid Metabolism of Growing Pigs. J. Anim. Sci. 2020, 98, skz369. [Google Scholar] [CrossRef] [PubMed]
- Gavalda-Navarro, A.; Pastor, J.J.; Mereu, A.; Villarroya, F.; Ipharraguerre, I.R. Developmental Regulation of the Intestinal FGF19 System in Domestic Pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G647–G654. [Google Scholar] [CrossRef] [PubMed]
- Merz, T.; Denoix, N.; Wigger, D.; Waller, C.; Wepler, M.; Vettorazzi, S.; Tuckermann, J.; Radermacher, P.; McCook, O. The Role of Glucocorticoid Receptor and Oxytocin Receptor in the Septic Heart in a Clinically Relevant, Resuscitated Porcine Model With Underlying Atherosclerosis. Front. Endocrinol. 2020, 11, 299. [Google Scholar] [CrossRef]
- Weber, T.E.; Kerr, B.J.; Spurlock, M.E. Regulation of Hepatic Peroxisome Proliferator-Activated Receptor Alpha Expression but Not Adiponectin by Dietary Protein in Finishing Pigs. J. Anim. Physiol. Anim. Nutr. 2008, 92, 569–577. [Google Scholar] [CrossRef]
- Jin, J.-X.; Lee, S.; Taweechaipaisankul, A.; Kim, G.A.; Lee, B.C. Melatonin Regulates Lipid Metabolism in Porcine Oocytes. J. Pineal Res. 2017, 62, e12388. [Google Scholar] [CrossRef]
- Niu, Y.-J.; Zhou, W.; Nie, Z.-W.; Shin, K.-T.; Cui, X.-S. Melatonin Enhances Mitochondrial Biogenesis and Protects against Rotenone-Induced Mitochondrial Deficiency in Early Porcine Embryos. J. Pineal Res. 2020, 68, e12627. [Google Scholar] [CrossRef]
- Duran-Montgé, P.; Theil, P.K.; Lauridsen, C.; Esteve-Garcia, E. Dietary Fat Source Affects Metabolism of Fatty Acids in Pigs as Evaluated by Altered Expression of Lipogenic Genes in Liver and Adipose Tissues. Animal 2009, 3, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, J.; Zhang, Y.; Liao, P.; Li, T.; Chen, L.; Yin, Y.; Wang, J.; Wu, G. Oral MSG Administration Alters Hepatic Expression of Genes for Lipid and Nitrogen Metabolism in Suckling Piglets. Amino Acids 2014, 46, 245–250. [Google Scholar] [CrossRef]
- Zhou, X.; Wan, D.; Zhang, Y.; Zhang, Y.; Long, C.; Chen, S.; He, L.; Tan, B.; Wu, X.; Yin, Y. Diurnal Variations in Polyunsaturated Fatty Acid Contents and Expression of Genes Involved in Their de Novo Synthesis in Pigs. Biochem. Biophys. Res. Commun. 2017, 483, 430–434. [Google Scholar] [CrossRef]
- He, D.; Ma, J.; Long, K.; Wang, X.; Li, X.; Jiang, A.; Li, M. Differential Expression of Genes Related to Glucose Metabolism in Domesticated Pigs and Wild Boar. Biosci. Biotechnol. Biochem. 2017, 81, 1478–1483. [Google Scholar] [CrossRef]
- Hu, H.; Li, Y.; Yang, Y.; Xu, K.; Yang, L.; Qiao, S.; Pan, H. Effect of a Plateau Environment on the Oxidation State of the Heart and Liver through AMPK/P38 MAPK/Nrf2-ARE Signaling Pathways in Tibetan and DLY Pigs. Animals 2022, 12, 1219. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, J.; Shi, S.J.; Zhou, X.; Wang, L.; Huang, L.; Gao, L.; Pang, W.; Yang, G.; Chu, G. Fibroblast Growth Factor 21 (FGF21) Promotes Porcine Granulosa Cell Estradiol Production and Proliferation via PI3K/AKT/mTOR Signaling. Theriogenology 2022, 194, 1–12. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Kim, T.; Long, Q.; Liu, J.; Wang, P.; Zhou, Y.; Ding, Y.; Prasain, J.; Wood, P.A.; Yang, Q. Carnitine Palmitoyltransferase-1b Deficiency Aggravates Pressure Overload-Induced Cardiac Hypertrophy Caused by Lipotoxicity. Circulation 2012, 126, 1705–1716. [Google Scholar] [CrossRef]
- Ying, Z.; Zhang, H.; Su, W.; Zhou, L.; Wang, F.; Li, Y.; Zhang, L.; Wang, T. Dietary Methionine Restriction Alleviates Hyperglycemia in Pigs with Intrauterine Growth Restriction by Enhancing Hepatic Protein Kinase B Signaling and Glycogen Synthesis. J. Nutr. 2017, 147, 1892–1899. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Wang, X.; Wang, Y.; Feng, J. Betaine Affects Muscle Lipid Metabolism via Regulating the Fatty Acid Uptake and Oxidation in Finishing Pig. J. Anim. Sci. Biotechnol. 2017, 8, 72. [Google Scholar] [CrossRef]
- Zorrilla, L.M.; Irvin, M.S.; Gadsby, J.E. Protein Kinase C Isoforms in the Porcine Corpus Luteum: Temporal and Spatial Expression Patterns. Domest. Anim. Endocrinol. 2009, 36, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Liu, M.; Ren, W.; Duan, J.; Yang, G.; Zhao, Y.; Fang, R.; Chen, L.; Li, T.; Yin, Y. Effects of Dietary Supplementation with Glutamate and Aspartate on Diquat-Induced Oxidative Stress in Piglets. PLoS ONE 2015, 10, e0122893. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, B.; Gao, J.; Htoo, J.K.; Chen, D. Regulation of Intestinal Health by Branched-Chain Amino Acids. Anim. Sci. J. 2018, 89, 3–11. [Google Scholar] [CrossRef]
- Zhong, H.; Fan, S.; Du, Y.; Zhang, Y.; Zhang, A.; Jiang, D.; Han, S.; Wan, B.; Zhang, G. African Swine Fever Virus MGF110-7L Induces Host Cell Translation Suppression and Stress Granule Formation by Activating the PERK/PKR-eIF2α Pathway. Microbiol. Spectr. 2022, 10, e0328222. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, B.; Guan, H.; Jiao, X.; Yang, J.; Cai, J.; Liu, Q.; Zhang, Z. Selenium Deficiency Causes Apoptosis through Endoplasmic Reticulum Stress in Swine Small Intestine. Biofactors 2021, 47, 788–800. [Google Scholar] [CrossRef]
- Guo, M.; Wu, M.H.; Korompai, F.; Yuan, S.Y. Upregulation of PKC Genes and Isozymes in Cardiovascular Tissues during Early Stages of Experimental Diabetes. Physiol. Genom. 2003, 12, 139–146. [Google Scholar] [CrossRef][Green Version]
- Pezeshki, A.; Chelikani, P.K. Effects of Roux-En-Y Gastric Bypass and Ileal Transposition Surgeries on Glucose and Lipid Metabolism in Skeletal Muscle and Liver. Surg. Obes. Relat. Dis. 2014, 10, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, A.; Zapata, R.C.; Singh, A.; Yee, N.J.; Chelikani, P.K. Low Protein Diets Produce Divergent Effects on Energy Balance. Sci. Rep. 2016, 6, 25145. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kido, J.; Matsumoto, S.; Shimizu, K.; Nakamura, K. Associations among Amino Acid, Lipid, and Glucose Metabolic Profiles in Childhood Obesity. BMC Pediatr. 2019, 19, 273. [Google Scholar] [CrossRef]
- Buyse, J.; Decuypere, E.; Berghman, L.; Kühn, E.R.; Vandesande, F. Effect of Dietary Protein Content on Episodic Growth Hormone Secretion and on Heat Production of Male Broiler Chickens. Br. Poult. Sci. 1992, 33, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Zapata, R.C.; Singh, A.; Pezeshki, A.; Avirineni, B.S.; Patra, S.; Chelikani, P.K. Low-Protein Diets with Fixed Carbohydrate Content Promote Hyperphagia and Sympathetically Mediated Increase in Energy Expenditure. Mol. Nutr. Food Res. 2019, 63, e1900088. [Google Scholar] [CrossRef]
- Zapata, R.C.; Singh, A.; Pezeshki, A.; Chelikani, P.K. Tryptophan Restriction Partially Recapitulates the Age-Dependent Effects of Total Amino Acid Restriction on Energy Balance in Diet-Induced Obese Rats. J. Nutr. Biochem. 2019, 65, 115–127. [Google Scholar] [CrossRef]
- Spring, S.; Singh, A.; Zapata, R.C.; Chelikani, P.K.; Pezeshki, A. Methionine Restriction Partly Recapitulates the Sympathetically Mediated Enhanced Energy Expenditure Induced by Total Amino Acid Restriction in Rats. Nutrients 2019, 11, 707. [Google Scholar] [CrossRef]
- Pezeshki, A.; Chelikani, P.K. Low Protein Diets and Energy Balance: Mechanisms of Action on Energy Intake and Expenditure. Front. Nutr. 2021, 8, 655833. [Google Scholar] [CrossRef]
- Trayhurn, P.; Temple, N.J.; Van Aerde, J. Evidence from Immunoblotting Studies on Uncoupling Protein That Brown Adipose Tissue Is Not Present in the Domestic Pig. Can. J. Physiol. Pharmacol. 1989, 67, 1480–1485. [Google Scholar] [CrossRef]
- Klaman, L.D.; Boss, O.; Peroni, O.D.; Kim, J.K.; Martino, J.L.; Zabolotny, J.M.; Moghal, N.; Lubkin, M.; Kim, Y.B.; Sharpe, A.H.; et al. Increased Energy Expenditure, Decreased Adiposity, and Tissue-Specific Insulin Sensitivity in Protein-Tyrosine Phosphatase 1B-Deficient Mice. Mol. Cell. Biol. 2000, 20, 5479–5489. [Google Scholar] [CrossRef] [PubMed]
- Chadt, A.; Al-Hasani, H. Glucose Transporters in Adipose Tissue, Liver, and Skeletal Muscle in Metabolic Health and Disease. Pflugers Arch.-Eur. J. Physiol. 2020, 472, 1273–1298. [Google Scholar] [CrossRef] [PubMed]
- Gould, G.W. Facilitative Glucose Transporters. In Molecular Biology Intelligence Unit; R.G. Landes: Austin, TX, USA, 1997; ISBN 978-0-412-13291-9. [Google Scholar]
- Santalucía, T.; Camps, M.; Castelló, A.; Muñoz, P.; Nuel, A.; Testar, X.; Palacin, M.; Zorzano, A. Developmental Regulation of GLUT-1 (Erythroid/Hep G2) and GLUT-4 (Muscle/Fat) Glucose Transporter Expression in Rat Heart, Skeletal Muscle, and Brown Adipose Tissue. Endocrinology 1992, 130, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.; Gannaban, R.B.; Haque, Z.F.; Dehghani, F.; Kramer, A.; Bowers, F.; Ta, M.; Huynh, T.; Ramezan, M.; Maniates, A.; et al. BCAAs Acutely Drive Glucose Dysregulation and Insulin Resistance: Role of AgRP Neurons. Nutr. Diabetes 2024, 14, 40. [Google Scholar] [CrossRef]
- De la Cruz, J.F.; Pacunla, K.W.M.; Hwang, S.G. Low Lysine Stimulates Adipogenesis through ZFP423 Upregulation in Bovine Stromal Vascular Cells. J. Anim. Sci. Technol. 2022, 64, 1173–1183. [Google Scholar] [CrossRef]
- Kovacs, P.; Hanson, R.L.; Lee, Y.-H.; Yang, X.; Kobes, S.; Permana, P.A.; Bogardus, C.; Baier, L.J. The Role of Insulin Receptor Substrate-1 Gene (IRS1) in Type 2 Diabetes in Pima Indians. Diabetes 2003, 52, 3005–3009. [Google Scholar] [CrossRef]
- Mackenzie, R.W.; Elliott, B.T. Akt/PKB Activation and Insulin Signaling: A Novel Insulin Signaling Pathway in the Treatment of Type 2 Diabetes. Diabetes Metab. Syndr. Obes. 2014, 7, 55–64. [Google Scholar] [CrossRef]
- Crossland, H.; Smith, K.; Idris, I.; Phillips, B.E.; Atherton, P.J.; Wilkinson, D.J. Exploring Mechanistic Links between Extracellular Branched-Chain Amino Acids and Muscle Insulin Resistance: An in Vitro Approach. Am. J. Physiol. Cell. Physiol. 2020, 319, C1151–C1157. [Google Scholar] [CrossRef]
- Guo, F.; Cavener, D.R. The GCN2 eIF2α Kinase Regulates Fatty-Acid Homeostasis in the Liver during Deprivation of an Essential Amino Acid. Cell Metab. 2007, 5, 103–114. [Google Scholar] [CrossRef]
- Cheng, Y.; Meng, Q.; Wang, C.; Li, H.; Huang, Z.; Chen, S.; Xiao, F.; Guo, F. Leucine Deprivation Decreases Fat Mass by Stimulation of Lipolysis in White Adipose Tissue and Upregulation of Uncoupling Protein 1 (UCP1) in Brown Adipose Tissue. Diabetes 2010, 59, 17–25. [Google Scholar] [CrossRef]
- Maida, A.; Chan, J.S.K.; Sjøberg, K.A.; Zota, A.; Schmoll, D.; Kiens, B.; Herzig, S.; Rose, A.J. Repletion of Branched Chain Amino Acids Reverses mTORC1 Signaling but Not Improved Metabolism during Dietary Protein Dilution. Mol. Metab. 2017, 6, 873–881. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, L.; Liu, Y.; Huang, P.; Song, H.; Zheng, P. The Potential Function and Clinical Application of FGF21 in Metabolic Diseases. Front. Pharmacol. 2022, 13, 1089214. [Google Scholar] [CrossRef] [PubMed]
- Berglund, E.D.; Li, C.Y.; Bina, H.A.; Lynes, S.E.; Michael, M.D.; Shanafelt, A.B.; Kharitonenkov, A.; Wasserman, D.H. Fibroblast Growth Factor 21 Controls Glycemia via Regulation of Hepatic Glucose Flux and Insulin Sensitivity. Endocrinology 2009, 150, 4084–4093. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a Novel Metabolic Regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Coskun, T.; Bina, H.A.; Schneider, M.A.; Dunbar, J.D.; Hu, C.C.; Chen, Y.; Moller, D.E.; Kharitonenkov, A. Fibroblast Growth Factor 21 Corrects Obesity in Mice. Endocrinology 2008, 149, 6018–6027. [Google Scholar] [CrossRef]
- Sarruf, D.A.; Thaler, J.P.; Morton, G.J.; German, J.; Fischer, J.D.; Ogimoto, K.; Schwartz, M.W. Fibroblast Growth Factor 21 Action in the Brain Increases Energy Expenditure and Insulin Sensitivity in Obese Rats. Diabetes 2010, 59, 1817–1824. [Google Scholar] [CrossRef]
- Xu, J.; Lloyd, D.J.; Hale, C.; Stanislaus, S.; Chen, M.; Sivits, G.; Vonderfecht, S.; Hecht, R.; Li, Y.-S.; Lindberg, R.A.; et al. Fibroblast Growth Factor 21 Reverses Hepatic Steatosis, Increases Energy Expenditure, and Improves Insulin Sensitivity in Diet-Induced Obese Mice. Diabetes 2009, 58, 250–259. [Google Scholar] [CrossRef]
- De Sousa-Coelho, A.L.; Marrero, P.F.; Haro, D. Activating Transcription Factor 4-Dependent Induction of FGF21 during Amino Acid Deprivation. Biochem. J. 2012, 443, 165–171. [Google Scholar] [CrossRef]
- Richardson, N.E.; Konon, E.N.; Schuster, H.S.; Mitchell, A.T.; Boyle, C.; Rodgers, A.C.; Finke, M.; Haider, L.R.; Yu, D.; Flores, V.; et al. Lifelong Restriction of Dietary Branched-Chain Amino Acids Has Sex-Specific Benefits for Frailty and Lifespan in Mice. Nat. Aging 2021, 1, 73–86. [Google Scholar] [CrossRef]
- Laeger, T.; Henagan, T.M.; Albarado, D.C.; Redman, L.M.; Bray, G.A.; Noland, R.C.; Münzberg, H.; Hutson, S.M.; Gettys, T.W.; Schwartz, M.W.; et al. FGF21 Is an Endocrine Signal of Protein Restriction. J. Clin. Investig. 2014, 124, 3913–3922. [Google Scholar] [CrossRef]
- Laeger, T.; Albarado, D.C.; Burke, S.J.; Trosclair, L.; Hedgepeth, J.W.; Berthoud, H.-R.; Gettys, T.W.; Collier, J.J.; Münzberg, H.; Morrison, C.D. Metabolic Responses to Dietary Protein Restriction Require an Increase in FGF21 That Is Delayed by the Absence of GCN2. Cell. Rep. 2016, 16, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.M.; Laeger, T.; Dehner, M.; Albarado, D.C.; Clarke, B.; Wanders, D.; Burke, S.J.; Collier, J.J.; Qualls-Creekmore, E.; Solon-Biet, S.M.; et al. FGF21 Signals Protein Status to the Brain and Adaptively Regulates Food Choice and Metabolism. Cell Rep. 2019, 27, 2934–2947.e3. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, O.; Tsuchida, K.; Ushiro, Y.; Hosoi, Y.; Hoshi, N.; Sugino, H.; Hasegawa, Y. cDNA Cloning and Expression of Human Activin betaE Subunit. Mol. Cell. Endocrinol. 2002, 194, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, S.Q.; Smiley, E.; Bonadio, J. Genes Coding for Mouse Activin Beta C and Beta E Are Closely Linked and Exhibit a Liver-Specific Expression Pattern in Adult Tissues. Biochem. Biophys. Res. Commun. 1997, 231, 655–661. [Google Scholar] [CrossRef]
- Namwanje, M.; Brown, C.W. Activins and Inhibins: Roles in Development, Physiology, and Disease. Cold Spring Harb. Perspect. Biol. 2016, 8, a021881. [Google Scholar] [CrossRef]
- Deli, A.; Kreidl, E.; Santifaller, S.; Trotter, B.; Seir, K.; Berger, W.; Schulte-Hermann, R.; Rodgarkia-Dara, C.; Grusch, M. Activins and Activin Antagonists in Hepatocellular Carcinoma. World. J. Gastroenterol. 2008, 14, 1699–1709. [Google Scholar] [CrossRef]
- Hashimoto, O.; Funaba, M.; Sekiyama, K.; Doi, S.; Shindo, D.; Satoh, R.; Itoi, H.; Oiwa, H.; Morita, M.; Suzuki, C.; et al. Activin E Controls Energy Homeostasis in Both Brown and White Adipose Tissues as a Hepatokine. Cell. Rep. 2018, 25, 1193–1203. [Google Scholar] [CrossRef]
- Sekiyama, K.; Ushiro, Y.; Kurisaki, A.; Funaba, M.; Hashimoto, O. Activin E Enhances Insulin Sensitivity and Thermogenesis by Activating Brown/Beige Adipocytes. J. Vet. Med. Sci. 2019, 81, 646–652. [Google Scholar] [CrossRef]
- Hashimoto, O.; Ushiro, Y.; Sekiyama, K.; Yamaguchi, O.; Yoshioka, K.; Mutoh, K.; Hasegawa, Y. Impaired Growth of Pancreatic Exocrine Cells in Transgenic Mice Expressing Human Activin betaE Subunit. Biochem. Biophys. Res. Commun. 2006, 341, 416–424. [Google Scholar] [CrossRef]
- Hashimoto, O.; Sekiyama, K.; Matsuo, T.; Hasegawa, Y. Implication of Activin E in Glucose Metabolism: Transcriptional Regulation of the Inhibin/Activin betaE Subunit Gene in the Liver. Life Sci. 2009, 85, 534–540. [Google Scholar] [CrossRef]
- Hashimoto, O.; Funaba, M. Activin in Glucose Metabolism. Vitam. Horm. 2011, 85, 217–234. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.D.; Buxton, J.M.; Culver, J.A.; Barnes, R.; Jordan, E.A.; White, A.R.; Flaherty, S.E.; Bernardo, B.; Ross, T.; Bence, K.K.; et al. Hepatic Activin E Mediates Liver-Adipose Inter-Organ Communication, Suppressing Adipose Lipolysis in Response to Elevated Serum Fatty Acids. Mol. Metab. 2023, 78, 101830. [Google Scholar] [CrossRef] [PubMed]
- Deaton, A.M.; Dubey, A.; Ward, L.D.; Dornbos, P.; Flannick, J.; AMP-T2D-GENES Consortium; Yee, E.; Ticau, S.; Noetzli, L.; Parker, M.M.; et al. Rare Loss of Function Variants in the Hepatokine Gene INHBE Protect from Abdominal Obesity. Nat. Commun. 2022, 13, 4319. [Google Scholar] [CrossRef] [PubMed]

| Ingredient % 2 | Diets 1 | ||
|---|---|---|---|
| NR | R50 | R75 | |
| Whey protein concentrate 36.17% | 6.30 | 6.30 | 6.30 |
| Dried whey powder | 10.00 | 10.00 | 10.00 |
| Corn oil | 14.80 | 14.80 | 14.80 |
| Sodium casein AMCO | 2.00 | 2.00 | 2.00 |
| Dextrose | 9.84 | 7.82 | 6.75 |
| Lactose | 28.39 | 28.39 | 28.39 |
| Dicalcium phosphate 18.5% | 3.50 | 3.50 | 3.50 |
| L-arginine | 0.60 | 0.23 | 0.04 |
| L-alanine | 0.90 | 0.31 | 0.05 |
| L-glutamic acid | 14.05 | 21.68 | 25.5 |
| L-histidine | 0.37 | 0.37 | 0.37 |
| L-isoleucine | 0.89 | 0.30 | 0.01 |
| L-leucine | 1.83 | 0.68 | 0.11 |
| L-lysine HCL | 1.83 | 0.69 | 0.13 |
| DL-methionine | 0.43 | 0.43 | 0.43 |
| L-phenylalanine | 0.87 | 0.34 | 0.08 |
| L-threonine | 0.92 | 0.33 | 0.04 |
| L-tryptophan | 0.26 | 0.26 | 0.26 |
| L-valine | 1.01 | 0.36 | 0.03 |
| Vitamin premix | 0.13 | 0.13 | 0.13 |
| Mineral premix | 0.18 | 0.18 | 0.18 |
| Salt | 0.90 | 0.90 | 0.90 |
| Calculated Chemical Composition | |||
| Dry matter, % | 96.65 | 96.88 | 97.00 |
| ME 3, Mcal/kg | 4.10 | 4.10 | 4.10 |
| Crude protein, % | 22.00 | 22.00 | 22.00 |
| Crude fat, % | 15.21 | 15.21 | 15.21 |
| Calcium, % | 0.98 | 0.98 | 0.98 |
| Total phosphorus, % | 0.75 | 0.75 | 0.75 |
| Potassium, % | 0.29 | 0.29 | 0.29 |
| SID 4 alanine, % | 1.02 | 0.51 | 0.25 |
| SID 4 arginine, % | 0.74 | 0.37 | 0.18 |
| SID 4 glutamic acid, % | 14.92 | 22.56 | 26.37 |
| SID 4 histidine, % | 0.48 | 0.48 | 0.48 |
| SID 4 isoleucine, % | 1.18 | 0.59 | 0.30 |
| SID 4 leucine, % | 2.30 | 1.15 | 0.58 |
| SID 4 lysine, % | 2.10 | 1.05 | 0.53 |
| SID 4 methionine, % | 0.54 | 0.54 | 0.54 |
| SID 4 phenylalanine, % | 1.05 | 0.52 | 0.26 |
| SID 4 threonine, % | 1.18 | 0.59 | 0.30 |
| SID 4 tryptophan, % | 0.34 | 0.34 | 0.34 |
| SID 4 valine, % | 1.31 | 0.66 | 0.33 |
| Items | Diets 1 | ||
|---|---|---|---|
| NR | R50 | R75 | |
| Arginine, % | 0.81 | 0.35 | 0.19 |
| Alanine, % | 0.71 | 0.30 | 0.24 |
| Aspartic acid, % | 0.62 | 0.51 | 0.59 |
| Cysteine, % | 0.11 | 0.10 | 0.10 |
| Glutamic acid, % | 16.28 | 22.69 | 25.06 |
| Glycine, % | 0.14 | 0.11 | 0.11 |
| Histidine, % | 0.59 | 0.43 | 0.36 |
| Hydroxylysine, % | 0.00 | 0.02 | 0.06 |
| Hydroxyproline, % | 0.00 | 0.02 | 0.00 |
| Isoleucine, % | 1.36 | 0.58 | 0.38 |
| Lanthionine 2, % | 0.18 | 0.21 | 0.07 |
| Leucine, % | 1.80 | 1.21 | 0.53 |
| Lysine, % | 1.07 | 0.58 | 0.45 |
| Methionine, % | 0.66 | 0.43 | 0.44 |
| Ornithine 2, % | 0.01 | 0.00 | 0.00 |
| Phenylalanine, % | 0.86 | 0.34 | 0.29 |
| Proline, % | 0.49 | 0.32 | 0.36 |
| Serine, % | 0.31 | 0.22 | 0.23 |
| Taurine 2, % | 0.16 | 0.16 | 0.14 |
| Threonine, % | 1.20 | 0.55 | 0.31 |
| Tryptophan, % | 0.33 | 0.35 | 0.32 |
| Tyrosine, % | 0.28 | 0.20 | 0.22 |
| Valine, % | 1.35 | 0.65 | 0.34 |
| Dry matter, % | 95.89 | 96.78 | 97.36 |
| Crude protein 3, % | 22.32 | 22.29 | 22.55 |
| Calcium, % | 0.91 | 0.90 | 0.98 |
| Phosphorus, % | 0.85 | 0.77 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorton, M.W.; Goodarzi, P.; Lei, X.; Anderson, M.; Habibi, M.; Wilson, N.; Pezeshki, A. Dietary Insulinogenic Amino Acid Restriction Improves Glucose Metabolism in a Neonatal Piglet Model. Nutrients 2025, 17, 1675. https://doi.org/10.3390/nu17101675
Gorton MW, Goodarzi P, Lei X, Anderson M, Habibi M, Wilson N, Pezeshki A. Dietary Insulinogenic Amino Acid Restriction Improves Glucose Metabolism in a Neonatal Piglet Model. Nutrients. 2025; 17(10):1675. https://doi.org/10.3390/nu17101675
Chicago/Turabian StyleGorton, Matthew W., Parniyan Goodarzi, Xia Lei, Michael Anderson, Mohammad Habibi, Nedra Wilson, and Adel Pezeshki. 2025. "Dietary Insulinogenic Amino Acid Restriction Improves Glucose Metabolism in a Neonatal Piglet Model" Nutrients 17, no. 10: 1675. https://doi.org/10.3390/nu17101675
APA StyleGorton, M. W., Goodarzi, P., Lei, X., Anderson, M., Habibi, M., Wilson, N., & Pezeshki, A. (2025). Dietary Insulinogenic Amino Acid Restriction Improves Glucose Metabolism in a Neonatal Piglet Model. Nutrients, 17(10), 1675. https://doi.org/10.3390/nu17101675






