Personalized Nutrition Strategies for Patients in the Intensive Care Unit: A Narrative Review on the Future of Critical Care Nutrition
Abstract
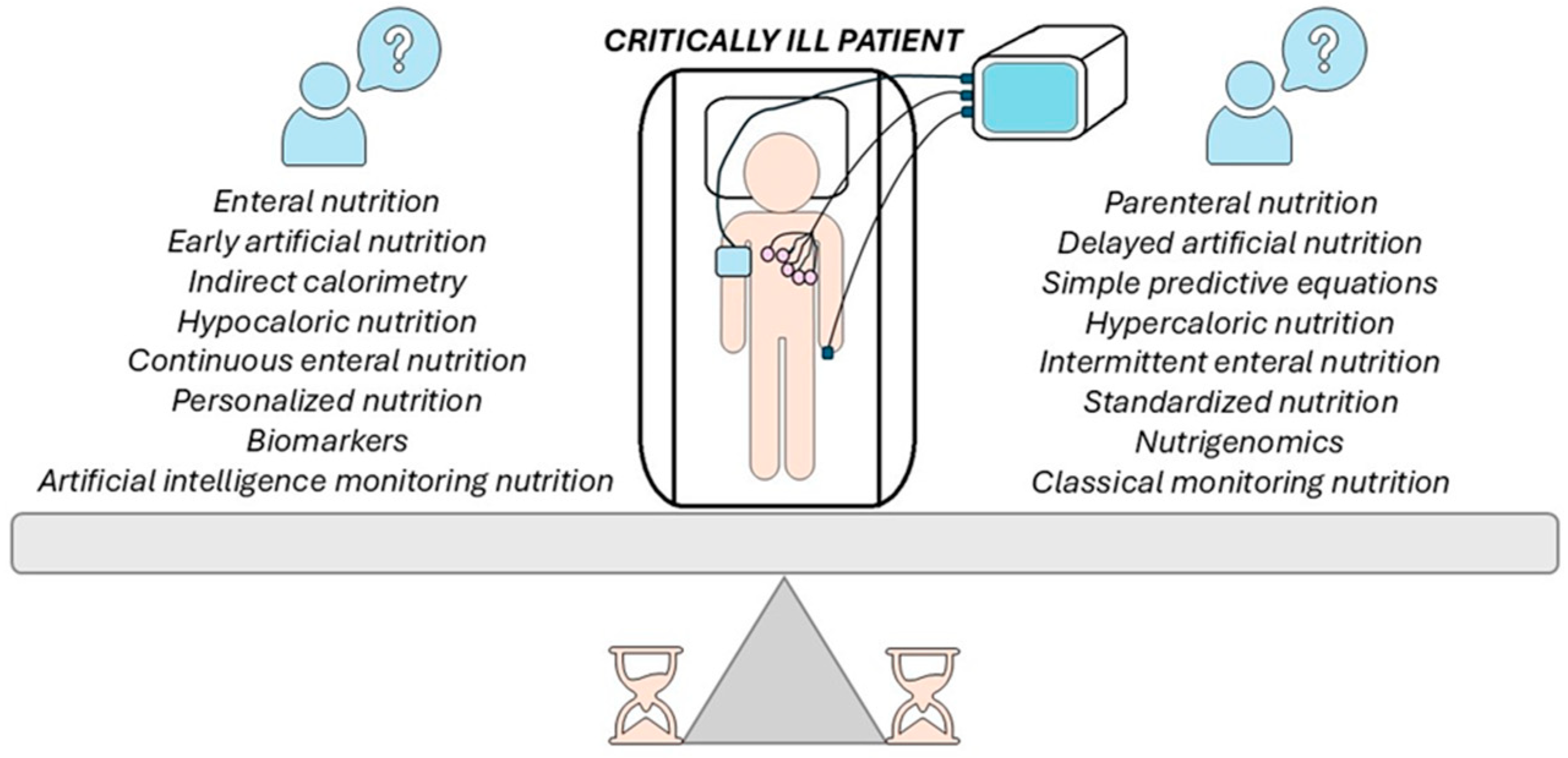
1. Introduction
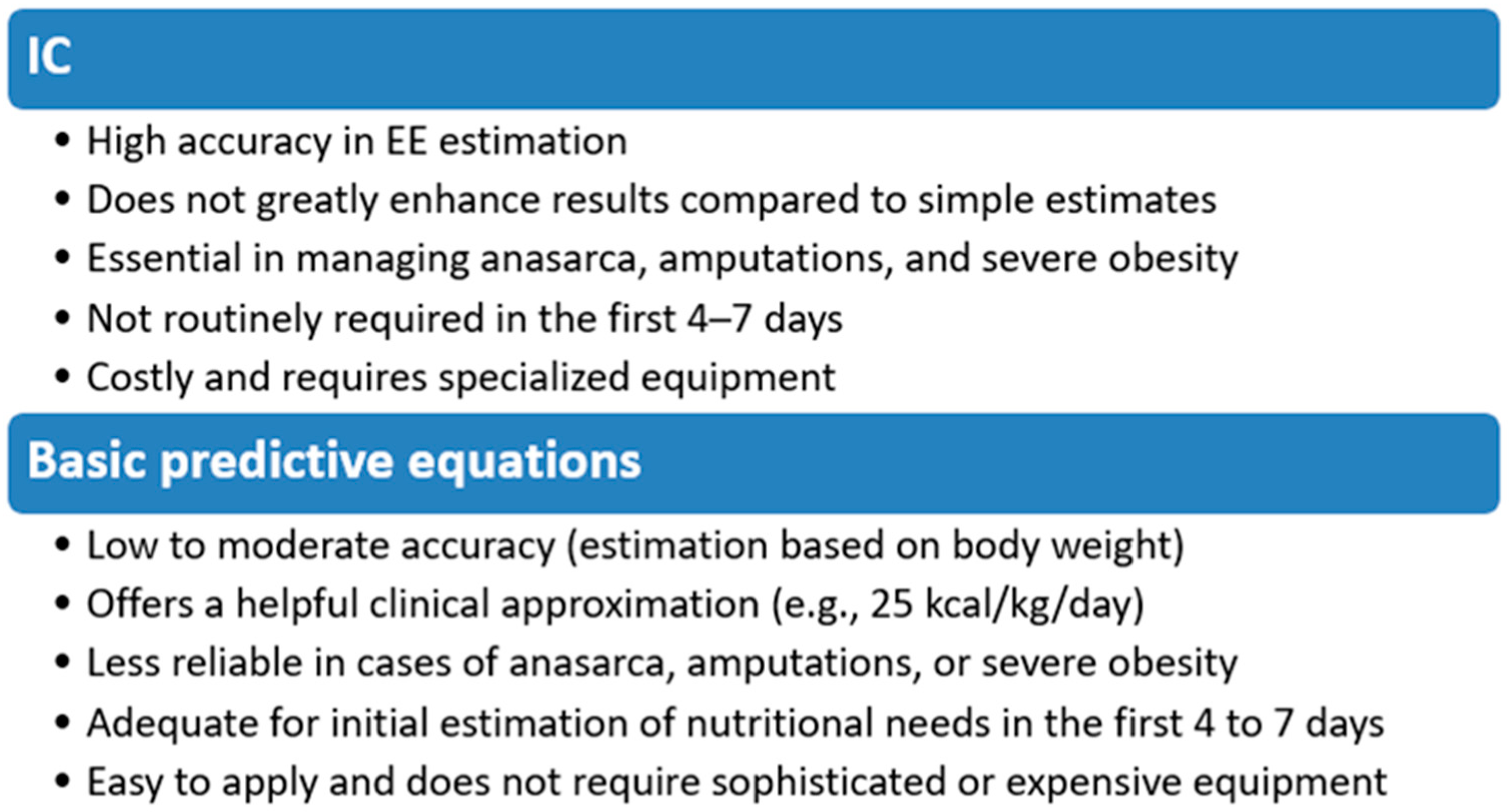
2. Indirect Calorimetry or Basic Predictive Equations
3. Enteral or Parenteral Nutrition
4. Early or Delayed Artificial Nutrition
5. Hypocaloric Versus Hypercaloric Nutrition
6. Continuous or Intermittent Enteral Nutrition
7. Personalized Versus Standardized Nutrition
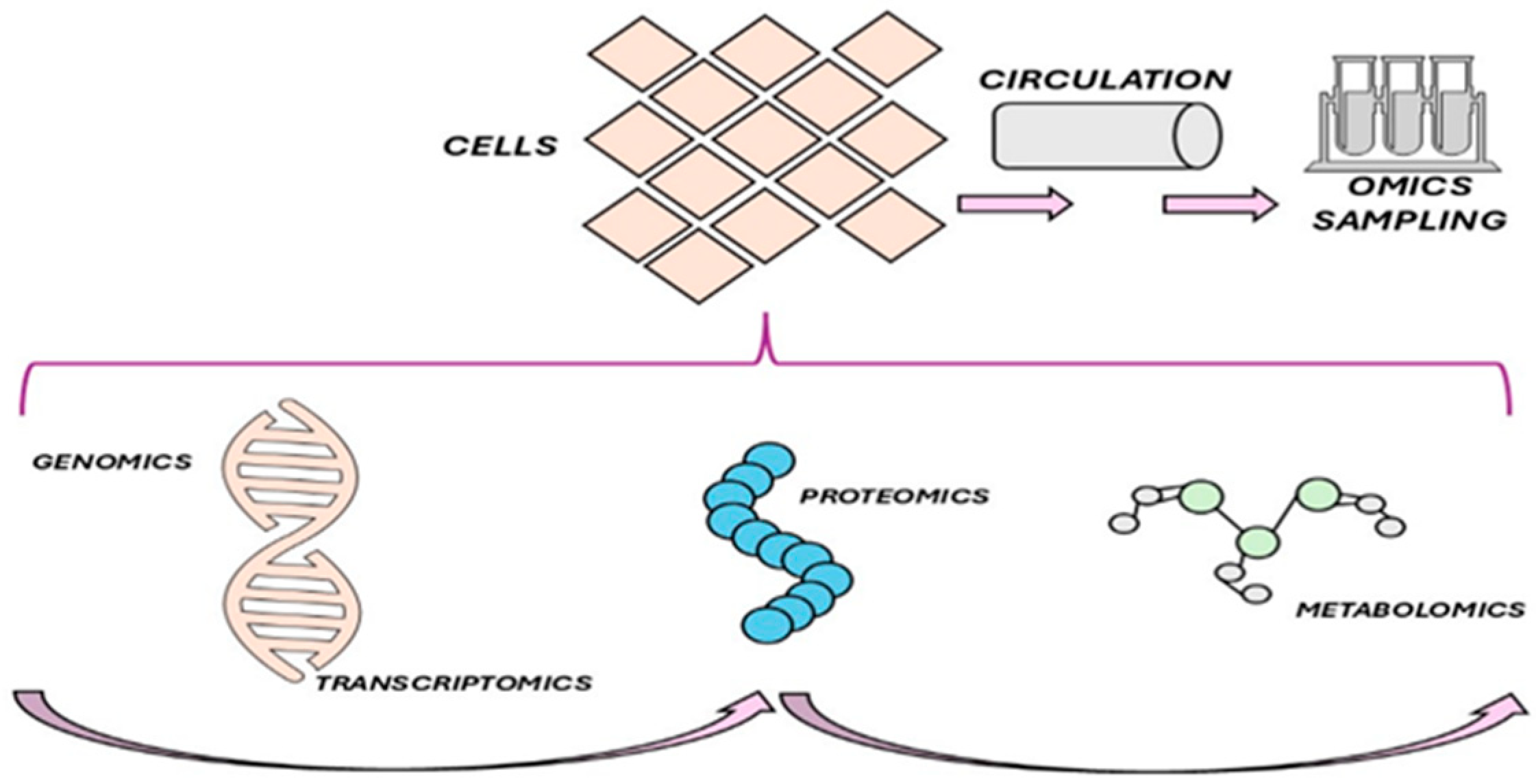
8. Biomarkers or Nutrigenomics
9. Artificial Intelligence (AI) or Traditional Methods for Monitoring and Evaluating Artificial Nutrition
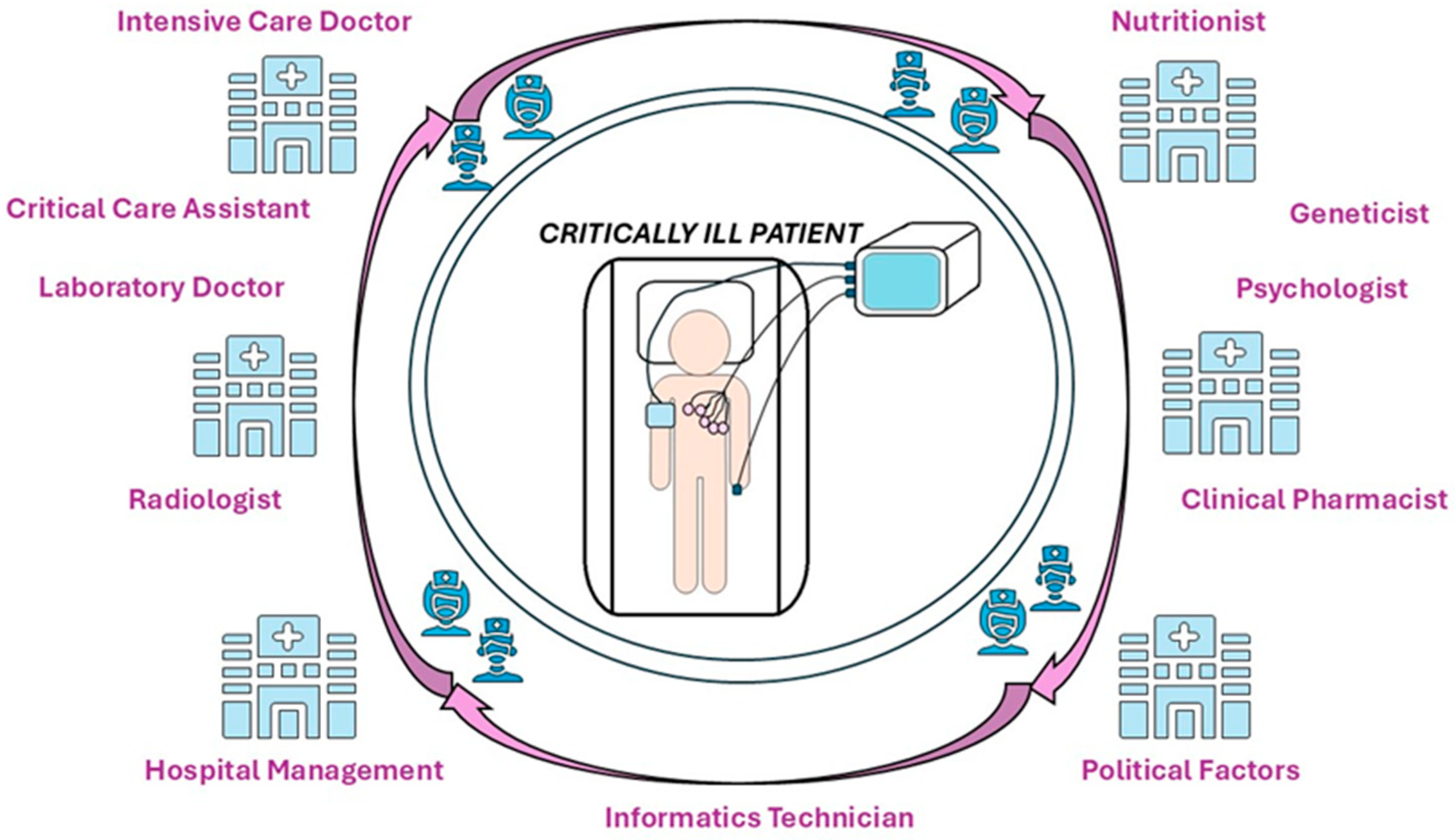
10. Multidisciplinary and Multiprofessional Collaborations in the Nutritional Therapy of Critically Ill Patients
11. Challenges and Barriers to Implementing Personalized Nutrition
12. From Theory to Practice: Implementing Personalized Nutrition in the ICU
13. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| ALB | Serum albumin |
| AMY1 | Amylase 1 |
| ASPEN | American Society for Parenteral and Enteral Nutrition |
| BPI | Bacterial permeability-increasing protein |
| CD14 | CD14 molecule |
| DNA | Deoxyribonucleic acid |
| EE | Energy expenditure |
| EN | Enteral nutrition |
| ESPEN | The European Society for Enteral and Parenteral Nutrition |
| IC | Indirect calorimetry |
| ICUAW | ICU-acquired weakness |
| ICUs | Intensive care units |
| IGF1 | Insulin-like growth factor 1 |
| IL6 | Interleukin 6 |
| IL10 | Interleukin 10 |
| LCT | Lactase |
| LBP | Lipopolysaccharide-binding protein |
| LTA | Lymphotoxin alpha |
| ML | Machine learning |
| MBL | Mannose-binding lectin |
| PAI1 | Plasminogen activator inhibitor 1 |
| PN | Parenteral nutrition |
| REE | Resting EE |
| RNA | Ribonucleic acid |
| SNPs | Single nucleotide polymorphisms |
| TLR4 | Toll-like receptor 4 |
| TNF | Tumor necrosis factor |
References
- Cass, A.R.; Charlton, K.E. Prevalence of Hospital-Acquired Malnutrition and Modifiable Determinants of Nutritional Deterioration during Inpatient Admissions: A Systematic Review of the Evidence. J. Hum. Nutr. Diet. Off. J. Br. Diet. Assoc. 2022, 35, 1043–1058. [Google Scholar] [CrossRef] [PubMed]
- Starace, E.; De Pasquale, G.; Morenghi, E.; Crippa, C.; Matteucci, S.; Pieri, G.; Soekeland, F.; Gibbi, S.M.; Lo Cricchio, G.; Reggiani, F.; et al. Hospital Malnutrition in the Medicine and Neurology Departments: A Complex Challenge. Nutrients 2023, 15, 5061. [Google Scholar] [CrossRef] [PubMed]
- White, J.V.; Guenter, P.; Jensen, G.; Malone, A.; Schofield, M.; Academy Malnutrition Work Group. ASPEN Malnutrition Task Force; A.S.P.E.N. Board of Directors. Consensus Statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: Characteristics Recommended for the Identification and Documentation of Adult Malnutrition (Undernutrition). JPEN J. Parenter. Enter. Nutr. 2012, 36, 275–283. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Dubois, M.-J.; Navickis, R.J.; Wilkes, M.M. Hypoalbuminemia in Acute Illness: Is There a Rationale for Intervention? A Meta-Analysis of Cohort Studies and Controlled Trials. Ann. Surg. 2003, 237, 319–334. [Google Scholar] [CrossRef]
- Lad, H.; Saumur, T.M.; Herridge, M.S.; Dos Santos, C.C.; Mathur, S.; Batt, J.; Gilbert, P.M. Intensive Care Unit-Acquired Weakness: Not Just Another Muscle Atrophying Condition. Int. J. Mol. Sci. 2020, 21, 7840. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Padilla, I.A.; Rodríguez-Moguel, N.C.; Rodríguez-Llamazares, S.; Orsso, C.E.; Prado, C.M.; Ríos-Ayala, M.A.; Villanueva-Camacho, O.; Aguilar-Vargas, A.; Pensado-Piedra, L.E.; Juárez-Hernández, F.; et al. Low Muscle Mass in COVID-19 Critically-Ill Patients: Prognostic Significance and Surrogate Markers for Assessment. Clin. Nutr. 2022, 41, 2910–2917. [Google Scholar] [CrossRef]
- Lew, C.C.H.; Wong, G.J.Y.; Cheung, K.P.; Fraser, R.J.L.; Chua, A.P.; Chong, M.F.F.; Miller, M. The Association between Nutritional Adequacy and 28-Day Mortality in the Critically Ill Is Not Modified by Their Baseline Nutritional Status and Disease Severity. Crit. Care 2019, 23, 222. [Google Scholar] [CrossRef]
- Yasuda, H.; Horikoshi, Y.; Kamoshita, S.; Kuroda, A.; Moriya, T. Associations between In-Hospital Mortality and Prescribed Parenteral Energy and Amino Acid Doses in Critically Ill Patients: A Retrospective Cohort Study Using a Medical Claims Database. Nutrients 2024, 16, 57. [Google Scholar] [CrossRef]
- Jubina, L.E.; Locke, A.; Fedder, K.R.; Slone, S.A.; Soper, M.K.; Kalema, A.G.; Montgomery-Yates, A.A.; Mayer, K.P. Nutrition in the Intensive Care Unit and Early Recovery Influence Functional Outcomes for Survivors of Critical Illness: A Prospective Cohort Study. JPEN J. Parenter. Enter. Nutr. 2023, 47, 888–895. [Google Scholar] [CrossRef]
- Vest, M.T.; Newell, E.; Shapero, M.; McGraw, P.; Jurkovitz, C.; Lennon, S.L.; Trabulsi, J. Energy Balance in Obese, Mechanically Ventilated Intensive Care Unit Patients. Nutrients 2019, 66, 48–53. [Google Scholar] [CrossRef]
- Stoian, M.; Andone, A.; Bândilă, S.R.; Onișor, D.; Laszlo, S.Ș.; Lupu, G.; Danielescu, A.; Baba, D.-F.; Văsieșiu, A.M.; Manea, A.; et al. Mechanical Ventilator-Associated Pneumonia in the COVID-19 Pandemic Era: A Critical Challenge in the Intensive Care Units. Antibiotics 2025, 14, 28. [Google Scholar] [CrossRef]
- Stoian, M.; Roman, A.; Boeriu, A.; Onișor, D.; Bandila, S.R.; Babă, D.F.; Cocuz, I.; Niculescu, R.; Costan, A.; Laszlo, S.Ș.; et al. Long-Term Radiological Pulmonary Changes in Mechanically Ventilated Patients with Respiratory Failure Due to SARS-CoV-2 Infection. Biomedicines 2023, 11, 2637. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN Guideline on Clinical Nutrition in the Intensive Care Unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Calder, P.C.; Casaer, M.; Hiesmayr, M.; Mayer, K.; Montejo-Gonzalez, J.C.; Pichard, C.; Preiser, J.-C.; et al. ESPEN Practical and Partially Revised Guideline: Clinical Nutrition in the Intensive Care Unit. Clin. Nutr. 2023, 42, 1671–1689. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E.; Bear, D.E.; Berger, M.M.; De Waele, E.; Gunst, J.; McClave, S.A.; Prado, C.M.; Puthucheary, Z.; Ridley, E.J.; Van den Berghe, G.; et al. Personalized Nutrition Therapy in Critical Care: 10 Expert Recommendations. Crit. Care 2023, 27, 261. [Google Scholar] [CrossRef]
- De Waele, E.; Jonckheer, J.; Wischmeyer, P.E. Indirect Calorimetry in Critical Illness: A New Standard of Care? Curr. Opin. Crit. Care 2021, 27, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zheng, J.; Cheng, J.; Zou, H.; Li, M.; Deng, B.; Luo, R.; Wang, F.; Huang, D.; Li, G.; et al. Personalized Nutrition: A Review of Genotype-Based Nutritional Supplementation. Front. Nutr. 2022, 9, 992986. [Google Scholar] [CrossRef]
- Kan, J.; Ni, J.; Xue, K.; Wang, F.; Zheng, J.; Cheng, J.; Wu, P.; Runyon, M.K.; Guo, H.; Du, J. Personalized Nutrition Intervention Improves Health Status in Overweight/Obese Chinese Adults: A Randomized Controlled Trial. Front. Nutr. 2022, 9, 919882. [Google Scholar] [CrossRef]
- McClave, S.A.; Omer, E. Point-Counterpoint: Indirect Calorimetry Is Not Necessary for Optimal Nutrition Therapy in Critical Illness. Nutr. Clin. Pract. 2021, 36, 268–274. [Google Scholar] [CrossRef]
- Casaer, M.P.; Mesotten, D.; Hermans, G.; Wouters, P.J.; Schetz, M.; Meyfroidt, G.; Van Cromphaut, S.; Ingels, C.; Meersseman, P.; Muller, J.; et al. Early versus Late Parenteral Nutrition in Critically Ill Adults. N. Engl. J. Med. 2011, 365, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Preiser, J.-C.; Arabi, Y.M.; Berger, M.M.; Casaer, M.; McClave, S.; Montejo-González, J.C.; Peake, S.; Reintam Blaser, A.; Van den Berghe, G.; van Zanten, A.; et al. A Guide to Enteral Nutrition in Intensive Care Units: 10 Expert Tips for the Daily Practice. Crit. Care 2021, 25, 424. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.; Demehri, F.R.; Teitelbaum, D.H. Intestine, Immunity, and Parenteral Nutrition in an Era of Preferred Enteral Feeding. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 496–500. [Google Scholar] [CrossRef]
- Asrani, V.M.; Brown, A.; Bissett, I.; Windsor, J.A. Impact of Intravenous Fluids and Enteral Nutrition on the Severity of Gastrointestinal Dysfunction: A Systematic Review and Meta-Analysis. J. Crit. Care Med. 2020, 6, 5–24. [Google Scholar] [CrossRef]
- McClave, S.A.; Martindale, R.G.; Rice, T.W.; Heyland, D.K. Feeding the Critically Ill Patient. Crit. Care Med. 2014, 42, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Elke, G.; van Zanten, A.R.H.; Lemieux, M.; McCall, M.; Jeejeebhoy, K.N.; Kott, M.; Jiang, X.; Day, A.G.; Heyland, D.K. Enteral versus Parenteral Nutrition in Critically Ill Patients: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Care 2016, 20, 117. [Google Scholar] [CrossRef]
- Martindale, R.G.; Warren, M. Should Enteral Nutrition Be Started in the First Week of Critical Illness? Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 202–206. [Google Scholar] [CrossRef]
- van den Berghe, G.; Wouters, P.; Weekers, F.; Verwaest, C.; Bruyninckx, F.; Schetz, M.; Vlasselaers, D.; Ferdinande, P.; Lauwers, P.; Bouillon, R. Intensive Insulin Therapy in Critically Ill Patients. N. Engl. J. Med. 2001, 345, 1359–1367. [Google Scholar] [CrossRef]
- Gramlich, L.; Kichian, K.; Pinilla, J.; Rodych, N.J.; Dhaliwal, R.; Heyland, D.K. Does Enteral Nutrition Compared to Parenteral Nutrition Result in Better Outcomes in Critically Ill Adult Patients? A Systematic Review of the Literature. Nutrients 2004, 20, 843–848. [Google Scholar] [CrossRef]
- Lewis, S.R.; Schofield-Robinson, O.J.; Alderson, P.; Smith, A.F. Enteral versus Parenteral Nutrition and Enteral versus a Combination of Enteral and Parenteral Nutrition for Adults in the Intensive Care Unit. Cochrane Database Syst. Rev. 2018, 6, CD012276. [Google Scholar] [CrossRef]
- Ramaswamy, T.; DeWane, M.P.; Dashti, H.S.; Lau, M.; Wischmeyer, P.E.; Nagrebetsky, A.; Sparling, J. Nine Myths about Enteral Feeding in Critically Ill Adults: An Expert Perspective. Adv. Nutr. 2025, 16, 100345. [Google Scholar] [CrossRef]
- Lambell, K.J.; Tatucu-Babet, O.A.; Chapple, L.-A.; Gantner, D.; Ridley, E.J. Nutrition Therapy in Critical Illness: A Review of the Literature for Clinicians. Crit. Care 2020, 24, 35. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Xie, Y.; Zhong, M.; Wang, F.; Huang, H.; Nie, L.; Liu, X.; Xiao, M.; Zhu, H. Comparison of the Initiation Time of Enteral Nutrition for Critically Ill Patients: At Admission vs. 24 to 48 Hours after Admission. Emerg. Med. Int. 2021, 2021, 3047732. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.L.; Biddle, M.; Thomas, T. Evaluation of Current Feeding Practices in the Critically Ill: A Retrospective Chart Review. Intensive Crit. Care Nurs. 2017, 38, 24–30. [Google Scholar] [CrossRef]
- Weijs, P.J.M.; Stapel, S.N.; de Groot, S.D.W.; Driessen, R.H.; de Jong, E.; Girbes, A.R.J.; Strack van Schijndel, R.J.M.; Beishuizen, A. Optimal Protein and Energy Nutrition Decreases Mortality in Mechanically Ventilated, Critically Ill Patients: A Prospective Observational Cohort Study. JPEN J. Parenter. Enter. Nutr. 2012, 36, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Fuentes Padilla, P.; Martínez, G.; Vernooij, R.W.; Urrútia, G.; Roqué, I.; Figuls, M.; Bonfill Cosp, X. Early Enteral Nutrition (within 48 Hours) versus Delayed Enteral Nutrition (after 48 Hours) with or without Supplemental Parenteral Nutrition in Critically Ill Adults. Cochrane Database Syst. Rev. 2019, 2019, CD012340. [Google Scholar] [CrossRef]
- Gunst, J.; Derese, I.; Aertgeerts, A.; Ververs, E.-J.; Wauters, A.; Van den Berghe, G.; Vanhorebeek, I. Insufficient Autophagy Contributes to Mitochondrial Dysfunction, Organ Failure, and Adverse Outcome in an Animal Model of Critical Illness. Crit. Care Med. 2013, 41, 182–194. [Google Scholar] [CrossRef]
- Lo, S.; Yuan, S.-S.F.; Hsu, C.; Cheng, Y.-J.; Chang, Y.-F.; Hsueh, H.-W.; Lee, P.-H.; Hsieh, Y.-C. Lc3 Over-Expression Improves Survival and Attenuates Lung Injury through Increasing Autophagosomal Clearance in Septic Mice. Ann. Surg. 2013, 257, 352–363. [Google Scholar] [CrossRef]
- Sharma, S.K.; Rani, R.; Thakur, K. Effect of Early Versus Delayed Parenteral Nutrition on the Health Outcomes of Critically Ill Adults: A Systematic Review. J. Crit. Care Med. 2021, 7, 160–169. [Google Scholar] [CrossRef]
- Hermans, G.; Casaer, M.P.; Clerckx, B.; Güiza, F.; Vanhullebusch, T.; Derde, S.; Meersseman, P.; Derese, I.; Mesotten, D.; Wouters, P.J.; et al. Effect of Tolerating Macronutrient Deficit on the Development of Intensive-Care Unit Acquired Weakness: A Subanalysis of the EPaNIC Trial. Lancet Respir. Med. 2013, 1, 621–629. [Google Scholar] [CrossRef]
- Preiser, J.-C.; van Zanten, A.R.H.; Berger, M.M.; Biolo, G.; Casaer, M.P.; Doig, G.S.; Griffiths, R.D.; Heyland, D.K.; Hiesmayr, M.; Iapichino, G.; et al. Metabolic and Nutritional Support of Critically Ill Patients: Consensus and Controversies. Crit. Care 2015, 19, 35. [Google Scholar] [CrossRef]
- Yuk, J.-M.; Yoshimori, T.; Jo, E.-K. Autophagy and Bacterial Infectious Diseases. Exp. Mol. Med. 2012, 44, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Deretic, V. Unveiling the Roles of Autophagy in Innate and Adaptive Immunity. Nat. Rev. Immunol. 2007, 7, 767–777. [Google Scholar] [CrossRef]
- Gutierrez, M.G.; Master, S.S.; Singh, S.B.; Taylor, G.A.; Colombo, M.I.; Deretic, V. Autophagy Is a Defense Mechanism Inhibiting BCG and Mycobacterium Tuberculosis Survival in Infected Macrophages. Cell 2004, 119, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Reintam Blaser, A.; Starkopf, J.; Alhazzani, W.; Berger, M.M.; Casaer, M.P.; Deane, A.M.; Fruhwald, S.; Hiesmayr, M.; Ichai, C.; Jakob, S.M.; et al. Early Enteral Nutrition in Critically Ill Patients: ESICM Clinical Practice Guidelines. Intensive Care Med. 2017, 43, 380–398. [Google Scholar] [CrossRef]
- Compher, C.; Bingham, A.L.; McCall, M.; Patel, J.; Rice, T.W.; Braunschweig, C.; McKeever, L. Guidelines for the Provision of Nutrition Support Therapy in the Adult Critically Ill Patient: The American Society for Parenteral and Enteral Nutrition. JPEN J. Parenter. Enter. Nutr. 2022, 46, 12–41. [Google Scholar] [CrossRef] [PubMed]
- Achamrah, N.; Delsoglio, M.; De Waele, E.; Berger, M.M.; Pichard, C. Indirect Calorimetry: The 6 Main Issues. Clin. Nutr. 2021, 40, 4–14. [Google Scholar] [CrossRef]
- Zusman, O.; Theilla, M.; Cohen, J.; Kagan, I.; Bendavid, I.; Singer, P. Resting Energy Expenditure, Calorie and Protein Consumption in Critically Ill Patients: A Retrospective Cohort Study. Crit. Care 2016, 20, 367. [Google Scholar] [CrossRef]
- Delsoglio, M.; Achamrah, N.; Berger, M.M.; Pichard, C. Indirect Calorimetry in Clinical Practice. J. Clin. Med. 2019, 8, 1387. [Google Scholar] [CrossRef]
- Reignier, J.; Plantefeve, G.; Mira, J.-P.; Argaud, L.; Asfar, P.; Aissaoui, N.; Badie, J.; Botoc, N.-V.; Brisard, L.; Bui, H.-N.; et al. Low versus Standard Calorie and Protein Feeding in Ventilated Adults with Shock: A Randomised, Controlled, Multicentre, Open-Label, Parallel-Group Trial (NUTRIREA-3). Lancet Respir. Med. 2023, 11, 602–612. [Google Scholar] [CrossRef]
- Van Dyck, L.; Vanhorebeek, I.; Wilmer, A.; Schrijvers, A.; Derese, I.; Mebis, L.; Wouters, P.J.; Van den Berghe, G.; Gunst, J.; Casaer, M.P. Towards a Fasting-Mimicking Diet for Critically Ill Patients: The Pilot Randomized Crossover ICU-FM-1 Study. Crit. Care 2020, 24, 249. [Google Scholar] [CrossRef] [PubMed]
- Goossens, C.; Weckx, R.; Derde, S.; Dufour, T.; Vander Perre, S.; Pauwels, L.; Thiessen, S.E.; Van Veldhoven, P.P.; Van den Berghe, G.; Langouche, L. Adipose Tissue Protects against Sepsis-Induced Muscle Weakness in Mice: From Lipolysis to Ketones. Crit. Care 2019, 23, 236. [Google Scholar] [CrossRef]
- Gunst, J. Recovery from Critical Illness-Induced Organ Failure: The Role of Autophagy. Crit. Care 2017, 21, 209. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.; Litwic, A.; Cooper, C.; Dennison, E. Determinants of Muscle and Bone Aging. J. Cell. Physiol. 2015, 230, 2618–2625. [Google Scholar] [CrossRef]
- Vanhorebeek, I.; Verbruggen, S.; Casaer, M.P.; Gunst, J.; Wouters, P.J.; Hanot, J.; Guerra, G.G.; Vlasselaers, D.; Joosten, K.; den Berghe, G.V. Effect of Early Supplemental Parenteral Nutrition in the Paediatric ICU: A Preplanned Observational Study of Post-Randomisation Treatments in the PEPaNIC Trial. Lancet Respir. Med. 2017, 5, 475–483. [Google Scholar] [CrossRef]
- Van Dyck, L.; Casaer, M.P. Intermittent or Continuous Feeding: Any Difference during the First Week? Curr. Opin. Crit. Care 2019, 25, 356–362. [Google Scholar] [CrossRef]
- Patel, J.J.; Rosenthal, M.D.; Heyland, D.K. Intermittent versus Continuous Feeding in Critically Ill Adults. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Bear, D.E.; Hart, N.; Puthucheary, Z. Continuous or Intermittent Feeding: Pros and Cons. Curr. Opin. Crit. Care 2018, 24, 256–261. [Google Scholar] [CrossRef]
- Qu, J.; Xu, X.; Xu, C.; Ding, X.; Zhang, K.; Hu, L. The Effect of Intermittent versus Continuous Enteral Feeding for Critically Ill Patients: A Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2023, 10, 1214774. [Google Scholar] [CrossRef]
- Beattie, C.E.; Thomas, M.; Borislavova, B.; Smith, H.A.; Ambler, M.; White, P.; Hayes, K.; Milne, D.; Ramesh, A.V.; Gonzalez, J.T.; et al. Does Intermittent Nutrition Enterally Normalise Hormonal and Metabolic Responses to Feeding in Critically Ill Adults? A Protocol for the DINE-Normal Proof-of-Concept Randomised Parallel-Group Study. BMJ Open 2024, 14, e086540. [Google Scholar] [CrossRef]
- Atherton, P.J.; Etheridge, T.; Watt, P.W.; Wilkinson, D.; Selby, A.; Rankin, D.; Smith, K.; Rennie, M.J. Muscle Full Effect after Oral Protein: Time-Dependent Concordance and Discordance between Human Muscle Protein Synthesis and mTORC1 Signaling. Am. J. Clin. Nutr. 2010, 92, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.H.; Murray, K.; Hoad, C.L.; Costigan, C.; Marciani, L.; Macdonald, I.A.; Bowling, T.E.; Lobo, D.N. Effects of Bolus and Continuous Nasogastric Feeding on Gastric Emptying, Small Bowel Water Content, Superior Mesenteric Artery Blood Flow, and Plasma Hormone Concentrations in Healthy Adults: A Randomized Crossover Study. Ann. Surg. 2016, 263, 450–457. [Google Scholar] [CrossRef]
- Tatsumi, H. Enteral Tolerance in Critically Ill Patients. J. Intensive Care 2019, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Sun, R.; Wu, A.; Ni, Y.; Liu, J.; Guo, F.; Ying, L.; Ge, G.; Ding, A.; Shi, Y.; et al. Prognostic Value of Prolonged Feeding Intolerance in Predicting All-Cause Mortality in Critically Ill Patients: A Multicenter, Prospective, Observational Study. JPEN J. Parenter. Enter. Nutr. 2020, 44, 855–865. [Google Scholar] [CrossRef]
- Oshima, T.; Berger, M.M.; De Waele, E.; Guttormsen, A.B.; Heidegger, C.-P.; Hiesmayr, M.; Singer, P.; Wernerman, J.; Pichard, C. Indirect Calorimetry in Nutritional Therapy. A Position Paper by the ICALIC Study Group. Clin. Nutr. 2017, 36, 651–662. [Google Scholar] [CrossRef] [PubMed]
- van der Merwe, N.J.; Masao, F.T.; Bamford, M.K. Isotopic Evidence for Contrasting Diets of Early Hominins Homo Habilis and Australopithecus Boisei of Tanzania. S. Afr. J. Sci. 2008, 104, 153–155. [Google Scholar]
- Dominy, N.J.; Vogel, E.R.; Yeakel, J.D.; Constantino, P.; Lucas, P.W. Mechanical Properties of Plant Underground Storage Organs and Implications for Dietary Models of Early Hominins. Evol. Biol. 2008, 35, 159–175. [Google Scholar] [CrossRef]
- Colagiuri, S.; Brand Miller, J. The ‘Carnivore Connection’—Evolutionary Aspects of Insulin Resistance. Eur. J. Clin. Nutr. 2002, 56 (Suppl. 1), S30–S35. [Google Scholar] [CrossRef]
- Luca, F.; Perry, G.H.; Di Rienzo, A. Evolutionary Adaptations to Dietary Changes. Annu. Rev. Nutr. 2010, 30, 291–314. [Google Scholar] [CrossRef]
- Coop, G.; Witonsky, D.; Di Rienzo, A.; Pritchard, J.K. Using Environmental Correlations to Identify Loci Underlying Local Adaptation. Genetics 2010, 185, 1411–1423. [Google Scholar] [CrossRef]
- McDonald, J.H.; Kreitman, M. Adaptive Protein Evolution at the Adh Locus in Drosophila. Nature 1991, 351, 652–654. [Google Scholar] [CrossRef]
- Bustamante, C.D.; Fledel-Alon, A.; Williamson, S.; Nielsen, R.; Hubisz, M.T.; Glanowski, S.; Tanenbaum, D.M.; White, T.J.; Sninsky, J.J.; Hernandez, R.D.; et al. Natural Selection on Protein-Coding Genes in the Human Genome. Nature 2005, 437, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Sebastio, G.; Villa, M.; Sartorio, R.; Guzzetta, V.; Poggi, V.; Auricchio, S.; Boll, W.; Mantei, N.; Semenza, G. Control of Lactase in Human Adult-Type Hypolactasia and in Weaning Rabbits and Rats. Am. J. Hum. Genet. 1989, 45, 489–497. [Google Scholar] [PubMed]
- Bersaglieri, T.; Sabeti, P.C.; Patterson, N.; Vanderploeg, T.; Schaffner, S.F.; Drake, J.A.; Rhodes, M.; Reich, D.E.; Hirschhorn, J.N. Genetic Signatures of Strong Recent Positive Selection at the Lactase Gene. Am. J. Hum. Genet. 2004, 74, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Perry, G.H.; Dominy, N.J.; Claw, K.G.; Lee, A.S.; Fiegler, H.; Redon, R.; Werner, J.; Villanea, F.A.; Mountain, J.L.; Misra, R.; et al. Diet and the Evolution of Human Amylase Gene Copy Number Variation. Nat. Genet. 2007, 39, 1256–1260. [Google Scholar] [CrossRef]
- Hancock, A.M.; Witonsky, D.B.; Ehler, E.; Alkorta-Aranburu, G.; Beall, C.; Gebremedhin, A.; Sukernik, R.; Utermann, G.; Pritchard, J.; Coop, G.; et al. Human Adaptations to Diet, Subsistence, and Ecoregion Are Due to Subtle Shifts in Allele Frequency. Proc. Natl. Acad. Sci. USA 2010, 107, 8924–8930. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Hausman-Cohen, S.; Pizano, J.; Schmidt, M.A.; Minich, D.M.; Joffe, Y.; Brandhorst, S.; Evans, S.J.; Brady, D.M. Personalized Nutrition: Translating the Science of Nutrigenomics into Practice: Proceedings From the 2018 American College of Nutrition Meeting. J. Am. Coll. Nutr. 2019, 38, 287–301. [Google Scholar] [CrossRef]
- Azamfirei, L. The Human Microbiome in Intensive Care—A Journey Forward? J. Crit. Care Med. 2023, 9, 205–207. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-Microbiota Interactions and Personalized Nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Zusman, O.; Kagan, I.; Bendavid, I.; Theilla, M.; Cohen, J.; Singer, P. Predictive Equations versus Measured Energy Expenditure by Indirect Calorimetry: A Retrospective Validation. Clin. Nutr. 2019, 38, 1206–1210. [Google Scholar] [CrossRef]
- Ndahimana, D.; Kim, E.-K. Energy Requirements in Critically Ill Patients. Clin. Nutr. Res. 2018, 7, 81–90. [Google Scholar] [CrossRef]
- Thawkar, V.N.; Taksande, K. Navigating Nutritional Strategies: Permissive Underfeeding in Critically Ill Patients. Cureus 2024, 16, e58083. [Google Scholar] [CrossRef]
- Sébédio, J.-L. Metabolomics, Nutrition, and Potential Biomarkers of Food Quality, Intake, and Health Status. Adv. Food Nutr. Res. 2017, 82, 83–116. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O.; Kopka, J.; Dörmann, P.; Altmann, T.; Trethewey, R.N.; Willmitzer, L. Metabolite Profiling for Plant Functional Genomics. Nat. Biotechnol. 2000, 18, 1157–1161. [Google Scholar] [CrossRef]
- Akyol, S.; Ashrafi, N.; Yilmaz, A.; Turkoglu, O.; Graham, S.F. Metabolomics: An Emerging “Omics” Platform for Systems Biology and Its Implications for Huntington Disease Research. Metabolites 2023, 13, 1203. [Google Scholar] [CrossRef] [PubMed]
- Putri, S.P.; Ikram, M.M.M.; Sato, A.; Dahlan, H.A.; Rahmawati, D.; Ohto, Y.; Fukusaki, E. Application of Gas Chromatography-Mass Spectrometry-Based Metabolomics in Food Science and Technology. J. Biosci. Bioeng. 2022, 133, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, D.; Johnson, C.H.; Nguyen, T.; Ivanisevic, J.; Benton, H.P.; Lloyd, J.; Arkin, A.P.; Deutschbauer, A.M.; Patti, G.J.; Siuzdak, G. Metabolomic Data Streaming for Biology-Dependent Data Acquisition. Nat. Biotechnol. 2014, 32, 524–527. [Google Scholar] [CrossRef]
- Wishart, D.S.; Jewison, T.; Guo, A.C.; Wilson, M.; Knox, C.; Liu, Y.; Djoumbou, Y.; Mandal, R.; Aziat, F.; Dong, E.; et al. HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res. 2013, 41, D801–D807. [Google Scholar] [CrossRef]
- Christopher, K.B. Nutritional Metabolomics in Critical Illness. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 121–125. [Google Scholar] [CrossRef]
- Langley, R.J.; Tsalik, E.L.; van Velkinburgh, J.C.; Glickman, S.W.; Rice, B.J.; Wang, C.; Chen, B.; Carin, L.; Suarez, A.; Mohney, R.P.; et al. An Integrated Clinico-Metabolomic Model Improves Prediction of Death in Sepsis. Sci. Transl. Med. 2013, 5, 195ra95. [Google Scholar] [CrossRef]
- Kiehntopf, M.; Nin, N.; Bauer, M. Metabolism, Metabolome, and Metabolomics in Intensive Care: Is It Time to Move beyond Monitoring of Glucose and Lactate? Am. J. Respir. Crit. Care Med. 2013, 187, 906–907. [Google Scholar] [CrossRef]
- Chung, L.P.; Waterer, G.W. Genetic Predisposition to Respiratory Infection and Sepsis. Crit. Rev. Clin. Lab. Sci. 2011, 48, 250–268. [Google Scholar] [CrossRef] [PubMed]
- Romero-Tapiador, S.; Lacruz-Pleguezuelos, B.; Tolosana, R.; Freixer, G.; Daza, R.; Fernández-Díaz, C.M.; Aguilar-Aguilar, E.; Fernández-Cabezas, J.; Cruz-Gil, S.; Molina, S.; et al. AI4FoodDB: A Database for Personalized e-Health Nutrition and Lifestyle through Wearable Devices and Artificial Intelligence. Database 2023, 2023, baad049. [Google Scholar] [CrossRef] [PubMed]
- Tsolakidis, D.; Gymnopoulos, L.P.; Dimitropoulos, K. Artificial Intelligence and Machine Learning Technologies for Personalized Nutrition: A Review. Informatics 2024, 11, 62. [Google Scholar] [CrossRef]
- Suresh, V.; Singh, K.K.; Vaish, E.; Gurjar, M.; Ambuli Nambi, A.; Khulbe, Y.; Muzaffar, S. Artificial Intelligence in the Intensive Care Unit: Current Evidence on an Inevitable Future Tool. Cureus 2024, 16, e59797. [Google Scholar] [CrossRef]
- Mougiakakou, S. Evaluation of a Novel Artificial Intelligence System to Monitor and Assess Energy and Macronutrient Intake in Hospitalised Older Patients. Nutrients 2021, 13, 4539. [Google Scholar] [CrossRef]
- Lin, Y.-R.; Chen, P.-C.; Li, W.-T.; Huang, M.-H.; Huang, S.-F.; Wang, C.-J.; Chien, Y.-W.; Kao, A.-W.; Shan, Y.-S. The Relationship between Caloric Intake and Clinical Outcomes in Critically Ill Patients: A Retrospective Study. Clin. Nutr. ESPEN 2025, 65, 9–15. [Google Scholar] [CrossRef]
- Shunxia, S.; Jin, Y.; Xiaoling, T.; Juan, H.; Jiangqiong, P. Effect of a Multidisciplinary Nutrition Management Model in Patients with Critical Illness: A Randomized Trial. Nurs. Crit. Care 2024, 29, 417–426. [Google Scholar] [CrossRef]
- Taberna, M.; Gil Moncayo, F.; Jané-Salas, E.; Antonio, M.; Arribas, L.; Vilajosana, E.; Peralvez Torres, E.; Mesía, R. The Multidisciplinary Team (MDT) Approach and Quality of Care. Front. Oncol. 2020, 10, 85. [Google Scholar] [CrossRef]
- Zhao, J.; Kan, Y.; Wu, X.; Yang, S.; Wang, G.; Bao, Y.; Li, J. Nutrition Management for Patients with Head and Neck Cancer during Peri-Radiotherapy: A Systematic Review and Quality Appraisal of Clinical Practice Guidelines Using the AGREE II Instrument. Front. Oncol. 2022, 12, 974059. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pang, D.; Lu, Y. The Role of Nurse in the Multidisciplinary Management of Cancer Cachexia. Asia-Pac. J. Oncol. Nurs. 2021, 8, 487–497. [Google Scholar] [CrossRef]
- Moore, J.H.; Asselbergs, F.W.; Williams, S.M. Bioinformatics Challenges for Genome-Wide Association Studies. Bioinformatics 2010, 26, 445–455. [Google Scholar] [CrossRef]
- Yngvadottir, B.; MacArthur, D.G.; Jin, H.; Tyler-Smith, C. The Promise and Reality of Personal Genomics. Genome Biol. 2009, 10, 237. [Google Scholar] [CrossRef]
- Verma, M.; Hontecillas, R.; Tubau-Juni, N.; Abedi, V.; Bassaganya-Riera, J. Challenges in Personalized Nutrition and Health. Front. Nutr. 2018, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- Fotiadis, D.I.; Glaros, C.; Likas, A. Wearable Medical Devices. In Wiley Encyclopedia of Biomedical Engineering; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; ISBN 978-0-471-74036-0. [Google Scholar]
- Jiang, N.; Mück, J.E.; Yetisen, A.K. The Regulation of Wearable Medical Devices. Trends Biotechnol. 2020, 38, 129–133. [Google Scholar] [CrossRef]
- Kittrell, H.D.; Shaikh, A.; Adintori, P.A.; McCarthy, P.; Kohli-Seth, R.; Nadkarni, G.N.; Sakhuja, A. Role of Artificial Intelligence in Critical Care Nutrition Support and Research. Nutr. Clin. Pract. 2024, 39, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Choi, E.-J.; Choi, E.-J. Evolving Paradigms in Sepsis Management: A Narrative Review. Cells 2024, 13, 1172. [Google Scholar] [CrossRef]
- van Zanten, A.R.H. Editorial: Personalized Nutrition Therapy in Critical Illness and Convalescence: Moving beyond One-Size-Fits-All to Phenotyping and Endotyping. Curr. Opin. Crit. Care 2023, 29, 281–285. [Google Scholar] [CrossRef]
- Bousie, E.; van Blokland, D.; van Zanten, A.R.H. Effects of Implementation of a Computerized Nutritional Protocol in Mechanically Ventilated Critically Ill Patients: A Single-Centre before and after Study. Clin. Nutr. ESPEN 2016, 11, e47–e54. [Google Scholar] [CrossRef]
- Singar, S.; Nagpal, R.; Arjmandi, B.H.; Akhavan, N.S. Personalized Nutrition: Tailoring Dietary Recommendations Through Genetic Insights. Nutrients 2024, 16, 2673. [Google Scholar] [CrossRef]
- Udin, I.; Habisreutinger, M.; Tappy, L.; Schneider, A.G.; Berger, M.M. Magnitude of Gluconeogenesis and Endogenous Glucose Production: Are They Predictable in Clinical Settings? Clin. Nutr. 2021, 40, 3807–3814. [Google Scholar] [CrossRef]
- Chapple, L.-A.S.; Kouw, I.W.K.; Summers, M.J.; Weinel, L.M.; Gluck, S.; Raith, E.; Slobodian, P.; Soenen, S.; Deane, A.M.; van Loon, L.J.C.; et al. Muscle Protein Synthesis after Protein Administration in Critical Illness. Am. J. Respir. Crit. Care Med. 2022, 206, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Middleton, F.A.; Tawil, R.; Chen, X.J. Cytosolic Adaptation to Mitochondria-Induced Proteostatic Stress Causes Progressive Muscle Wasting. iScience 2022, 25, 103715. [Google Scholar] [CrossRef]
- Haines, R.W.; Fowler, A.J.; Wan, Y.I.; Flower, L.; Heyland, D.K.; Day, A.; Pearse, R.M.; Prowle, J.R.; Puthucheary, Z. Catabolism in Critical Illness: A Reanalysis of the REducing Deaths Due to OXidative Stress (REDOXS) Trial. Crit. Care Med. 2022, 50, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Yinusa, G.; Scammell, J.; Murphy, J.; Ford, G.; Baron, S. Multidisciplinary Provision of Food and Nutritional Care to Hospitalized Adult In-Patients: A Scoping Review. J. Multidiscip. Healthc. 2021, 14, 459–491. [Google Scholar] [CrossRef] [PubMed]
- Legea, nr. 256/2015-Updated. Colegiul Dieteticienilor din Romania. Available online: https://www.colegiuldieteticienilor.ro/legea-nr-256-2015-updated/ (accessed on 15 April 2025).
- Azamfirei, R. The Implementation Gap in Critical Care: From Nutrition to Ventilation. J. Crit. Care Med. 2025, 11, 3–4. [Google Scholar] [CrossRef]
- Cahill, N.E.; Dhaliwal, R.; Day, A.G.; Jiang, X.; Heyland, D.K. Nutrition Therapy in the Critical Care Setting: What Is “Best Achievable” Practice? An International Multicenter Observational Study. Crit. Care Med. 2010, 38, 395–401. [Google Scholar] [CrossRef]
- Bell, J.J.; Young, A.; Hill, J.; Banks, M.; Comans, T.; Barnes, R.; Keller, H.H. Rationale and Developmental Methodology for the SIMPLE Approach: A Systematised, Interdisciplinary Malnutrition Pathway for Implementation and Evaluation in Hospitals. Nutr. Diet. J. Dietit. Assoc. Aust. 2018, 75, 226–234. [Google Scholar] [CrossRef]
- Reber, E.; Gomes, F.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Nutritional Risk Screening and Assessment. J. Clin. Med. 2019, 8, 1065. [Google Scholar] [CrossRef]
- Holmes, C.J.; Racette, S.B. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: An Overview of Current Methodology. Nutrients 2021, 13, 2493. [Google Scholar] [CrossRef] [PubMed]
- Kassem, H.; Beevi, A.A.; Basheer, S.; Lutfi, G.; Cheikh Ismail, L.; Papandreou, D. Investigation and Assessment of AI’s Role in Nutrition—An Updated Narrative Review of the Evidence. Nutrients 2025, 17, 190. [Google Scholar] [CrossRef] [PubMed]
- Turner, H.C.; Hao, N.V.; Yacoub, S.; Hoang, V.M.T.; Clifton, D.A.; Thwaites, G.E.; Dondorp, A.M.; Thwaites, C.L.; Chau, N.V.V. Achieving Affordable Critical Care in Low-Income and Middle-Income Countries. BMJ Glob. Health 2019, 4, e001675. [Google Scholar] [CrossRef]
- Cubro, H.; Somun-Kapetanovic, R.; Thiery, G.; Talmor, D.; Gajic, O. Cost Effectiveness of Intensive Care in a Low Resource Setting: A Prospective Cohort of Medical Critically Ill Patients. World J. Crit. Care Med. 2016, 5, 150–164. [Google Scholar] [CrossRef]
- Dat, V.Q.; Long, N.T.; Giang, K.B.; Diep, P.B.; Giang, T.H.; Diaz, J.V. Healthcare Infrastructure Capacity to Respond to Severe Acute Respiratory Infection (SARI) and Sepsis in Vietnam: A Low-Middle Income Country. J. Crit. Care 2017, 42, 109–115. [Google Scholar] [CrossRef] [PubMed]
| Criteria | EN | PN |
|---|---|---|
| Management and expenses | It is more challenging to manage, but it is less expensive | It is easy to administer, but it is more expensive |
| Tolerance | Reduced digestive tolerance during the acute phase | Generally, well tolerated |
| Common complications | Nausea and vomiting, temporary malnutrition, a reduction in fistulas, and instances of peritonitis | Infections, elevated blood sugar levels, and intestinal atrophy |
| Nutritional benefits | Preserves the integrity of the intestines | There are no benefits for gut health |
| Effect on infections and mortality | Reduces the risk of infections and death | There is an increased risk of infections, but no clear differences in mortality rates |
| The best time to begin | In the first 48 h | Day 7 after surgery |
| Indications, long-term use | The digestive tract is functioning properly; post-discharge monitoring might be necessary | Intestinal obstruction, can be sometimes managed at home, depending on certain conditions |
| Efficiency of caloric intake, recommendations | Sometimes it is insufficient, choose as the first option if possible | Offers a more efficient way to meet energy needs and is an alternative when EN is unavailable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoian, M.; Andone, A.; Bândilă, S.R.; Onișor, D.; Babă, D.-F.; Niculescu, R.; Stoian, A.; Azamfirei, L. Personalized Nutrition Strategies for Patients in the Intensive Care Unit: A Narrative Review on the Future of Critical Care Nutrition. Nutrients 2025, 17, 1659. https://doi.org/10.3390/nu17101659
Stoian M, Andone A, Bândilă SR, Onișor D, Babă D-F, Niculescu R, Stoian A, Azamfirei L. Personalized Nutrition Strategies for Patients in the Intensive Care Unit: A Narrative Review on the Future of Critical Care Nutrition. Nutrients. 2025; 17(10):1659. https://doi.org/10.3390/nu17101659
Chicago/Turabian StyleStoian, Mircea, Adina Andone, Sergiu Rareș Bândilă, Danusia Onișor, Dragoș-Florin Babă, Raluca Niculescu, Adina Stoian, and Leonard Azamfirei. 2025. "Personalized Nutrition Strategies for Patients in the Intensive Care Unit: A Narrative Review on the Future of Critical Care Nutrition" Nutrients 17, no. 10: 1659. https://doi.org/10.3390/nu17101659
APA StyleStoian, M., Andone, A., Bândilă, S. R., Onișor, D., Babă, D.-F., Niculescu, R., Stoian, A., & Azamfirei, L. (2025). Personalized Nutrition Strategies for Patients in the Intensive Care Unit: A Narrative Review on the Future of Critical Care Nutrition. Nutrients, 17(10), 1659. https://doi.org/10.3390/nu17101659








