Zinc Supplementation, Inflammation, and Gut Integrity Markers in HIV Infection: A Randomized Placebo-Controlled Trial
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Ethical Considerations
2.3. Intervention Details
2.4. Study Measurements
2.4.1. Clinical Assessment of Participants
2.4.2. Metabolic and Cardiovascular Biomarkers
2.4.3. Inflammatory and Gut Biomarkers
2.5. Statistical Analysis
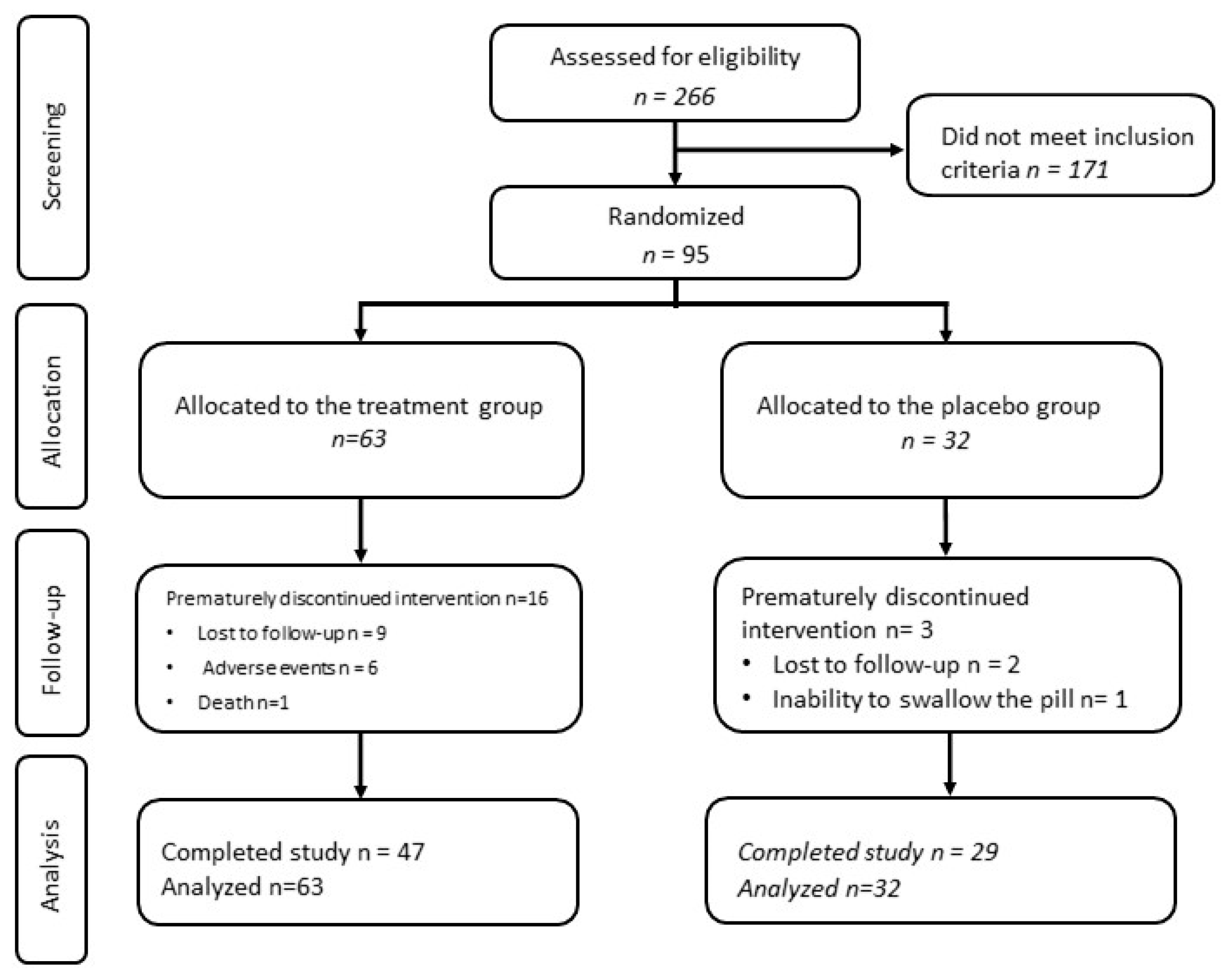
3. Results
3.1. Baseline Characteristics
3.2. Safety Analysis and Adverse Events
3.3. Primary Analysis
3.4. Stratified Analysis
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seid, A.; Seid, O.; Workineh, Y.; Dessie, G.; Bitew, Z.W. Prevalence of Undernutrition and Associated Factors among Adults Taking Antiretroviral Therapy in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. PLoS ONE 2023, 18, e0283502. [Google Scholar] [CrossRef] [PubMed]
- Sindhughosa, D.A.; Somia, I.K.A.; Merati, K.T.P.; Suryana, K. Adjunct Therapy of Zinc Supplementation Increases Immunological Response in HIV-Infected Patients: A Systematic Review and Meta-Analysis. Open AIDS J. 2022, 16, e187461362204120. [Google Scholar] [CrossRef]
- Duggal, S.; Chugh, T.D.; Duggal, A.K. HIV and Malnutrition: Effects on Immune System. J. Immunol. Res. 2012, 2012, 784740. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.K.; Lai, S.; Sales, S.; Page, J.B.; Campa, A. Randomized Controlled Clinical Trial of Zinc Supplementation to Prevent Immunological Failure in HIV-Positive Adults. Clin. Infect. Dis. 2010, 50, 1653. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Lai, S.; Shor-Posner, G.; Ma, F.; Trapido, E.; Baum, M.K. Plasma Zinc, Copper, Copper:Zinc Ratio, and Survival in a Cohort of HIV-1–Infected Homosexual Men. J. Acquir. Immune Defic. Syndr. 2001, 27, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK222310/ (accessed on 10 May 2025).
- Saper, R.B.; Rash, R. Zinc: An Essential Micronutrient. Am. Fam. Physician 2009, 79, 768–772. [Google Scholar] [PubMed]
- Ezzati, M. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors; World Health Organization: Geneva, Switzerland, 2004; Volume 1, p. 2248. ISBN 9241580313. [Google Scholar]
- Bonaventura, P.; Benedetti, G.; Albarède, F.; Miossec, P. Zinc and Its Role in Immunity and Inflammation. Autoimmun. Rev. 2015, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, R.; Ferns, G.A.; Sahebkar, A.; Mirshekar, M.A.; Jalali, M. Zinc Supplementation Is Associated with a Reduction in Serum Markers of Inflammation and Oxidative Stress in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cytokine 2021, 138, 155396. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Liu, M.J.; Lee, B.; Besecker, B.; Lai, J.P.; Guttridge, D.C.; Knoell, D.L. Zinc Modulates the Innate Immune Response in Vivo to Polymicrobial Sepsis through Regulation of NF-ΚB. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 298, L744–L754. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc Is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front. Nutr. 2014, 1, 100515. [Google Scholar] [CrossRef] [PubMed]
- Dirajlal-Fargo, S.; Yu, J.; Kulkarni, M.; Sattar, A.; Funderburg, N.; Barkoukis, H.; McComsey, G.A. Brief Report: Zinc Supplementation and Inflammation in Treated HIV. J. Acquir. Immune Defic. Syndr. 2019, 82, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulos, S.F.; Dimitropoulou, D.; Marangos, M.; Gogos, C.A. Intestinal Barrier Dysfunction in HIV Infection: Pathophysiology, Clinical Implications and Potential Therapies. Infection 2014, 42, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Bedimo, R.J.; Diaz, R.S.; Guaraldi, G.; Lo, J.; Martínez, E.; McComsey, G.A.; Milinkovic, A.; Naito, T.; Noe, S.; et al. Cardiometabolic Health in People with HIV: Expert Consensus Review. J. Antimicrob. Chemother. 2024, 79, 1218–1233. [Google Scholar] [CrossRef] [PubMed]
- Mouchati, C.; Durieux, J.C.; Zisis, S.N.; McComsey, G.A. HIV and Race Are Independently Associated with Endothelial Dysfunction. AIDS 2023, 37, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc Status Is Associated with Inflammation, Oxidative Stress, Lipid, and Glucose Metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef] [PubMed]
- ASCVD Risk Estimator+. Available online: https://tools.acc.org/ascvd-risk-estimator-plus/#!/calculate/estimate/ (accessed on 1 December 2024).
- Chevalier, M.F.; Petitjean, G.; Dunyach-Rémy, C.; Didier, C.; Girard, P.M.; Manea, M.E.; Campa, P.; Meyer, L.; Rouzioux, C.; Lavigne, J.P.; et al. The Th17/Treg Ratio, IL-1RA and SCD14 Levels in Primary HIV Infection Predict the T-Cell Activation Set Point in the Absence of Systemic Microbial Translocation. PLoS Pathog. 2013, 9, e1003453. [Google Scholar] [CrossRef] [PubMed]
- Sandler, N.G.; Wand, H.; Roque, A.; Law, M.; Nason, M.C.; Nixon, D.E.; Pedersen, C.; Ruxrungtham, K.; Lewin, S.R.; Emery, S.; et al. Plasma Levels of Soluble CD14 Independently Predict Mortality in HIV Infection. J. Infect. Dis. 2011, 203, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Novelli, S.; Lécuroux, C.; Goujard, C.; Reynes, J.; Villemant, A.; Blum, L.; Essat, A.; Avettand-Fenoël, V.; Launay, O.; Molina, J.M.; et al. Persistence of Monocyte Activation under Treatment in People Followed since Acute HIV-1 Infection Relative to Participants at High or Low Risk of HIV Infection. eBioMedicine 2020, 62, 103129. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, M.S.; Cheng, D.M.; Gnatienko, N.; Blokhina, E.; Coleman, S.M.; Doyle, M.F.; Yaroslavtseva, T.; Bridden, C.; So-Armah, K.; Tracy, R.; et al. Effect of Zinc Supplementation vs Placebo on Mortality Risk and HIV Disease Progression Among HIV-Positive Adults with Heavy Alcohol Use: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e204330. [Google Scholar] [CrossRef] [PubMed]
| Both Groups | Placebo | Zinc 90 mg | p-Value * | ||
|---|---|---|---|---|---|
| N = 95 | N = 32 | N = 63 | |||
| Age (years) | 52.42 (40.34–60.12) | 53.29 (45.31–58.90) | 52.42 (38.92–60.55) | 0.65 | |
| Male | 70 (74%) | 26 (81%) | 44 (70%) | 0.23 | |
| Race | 0.80 | ||||
| African American | 56 (59%) | 18 (56%) | 38 (60%) | ||
| Caucasian | 37 (39%) | 14 (44%) | 23 (36%) | ||
| Native American | 1 (1%) | 0 (0%) | 1 (2%) | ||
| Other | 1 (1%) | 0 (0%) | 1 (2%) | ||
| Ethnicity | 0.38 | ||||
| Hispanic or Latino | 11 (12%) | 5 (16%) | 6 (10%) | ||
| Alcohol (current) | 66 (69%) | 21 (66%) | 45 (71%) | 0.19 | |
| Smoking (current) | 29 (31%) | 13 (41%) | 16 (25%) | 0.31 | |
| Zinc Level | |||||
| Zinc (μg/dL) | 69.80 (64.30–73.00) | 69.00 (61.20–73.00) | 70.00 (66.00–72.20) | 0.61 | |
| HIV Profile | |||||
| CD4+ T-cell count | 722.00 (526.00–973.00) | 668.00 (507.00–775.00) | 747.00 (529.00–1024.00) | 0.18 | |
| HIV RNA (<20 copies) (%) | 74 (80%) | 23 (72%) | 51 (81%) | 0.31 | |
| ART duration (months) | 171.86 (108.22–236.36) | 167.86 (115.14–239.51) | 178.76 (107.20–232.51) | 0.88 | |
| PI USE | 15 (16%) | 7 (23%) | 8 (13%) | 0.25 | |
| Metabolic Markers | |||||
| BMI (kg/m2) | 28.08 (24.62–32.70) | 27.45 (23.23–32.40) | 28.34 (24.80–32.70) | 0.46 | |
| Waist circumference (cm) | 97.00 (89.17–106.33) | 97.83 (87.50–106.42) | 96.67 (89.83–106.33) | 0.94 | |
| Systolic blood pressure (mmHg) | 126.00 (117.00–141.00) | 129.00 (118.50–141.00) | 125.00 (117.00–139.00) | 0.55 | |
| Diastolic blood pressure (mmHg) | 81.00 (75.00–86.00) | 82.00 (77.00–88.50) | 80.00 (75.00–85.00) | 0.30 | |
| Non-HDL cholesterol (mg/dL) | 115.30 (95.70–149.20) | 115.50 (93.90–166.25) | 115.30 (98.00–147.60) | 0.79 | |
| HDL (mg/dL) | 47.00 (39.90–61.10) | 48.15 (41.30–62.70) | 45.40 (39.80–60.80) | 0.48 | |
| LDL (mg/dL) | 96.00 (71.00–118.00) | 89.00 (66.50–127.50) | 96.00 (74.00–114.00) | 0.81 | |
| VLDL (mg/dL) | 21.00 (15.00–28.50) | 20.00 (15.00–27.00) | 22.00 (15.00–29.00) | 0.69 | |
| Cholesterol (mg/dL) | 169.00 (144.00–202.00) | 165.50 (147.50–213.50) | 171.00 (144.00–192.00) | 0.81 | |
| Chol:HDL ratio | 3.40 (2.70–4.40) | 3.20 (2.55–4.85) | 3.50 (2.80–4.40) | 0.47 | |
| Triglycerides (mg/dL) | 105.00 (74.00–146.00) | 103.00 (76.50–136.00) | 108.00 (71.00–146.00) | 0.89 | |
| Insulin (uIU/mL) | 10.50 (6.00–18.00) | 11.50 (6.50–18.50) | 10.00 (6.00–17.00) | 0.84 | |
| 10-year ASCVD (%) | 6.50 (2.90–11.80) | 6.70 (3.85–12.50) | 6.10 (2.70–11.10) | 0.44 | |
| Presence of metabolic syndrome | 40 (42%) | 14 (44%) | 26 (41%) | ||
| Endothelial Function | |||||
| Reactive Hyperemic Index | 1.90 (1.60–2.19) | 1.89 (1.62–2.04) | 1.91 (1.60–2.21) | 0.47 | |
| Augmentation Index | 10.00 (−1.00–15.00) | 11.00 (4.00–16.00) | 7.50 (−2.00–14.00) | 0.22 | |
| Inflammatory Markers | |||||
| hsCRP (ng/mL) | 2202.14 (993.51–7795.00) | 1998.85 (967.08–6778.08) | 2240.00 (1075.40–7930.31) | 0.66 | |
| sCD14 (ng/mL) | 1681.63 (1468.60–1966.05) | 1727.05 (1525.77–1963.90) | 1671.20 (1424.41–2001.68) | 0.68 | |
| sCD163 (ng/mL) | 568.55 (420.62–780.63) | 593.61 (461.03–784.74) | 543.40 (366.03–778.58) | 0.26 | |
| sTNF-RI (pg/mL) | 1006.42 (881.12–1195.08) | 977.39 (819.30–1200.68) | 1036.85 (890.50–1183.57) | 0.54 | |
| sTNF-RII (pg/mL) | 2234.54 (1907.77–2912.41) | 2253.23 (1923.78–3031.33) | 2162.27 (1889.96–2867.83) | 0.63 | |
| D-dimer (ng/mL) | 536.22 (366.79–809.72) | 520.93 (293.47–717.11) | 558.43 (389.36–883.46) | 0.67 | |
| oxLDL (U/L) | 50145.48 (39945.47–70630.87) | 47606.57 (37342.25–62313.38) | 51731.12 (41091.65–75262.57) | 0.24 | |
| IL−6 (pg/mL) | 1.94 (1.31–3.46) | 1.89 (1.42–3.51) | 2.11 (1.26–3.46) | 0.70 | |
| VCAM (ng/mL) | 783.99 (671.49–970.46) | 857.01 (693.53–1013.78) | 758.04 (658.95–950.49) | 0.22 | |
| ICAM (ng/mL) | 247.37 (180.09–293.93) | 250.13 (175.81–290.17) | 244.28 (180.09–298.24) | 0.70 | |
| IP−10 (pg/mL) | 134.48 (99.61–199.31) | 134.70 (90.04–190.78) | 130.52 (102.35–207.19) | 0.55 | |
| Gut Integrity Markers | |||||
| LBP (μg/mL) | 16.6 (12.77–25.77) | 16.44 (12.55–20.35) | 16.6 (13.16–30.47) | 0.33 | |
| IFAB (pg/mL) | 1730.93 (1087.46–2681.82) | 1616.94 (996.70–2295.69) | 1826.49 (1131.22–2741.16) | 0.24 | |
| BDG (pg/mL) | 106.08 (77.72–168.20) | 122.51 (87.07–197.11) | 98.46 (72.89–164.33) | 0.24 | |
| Zonulin (μg/mL) | 1030 (674.68–1440) | 1040 (665.92–1460) | 1020 (674.68–1390) | 0.80 | |
| Placebo | Zinc 90 mg | p-Value | ||
|---|---|---|---|---|
| Absolute change | ||||
| Zinc Level | ||||
| Zinc (μg/dL) | 8.60 (1.65–20.00) | 33.50 (14.00–62.70) | <0.01 | |
| HIV Profile | ||||
| CD4+ T-cell count | 0.00 (−109.00–73.00) | 0.00 (−63.00–38.00) | 0.79 | |
| Metabolic Markers | ||||
| BMI (kg/m2) | 0.28 (−0.52–1.16) | 0.10 (−0.58–0.95) | 0.67 | |
| Weight (lbs) | 2.20 (−3.50–7.60) | 0.00 (−4.00–4.40) | 0.42 | |
| Waist-umbilicus (cm) | 0.50 (−2.53–2.50) | 1.67 (−2.67–3.00) | 0.39 | |
| Systolic blood pressure (mmHg) | 6.00 (−4.00–12.00) | 0.00 (−9.00–9.00) | 0.57 | |
| Diastolic blood pressure (mmHg) | 3.00 (−5.00–6.00) | 0.00 (−5.00–5.00) | 0.89 | |
| Non-HDL cholesterol (mg/dL) | 3.10 (−28.20–26.50) | 1.35 (−12.30–10.85) | 0.64 | |
| HDL (mg/dL) | −1.70 (−4.60–1.40) | −2.45 (−8.45–1.25) | 0.49 | |
| LDL (mg/dL) | 6.00 (−28.00–16.00) | −1.50 (−19.50–11.50) | 0.38 | |
| VLDL (mg/dL) | 0.50 (−6.00–3.50) | 2.00 (−2.00–6.00) | 0.23 | |
| Cholesterol (mg/dL) | 2.00 (−25.00–27.00) | −3.50 (−23.50–12.00) | 0.47 | |
| Chol:HDL ratio | 0.10 (−0.20–0.40) | 0.10 (−0.10–0.45) | 0.52 | |
| Triglycerides (mg/dL) | 2.00 (−29.00–12.00) | 7.00 (−12.50–34.50) | 0.21 | |
| 10-year ASCVD (%) | 0.40 (−1.10–2.00) | 0.00 (−1.35–1.55) | 0.61 | |
| Endothelial Function | ||||
| Reactive Hyperemic Index | −0.34 (−0.77–0.16) | −0.18 (−0.60–0.23) | 0.77 | |
| Augmentation Index | 0.00 (−8.00–5.50) | −1.00 (−7.00–6.00) | 0.48 | |
| Inflammatory Markers | ||||
| hsCRP (ng/mL) | −22.86 (−689.27–1744.48) | 1.33 (−602.56–1174.87) | 0.97 | |
| sCD14 (ng/mL) | 101.71 (−90.50–243.20) | −56.31 (−263.24–134.19) | 0.02 | |
| sCD163 (ng/mL) | −50.11 (−153.06–89.80) | 43.69 (−102.27–126.91) | 0.73 | |
| sTNF-RI (pg/mL) | −25.08 (−209.51–186.66) | 1.27 (−236.80–168.27) | 0.92 | |
| sTNF-RII (pg/mL) | −121.65 (−457.93–467.29) | 99.33 (−484.31–477.72) | 0.93 | |
| D-dimer (ng/mL) | −149.05 (−294.46–58.72) | −45.85 (−295.57–142.43) | 0.61 | |
| oxLDL (U/L) | 20,833.33 (2397.72–61,644.46) | 8639.79 (−1716.35–43,693.14) | 0.31 | |
| IL−6 (pg/mL) | −0.47 (−0.85–0.61) | −0.02 (−0.88–0.72) | 0.51 | |
| VCAM (ng/mL) | 66.69 (−2.65–187.38) | 49.53 (−55.61–170.15) | 0.38 | |
| ICAM (ng/mL) | −2.03 (−28.37–22.30) | 2.35 (−29.19–42.46) | 0.45 | |
| IP−10 (pg/mL) | 0.23 (−44.28–29.08) | −9.99 (−31.65–20.27) | 0.51 | |
| Gut Integrity Markers | ||||
| LBP (μg/mL) | 0.47(−4.8–8.35) | 0.72 (−4.52–6.9) | 0.94 | |
| IFAB (pg/mL) | 100.82 (−747.86–953.32) | 58.89 (−1049.29–654.96) | 0.46 | |
| BDG (pg/mL) | −28.19 (−100.43–4.02) | 8.36 (−63.26–76.12) | 0.14 | |
| Zonulin (μg/mL) | 78.2 (−138–506.86) | 270.54 (59.36–600.45) | 0.15 | |
| Relative change (%) | ||||
| Zinc Level | ||||
| Zinc (μg/dL) | 12.92 (2.36–31.15) | 53.17 (19.44–87.08) | 0.0001 | |
| HIV Profile | ||||
| CD4+ T-cell count | 0.00 (−14.12–9.81) | 0.00 (−13.26–4.91) | 0.80 | |
| Metabolic Markers | ||||
| BMI (kg/m2) | 0.78 (−2.30–3.23) | 0.42 (−2.83–3.36) | 0.68 | |
| Weight (lbs) | 1.36 (−2.13–4.25) | 0.00 (−2.83–3.05) | 0.46 | |
| Waist-umbilicus (cm) | 0.62 (−1.90–2.43) | 1.67 (−2.76–3.49) | 0.52 | |
| Systolic blood pressure (mmHg) | 4.65 (−3.10–9.85) | 0.00 (−7.26–7.83) | 0.59 | |
| Diastolic blood pressure (mmHg) | 3.49 (−6.41–7.23) | 0.00 (−6.49–6.49) | 0.90 | |
| Non-HDL cholesterol (mg/dL) | 3.97 (−16.95–30.09) | 0.78 (−9.91–10.67) | 0.60 | |
| HDL (mg/dL) | −3.51 (−9.81–3.51) | −4.94 (−16.16–2.44) | 0.42 | |
| LDL (mg/dL) | 9.23 (−25.23–25.40) | −1.95 (−18.27–14.05) | 0.32 | |
| VLDL (mg/dL) | 3.33 (−22.47–22.05) | 12.50 (−7.27–36.36) | 0.23 | |
| Cholesterol (mg/dL) | 1.72 (−12.50–17.59) | −1.59 (−12.82–6.91) | 0.73 | |
| Chol:HDL ratio | 3.45 (−7.41–17.39) | 4.08 (−3.47–13.13) | 0.64 | |
| Triglycerides (mg/dL) | 0.73 (−23.18–20.45) | 10.66 (−8.42–36.46) | 0.19 | |
| 10-year ASCVD (%) | 10.00 (−15.94–25.51) | 0.00 (−27.87–28.00) | 0.91 | |
| Endothelial Function | ||||
| Reactive Hyperemic Index | −18.54 (−31.73–10.74) | −10.16 (−27.27–13.79) | 0.74 | |
| Augmentation Index | 0.00 (−69.70–75.00) | −7.14 (−52.94–50.00) | 0.99 | |
| Inflammatory Markers | ||||
| hsCRP (ng/mL) | −1.58 (−41.77–76.81) | 1.10 (−33.16–79.09) | 0.84 | |
| sCD14 (ng/mL) | 6.57 (−5.25–17.33) | −2.87 (−15.74–7.84) | 0.02 | |
| sCD163 (ng/mL) | −6.91 (−19.48–14.43) | 7.63 (−17.77–28.19) | 0.25 | |
| sTNF-RI (pg/mL) | −2.19 (−22.55–17.72) | 0.11 (−15.68–16.26) | 0.71 | |
| sTNF-RII (pg/mL) | −4.32 (−20.66–24.72) | 5.48 (−21.53–19.40) | 0.69 | |
| D-dimer (ng/mL) | −22.00 (−54.24–12.08) | −16.75 (−45.32–32.14) | 0.32 | |
| oxLDL (U/L) | 50.91 (4.50–171.86) | 15.24 (−4.46–81.64) | 0.20 | |
| IL−6 (pg/mL) | −26.59 (−50.50–32.77) | −1.67 (−43.43–45.02) | 0.29 | |
| VCAM (ng/mL) | 7.95 (−0.27–26.61) | 6.44 (−4.73–21.81) | 0.39 | |
| ICAM (ng/mL) | −1.64 (−13.54–6.81) | 1.52 (−10.99–17.82) | 0.10 | |
| IP−10 (pg/mL) | 0.20 (−30.21–25.82) | −9.95 (−18.18–18.14) | 0.56 | |
| Gut Integrity Markers | ||||
| LBP (ng/mL) | 3.56 (−28.23–55.41) | 6.80 (−25.55–53.60) | 0.89 | |
| IFAB (pg/mL) | 4.74 (−37.57–75.31) | 3.95 (−39.73–52.54) | 0.42 | |
| BDG (pg/mL) | −25.89 (−51.03–3.45) | 9.71 (−38.45–85.52) | 0.13 | |
| Zonulin (ng/mL) | 15.70 (−14.77–56.66) | 25.70 (10.18–84.41) | 0.17 | |
| Outcome Variable | SE () | p-Value | |||
|---|---|---|---|---|---|
| Zinc Level | |||||
| Ln zinc (μg/dL) | 0.71 | 0.14 | 0.43, 0.98 | <0.01 | |
| Metabolic Markers | |||||
| BMI (kg/m2) | −0.35 | 2.01 | −4.29, 3.60 | 0.86 | |
| Weight (lbs) | −2.99 | 12.23 | −26.97, 20.99 | 0.81 | |
| Ln waist-umbilicus (cm) | −0.01 | 0.03 | −0.08, 0.05 | 0.74 | |
| Ln systolic blood pressure (mmHg) | −0.02 | 0.03 | −0.07, 0.03 | 0.41 | |
| Diastolic blood pressure (mmHg) | −1.68 | 2.06 | −5.71, 2.36 | 0.42 | |
| Ln non-HDL cholesterol (mg/dL) | 0.01 | 0.07 | −0.12, 0.15 | 0.84 | |
| Ln HDL (mg/dL) | −0.08 | 0.06 | −0.19, 0.04 | 0.18 | |
| Ln LDL (mg/dL) | −0.02 | 0.09 | −0.19, 0.15 | 0.84 | |
| Ln VLDL (mg/dL) | 0.07 | 0.11 | −0.15, 0.29 | 0.55 | |
| Ln cholesterol (mg/dl) | −0.01 | 0.05 | −0.11, 0.09 | 0.78 | |
| Ln Chol:HDL ratio | 0.06 | 0.07 | −0.07, 0.20 | 0.36 | |
| Ln triglycerides (mg/dL) | 0.07 | 0.12 | −0.17, 0.31 | 0.58 | |
| Ln 10-year ASCVD (%) | −0.04 | 0.11 | −0.26, 0.17 | 0.70 | |
| Metabolic syndrome | −0.44 | 0.47 | −1.36, 0.48 | 0.35 | |
| Endothelial Function | |||||
| Reactive Hyperemic Index | −0.03 | 0.07 | −0.17, 0.10 | 0.62 | |
| Augmentation Index | 0.59 | 2.43 | −4.19, 5.36 | 0.81 | |
| Inflammatory Markers | |||||
| Ln hsCRP (ng/mL) | −0.002 | 0.29 | −0.57, 0.57 | 1.00 | |
| Ln sCD14 (ng/mL) | −0.08 | 0.05 | −0.18, 0.01 | 0.08 | |
| Ln sCD163 (ng/mL) | −0.03 | 0.09 | −0.21, 0.15 | 0.77 | |
| Ln sTNF-RI (pg/mL) | 0.04 | 0.06 | −0.09, 0.16 | 0.55 | |
| Ln sTNF-RII (pg/mL) | −0.004 | 0.07 | −0.13, 0.13 | 0.95 | |
| Ln D-dimer (ng/mL) | 0.2 | 0.15 | −0.10, 0.50 | 0.19 | |
| oxLDL (U/L) | 1819.18 | 8530.03 | −14,899.37, 18,537.74 | 0.83 | |
| Ln IL−6 (pg/mL) | 0.07 | 0.19 | −0.30, 0.43 | 0.73 | |
| Ln VCAM (ng/mL) | −0.19 | 0.11 | −0.41, 0.04 | 0.10 | |
| ICAM (ng/mL) | 0.41 | 20.19 | −39.16, 39.98 | 0.98 | |
| Ln IP−10 (pg/mL) | 0.12 | 0.18 | −0.22, 0.47 | 0.48 | |
| Gut Integrity Markers | |||||
| Ln LBP (ng/mL) | 0.05 | 0.14 | −0.21, 0.32 | 0.69 | |
| Ln IFAB (pg/mL) | 0.02 | 0.14 | −0.26, 0.30 | 0.90 | |
| Ln BDG (pg/mL) | 0.18 | 0.15 | −0.12, 0.47 | 0.24 | |
| Ln zonulin (ng/mL) | 0.09 | 0.13 | −0.16, 0.34 | 0.49 | |
| Above-Median Zinc Group, Beta (p-Value) | Below-Median Zinc Group, Beta (p-Value) | ||||
|---|---|---|---|---|---|
| N = 46 | N = 47 | ||||
| Zinc Level | |||||
| Zinc (μg/dL) | 23.8 | 0.04 | 32.02 | 0.001 | |
| Metabolic Markers | |||||
| BMI (kg/m2) | −2.67 | 0.37 | 2.8 | 0.31 | |
| Ln weight (lbs) | −0.2 | 0.12 | 0.17 | 0.15 | |
| Ln waist-umbilicus (cm) | −0.06 | 0.24 | 0.04 | 0.35 | |
| Ln systolic blood pressure (mmHg) | −0.05 | 0.18 | −0.001 | 0.97 | |
| Diastolic blood pressure (mmHg) | −5.67 | 0.06 | 1.48 | 0.60 | |
| Ln non-HDL cholesterol (mg/dL) | −0.06 | 0.58 | 0.1 | 0.30 | |
| Ln HDL (mg/dL) | 0.02 | 0.85 | −0.21 | 0.01 | |
| Ln LDL (mg/dL) | −0.08 | 0.55 | 0.03 | 0.77 | |
| Ln VLDL (mg/dL) | −0.01 | 0.97 | 0.21 | 0.23 | |
| Ln cholesterol (mg/dL) | −0.04 | 0.58 | 0.01 | 0.90 | |
| Chol:HDL ratio | 0.26 | 0.72 | 0.71 | 0.25 | |
| Triglycerides (mg/dL) | 15.76 | 0.66 | 30.66 | 0.4 | |
| 10-year ASCVD (%) | −2.59 | 0.29 | 2.1 | 0.47 | |
| Metabolic syndrome | −0.87 | 0.22 | 0.12 | 0.85 | |
| Endothelial Function | |||||
| Reactive Hyperemic Index | −0.08 | 0.49 | 0.10 | 0.55 | |
| Augmentation Index | 4.95 | 0.30 | −4.25 | 0.38 | |
| Inflammatory Markers | |||||
| hsCRP (ng/mL) | −223.09 | 0.79 | 7.96 | 1.00 | |
| Ln sCD14 (ng/mL) | −0.08 | 0.27 | −0.12 | 0.07 | |
| Ln sCD163 (ng/mL) | −0.06 | 0.63 | −0.05 | 0.74 | |
| Ln sTNF-RI (pg/mL) | 0.07 | 0.41 | 0.02 | 0.83 | |
| Ln sTNF-RII (pg/mL) | 0.07 | 0.51 | −0.03 | 0.75 | |
| Ln D-dimer (ng/mL) | 0.10 | 0.65 | 0.24 | 0.27 | |
| Ln oxLDL (U/L) | 0.01 | 0.96 | −0.06 | 0.70 | |
| Ln IL−6 (pg/mL) | 0.08 | 0.75 | 0.07 | 0.81 | |
| VCAM (ng/mL) | −84.53 | 0.48 | −99.09 | 0.32 | |
| ICAM (ng/mL) | 22.08 | 0.31 | −20.7 | 0.54 | |
| Ln IP−10 (pg/mL) | 0.2 | 0.44 | 0.11 | 0.62 | |
| Gut Integrity Markers | |||||
| LBP (ng/mL) | −2.64 | 0.51 | 4.25 | 0.24 | |
| Ln IFAB (pg/mL) | 0.23 | 0.23 | −0.21 | 0.32 | |
| Ln BDG (pg/mL) | 0.31 | 0.11 | 0.15 | 0.50 | |
| Ln zonulin (ng/mL) | 0.31 | 0.03 | −0.13 | 0.53 | |
| Other Biomarkers | At Baseline | At Week 24 | |||
|---|---|---|---|---|---|
| Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | ||
| Metabolic Markers | |||||
| BMI (kg/m2) | 0.0511 | 0.63 | −0.0771 | 0.50 | |
| Weight (lbs) | 0.0863 | 0.41 | −0.0844 | 0.46 | |
| Waist-umbilicus (cm) | −0.0115 | 0.91 | 0.0191 | 0.87 | |
| Systolic blood pressure (mmHg) | 0.1029 | 0.33 | 0.1457 | 0.21 | |
| Diastolic blood pressure (mmHg) | 0.1896 | 0.07 | 0.1668 | 0.15 | |
| Non-HDL cholesterol (mg/dL) | −0.0061 | 0.95 | 0.1146 | 0.32 | |
| HDL (mg/dL) | 0.1047 | 0.32 | −0.1329 | 0.25 | |
| LDL (mg/dL) | 0.016 | 0.88 | 0.0113 | 0.92 | |
| VLDL (mg/dl) | −0.0404 | 0.71 | 0.2529 | 0.03 | |
| Cholesterol (mg/dL) | 0.0392 | 0.71 | 0.0711 | 0.54 | |
| Chol:HDL ratio | −0.0338 | 0.75 | 0.1935 | 0.09 | |
| Triglycerides (mg/dL) | −0.0227 | 0.83 | 0.2715 | 0.02 | |
| 10-year ASCVD (%) | 0.1096 | 0.29 | 0.286 | 0.01 | |
| Metabolic syndrome | −0.0715 | 0.49 | −0.0696 | 0.55 | |
| Endothelial Function | |||||
| Reactive Hyperemic Index | 0.0013 | 0.99 | 0.0572 | 0.64 | |
| Augmentation Index | 0.0335 | 0.75 | 0.1219 | 0.30 | |
| Inflammatory Markers | |||||
| hsCRP (ng/mL) | 0.068 | 0.52 | −0.0551 | 0.65 | |
| sCD14 (ng/mL) | −0.0835 | 0.43 | −0.1329 | 0.27 | |
| sCD163 (ng/mL) | −0.0687 | 0.51 | 0.0484 | 0.69 | |
| sTNF-RI (pg/mL) | −0.0059 | 0.96 | −0.0052 | 0.97 | |
| sTNF-RII (pg/mL) | 0.031 | 0.77 | −0.0266 | 0.82 | |
| D-dimer (ng/mL) | 0.0908 | 0.39 | 0.0623 | 0.60 | |
| oxLDL (U/L) | −0.0415 | 0.69 | 0.1365 | 0.25 | |
| IL−6 (pg/mL) | −0.0262 | 0.80 | −0.149 | 0.21 | |
| VCAM (ng/mL) | −0.2043 | 0.05 | 0.0891 | 0.46 | |
| ICAM (ng/mL) | −0.0278 | 0.79 | −0.0419 | 0.73 | |
| IP−10 (pg/mL) | 0.0874 | 0.40 | −0.0372 | 0.76 | |
| Gut Integrity Markers | |||||
| LBP (ng/mL) | −0.0923 | 0.38 | 0.0987 | 0.41 | |
| IFAB (pg/mL) | −0.2286 | 0.03 | −0.0082 | 0.95 | |
| BDG (pg/mL) | −0.0639 | 0.54 | 0.1719 | 0.15 | |
| Zonulin (ng/mL) | 0.0422 | 0.69 | −0.0699 | 0.56 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baissary, J.; Koberssy, Z.; Wu, Q.; Sattar, A.; Atieh, O.; Daher, J.; Ailstock, K.; Cummings, M.; Labbato, D.; Funderburg, N.T.; et al. Zinc Supplementation, Inflammation, and Gut Integrity Markers in HIV Infection: A Randomized Placebo-Controlled Trial. Nutrients 2025, 17, 1671. https://doi.org/10.3390/nu17101671
Baissary J, Koberssy Z, Wu Q, Sattar A, Atieh O, Daher J, Ailstock K, Cummings M, Labbato D, Funderburg NT, et al. Zinc Supplementation, Inflammation, and Gut Integrity Markers in HIV Infection: A Randomized Placebo-Controlled Trial. Nutrients. 2025; 17(10):1671. https://doi.org/10.3390/nu17101671
Chicago/Turabian StyleBaissary, Jhony, Ziad Koberssy, Qian Wu, Abdus Sattar, Ornina Atieh, Joviane Daher, Kate Ailstock, Morgan Cummings, Danielle Labbato, Nicholas T. Funderburg, and et al. 2025. "Zinc Supplementation, Inflammation, and Gut Integrity Markers in HIV Infection: A Randomized Placebo-Controlled Trial" Nutrients 17, no. 10: 1671. https://doi.org/10.3390/nu17101671
APA StyleBaissary, J., Koberssy, Z., Wu, Q., Sattar, A., Atieh, O., Daher, J., Ailstock, K., Cummings, M., Labbato, D., Funderburg, N. T., & McComsey, G. A. (2025). Zinc Supplementation, Inflammation, and Gut Integrity Markers in HIV Infection: A Randomized Placebo-Controlled Trial. Nutrients, 17(10), 1671. https://doi.org/10.3390/nu17101671





