The Interplay Between Psychological and Neurobiological Predictors of Weight Regain: A Narrative Review
Abstract
:1. Introduction
2. Methods
- “Personality Traits” [MeSH] OR “Personality” OR “Emotional regulation” OR “Mental Health” [MeSH] OR “Emotional States” OR “Cognitive Distortions” OR “Dichotomous Thinking”.
- “Brain” [MeSH] OR “Brain Activity” OR “Neural Activation” OR “Neural Mechanisms” OR “Food Addiction” [MeSH] OR “Dopamine” [MeSH] OR “Reward System” OR “Metabolic Adaptations”.
- ”Weight Gain” [MeSH] OR “Weight Regain” OR “Body Weight” [MeSH] OR “Weight Loss” [MeSH] OR “Weight Maintenance” OR “Weight Control” OR “Obesity” [MeSH] OR “Weight Management”.
- “Bariatric Surgery” [MeSH] OR “Metabolic Surgery” OR “Pharmacotherapy” [MeSH] OR “Anti-Obesity Agents” [MeSH] OR “Pharmacological Treatment”.
- (1 AND 3) OR (2 AND 3) OR (3 AND 4).
3. Results
3.1. Psychological Determinants of WR
3.1.1. Personality Traits and WR
3.1.2. Emotional Component
3.1.3. Dichotomous Thinking
3.2. Neurobiological Factors in WR
3.2.1. Food Addiction, Reward Sensitivity, and Neural Responses to Food Stimuli
3.2.2. Metabolic Adaptations
3.3. The Effects of Dietary, Pharmacological, and Bariatric Approaches on WR
3.3.1. Dietary Weight Loss Interventions
3.3.2. Bariatric Surgery
3.3.3. Pharmacotherapy
| AOMs | Weight-Lowering Mechanisms of Action | Influence of WR |
|---|---|---|
| Orlistat | Inhibits lipase—blocks fat absorption in the intestine | after diet-induced weight loss: Richelsen et al. (2007) [224]: Following initial very-low-energy diet-induced weight loss (14.4 ± 2.0 kg), orlistat treatment (120 mg/3 times a day over 3 years) resulted in lower weight regain (4.6 ± 8.6 kg vs. 7.0 ± 7.1 kg; p < 0.02) and a higher proportion of patients achieving ≥5% weight loss compared to placebo. Sjöström et al. (1998) [225]: In a 2-year randomized controlled trial, orlistat (120 mg/3 times a day) resulted in greater weight loss compared to placebo (10.2% vs. 6.1%; p < 0.001) during the first year on a slightly hypocaloric diet (600 kcal/day deficit) and less weight regain in the second year (0.9 kg loss vs. 2.5 kg regain; p < 0.001). after BS-induced weight loss: Zoss I et al. (2002) [226]: Orlistat (120 mg/3 times a day for 8 months) resulted in greater weight loss compared to controls (8 ± 3 kg vs. 3 ± 2 kg; p < 0.01) in patients with adjustable gastric banding who had stopped losing weight. |
| Phentermine-topiramate | Phentermine suppresses appetite via norepinephrine; topiramate affects satiety through CNS effects | after BS-induced weight loss: Istfan, N.W. et al. (2020) [221]: WR after RYGB in patients treated with phentermine/topiramate, topiramate, or phentermine was approximately 10% lower at the end of the 6-year observation period compared to placebo. Schwartz, J. et al. (2016) [227]: Phentermine and topiramate effectively reduced weight loss (12–13%) in patients who experienced WR or a weight loss plateau after RYBG/LAGB. |
| Naltrexone-bipropion | Bupropion modulates dopamine/norepinephrine; naltrexone blocks food-related reward pathways | no data |
| Liraglutide [193] | GLP-1 receptor agonist—increases satiety, delays gastric emptying, and reduces appetite | after BS-induced weight loss: de Moraes et al. (2024) [228]: A systematic review and meta-analysis (16 studies, 881 individuals, mean follow-up time from 3 months to 4 years) showed that liraglutide led to significant reductions in BMI (−8.56 kg/m2; p < 0.01) and a mean reduction in total weight (−16.03 kg; p = 0.05) in patients who experienced WR after BS. Vinciguerra et al. (2024) [229]: Meta-analysis (119 individuals) showed that liraglutide at 3 mg led to weight loss (5.6 ± 2.6% at 12 weeks and 9.3 ± 3.6% at 24 weeks) with a significant reduction in waist circumference (p < 0.0001) in patients who experienced inadequate weight loss or WR after BS. Jamal et al. (2024) [230]: In patients who underwent SG, 3-month liraglutide treatment resulted in a significant mean weight loss of 5.94 kg (6.2% of pre-treatment weight; p < 0.001), with greater weight reduction observed in patients aged 31–40 years and those tolerating doses ≥2.4 mg, suggesting liraglutide as an effective and dose-responsive adjunct therapy for managing WR or inadequate weight loss post-surgery. |
| Semaglutide | GLP-1 receptor agonist—increases satiety, delays gastric emptying, and reduces appetite | after BS-induced weight loss: Lautenbach, A. et al. (2022) [231]: In patients who experienced WR or inadequate weight loss after BS, semaglutide treatment led to a mean total weight loss of 6.0% at 3 months and 10.3% at 6 months. Murvelashvili, N. et al. (2023) [232]: In patients with post-bariatric surgery WR, semaglutide (1.0 mg weekly) led to superior weight loss (−12.92%) compared with liraglutide (−8.77%). Kanai, R. et al. (2024) [233]: In patients with obesity and T2D after LSG, semaglutide treatment (1.0 mg weekly) resulted in additional BMI reductions (−1.6 kg/m2) and improved glycemic control. |
| Tirzepatide | Combination of glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonist synergistically improves appetite control and insulin sensitivity | Stoll, F. et al. (2025) [234]: In post-bariatric (SG, RYGB) patients who experienced WR or insufficient weight loss (12.0 ± 3.4%; p < 0.001), tirzepatide treatment led to significant weight loss and improvements in metabolic health, regardless of surgery type or sex. |
4. Discussion
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AOMs | Anti-obesity medications |
| ACC | Anterior cingulate cortex |
| AgRP | Agouti-related peptide |
| AN | Anorexia nervosa |
| ARC | Arcuate nucleus |
| BLA | Basolateral nucleus of the amygdale |
| BMI | Body mass index |
| BS | Bariatric surgery |
| CART | Cocaine- and amphetamine-regulated transcript |
| CBT | Cognitive behavioral therapy |
| CCK | Cholecystokinin |
| CeA | Central nucleus of the amygdala |
| CNS | Central nervous system |
| CR | Cognitive restructuring |
| D2R | Dopamine D2 receptor |
| DLPFC | Dorsolateral prefrontal cortex |
| DMV | Dorsomedial region |
| FDA | U.S. Food and Drug Administration |
| fMRI | Functional magnetic resonance imaging |
| GLP-1 | Glucagon-like peptide 1 |
| GM | Gray matter |
| HIPP | Hippocampus |
| hs-CRP | High-sensitivity C-reactive protein |
| LAGB | Laparoscopic adjustable gastric banding |
| LHA | Lateral hypothalamic area |
| LSG | Laparoscopic sleeve gastrectomy |
| MeA | Medial nucleus of the amygdala |
| NAc | Nucleus accumbens |
| NB | Naltrexone/bupropion |
| NPY | Neuropeptide Y |
| NWCR | National Weight Control Registry |
| OFC | Orbitofrontal cortex |
| PCG | Precentral gyrus |
| PET | Positron emission tomography |
| PFC | Prefrontal cortex |
| POMC | Pro-opiomelanocortin |
| PYY | Peptide YY |
| PVN | Paraventricular nucleus |
| RYGB | Roux-en-Y gastric bypass |
| T2DM | Type 2 diabetes |
| WM | White matter |
| WR | Weight regain |
| VMN | Ventromedial nucleus |
| VS | Ventral striatum |
| VTA | Ventral tegmental area |
References
- Boutari, C.; Mantzoros, C.S. A 2022 Update on the Epidemiology of Obesity and a Call to Action: As Its Twin COVID-19 Pandemic Appears to Be Receding, the Obesity and Dysmetabolism Pandemic Continues to Rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef] [PubMed]
- Jebeile, H.; Kelly, A.S.; O’Malley, G.; Baur, L.A. Obesity in Children and Adolescents: Epidemiology, Causes, Assessment, and Management. Lancet Diabetes Endocrinol. 2022, 10, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Gakidou, E.; Lo, J.; Abate, Y.H.; Abbafati, C.; Abbas, N.; Abbasian, M.; Abd ElHafeez, S.; Abdel-Rahman, W.M.; Abd-Elsalam, S.; et al. Global, Regional, and National Prevalence of Adult Overweight and Obesity, 1990–2021, with Forecasts to 2050: A Forecasting Study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 813–838. [Google Scholar] [CrossRef]
- Kerr, J.A.; Patton, G.C.; Cini, K.I.; Abate, Y.H.; Abbas, N.; Abd Al Magied, A.H.A.; Abd ElHafeez, S.; Abd-Elsalam, S.; Abdollahi, A.; Abdoun, M.; et al. Global, Regional, and National Prevalence of Child and Adolescent Overweight and Obesity, 1990–2021, with Forecasts to 2050: A Forecasting Study for the Global Burden of Disease Study 2021. Lancet 2025, 405, 785–812. [Google Scholar] [CrossRef]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation 2013, 129, S102. [Google Scholar] [PubMed]
- Wharton, S.; Lau, D.C.W.; Vallis, M.; Sharma, A.M.; Biertho, L.; Campbell-Scherer, D.; Adamo, K.; Alberga, A.; Bell, R.; Boulé, N.; et al. Obesity in Adults: A Clinical Practice Guideline. Can. Med. Assoc. J. 2020, 192, E875–E891. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical Practice Guidelines For The Perioperative Nutrition, Metabolic, and Nonsurgical Support of Patients Undergoing Bariatric Procedures–2019 Update: Cosponsored By American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society For Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Endocr. Pract. 2019, 25, 1346–1359. [Google Scholar] [CrossRef]
- Higgs, S. Is There a Role for Higher Cognitive Processes in the Development of Obesity in Humans? Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220208. [Google Scholar] [CrossRef]
- Dakanalis, A.; Mentzelou, M.; Papadopoulou, S.K.; Papandreou, D.; Spanoudaki, M.; Vasios, G.K.; Pavlidou, E.; Mantzorou, M.; Giaginis, C. The Association of Emotional Eating with Overweight/Obesity, Depression, Anxiety/Stress, and Dietary Patterns: A Review of the Current Clinical Evidence. Nutrients 2023, 15, 1173. [Google Scholar] [CrossRef]
- El Ansari, W.; Elhag, W. Weight Regain and Insufficient Weight Loss After Bariatric Surgery: Definitions, Prevalence, Mechanisms, Predictors, Prevention and Management Strategies, and Knowledge Gaps—A Scoping Review. Obes. Surg. 2021, 31, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Blomain, E.S.; Dirhan, D.A.; Valentino, M.A.; Kim, G.W.; Waldman, S.A. Mechanisms of Weight Regain Following Weight Loss. ISRN Obes. 2013, 2013, 210524. [Google Scholar] [CrossRef]
- Thonusin, C.; Shinlapawittayatorn, K.; Chattipakorn, S.C.; Chattipakorn, N. The Impact of Genetic Polymorphisms on Weight Regain after Successful Weight Loss. Br. J. Nutr. 2020, 124, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Busetto, L.; Bettini, S.; Makaronidis, J.; Roberts, C.A.; Halford, J.C.G.; Batterham, R.L. Mechanisms of Weight Regain. Eur. J. Intern. Med. 2021, 93, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Shiner, R.L.; Buss, K.A.; McClowry, S.G.; Putnam, S.P.; Saudino, K.J.; Zentner, M. What Is Temperament Now? Assessing Progress in Temperament Research on the Twenty-Fifth Anniversary of Goldsmith et al. Child Dev. Perspect. 2012, 6, 436–444. [Google Scholar] [CrossRef]
- Van Eeden, A.E.; Hoek, H.W.; Van Hoeken, D.; Deen, M.; Oldehinkel, A.J. Temperament in Preadolescence Is Associated with Weight and Eating Pathology in Young Adulthood. Int. J. Eat. Disord. 2020, 53, 736–745. [Google Scholar] [CrossRef]
- Academy of Nutrition and Dietetics ANDJ. Narrative Review Checklist. 2021. Available online: https://cdn.amegroups.cn/journals/tgh/files/journals/28/articles/6919/public/6919-PB2-5118-R1.pdf (accessed on 24 March 2025).
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Van Der Valk, E.S.; Savas, M.; Van Rossum, E.F.C. Stress and Obesity: Are There More Susceptible Individuals? Curr. Obes. Rep. 2018, 7, 193–203. [Google Scholar] [CrossRef]
- Sullivan, S.; Cloninger, C.R.; Przybeck, T.R.; Klein, S. Personality Characteristics in Obesity and Relationship with Successful Weight Loss. Int. J. Obes. 2007, 31, 669–674. [Google Scholar] [CrossRef]
- Cloninger, C.R. A Psychobiological Model of Temperament and Character. Arch. Gen. Psychiatry 1993, 50, 975. [Google Scholar] [CrossRef]
- Varkevisser, R.D.M.; Van Stralen, M.M.; Kroeze, W.; Ket, J.C.F.; Steenhuis, I.H.M. Determinants of Weight Loss Maintenance: A Systematic Review. Obes. Rev. 2019, 20, 171–211. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, K.; Sheffield, J.; Tan, W.H. Predictors of Diet Failure: A Multifactorial Cognitive and Behavioural Model. J. Health Psychol. 2019, 24, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Palmeira, A.L.; Sánchez-Oliva, D.; Encantado, J.; Marques, M.M.; Santos, I.; Duarte, C.; Matos, M.; Larsen, S.C.; Horgan, G.; Teixeira, P.J.; et al. Motivational and Self-efficacy Reciprocal Effects during a 12-month’ Weight Regain Prevention Program. Br. J. Health Psychol. 2023, 28, 467–481. [Google Scholar] [CrossRef]
- Elfhag, K.; Rössner, S. Who Succeeds in Maintaining Weight Loss? A Conceptual Review of Factors Associated with Weight Loss Maintenance and Weight Regain. Obes. Rev. 2005, 6, 67–85. [Google Scholar] [CrossRef]
- Ross, K.M.; Qiu, P.; You, L.; Wing, R.R. Week-to-Week Predictors of Weight Loss and Regain. Health Psychol. 2019, 38, 1150–1158. [Google Scholar] [CrossRef]
- Wadden, T.A.; Webb, V.L.; Moran, C.H.; Bailer, B.A. Lifestyle Modification for Obesity: New Developments in Diet, Physical Activity, and Behavior Therapy. Circulation 2012, 125, 1157–1170. [Google Scholar] [CrossRef]
- Gold, J.M.; Carr, L.J.; Thomas, J.G.; Burrus, J.; O’Leary, K.C.; Wing, R.; Bond, D.S. Conscientiousness in Weight Loss Maintainers and Regainers. Health Psychol. 2020, 39, 421–429. [Google Scholar] [CrossRef]
- Sutin, A.R.; Ferrucci, L.; Zonderman, A.B.; Terracciano, A. Personality and Obesity across the Adult Life Span. J. Pers. Soc. Psychol. 2011, 101, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; Young, H.A. A Meta-Analysis of the Relationship between Brain Dopamine Receptors and Obesity: A Matter of Changes in Behavior Rather than Food Addiction? Int. J. Obes. 2016, 40, S12–S21. [Google Scholar] [CrossRef]
- Bénard, M.; Camilleri, G.; Etilé, F.; Méjean, C.; Bellisle, F.; Reach, G.; Hercberg, S.; Péneau, S. Association between Impulsivity and Weight Status in a General Population. Nutrients 2017, 9, 217. [Google Scholar] [CrossRef]
- Milsom, V.; Ross Middleton, K.; Perri, M. Successful Long-Term Weight Loss Maintenance in a Rural Population. Clin. Interv. Aging 2011, 2011, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Konttinen, H.; Van Strien, T.; Männistö, S.; Jousilahti, P.; Haukkala, A. Depression, Emotional Eating and Long-Term Weight Changes: A Population-Based Prospective study. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.E.; Latendresse, S.J.; Bartholome, L.T.; Warren, C.S.; Raymond, N.C. Binge Eating Disorder Mediates Links between Symptoms of Depression, Anxiety, and Caloric Intake in Overweight and Obese Women. J. Obes. 2012, 2012, 407103. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Strachan, S.; Berkson, M. Sensitivity to Reward: Implications for Overeating and Overweight. Appetite 2004, 42, 131–138. [Google Scholar] [CrossRef]
- Schienle, A.; Schäfer, A.; Hermann, A.; Vaitl, D. Binge-Eating Disorder: Reward Sensitivity and Brain Activation to Images of Food. Biol. Psychiatry 2009, 65, 654–661. [Google Scholar] [CrossRef]
- Gross, J.J. The Emerging Field of Emotion Regulation: An Integrative Review. Emot. Regul. 1998, 2, 271–299. [Google Scholar] [CrossRef]
- Jackson, S.E.; Kirschbaum, C.; Steptoe, A. Hair Cortisol and Adiposity in a Population-based Sample of 2,527 Men and Women Aged 54 to 87 Years. Obesity 2017, 25, 539–544. [Google Scholar] [CrossRef]
- Block, J.P.; He, Y.; Zaslavsky, A.M.; Ding, L.; Ayanian, J.Z. Psychosocial Stress and Change in Weight Among US Adults. Am. J. Epidemiol. 2009, 170, 181–192. [Google Scholar] [CrossRef]
- Siep, N.; Roefs, A.; Roebroeck, A.; Havermans, R.; Bonte, M.; Jansen, A. Fighting Food Temptations: The Modulating Effects of Short-Term Cognitive Reappraisal, Suppression and up-Regulation on Mesocorticolimbic Activity Related to Appetitive Motivation. NeuroImage 2012, 60, 213–220. [Google Scholar] [CrossRef]
- Steward, T.; Picó-Pérez, M.; Mata, F.; Martínez-Zalacaín, I.; Cano, M.; Contreras-Rodríguez, O.; Fernández-Aranda, F.; Yucel, M.; Soriano-Mas, C.; Verdejo-García, A. Emotion Regulation and Excess Weight: Impaired Affective Processing Characterized by Dysfunctional Insula Activation and Connectivity. PLoS ONE 2016, 11, e0152150. [Google Scholar] [CrossRef]
- Nelson, S.M.; Dosenbach, N.U.F.; Cohen, A.L.; Wheeler, M.E.; Schlaggar, B.L.; Petersen, S.E. Role of the Anterior Insula in Task-Level Control and Focal Attention. Brain Struct. Funct. 2010, 214, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.W.; Omura, K.; Constable, R.T.; Canli, T. Emotional Conflict and Neuroticism: Personality-Dependent Activation in the Amygdala and Subgenual Anterior Cingulate. Behav. Neurosci. 2007, 121, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Cremers, H.R.; Demenescu, L.R.; Aleman, A.; Renken, R.; Van Tol, M.-J.; Van Der Wee, N.J.A.; Veltman, D.J.; Roelofs, K. Neuroticism Modulates Amygdala—Prefrontal Connectivity in Response to Negative Emotional Facial Expressions. NeuroImage 2010, 49, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Nolen-Hoeksema, S.; Stice, E.; Wade, E.; Bohon, C. Reciprocal Relations between Rumination and Bulimic, Substance Abuse, and Depressive Symptoms in Female Adolescents. J. Abnorm. Psychol. 2007, 116, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Aldao, A.; Nolen-Hoeksema, S.; Schweizer, S. Emotion-Regulation Strategies across Psychopathology: A Meta-Analytic Review. Clin. Psychol. Rev. 2010, 30, 217–237. [Google Scholar] [CrossRef]
- McRae, K.; Ochsner, K.N.; Mauss, I.B.; Gabrieli, J.J.D.; Gross, J.J. Gender Differences in Emotion Regulation: An fMRI Study of Cognitive Reappraisal. Group Process. Intergroup Relat. 2008, 11, 143–162. [Google Scholar] [CrossRef]
- Eldesouky, L.; English, T. Another Year Older, Another Year Wiser? Emotion Regulation Strategy Selection and Flexibility across Adulthood. Psychol. Aging 2018, 33, 572–585. [Google Scholar] [CrossRef]
- Whitmoyer, P.; Fisher, M.E.; Duraney, E.J.; Manzler, C.; Isaacowitz, D.M.; Andridge, R.; Prakash, R.S. Age Differences in Emotion Regulation Strategy Use and Flexibility in Daily Life. Aging Ment. Health 2024, 28, 330–343. [Google Scholar] [CrossRef]
- Sainsbury, K.; Evans, E.H.; Pedersen, S.; Marques, M.M.; Teixeira, P.J.; Lähteenmäki, L.; Stubbs, R.J.; Heitmann, B.L.; Sniehotta, F.F. Attribution of Weight Regain to Emotional Reasons amongst European Adults with Overweight and Obesity Who Regained Weight Following a Weight Loss Attempt. Eat. Weight Disord.-Stud. Anorex. Bulim. Obes. 2019, 24, 351–361. [Google Scholar] [CrossRef]
- Blomquist, K.K.; Barnes, R.D.; White, M.A.; Masheb, R.M.; Morgan, P.T.; Grilo, C.M. Exploring Weight Gain in Year before Treatment for Binge Eating Disorder: A Different Context for Interpreting Limited Weight Losses in Treatment Studies. Int. J. Eat. Disord. 2011, 44, 435–439. [Google Scholar] [CrossRef]
- Palascha, A.; Van Kleef, E.; Van Trijp, H.C. How Does Thinking in Black and White Terms Relate to Eating Behavior and Weight Regain? J. Health Psychol. 2015, 20, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Fairburn, C.G.; Cooper, Z.; Shafran, R. Cognitive Behaviour Therapy for Eating Disorders: A “Transdiagnostic” Theory and Treatment. Behav. Res. Ther. 2003, 41, 509–528. [Google Scholar] [CrossRef]
- Lewis, J.A.; Fraga, K.J.; Erickson, T.M. Dichotomous Thinking. In Encyclopedia of Personality and Individual Differences; Springer: Cham, Switzerland, 2019; pp. 1–5. ISBN 978-3-319-28099-8. [Google Scholar]
- Byrne, S.M.; Cooper, Z.; Fairburn, C.G. Psychological Predictors of Weight Regain in Obesity. Behav. Res. Ther. 2004, 42, 1341–1356. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.; Reay, R.; Bowman, A.R. Weight Loss After Weight-Loss Surgery: The Mediating Role of Dichotomous Thinking. Obes. Surg. 2024, 34, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Lethbridge, J.; Watson, H.J.; Egan, S.J.; Street, H.; Nathan, P.R. The Role of Perfectionism, Dichotomous Thinking, Shape and Weight Overvaluation, and Conditional Goal Setting in Eating Disorders. Eat. Behav. 2011, 12, 200–206. [Google Scholar] [CrossRef]
- Antoniou, E.E.; Bongers, P.; Jansen, A. The Mediating Role of Dichotomous Thinking and Emotional Eating in the Relationship Between Depression and BMI. Eat. Behav. 2017, 26, 55–60. [Google Scholar] [CrossRef]
- Westenhoefer, J.; Engel, D.; Holst, C.; Lorenz, J.; Peacock, M.; Stubbs, J.; Whybrow, S.; Raats, M. Cognitive and Weight-Related Correlates of Flexible and Rigid Restrained Eating Behaviour. Eat. Behav. 2013, 14, 69–72. [Google Scholar] [CrossRef]
- Sairanen, E.; Lappalainen, R.; Lapveteläinen, A.; Tolvanen, A.; Karhunen, L. Flexibility in Weight Management. Eat. Behav. 2014, 15, 218–224. [Google Scholar] [CrossRef]
- Cristea, I.A.; Huibers, M.J.H.; David, D.; Hollon, S.D.; Andersson, G.; Cuijpers, P. The Effects of Cognitive Behavior Therapy for Adult Depression on Dysfunctional Thinking: A Meta-Analysis. Clin. Psychol. Rev. 2015, 42, 62–71. [Google Scholar] [CrossRef]
- Ezawa, I.D.; Hollon, S.D. Cognitive Restructuring and Psychotherapy Outcome: A Meta-Analytic Review. Psychotherapy 2023, 60, 396–406. [Google Scholar] [CrossRef]
- Roberts, B.W.; Walton, K.E.; Viechtbauer, W. Patterns of Mean-Level Change in Personality Traits across the Life Course: A Meta-Analysis of Longitudinal Studies. Psychol. Bull. 2006, 132, 1–25. [Google Scholar] [CrossRef]
- Zheng, H.; Lenard, N.R.; Shin, A.C.; Berthoud, H.-R. Appetite Control and Energy Balance Regulation in the Modern World: Reward-Driven Brain Overrides Repletion Signals. Int. J. Obes. 2009, 33, S8–S13. [Google Scholar] [CrossRef]
- Lenard, N.R.; Berthoud, H. Central and Peripheral Regulation of Food Intake and Physical Activity: Pathways and Genes. Obesity 2008, 16, S11–S22. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Dixon, J.B. Food for Thought: Reward Mechanisms and Hedonic Overeating in Obesity. Curr. Obes. Rep. 2017, 6, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.N.; Knutson, B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. Taste, Olfactory and Food Texture Reward Processing in the Brain and Obesity. Int. J. Obes. 2011, 35, 550–561. [Google Scholar] [CrossRef]
- Zapparoli, L.; Devoto, F.; Giannini, G.; Zonca, S.; Gallo, F.; Paulesu, E. Neural Structural Abnormalities Behind Altered Brain Activation in Obesity: Evidence from Meta-Analyses of Brain Activation and Morphometric Data. NeuroImage Clin. 2022, 36, 103179. [Google Scholar] [CrossRef]
- Szmygin, H.; Szmygin, M.; Cheda, M.; Kłobuszewski, B.; Drelich-Zbroja, A.; Matyjaszek-Matuszek, B. Current Insights into the Potential Role of fMRI in Discovering the Mechanisms Underlying Obesity. J. Clin. Med. 2023, 12, 4379. [Google Scholar] [CrossRef]
- Drelich-Zbroja, A.; Matuszek, M.; Kaczor, M.; Kuczyńska, M. Functional Magnetic Resonance Imaging and Obesity—Novel Ways to Seen the Unseen. J. Clin. Med. 2022, 11, 3561. [Google Scholar] [CrossRef]
- Rothemund, Y.; Preuschhof, C.; Bohner, G.; Bauknecht, H.-C.; Klingebiel, R.; Flor, H.; Klapp, B.F. Differential Activation of the Dorsal Striatum by High-Calorie Visual Food Stimuli in Obese Individuals. NeuroImage 2007, 37, 410–421. [Google Scholar] [CrossRef]
- Lewis, R.G.; Florio, E.; Punzo, D.; Borrelli, E. The Brain’s Reward System in Health and Disease. In Circadian Clock in Brain Health and Disease; Engmann, O., Brancaccio, M., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; Volume 1344, pp. 57–69. ISBN 978-3-030-81146-4. [Google Scholar]
- Gryz, M.; Lehner, M.; Wisłowska-Stanek, A.; Płaźnik, A. Dopaminergic System Activity under Stress Condition–Seeking Individual Differences, Preclinical Studies. Psychiatr. Pol. 2018, 52, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Colvin, K.J.; Killen, H.S.; Kanter, M.J.; Halperin, M.C.; Engel, L.; Currie, P.J. Brain Site-Specific Inhibitory Effects of the GLP-1 Analogue Exendin-4 on Alcohol Intake and Operant Responding for Palatable Food. Int. J. Mol. Sci. 2020, 21, 9710. [Google Scholar] [CrossRef] [PubMed]
- Farr, O.M.; Li, C.R.; Mantzoros, C.S. Central Nervous System Regulation of Eating: Insights from Human Brain Imaging. Metabolism 2016, 65, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Courtney, K.E.; Schacht, J.P.; Hutchison, K.; Roche, D.J.O.; Ray, L.A. Neural Substrates of Cue Reactivity: Association with Treatment Outcomes and Relapse. Addict. Biol. 2016, 21, 3–22. [Google Scholar] [CrossRef]
- Han, P.; Roitzsch, C.; Horstmann, A.; Pössel, M.; Hummel, T. Increased Brain Reward Responsivity to Food-Related Odors in Obesity. Obesity 2021, 29, 1138–1145. [Google Scholar] [CrossRef]
- Rolls, E.T.; Feng, R.; Cheng, W.; Feng, J. Orbitofrontal Cortex Connectivity is Associated with Food Reward and Body Weight in Humans. Soc. Cogn. Affect. Neurosci. 2023, 18, nsab083. [Google Scholar] [CrossRef]
- Spetter, M.S.; Malekshahi, R.; Birbaumer, N.; Lührs, M.; Van Der Veer, A.H.; Scheffler, K.; Spuckti, S.; Preissl, H.; Veit, R.; Hallschmid, M. Volitional Regulation of Brain Responses to Food Stimuli in Overweight and Obese Subjects: A Real-Time fMRI Feedback Study. Appetite 2017, 112, 188–195. [Google Scholar] [CrossRef]
- Hogenkamp, P.S.; Zhou, W.; Dahlberg, L.S.; Stark, J.; Larsen, A.L.; Olivo, G.; Wiemerslage, L.; Larsson, E.-M.; Sundbom, M.; Benedict, C.; et al. Higher Resting-State Activity in Reward-Related Brain Circuits in Obese Versus Normal-Weight Females Independent of Food Intake. Int. J. Obes. 2016, 40, 1687–1692. [Google Scholar] [CrossRef]
- Murdaugh, D.L.; Cox, J.E.; Cook, E.W.; Weller, R.E. fMRI Reactivity to High-Calorie Food Pictures Predicts Short- and Long-Term Outcome in a Weight-Loss Program. NeuroImage 2012, 59, 2709–2721. [Google Scholar] [CrossRef]
- Yokum, S.; Ng, J.; Stice, E. Attentional Bias to Food Images Associated With Elevated Weight and Future Weight Gain: An fMRI Study. Obesity 2011, 19, 1775–1783. [Google Scholar] [CrossRef]
- Stopyra, M.A.; Friederich, H.-C.; Lavandier, N.; Mönning, E.; Bendszus, M.; Herzog, W.; Simon, J.J. Homeostasis and Food Craving in Obesity: A Functional MRI Study. Int. J. Obes. 2021, 45, 2464–2470. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.; Gál, V.; Kóbor, I.; Kirwan, C.B.; Kovács, P.; Kitka, T.; Lengyel, Z.; Bálint, E.; Varga, B.; Csekő, C.; et al. Efficacy of Weight Loss Intervention Can Be Predicted Based on Early Alterations of fMRI Food Cue Reactivity in the Striatum. NeuroImage Clin. 2019, 23, 101803. [Google Scholar] [CrossRef] [PubMed]
- Stice, E.; Burger, K. Neural Vulnerability Factors for Obesity. Clin. Psychol. Rev. 2019, 68, 38–53. [Google Scholar] [CrossRef]
- Devoto, F.; Zapparoli, L.; Bonandrini, R.; Berlingeri, M.; Ferrulli, A.; Luzi, L.; Banfi, G.; Paulesu, E. Hungry Brains: A Meta-Analytical Review of Brain Activation Imaging Studies on Food Perception and Appetite in Obese Individuals. Neurosci. Biobehav. Rev. 2018, 94, 271–285. [Google Scholar] [CrossRef]
- Li, G.; Hu, Y.; Zhang, W.; Wang, J.; Ji, W.; Manza, P.; Volkow, N.D.; Zhang, Y.; Wang, G.-J. Brain Functional and Structural Magnetic Resonance Imaging of Obesity and Weight Loss Interventions. Mol. Psychiatry 2023, 28, 1466–1479. [Google Scholar] [CrossRef]
- Le, D.S.N.; Pannacciulli, N.; Chen, K.; Del Parigi, A.; Salbe, A.D.; Reiman, E.M.; Krakoff, J. Less Activation of the Left Dorsolateral Prefrontal Cortex in Response to a Meal: A Feature of Obesity. Am. J. Clin. Nutr. 2006, 84, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Gluck, M.E.; Alonso-Alonso, M.; Piaggi, P.; Weise, C.M.; Jumpertz-von Schwartzenberg, R.; Reinhardt, M.; Wassermann, E.M.; Venti, C.A.; Votruba, S.B.; Krakoff, J. Neuromodulation Targeted to the Prefrontal Cortex Induces Changes in Energy Intake and Weight Loss in Obesity: tDCS and Weight Loss. Obesity 2015, 23, 2149–2156. [Google Scholar] [CrossRef]
- García-García, I.; Michaud, A.; Dadar, M.; Zeighami, Y.; Neseliler, S.; Collins, D.L.; Evans, A.C.; Dagher, A. Neuroanatomical Differences in Obesity: Meta-Analytic Findings and Their Validation in an Independent Dataset. Int. J. Obes. 2019, 43, 943–951. [Google Scholar] [CrossRef]
- Li, L.; Yu, H.; Zhong, M.; Liu, S.; Wei, W.; Meng, Y.; Li, M.; Li, T.; Wang, Q. Gray Matter Volume Alterations in Subjects with Overweight and Obesity: Evidence from a Voxel-Based Meta-Analysis. Front. Psychiatry 2022, 13, 955741. [Google Scholar] [CrossRef]
- Yokum, S.; Ng, J.; Stice, E. Relation of Regional Gray and White Matter Volumes to Current BMI and Future Increases in BMI: A Prospective MRI Study. Int. J. Obes. 2012, 36, 656–664. [Google Scholar] [CrossRef]
- Tuulari, J.J.; Karlsson, H.K.; Antikainen, O.; Hirvonen, J.; Pham, T.; Salminen, P.; Helmiö, M.; Parkkola, R.; Nuutila, P.; Nummenmaa, L. Bariatric Surgery Induces White and Grey Matter Density Recovery in the Morbidly Obese: A Voxel-Based Morphometric Study. Hum. Brain Mapp. 2016, 37, 3745–3756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, G.; Xu, M.; Cai, W.; Zhu, Q.; Qian, L.; Zhang, Y.E.; Yuan, K.; Liu, J.; Li, Q.; et al. Recovery of Brain Structural Abnormalities in Morbidly Obese Patients after Bariatric Surgery. Int. J. Obes. 2016, 40, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, S.; Gilbert, S.; Serpell, L. Systematic Review: Are Overweight and Obese Individuals Impaired on Behavioural Tasks of Executive Functioning? Neuropsychol. Rev. 2013, 23, 138–156. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Tomasi, D.; Baler, R.D. Obesity and Addiction: Neurobiological Overlaps. Obes. Rev. 2013, 14, 2–18. [Google Scholar] [CrossRef]
- Mahapatra, A. Overeating, Obesity, and Dopamine Receptors. ACS Chem. Neurosci. 2010, 1, 346–347. [Google Scholar] [CrossRef]
- De Weijer, B.A.; Van De Giessen, E.; Van Amelsvoort, T.A.; Boot, E.; Braak, B.; Janssen, I.M.; Van De Laar, A.; Fliers, E.; Serlie, M.J.; Booij, J. Lower Striatal Dopamine D2/3 Receptor Availability in Obese Compared with Non-Obese Subjects. EJNMMI Res. 2011, 1, 37. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.-J.; Telang, F.; Fowler, J.S.; Thanos, P.K.; Logan, J.; Alexoff, D.; Ding, Y.-S.; Wong, C.; Ma, Y.; et al. Low Dopamine Striatal D2 Receptors Are Associated with Prefrontal Metabolism in Obese Subjects: Possible Contributing Factors. NeuroImage 2008, 42, 1537–1543. [Google Scholar] [CrossRef]
- Blumenthal, D.M.; Gold, M.S. Neurobiology of Food Addiction. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 359–365. [Google Scholar] [CrossRef]
- Ahmed, S.; Kashem, M.A.; Sarker, R.; Ahmed, E.U.; Hargreaves, G.A.; McGregor, I.S. Neuroadaptations in the Striatal Proteome of the Rat Following Prolonged Excessive Sucrose Intake. Neurochem. Res. 2014, 39, 815–824. [Google Scholar] [CrossRef]
- Parylak, S.L.; Koob, G.F.; Zorrilla, E.P. The Dark Side of Food Addiction. Physiol. Behav. 2011, 104, 149–156. [Google Scholar] [CrossRef]
- Baik, J.-H. Dopamine Signaling in Food Addiction: Role of Dopamine D2 Receptors. BMB Rep. 2013, 46, 519–526. [Google Scholar] [CrossRef] [PubMed]
- De Macedo, I.C.; De Freitas, J.S.; Da Silva Torres, I.L. The Influence of Palatable Diets in Reward System Activation: A Mini Review. Adv. Pharmacol. Sci. 2016, 2016, 7238679. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.J.; Tesar, A.; Beier, J.; Berg, M.; Warrings, B. Grey Matter Alterations in Obesity: A Meta-analysis of Whole-brain Studies. Obes. Rev. 2019, 20, 464–471. [Google Scholar] [CrossRef]
- Matsuo, K.; Nicoletti, M.; Nemoto, K.; Hatch, J.P.; Peluso, M.A.M.; Nery, F.G.; Soares, J.C. A Voxel-based Morphometry Study of Frontal Gray Matter Correlates of Impulsivity. Hum. Brain Mapp. 2009, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Love, T.M.; Stohler, C.S.; Zubieta, J.-K. Positron Emission Tomography Measures of Endogenous Opioid Neurotransmission and Impulsiveness Traits in Humans. Arch. Gen. Psychiatry 2009, 66, 1124. [Google Scholar] [CrossRef]
- Korponay, C.; Dentico, D.; Kral, T.; Ly, M.; Kruis, A.; Goldman, R.; Lutz, A.; Davidson, R.J. Neurobiological Correlates of Impulsivity in Healthy Adults: Lower Prefrontal Gray Matter Volume and Spontaneous Eye-Blink Rate but Greater Resting-State Functional Connectivity in Basal Ganglia-Thalamo-Cortical Circuitry. NeuroImage 2017, 157, 288–296. [Google Scholar] [CrossRef]
- Holmes, A.J.; Hollinshead, M.O.; Roffman, J.L.; Smoller, J.W.; Buckner, R.L. Individual Differences in Cognitive Control Circuit Anatomy Link Sensation Seeking, Impulsivity, and Substance Use. J. Neurosci. 2016, 36, 4038–4049. [Google Scholar] [CrossRef]
- Kerr, K.L.; Avery, J.A.; Barcalow, J.C.; Moseman, S.E.; Bodurka, J.; Bellgowan, P.S.F.; Simmons, W.K. Trait Impulsivity Is Related to Ventral ACC and Amygdala Activity during Primary Reward Anticipation. Soc. Cogn. Affect. Neurosci. 2015, 10, 36–42. [Google Scholar] [CrossRef]
- Pape, M.; Herpertz, S.; Schroeder, S.; Seiferth, C.; Färber, T.; Wolstein, J.; Steins-Loeber, S. Food Addiction and Its Relationship to Weight- and Addiction-Related Psychological Parameters in Individuals With Overweight and Obesity. Front. Psychol. 2021, 12, 736454. [Google Scholar] [CrossRef]
- Sawamoto, R.; Nozaki, T.; Nishihara, T.; Furukawa, T.; Hata, T.; Komaki, G.; Sudo, N. Predictors of Successful Long-Term Weight Loss Maintenance: A Two-Year Follow-Up. Biopsychosoc. Med. 2017, 11, 14. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Wang, G.; Hitzemann, R.; Logan, J.; Schlyer, D.J.; Dewey, S.L.; Wolf, A.P. Decreased Dopamine D 2 Receptor Availability Is Associated with Reduced Frontal Metabolism in Cocaine Abusers. Synapse 1993, 14, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-J.; Volkow, N.D.; Logan, J.; Pappas, N.R.; Wong, C.T.; Zhu, W.; Netusll, N.; Fowler, J.S. Brain Dopamine and Obesity. Lancet 2001, 357, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-J.; Volkow, N.D.; Felder, C.; Fowler, J.S.; Levy, A.V.; Pappas, N.R.; Wong, C.T.; Zhu, W.; Netusil, N. Enhanced Resting Activity of the Oral Somatosensory Cortex in Obese Subjects. Neuroreport 2002, 13, 1151–1155. [Google Scholar] [CrossRef]
- Wang, G.-J.; Volkow, N.D.; Thanos, P.K.; Fowler, J.S. Similarity Between Obesity and Drug Addiction as Assessed by Neurofunctional Imaging: A Concept Review. J. Addict. Dis. 2004, 23, 39–53. [Google Scholar] [CrossRef]
- Beeler, J.A.; Faust, R.P.; Turkson, S.; Ye, H.; Zhuang, X. Low Dopamine D2 Receptor Increases Vulnerability to Obesity Via Reduced Physical Activity, Not Increased Appetitive Motivation. Biol. Psychiatry 2016, 79, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Leibel, R.L. Changes in Energy Expenditure Resulting from Altered Body Weight. N. Engl. J. Med. 1995, 332, 621–628. [Google Scholar] [CrossRef]
- Doucet, É.; McInis, K.; Mahmoodianfard, S. Compensation in Response to Energy Deficits Induced by Exercise or Diet. Obes. Rev. 2018, 19, 36–46. [Google Scholar] [CrossRef]
- Roger, C.; Lasbleiz, A.; Guye, M.; Dutour, A.; Gaborit, B.; Ranjeva, J.-P. The Role of the Human Hypothalamus in Food Intake Networks: An MRI Perspective. Front. Nutr. 2022, 8, 760914. [Google Scholar] [CrossRef]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central Nervous System Control of Food Intake and Body Weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Jais, A.; Brüning, J.C. Arcuate Nucleus-Dependent Regulation of Metabolism—Pathways to Obesity and Diabetes Mellitus. Endocr. Rev. 2022, 43, 314–328. [Google Scholar] [CrossRef]
- Yu, J.H.; Kim, M.-S. Molecular Mechanisms of Appetite Regulation. Diabetes Metab. J. 2012, 36, 391. [Google Scholar] [CrossRef]
- Ochner, C.N.; Barrios, D.M.; Lee, C.D.; Pi-Sunyer, F.X. Biological Mechanisms That Promote Weight Regain Following Weight Loss in Obese Humans. Physiol. Behav. 2013, 120, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Hanson, P.; Weickert, M.O.; Barber, T.M. Obesity: Novel and Unusual Predisposing Factors. Ther. Adv. Endocrinol. Metab. 2020, 11, 204201882092201. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Sy, M.; Pavlovich, K.; Leibel, R.L.; Hirsch, J. Leptin Reverses Weight Loss–Induced Changes in Regional Neural Activity Responses to Visual Food Stimuli. J. Clin. Investig. 2008, 118, 2583–2591. [Google Scholar] [CrossRef]
- Camps, S.G.J.A.; Verhoef, S.P.M.; Westerterp, K.R. Leptin and Energy Restriction Induced Adaptation in Energy Expenditure. Metabolism 2015, 64, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-D. Leptin and Beyond: An Odyssey to the Central Control of Body Weight. Yale J. Biol. Med. 2011, 84, 1–7. [Google Scholar] [PubMed]
- Cummings, D.E.; Weigle, D.S.; Frayo, R.S.; Breen, P.A.; Ma, M.K.; Dellinger, E.P.; Purnell, J.Q. Plasma Ghrelin Levels after Diet-Induced Weight Loss or Gastric Bypass Surgery. N. Engl. J. Med. 2002, 346, 1623–1630. [Google Scholar] [CrossRef]
- Martins, C.; Roekenes, J.A.; Rehfeld, J.F.; Hunter, G.R.; Gower, B.A. Metabolic Adaptation Is Associated with a Greater Increase in Appetite Following Weight Loss: A Longitudinal Study. Am. J. Clin. Nutr. 2023, 118, 1192–1201. [Google Scholar] [CrossRef]
- Sumithran, P.; Delbridge, E.; Kriketos, A. Long-Term Persistence of Hormonal Adaptations to Weight Loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef]
- Trexler, E.T.; Smith-Ryan, A.E.; Norton, L.E. Metabolic Adaptation to Weight Loss: Implications for the Athlete. J. Int. Soc. Sports Nutr. 2014, 11, 7. [Google Scholar] [CrossRef]
- Zanchi, D.; Depoorter, A.; Egloff, L.; Haller, S.; Mählmann, L.; Lang, U.E.; Drewe, J.; Beglinger, C.; Schmidt, A.; Borgwardt, S. The Impact of Gut Hormones on the Neural Circuit of Appetite and Satiety: A Systematic Review. Neurosci. Biobehav. Rev. 2017, 80, 457–475. [Google Scholar] [CrossRef] [PubMed]
- Slouha, E.; Elkersh, E.M.; Shay, A.; Ghosh, S.; Mahmood, A.; Gorantla, V.R. Significance of Hormone Alteration Following Bariatric Surgery. Cureus 2023, 15, e49053. [Google Scholar] [CrossRef]
- Dimitriadis, G.K.; Randeva, M.S.; Miras, A.D. Potential Hormone Mechanisms of Bariatric Surgery. Curr. Obes. Rep. 2017, 6, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Stoeckli, R.; Chanda, R.; Langer, I.; Keller, U. Changes of Body Weight and Plasma Ghrelin Levels after Gastric Banding and Gastric Bypass. Obes. Res. 2004, 12, 346–350. [Google Scholar] [CrossRef]
- Frühbeck, G.; Rotellar, F.; Hernández-Lizoain, J.L.; Gil, M.J.; Gómez-Ambrosi, J.; Salvador, J.; Cienfuegos, J.A. Fasting Plasma Ghrelin Concentrations 6 Months after Gastric Bypass Are Not Determined by Weight Loss or Changes in Insulinemia. Obes. Surg. 2004, 14, 1208–1215. [Google Scholar] [CrossRef]
- Schindler, K.; Prager, G.; Ballaban, T.; Kretschmer, S.; Riener, R.; Buranyi, B.; Maier, C.; Luger, A.; Ludvik, B. Impact of Laparoscopic Adjustable Gastric Banding on Plasma Ghrelin, Eating Behaviour and Body Weight. Eur. J. Clin. Investig. 2004, 34, 549–554. [Google Scholar] [CrossRef]
- Nijhuis, J.; Van Dielen, F.M.H.; Buurman, W.A.; Greve, J.W.M. Ghrelin, Leptin and Insulin Levels after Restrictive Surgery: A 2-Year Follow-up Study. Obes. Surg. 2004, 14, 783–787. [Google Scholar] [CrossRef]
- Dadan, J.; Hady, H.R.; Zbucki, R.L.; Iwacewicz, P.; Bossowski, A.; Kasacka, I. The Activity of Gastric Ghrelin Positive Cells in Obese Patients Treated Surgically. Folia Histochem. Cytobiol. 2009, 47, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Langer, F.B.; Reza Hoda, M.A.; Bohdjalian, A.; Felberbauer, F.X.; Zacherl, J.; Wenzl, E.; Schindler, K.; Luger, A.; Ludvik, B.; Prager, G. Sleeve Gastrectomy and Gastric Banding: Effects on Plasma Ghrelin Levels. Obes. Surg. 2005, 15, 1024–1029. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, G.; Li, G.; Hu, Y.; Liu, L.; Jin, Q.; Meng, Q.; Zhao, J.; Yuan, K.; Liu, J.; et al. Ghrelin Reductions Following Bariatric Surgery Were Associated with Decreased Resting State Activity in the Hippocampus. Int. J. Obes. 2019, 43, 842–851. [Google Scholar] [CrossRef]
- Bohdjalian, A.; Langer, F.B.; Shakeri-Leidenmühler, S.; Gfrerer, L.; Ludvik, B.; Zacherl, J.; Prager, G. Sleeve Gastrectomy as Sole and Definitive Bariatric Procedure: 5-Year Results for Weight Loss and Ghrelin. Obes. Surg. 2010, 20, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Ramón, J.M.; Salvans, S.; Crous, X.; Puig, S.; Goday, A.; Benaiges, D.; Trillo, L.; Pera, M.; Grande, L. Effect of Roux-En-Y Gastric Bypass vs Sleeve Gastrectomy on Glucose and Gut Hormones: A Prospective Randomised Trial. J. Gastrointest. Surg. 2012, 16, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ji, G.; Li, G.; Manza, P.; Zhang, W.; Wang, J.; Lv, G.; He, Y.; Zhang, Z.; Yuan, K.; et al. Brain Connectivity, and Hormonal and Behavioral Correlates of Sustained Weight Loss in Obese Patients after Laparoscopic Sleeve Gastrectomy. Cereb. Cortex 2021, 31, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.R.; Jirapinyo, P.; Thompson, C.C. Effect of Sleeve Gastrectomy on Ghrelin, GLP-1, PYY, and GIP Gut Hormones: A Systematic Review and Meta-Analysis. Ann. Surg. 2020, 272, 72–80. [Google Scholar] [CrossRef]
- Tsouristakis, A.I.; Febres, G.; McMahon, D.J.; Tchang, B.; Conwell, I.M.; Tsang, A.J.; Ahmed, L.; Bessler, M.; Korner, J. Long-Term Modulation of Appetitive Hormones and Sweet Cravings After Adjustable Gastric Banding and Roux-En-Y Gastric Bypass. Obes. Surg. 2019, 29, 3698–3705. [Google Scholar] [CrossRef]
- Hansen, T.K.; Dall, R.; Hosoda, H.; Kojima, M.; Kangawa, K.; Christiansen, J.S.; Jørgensen, J.O.L. Weight Loss Increases Circulating Levels of Ghrelin in Human Obesity. Clin. Endocrinol. 2002, 56, 203–206. [Google Scholar] [CrossRef]
- Hooper, L.E.; Foster-Schubert, K.E.; Weigle, D.S.; Sorensen, B.; Ulrich, C.M.; McTiernan, A. Frequent Intentional Weight Loss is Associated with Higher Ghrelin and Lower Glucose and Androgen Levels in Postmenopausal Women. Nutr. Res. 2010, 30, 163–170. [Google Scholar] [CrossRef]
- Shak, J.R.; Roper, J.; Perez-Perez, G.I.; Tseng, C.; Francois, F.; Gamagaris, Z.; Patterson, C.; Weinshel, E.; Fielding, G.A.; Ren, C.; et al. The Effect of Laparoscopic Gastric Banding Surgery on Plasma Levels of Appetite-Control, Insulinotropic, and Digestive Hormones. Obes. Surg. 2008, 18, 1089–1096. [Google Scholar] [CrossRef]
- Terra, X.; Auguet, T.; Guiu-Jurado, E.; Berlanga, A.; Orellana-Gavaldà, J.M.; Hernández, M.; Sabench, F.; Porras, J.A.; Llutart, J.; Martinez, S.; et al. Long-Term Changes in Leptin, Chemerin and Ghrelin Levels Following Different Bariatric Surgery Procedures: Roux-En-Y Gastric Bypass and Sleeve Gastrectomy. Obes. Surg. 2013, 23, 1790–1798. [Google Scholar] [CrossRef]
- Korner, J.; Bessler, M.; Inabnet, W.; Taveras, C.; Holst, J.J. Exaggerated GLP-1 and Blunted GIP Secretion Are Associated with Roux-En-Y Gastric Bypass but Not Adjustable Gastric Banding. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2007, 3, 597. [Google Scholar] [CrossRef]
- Van Rossum, E.F.C.; Nicklas, B.J.; Dennis, K.E.; Berman, D.M.; Goldberg, A.P. Leptin Responses to Weight Loss in Postmenopausal Women: Relationship to Sex-Hormone Binding Globulin and Visceral Obesity. Obes. Res. 2000, 8, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Tussing, L.; Bhutani, S.; Braunschweig, C.L. Degree of Weight Loss Required to Improve Adipokine Concentrations and Decrease Fat Cell Size in Severely Obese Women. Metabolism 2009, 58, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, B.J.; Katzel, L.I.; Ryan, A.S.; Dennis, K.E.; Goldberg, A.P. Gender Differences in the Response of Plasma Leptin Concentrations to Weight Loss in Obese Older Individuals. Obes. Res. 1997, 5, 62–68. [Google Scholar] [CrossRef] [PubMed]
- De Luis, D.A.; Gonzalez Sagrado, M.; Conde, R.; Aller, R.; Izaola, O. Decreased Basal Levels of Glucagon-Like Peptide-1 after Weight Loss in Obese Subjects. Ann. Nutr. Metab. 2007, 51, 134–138. [Google Scholar] [CrossRef]
- Peterli, R.; Steinert, R.E.; Woelnerhanssen, B.; Peters, T.; Christoffel-Courtin, C.; Gass, M.; Kern, B.; Von Fluee, M.; Beglinger, C. Metabolic and Hormonal Changes After Laparoscopic Roux-En-Y Gastric Bypass and Sleeve Gastrectomy: A Randomized, Prospective Trial. Obes. Surg. 2012, 22, 740–748. [Google Scholar] [CrossRef]
- Svane, M.S.; Bojsen-Møller, K.N.; Martinussen, C.; Dirksen, C.; Madsen, J.L.; Reitelseder, S.; Holm, L.; Rehfeld, J.F.; Kristiansen, V.B.; Van Hall, G.; et al. Postprandial Nutrient Handling and Gastrointestinal Hormone Secretion After Roux-En-Y Gastric Bypass vs Sleeve Gastrectomy. Gastroenterology 2019, 156, 1627–1641.e1. [Google Scholar] [CrossRef]
- Jacobsen, S.H.; Olesen, S.C.; Dirksen, C.; Jørgensen, N.B.; Bojsen-Møller, K.N.; Kielgast, U.; Worm, D.; Almdal, T.; Naver, L.S.; Hvolris, L.E.; et al. Changes in Gastrointestinal Hormone Responses, Insulin Sensitivity, and Beta-Cell Function Within 2 Weeks After Gastric Bypass in Non-Diabetic Subjects. Obes. Surg. 2012, 22, 1084–1096. [Google Scholar] [CrossRef]
- Chearskul, S.; Delbridge, E.; Shulkes, A.; Proietto, J.; Kriketos, A. Effect of Weight Loss and Ketosis on Postprandial Cholecystokinin and Free Fatty Acid Concentrations. Am. J. Clin. Nutr. 2008, 87, 1238–1246. [Google Scholar] [CrossRef]
- Papamargaritis, D.; Le Roux, C.W.; Sioka, E.; Koukoulis, G.; Tzovaras, G.; Zacharoulis, D. Changes in Gut Hormone Profile and Glucose Homeostasis after Laparoscopic Sleeve Gastrectomy. Surg. Obes. Relat. Dis. 2013, 9, 192–201. [Google Scholar] [CrossRef]
- Adam, T.C.M.; Jocken, J.; Westerterp-Plantenga, M.S. Decreased Glucagon-like Peptide 1 Release after Weight Loss in Overweight/Obese Subjects. Obes. Res. 2005, 13, 710–716. [Google Scholar] [CrossRef]
- Bose, M.; Machineni, S.; Oliván, B.; Teixeira, J.; McGinty, J.J.; Bawa, B.; Koshy, N.; Colarusso, A.; Laferrère, B. Superior Appetite Hormone Profile After Equivalent Weight Loss by Gastric Bypass Compared to Gastric Banding. Obesity 2010, 18, 1085–1091. [Google Scholar] [CrossRef]
- Korner, J.; Inabnet, W.; Conwell, I.M.; Taveras, C.; Daud, A.; Olivero-Rivera, L.; Restuccia, N.L.; Bessler, M. Differential Effects of Gastric Bypass and Banding on Circulating Gut Hormone and Leptin Levels. Obesity 2006, 14, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Roux, C.W.L.; Aylwin, S.J.B.; Batterham, R.L.; Borg, C.M.; Coyle, F.; Prasad, V.; Shurey, S.; Ghatei, M.A.; Patel, A.G.; Bloom, S.R. Gut Hormone Profiles Following Bariatric Surgery Favor an Anorectic State, Facilitate Weight Loss, and Improve Metabolic Parameters. Ann. Surg. 2006, 243, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Korner, J.; Inabnet, W.; Febres, G.; Conwell, I.M.; McMahon, D.J.; Salas, R.; Taveras, C.; Schrope, B.; Bessler, M. Prospective Study of Gut Hormone and Metabolic Changes after Adjustable Gastric Banding and Roux-En-Y Gastric Bypass. Int. J. Obes. 2009, 33, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Kokkinos, A.; Peradze, N.; Tentolouris, N.; Ghaly, W.; Pilitsi, E.; Upadhyay, J.; Alexandrou, A.; Mantzoros, C.S. Circulating Levels of Gastrointestinal Hormones in Response to the Most Common Types of Bariatric Surgery and Predictive Value for Weight Loss over One Year: Evidence from Two Independent Trials. Metabolism 2019, 101, 153997. [Google Scholar] [CrossRef]
- Essah, P.A.; Levy, J.R.; Sistrun, S.N.; Kelly, S.M.; Nestler, J.E. Effect of Weight Loss by a Low-Fat Diet and a Low-Carbohydrate Diet on Peptide YY Levels. Int. J. Obes. 2010, 34, 1239–1242. [Google Scholar] [CrossRef]
- Pfluger, P.T.; Kampe, J.; Castaneda, T.R.; Vahl, T.; D’Alessio, D.A.; Kruthaupt, T.; Benoit, S.C.; Cuntz, U.; Rochlitz, H.J.; Moehlig, M.; et al. Effect of Human Body Weight Changes on Circulating Levels of Peptide YY and Peptide YY3–36. J. Clin. Endocrinol. Metab. 2007, 92, 583–588. [Google Scholar] [CrossRef]
- Van Opstal, A.M.; Wijngaarden, M.A.; Van Der Grond, J.; Pijl, H. Changes in Brain Activity after Weight Loss. Obes. Sci. Pract. 2019, 5, 459–467. [Google Scholar] [CrossRef]
- Honea, R.A.; Szabo-Reed, A.N.; Lepping, R.J.; Perea, R.; Breslin, F.; Martin, L.E.; Brooks, W.M.; Donnelly, J.E.; Savage, C.R. Voxel-based Morphometry Reveals Brain Gray Matter Volume Changes in Successful Dieters. Obesity 2016, 24, 1842–1848. [Google Scholar] [CrossRef]
- Haltia, L.T.; Viljanen, A.; Parkkola, R.; Kemppainen, N.; Rinne, J.O.; Nuutila, P.; Kaasinen, V. Brain White Matter Expansion in Human Obesity and the Recovering Effect of Dieting. J. Clin. Endocrinol. Metab. 2007, 92, 3278–3284. [Google Scholar] [CrossRef]
- DelParigi, A.; Chen, K.; Salbe, A.D.; Hill, J.O.; Wing, R.R.; Reiman, E.M.; Tataranni, P.A. Successful Dieters Have Increased Neural Activity in Cortical Areas Involved in the Control of Behavior. Int. J. Obes. 2007, 31, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Kiem, L.; Wing, R.; McGuire, T. A Descriptive Study of Individuals Successful at Long-Term Maintenance of Substantial Weight. Am. J. Clin. Nutr. 1997, 66, 239–246. [Google Scholar]
- Su, Y.; Bi, T.; Gong, G.; Jiang, Q.; Chen, H. Why Do Most Restrained Eaters Fail in Losing Weight?: Evidence from an fMRI Study. Psychol. Res. Behav. Manag. 2019, 12, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Cornier, M.-A.; Salzberg, A.K.; Endly, D.C.; Bessesen, D.H.; Rojas, D.C.; Tregellas, J.R. The Effects of Overfeeding on the Neuronal Response to Visual Food Cues in Thin and Reduced-Obese Individuals. PLoS ONE 2009, 4, e6310. [Google Scholar] [CrossRef]
- DelParigi, A.; Chen, K.; Salbe, A.D.; Hill, J.O.; Wing, R.R.; Reiman, E.M.; Tataranni, P.A. Persistence of Abnormal Neural Responses to a Meal in Postobese Individuals. Int. J. Obes. 2004, 28, 370–377. [Google Scholar] [CrossRef]
- Savic, I.; Gulyas, B. PET Shows That Odors Are Processed Both Ipsilaterally and Contralaterally to the Stimulated Nostril. NeuroReport 2000, 11, 2861–2866. [Google Scholar] [CrossRef]
- Hamdy, S.; Rothwell, J.C.; Brooks, D.J.; Bailey, D.; Aziz, Q.; Thompson, D.G. Identification of the Cerebral Loci Processing Human Swallowing With H 215 O PET Activation. J. Neurophysiol. 1999, 81, 1917–1926. [Google Scholar] [CrossRef]
- Nunn, K.; Frampton, I.; Fuglset, T.S.; Törzsök-Sonnevend, M.; Lask, B. Anorexia Nervosa and the Insula. Med. Hypotheses 2011, 76, 353–357. [Google Scholar] [CrossRef]
- Kerr, K.L.; Moseman, S.E.; Avery, J.A.; Bodurka, J.; Zucker, N.L.; Simmons, W.K. Altered Insula Activity during Visceral Interoception in Weight-Restored Patients with Anorexia Nervosa. Neuropsychopharmacology 2016, 41, 521–528. [Google Scholar] [CrossRef]
- Curzio, O.; Calderoni, S.; Maestro, S.; Rossi, G.; De Pasquale, C.F.; Belmonti, V.; Apicella, F.; Muratori, F.; Retico, A. Lower Gray Matter Volumes of Frontal Lobes and Insula in Adolescents with Anorexia Nervosa Restricting Type: Findings from a Brain Morphometry Study. Eur. Psychiatry 2020, 63, e27. [Google Scholar] [CrossRef]
- Tomiyama, A.J.; Mann, T.; Vinas, D.; Hunger, J.M.; DeJager, J.; Taylor, S.E. Low Calorie Dieting Increases Cortisol. Psychosom. Med. 2010, 72, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Tryon, M.S.; Carter, C.S.; DeCant, R.; Laugero, K.D. Chronic Stress Exposure May Affect the Brain’s Response to High Calorie Food Cues and Predispose to Obesogenic Eating Habits. Physiol. Behav. 2013, 120, 233–242. [Google Scholar] [CrossRef]
- Morales, I.; Berridge, K.C. ‘Liking’ and ‘Wanting’ in Eating and Food Reward: Brain Mechanisms and Clinical Implications. Physiol. Behav. 2020, 227, 113152. [Google Scholar] [CrossRef]
- Kroemer, N.B.; Krebs, L.; Kobiella, A.; Grimm, O.; Pilhatsch, M.; Bidlingmaier, M.; Zimmermann, U.S.; Smolka, M.N. Fasting Levels of Ghrelin Covary with the Brain Response to Food Pictures: Ghrelin and Food-Cue Reactivity. Addict. Biol. 2013, 18, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, H.; Guo, F. Amygdala, an Important Regulator for Food Intake. Front. Biol. 2011, 6, 82–85. [Google Scholar] [CrossRef]
- Ip, C.K.; Zhang, L.; Farzi, A.; Qi, Y.; Clarke, I.; Reed, F.; Shi, Y.-C.; Enriquez, R.; Dayas, C.; Graham, B.; et al. Amygdala NPY Circuits Promote the Development of Accelerated Obesity under Chronic Stress Conditions. Cell Metab. 2019, 30, 111–128.e6. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Lacadie, C.; Seo, D.; Kubat, J.; Van Name, M.A.; Giannini, C.; Savoye, M.; Constable, R.T.; Sherwin, R.S.; Caprio, S.; et al. Leptin Is Associated With Exaggerated Brain Reward and Emotion Responses to Food Images in Adolescent Obesity. Diabetes Care 2014, 37, 3061–3068. [Google Scholar] [CrossRef]
- Eik-Nes, T.T.; Tokatlian, A.; Raman, J.; Spirou, D.; Kvaløy, K. Depression, Anxiety, and Psychosocial Stressors across BMI Classes: A Norwegian Population Study-The HUNT Study. Front. Endocrinol. 2022, 13, 886148. [Google Scholar] [CrossRef]
- Roozendaal, B.; McEwen, B.S.; Chattarji, S. Stress, Memory and the Amygdala. Nat. Rev. Neurosci. 2009, 10, 423–433. [Google Scholar] [CrossRef]
- Vyas, A.; Pillai, A.G.; Chattarji, S. Recovery after Chronic Stress Fails to Reverse Amygdaloid Neuronal Hypertrophy and Enhanced Anxiety-like Behavior. Neuroscience 2004, 128, 667–673. [Google Scholar] [CrossRef]
- Salminen, P.; Grönroos, S.; Helmiö, M.; Hurme, S.; Juuti, A.; Juusela, R.; Peromaa-Haavisto, P.; Leivonen, M.; Nuutila, P.; Ovaska, J. Effect of Laparoscopic Sleeve Gastrectomy vs Roux-En-Y Gastric Bypass on Weight Loss, Comorbidities, and Reflux at 10 Years in Adult Patients With Obesity: The SLEEVEPASS Randomized Clinical Trial. JAMA Surg. 2022, 157, 656. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Ji, G.; Li, G.; Hu, Y.; Zhang, W.; Wang, J.; Tomasi, D.; Volkow, N.D.; Nie, Y.; Cui, G.; et al. Bariatric Surgery Induces Alterations in Effective Connectivity between the Orbitofrontal Cortex and Limbic Regions in Obese Patients. Sci. China Inf. Sci. 2020, 63, 170104. [Google Scholar] [CrossRef]
- Tao, B.; Tian, P.; Hao, Z.; Qi, Z.; Zhang, J.; Liu, J.; Liu, J.; Li, M.; Zhang, Z.; Zhang, P. Bariatric Surgery Improves Cognition Function in the Patients with Obesity: A Meta-Analysis. Obes. Surg. 2024, 34, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Michaud, A.; Dadar, M.; Pelletier, M.; Zeighami, Y.; Garcia-Garcia, I.; Iceta, S.; Yau, Y.; Nadeau, M.; Marceau, S.; Biertho, L.; et al. Neuroanatomical Changes in White and Grey Matter after Sleeve Gastrectomy. NeuroImage 2020, 213, 116696. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, G.; Hu, Y.; Li, G.; Ding, Y.; Hu, C.; Liu, L.; Zhang, W.; Von Deneen, K.M.; Han, Y.; et al. Laparoscopic Sleeve Gastrectomy Induces Sustained Changes in Gray and White Matter Brain Volumes and Resting Functional Connectivity in Obese Patients. Surg. Obes. Relat. Dis. 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Zeighami, Y.; Iceta, S.; Dadar, M.; Pelletier, M.; Nadeau, M.; Biertho, L.; Lafortune, A.; Tchernof, A.; Fulton, S.; Evans, A.; et al. Spontaneous Neural Activity Changes after Bariatric Surgery: A Resting-State fMRI Study. NeuroImage 2021, 241, 118419. [Google Scholar] [CrossRef]
- Daniele, G.; Dardano, A.; Lunghi, C.; Binda, P.; Ciccarone, A.; Santini, F.; Ceccarini, G.; Giusti, L.; Bellini, R.; Seghieri, M.; et al. Effect of Bariatric Surgery on Neuroplasticity in Humans. Diabetes 2018, 67, 1791-P. [Google Scholar] [CrossRef]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.; Proies, W.; Fahrbach, K. Bariatric Surgery. A Systematic Review and Meta-Analysis. JAMA 2004, 292, 1724–1737. [Google Scholar] [CrossRef] [PubMed]
- Karmali, S.; Brar, B.; Shi, X.; Sharma, A.M.; De Gara, C.; Birch, D.W. Weight Recidivism Post-Bariatric Surgery: A Systematic Review. Obes. Surg. 2013, 23, 1922–1933. [Google Scholar] [CrossRef]
- Wharton, S.; Kuk, J.L.; Luszczynski, M.; Kamran, E.; Christensen, R.A.G. Liraglutide 3.0 Mg for the Management of Insufficient Weight Loss or Excessive Weight Regain Post-bariatric Surgery. Clin. Obes. 2019, 9, e12323. [Google Scholar] [CrossRef]
- Van Hout, G.C.M.; Verschure, S.K.M.; Van Heck, G.L. Psychosocial Predictors of Success Following Bariatric Surgery. Obes. Surg. 2005, 15, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Sheets, C.S.; Peat, C.M.; Berg, K.C.; White, E.K.; Bocchieri-Ricciardi, L.; Chen, E.Y.; Mitchell, J.E. Post-Operative Psychosocial Predictors of Outcome in Bariatric Surgery. Obes. Surg. 2015, 25, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Odom, J.; Zalesin, K.C.; Washington, T.L.; Miller, W.W.; Hakmeh, B.; Zaremba, D.L.; Altattan, M.; Balasubramaniam, M.; Gibbs, D.S.; Krause, K.R.; et al. Behavioral Predictors of Weight Regain after Bariatric Surgery. Obes. Surg. 2010, 20, 349–356. [Google Scholar] [CrossRef]
- Saunders, R. “Grazing”: A High-Risk Behavior. Obes. Surg. 2004, 14, 98–102. [Google Scholar] [CrossRef]
- Kalarchian, M.A.; Marcus, M.D.; Wilson, G.T.; Labouvie, E.W.; Brolin, R.E.; LaMarca, L.B. Binge Eating Among Gastric Bypass Patients at Long-Term Follow-Up. Obes. Surg. 2002, 12, 270–275. [Google Scholar] [CrossRef]
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.H.; Still, C.D. Pharmacological Management of Obesity: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Z.; Ye, W.; Peng, P.; Wang, Y.; Wan, L.; Li, J.; Zhang, M.; Wang, Y.; Liu, R.; et al. Safety and Effects of Anti-Obesity Medications on Weight Loss, Cardiometabolic, and Psychological Outcomes in People Living with Overweight or Obesity: A Systematic Review and Meta-Analysis. eClinicalMedicine 2025, 79, 103020. [Google Scholar] [CrossRef]
- Tak, Y.J.; Lee, S.Y. Long-Term Efficacy and Safety of Anti-Obesity Treatment: Where Do We Stand? Curr. Obes. Rep. 2021, 10, 14–30. [Google Scholar] [CrossRef]
- Khera, R.; Pandey, A.; Chandar, A.K.; Murad, M.H.; Prokop, L.J.; Neeland, I.J.; Berry, J.D.; Camilleri, M.; Singh, S. Effects of Weight-Loss Medications on Cardiometabolic Risk Profiles: A Systematic Review and Network Meta-Analysis. Gastroenterology 2018, 154, 1309–1319.e7. [Google Scholar] [CrossRef]
- Horber, F.F.; Steffen, R. Reversal of Long-Term Weight Regain After Roux-En-Y Gastric Bypass Using Liraglutide or Surgical Revision. A Prospective Study. Obes. Surg. 2021, 31, 93–100. [Google Scholar] [CrossRef]
- Firkins, S.; Chittajallu, V.; Flora, B.; Yoo, H.; Simons-Linares, R. Utilization of Anti-Obesity Medications After Bariatric Surgery: Analysis of a Large National Database. Obes. Surg. 2024, 34, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Cifuentes, L.; Galbiati, F.; Mahmud, H.; Rometo, D. Weight Regain after Total Meal Replacement Very Low-calorie Diet Program with and With-out Anti-obesity Medications. Obes. Sci. Pract. 2024, 10, e722. [Google Scholar] [CrossRef]
- Edgerton, C.; Mehta, M.; Mou, D.; Dey, T.; Khaodhiar, L.; Tavakkoli, A. Patterns of Weight Loss Medication Utilization and Outcomes Following Bariatric Surgery. J. Gastrointest. Surg. 2021, 25, 369–377. [Google Scholar] [CrossRef]
- Gazda, C.; Clark, J.; Lingvay, I.; Almandoz, J. Pharmacotherapies for Post-Bariatric Weight Regain: Real-World Comparative Outcomes. Obesity 2021, 29, 829–836, Erratum in Obesity 2021, 29, 1567. [Google Scholar] [CrossRef]
- Jensen, A.B.; Renström, F.; Aczél, S.; Folie, P.; Biraima-Steinemann, M.; Beuschlein, F.; Bilz, S. Efficacy of the Glucagon-Like Peptide-1 Receptor Agonists Liraglutide and Semaglutide for the Treatment of Weight Regain After Bariatric Surgery: A Retrospective Observational Study. Obes. Surg. 2023, 33, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Stanford, F.C.; Alfaris, N.; Gomez, G.; Ricks, E.T.; Shukla, A.P.; Corey, K.E.; Pratt, J.S.; Pomp, A.; Rubino, F.; Aronne, L.J. The Utility of Weight Loss Medications after Bariatric Surgery for Weight Regain or Inadequate Weight Loss: A Multi-Center Study. Surg. Obes. Relat. Dis. 2017, 13, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.T.; Gomez, G.; Shukla, A.P.; Pratt, J.S.; Cena, H.; Biino, G.; Aronne, L.J.; Stanford, F.C. Weight Loss Medications in Young Adults after Bariatric Surgery for Weight Regain or Inadequate Weight Loss: A Multi-Center Study. Children 2018, 5, 116. [Google Scholar] [CrossRef]
- Istfan, N.W.; Anderson, W.A.; Hess, D.T.; Yu, L.; Carmine, B.; Apovian, C.M. The Mitigating Effect of Phentermine and Topiramate on Weight Regain After Roux-en-Y Gastric Bypass Surgery. Obesity 2020, 28, 1023–1030. [Google Scholar] [CrossRef]
- Srivastava, G.; Buffington, C. A Specialized Medical Management Program to Address Post-Operative Weight Regain in Bariatric Patients. Obes. Surg. 2018, 28, 2241–2246. [Google Scholar] [CrossRef]
- Stanford, F.C.; Toth, A.T.; Shukla, A.P.; Pratt, J.S.; Cena, H.; Biino, G.; Aronne, L.J. Weight Loss Medications in Older Adults After Bariatric Surgery for Weight Regain or Inadequate Weight Loss: A Multicenter Study. Bariatr. Surg. Pract. Patient Care 2018, 13, 171–178. [Google Scholar] [CrossRef]
- Richelsen, B.; Tonstad, S.; Rössner, S.; Toubro, S.; Niskanen, L.; Madsbad, S.; Mustajoki, P.; Rissanen, A. Effect of Orlistat on Weight Regain and Cardiovascular Risk Factors Following a Very-Low-Energy Diet in Abdominally Obese Patients. Diabetes Care 2007, 30, 27–32. [Google Scholar] [CrossRef]
- Sjöström, L.; Rissanen, A.; Andersen, T.; Boldrin, M.; Golay, A.; Koppeschaar, H.P.; Krempf, M. Randomised Placebo-Controlled Trial of Orlistat for Weight Loss and Prevention of Weight Regain in Obese Patients. Lancet 1998, 352, 167–172. [Google Scholar] [CrossRef]
- Zoss, I.; Piec, G.; Horber, F.F. Impact of Orlistat Therapy on Weight Reduction in Morbidly Obese Patients After Implantation of the Swedish Adjustable Gastric Band. Obes. Surg. 2002, 12, 113–117. [Google Scholar] [CrossRef]
- Schwartz, J.; Suzo, A.; Wehr, A.M.; Foreman, K.S.; Mikami, D.J.; Needleman, B.J.; Noria, S.F. Pharmacotherapy in Conjunction with a Diet and Exercise Program for the Treatment of Weight Recidivism or Weight Loss Plateau Post-Bariatric Surgery: A Retrospective Review. Obes. Surg. 2016, 26, 452–458. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, F.C.A.; Morbach, V.; Sano, V.K.T.; Fernandes, L.R.; Kreuz, M.; Kelly, F.A. Liraglutide for the Treatment of Weight Regain After Bariatric Surgery: A Systematic Review and Meta-Analysis. Obes. Surg. 2024, 34, 2844–2853. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, F.; Di Stefano, C.; Baratta, R.; Pulvirenti, A.; Mastrandrea, G.; Piazza, L.; Guccione, F.; Navarra, G.; Frittitta, L. Efficacy of High-Dose Liraglutide 3.0 Mg in Patients with Poor Response to Bariatric Surgery: Real-World Experience and Updated Meta-Analysis. Obes. Surg. 2024, 34, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Qasem, W.; Hamshari, F.; Dsouza, C.; Alqallaf, N.; Otiku, P.; Nnaji, C.A. Effectiveness and Tolerability of Liraglutide for the Management of Weight Regain Following Sleeve Gastrectomy. Obes. Sci. Pract. 2024, 10, e706. [Google Scholar] [CrossRef]
- Lautenbach, A.; Wernecke, M.; Huber, T.B.; Stoll, F.; Wagner, J.; Meyhöfer, S.M.; Meyhöfer, S.; Aberle, J. The Potential of Semaglutide Once-Weekly in Patients Without Type 2 Diabetes with Weight Regain or Insufficient Weight Loss After Bariatric Surgery—A Retrospective Analysis. Obes. Surg. 2022, 32, 3280–3288. [Google Scholar] [CrossRef]
- Murvelashvili, N.; Xie, L.; Schellinger, J.N.; Mathew, M.S.; Marroquin, E.M.; Lingvay, I.; Messiah, S.E.; Almandoz, J.P. Effectiveness of Semaglutide versus Liraglutide for Treating Post-metabolic and Bariatric Surgery Weight Recurrence. Obesity 2023, 31, 1280–1289. [Google Scholar] [CrossRef]
- Kanai, R.; Kinoshita, S.; Kanbe, I.; Sameda, M.; Yamaoka, S.; Horikawa, O.; Watanabe, Y.; Tatsuno, I.; Shirai, K.; Oshiro, T.; et al. Once-Weekly Semaglutide Administered after Laparoscopic Sleeve Gastrectomy: Effects on Body Weight, Glycemic Control, and Measured Nutritional Metrics in Japanese Patients Having Both Obesity and Type 2 Diabetes. Obes. Pillars 2024, 9, 100098. [Google Scholar] [CrossRef]
- Stoll, F.; Kantowski, T.; Laaser, J.; Kloiber, U.; Plitzko, G.; Mann, O.; Aberle, J.; Lautenbach, A. Tackling Suboptimal Clinical Response after Metabolic Bariatric Surgery: Impact of Tirzepatide on Weight Loss and Body Composition. Obes. Res. Clin. Pract. 2025, 19, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Davies, M.; Van Gaal, L.F.; Kandler, K.; Konakli, K.; Lingvay, I.; McGowan, B.M.; Oral, T.K.; Rosenstock, J.; et al. Weight Regain and Cardiometabolic Effects after Withdrawal of Semaglutide: The STEP 1 Trial Extension. Diabetes Obes. Metab. 2022, 24, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.K.; Blond, M.B.; Sandsdal, R.M.; Olsen, L.M.; Juhl, C.R.; Lundgren, J.R.; Janus, C.; Stallknecht, B.M.; Holst, J.J.; Madsbad, S.; et al. Healthy Weight Loss Maintenance with Exercise, GLP-1 Receptor Agonist, or Both Combined Followed by One Year without Treatment: A Post-Treatment Analysis of a Randomised Placebo-Controlled Trial. eClinicalMedicine 2024, 69, 102475. [Google Scholar] [CrossRef]
- Aronne, L.; Sattar, N.; Horn, D. Continued Treatment With Tirzepatide for Maintenance of Weight Reduction in Adults With Obesity: The SURMOUNT-4 Randomized Clinical Trial. JAMA 2024, 331, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, M.A.; D’Angelo, D.; Tchang, B.G.; Sahagun, A.D.; Andre, C.; Aronne, L.J.; Shukla, A.P. Five-Year Weight Loss Maintenance With Obesity Pharmacotherapy. J. Clin. Endocrinol. Metab. 2023, 108, e832–e841. [Google Scholar] [CrossRef]
- Wang, X.-F.; Liu, J.-J.; Xia, J.; Liu, J.; Mirabella, V.; Pang, Z.P. Endogenous Glucagon-like Peptide-1 Suppresses High-Fat Food Intake by Reducing Synaptic Drive onto Mesolimbic Dopamine Neurons. Cell Rep. 2015, 12, 726–733. [Google Scholar] [CrossRef]
- Dickson, S.L.; Shirazi, R.H.; Hansson, C.; Bergquist, F.; Nissbrandt, H.; Skibicka, K.P. The Glucagon-Like Peptide 1 (GLP-1) Analogue, Exendin-4, Decreases the Rewarding Value of Food: A New Role for Mesolimbic GLP-1 Receptors. J. Neurosci. 2012, 32, 4812–4820. [Google Scholar] [CrossRef]
- Péterfi, Z.; Szilvásy-Szabó, A.; Farkas, E.; Ruska, Y.; Pyke, C.; Knudsen, L.B.; Fekete, C. Glucagon-Like Peptide-1 Regulates the Proopiomelanocortin Neurons of the Arcuate Nucleus Both Directly and Indirectly via Presynaptic Action. Neuroendocrinology 2021, 111, 986–997. [Google Scholar] [CrossRef]
- Dong, Y.; Carty, J.; Goldstein, N.; He, Z.; Hwang, E.; Chau, D.; Wallace, B.; Kabahizi, A.; Lieu, L.; Peng, Y.; et al. Time and Metabolic State-Dependent Effects of GLP-1R Agonists on NPY/AgRP and POMC Neuronal Activity In Vivo. Mol. Metab. 2021, 54, 101352. [Google Scholar] [CrossRef]
- Van Bloemendaal, L.; Veltman, D.J.; Ten Kulve, J.S.; Drent, M.L.; Barkhof, F.; Diamant, M.; IJzerman, R.G. Emotional Eating Is Associated with Increased Brain Responses to Food-cues and Reduced Sensitivity to GLP-1 Receptor Activation. Obesity 2015, 23, 2075–2082. [Google Scholar] [CrossRef]
- Van Ruiten, C.C.; Ten Kulve, J.S.; Van Bloemendaal, L.; Nieuwdorp, M.; Veltman, D.J.; IJzerman, R.G. Eating Behavior Modulates the Sensitivity to the Central Effects of GLP-1 Receptor Agonist Treatment: A Secondary Analysis of a Randomized Trial. Psychoneuroendocrinology 2022, 137, 105667. [Google Scholar] [CrossRef] [PubMed]
- Greenway, F.L.; Dunayevich, E.; Tollefson, G.; Erickson, J.; Guttadauria, M.; Fujioka, K.; Cowley, M.A.; for the NB-201 Study Group. Comparison of Combined Bupropion and Naltrexone Therapy for Obesity with Monotherapy and Placebo. J. Clin. Endocrinol. Metab. 2009, 94, 4898–4906. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-J.; Zhao, J.; Tomasi, D.; Kojori, E.S.; Wang, R.; Wiers, C.E.; Caparelli, E.C.; Volkow, N.D. Effect of Combined Naltrexone and Bupropion Therapy on the Brain’s Functional Connectivity. Int. J. Obes. 2018, 42, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Carbone, E.A.; Caroleo, M.; Rania, M.; Calabrò, G.; Staltari, F.A.; De Filippis, R.; Aloi, M.; Condoleo, F.; Arturi, F.; Segura-Garcia, C. An Open-Label Trial on the Efficacy and Tolerability of Naltrexone/Bupropion SR for Treating Altered Eating Behaviours and Weight Loss in Binge Eating Disorder. Eat. Weight Disord.-Stud. Anorex. Bulim. Obes. 2021, 26, 779–788. [Google Scholar] [CrossRef]
- Grilo, C.M.; Lydecker, J.A.; Fineberg, S.K.; Moreno, J.O.; Ivezaj, V.; Gueorguieva, R. Naltrexone-Bupropion and Behavior Therapy, Alone and Combined, for Binge-Eating Disorder: Randomized Double-Blind Placebo-Controlled Trial. Am. J. Psychiatry 2022, 179, 927–937. [Google Scholar] [CrossRef]
- Grilo, C.M.; Lydecker, J.A.; Gueorguieva, R. Naltrexone plus Bupropion Combination Medication Maintenance Treatment for Binge-Eating Disorder Following Successful Acute Treatments: Randomized Double-Blind Placebo-Controlled Trial. Psychol. Med. 2023, 53, 7775–7784. [Google Scholar] [CrossRef]
- Moawad, M.H.-E.; Sadeq, M.A.; Abbas, A.; Ghorab, R.M.F.; Serag, I.; Hendawy, M.; Alkasaby, M. Efficacy of Naltrexone/Bupropion in Treatment of Binge Eating: A Systematic Review and Meta-Analysis. Psychiatry Int. 2024, 5, 323–337. [Google Scholar] [CrossRef]

 decrease activity
decrease activity  increase activity.
increase activity.
 decrease activity
decrease activity  increase activity.
increase activity.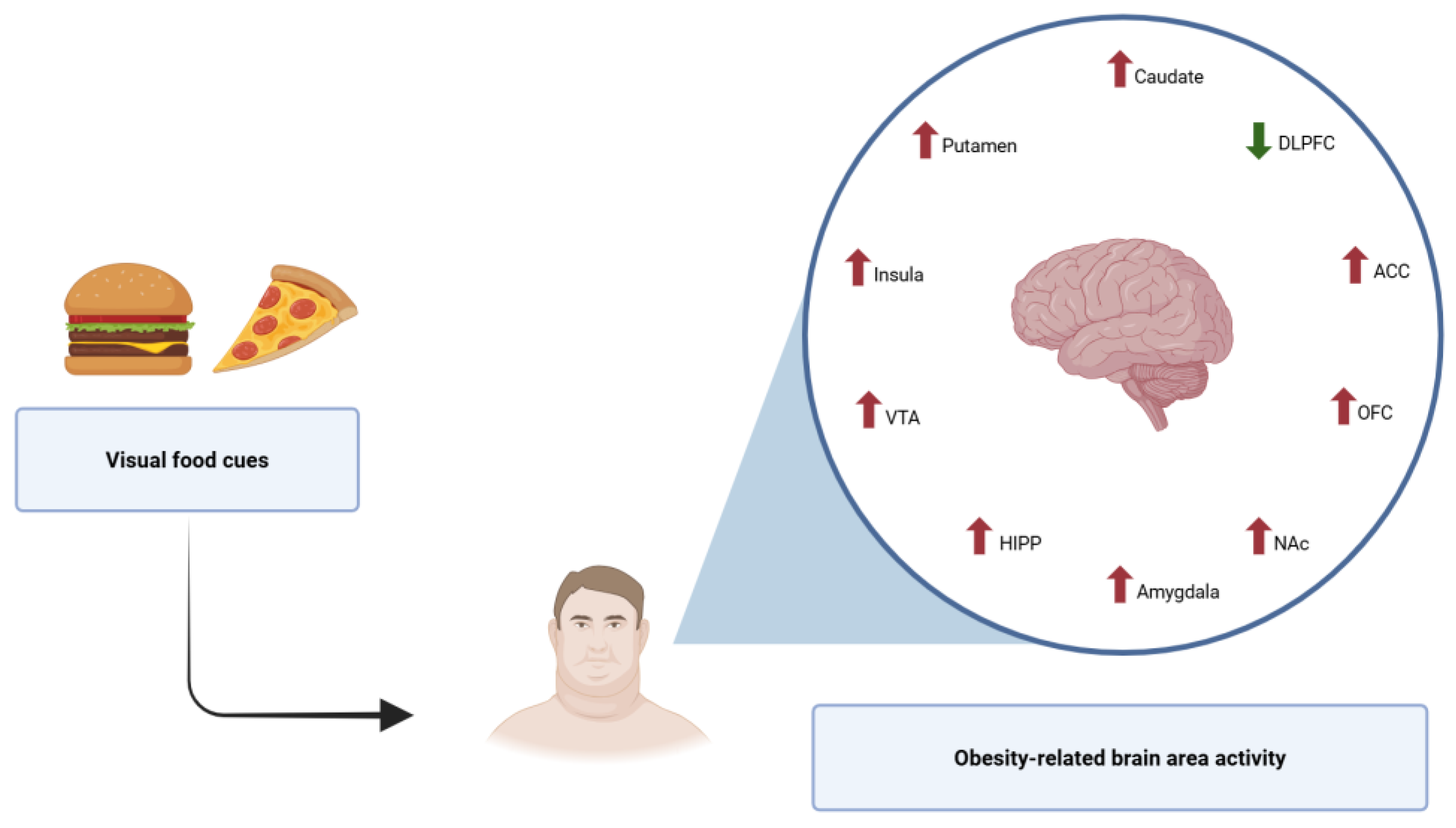

| Psychological Factor | Effect on Weight Regain | Mechanism of Weight Control |
|---|---|---|
| Impulsiveness | ↑ WR risk | Increased impulsiveness leads to heightened activation in the ACC and bilateral amygdala during reward anticipation, promoting overeating. Hypodensity of OFC, ACC, amygdala, and medial PFC is also correlated with higher levels of impulsiveness. |
| Neuroticism | ↑ WR risk | Higher neuroticism correlates with emotional eating and increased susceptibility to weight regain due to heightened amygdala activity and reduced connectivity between the amygdala and ACC, which may favor anxiety and depressive symptoms. |
| Dichotomous thinking | ↑ WR risk | Dichotomous thinking contributes to unhealthy eating patterns, overeating, and weight cycling through highly rigid “black and white” dietary restraint. This cognitive style also mediates the relationship between depression and obesity and may be closely associated with perfectionism. |
| High sensitivity to reward | ↑ WR risk | Sensitivity to reward is associated with impairments in dopaminergic system pathways and may favor an increased risk of food addiction. At higher levels, it promotes compensatory overeating in response to negative stimuli, as this feature is related to emotional regulation problems. |
| Conscientiousness | ↓ WR risk | Conscientiousness promotes sustained efforts in maintaining weight, linked to stronger prefrontal cortex activity supporting self-regulation. |
| High self-control | ↓ WR risk | Higher levels of self-control predict successful weight maintenance, which is associated with better regulation of eating behavior and reduced activation of reward areas. |
| Persistence | ↓ WR risk | It prevents weight regain and enables long-term weight maintenance through consistent use of weight management strategies. |
| High self-efficacy | ↓ WR risk | It fosters persistence in pursuing defined goals and makes it easier to engage in behaviors that promote a healthy lifestyle. In combination with higher self-control, it can increase motivation. |
| Brain Region | Behavioral Outcomes | Impact on Weight Regain (WR) |
|---|---|---|
| Orbitofrontal cortex (OFC) | Hedonic evaluation of food; reward valuation; decision-making | Hyperactivity leads to excessive food reward seeking and overeating; lower density is associated with higher levels of impulsiveness |
| Ventral striatum (VS) | Reward anticipation and habit learning | Increased activation enhances susceptibility to food cues |
| Prefrontal cortex (PFC) | Executive control; inhibition of impulsive behaviors | Reduced activity impairs self-regulation, facilitating overeating; lower density is associated with higher levels of impulsiveness |
| Anterior cingulate cortex (ACC) | Cognitive and emotional processing; motivation and decision-making; learning and cost–benefit analysis | Impairments associated with higher impulsivity and food addiction |
| Amygdala | Emotional regulation (processing of emotions, particularly fear and anxiety); emotional eating | Hyperresponsivity enhances emotional eating and craving; lower density is associated with higher levels of impulsiveness; increased activity is positively correlated with neuroticism |
| Ventral tegmental area (VTA) | Reward processing; motivation and goal-directed behavior; learning and memory; addictive behaviors | Dysregulation reduces reward sensitivity, promoting compensatory overeating |
| Nucleus accumbens (NAc) | Incentive salience; reinforcement learning; hedonic value | Overactivation reinforces habitual overeating patterns |
| Parahippocampal gyrus | Emotional memory; emotional processing; contextual modulation of food cues | Altered activation in response to high-calorie food cues may enhance context-driven food craving and emotional eating; dysfunctional activity is associated with impaired inhibitory control over eating behaviors |
| Insula | Interoceptive awareness; sensory integration (taste and odor perception); cognitive control and decision-making | Altered interoception linked to dysregulated eating; heightened insular activation in response to food cues is associated with stronger cravings, greater emotional reactivity, and reduced control over eating behavior |
| Thalamus and midbrain | Sensory relay; reward processing integration | Structural deficits are associated with impaired motivated behavior |
| Hormone | Mechanism of Action | Serum Concentrations in Obesity | Changes After Bariatric Surgery | Changes After Calorie Reduction Diet |
|---|---|---|---|---|
| Ghrelin | ↑ appetite | ↓ | ↑ LAGB [137,138,139,140,141,142] ↓ LSG [143,144,145,146,147] RYGB: ↓ in short-term/↑ in long-term [148] | ↑ [130,132,149,150] |
| Leptin | ↓ appetite improves satiation | ↑ (leptin resistance) | ↓ LAGB [138,140,148,151] ↓ LSG [143,145,146,152] ↓ RYGB [138,145,148,152,153] | ↓ [132,154,155] |
| Insulin | ↓ appetite | ↑ (insulin resistance) | ↓ LAGB [138,140] ↓ LSG [145,146,152] ↓ RYGB [138,145,152] | ↓ [132,150,154,156,157] |
| CCK | slows gastric emptying induces satiation | postprandial ↓ | ? LAGB ↑ LSG [158,159] ↑ RYGB [159,160] | ↓ [132,161] |
| GLP-1 | ↓ appetite slows gastric emptying | postprandial ↓ | - LAGB [148,153] ↑ LSG [145,147,162] ↑ RYGB [145,148] | ↓ [132,157,163] |
| PYY | ↓ appetite slows gastric emptying | postprandial ↓ | ↑/- LAGB [164,165,166,167,168] ↑ LSG [145,147,162] ↑ RYGB [145,148] | ↓ [132,169,170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moszak, M.; Marcickiewicz, J.; Pelczyńska, M.; Bogdański, P. The Interplay Between Psychological and Neurobiological Predictors of Weight Regain: A Narrative Review. Nutrients 2025, 17, 1662. https://doi.org/10.3390/nu17101662
Moszak M, Marcickiewicz J, Pelczyńska M, Bogdański P. The Interplay Between Psychological and Neurobiological Predictors of Weight Regain: A Narrative Review. Nutrients. 2025; 17(10):1662. https://doi.org/10.3390/nu17101662
Chicago/Turabian StyleMoszak, Małgorzata, Justyna Marcickiewicz, Marta Pelczyńska, and Paweł Bogdański. 2025. "The Interplay Between Psychological and Neurobiological Predictors of Weight Regain: A Narrative Review" Nutrients 17, no. 10: 1662. https://doi.org/10.3390/nu17101662
APA StyleMoszak, M., Marcickiewicz, J., Pelczyńska, M., & Bogdański, P. (2025). The Interplay Between Psychological and Neurobiological Predictors of Weight Regain: A Narrative Review. Nutrients, 17(10), 1662. https://doi.org/10.3390/nu17101662







