Association of Dietary Advanced Glycation End Products with Overall and Site-Specific Cancer Risk and Mortality: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion/Exclusion Criteria
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Grading the Quality of Evidence
2.6. Statistical Analysis
2.7. Sensitivity Analyses
3. Results
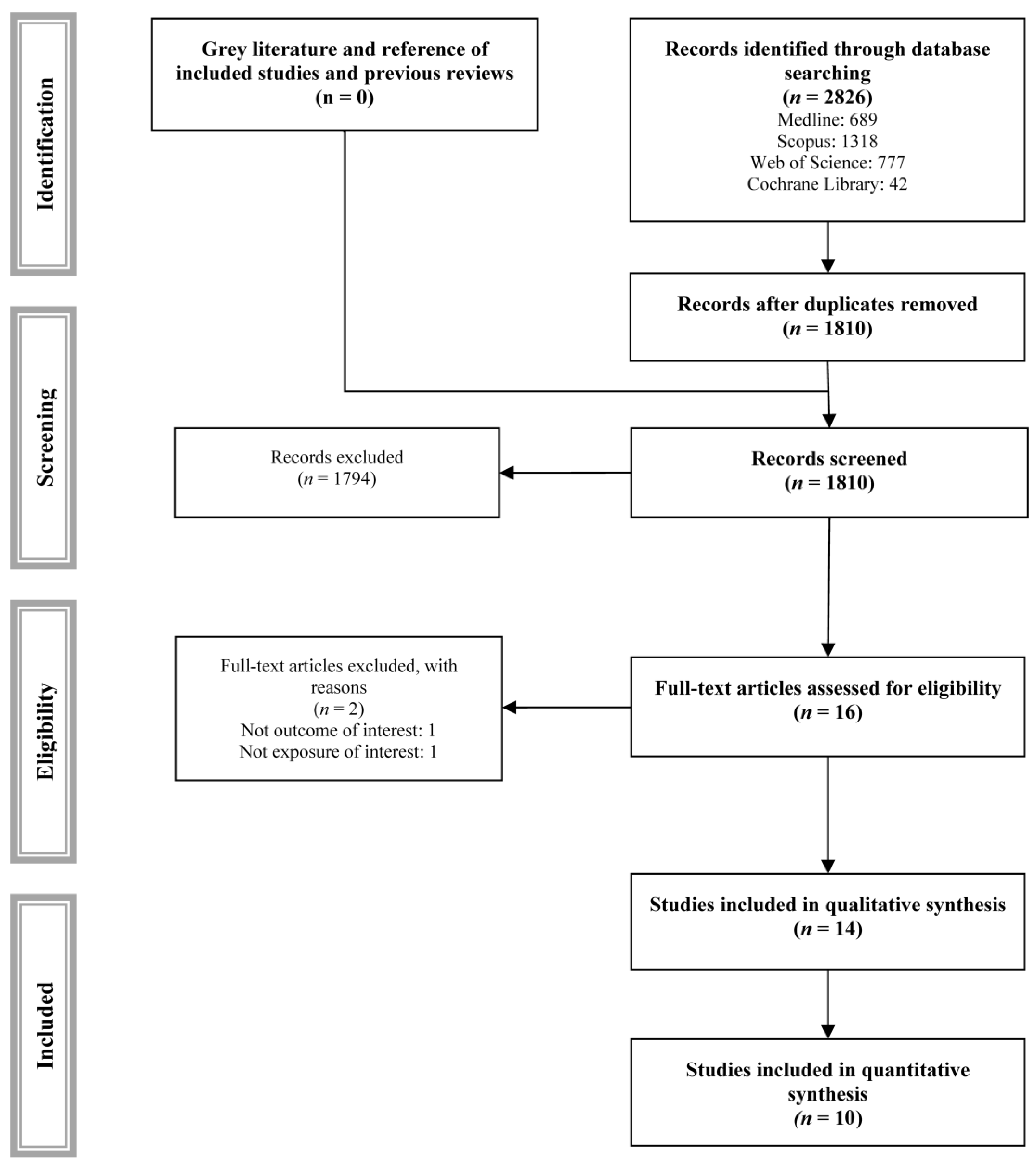
3.1. Systematic Review
3.2. Risk of Bias Assessment
3.3. Quality of Evidence Assessment
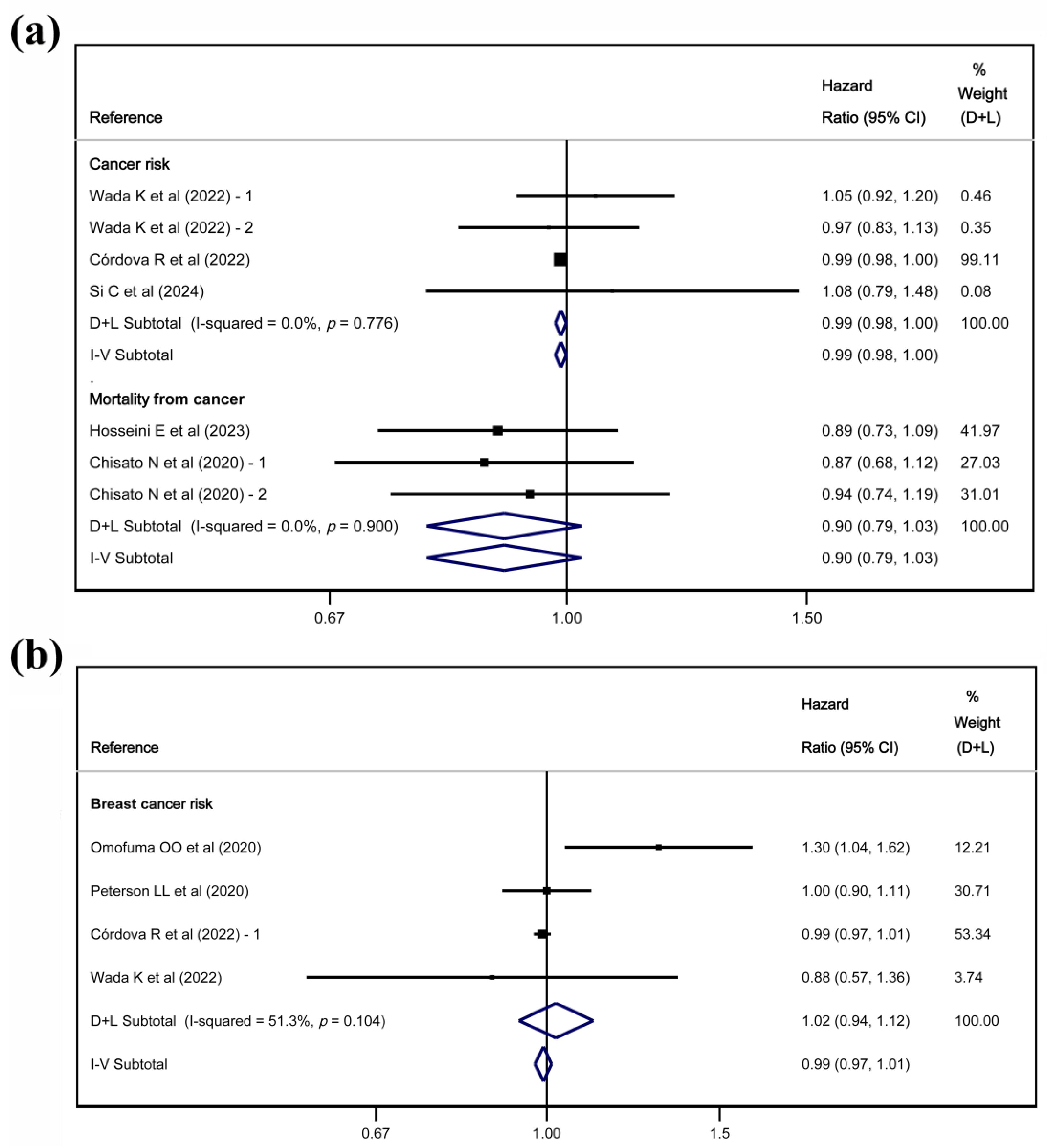
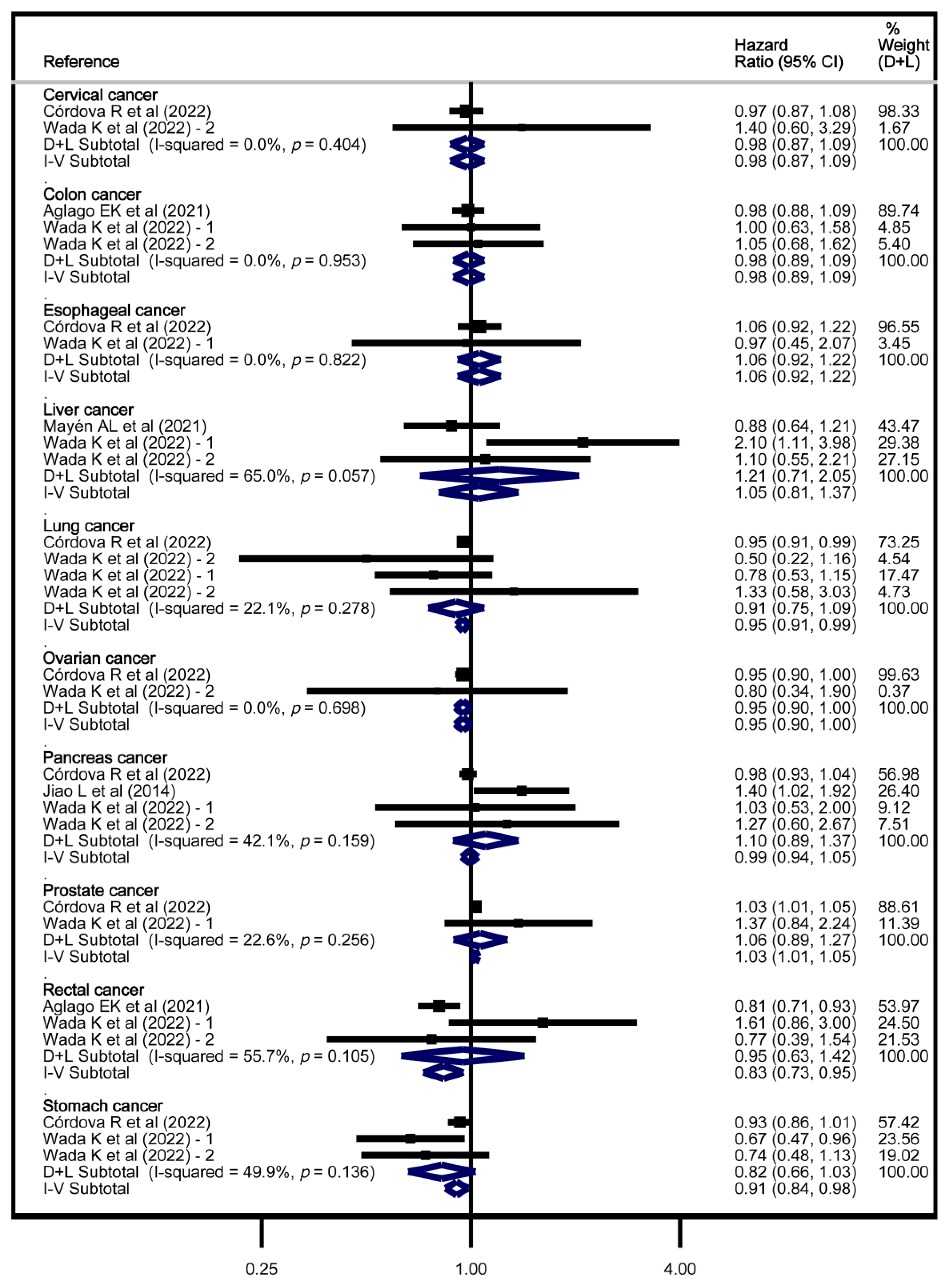
3.4. Meta-Analyses
3.5. Sensitivity Analyses
4. Discussion
4.1. Main Findings
4.2. Findings in Overall Cancer
4.3. Findings in Breast Cancer
4.4. Findings in Prostate Cancer
4.5. Findings in Other Tumour Sites
4.6. Implications
4.7. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 95% CI | 95% confidence intervals |
| AGEs | Advanced glycation end products |
| BC | Breast Cancer |
| CML | N-ε-[carboxymethyl]-L-lysine |
| dAGEs | Dietary advanced glycation end products |
| GRADE | Grading of Recommendations, Assessment, Development and Evaluation |
| MH-H1 | N-δ-[5-hydro-5-methyl-4-imidazolon-2-yl]-ornithine |
| MOOSE | Meta-analysis of Observational Studies in Epidemiology |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RAGE | Receptor for advanced glycation end products |
| SE | Standard error |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, M.; Cannon, G.; Butrum, R.; Martin, G.; Higginbotham, S.; Heggie, S.; Jones, C.; Fletcher, M. Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective; Amer Inst for Cancer Research: Washington, DC, USA, 2007. [Google Scholar]
- Murphy, N.; Moreno, V.; Hughes, D.J.; Vodicka, L.; Vodicka, P.; Aglago, E.K.; Gunter, M.J.; Jenab, M. Lifestyle and Dietary Environmental Factors in Colorectal Cancer Susceptibility. Mol. Asp. Med. 2019, 69, 2–9. [Google Scholar] [CrossRef]
- Cifu, G.; Arem, H. Adherence to Lifestyle-Related Cancer Prevention Guidelines and Breast Cancer Incidence and Mortality. Ann. Epidemiol. 2018, 28, 767–773.e1. [Google Scholar] [CrossRef]
- Mehta, R.S.; Song, M.; Nishihara, R.; Drew, D.A.; Wu, K.; Qian, Z.R.; Fung, T.T.; Hamada, T.; Masugi, Y.; da Silva, A.; et al. Dietary Patterns and Risk of Colorectal Cancer: Analysis by Tumor Location and Molecular Subtypes. Gastroenterology 2017, 152, 1944–1953.e1. [Google Scholar] [CrossRef]
- Aragno, M.; Mastrocola, R. Dietary Sugars and Endogenous Formation of Advanced Glycation Endproducts: Emerging Mechanisms of Disease. Nutrients 2017, 9, 385. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced Glycation End Products in Foods and a Practical Guide to Their Reduction in the Diet. J. Am. Diet. Assoc. 2010, 110, 911–916.e12. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced Glycation Endproducts in Food and Their Effects on Health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef]
- Schröter, D.; Höhn, A. Role of Advanced Glycation End Products in Carcinogenesis and Their Therapeutic Implications. Curr. Pharm. Des. 2018, 24, 5245–5251. [Google Scholar] [CrossRef]
- Turner, D.P. The Role of Advanced Glycation End-Products in Cancer Disparity. Adv. Cancer Res. 2017, 133, 1–22. [Google Scholar] [CrossRef]
- Fritz, G. RAGE: A Single Receptor Fits Multiple Ligands. Trends Biochem. Sci. 2011, 36, 625–632. [Google Scholar] [CrossRef]
- Scheijen, J.L.J.M.; Clevers, E.; Engelen, L.; Dagnelie, P.C.; Brouns, F.; Stehouwer, C.D.A.; Schalkwijk, C.G. Analysis of Advanced Glycation Endproducts in Selected Food Items by Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry: Presentation of a Dietary AGE Database. Food Chem. 2016, 190, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Zahabi, E.; Soltani, S.; Hajizadeh-Sharafabad, F.; Abdollahzad, H. Dietary Advanced Glycation End-Products (DAGEs) Are Not Associated with the Risk of Cancer Incidence. A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Food Sci. Nutr. 2024, 12, 7788–7797. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. J. Am. Med. Assoc. 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 1–694. [Google Scholar] [CrossRef]
- Study Quality Assessment Tools|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 29 April 2025).
- Ma, L.-L.; Wang, Y.-Y.; Yang, Z.-H.; Huang, D.; Weng, H.; Zeng, X.-T. Methodological Quality (Risk of Bias) Assessment Tools for Primary and Secondary Medical Studies: What Are They and Which Is Better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading Quality of Evidence and Strength of Recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. How to Obtain the Confidence Interval from a P Value. BMJ 2011, 343, d2090. [Google Scholar] [CrossRef]
- Tufanaru, C.; Munn, Z.; Stephenson, M.; Aromataris, E. Fixed or Random Effects Meta-Analysis? Common Methodological Issues in Systematic Reviews of Effectiveness. Int. J. Evid. Based Healthc. 2015, 13, 196–207. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Egger, M.; Smith, G.D. Systematic Reviews in Health Care: Investigating and Dealing with Publication and Other Biases in Meta-Analysis. Br. Med. J. 2001, 323, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Stolzenberg-Solomon, R.; Zimmerman, T.P.; Duan, Z.; Chen, L.; Kahle, L.; Risch, A.; Subar, A.F.; Cross, A.J.; Hollenbeck, A.; et al. Dietary Consumption of Advanced Glycation End Products and Pancreatic Cancer in the Prospective NIH-AARP Diet and Health Study. Am. J. Clin. Nutr. 2015, 101, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.Y.; Takeuchi, M.; Hyogo, H.; McKeown-Eyssen, G.; Yamagishi, S.-I.; Chayama, K.; O’Brien, P.J.; Ferrari, P.; Overvad, K.; Olsen, A.; et al. The Association between Glyceraldehyde-Derived Advanced Glycation End-Products and Colorectal Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Omofuma, O.O.; Turner, D.P.; Peterson, L.L.; Merchant, A.T.; Zhang, J.; Steck, S.E. Dietary Advanced Glycation End-Products (AGE) and Risk of Breast Cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO). Cancer Prev. Res. 2020, 13, 601–610. [Google Scholar] [CrossRef]
- Peterson, L.L.; Park, S.; Park, Y.; Colditz, G.A.; Anbardar, N.; Turner, D.P. Dietary Advanced Glycation End Products and the Risk of Postmenopausal Breast Cancer in the National Institutes of Health-AARP Diet and Health Study. Cancer 2020, 126, 2648–2657. [Google Scholar] [CrossRef]
- Aglago, E.K.; Mayén, A.-L.; Knaze, V.; Freisling, H.; Fedirko, V.; Hughes, D.J.; Jiao, L.; Eriksen, A.K.; Tjønneland, A.; Boutron-Ruault, M.-C.; et al. Dietary Advanced Glycation End-Products and Colorectal Cancer Risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Nutrients 2021, 13, 3132. [Google Scholar] [CrossRef]
- Mayén, A.-L.; Aglago, E.K.; Knaze, V.; Cordova, R.; Schalkwijk, C.G.; Wagner, K.-H.; Aleksandrova, K.; Fedirko, V.; Keski-Rahkonen, P.; Leitzmann, M.F.; et al. Dietary Intake of Advanced Glycation Endproducts and Risk of Hepatobiliary Cancers: A Multinational Cohort Study. Int. J. Cancer 2021, 149, 854–864. [Google Scholar] [CrossRef]
- Córdova, R.; Mayén, A.-L.; Knaze, V.; Aglago, E.K.; Schalkwijk, C.; Wagner, K.-H.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Katzke, V.A.; et al. Dietary Intake of Advanced Glycation Endproducts (AGEs) and Cancer Risk across More than 20 Anatomical Sites: A Multinational Cohort Study. Cancer Commun. 2022, 42, 1041–1045. [Google Scholar] [CrossRef]
- Wada, K.; Nakashima, Y.; Yamakawa, M.; Hori, A.; Seishima, M.; Tanabashi, S.; Matsushita, S.; Tokimitsu, N.; Nagata, C. Dietary Advanced Glycation End Products and Cancer Risk in Japan: From the Takayama Study. Cancer Sci. 2022, 113, 2839–2848. [Google Scholar] [CrossRef]
- Jahromi, M.K.; Tehrani, A.N.; Farhadnejad, H.; Emamat, H.; Ahmadirad, H.; Teymoori, F.; Heidari, Z.; Saber, N.; Rashidkhani, B.; Mirmiran, P. Dietary Advanced Glycation End Products Are Associated with an Increased Risk of Breast Cancer in Iranian Adults. BMC Cancer 2023, 23, 932. [Google Scholar] [CrossRef]
- Si, C.; Liu, F.; Peng, Y.; Qiao, Y.; Wang, P.; Wang, X.; Gong, J.; Zhou, H.; Zhang, M.; Song, F. Association of Total and Different Food-Derived Advanced Glycation End-Products with Risks of All-Cause and Cause-Specific Mortality. Food Funct. 2024, 15, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Wada, K.; Yamakawa, M.; Nakashima, Y.; Koda, S.; Uji, T.; Oba, S. Dietary Intake of Nε-Carboxymethyl-Lysine, a Major Advanced Glycation End Product, Is Not Associated with Increased Risk of Mortality in Japanese Adults in the Takayama Study. J. Nutr. 2020, 150, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Omofuma, O.O.; Peterson, L.L.; Turner, D.P.; Merchant, A.T.; Zhang, J.; Thomson, C.A.; Neuhouser, M.L.; Snetselaar, L.G.; Caan, B.J.; Shadyab, A.H.; et al. Dietary Advanced Glycation End-Products and Mortality after Breast Cancer in the Women’s Health Initiative. Cancer Epidemiol. Biomark. Prev. 2021, 30, 2217–2226. [Google Scholar] [CrossRef]
- Mao, Z.; Aglago, E.K.; Zhao, Z.; Schalkwijk, C.; Jiao, L.; Freisling, H.; Weiderpass, E.; Hughes, D.J.; Eriksen, A.K.; Tjønneland, A.; et al. Dietary Intake of Advanced Glycation End Products (AGEs) and Mortality among Individuals with Colorectal Cancer. Nutrients 2021, 13, 4435. [Google Scholar] [CrossRef]
- Hosseini, E.; Mokhtari, Z.; Poustchi, H.; Khoshnia, M.; Dawsey, S.M.; Boffetta, P.; Abnet, C.C.; Kamangar, F.; Etemadi, A.; Pourshams, A.; et al. Dietary Advanced Glycation End Products and Risk of Overall and Cause-Specific Mortality: Results from the Golestan Cohort Study. Int. J. Environ. Res. Public Health 2023, 20, 3788. [Google Scholar] [CrossRef]
- Sfeir, M.; Jacobs, E.T.; Kohler, L.N.; Steck, S.E.; Yung, A.K.; Thomson, C.A. Characterizing Dietary Advanced Glycation End-Product (DAGE) Exposure and the Relationship to Colorectal Adenoma Recurrence: A Secondary Analysis. Nutrients 2023, 15, 1126. [Google Scholar] [CrossRef]
- Agnoli, C.; Perlino, F.; Guerra, G.; Quartiroli, M.; Vener, C.; Mauri, P.; de Palma, A.; Venturelli, E.; Sieri, S. Advanced Glycation End Products and Breast Cancer Risk in a Sample of the ORDET Cohort. Int. J. Biol. Markers 2025, 40, 75–79. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Fu, L. Dietary Advanced Glycation End-Products: Perspectives Linking Food Processing with Health Implications. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2559–2587. [Google Scholar] [CrossRef]
- Prasad, K.; Mishra, M. AGE-RAGE Stress, Stressors, and Antistressors in Health and Disease. Int..J Angiol. 2018, 27, 1–12. [Google Scholar] [CrossRef]
- Walter, K.R.; Ford, M.E.; Gregoski, M.J.; Kramer, R.M.; Knight, K.D.; Spruill, L.; Nogueira, L.M.; Krisanits, B.A.; Phan, V.; La Rue, A.C.; et al. Advanced Glycation End Products Are Elevated in Estrogen Receptor-Positive Breast Cancer Patients, Alter Response to Therapy, and Can Be Targeted by Lifestyle Intervention. Breast Cancer Res. Treat. 2019, 173, 559–571. [Google Scholar] [CrossRef]
- Radia, A.-M.; Yaser, A.-M.; Ma, X.; Zhang, J.; Yang, C.; Dong, Q.; Rong, P.; Ye, B.; Liu, S.; Wang, W. Specific SiRNA Targeting Receptor for Advanced Glycation End Products (RAGE) Decreases Proliferation in Human Breast Cancer Cell Lines. Int. J. Mol. Sci. 2013, 14, 7959–7978. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Li, H.; Zhang, B.; Liu, Y.; Lu, G.; Lu, S.; Sun, L.; Qi, Y.; Li, X.; Chen, W. RAGE-Binding S100A8/A9 Promotes the Migration and Invasion of Human Breast Cancer Cells through Actin Polymerization and Epithelial-Mesenchymal Transition. Breast Cancer Res. Treat. 2013, 142, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Yoo, J.W.; Kim, Y.K.; Choi, J.H.; Ha, T.-Y.; Gil, M. Advanced Glycation End Products Promote Triple Negative Breast Cancer Cells via ERK and NF-ΚB Pathway. Biochem. Biophys. Res. Commun. 2018, 495, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, H.; Matou-Nasri, S.; Wang, Q.; Rabhan, Z.; Al-Eidi, H.; Al Abdulrahman, A.; Ahmed, N. Advanced Glycation Endproducts Increase Proliferation, Migration and Invasion of the Breast Cancer Cell Line MDA-MB-231. Biochim. Biophys. Acta 2015, 1852, 429–441. [Google Scholar] [CrossRef]
- Matou-Nasri, S.; Sharaf, H.; Wang, Q.; Almobadel, N.; Rabhan, Z.; Al-Eidi, H.; Yahya, W.; Trivilegio, T.; Ali, R.; Al-Shanti, N.; et al. Biological Impact of Advanced Glycation Endproducts on Estrogen Receptor-Positive MCF-7 Breast Cancer Cells. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2808–2820. [Google Scholar] [CrossRef]
- Khoo, S.-H.; Wu, P.-R.; Yeh, K.-T.; Hsu, S.-L.; Wu, C.-H. Biological and Clinical Significance of the AGE-RAGE Axis in the Aggressiveness and Prognosis of Prostate Cancer. J. Food Drug Anal. 2023, 31, 664–682. [Google Scholar] [CrossRef]
- Konopka, C.; Paton, A.; Skokowska, A.; Rowles, J.I.I.I.; Erdman, J.J.; Dobrucka, I.; Dobrucki, L. Examining the Role of Dietary Advanced Gycation End Products in Prostate Cancer Using Molecular Imaging Techniques (OR04-08-19). Curr. Dev. Nutr. 2019, 3, nzz030. [Google Scholar] [CrossRef]
- Semba, R.D.; Ang, A.; Talegawkar, S.; Crasto, C.; Dalal, M.; Jardack, P.; Traber, M.G.; Ferrucci, L.; Arab, L. Dietary Intake Associated with Serum versus Urinary Carboxymethyl-Lysine, a Major Advanced Glycation End Product, in Adults: The Energetics Study. Eur. J. Clin. Nutr. 2012, 66, 3–9. [Google Scholar] [CrossRef]
- Bartling, B.; Demling, N.; Silber, R.-E.; Simm, A. Proliferative Stimulus of Lung Fibroblasts on Lung Cancer Cells Is Impaired by the Receptor for Advanced Glycation End-Products. Am. J. Respir. Cell Mol. Biol. 2006, 34, 83–91. [Google Scholar] [CrossRef]
- Riehl, A.; Németh, J.; Angel, P.; Hess, J. The Receptor RAGE: Bridging Inflammation and Cancer. Cell Commun. Signal 2009, 7, 12. [Google Scholar] [CrossRef]
- Gu, H.; Yang, L.; Sun, Q.; Zhou, B.; Tang, N.; Cong, R.; Zeng, Y.; Wang, B. Gly82Ser Polymorphism of the Receptor for Advanced Glycation End Products Is Associated with an Increased Risk of Gastric Cancer in a Chinese Population. Clin. Cancer Res. 2008, 14, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, S.-X.; Zhou, Y.-N.; Wang, J.; Ji, R. Research on the Relationship between RAGE and Its Ligand HMGB1, and Prognosis and Pathogenesis of Gastric Cancer with Diabetes Mellitus. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Nishiguchi, Y.; Honoki, K.; Mori, S.; Fujiwara-Tani, R.; Sasaki, T.; Fujii, K.; Kawahara, I.; Goto, K.; Nakashima, C.; et al. Role of Glycated High Mobility Group Box-1 in Gastric Cancer. Int. J. Mol. Sci. 2021, 22, 5185. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, T.; Ye, G.; Shen, Z.; Hu, Y.; Mou, T.; Yu, J.; Li, S.; Liu, H.; Li, G. Overexpression of the Receptor for Advanced Glycation Endproducts (RAGE) Is Associated with Poor Prognosis in Gastric Cancer. PLoS ONE 2015, 10, e0122697. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Bellesia, A. The Gastro-Intestinal Tract as the Major Site of Biological Action of Dietary Melanoidins. Amino Acids 2015, 47, 1077–1089. [Google Scholar] [CrossRef]
- Hiramoto, S.; Itoh, K.; Shizuuchi, S.; Kawachi, Y.; Morishita, Y.; Nagase, M.; Suzuki, Y.; Nobuta, Y.; Sudou, Y.; Nakamura, O.; et al. Melanoidin, a Food Protein-Derived Advanced Maillard Reaction Product, Suppresses Helicobacter Pylori in Vitro and in Vivo. Helicobacter 2004, 9, 429–435. [Google Scholar] [CrossRef]
- Martínez-Ortega, I.A.; Mesas, A.E.; Bizzozero-Peroni, B.; Garrido-Miguel, M.; Jiménez-López, E.; Martínez-Vizcaíno, V.; Fernández-Rodríguez, R. Can Different Types of Tree Nuts and Peanuts Induce Varied Effects on Specific Blood Lipid Parameters? A Systematic Review and Network Meta-Analysis. Crit. Rev. Food Sci. Nutr. 2023, 65, 1538–1552. [Google Scholar] [CrossRef]
| Reference | Region | Design | Sample Size | Females (%) | Age (years) | Comparison | Length (years) | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | BC | Others | ||||||||
| Cancer risk | ||||||||||
| Jiao L et al. (2014) [25] | US | Cohorts (Prospective) | 528,251 | 41.23 | 62.1 ± 5.4 | Quintile 5 vs. Quintile 1 | 10.5 | - | - | ✓ |
| Kong SY et al. (2015) [26] | Denmark, France, Greece, Germany, Italy, Netherlands, Norway, Spain, Sweden, UK | Case-control | 2110 | 51.7 | 58.5 | Quartile 4 vs. Quartile 1 | - | - | - | ✓ |
| Omofuma OO et al. (2020)—1 [27] | US | Cohorts (Prospective) | 27,464 | 100 | 62.4 | Quintile 5 vs. Quintile 1 | 11.5 | - | ✓ | - |
| Peterson LL et al. (2020) [28] | US | Cohorts (Prospective) | 183,548 | 100 | 61.6 | Quintile 5 vs. Quintile 1 | 12.8 | - | ✓ | - |
| Aglago EK et al. (2021) [29] | Europe | Cohorts (Prospective) | 450,111 | 70.80 | 50.6 | Quintile 5 vs. Quintile 1 | 14.1 | - | - | ✓ |
| Mayén AL et al. (2021) [30] | Europe | Cohorts (Prospective) | 450,111 | 70.80 | 50.6 | Tertile 3 vs. Tertile 1 | 14.9 | - | - | ✓ |
| Córdova R et al. (2022) [31] | Europe | Cohorts (Prospective) | 450,111 | 70.80 | 50.6 | Quintile 5 vs. Quintile 1 | 14.9 | ✓ | ✓ | ✓ |
| Wada K et al. (2022) [32] | Japan | Cohorts (Prospective) | 30,722 | 53.87 | M: 55.1 F: 56.3 | Quartile 4 vs. Quartile 1 | - | ✓ | ✓ | ✓ |
| Jahromi MK et al. (2023) [33] | Iran | Case-control | 401 | 100 | 47.9 ± 10.3 | Tertile 3 vs. Tertile 1 | - | - | ✓ | - |
| Si C et al. (2024) [34] | US | Cohorts (Prospective) | 22,124 | 57.5 | 45.6 ± 0.4 | Quartile 4 vs. Quartile 1 | 27.1 | ✓ | - | - |
| Mortality | ||||||||||
| Chisato N et al. (2020) [35] | Japan | Cohorts | 29,079 | 54.07 | 54.56 | Quartile 4 vs. Quartile 1 | 14.1 | ✓ | - | - |
| Omofuma OO et al. (2020)—2 [36] | US | Cohorts | 2023 | 100 | - | Tertile 3 vs. Tertile 1 | 15.1 | - | ✓ | - |
| Mao Z et al. (2021) [37] | Europe | Cohorts | 5801 | 57.2 | 66.3 | Quintile 5 vs. Quintile 1 | 4.8 | - | - | ✓ |
| Hosseini E et al. (2023) [38] | Iran | Cohorts | 48,632 | 57.52 | 52.0 ± 8.9 | Quintile 5 vs. Quintile 1 | 13.5 | ✓ | - | ✓ |
| Reference | Comparison | Type of Association | Overall Cancer (95% CI) | Breast Cancer (95% CI) |
|---|---|---|---|---|
| Cancer risk | ||||
| Omofuma OO et al. (2020)—1 [27] | Q5 vs. Q1 | Hazard Ratio | - | 1.30 (1.04, 1.62) * |
| Peterson LL et al. (2020) [28] | Q5 vs. Q1 | Hazard Ratio | - | 1.00 (0.90, 1.11) |
| Córdova R et al. (2022) [31] | Q5 vs. Q1 | Hazard Ratio | 0.99 (0.98, 1.00) * | 0.99 (0.97, 1.01) |
| Wada K et al. (2022) [32] | Q4 vs. Q1—males | Hazard Ratio | 1.05 (0.92, 1.20 | - |
| Wada K et al. (2022) [32] | Q4 vs. Q1—females | Hazard Ratio | 0.97 (0.83, 1.13) | 0.88 (0.57, 1.36) |
| Jahromi MK et al. (2023) [33] | T3 vs. T1 | Odds Ratio | - | 2.33 (1.18, 4.60) * |
| Si C et al. (2024) [34] | Q4 vs. Q1 | Hazard Ratio | 1.08 (0.78, 1.48) | - |
| Mortality | ||||
| Chisato N et al. (2020)—1 [35] | Q4 vs. Q1—males | Hazard Ratio | 0.87 (0.67, 1.12) | - |
| Chisato N et al. (2020)—2 [35] | Q4 vs. Q1—females | Hazard Ratio | 0.94 (0.74, 1.19) | - |
| Omofuma OO et al. (2020)—2 [36] | T3 vs. T1 | Hazard Ratio | - | 1.49 (0.98–2.24) |
| Hosseini E et al. (2023) [38] | Q5 vs. Q1 | Hazard Ratio | 0.89 (0.76, 1.03) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual-Morena, C.; Garrido-Miguel, M.; Martínez-García, I.; Lucerón-Lucas-Torres, M.; Rodríguez-Gutiérrez, E.; Berlanga-Macías, C.; Fernández-Bravo-Rodrigo, J.; Patiño-Cardona, S. Association of Dietary Advanced Glycation End Products with Overall and Site-Specific Cancer Risk and Mortality: A Systematic Review and Meta-Analysis. Nutrients 2025, 17, 1638. https://doi.org/10.3390/nu17101638
Pascual-Morena C, Garrido-Miguel M, Martínez-García I, Lucerón-Lucas-Torres M, Rodríguez-Gutiérrez E, Berlanga-Macías C, Fernández-Bravo-Rodrigo J, Patiño-Cardona S. Association of Dietary Advanced Glycation End Products with Overall and Site-Specific Cancer Risk and Mortality: A Systematic Review and Meta-Analysis. Nutrients. 2025; 17(10):1638. https://doi.org/10.3390/nu17101638
Chicago/Turabian StylePascual-Morena, Carlos, Miriam Garrido-Miguel, Irene Martínez-García, Maribel Lucerón-Lucas-Torres, Eva Rodríguez-Gutiérrez, Carlos Berlanga-Macías, Jaime Fernández-Bravo-Rodrigo, and Silvana Patiño-Cardona. 2025. "Association of Dietary Advanced Glycation End Products with Overall and Site-Specific Cancer Risk and Mortality: A Systematic Review and Meta-Analysis" Nutrients 17, no. 10: 1638. https://doi.org/10.3390/nu17101638
APA StylePascual-Morena, C., Garrido-Miguel, M., Martínez-García, I., Lucerón-Lucas-Torres, M., Rodríguez-Gutiérrez, E., Berlanga-Macías, C., Fernández-Bravo-Rodrigo, J., & Patiño-Cardona, S. (2025). Association of Dietary Advanced Glycation End Products with Overall and Site-Specific Cancer Risk and Mortality: A Systematic Review and Meta-Analysis. Nutrients, 17(10), 1638. https://doi.org/10.3390/nu17101638







