Protective Role of Polyphenols from Aronia Berry (Aronia melanocarpa) Against LPS-Induced Inflammation in Colon Cells and Macrophages
Abstract
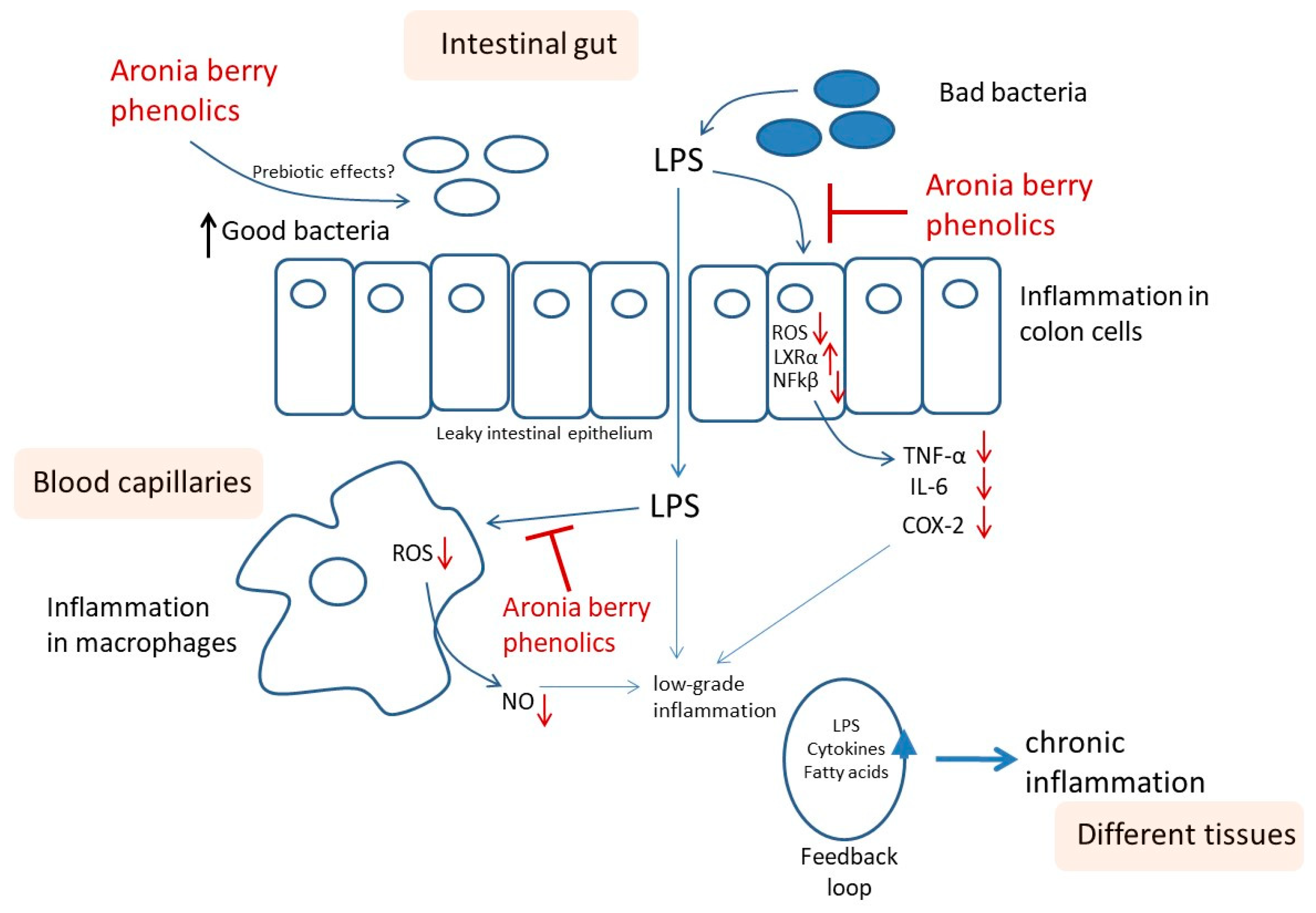
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Aronia Berry Samples
2.3. Sample Preparation for Polyphenol Analysis and LC-MS Profiling
2.4. Cell Culture for Inflammation Studies
2.5. Cell Viability Test
2.6. Detection of Extracellular Nitric Oxide and Intracellular Reactive Oxygen Species Production
2.7. Total RNA and Gene Expression Analysis (Real-Time qRT-PCR)
2.8. Statistical Analysis
3. Results and Discussion
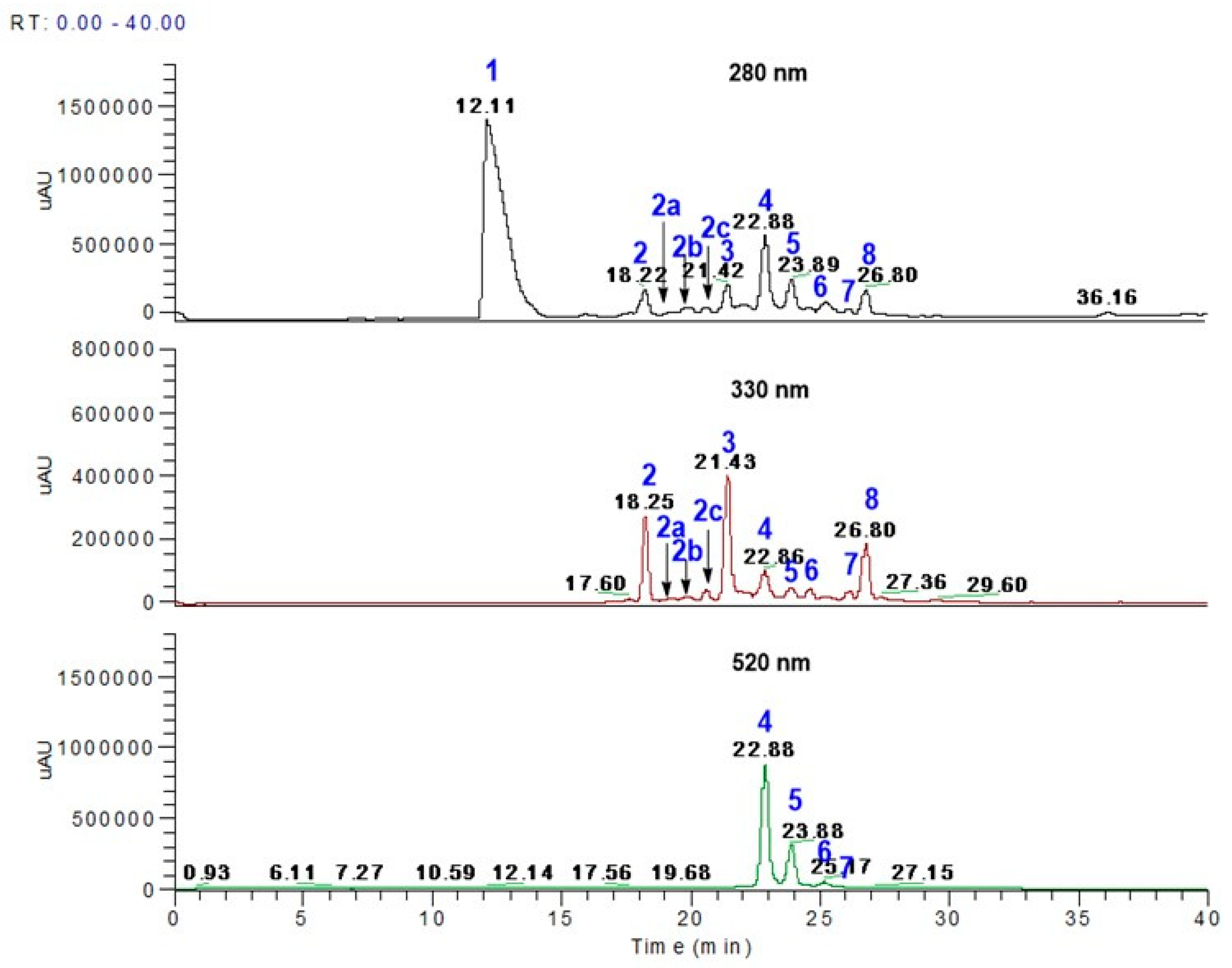
3.1. Polyphenol Profiles from Aronia Berries Using LC-MS
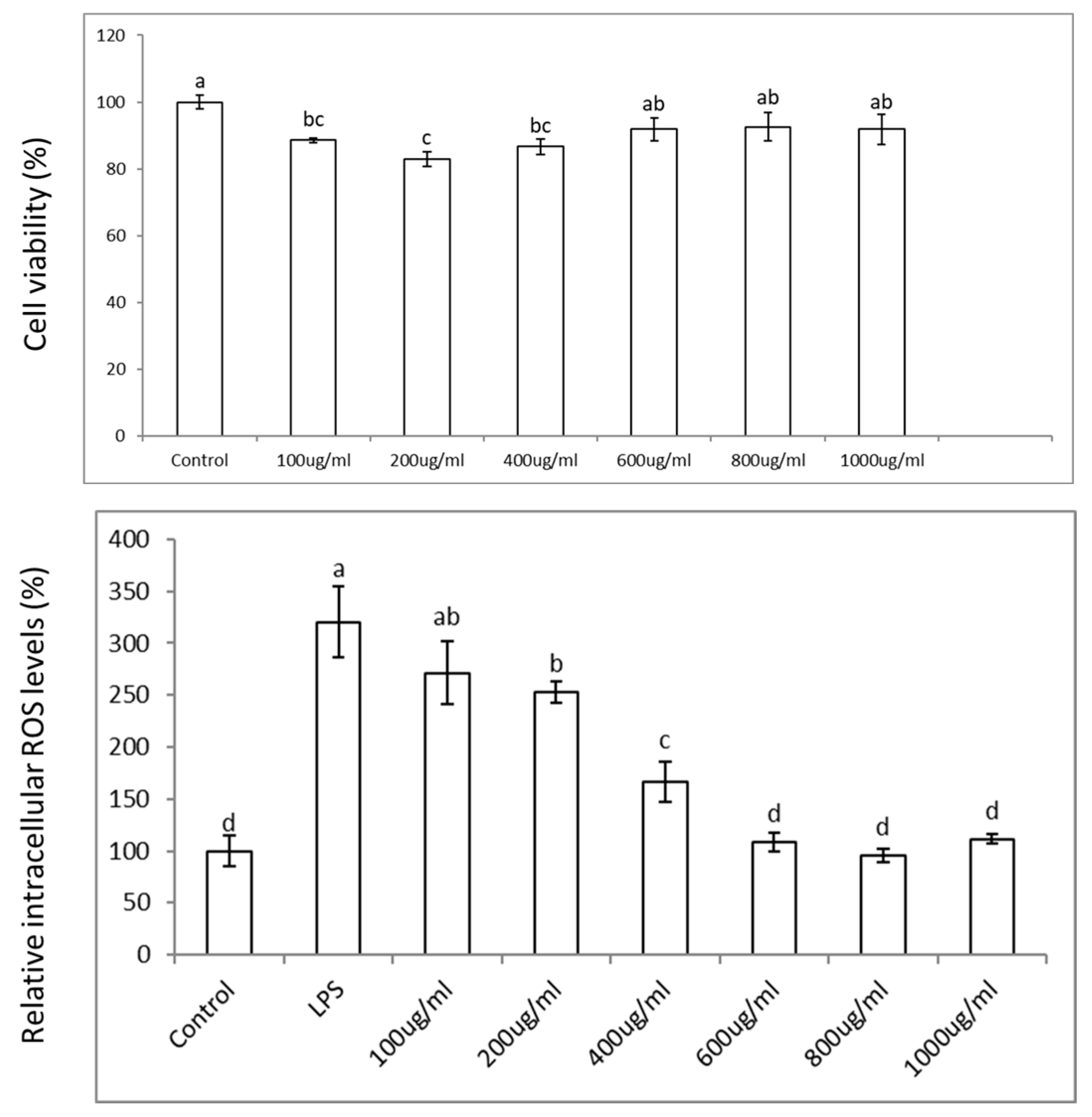
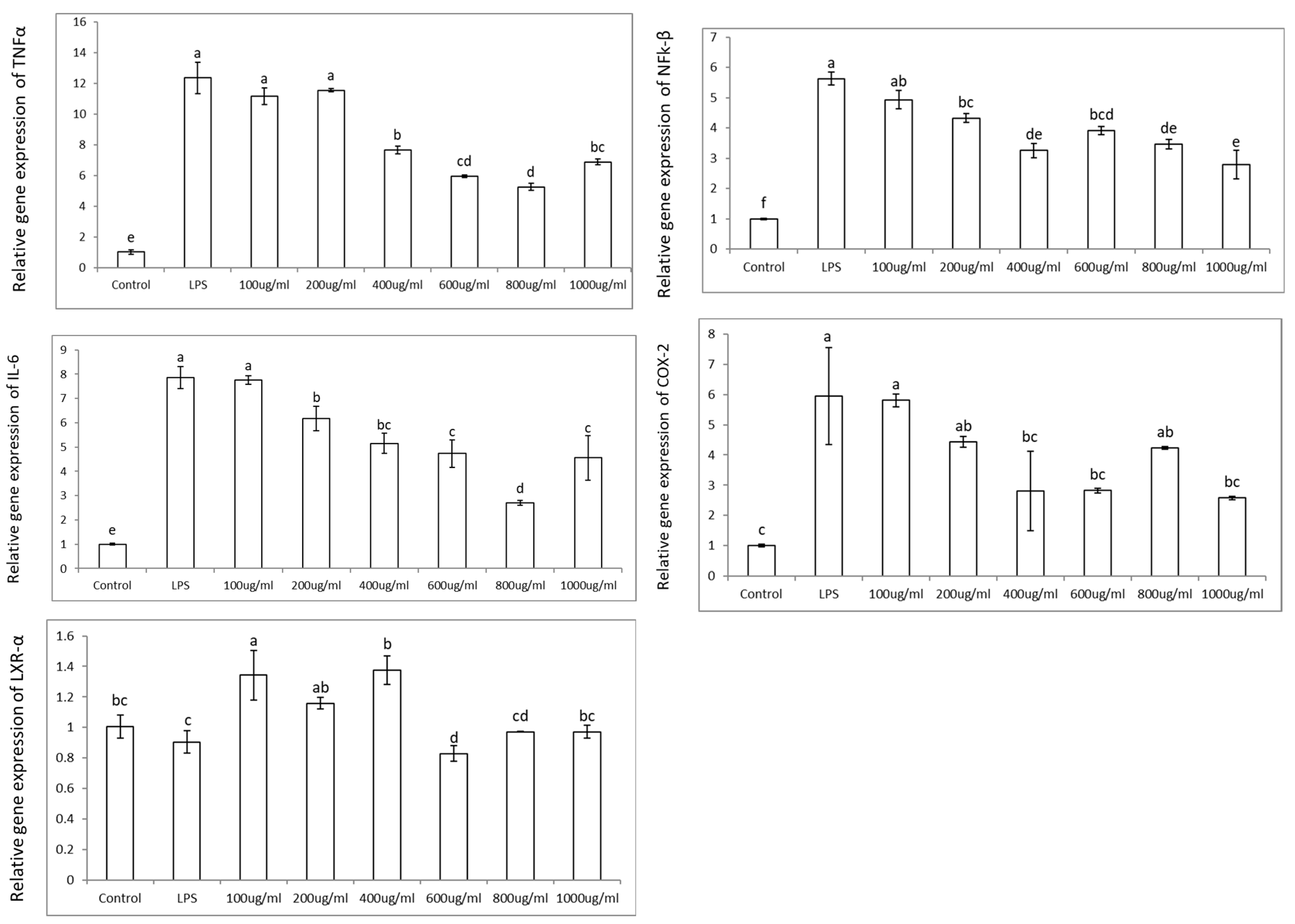
3.2. Colon Cell Viability, Reactive Oxygen Species (ROS), and Effects in Pro-Inflammatory Genes
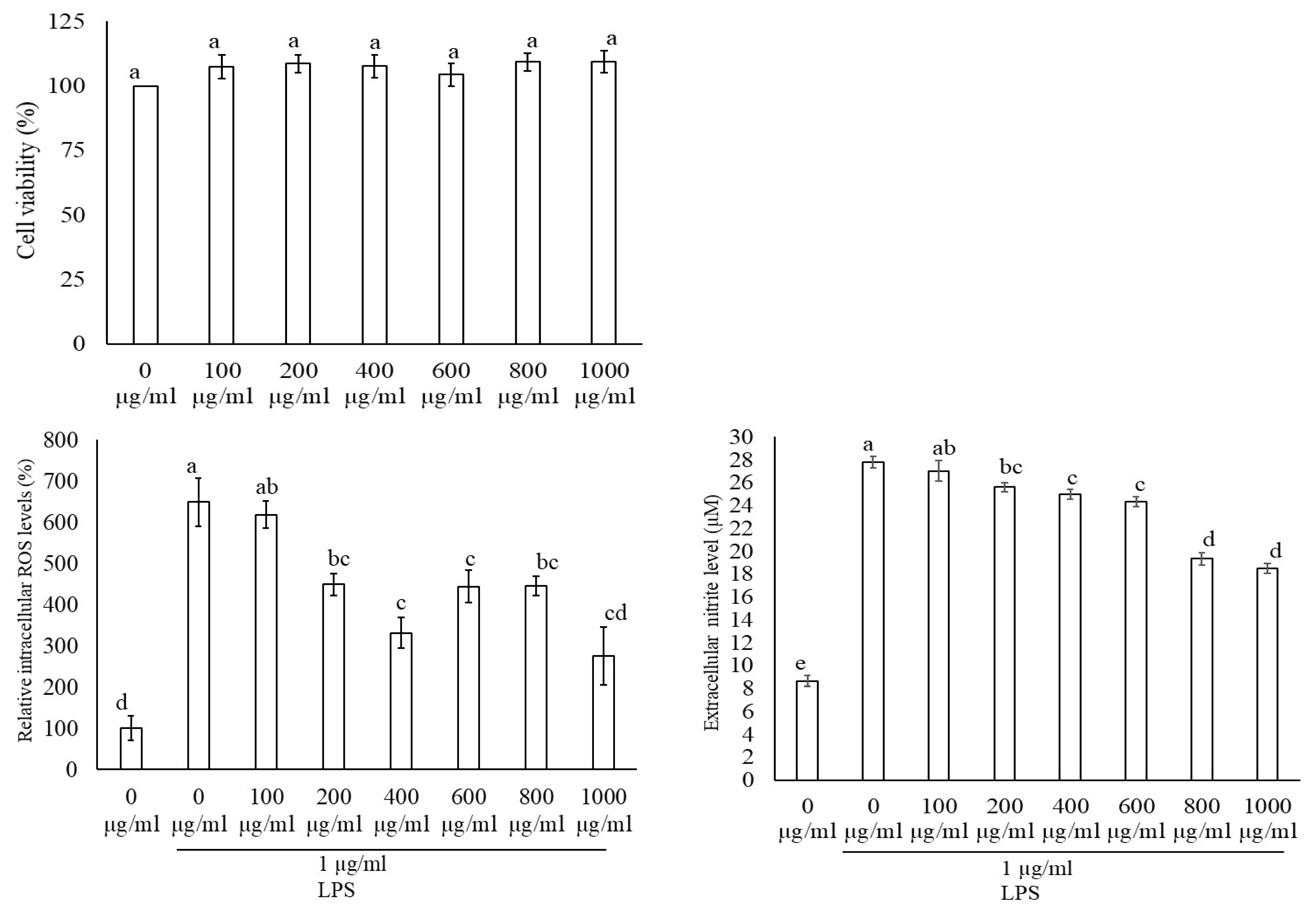
3.3. Macrophage Cell Viability, Reactive Oxygen Species (ROS), and Nitric Oxide (NO) Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| NO | Nitric oxide |
| LPS | Lipopolysaccharide |
| NFkβ | Nuclear factor kappa β |
| TNFα | Tumor necrosis factor alpha |
| IL-6 | Interleukin 6 |
| COX2 | Cyclooxygenase 2 |
| LXRα | Liver x receptor alpha |
| RNA | Ribonucleic acid |
| PCR | Polymerase chain reaction |
| DMSO | Dimethyl sulfoxide |
| NOX | NADPH oxidase (nicotinamide adenine dinucleotide phosphate oxidase) |
| iNOS | Inducible nitric oxide synthase |
| TLR4 | Toll-like receptor 4 |
References
- McNally, A. Demand for Superfruit Aronia Rockets. Available online: https://www.nutraingredients.com/Article/2008/01/08/demand-for-superfruit-aronia-rockets (accessed on 11 June 2024).
- Sueiro, L.; Yousef, G.G.; Seigler, D.; De Mejia, E.G.; Grace, M.H.; Lila, M.A. Chemopreventive Potential of Flavonoid Extracts from Plantation-Bred and Wild Aronia melanocarpa (Black Chokeberry) Fruits. J. Food Sci. 2006, 71, C480–C488. [Google Scholar] [CrossRef]
- Ochmian, I.D.; Grajkowski, J.; Smolik, M. Comparison of Some Morphological Features, Quality and Chemical Content of Four Cultivars of Chokeberry Fruits (Aronia melanocarpa). Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 253–260. [Google Scholar] [CrossRef]
- Benvenuti, S.; Pellati, F.; Melegari, M.; Bertelli, D. Polyphenols, Anthocyanins, Ascorbic Acid, and Radical Scavenging Activity of Rubus, Ribes, and Aronia. J. Food Sci. 2004, 69, FCT164–FCT169. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Törrönen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef]
- Ovaskainen, M.L.; Törrönen, R.; Koponen, J.M.; Sinkko, H.; Hellström, J.; Reinivuo, H.; Mattila, P. Dietary intake and major food sources of polyphenols in Finnish adults. J. Nutr. 2008, 138, 562–566. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Bräunlich, M.; Slimestad, R.; Wangensteen, H.; Brede, C.; Malterud, K.E.; Barsett, H. Extracts, anthocyanins and procyanidins from Aronia melanocarpa as radical scavengers and enzyme inhibitors. Nutrients 2013, 5, 663–678. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Jaremicz, Z.; Luczkiewicz, M. Aronia plants: A review of traditional use, biological activities, and perspectives for modern medicine. J. Med. Food 2010, 13, 255–269. [Google Scholar] [CrossRef]
- Broncel, M.; Kozirog, M.; Duchnowicz, P.; Koter-Michalak, M.; Sikora, J.; Chojnowska-Jezierska, J. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med. Sci. Monit. 2010, 16, Cr28-34. [Google Scholar]
- Simeonov, S.B.; Botushanov, N.P.; Karahanian, E.B.; Pavlova, M.B.; Husianitis, H.K.; Troev, D.M. Effects of Aronia melanocarpa juice as part of the dietary regimen in patients with diabetes mellitus. Folia Med. 2002, 44, 20–23. [Google Scholar]
- Malik, M.; Zhao, C.; Schoene, N.; Guisti, M.M.; Moyer, M.P.; Magnuson, B.A. Anthocyanin-rich extract from Aronia meloncarpa E induces a cell cycle block in colon cancer but not normal colonic cells. Nutr. Cancer 2003, 46, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Giusti, M.M.; Malik, M.; Moyer, M.P.; Magnuson, B.A. Effects of commercial anthocyanin-rich extracts on colonic cancer and nontumorigenic colonic cell growth. J. Agric. Food Chem. 2004, 52, 6122–6128. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.J.; Larrosa, M.; Garcia-Cantalejo, J.M.; Espín, J.C.; Tomás-Barberan, F.A.; García-Conesa, M.T. Up-regulation of tumor suppressor carcinoembryonic antigen-related cell adhesion molecule 1 in human colon cancer Caco-2 cells following repetitive exposure to dietary levels of a polyphenol-rich chokeberry juice. J. Nutr. Biochem. 2007, 18, 259–271. [Google Scholar] [CrossRef]
- Kowalczyk, E.; Kopff, A.; Fijałkowski, P.; Kopff, M.; Niedworok, J.; Błaszczyk, J.; Kedziora, J.; Tyślerowicz, P. Effect of anthocyanins on selected biochemical parameters in rats exposed to cadmium. Acta Biochim. Pol. 2003, 50, 543–548. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Kuzmanov, K.; Tancheva, S.; Belcheva, A. Hypoglycemic and hypolipidemic effects of Aronia melanocarpa fruit juice in streptozotocin-induced diabetic rats. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 101–105. [Google Scholar] [CrossRef]
- Zapolska-Downar, D.K.A.; Naruszewicz, M. Flavonoid-rich chokeberry fruit extract inhibits endothelial progenitor cells senescence induced by oxidized ldl. J. Clin. Lipidol. 2006, 1, 347. [Google Scholar]
- Sreedharan, S.; Nair, V.; Cisneros-Zevallos, L. Protective Role of Phenolic Compounds from Whole Cardamom (Elettaria cardamomum (L.) Maton) against LPS-Induced Inflammation in Colon and Macrophage Cells. Nutrients 2023, 15, 2965. [Google Scholar] [CrossRef]
- Ryszawa, N.; Kawczyńska-Drózdz, A.; Pryjma, J.; Czesnikiewicz-Guzik, M.; Adamek-Guzik, T.; Naruszewicz, M.; Korbut, R.; Guzik, T.J. Effects of novel plant antioxidants on platelet superoxide production and aggregation in atherosclerosis. J. Physiol. Pharmacol. 2006, 57, 611–626. [Google Scholar]
- Bell, D.R.; Burt, T.D. Phenolic acids contained in anthocyanin enriched extracts from Elderberry, Bilberry and Chokeberry possess endothelium dependent and independent vasorelaxation properties in porcine coronary arteries. FASEB J. 2007, 21, A366. [Google Scholar] [CrossRef]
- Naruszewicz, M.; Laniewska, I.; Millo, B.; Dłuzniewski, M. Combination therapy of statin with flavonoids rich extract from chokeberry fruits enhanced reduction in cardiovascular risk markers in patients after myocardial infraction (MI). Atherosclerosis 2007, 194, e179–e184. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Przyjemska, M.; Olejnik, A.; Dobrowolska-Zachwieja, A.; Grajek, W. Effects of Aronia melanocarpa polyphenols on oxidative metabolism and apoptosis of neutrophils from obese and non-obese individuals. Acta Sci. Pol. Technol. Aliment. 2007, 6, 75–86. [Google Scholar]
- Cisneros-Zevallos, L. The power of plants: How fruit and vegetables work as source of nutraceuticals and supplements. Int. J. Food Sci. Nutr. 2021, 72, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Goto, T.; Hirai, S.; Yu, R.; Takahashi, N. Obesity and Nuclear Receptors: Effective Genomic Strategies in Functional Foods. In Nutrigenomics and Proteomics in Health and Disease; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2009; pp. 47–58. [Google Scholar]
- Boulebd, H.; Carmena-Bargueño, M.; Pérez-Sánchez, H. Exploring the Antioxidant Properties of Caffeoylquinic and Feruloylquinic Acids: A Computational Study on Hydroperoxyl Radical Scavenging and Xanthine Oxidase Inhibition. Antioxidants 2023, 12, 1669. [Google Scholar] [CrossRef]
- Nakatani, N.; Kayano, S.; Kikuzaki, H.; Sumino, K.; Katagiri, K.; Mitani, T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.). J. Agric. Food Chem. 2000, 48, 5512–5516. [Google Scholar] [CrossRef]
- Kayano, S.; Kikuzaki, H.; Fukutsuka, N.; Mitani, T.; Nakatani, N. Antioxidant activity of prune (Prunus domestica L.) constituents and a new synergist. J. Agric. Food Chem. 2002, 50, 3708–3712. [Google Scholar] [CrossRef]
- Kim, T.Y.; Lee, B.S.; Jo, B.G.; Heo, S.P.; Keem, M.J.; Kwon, T.H.; Kim, S.N.; Kim, K.H.; Yang, M.H. 3‴-O-Foliamenthoyl-Rutin, a New Flavonoid Glycoside from the Roots of Nymphoides peltata. Plants 2023, 12, 4083. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Lee, M.; Shim, S.Y. Inhibitory Effects of Eriodictyol-7-O-β-d-glucuronide and 5,7-Dihydroxy-4-chromene Isolated from Chrysanthemum zawadskii var. latilobum in FcεRI-Mediated Human Basophilic KU812F Cell Activation. Molecules 2020, 25, 994. [Google Scholar] [CrossRef]
- Liang, Z.; Liang, H.; Guo, Y.; Yang, D. Cyanidin 3-O-galactoside: A Natural Compound with Multiple Health Benefits. Int. J. Mol. Sci. 2021, 22, 2261. [Google Scholar] [CrossRef]
- Baek, H.; Sanjay; Park, M.; Lee, H.J. Cyanidin-3-O-glucoside protects the brain and improves cognitive function in APPswe/PS1ΔE9 transgenic mice model. J. Neuroinflamm. 2023, 20, 268. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Chae, C.W.; Choi, G.E.; Shin, H.C.; Lim, J.R.; Chang, H.S.; Park, J.; Cho, J.H.; Park, M.R.; Lee, H.J.; et al. Cyanidin 3-O-arabinoside suppresses DHT-induced dermal papilla cell senescence by modulating p38-dependent ER-mitochondria contacts. J. Biomed. Sci. 2022, 29, 17. [Google Scholar] [CrossRef] [PubMed]
- Kaloudi, T.; Tsimogiannis, D.; Oreopoulou, V. Aronia Melanocarpa: Identification and Exploitation of Its Phenolic Components. Molecules 2022, 27, 4375. [Google Scholar] [CrossRef] [PubMed]
- King, E.S.; Bolling, B.W. Composition, polyphenol bioavailability, and health benefits of aronia berry: A review. J. Food Bioact. 2020, 11, 13–30. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Black Chokeberry Aronia melanocarpa L.—A Qualitative Composition, Phenolic Profile and Antioxidant Potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef]
- Ren, Y.; Frank, T.; Meyer, G.; Lei, J.; Grebenc, J.R.; Slaughter, R.; Gao, Y.G.; Kinghorn, A.D. Potential Benefits of Black Chokeberry (Aronia melanocarpa) Fruits and Their Constituents in Improving Human Health. Molecules 2022, 27, 7823. [Google Scholar] [CrossRef]
- Slimestad, R.; Torskangerpoll, K.; Nateland, H.S.; Johannessen, T.; Giske, N.H. Flavonoids from black chokeberries, Aronia melanocarpa. J. Food Compos. Anal. 2005, 18, 61–68. [Google Scholar] [CrossRef]
- Brand, M.H.; Connolly, B.A.; Levine, L.H.; Richards, J.T.; Shine, S.M.; Spencer, L.E. Anthocyanins, total phenolics, ORAC and moisture content of wild and cultivated dark-fruited Aronia species. Sci. Hortic. 2017, 224, 332–334. [Google Scholar] [CrossRef]
- Delgadillo-Puga, C.; Torre-Villalvazo, I.; Noriega, L.G.; Rodríguez-López, L.A.; Alemán, G.; Torre-Anaya, E.A.; Cariño-Cervantes, Y.Y.; Palacios-Gonzalez, B.; Furuzawa-Carballeda, J.; Tovar, A.R.; et al. Pecans and Its Polyphenols Prevent Obesity, Hepatic Steatosis and Diabetes by Reducing Dysbiosis, Inflammation, and Increasing Energy Expenditure in Mice Fed a High-Fat Diet. Nutrients 2023, 15, 2591. [Google Scholar] [CrossRef]
- Nair, V.; Bang, W.Y.; Schreckinger, E.; Andarwulan, N.; Cisneros-Zevallos, L. Protective Role of Ternatin Anthocyanins and Quercetin Glycosides from Butterfly Pea (Clitoria ternatea Leguminosae) Blue Flower Petals against Lipopolysaccharide (LPS)-Induced Inflammation in Macrophage Cells. J. Agric. Food Chem. 2015, 63, 6355–6365. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Bang, W.Y.; Delgadillo-Puga, C. Ellagic Acid and Urolithins A and B Differentially Regulate Fat Accumulation and Inflammation in 3T3-L1 Adipocytes While Not Affecting Adipogenesis and Insulin Sensitivity. Int. J. Mol. Sci. 2020, 21, 2086. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Polyphenolics from peach (Prunus persica var. Rich Lady) inhibit tumor growth and metastasis of MDA-MB-435 breast cancer cells in vivo. J. Nutr. Biochem. 2014, 25, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo-Puga, C.; Noriega, L.G.; Morales-Romero, A.M.; Nieto-Camacho, A.; Granados-Portillo, O.; Rodríguez-López, L.A.; Alemán, G.; Furuzawa-Carballeda, J.; Tovar, A.R.; Cisneros-Zevallos, L. Goat’s milk intake prevents obesity, hepatic steatosis and insulin resistance in mice fed a high-fat diet by reducing inflammatory markers and increasing energy expenditure and mitochondrial content in skeletal muscle. Int. J. Mol. Sci. 2020, 21, 5530. [Google Scholar] [CrossRef] [PubMed]

| COX2 | Fw-ACATCGATGTCATGGAACTG Rv-GGACACCCCTTCACATTATT |
| IL-6 | Fw-TGACAACCACGGCCTTCCCT Rv-AGCCTCCGACTTGTGAAGTGGT |
| LXRα | Fw-AAGCCCTGCATGCCTACGT Rv-TGCAGACGCAGTGCAAACA |
| β-actin | Fw-CCCAGGCATTGCTGACAGG Rv-TGGAAGGTGGACAGTGAGGC |
| TNFα | Fw-ACTGGCAGAAGAGGCACTCC Rv-CGATCACCCCGAAGTTCA |
| NFKβ | Fw-GGTGGAGGCATGTTCGGTA Rv-TGACCCCTGCGTTGGATT |
| Peak No. | Retention Time (min) | M-H | * MS Fragments | Aronia Berry Phenolics (mg/100 g) | Identification |
|---|---|---|---|---|---|
| 1 | 12.11 | 353 | 191, 179 | 3114.8 ± 4 | 5-O-caffeoylquinic acid |
| 2 | 18.22–18.25 | 353 | 191, 179 | 177.3 ± 2.4 | 3-O-caffeoylquinic acid |
| 2a | 19.20–19.22 | 625 | 301, 463 | 139.9 + 0.4 | Quercetin dihexose |
| 2b | 19.85–19.88 | 595 | 301, 463 | 11.5 ± 0.3 | Quercetin-3-O-vicianoside |
| 2c | 20.35–20.36 | 609 | 301 | 25.7 ± 0.0 | Quercetin-3-O-rutinoside |
| 3 | 21.43 | 463 | 287, 421 | 289.4 ± 0.9 | Eriodictyol 7-O-β-glucuronide |
| 4 | 22.88 | 447 | 287, 211 | 2890.3 ± 16.1 | Cyanidin-3-O-galactoside |
| 5 | 23.88–23.89 | 447 | 287, 225 | 839.6 ± 2.3 | Cyanidin-3-O-glucoside |
| 6 | 25.17 | 417 | 287, 225 | 129.9 ± 0.2 | Cyanidin-3-O-arabinoside |
| 7 | 26.18 | 417 | 287 | 100.4 ± 0.4 | Cyanidin-3-O- xyloside |
| 8 | 26.80 | 463 | 301 | 172.8 ± 0.7 | Quercetin hexoside |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sreedharan, S.; Nair, V.; Bhargava, P.; Cisneros-Zevallos, L. Protective Role of Polyphenols from Aronia Berry (Aronia melanocarpa) Against LPS-Induced Inflammation in Colon Cells and Macrophages. Nutrients 2025, 17, 1652. https://doi.org/10.3390/nu17101652
Sreedharan S, Nair V, Bhargava P, Cisneros-Zevallos L. Protective Role of Polyphenols from Aronia Berry (Aronia melanocarpa) Against LPS-Induced Inflammation in Colon Cells and Macrophages. Nutrients. 2025; 17(10):1652. https://doi.org/10.3390/nu17101652
Chicago/Turabian StyleSreedharan, Shareena, Vimal Nair, Prerna Bhargava, and Luis Cisneros-Zevallos. 2025. "Protective Role of Polyphenols from Aronia Berry (Aronia melanocarpa) Against LPS-Induced Inflammation in Colon Cells and Macrophages" Nutrients 17, no. 10: 1652. https://doi.org/10.3390/nu17101652
APA StyleSreedharan, S., Nair, V., Bhargava, P., & Cisneros-Zevallos, L. (2025). Protective Role of Polyphenols from Aronia Berry (Aronia melanocarpa) Against LPS-Induced Inflammation in Colon Cells and Macrophages. Nutrients, 17(10), 1652. https://doi.org/10.3390/nu17101652







