Highlights
- Adolescent girls exhibit higher levels of internalizing symptoms than boys.
- Greater responsiveness to bitterness and astringency is associated with a higher prevalence of depressive symptoms, exclusively in girls.
- The severity of depressive symptoms is correlated with lower intake of beneficial nutrients, particularly in girls.
- In girls, comorbidities of depression moderate the link between sensory acuity and dietary habits.
Abstract
Background/Objectives: As a peripheral effect of depression-related traits, sensory responses may predispose individuals to depressive symptoms by prompting suboptimal dietary patterns with long-term effects on mood. Mood disturbances in adolescence are strong predictors of adult mental illness, making it crucial to identify factors that may shift transient mood fluctuations into more severe mental health issues during this vulnerable period. Given the substantial gender differences in susceptibility to comorbidities of depression, we examined whether the link between sensory perception and depressive symptoms in nonclinical adolescents varied by gender and was related to dietary habits. Methods: In this cross-sectional study, 232 healthy adolescents (41.8% girls, aged 13–17) reported their diet over the past year using the EPIC Food Frequency Questionnaire and rated their liking and perceived intensity of oral sensations from four grapefruit juices and dark chocolate puddings with varying sucrose levels. Additionally, participants completed assessments of anxiety, neuroticism, pickiness, body dissatisfaction, and the Patient Health Questionnaire (PHQ-9) to evaluate depressive symptoms. Results: We found that girls exhibited higher levels of depression, anxiety, neuroticism, and pickiness compared to boys (Wilcoxon Rank Sum Test), and that greater responsiveness to bitterness (e.g., β = 0.264, p = 0.037) and astringency (β = 0.269, p = 0.029) predicted higher depressive symptoms exclusively in girls. PHQ-9 scores were positively associated with alcohol use in both girls (ρ = 0.176, p = 0.003) and boys (ρ = 0.148, p = 0.004) and inversely related to the intake of beneficial nutrients (e.g., fiber, polyunsaturated fats), particularly in girls. Intriguingly, moderation analyses suggested that associations between nutrient intake and acuity for alarming oral sensations were largely moderated by depression-related traits in girls, but not in boys. Conclusions: Our findings suggest that gender moderates the links between depressive symptoms, sensory perception, and dietary habits in healthy adolescents, possibly reflecting gender-specific coping strategies for comorbidities of depression.
1. Introduction
Adolescence is a transient stage of life marked by profound physiological and psychosocial changes that can increase vulnerability to mood disorders. As emotional challenges intensify, mood disturbances may escalate to more significant mental health conditions, including depression. Characterized by persistent loss of pleasure or interest in activities and depressed mood [1], depressive disorders are one of the leading causes of morbidity globally among adolescents, contributing to 15% of the disease burden in this age group [2]. Approximately 34% of adolescents aged 10–19 are thought to experience intense depressive symptoms [3], with prevalence doubling during the first year of the COVID-19 pandemic [4] and increasing by 14% from the first (24%) to the second (38%) decade of the 21st century [3]. In particular, girls are twice as likely as boys to experience severe depressive symptoms, e.g., [5,6,7]. This gap typically emerges during preadolescence and peaks at ages 15–18, e.g., [6], likely due to factors such as earlier onset of puberty, increased exposure to severe stressors, intensified societal and academic pressures, and heightened susceptibility to the psychosocial correlates of depression (for review, see [7]). This is inevitably a cause for concern, as severe mood disturbances during adolescence are a strong predictor of mental illness in adulthood [8], and early symptoms often go undetected and untreated [2,9]. Therefore, identifying factors that may facilitate the progression from short-term mood fluctuations to significant mental health problems during adolescence is a critical public health priority.
In this context, changes in dietary habits are increasingly recognized as one of the most promising modifiable risk factors for preventing depression (for review, see [10]). In particular, an adequate intake of vegetables, fruits, vegetable oils, fish, and whole grains, along with limited consumption of refined grains, simple sugars, red and processed meats, and full-fat dairy products, has been consistently associated with a reduced risk of depression among adolescents worldwide, e.g., [11,12,13]. Indeed, diets rich in plant-based nutrients, such as the Mediterranean Diet, have been shown to reduce plasma levels of pro-inflammatory cytokines (e.g., IL-6, INF-γ, TNF-α), thereby counteracting biological mechanisms implicated in the etiology of depression, including systemic oxidative stress and dysregulation in the production of glucocorticoids, monoamines, and brain-derived neurotrophic factor (for review, see [14]). Therefore, uncovering barriers to adherence to dietary patterns beneficial for mood is one of the essential steps in addressing the rising rates of depression among youth.
Despite greater independence compared to childhood and significant familial and social influences, the sensory properties of food (taste, smell, flavor, texture, appearance) remain one of the key determinants of dietary choices during this developmental stage (for review, see [15]). Adolescents generally favor more intensely sweet tastes relative to adults [16] and tend to avoid foods that evoke inherently disliked oral sensations, such as bitterness [15]. However, there are substantial interpersonal differences in taste perception, which can influence adherence to or deviation from healthy dietary patterns, e.g., [17,18]. An example is the genetic ability to perceive bitter thiourea compounds, such as phenylthiocarbamide or 6-n-propylthiouracil (PROP), which can have downstream effects on food preferences (for review, see [18]). While PROP perception is a continuous trait, individuals are typically classified as non-tasters (NTs), medium-tasters (MTs), or super-tasters (STs) based on cut-offs that reflect varying levels of perceived bitterness, from null to extreme, with haplotypes in the TAS2R38 gene largely responsible for this phenotypic variation [18,19].
In adolescents, PROP status appears to influence food liking more than actual intake. For instance, NTs rate cruciferous vegetables as more pleasant than STs [20], whereas STs find animal-based foods (e.g., bacon, fried chicken, and herring), sauces, condiments [21], and sweetened foods more appealing [22]. While these findings might suggest that STs are more likely to adopt suboptimal dietary patterns, previous reports yielded mixed results. Only one study reported a higher sugar intake among STs relative to NTs [22], while others failed to link PROP phenotypes and/or genotypes to diet [20,21,23]. Thus, although the evidence remains insufficient to draw definitive conclusions due to small sample sizes and varying methods of operationalizing PROP status and dietary habits [24], other factors underlying variations in taste perception may contribute more to undesired dietary patterns during this phase of life.
In addition to PROP, notable gender differences in taste perception have been documented across the lifespan [22,25]. Girls at various stages of adolescence generally show greater responsiveness to oral stimuli than boys, e.g., [22,26], and are more likely to be STs [19]. However, whether these differences are primarily psychological or physiological remains debated [27]. In fact, a large body of literature suggests that sensory perception can also be influenced by mood states and personality traits (for reviews, see [17,28]). In nonclinical adults, anxiety and exposure to stressors (e.g., airhorn sounds, cold pressor test) tend to express higher intensity ratings to sweet, salty, and bitter stimuli [29,30,31], and being neurotic [32] or a picky eater [33] has been positively associated with acuity for bitter and for sweet and bitter aqueous solutions, respectively.
Interestingly, similar findings have been reported for depression. Dess and Chapman [34] found a positive correlation between depressive symptoms and bitterness ratings of quinine solutions, and Platte et al. [31] extended the same findings to the sweet taste elicited by varying levels of sucrose in water. Nevertheless, a link between greater responsiveness to oral stimuli and pronounced internalizing symptoms (anxiety, depression, stress) has not always been supported [35,36,37], and the opposite has often been observed in clinically depressed adults (for review, see [28]). Although findings are inconclusive and largely based on small adult samples, there is evidence that vulnerability to correlates of depression may drive variations in taste perception, potentially shaping dietary choices with long-term negative effects on mood.
Building on this, adolescents worldwide often cite the unpleasant sensory properties of healthy foods as a key barrier to healthy eating, leading them to opt for less nutritious options [15]. This is evident because prototypical healthy foods (e.g., vegetables, fruits, nuts, vegetable oils), whether raw or cooked, can evoke a range of sensory qualities such as bitterness, sourness, or astringency (hereafter referred to as alarming oral sensations), which elicit psychobiological states (i.e., arousal) that enhance alertness and facilitate rejection due to their perceived dangerousness [38]. However, the impact of arousal on food choices varies widely among individuals and depends on how well a sensation aligns with their optimal level of activation [38]. Consequently, an individual’s susceptibility to sources of arousal in foods (stimulus intensity, novelty, and complexity) is contingent upon their responsiveness to exogenous stimuli and mood state (for review, see [39]). Individuals with traits that heighten responses to external inputs, such as those related to depression, may thus be more reactive to negative arousal from food and potentially reinforce undesired dietary choices with long-term effects on mood. Given the increased vulnerability of girls to depression and its comorbidities [3,7], this holds the potential to yield new insights into gender-based antecedents of depressive symptoms and warrants further investigation.
While taste plays a crucial role in shaping food habits among adolescents [15], and diet may prevent depressive disorders [10], no studies have yet simultaneously examined the links between sensory perception, depressive symptoms, and dietary habits in healthy adolescents, particularly concerning variations in vulnerability to correlates of depression. Additionally, the current knowledge has focused on either small adult samples, e.g., [31,34,35], or documented inconclusive results on the impact of taste on dietary habits due to a paucity of studies [24]. Also, the majority of previous research has relied on detection or recognition thresholds of diluted tastants in simple solutions, which have shown minimal correlations with everyday perceptions at suprathreshold levels [40]. To address this gap, the use of actual or model foods with varying tastant concentrations could offer more ecologically relevant insights, e.g., [41].
Against this backdrop, we examined whether the link between sensory perception and depressive symptoms in nonclinical adolescents varied by gender and was related to dietary habits. Additionally, we explored whether these associations were moderated by common psychosocial correlates of depression, such as anxiety [42], neuroticism [43], picky eating [33], and body dissatisfaction [44].
In this study, 232 nonclinical adolescents provided data on PROP responsiveness, as well as hedonic and psychophysical ratings to oral stimuli elicited by four variants of two food models with varying sucrose levels. Participants also completed the European Prospective Investigation into Cancer and Nutrition Food Frequency Questionnaire (EPIC-FFQ) [45] to monitor their habitual diet, a series of questionnaires assessing the aforementioned correlates of depression, and reported their depressive symptoms over the previous two weeks using the nine-item Patient Health Questionnaire (PHQ-9) [46].
2. Materials and Methods
2.1. Participants
This work builds on a broader project aimed at elucidating the genetic, non-genetic, and psychosocial determinants of individual variations in sensory perception among adolescents and their impact on diet. Due to the lack of comparable studies, sample size was estimated based on a previous study from our group involving healthy young adults (aged 18–30) from the same location (Autonomous Province of Trento, Italy) and engaged in similar experimental tasks [47]. In that study, we found significant differences (Cohen’s d = 0.402) in responsiveness to bitterness and sourness elicited by commercially available foods between groups with comparable salivary microbial profiles. Accordingly, we conducted a power analysis using the pwr.t.test R function [48], which indicated that 198 participants would be needed to detect such an effect with 80% power at α = 0.05 (two-tailed). To account for deviations from normality and potential dropouts, the target sample was increased by 15%, resulting in an expected sample size of 228 participants.
This was later accommodated with a final sample of 232 healthy adolescents (41.8% girls, 13–17 years; mean age ± SD = 14.5 ± 0.6 years; mean BMI ± SD = 21.0 ± 3.2 kg/m2), who were recruited via a series of promotional events targeting both potential participants and their parents from two high schools in the Autonomous Province of Trento, Italy.
No formal exclusion criteria were applied. Nonetheless, participants reported no current diagnosis of major depressive disorder, nor were they undergoing treatment or had used medications in the past 6 months that could affect mood or taste function. Furthermore, girls and boys did not differ (p > 0.05) in terms of age, BMI, smoking habits, weekly alcohol consumption, food allergies, and habitual diet. In contrast, boys engaged in more physical activity than girls (t = 2.532, p = 0.012). A full demographic description of our sample is provided in Table S1.
Before data collection, parents or legal guardians provided written consent, and adolescents gave written assent. The study was approved by the Research Ethics Committee of the University of Trento (n° prot. 2023-047, approved on 28 September 2023) and followed the principles of the Declaration of Helsinki (as amended in Fortaleza, Brazil, 2013).
2.2. Overview of Data Collection
To align with the project objectives, a 7-day cross-sectional protocol was designed to collect a broad range of sensory, psychometric, demographic, health-related, and dietary data, as well as biological samples (saliva and tongue swabs) for metagenomic and genetic analyses. Key variables used in this study are highlighted in bold in the graphical overview of the experimental design (Figure 1), while Table 1 and Table 2 provide details on tasks and measures used during data collection.
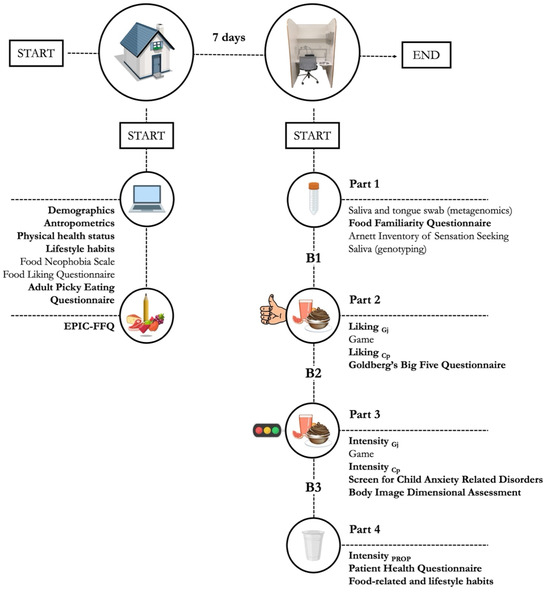
Figure 1.
Graphical overview of data collection, with measures employed in this study highlighted in bold. Gj: grapefruit juice series; Cp: dark chocolate pudding series.
To optimize logistics, participants took part in two distinct work sessions: one remote and one conducted in an ISO 8589:2007 [49] compliant sensory lab. At least 7 days before the lab session, a researcher visited participants during school hours to provide detailed instructions for the home tasks (Table 1). These included an online questionnaire assessing demographics, anthropometrics, physical health status, lifestyle habits, stated preferences for a list of 57 food items, and selective/avoidant feeding attitudes using the Food Neophobia Scale and the Adult Picky Eating Questionnaire. Additionally, to assess their habitual diet, participants completed a paper version of the EPIC-FFQ, which they returned at the beginning of the lab session.
After the remote session, participants were instructed to refrain from eating, drinking (except water), smoking, and brushing their teeth for at least 2 h before visiting the sensory lab at the Edmund Mach Foundation (San Michele all’Adige, Trento, Italy). Due to the extensive data collection, the lab session was divided into four slots (Parts 1–4) to minimize participants’ burden and ensure data quality (Figure 1).
Part 1 (Table 2) began with the autonomous collection of a saliva sample and a tongue swab for metagenomic analyses. Participants then rated their familiarity with the same 57 food items assessed remotely and completed the Arnett Inventory of Sensation Seeking to evaluate their inclination for novel, varied, and intense experiences. An additional salivary sample was collected for genotyping before concluding Part 1 (Figure 1).
In Part 2 (Table 2), participants evaluated their liking of two independent sets of four variants each of two model foods (grapefruit juice, dark chocolate pudding) with varying sucrose concentrations. Sensory evaluations were interspersed with a 5 min break featuring logic games to maintain motivation. The following assessment of personality dimensions through the Goldberg’s Big Five Inventory marked the end of this slot.
Part 3 (Table 2) focused on psychophysical responses to oral sensations elicited by the same model foods from Part 2. Participants then completed the Screen for Child Anxiety Related Disorders and the Body Image Dimensional Assessment to assess their generalized, social, and school anxiety and estimate their level of body dissatisfaction, respectively.
In Part 4 (Table 2), participants rated the perceived bitterness of two aqueous solutions containing PROP and reported the severity of depressive symptoms experienced in the past 2 weeks through the Patient Health Questionnaire. Next, they were asked about their smoking habits, alcohol, snacks, and sweetened beverages consumption, and habitual use of social networks before concluding the study.
Each task slot (Parts 1–4) was separated by a break (B1–B3) of at least 15 min (Figure 1), during which detailed verbal and practical instructions for the upcoming tasks were provided. Data were collected using the EyeQuestion software (version 5.5.0, Elst, The Netherlands), except for the EPIC-FFQ, which was completed in paper form. Data collection took place between November and December 2023, with lab sessions held from 9:00 AM to 12:00 PM. The following sections provide details on the measures used to achieve the aims of the current work.

Table 1.
Data collected during the remote work session. Relevant questionnaires, response options, number of items, rating scales, and references are listed. † Data collected in paper form.
Table 1.
Data collected during the remote work session. Relevant questionnaires, response options, number of items, rating scales, and references are listed. † Data collected in paper form.
| Questionnaire | Output | Options (Scale) | References |
|---|---|---|---|
| Demographics | Age | Years old at the moment of the test | |
| Gender | Girl/Boy | ||
| Anthropometrics | Weight | 40–180 kg | |
| Height | 100–220 cm | ||
| Physical health status (ongoing diagnosis or within the last 6 months) | Chronic diseases | Asthma; COVID-19; Type I or II diabetes; Celiac disease; Crohn’s disease; Gastroesophageal reflux; Hiatal hernia; Irritable bowel syndrome | |
| Oral diseases | Aphthous ulcers; Halitosis; Oral candidiasis; Gingivitis; Periodontitis; Xerostomia | ||
| Psychiatric disorders | Anorexia nervosa; Bulimia nervosa; Binge-eating disorder; Generalized anxiety disorder; Major depressive disorder; Autism spectrum disorder | ||
| Taste and smell disorders | Ageusia; Anosmia; Hypogeusia; Hyposmia | ||
| Food allergies | Yes/No [if yes, details asked] | ||
| Food intolerances | Yes/No [if yes, details asked] | ||
| Medications and supplements use (past 6 month) | Antibiotics use | Yes/No | |
| Medicines use | Yes/No [if yes, details asked] | ||
| Probiotics use | Yes/No | ||
| Lifestyle habits | Diet | 10 items | [50] |
| International Physical Activity Questionnaire (IPAQ) | 7 items | [51] | |
| Oral Hygiene Behavior Questionnaire | 8 items | [52] | |
| Food Liking Questionnaire | Stated liking for a range of foods | 57 items rated (9-point hedonic scale; 1 = extremely disliked, 9 = extremely liked) | Adapted from [53] |
| Food Neophobia Scale | Selective and avoidant feeding attitudes | 10 items (7-point Likert scale; 1 = strongly disagree; 7 = strongly agree) | [54] |
| Adult Picky Eating Questionnaire | 20 items (5-point Likert scale; 1 = never; 5 = always) | [55] | |
| Dietary habits | EPIC Food Frequency Questionnaire † | 163 items | [45] |

Table 2.
Data collected during the four task slots (Part) designed for the lab session. Relevant tasks, expected outputs, number of items, rating scales, and references are listed. Gj: grapefruit juice series; Cp: dark chocolate pudding series.
Table 2.
Data collected during the four task slots (Part) designed for the lab session. Relevant tasks, expected outputs, number of items, rating scales, and references are listed. Gj: grapefruit juice series; Cp: dark chocolate pudding series.
| Part | Task | Output | Items | Scale | References |
|---|---|---|---|---|---|
| 1 | Saliva and tongue swab (metagenomics) | One salivary and one tongue swab sample for shotgun metagenomics | |||
| Food familiarity questionnaire | Familiarity with a range of foods | 57 items | 5-point Likert scale (1 = I do not recognize it; 5 = I regularly eat it) | Adapted from [53] | |
| Arnett Inventory of Sensation Seeking questionnaire | Inclination for novel, varied, and intense experiences | 20 items | 4-point Likert scale (1 = describes me very well; 4 = does not describe me at all) | [56] | |
| Saliva (genotyping) | One salivary sample for SNP-array | ||||
| 2 | Liking Gj | Liking ratings for the four variants of Gj | Labeled Affective Magnitude scale (0 = greatest imaginable dislike; 100 = greatest imaginable like) | [57] | |
| Liking Cp | Liking ratings for the four variants of Cp | ||||
| Goldberg’s Big Five Inventory questionnaire | Personality traits | 30 items | 7-point Likert scale (1 = does not apply to me at all, 7 = applies to me very well) | [58] | |
| 3 | Intensity Gj | Intensity ratings for oral sensations from the four Variants of Gj | generalized Labeled Magnitude Scale (0 = no sensation, 100 = the strongest imaginable sensation of any kind) | [59] | |
| Intensity Cp | Intensity ratings for oral sensations from the four variants of Cp | ||||
| Screen for Child Anxiety Related Disorders questionnaire | Level of generalized, social, and school anxiety | 17 items | 3-point Likert scale (1 = almost never, 3 = often) | [60] | |
| Body Image Dimensional Assessment questionnaire | Level of body dissatisfaction | 4 items | Line scale from 1.8 to 5.2 | [61] | |
| 4 | Intensity PROP | Intensity ratings from two PROP aqueous solutions | generalized Labeled Magnitude Scale (0 = no sensation, 100 = the strongest imaginable sensation of any kind) | [59] | |
| Patient Health Questionnaire | Severity of depressive symptoms | 9 items | 4-point Likert scale (0 = never; 3 = nearly every day) | [46] | |
| Food-related and lifestyle habits | Monthly frequency of smoking cigarettes and e-cigs | 3 items | 7-point Likert scale (1 = never; 7 = 30 days) | ||
| Weekly intake of beer, wine, liquors, and cocktails | 4 items | 7-point Likert scale (1 = never; 7 = more than once a day) | |||
| Weekly intake of snacks and sweetened beverages | 14 items | 7-point Likert scale (1 = never; 7 = more than once a day) | |||
| Daily use of social networks | 2 items | 5-point Likert scale (1 = less than 1 h; 5 = more than 4 h) |
2.3. Sensory Tasks
2.3.1. Stimuli
We aimed to develop four variants of two food models that would elicit a range of oral sensations, including sweet, bitter, sour, astringent, and flavors (grapefruit and chocolate), with the potential to mimic the spectrum of intensities experienced in everyday foods. Given its strong acceptance at high intensities among adolescents [15], sweetness was selected as the target sensation for modulation. Each food model had to (a) be widely available on the Italian market and familiar to our target population; (b) evoke alarming oral sensations that could be masked by increasing sweetness levels while maintaining a congruent sensory profile; (c) be simple to prepare, store, portion, and consume at room temperature; (d) be accepted by omnivores, vegetarians, and vegans. As a result, commercially available 100% grapefruit juice (Gj) and dark chocolate pudding (Cp) base formulations [53] were deemed the most suitable options (Table 3).

Table 3.
Food models and relative variants used in the present study. Ingredients (Brand), order of presentation of each variant within the liking and intensity tasks, sucrose concentrations added to each variant of Gj and Cp (g/kg), and the sensory ballot evaluated are listed. Target sensation is highlighted in bold.
For each food model, four different sucrose concentrations were initially chosen based on previous studies using Gj [41] and Cp [53], and expected to elicit an increasingly higher level of sweetness while progressively decreasing relevant alarming oral sensations. Given that these sucrose levels had been tested either in a different Gj base [41] or in adults (Cp) [53], an initial evaluation was conducted with a panel of 39 individuals (58.9% girls, mean age ± SD = 44.7 ± 14.5 y) experienced in sensory analysis (Pilot 1). Results (Figure S1a) indicated that slight adaptations to the sucrose span in both Gj and Cp were necessary to more effectively differentiate each series of products. Following these adjustments, a group of adolescents (Pilot 2, n = 16; 26.6% girls, mean age ± SD = 17.3 ± 0.4 y) rated the perceived intensities of oral sensations evoked by the optimized food models (Table 3) using the generalized Labeled Magnitude Scale (gLMS, 0 = no sensation, 100 = the strongest imaginable sensation of any kind), which revealed clearer sensory differences among variants of both Gj and Cp (Figure S1b). The suitability of both food models was later corroborated by our population (Part 3, Figure 1), as psychophysical responses (gLMS) to sweetness exhibited a systematic increase, while bitterness, sourness, and astringency decreased as sucrose concentration increased (Figure S2). For all testing, Gj was prepared by dissolving sucrose in the base juice, while the same methodology developed by Monteleone et al. [53] was used for preparation of the Cp variants.
Phenotypic responses to PROP (Part 4, Figure 1) were instead collected in duplicate from 10 mL aqueous solutions with 0.5447 g/L of PROP [53]. All stimuli were prepared the day before, stored at 4 °C overnight, and brought to ambient temperature 2 h prior to testing. Both food models and PROP solutions were served in 80 cc plastic glasses at ~20 °C, coded with a random 3-digit code, and tested under white warm light for liking and red light for intensity tasks (Figure 1).
2.3.2. Training
Each sensory assessment was preceded by detailed instructions on the psychophysical scaling methods to be used for the upcoming tasks. Before the liking task (Part 2, Figure 1), participants were trained to use the Labeled Affective Magnitude scale (LAM, 0 = greatest imaginable dislike, 100 = greatest imaginable like) according to common practices [57]. Subsequently, before the intensity tasks outlined in Parts 3 and 4 (Figure 1), special attention was given to minimizing potential artifacts in the use of the gLMS (Table 2) by (a) clarifying the meaning of the scale anchors and the sensory attributes being evaluated, providing simple descriptions (e.g., a dry mouth feeling led by unripe fruits for astringency) along with relevant food examples (e.g., lemon juice for sourness), (b) encouraging the use of the full scale to avoid categorical behaviors and to distinguish the intensity of a stimulus from its hedonic value, and (c) guiding participants to base the ratings on their daily sensory experiences across various modalities [40,59,62].
For individual calibration, participants provided psychophysical responses to 10 extraoral stimuli shortly after rating the intensity of oral sensations from each variant of both Gj and Cp series (n = 4 × 2) or PROP solution (n = 2). The items were presented in a fixed order, and stimuli representing various theoretical ranges on the gLMS were included in each set of products. Collectively, systematic differences were observed in parallel with the expected magnitude of the orientation stimuli (Figure S3). Furthermore, the effectiveness of the gLMS training was supported by both girls and boys rating all extraoral stimuli as equally intense, indicating consistent use and interpretation of the scale (Figure S4).
2.3.3. Sensory Assessments
In the hedonic task (Part 2, Figure 1), ratings were obtained from two independent sets of four variants each of Gj and Cp (Table 3) using the LAM scale (Table 2). Participants were instructed to hold the whole sample of Gj (20 mL) or a full spoon of Cp (20 g) in their mouth for 5 s before swallowing and then rate their liking.
The same quantity of sample was used for the intensity assessment (Part 3, Figure 1). Unlike the preceding task, participants were asked to keep each variant of Gj and Cp in their mouth for 7 s, then swallow and wait 5 s before evaluating the perceived intensities using the gLMS scale (Table 2). The Gj series was always presented first, followed by the Cp series after a 5 min break during which participants engaged in logic games to maintain motivation (Figure 1). In contrast, the variants were presented in different fixed orders across the liking and intensity tasks (Table 3). This approach was designed to minimize excessive fluctuations in perceived intensities that could lead to inflated responses, prevent participants from associating the same presentation order with both tasks, and induce similar perceptual biases across individuals, thereby facilitating subsequent comparisons of sensory responses [63]. For the same purpose, the sensory ballot was presented with a fixed sequence (Table 3): the target sensation (sweetness) was rated first, followed by relevant attributes to Gj (sour, bitter) or Cp (bitter, astringency), with flavor evaluated last [63]. Before sensory evaluations, participants were asked to declare any allergies and/or intolerances to Gj and Cp ingredients (Table 3).
For PROP phenotyping (Part 4, Figure 1), participants retained each solution in their mouth for 10 s, then expectorated and waited 20 s before reporting the perceived bitterness (gLMS, Table 2). The average PROP values were used after confirming similar ratings among replicates (W = 13,858, p = 0.295).
A 60 s break was enforced after each tasting, during which participants were provided with mineral water and plain crackers to cleanse their palate.
2.4. Questionnaires
2.4.1. The Patient Health Questionnaire (PHQ-9)
To assess depressive symptoms, we utilized the Italian version of the 9-item Patient Health Questionnaire [46], which has demonstrated psychometric soundness with diverse Italian adolescent populations, e.g., [64,65]. The PHQ-9 is widely recognized as the gold standard for diagnosing depressive disorders, exhibiting high sensitivity (89%) and specificity (88%) in primary care settings [66]. A 4-point Likert scale (0 = never, 3 = nearly every day) is employed to quantify the frequency of depressive symptoms experienced over the past 2 weeks. The sum of the nine items yields a total score ranging from 0 to 27, with higher values reflecting greater severity of depressive symptoms. In this study, the PHQ-9 demonstrated good internal consistency (α = 0.818).
2.4.2. The Screen for Child Anxiety Related Emotional Disorders (SCARED)
Anxiety levels were operationalized using a subset of the 38-item Screen for Child Anxiety Related Emotional Disorders adapted for the Italian adolescent population [60,67]. The SCARED employs a 3-point Likert scale (1 = almost never, 3 = often) to measure the frequency of anxiety-related symptoms across five factors: generalized anxiety (9 items), social anxiety (4 items), school anxiety (4 items), panic disorders (13 items), and separation anxiety (8 items). In the present study, only the generalized, school, and social anxiety subscales were collected. Each domain was scored by summing up the relevant items, with higher values indicating a greater inclination toward anxiety-related symptoms. The internal consistency of the subscales was acceptable to good, with Cronbach’s αs of 0.821, 0.747, and 0.684 for the generalized, social, and school anxiety, respectively.
2.4.3. The Goldberg’s Big Five Questionnaire (BIG-5)
The facets of personality were assessed using the 30-item Goldberg’s Big Five questionnaire [68], which has been validated for use with Italian adolescents by Klimstra et al. [58]. Each personality dimension (agreeableness, conscientiousness, emotional stability, extraversion, and openness) was evaluated with six items on a 7-point Likert scale (1 = does not apply to me at all, 7 = applies to me very well). Composite subscale scores were then computed by averaging the ratings for each subscale, with the control items reversed. Each domain showed acceptable to good internal consistency, with Cronbach’s αs of 0.801 for agreeableness, 0.838 for conscientiousness, 0.778 for emotional stability, 0.845 for extraversion, and 0.671 for openness. For the purposes of this study, the emotional stability dimension was used and reversed to represent its opposite, neuroticism, which reflects the tendency to experience negative affective states [43].
2.4.4. The Adult Picky Eating Questionnaire (APEQ)
As a food-related comorbidity of depressive symptoms [33], the Italian version of the 20-item Adult Picky Eating Questionnaire [55,69,70] was used to evaluate picky eating, defined as the reluctance to try familiar and novel foods [33]. Participants were asked to indicate how frequently they engage in picky eating behaviors on a 5-point Likert scale (1 = “Never”; 5 = “Always”) across four dimensions: meal presentation (7 items), food variety (4 items), meal disengagement (3 items), and taste aversion (6 items). Scores were calculated as the average of all 20 items or within each subscale, with higher values indicating greater pickiness.
Due to the absence of validated measures for assessing picky eating among Italian adolescents and our prior validation being specific to adults over 18 [55], only the APEQ global score was used in this study. To preliminarily assess its psychometric properties, we tested its internal consistency (α = 0.808), convergent and discriminant validity (Figure S5), and test-retest reliability, all yielding adequate results. For further details, please refer to Appendix A.
2.4.5. The Body Image Dimensional Assessment (BIDA)
Lastly, participants completed the 4-item Body Image Dimensional Assessment [61], which measures the discrepancy between an individual’s current body image and their idealized physique. Participants viewed four anonymized body shapes representing increasing weights along with a line scale from 1.8 to 5.2, before rating how closely their actual body shape aligned with their ideal figure (body dissatisfaction), the most attractive shape for the opposite sex (sexual body dissatisfaction), and the appearance of same-sex and -age peers (compared body dissatisfaction). Three sub-scores (ranging from −100 to 100) can be computed using the formulas provided by Sánchez-Miguel et al. [61], and a total BIDA score was derived as the mean of their absolute values, with higher scores reflecting greater body dissatisfaction [61].
Although validated only for Spanish adolescents aged 12–15 [61], the BIDA was still preferred over the recently validated 8-item BIBA [71] in the Italian context, as the latter was designed for younger children (ages 6–13). After an initial psychometric check, the BIDA total score showed good internal consistency (α = 0.763), strong inter-item correlations (Table S2), and evidence of convergent and discriminant validity (Figure S6). Further details are provided in Appendix B.
2.4.6. Demographic and Lifestyle Variables
Alongside the remote (Table 1) and lab (Table 2) sessions, a range of demographic and lifestyle-related measures were collected. These included, but were not limited to, gender, age, and weight and height, with the latter used to calculate body mass index (BMI) in kg/m2. Additionally, physical activity levels were estimated using the Italian short form of the International Physical Activity Questionnaire (IPAQ) [51].
2.4.7. The EPIC Food Frequency Questionnaire (EPIC-FFQ)
In the week preceding the lab session (Figure 1), participants filled out a paper-based version of the 163-item EPIC-FFQ, which was recently validated for Italian adolescents [45], to monitor their habitual diet over the past year. Participants were asked to report consumption frequencies for both individual foods (e.g., vegetables) and recipes (e.g., pasta, pizza), along with habitual cooking methods (e.g., baked, boiled, fried, grilled) across daily, weekly, monthly, and yearly intervals. To ensure accuracy, the questionnaire included images depicting portion sizes (small, medium, large) for various recipes (e.g., soups, stew) or food items within each food group (e.g., carrots for vegetables, cod for fish). Dietary data were treated according to Pala et al. [72] to estimate daily intake of energy (Kcal) and a list of macro- (e.g., carbohydrates, fats, proteins) and micronutrients (e.g., B vitamins, minerals) before further processing (Section 2.5).
2.5. Data Analysis
Given that the majority of variables did not meet the normality assumptions, gender differences in severity of depressive symptoms and their psychosocial correlates (generalized anxiety, neuroticism, picky eating, and body dissatisfaction) were first assessed using the Wilcoxon Rank Sum Test (W). To prepare for further analyses, the PHQ-9 scores, which exhibited right skewness (γ = 1.421), were transformed using the Yeo–Johnson method to approximate normality (γ = 0.030). Additionally, psychophysical responses to oral sensations evoked by each variant of Gj (sweet, sour, bitter) and Cp (sweet, bitter, astringent) were individually summed to derive six scores of global responsiveness (Ʃ), as outlined by Piochi et al. [73]. Importantly, hedonic and intensity ratings were available for 231 and 227 individuals, as one participant reported constraints to ingredients in Gj, and five in Cp.
Separate general linear models (GLMs) were then fitted to evaluate whether the associations between sensory perception and depressive symptoms differed by gender. In these models, the PHQ-9 scores served as the dependent variable, while both the main effects and the interactions between gender and each of the six taste global scores were included as predictors. To control for possible psychosocial confounders, each GLM was re-run with comorbid states or traits of depression included as covariates. According to the results, the global taste scores that exhibited significant interactions with gender as predictors of depressive symptoms, either with or without adjustments, were retained for further analysis. Data were then stratified by gender, and subsequent analyses were conducted separately for girls and boys.
Next, partial Spearman’s rank correlation coefficients (ρ) were computed to examine gender-specific relationships between depressive symptoms and dietary habits, adjusting for age, BMI, and physical activity. Before analysis, nutrient data were screened for misreporting and implausible extreme values, as recommended by Welch et al. [74]. In brief, participants with more than 10 missing items (n = 8) due to misreporting or duplicate responses were excluded [74]. Next, basal metabolic rates specific to gender and age were estimated for each participant using the Schofield equation [75], and outliers (n = 6) were identified as those whose ratio of habitual energy intake to basal metabolic rate fell within the top or bottom 1% of the distribution [76]. As a result, data from 218 participants who had a demographic and lifestyle background similar to that of the original population (Table S3), were retained for analysis. Nutrient data were then individually adjusted for daily energy intake (Kcal) using the residual method [77] to account for variations in energy needs and to prevent inflation of the results.
Lastly, moderation analysis was applied to test whether varying vulnerabilities to correlates of depression led to variations in oral acuity that may prompt undesired dietary choices with long-term effects on mood. Moderation analysis evaluates how the effect of an independent variable (oral acuity) on a dependent variable (nutrient intake) varies with the level of a moderating variable (correlates of depression) [78]. Accordingly, each energy-adjusted nutrient was treated as a dependent variable, with both main effects and interaction terms between each global taste score (independent variable) and each comorbidity of depression (moderator) included as predictors. Again, these analyses were adjusted for age, BMI, and physical activity.
Bootstrapping with 10,000 iterations was used to robustly estimate 95% confidence intervals for all models, and continuous variables were scaled to unit variance prior to analysis to facilitate interpretability. Effect sizes are presented as bootstrapped standardized β coefficients, with squared semi-partial correlations (sr2) included in moderation analysis to quantify the unique variance explained by each predictor [78]. The absence of severe multicollinearity and autocorrelation was confirmed by Variance Inflation Factor (VIF) values < 10 [79] and non-significant results from the Durbin–Watson test (p > 0.05), respectively. Lastly, data are summarized as median ± interquartile range (IQR) where applicable, with all tests being two-tailed and statistical significance set at p < 0.05. Data analysis was conducted using R version 4.3.1 [80].
3. Results
3.1. Rates of Depression and Gender Differences in Depressive Symptoms and Comorbid Traits
According to standard clinical cut-offs [46], our sample overall showed mild (PHQ-9 = 5–9) depressive symptoms (median ± IQR = 7 ± 5). Nonetheless, at least moderate depressive symptoms (PHQ-9 ≥ 10) were observed in 21.6% of participants. In detail, 1.7%, 27.6%, 49.1%, 13.4%, 6.0%, and 2.2% of adolescents reported experiencing no (PHQ-9 = 0), minimal (PHQ-9 = 1–4), mild (PHQ-9 = 5–9), moderate (PHQ-9 = 10–14), moderately severe (PHQ-9 = 15–19), and severe (PHQ-9 ≥ 20) depressive symptoms over the past two weeks, respectively. Notably, 36.1% of adolescent girls exhibited at least moderate depressive symptoms compared to 11.1% of boys.
As a result, significant (p < 0.001) gender differences in depression and related comorbidities emerged (Figure 2). Girls reported greater depressive symptoms, a higher inclination toward generalized anxiety, increased levels of neuroticism, and more engagement in picky eating behaviors compared to boys. In contrast, no significant differences were found between genders regarding overall body dissatisfaction (W = 6532.5, p = 0.977).
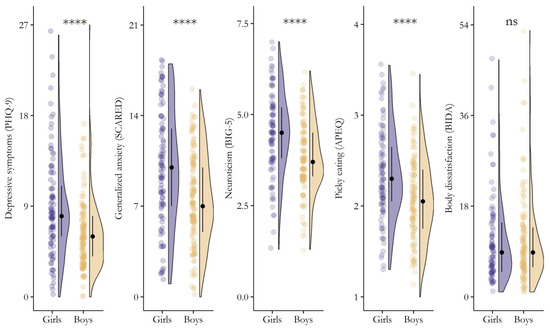
Figure 2.
Differences (Wilcoxon Rank Sum Test) in the severity of depressive symptoms, generalized anxiety, neuroticism, picky eating behaviors, and body dissatisfaction between girls (slate blue) and boys (light tan). The plot shows raw data points (the “rain”), the kernel density estimate (the “cloud”), and the median (black filled circle) ± IQR (perpendicular black line). **** = p < 0.0001, ns = p > 0.05.
3.2. Associations Between Sensory Perception and Depression by Gender
Next, we examined whether the associations between PHQ-9 scores and the six global scores of oral acuity varied by gender. Across all models, gender consistently showed a significant main effect on depressive symptoms (p < 0.001), whereas the opposite was true for all global taste scores (Table 4). This suggests that gender was a more influential predictor of PHQ-9 scores than orosensory responsiveness.

Table 4.
Associations between global sensory responsiveness (Ʃ) and depressive symptoms as a function of gender. Bootstrapped β estimates, 95% confidence intervals, and p-values are provided. Statistically significant main and interaction effects are highlighted in bold. Gender [G]: girls, Gj: grapefruit juice, Cp: dark chocolate pudding.
However, the relationship between sensory perception and depression was gender-specific. Notably, significant interaction effects were found in girls for global acuity for bitterness in Gj and astringency in Cp. In contrast, no significant or almost significant interactions were observed for sweetness in either food model, sourness in Gj (p = 0.055), or bitterness in Cp (Table 4). These findings indicate that greater responsiveness to alarming oral sensations predicted higher depressive symptoms in girls, but not in boys.
Similar patterns emerged after adjusting for psychosocial correlates of depression (Table 5). The main effects of global taste scores remained non-significant, while the predictive value of gender lost significance after adjustment. As expected, generalized anxiety and neuroticism were the strongest predictors of PHQ-9 scores across all models, with higher internalizing symptoms systematically linked to higher levels of depression.

Table 5.
Associations between global responsiveness (Ʃ) to oral sensations and depressive symptoms by gender, controlling for psychosocial comorbidities of depression. Bootstrapped standardized β coefficients and 95% confidence intervals, as well as p-values, are listed. Statistically significant main and interaction effects are highlighted in bold. Gender [G]: girls, Gj: grapefruit juice Cp: dark chocolate pudding, SCARED: generalized anxiety, BIG-5 [N]: neuroticism, APEQ: picky eating, BIDA: body dissatisfaction.
Consistent with our previous findings, no significant interactions between gender and sweetness were observed in either food model. Similarly, the positive association between acuity for alarming oral sensations and PHQ-9 scores remained exclusive to girls, with significant interaction effects found for bitterness in Gj and for both bitterness and astringency in Cp (Table 5). Notably, both the crude (Table S4) and adjusted (Table S5) models showed no evidence of severe multicollinearity or autocorrelation (Durbin–Watson tests, p > 0.05), with VIFs well below the commonly used threshold of 10 [79]. Based on these results, only the global scores related to alarming oral sensations were retained for further analysis.
3.3. Associations Between Dietary Habits and Depressive Symptoms by Gender
After stratifying by gender, we examined the correlations between depressive symptoms and dietary habits. Several commonalities emerged between girls and boys, though depressive symptoms were associated with a greater number of dietary outcomes in girls (Table 6).

Table 6.
Partial correlation between the severity of depressive symptoms and nutrient intake by gender. Spearman’s ρ coefficients, adjusted for age, BMI, and physical activity, along with p-values, are listed. Statistically significant values (p < 0.05) are indicated in bold.
In both genders, PHQ-9 scores were positively correlated (p < 0.05) with alcohol intake and exhibited inverse associations with daily consumption of proteins, vegetable fats, oleic acid, and vitamin E (Table 6).
It is noteworthy that, in addition to a positive correlation with carbohydrate intake, depressive symptoms were inversely correlated (p < 0.05) with the intake of a lengthy list of beneficial nutrients exclusively in girls. These included, but were not limited to, fibers, polyunsaturated fats, vitamin B6, vitamin D, and zinc. In contrast, habitual intake of vegetable proteins showed an inverse correlation with PHQ-9 scores in boys, but not in girls (Table 6).
3.4. Moderation Analysis
Lastly, we performed several moderation analyses to examine whether varying vulnerabilities to comorbidities of depression influenced the link between global responsiveness to alarming oral sensations and diet. In general, psychosocial correlates of depression primarily moderated the associations between dietary habits and responsiveness to alarming oral sensations in girls (Figure 3). For girls, the moderation effect from all significant models (F p-value < 0.05) accounted for additional variance (sr2) ranging from 1.8% to 7.8% for generalized anxiety, 0.0% to 7.7% for neuroticism, 1.0% to 6.8% for picky eating, and 4.7% to 9.8% for body dissatisfaction. In contrast, for boys, the incremental variance attributed to these variables was generally lower and often non-significant (p > 0.05), ranging from 0.0% to 5.5% for generalized anxiety, 0.5% to 4.1% for neuroticism, 0.0% to 3.1% for picky eating, and 0.0% to 0.9% for body dissatisfaction.
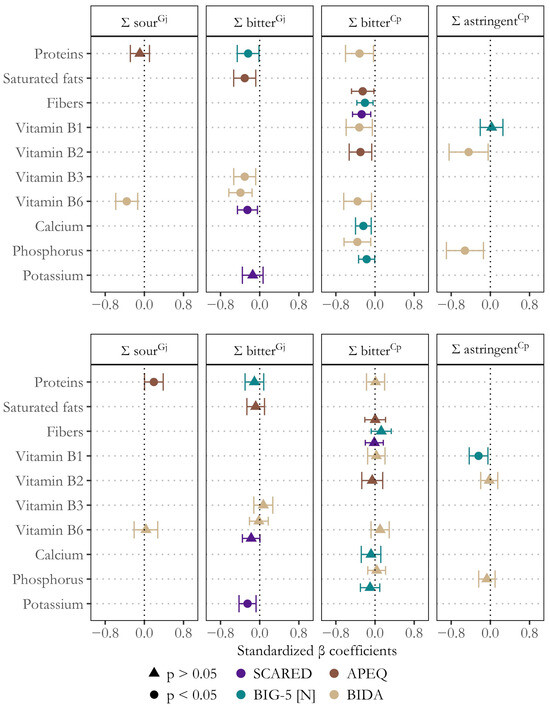
Figure 3.
Significant moderating effects of depression comorbidities on the links between global responsiveness (Σ) to alarming oral sensations and nutrient intake in girls (top) and boys (bottom). The plot shows bootstrapped standardized β coefficients and 95% confidence intervals, along with p-values (circles: p < 0.05, triangles: p > 0.05). SCARED (generalized anxiety), BIG-5 [N] (neuroticism), APEQ (picky eating), and BIDA (body dissatisfaction).
Specifically, comorbidities of depression moderated (p < 0.05) the association between responsiveness to bitterness, astringency, and (to a lesser extent) sourness and the consumption of various healthful nutrients (Figure 3). For instance, generalized anxiety (sr2 = 0.078), neuroticism (sr2 = 0.057), and picky eating (sr2 = 0.045) moderated the negative association between bitter taste acuity and habitual fiber intake. Similarly, neuroticism exerted a moderating role on the inverse association between bitterness perception and habitual intake of proteins (sr2 = 0.040), calcium (sr2 = 0.077), and phosphorus (sr2 = 0.040). Additionally, body dissatisfaction was found to moderate the negative association between the intake of vitamins B1 (sr2 = 0.050), B2 (sr2 = 0.050), B3 (sr2 = 0.062), and B6 (sr2 = 0.092) and responsiveness to bitterness (in Cp), astringency, bitterness (in Gj), and sourness, respectively (Figure 3). In other words, adolescent girls with higher levels of comorbid traits of depression showed stronger negative associations between responsiveness to alarming oral sensations and the consumption of these nutrients.
Importantly, all models were free from multicollinearity (VIF < 10) [79] and autocorrelation issues, with VIFs ranging from 1.005 to 1.837 for girls and 1.014 to 1.259 for boys. Additionally, the results from the Durbin–Watson test were non-significant (p > 0.05) across all analyses.
To facilitate comprehension of the moderating effects, Figure 4 depicts examples of how varying levels of correlates of depression influence the strength of associations between sensory perception and dietary intake by gender. Full details on bootstrapped standardized β coefficients and 95% confidence intervals, as well as p-values, sr2, and adjusted R2 for all significant models (F p-value < 0.05) highlighting a moderating effect of generalized anxiety, neuroticism, picky eating, and body dissatisfaction on the relationship between acuity for alarming oral sensations and nutrient intake by gender are provided in Tables S6–S9.
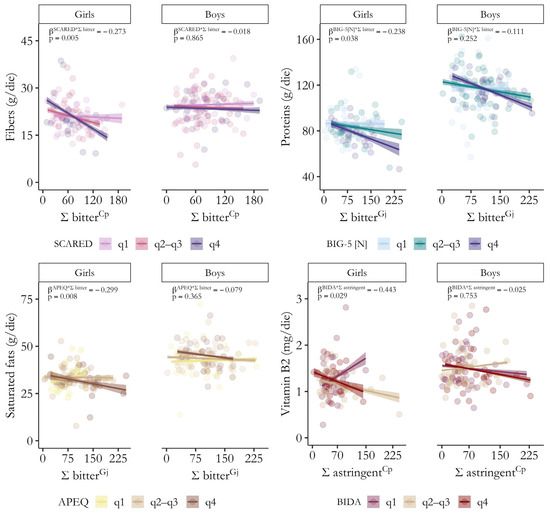
Figure 4.
Significant moderating effects of depression comorbidities on the relationship between global responsiveness (Ʃ) to alarming oral sensations and dietary habits by gender. Simple slopes illustrate how internalizing symptom severity (q1: lowest quartile, q2–q3: second and third quartiles, q4: highest quartile) may exacerbate the association between sensory perception and nutrient intake (fibers: top left, proteins: top right, saturated fats: bottom left, vitamin B2: bottom right). Bootstrapped standardized β coefficients and p-values for the interaction term (Ʃ taste * correlates of depression) from moderation analyses are shown. SCARED (generalized anxiety), BIG-5 [N] (neuroticism), APEQ (picky eating), and BIDA (body dissatisfaction).
4. Discussion
In this study, we investigated how gender influences the associations between sensory perception, depression, and diet during adolescence. Given the differing vulnerabilities between girls and boys, e.g., [7], we further examined whether common comorbidities of depression moderated the impact of sensory perception on dietary choices. Our findings first confirmed that girls are more susceptible to mood disturbances and revealed that acuity for bitterness and astringency is positively associated with depressive symptoms exclusively in this group. Additionally, we corroborated known associations between depression and poor dietary habits and found that higher levels of internalizing symptoms in girls exacerbated the inverse relationship between heightened responsiveness to alarming oral sensations and the intake of several nutrients.
4.1. Adolescent Girls Exhibit Higher Levels of Internalizing Symptoms than Boys
The concerning global trends in the prevalence of depression among adolescents, e.g., [3,7], were also evident in our sample. Using a PHQ-9 score ≥ 10 [46] as a sensitive (85%) and specific (89%) indicator of early major depressive disorders [66], 21.6% of adolescents exceeded this threshold. This finding is consistent with recent data from similar regions, where 16% of European [3] and 20.8% of Italian adolescents [81] are currently at risk of developing clinical depression. Moreover, we corroborated established gender differences in susceptibility to internalizing symptoms. In our sample, girls reported depressive symptoms at a rate three times higher than boys (36.1% vs. 11.1%), thus slightly surpassing the 2017–2018 estimates (28.7% vs. 10.4%) from a larger Italian sample [81] and underscoring the growing incidence of mental health difficulties among adolescents [3,7].
It was therefore unsurprising to observe girls exhibiting higher levels of common comorbid traits of depression, such as generalized anxiety, neuroticism, and picky eating [7,33,42,43]. However, interpreting the latter finding is less straightforward. The literature on variations in the engagement of picky eating behaviors by gender is sparse and inconsistent, often focusing on younger children and varying in assessment tools and definitions of picky eating itself [82]. For instance, Marchi and Cohen [83] found that pickiness was more common in girls (n = 326) than in boys (n = 333) across the ages of 1–10, 9–18, and 11–21 over a 10-year follow-up. In contrast, no significant gender differences were found in other cross-sectional [84] or prospective [85] studies.
Despite this mixed evidence, it is reasonable to speculate that our findings reflect underlying gender differences in domains of anxiety, which have been reported to prompt selective eating behaviors, e.g., [33]. This is further supported by systematic gender disparities observed in the links between pickiness and domains of anxiety within our sample, with stronger associations in girls for generalized (girls: ρ = 0.334, p < 0.001; boys: ρ = 0.205, p = 0.017), social (girls: ρ = 0.313, p = 0.002; boys: ρ = 0.193, p = 0.025), and school anxiety (girls: ρ = 0.371, p < 0.001; boys: ρ = 0.205, p = 0.017). However, further research is needed to confirm or refute this hypothesis within diverse nonclinical adolescent populations.
Surprisingly, our data also revealed no gender variations in overall body dissatisfaction. This may tentatively be attributed to the multifaceted nature of the BIDA total score, which assesses various aspects of body dissatisfaction. Indeed, girls felt their actual body shape was more rounded compared to their ideal figure, whereas boys perceived their physique to be leaner than what they viewed as most attractive to the opposite sex (Figure S7). Hence, while our findings contrast with those of Sánchez-Miguel et al. [61], they align with the current understanding that weight concerns and the pursuit of muscularity are key contributors of body dissatisfaction for girls and boys, respectively (for review, see [86]).
4.2. Greater Responsiveness to Bitterness and Astringency Is Associated with Higher Severity of Depressive Symptoms in Adolescent Girls, but Not in Boys
We found no main effects of sensory responsiveness on depressive symptoms, thus suggesting that taste per se is not a relevant predictor of depressive symptoms in healthy adolescents. While comparisons with the existing literature should be made cautiously due to differing methods for assessing taste function that may not be correlated [40], our result aligns with prior research involving nonclinical adults showing no associations between depression and thresholds for sweet, sour, bitter, or salty tastes [35], intensity ratings of chocolate and vanilla milks [35], or recalled saltiness intensities for 10 food items [36]. However, it contrasts with studies observing either positive [31,34] or negative [37] associations between depressive symptoms and intensity ratings for sweet and bitter-tasting aqueous solutions.
Nonetheless, we provide evidence that sensory perception may differentially predict mood disturbances by gender. Specifically, responsiveness to bitterness and astringency was associated with the severity of depressive symptoms exclusively in girls, an effect observed in both the crude and adjusted models. This finding is somewhat consistent with a Japanese work involving young adults (n = 70, mean age ± SD = 20.7 ± 0.3 y) that explored gender- and menstrual-cycle-related associations between internalizing symptoms (depression, anxiety) and sweet taste recognition thresholds [87]. In that study, depression was positively correlated with sweet taste sensitivity (i.e., decreased thresholds) uniquely in women during the luteal phase, with no correlations detected in men or in women during the follicular phase.
However, a similarity with the findings of Nagai et al. [87] exists in the gender-specific association between a high responsiveness to oral stimuli and the severity of internalizing symptoms. Intriguingly, the luteal phase group was the sole group to show a positive correlation between sweet sensitivity and anxiety, which was independent of baseline differences in anxiety between genders or menstrual cycle groups [87]. While the authors hypothesized that this pattern might be attributed to higher levels of luteinizing hormones [87], we can further speculate that the predictive value of sensory perception regarding depressive symptoms might become more evident when comparing individuals experiencing distinct physiological states and/or possessing varying vulnerabilities to psychological constructs that predispose them to heightened affective responses [88,89].
Although the menstrual cycle was not considered in our study, we still lend support to this notion through three key points. First, adolescent girls exhibited higher anxiety-related traits relative to boys. Second, putative physiological influences on findings can be reasonably ruled out, as no gender differences were observed in either PROP acuity (Figure S8) or global responsiveness to oral sensations (Figure S9). Lastly, external cues are unlikely to fully account for the observed differences, as both genders had similar demographic and lifestyle backgrounds (Table S1), were equally familiar (Food Familiarity Questionnaire, Figure 1) with grapefruit juice (W = 6158, p = 0.423) and dark chocolate (W = 6051, p = 0.294), and reported similar hedonic ratings for all variants of Gj and Cp, except for the highest sucrose concentration in both food models (Table S1).
4.3. Comorbidities of Depression Moderate the Links Between Sensory Perception and Dietary Habits with Long-Term Adverse Effects on Mood in Adolescent Girls
Consistent with the existing literature, e.g., [10,11,12,13], we noted that various dietary outcomes were inversely correlated with PHQ-9 scores, with this relationship being more pronounced in girls. Specifically, alcohol use combined with low intake of proteins, vegetable fats, oleic acid, and vitamin E was associated with depressive symptoms in both genders. Similar patterns, though not statistically significant in boys, were observed with low daily consumption of animal proteins, mono- and polyunsaturated fats, linoleic acid, B vitamins (B1, B6, B9), iron, phosphorus, and zinc.
Several mechanisms have been proposed to explain how inadequate intake of these nutrients might exacerbate depression, and vice versa. Depression may prompt unhealthy eating habits, and nutrient deficiencies may impair brain function by disrupting the synthesis and activity of monoamines (serotonin, norepinephrine, dopamine) or by increasing oxidative stress, both of which are thought to be involved in the pathophysiology of depression (for review, see [14]). For example, amino acids from both animal and plant proteins are key for monoamine production, while omega-3 fatty acids (e.g., linolenic acid) enhance serotonin function and are believed to counteract oxidative stress through their potent anti-inflammatory effects. Similarly, B-group vitamins and iron support neurotransmitter metabolism, and zinc is essential for the optimal functioning of brain regions involved in mood regulation and cognitive functions [10]. Consequently, it is not surprising that dietary supplementation with nutrients involved in the homeostasis of brain functions has frequently led to significant improvements in depressive symptoms across diverse adolescent populations [10]. Our findings thus reinforce the substantial evidence supporting a mutual link between adequate nutrition and mental health during adolescence.
In this context, we observed that comorbidities of depression exacerbated the negative relationships between acuity for alarming oral stimuli and the intake of various nutrients associated with higher PHQ-9 scores, particularly among girls. Importantly, the overall direction of these effects was consistent across genders and aligned with current knowledge, e.g., [15,90], with greater responsiveness generally serving as a barrier to consuming beneficial nutrients that also support mood regulation (Tables S6–S9). However, in models accounting for the moderating effects of correlates of depression, significant associations emerged almost exclusively in girls. This suggests that other biological, non-biological, or psychosocial variables may better explain how variations in perception of alarming oral sensations affect dietary habits in adolescent boys, warranting further investigation.
Conversely, we can tentatively discuss why depression-related states or traits might strengthen the association between sensory perception and suboptimal dietary choices in girls. Alarming oral sensations, which signal potentially aversive post-ingestive effects, naturally elicit negative arousal. Even minor stimulation can trigger a cascade of psychobiological responses, which individuals may cope with differently before deciding whether to accept or reject the stimulus. Beyond intensity, the complexity and novelty of sensory stimuli also contribute to arousal, with responses shaped by expectations about the upcoming experience. A discrepancy between expected and perceived sensations may therefore lead to exaggerated attention on specific product cues, thereby intensifying negative emotional states [39]. This might explain why the associations between bitterness in Cp and diet were more pronounced than those observed in Gj. It is possible that participants had well-formed expectations about the taste qualities of both food models, with bitterness being more anticipated in Gj than in Cp. Therefore, a mismatch between expected and actual taste might have evoked negative arousal, which was possibly further promoted by participants’ greater familiarity with chocolate puddings (W = 838, p < 0.001). This, in turn, would have magnified the discrepancy between anxiety-related traits and highlighted stronger moderating effects. However, further studies are needed to confirm this hypothesis.
Alternatively, it is noteworthy that a higher vulnerability to internalizing symptoms often reflects a generalized hyperresponsiveness to environmental inputs and stressors, such as noise, tactile stimuli [91], pain [92] or danger-signaling smells [93]. While this trend is observed in both girls and boys, notable gender differences in coping strategies for stress exist [89]. Generally speaking, girls are more likely to notice and report even subtle emotional changes, while boys tend to exhibit more passive behaviors that shift toward a more feminine coping style only once signs of distress become evident [89,94]. Such heightened reactivity to stressors might thus translate into intensified attentional bias and negative affective states in response to negatively arousing stimuli, thereby reinforcing avoidant behaviors when alarming oral sensations are experienced in food. Conversely, a similar pattern might only emerge in boys when depression-related states or traits and extremely intense arousing sensations occur concurrently. To either confirm or refute this hypothesis, future studies with adolescent populations exhibiting similar levels of psychosocial correlates of depression among genders and exposed to food stimuli that combine multiple sources of arousal are necessary.
4.4. Strengths and Limitations
To our knowledge, this study is the first to examine the associations between depressive symptoms, taste perception, and dietary habits in a nonclinical adolescent population. Its key strengths include the use of one of the largest samples ever employed in this line of research, a thorough data collection protocol, and the use of real food models to assess a range of oral sensations at intensities that closely mirror real-life experiences. In response to calls for novel research on the role of taste in adolescent food choices [24], we also extend the existing literature by testing previously overlooked oral sensations (sourness, astringency). Furthermore, this work presents the first empirical evidence linking astringency perception with depressive symptoms in healthy adolescent girls. Lastly, we provide preliminary support for the psychometric validity of the APEQ and the BIDA for use with Italian adolescents, which have the potential to offer deeper insights into the determinants of eating habits in this age group, pending their comprehensive psychometric validation.
Nevertheless, our results should also be interpreted in light of several limitations. Firstly, while we employed ecologically valid food stimuli and sensory assessments, we did not test other relevant tastes (salty, umami) or chemesthetic sensations (e.g., pungency) that may influence adolescents’ dietary habits. Additionally, our focus was on a limited set of food models, which elicited a narrow range of sensory qualities typically encountered in daily experiences. Whether the results can be replicated with a broader variety of foods differing in sensory, chemical, and physical properties should thus be probed in future investigations.
Secondly, the dietary data were based on self-reports, which can be subject to motivational and recall biases, as well as under- or over-reporting practices [95]. Despite meticulous data preprocessing to minimize these biases and a strong alignment with existing knowledge, the potential for inaccuracies remains. In order to enhance the reliability of data, future research should incorporate more precise and less burdensome approaches, such as technological aids that facilitate memory and portion estimation, ideally within repeated measures frameworks. In this vein, the integration of 24-hour recalls with digital tools (e.g., mobile apps, image-based assessments) holds promise in mitigating prevalent sources of error in adolescent populations.
Thirdly, the gender-based sensitivity analysis may have lacked sufficient statistical power to detect subtle yet meaningful effects, particularly given the relatively modest variance explained by the moderation models (Tables S6–S9). Besides suggesting that other factors, including genetic, non-genetic (e.g., oral microbiome), and psychosocial variables not included in this study, might further elucidate how taste perception influences dietary outcomes and potentially affects mood in the long term, this prompts the need to devote future efforts to include larger and adequately powered samples.
Fourthly, the internalizing symptoms investigated herein are inherently interrelated and might have acted as confounding or overlapping variables in the observed associations. Case–control studies or novel research involving non-clinically gender-stratified groups that differ in key psychosocial traits will be instrumental in further validating and extending our findings. Lastly, due to the study’s design, causality can not be inferred. Novel longitudinal studies are thus needed to determine whether taste perception indirectly exacerbates depressive symptoms through inadequate food choices or whether the opposite is true.
4.5. Conclusions
In addition to confirming a greater susceptibility to mood disturbances in girls compared to boys, along with associations between internalizing symptoms and habitual nutrient intake, this study methodologically contributes to expanding knowledge on picky eating and body image in Italian adolescents by supporting the initial psychometric validity of tools currently used across different age groups (APEQ) or geographic contexts (BIDA). More importantly, we present the first evidence linking heightened responsiveness to bitterness and astringency, evoked by variants of food models designed to mimic a range of intensities encountered in daily food experiences, with greater severity of depressive symptoms exclusively in girls. While our findings require replication across a broader range of food stimuli, oral sensations, adolescent populations, and longitudinal frameworks, they remain highly relevant in supporting future gender-tailored, sensory-based strategies aimed at promoting adherence to dietary patterns that may confer long-term benefits for mental health. In conclusion, this study advances our understanding of gender-specific vulnerabilities to mood disturbances during adolescence and suggests that the links between depressive symptoms, taste perception, and dietary habits are gender-specific, possibly shaped by differing coping strategies for internalizing symptoms.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu17101653/s1, Figure S1: Sensory profiles of variants for each food model (Gj, Cp) evaluated in Pilot 1 and Pilot 2; Figure S2: Variations in psychophysical responses (gLMS) to oral sensations evoked by Gj and Cp variants across increasing sucrose concentrations; Figure S3: Differences in recalled intensities between the extraoral stimuli employed during the gLMS training; Figure S4: Girls and boys rated the ten extraoral stimuli employed during the gLMS training as equally intense; Figure S5: Correlations between the APEQ total score and both theoretically similar and dissimilar constructs; Figure S6: Correlations between the BIDA total and subscale scores and theoretically similar and dissimilar factors; Figure S7: Differences in body dissatisfaction and sexual body dissatisfaction between girls and boys; Figure S8: Variations in PROP responsiveness between genders; Figure S9: Differences in global sensory acuity by gender; Figure S10: Differences between genders in liking for all variants of grapefruit juice and dark chocolate pudding; Table S1: Demographic, lifestyle, and diet-related characteristics of participants; Table S2: Inter-item correlations between subscale scores of the Body Image Dimensional Assessment (BIDA); Table S3: Demographic, lifestyle, and diet-related characteristics of participants whose FFQ data passed the screening for misreporting and outliers; Table S4: Variance Inflation Factor and results from the Durbin-Watson test for the unadjusted general linear models assessing whether the relationship between global taste scores and depressive symptoms varies by gender; Table S5: Variance Inflation Factor and results from the Durbin-Watson test for the adjusted general linear models assessing whether the relationship between global scores of sensory responsiveness and depressive symptoms varies by gender; Table S6: Moderating effects of generalized anxiety on the link between global responsiveness to alarming oral sensations and habitual nutrient intake, as a function of gender; Table S7: Moderating effects of neuroticism on the link between global responsiveness to alarming oral sensations and habitual nutrient intake, as a function of gender; Table S8: Moderating effects of picky eating on the link between global responsiveness to alarming oral sensations and habitual nutrient intake, as a function of gender; Table S9: Moderating effects of body dissatisfaction on the link between global responsiveness to alarming oral sensations and habitual nutrient intake, as a function of gender.
Author Contributions
Conceptualization, L.M., L.F., S.C., I.E., M.P.C., P.G. and F.G.; Data curation, L.M.; Formal analysis, L.M.; Funding acquisition, L.M., P.G. and F.G.; Investigation, L.M., L.F., S.C., I.E. and F.G.; Methodology, L.M., L.F., I.E. and F.G.; Project administration, F.G.; Software, L.M.; Supervision, F.G.; Validation, L.M.; Visualization, L.M.; Writing—original draft, L.M.; Writing—review and editing, L.M., L.F., S.C., I.E., M.P.C., P.G. and F.G. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge the support of the MUR PNRR project iNEST—Interconnected Nord-Est Innovation Ecosystem (ECS00000043), funded by the European Union under NextGenerationEU. In addition, we recognize the funding provided by the PROmedLIFE project—Novel food products for the promotion of a Mediterranean lifestyle and healthy diet—grant agreement nr. 2132 (PRIMA programme supported by the European Union) for the support given to Lara Fontana.
Institutional Review Board Statement
Ethical approval for the involvement of human subjects in this study was granted by the Research Ethics Committee of the University of Trento, Reference number 2023-047, 28 September 2023.
Informed Consent Statement
Written informed consent was obtained from parents or legal guardians of participants, while written assent was secured from all adolescents involved in this study.
Data Availability Statement
Data are available from the corresponding author upon reasonable requests.
Acknowledgments
We extend our sincere thanks to all the volunteers and their families who participated in this study. Special thanks go to Luana Budano and the teachers from Istituto Tecnico Agrario, San Michele all’Adige (Trento, Italy), as well as to Caterina Bonapace and Federico Andermarcher from Istituto Martino Martini, Mezzolombardo (Trento, Italy), for their invaluable assistance in optimizing logistics throughout the project phases. We also wish to express our gratitude to Martina Moretton and Jessica Zambanini (Edmund Mach Foundation, San Michele all’Adige, Trento, Italy) for their significant contribution during data collection. Furthermore, we greatly appreciate the support of Sabina Sieri and Chiara Roncallo (Istituto Nazionale dei Tumori, Milan) for their indispensable help in processing the EPIC-FFQ.
Conflicts of Interest
The authors declare no conflicts of interest.
Disclaimer
During the preparation of this work the authors used ChatGPT (version 4o mini) in order to improve grammar and readability of the manuscript. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Appendix A
In this work, we only used the APEQ total score due to our previous validation being specific to adults over 18 [55] and the lack of validated measures for picky eating in Italian adolescents. Hence, to preliminarily assess its suitability for this age group, we tested the APEQ score for internal reliability (Cronbach’s α), convergent and discriminant validity (Spearman’s rho coefficients), and for test-retest reliability (Intra-Class Coefficients, ICC). Overall, we found good internal consistency (α = 0.808), adequate evidence of convergent or discriminant validity (Figure S5), and test-retest reliability. In line with previous reports, e.g., [33,55,69,70], the APEQ total score was positively correlated (p < 0.001) with theoretically similar states or traits such as depressive symptoms, domains of anxiety, food neophobia, and neuroticism, while showing no significant correlations with either BMI (ρ = 0.012, p = 0.861) or age (ρ = 0.014, p = 0.829). Additionally, several inverse correlations between picky eating and familiarity with plant-based foods (Figure S5) further supported the discriminant validity of the APEQ. Finally, the APEQ total score exhibited good test-retest reliability (ICC = 0.751; 95% CI: 0.669–0.813, p < 0.001) when reassessed in a subset of our sample (n = 190, 34.2% girls, mean age ± SD = 14.4 ± 0.64 y, mean BMI ± SD = 21.1 ± 3.2 kg/m2) 30 days after data collection.
Appendix B
Although the BIDA has been validated only for Spanish adolescents (ages 12–15) [61], it was still favored over the recently validated 8-item BIBA in the Italian context [71], as the latter was designed for children and early adolescents (ages 6–13). Despite this, the BIDA showed preliminary psychometric soundness, as evidenced by (a) a good internal consistency (α = 0.763); (b) an acceptable 30-day test-retest reliability (n = 190, ICC = 0.557, 95% CI: 0.410–0.668, p < 0.001); (c) strong inter-item correlations (ρ > 0.500, p < 0.001) (Table S2); and (d) an initial proof of its convergent and divergent validity (Figure S6). Particularly, convergent validity was supported by positive correlations between the BIDA composite score and its subscales with BMI [61] and anxiety domains, while divergent validity was corroborated by a negative association between the BIDA total score and engagement in physical activity [96] (Figure S6).
References
- World Health Organization. Depressive Disorder (Depression). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 19 February 2025).
- World Health Organization. Mental Health of Adolescents. Available online: https://www.who.int/news-room/fact-sheets/detail/adolescent-mental-health (accessed on 24 July 2024).
- Shorey, S.; Ng, E.D.; Wong, C.H.J. Global Prevalence of Depression and Elevated Depressive Symptoms among Adolescents: A Systematic Review and Meta-analysis. Br. J. Clin. Psychol. 2022, 61, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Racine, N.; McArthur, B.A.; Cooke, J.E.; Eirich, R.; Zhu, J.; Madigan, S. Global Prevalence of Depressive and Anxiety Symptoms in Children and Adolescents During COVID-19. JAMA Pediatr. 2021, 175, 1142. [Google Scholar] [CrossRef] [PubMed]
- Essau, C.A.; Lewinsohn, P.M.; Seeley, J.R.; Sasagawa, S. Gender Differences in the Developmental Course of Depression. J. Affect. Disord. 2010, 127, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Hankin, B.L.; Abramson, L.Y.; Moffitt, T.E.; Silva, P.A.; McGee, R.; Angell, K.E. Development of Depression from Preadolescence to Young Adulthood: Emerging Gender Differences in a 10-Year Longitudinal Study. J. Abnorm. Psychol. 1998, 107, 128–140. [Google Scholar] [CrossRef]
- Keyes, K.M.; Platt, J.M. Annual Research Review: Sex, Gender, and Internalizing Conditions among Adolescents in the 21st Century—Trends, Causes, Consequences. J. Child Psychol. Psychiatry 2024, 65, 384–407. [Google Scholar] [CrossRef]
- Caspi, A.; Houts, R.M.; Ambler, A.; Danese, A.; Elliott, M.L.; Hariri, A.; Harrington, H.L.; Hogan, S.; Poulton, R.; Ramrakha, S.; et al. Longitudinal Assessment of Mental Health Disorders and Comorbidities Across 4 Decades Among Participants in the Dunedin Birth Cohort Study. JAMA Netw. Open 2020, 3, e203221. [Google Scholar] [CrossRef]
- Ghafari, M.; Nadi, T.; Bahadivand-Chegini, S.; Doosti-Irani, A. Global Prevalence of Unmet Need for Mental Health Care among Adolescents: A Systematic Review and Meta-Analysis. Arch. Psychiatr. Nurs. 2022, 36, 1–6. [Google Scholar] [CrossRef]
- Chopra, C.; Mandalika, S.; Kinger, N. Does Diet Play a Role in the Prevention and Management of Depression among Adolescents? A Narrative Review. Nutr. Health 2021, 27, 243–263. [Google Scholar] [CrossRef]
- Oddy, W.H.; Hickling, S.; Smith, M.A.; O’Sullivan, T.A.; Robinson, M.; de Klerk, N.H.; Beilin, L.J.; Mori, T.A.; Syrette, J.; Zubrick, S.R.; et al. Dietary Intake of Omega-3 Fatty Acids and Risk of Depressive Symptoms in Adolescents. Depress. Anxiety 2011, 28, 582–588. [Google Scholar] [CrossRef]
- Sinclair, R.; Millar, L.; Allender, S.; Snowdon, W.; Waqa, G.; Jacka, F.; Moodie, M.; Petersen, S.; Swinburn, B. The Cross-Sectional Association between Diet Quality and Depressive Symptomology amongst Fijian Adolescents. PLoS ONE 2016, 11, e0161709. [Google Scholar] [CrossRef]
- Huang, P.; O’Keeffe, M.; Elia, C.; Karamanos, A.; Goff, L.M.; Maynard, M.; Cruickshank, J.K.; Harding, S. Fruit and Vegetable Consumption and Mental Health across Adolescence: Evidence from a Diverse Urban British Cohort Study. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Pano, O.; Martínez-Lapiscina, E.H.; Sayón-Orea, C.; Martinez-Gonzalez, M.A.; Martinez, J.A.; Sanchez-Villegas, A. Healthy Diet, Depression and Quality of Life: A Narrative Review of Biological Mechanisms and Primary Prevention Opportunities. World J. Psychiatry 2021, 11, 997–1016. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.N.; O’Sullivan, E.J.; Kearney, J.M. Considerations for Health and Food Choice in Adolescents. Proc. Nutr. Soc. 2022, 81, 75–86. [Google Scholar] [CrossRef]
- Drewnowski, A.; Mennella, J.A.; Johnson, S.L.; Bellisle, F. Sweetness and Food Preference. J. Nutr. 2012, 142, 1142S–1148S. [Google Scholar] [CrossRef] [PubMed]
- Köster, E.P. Diversity in the Determinants of Food Choice: A Psychological Perspective. Food Qual. Prefer. 2009, 20, 70–82. [Google Scholar] [CrossRef]
- Diószegi, J.; Llanaj, E.; Ádány, R. Genetic Background of Taste Perception, Taste Preferences, and Its Nutritional Implications: A Systematic Review. Front. Genet. 2019, 10, 1272. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Miller, I.J. PTC/PROP Tasting: Anatomy, Psychophysics, and Sex Effects. Physiol. Behav. 1994, 56, 1165–1171. [Google Scholar] [CrossRef]
- Feeney, E.L.; O’Brien, S.A.; Scannell, A.G.M.; Markey, A.; Gibney, E.R. Genetic and Environmental Influences on Liking and Reported Intakes of Vegetables in Irish Children. Food Qual. Prefer. 2014, 32, 253–263. [Google Scholar] [CrossRef]
- Borazon, E.Q.; Villarino, B.J.; Magbuhat, R.M.T.; Sabandal, M.L. Relationship of PROP (6-n-Propylthiouracil) Taster Status with Body Mass Index, Food Preferences, and Consumption of Filipino Adolescents. Food Res. Int. 2012, 47, 229–235. [Google Scholar] [CrossRef]
- Joseph, P.V.; Reed, D.R.; Mennella, J.A. Individual Differences Among Children in Sucrose Detection Thresholds. Nurs. Res. 2016, 65, 3–12. [Google Scholar] [CrossRef]
- Baranowski, T.; Baranowski, J.C.; Watson, K.B.; Jago, R.; Islam, N.; Beltran, A.; Martin, S.J.; Nguyen, N.; Tepper, B.J. 6-n-Propylthiouracil Taster Status Not Related to Reported Cruciferous Vegetable Intake among Ethnically Diverse Children. Nutr. Res. 2011, 31, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Bawajeeh, A.O.; Albar, S.A.; Zhang, H.; Zulyniak, M.A.; Evans, C.E.L.; Cade, J.E. Impact of Taste on Food Choices in Adolescence—Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1985. [Google Scholar] [CrossRef] [PubMed]
- Barragán, R.; Coltell, O.; Portolés, O.; Asensio, E.M.; Sorlí, J.V.; Ortega-Azorín, C.; González, J.I.; Sáiz, C.; Fernández-Carrión, R.; Ordovas, J.M.; et al. Bitter, Sweet, Salty, Sour and Umami Taste Perception Decreases with Age: Sex-Specific Analysis, Modulation by Genetic Variants and Taste-Preference Associations in 18 to 80 Year-Old Subjects. Nutrients 2018, 10, 1539. [Google Scholar] [CrossRef]
- Overberg, J.; Hummel, T.; Krude, H.; Wiegand, S. Differences in Taste Sensitivity between Obese and Non-Obese Children and Adolescents. Arch. Dis. Child. 2012, 97, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Spence, C. Do Men and Women Really Live in Different Taste Worlds? Food Qual. Prefer. 2019, 73, 38–45. [Google Scholar] [CrossRef]
- Spence, C. What Is the Link between Personality and Food Behavior? Curr. Res. Food Sci. 2022, 5, 19–27. [Google Scholar] [CrossRef]
- Ileri-Gurel, E.; Pehlivanoglu, B.; Dogan, M. Effect of Acute Stress on Taste Perception: In Relation with Baseline Anxiety Level and Body Weight. Chem. Senses 2013, 38, 27–34. [Google Scholar] [CrossRef]
- Dess, N.K.; Edelheit, D. The Bitter with the Sweet: The Taste/Stress/Temperament Nexus. Biol. Psychol. 1998, 48, 103–119. [Google Scholar] [CrossRef]
- Platte, P.; Herbert, C.; Pauli, P.; Breslin, P.A.S. Oral Perceptions of Fat and Taste Stimuli Are Modulated by Affect and Mood Induction. PLoS ONE 2013, 8, e65006. [Google Scholar] [CrossRef]
- Spinelli, S.; Pierguidi, L.; Gavazzi, G.; Dinnella, C.; De Toffoli, A.; Prescott, J.; Monteleone, E. Skin Conductance Responses to Oral Stimuli: The Role of Taste Quality and Intensity, and Personality Traits. Food Qual. Prefer. 2023, 109, 104917. [Google Scholar] [CrossRef]
- Kauer, J.; Pelchat, M.L.; Rozin, P.; Zickgraf, H.F. Adult Picky Eating. Phenomenology, Taste Sensitivity, and Psychological Correlates. Appetite 2015, 90, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Dess, N.K.; Chapman, C.D. Individual Differences in Taste, Body Weight, and Depression in the “Helplessness” Rat Model and in Humans. Brain Res. Bull. 1990, 24, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Scinska, A.; Sienkiewicz-Jarosz, H.; Kuran, W.; Ryglewicz, D.; Rogowski, A.; Wrobel, E.; Korkosz, A.; Kukwa, A.; Kostowski, W.; Bienkowski, P. Depressive Symptoms and Taste Reactivity in Humans. Physiol. Behav. 2004, 82, 899–904. [Google Scholar] [CrossRef]
- Ferraris, C.; Scarlett, C.J.; Bucher, T.; Beckett, E.L. Liking of Salt Is Associated with Depression, Anxiety, and Stress. Chem. Senses 2023, 48, 1–13. [Google Scholar] [CrossRef]
- Al’absi, M.; Nakajima, M.; Hooker, S.; Wittmers, L.; Cragin, T. Exposure to Acute Stress Is Associated with Attenuated Sweet Taste. Psychophysiology 2012, 49, 96–103. [Google Scholar] [CrossRef]
- Berlyne, D.E. Arousal and Reinforcement. In Nebraska Symposium on Motivation; University of Nebraska Press: Lincoln, NE, USA, 1967. [Google Scholar]
- Prescott, J.; Spinelli, S. Arousal and the Modulation of Sensory Experience: Evidence from Food-Related Emotions. Philos. Trans. R. Soc. B Biol. Sci. 2024, 379, 20230255. [Google Scholar] [CrossRef]
- Webb, J.; Bolhuis, D.P.; Cicerale, S.; Hayes, J.E.; Keast, R. The Relationships Between Common Measurements of Taste Function. Chemosens. Percept. 2015, 8, 11–18. [Google Scholar] [CrossRef]
- Ervina, E.; Almli, V.L.; Berget, I.; Spinelli, S.; Sick, J.; Dinnella, C. Does Responsiveness to Basic Tastes Influence Preadolescents’ Food Liking? Investigating Taste Responsiveness Segment on Bitter-Sour-Sweet and Salty-Umami Model Food Samples. Nutrients 2021, 13, 2721. [Google Scholar] [CrossRef]
- Cummings, C.M.; Caporino, N.E.; Kendall, P.C. Comorbidity of Anxiety and Depression in Children and Adolescents: 20 Years After. Psychol. Bull. 2014, 140, 816. [Google Scholar] [CrossRef]
- Kuyken, W.; Watkins, E.; Holden, E.; Cook, W. Rumination in Adolescents at Risk for Depression. J. Affect. Disord. 2006, 96, 39–47. [Google Scholar] [CrossRef]
- Goldfield, G.S.; Moore, C.; Henderson, K.; Buchholz, A.; Obeid, N.; Flament, M.F. Body Dissatisfaction, Dietary Restraint, Depression, and Weight Status in Adolescents. J. Sch. Health 2010, 80, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, I.; Miglio, R.; Mistura, L.; Grioni, S.; Pozzebon, I.; Odorifero, C.; Borea, R.; Gitto, A.; Terrafino, M.; Scipioni, M. Relative Validity of an Italian EPIC Food Frequency Questionnaire for Dietary Factors in Children and Adolescents. A Rizzoli Orthopedic Institute Study. Nutrients 2021, 13, 1245. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Menghi, L.; Cliceri, D.; Fava, F.; Pindo, M.; Gaudioso, G.; Giacalone, D.; Gasperi, F. Salivary Microbial Profiles Associate with Responsiveness to Warning Oral Sensations and Dietary Intakes. Food Res. Int. 2023, 171, 113072. [Google Scholar] [CrossRef]
- Champely, S. pwr: Basic Functions for Power Analysis; R package Version 1.3-0; Stephane Champely: Lyon, France, 2020; Volume 1. [Google Scholar]
- ISO 8589; Sensory analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: Genève, Switzerland, 2007.
- De Backer, C.J.S.; Hudders, L. Meat Morals: Relationship between Meat Consumption Consumer Attitudes towards Human and Animal Welfare and Moral Behavior. Meat Sci. 2015, 99, 68–74. [Google Scholar] [CrossRef]
- Mannocci, A.; Di Thiene, D.; Del Cimmuto, A.; Masala, D.; Boccia, A.; De Vito, E. International Physical Activity Questionnaire: Validation and Assessment in an Italian Sample. Ital. J. Public Health 2010, 7, 369–376. [Google Scholar] [CrossRef]
- Buunk-Werkhoven, Y.A.B.; Dijkstra, A.; Van Der Schans, C.P. Determinants of Oral Hygiene Behavior: A Study Based on the Theory of Planned Behavior. Community Dent. Oral Epidemiol. 2011, 39, 250–259. [Google Scholar] [CrossRef]
- Monteleone, E.; Spinelli, S.; Dinnella, C.; Endrizzi, I.; Laureati, M.; Pagliarini, E.; Sinesio, F.; Gasperi, F.; Torri, L.; Aprea, E.; et al. Exploring Influences on Food Choice in a Large Population Sample: The Italian Taste Project. Food Qual. Prefer. 2017, 59, 123–140. [Google Scholar] [CrossRef]
- Laureati, M.; Spinelli, S.; Monteleone, E.; Dinnella, C.; Prescott, J.; Cattaneo, C.; Proserpio, C.; De Toffoli, A.; Gasperi, F.; Endrizzi, I.; et al. Associations between Food Neophobia and Responsiveness to “Warning” Chemosensory Sensations in Food Products in a Large Population Sample. Food Qual. Prefer. 2018, 68, 113–124. [Google Scholar] [CrossRef]
- Menghi, L.; Endrizzi, I.; Cliceri, D.; Zampini, M.; Giacalone, D.; Gasperi, F. Validating the Italian Version of the Adult Picky Eating Questionnaire. Food Qual. Prefer. 2022, 101, 104647. [Google Scholar] [CrossRef]
- Smorti, M.; Guarnieri, S. A Study on the Validity of the Arnett Inventory of Sensation Seeking (AISS) in an Italian Adolescent Sample. Int. J. Adv. Psychol. 2013, 2, 10–18. [Google Scholar]
- Schutz, H.G.; Cardello, A.V. A Labeled Affective Magnitude (LAM) Scale for Assessing Food Liking/Disliking. J. Sens. Stud. 2001, 16, 117–159. [Google Scholar] [CrossRef]
- Klimstra, T.A.; Crocetti, E.; Hale, W.W., III; Fermani, A.; Meeus, W.H.J. Big Five Personality Dimensions in Italian and Dutch Adolescents: A Cross-Cultural Comparison of Mean-Levels, Sex Differences, and Associations with Internalizing Symptoms. J. Res. Pers. 2011, 45, 285–296. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Green, B.G.; Hoffman, H.J.; Ko, C.W.; Lucchina, L.A.; Marks, L.E.; Snyder, D.J.; Weiffenbach, J.M. Valid Across-Group Comparisons with Labeled Scales: The GLMS versus Magnitude Matching. Physiol. Behav. 2004, 82, 109–114. [Google Scholar] [CrossRef]
- Crocetti, E.; Hale, W.W.; Fermani, A.; Raaijmakers, Q.; Meeus, W. Psychometric Properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED) in the General Italian Adolescent Population: A Validation and a Comparison between Italy and The Netherlands. J. Anxiety Disord. 2009, 23, 824–829. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Miguel, P.A.; Vaquero-Solís, M.; Sánchez-Oliva, D.; Pulido-González, J.J.; Segura-García, C.; Tapia-Serrano, M.A. Validation of the Body Image Dimensional Assessment in Adolescents from Spanish High School. Eat. Weight. Disord. 2021, 26, 1749–1756. [Google Scholar] [CrossRef]
- Hayes, J.E.; Allen, A.L.; Bennett, S.M. Direct Comparison of the Generalized Visual Analog Scale (GVAS) and General Labeled Magnitude Scale (GLMS). Food Qual. Prefer. 2013, 28, 36–44. [Google Scholar] [CrossRef]
- Menghi, L.; Cliceri, D.; Fava, F.; Pindo, M.; Gaudioso, G.; Stefani, E.; Giacalone, D.; Gasperi, F. Variations in Oral Responsiveness Associate with Specific Signatures in the Gut Microbiota and Modulate Dietary Habits. Food Qual. Prefer. 2023, 106, 104790. [Google Scholar] [CrossRef]
- Graziano, S.; Spanò, B.; Majo, F.; Righelli, D.; Vincenzina, L.; Quittner, A.; Tabarini, P. Rates of Depression and Anxiety in Italian Patients with Cystic Fibrosis and Parent Caregivers: Implementation of the Mental Health Guidelines. Respir. Med. 2020, 172, 106147. [Google Scholar] [CrossRef]
- Correale, C.; Falamesca, C.; Tondo, I.; Borgi, M.; Cirulli, F.; Truglio, M.; Papa, O.; Vagnoli, L.; Arzilli, C.; Venturino, C.; et al. Depressive Anxiety Symptoms in Hospitalized Children with Chronic Illness during the First Italian COVID-19 Lockdown. Children 2022, 9, 1156. [Google Scholar] [CrossRef]
- Manea, L.; Gilbody, S.; McMillan, D. Optimal Cut-off Score for Diagnosing Depression with the Patient Health Questionnaire (PHQ-9): A Meta-Analysis. Can. Med. Assoc. J. 2012, 184, E191–E196. [Google Scholar] [CrossRef] [PubMed]
- Birmaher, B.; Khetarpal, S.; Brent, D.; Cully, M.; Balach, L.; Kaufman, J.; Neer, S.M.K. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale Construction and Psychometric Characteristics. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, L.R. The Development of Markers for the Big-Five Factor Structure. Psychol. Assess. 1992, 4, 26. [Google Scholar] [CrossRef]
- Ellis, J.M.; Galloway, A.T.; Mary Webb, R.; Martz, D.M. Measuring Adult Picky Eating: The Development of a Multidimensional Self-Report Instrument. Psychol. Assess. 2017, 29, 955–966. [Google Scholar] [CrossRef]
- He, J.; Ellis, J.M.; Zickgraf, H.F.; Fan, X. Translating, Modifying, and Validating the Adult Picky Eating Questionnaire for Use in China. Eat. Behav. 2019, 33, 78–84. [Google Scholar] [CrossRef]
- Segura-Garcia, C.; Aloi, M.; Carbone, E.A.; Staltari, F.A.; Rania, M.; Papaianni, M.C.; Vaquero-Solís, M.; Tapia-Serrano, M.Á.; Sánchez-Miguel, P.A.; Liuzza, M.T. Development, Validation, and Measurement Invariance of the Body Image Bidimensional Assessment (BIBA) in Italian and Spanish Children and Early Adolescent Samples. Int. J. Environ. Res. Public Health 2023, 20, 3626. [Google Scholar] [CrossRef]
- Pala, V.; Sieri, S.; Palli, D.; Salvini, S.; Berrino, F.; Bellegotti, M.; Frasca, G.; Tumino, R.; Sacerdote, C.; Fiorini, L.; et al. Diet in the Italian Epic Cohorts: Presentation of Data and Methodological Issues. Tumori J. 2003, 89, 594–607. [Google Scholar] [CrossRef]
- Piochi, M.; Dinnella, C.; Spinelli, S.; Monteleone, E.; Torri, L. Individual Differences in Responsiveness to Oral Sensations and Odours with Chemesthetic Activity: Relationships between Sensory Modalities and Impact on the Hedonic Response. Food Qual. Prefer. 2021, 88, 104112. [Google Scholar] [CrossRef]
- Welch, A.A.; Luben, R.; Khaw, K.T.; Bingham, S.A. The CAFE Computer Program for Nutritional Analysis of the EPIC-Norfolk Food Frequency Questionnaire and Identification of Extreme Nutrient Values. J. Hum. Nutr. Diet. 2005, 18, 99–116. [Google Scholar] [CrossRef]
- Schofield, W.N. Predicting Basal Metabolic Rate, New Standards and Review of Previous Work. Hum. Nutr. Clin. Nutr. 1985, 39, 5–41. [Google Scholar]
- Mazzeo, T.; Roncoroni, L.; Lombardo, V.; Tomba, C.; Elli, L.; Sieri, S.; Grioni, S.; Bardella, M.T.; Agostoni, C.; Doneda, L.; et al. Evaluation of a Modified Italian European Prospective Investigation into Cancer and Nutrition Food Frequency Questionnaire for Individuals with Celiac Disease. J. Acad. Nutr. Diet. 2016, 116, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Howe, G.; Kushi, L. Adjustment for Total Energy Intake in Epidemiologic Studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach; Guilford publications: New York, NY, USA, 2017; ISBN 146253466X. [Google Scholar]
- O’Brien, R.M. A Caution Regarding Rules of Thumb for Variance Inflation Factors. Qual. Quant. 2007, 41, 673–690. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Donato, F.; Triassi, M.; Loperto, I.; Maccaro, A.; Mentasti, S.; Crivillaro, F.; Elvetico, A.; Croce, E.; Raffetti, E. Symptoms of Mental Health Problems among Italian Adolescents in 2017–2018 School Year: A Multicenter Cross-Sectional Study. Environ. Health Prev. Med. 2021, 26, 67. [Google Scholar] [CrossRef]
- Taylor, C.M.; Wernimont, S.M.; Northstone, K.; Emmett, P.M. Picky/Fussy Eating in Children: Review of Definitions, Assessment, Prevalence and Dietary Intakes. Appetite 2015, 95, 349–359. [Google Scholar] [CrossRef]
- Marchi, M.; Cohen, P. Early Childhood Eating Behaviors and Adolescent Eating Disorders. J. Am. Acad. Child Adolesc. Psychiatry 1990, 29, 112–117. [Google Scholar] [CrossRef]
- Jacobi, C.; Schmitz, G.; Stewart Agras, W. Is Picky Eating an Eating Disorder? Int. J. Eat. Disord. 2008, 41, 626–634. [Google Scholar] [CrossRef]
- Mascola, A.J.; Bryson, S.W.; Agras, W.S. Picky Eating during Childhood: A Longitudinal Study to Age 11 years. Eat. Behav. 2010, 11, 253–257. [Google Scholar] [CrossRef]
- Karazsia, B.T.; Murnen, S.K.; Tylka, T.L. Is Body Dissatisfaction Changing across Time? A Cross-Temporal Meta-Analysis. Psychol. Bull. 2017, 143, 293–320. [Google Scholar] [CrossRef]
- Nagai, M.; Matsumoto, S.; Endo, J.; Sakamoto, R.; Wada, M. Sweet Taste Threshold for Sucrose Inversely Correlates with Depression Symptoms in Female College Students in the Luteal Phase. Physiol. Behav. 2015, 141, 92–96. [Google Scholar] [CrossRef]
- Handy, A.B.; Greenfield, S.F.; Yonkers, K.A.; Payne, L.A. Psychiatric Symptoms Across the Menstrual Cycle in Adult Women: A Comprehensive Review. Harv. Rev. Psychiatry 2022, 30, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Hankin, B.L.; Mermelstein, R.; Roesch, L. Sex Differences in Adolescent Depression: Stress Exposure and Reactivity Models. Child Dev. 2007, 78, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Dinnella, C.; Morizet, D.; Masi, C.; Cliceri, D.; Depezay, L.; Appleton, K.M.; Giboreau, A.; Perez-Cueto, F.J.A.; Hartwell, H.; Monteleone, E. Sensory Determinants of Stated Liking for Vegetable Names and Actual Liking for Canned Vegetables: A Cross-Country Study among European Adolescents. Appetite 2016, 107, 339–347. [Google Scholar] [CrossRef]
- Ben-Sasson, A.; Carter, A.S.; Briggs-Gowan, M.J. Sensory Over-Responsivity in Elementary School: Prevalence and Social-Emotional Correlates. J. Abnorm. Child Psychol. 2009, 37, 705–716. [Google Scholar] [CrossRef]
- Wilner, J.G.; Vranceanu, A.-M.; Blashill, A.J. Neuroticism Prospectively Predicts Pain among Adolescents: Results from a Nationally Representative Sample. J. Psychosom. Res. 2014, 77, 474–476. [Google Scholar] [CrossRef]
- Cortese, B.M.; Uhde, T.W.; Schumann, A.Y.; McTeague, L.M.; Sege, C.T.; Calhoun, C.D.; Danielson, C.K. Anxiety-Related Shifts in Smell Function in Children and Adolescents. Chem. Senses 2021, 46, bjab051. [Google Scholar] [CrossRef]
- Shi, P.; Yang, A.; Zhao, Q.; Chen, Z.; Ren, X.; Dai, Q. A Hypothesis of Gender Differences in Self-Reporting Symptom of Depression: Implications to Solve Under-Diagnosis and Under-Treatment of Depression in Males. Front. Psychiatry 2021, 12, 589687. [Google Scholar] [CrossRef]
- Thompson, F.E.; Subar, A.F. Dietary Assessment Methodology. In Nutrition in the Prevention and Treatment of Disease; Elsevier: Amsterdam, The Netherlands, 2017; pp. 5–48. [Google Scholar]
- Añez, E.; Fornieles-Deu, A.; Fauquet-Ars, J.; López-Guimerà, G.; Puntí-Vidal, J.; Sánchez-Carracedo, D. Body Image Dissatisfaction, Physical Activity and Screen-Time in Spanish Adolescents. J. Health Psychol. 2018, 23, 36–47. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).