Hyperhomocysteinemia: Underlying Links to Stroke and Hydrocephalus, with a Focus on Polyphenol-Based Therapeutic Approaches
Highlights
- Mutations (e.g., MTHFR) and B-vitamin deficiencies elevate homocysteine (HCys) levels.
- Elevated HCys levels induce oxidative stress, inflammation and neurodegeneration.
- HCys modulates endothelial dysfunction and fluid dynamics, with implications for stroke and hydrocephalus pathogenesis.
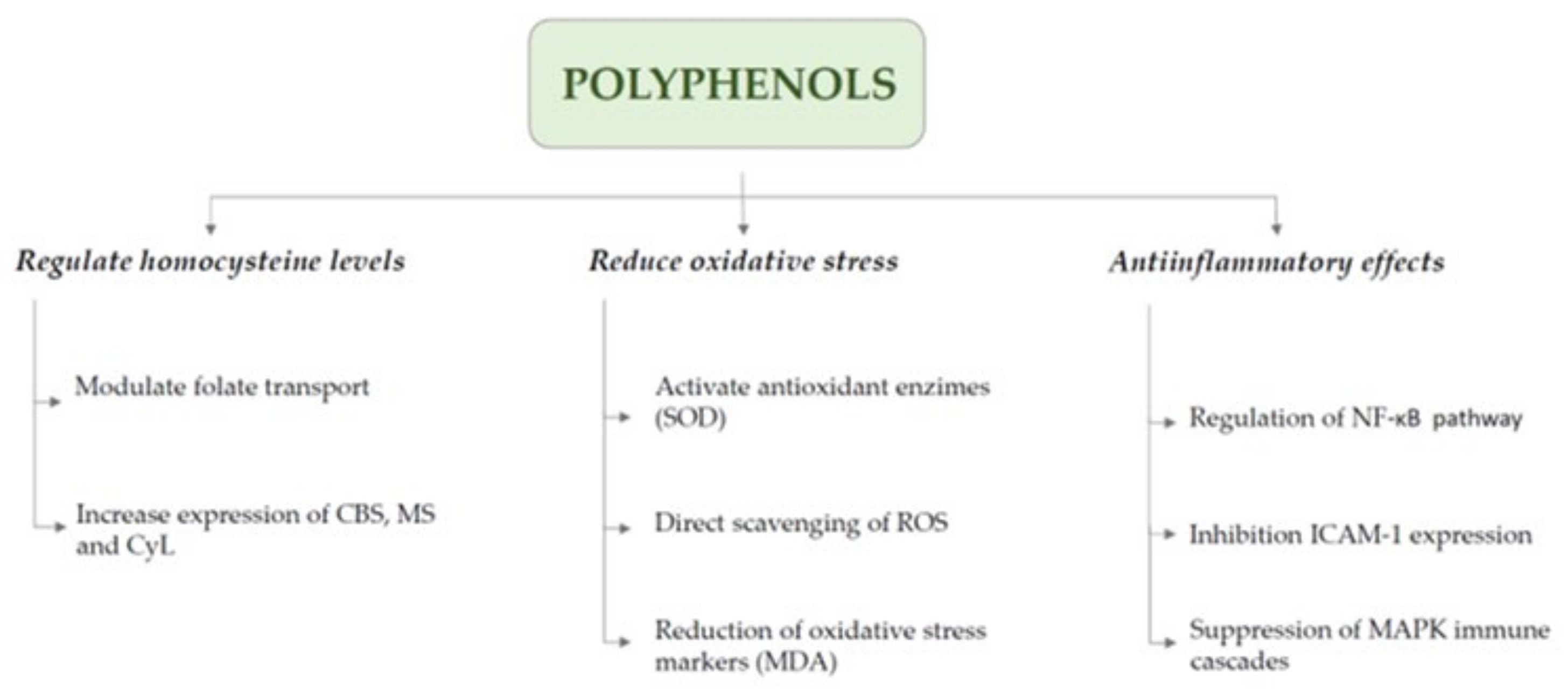
- Polyphenols can reduce HCys levels by enhancing key metabolic enzymes and counteracting oxidative and inflammatory damage, offering neurovascular protection.
- Polyphenol-rich diets and supplementation show potential in mitigating neurovascular damage.
Abstract
1. Introduction
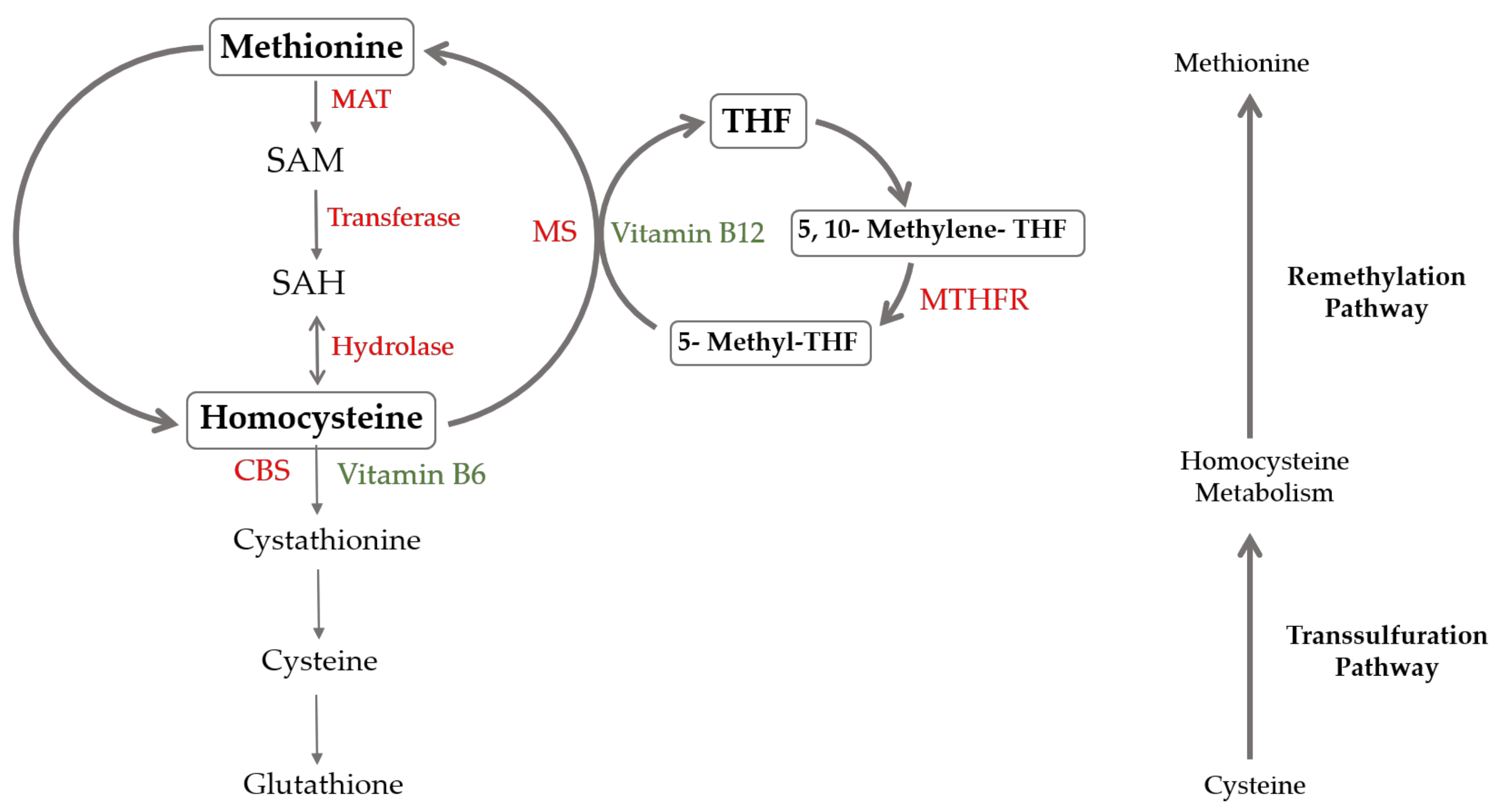
2. Hyperhomocysteinemia
3. Vascular Effects of Homocysteine
4. Hyperhomocysteinemia and Stroke
5. Hyperhomocysteinemia and Hydrocephalus
6. Homocysteine and Neurological Diseases
7. Therapeutic Perspectives on Homocysteine Modulation
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guieu, R.; Ruf, J.; Mottola, G. Hyperhomocysteinemia and cardiovascular diseases. Ann. Biol. Clin. 2022, 80, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Tiwari, M.; Tiwari, R.K. Hyperhomocysteinemia: Impact on Neurodegenerative Diseases. Basic. Clin. Pharmacol. Toxicol. 2015, 117, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Valentino, F.; Bivona, G.; Butera, D.; Paladino, P.; Fazzari, M.; Piccoli, T.; La Bella, V. Elevated cerebrospinal fluid and plasma homocysteine levels in ALS. Eur. J. Neurol. 2010, 17, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.F.; Kumar, S.; Ganguly, P. Measurement of homocysteine: A historical perspective. J. Clin. Biochem. Nutr. 2019, 65, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Paprotny, Ł.; Wianowska, D.; Izdebska, M.; Celejewska, A.; Szewczak, D.; Solski, J. Analysis of serum homocysteine in the laboratory practice—comparison of the direct chemiluminescence immunoassay and high performance liquid chromatography coupled with fluorescent detection. Biochem. Med. 2020, 30, 030703. [Google Scholar] [CrossRef]
- Rasmussen, K.; Moller, J. Total homocysteine measurement in clinical practice. Ann. Clin. Biochem. 2000, 37, 627–648. [Google Scholar] [CrossRef]
- Stanger, O.; Herrmann, W.; Pietrzik, K.; Fowler, B.; Geisel, J.; Dierkes, J.; Weger, M. DACH-LIGA homocystein (german, austrian and swiss homocysteine society): Consensus paper on the rational clinical use of homocysteine, folic acid and B-vitamins in cardiovascular and thrombotic diseases: Guidelines and recommendations. Clin. Chem. Lab. Med. 2003, 41, 1392–1403. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H. Homocysteine—from disease biomarker to disease prevention. J. Intern. Med. 2021, 290, 826–854. [Google Scholar] [CrossRef]
- Wilcken, B.; Bamforth, F.; Li, Z.; Zhu, H.; Ritvanen, A.; Redlund, M.; Botto, L.D. Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): Findings from over 7000 newborns from 16 areas world wide. J. Med. Genet. 2003, 40, 619–625. [Google Scholar] [CrossRef]
- Weisberg, I.; Tran, P.; Christensen, B.; Sibani, S.; Rozen, R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol. Genet. Metab. 1998, 64, 169–172. [Google Scholar] [CrossRef]
- Gupta, S.; Wang, L.; Hua, X.; Krijt, J.; Kozich, V.; Kruger, W.D. Cystathionine beta-synthase p.S466L mutation causes hyperhomocysteinemia in mice. Hum. Mutat. 2008, 29, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.; Maqbool, S.; Azam, M.; Iqbal, M.P.; Qamar, R. CBS mutations and MTFHR SNPs causative of hyperhomocysteinemia in Pakistani children. Mol. Biol. Rep. 2018, 45, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Mudd, S.H.; Skovby, F.; Levy, H.L.; Pettigrew, K.D.; Wilcken, B.; Pyeritz, R.E.; Andria, G.; Boers, G.H.; Bromberg, I.L.; Cerone, R.; et al. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am. J. Hum. Genet. 1985, 37, 1–31. [Google Scholar] [PubMed]
- Obeid, R.; McCaddon, A.; Herrmann, W. The role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric diseases. Clin. Chem. Lab. Med. 2007, 45, 1590–1606. [Google Scholar] [CrossRef]
- Vatsalya, V.; Gala, K.S.; Hassan, A.Z.; Frimodig, J.; Kong, M.; Sinha, N.; Schwandt, M.L. Characterization of Early-Stage Alcoholic Liver Disease with Hyperhomocysteinemia and Gut Dysfunction and Associated Immune Response in Alcohol Use Disorder Patients. Biomedicines 2020, 9, 7. [Google Scholar] [CrossRef]
- Okumura, K.; Tsukamoto, H. Folate in smokers. Clin. Chim. Acta 2011, 412, 521–526. [Google Scholar] [CrossRef]
- Shenoy, V.; Mehendale, V.; Prabhu, K.; Shetty, R.; Rao, P. Correlation of serum homocysteine levels with the severity of coronary artery disease. Indian J. Clin. Biochem. 2014, 29, 339–344. [Google Scholar] [CrossRef]
- Feletou, M. The Endothelium: Part 1: Multiple Functions of the Endothelial Cells-Focus on Endothelium-Derived Vasoactive Mediators. In Colloquium Series on Integrated Systems Physiology; Morgan & Claypool Life Sciences: Williston, VT, USA, 2011. [Google Scholar]
- Deanfield, J.; Donald, A.; Ferri, C.; Giannattasio, C.; Halcox, J.; Halligan, S.; Lerman, A.; Mancia, G.; Oliver, J.J.; Pessina, A.C.; et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: A statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J. Hypertens. 2005, 23, 7–17. [Google Scholar] [CrossRef]
- Topal, G.; Brunet, A.; Millanvoye, E.; Boucher, J.L.; Rendu, F.; Devynck, M.A.; David-Dufilho, M. Homocysteine induces oxidative stress by uncoupling of NO synthase activity through reduction of tetrahydrobiopterin. Free Radic. Biol. Med. 2004, 36, 1532–1541. [Google Scholar] [CrossRef]
- Jin, L.; Caldwell, R.B.; Li-Masters, T.; Caldwell, R.W. Homocysteine induces endothelial dysfunction via inhibition of arginine transport. J. Physiol. Pharmacol. 2007, 58, 191–206. [Google Scholar]
- Lai, W.K.; Kan, M.Y. Homocysteine-Induced Endothelial Dysfunction. Ann. Nutr. Metab. 2015, 67, 1–12. [Google Scholar] [CrossRef]
- Shin, W.S.; Berkowitz, D.E.; Ryoo, S.W. Increased arginase II activity contributes to endothelial dysfunction through endothelial nitric oxide synthase uncoupling in aged mice. Exp. Mol. Med. 2012, 44, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wu, H.; Li, W.; Gao, P. Protective effects of genistein in homocysteine-induced endothelial cell inflammatory injury. Mol. Cell. Biochem. 2015, 403, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Zhang, L.; Zhang, D.; Bai, L.; Kong, W.; Huang, Y.; Tang, C.; Du, J.; Jin, H. L-Cystathionine Protects against Homocysteine-Induced Mitochondria-Dependent Apoptosis of Vascular Endothelial Cells. Oxid. Med. Cell. Longev. 2019, 2019, 1253289. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; Zhang, Y.; Xu, Y.; Yang, W.Y.; Jiang, X.; Sha, X.; Cheng, X.; Wang, J.; Qin, X.; Yu, J.; et al. Caspase-1 Inflammasome Activation Mediates Homocysteine-Induced Pyrop-Apoptosis in Endothelial Cells. Circ. Res. 2016, 118, 1525–1539. [Google Scholar] [CrossRef]
- Shi, J.; Chen, D.; Wang, Z.; Li, S.; Zhang, S. Homocysteine induces ferroptosis in endothelial cells through the systemXc(-)/GPX4 signaling pathway. BMC Cardiovasc. Disord. 2023, 23, 316. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, X.; Liu, J.; Xie, X.; Cui, W.; Zhu, Y. Homocysteine accelerates senescence of endothelial cells via DNA hypomethylation of human telomerase reverse transcriptase. Arter. Thromb. Vasc. Biol. 2015, 35, 71–78. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, X.; Kong, W. Hyperhomocysteinaemia and vascular injury: Advances in mechanisms and drug targets. Br. J. Pharmacol. 2018, 175, 1173–1189. [Google Scholar] [CrossRef]
- Andrews, S.G.; Koehle, A.M.; Paudel, D.; Neuberger, T.; Ross, A.C.; Singh, V.; Bottiglieri, T.; Castro, R. Diet-Induced Severe Hyperhomocysteinemia Promotes Atherosclerosis Progression and Dysregulates the Plasma Metabolome in Apolipoprotein-E-Deficient Mice. Nutrients 2024, 16, 330. [Google Scholar] [CrossRef]
- Al Hageh, C.; Alefishat, E.; Ghassibe-Sabbagh, M.; Platt, D.E.; Hamdan, H.; Tcheroyan, R.; Chammas, E.; O’Sullivan, S.; Abchee, A.; Wang, B.; et al. Homocysteine levels, H-Hypertension, and the MTHFR C677T genotypes: A complex interaction. Heliyon 2023, 9, e16444. [Google Scholar] [CrossRef]
- Zhu, J.; Xie, R.; Piao, X.; Hou, Y.; Zhao, C.; Qiao, G.; Yang, B.; Shi, J.; Lu, Y. Homocysteine enhances clot-promoting activity of endothelial cells via phosphatidylserine externalization and microparticles formation. Amino Acids 2012, 43, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, R.I.; Peshkova, A.D.; Le Minh, G.; Khaertdinov, N.N.; Evtugina, N.G.; Sitdikova, G.F.; Weisel, J.W. Effects of Hyperhomocysteinemia on the Platelet-Driven Contraction of Blood Clots. Metabolites 2021, 11, 354. [Google Scholar] [CrossRef] [PubMed]
- Zipser, B.D.; Johanson, C.E.; Gonzalez, L.; Berzin, T.M.; Tavares, R.; Hulette, C.M.; Vitek, M.P.; Hovanesian, V.; Stopa, E.G. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol. Aging 2007, 28, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Candelario-Jalil, E.; Dijkhuizen, R.M.; Magnus, T. Neuroinflammation, Stroke, Blood-Brain Barrier Dysfunction, and Imaging Modalities. Stroke 2022, 53, 1473–1486. [Google Scholar] [CrossRef]
- Filippidis, A.S.; Kalani, M.Y.; Rekate, H.L. Hydrocephalus and aquaporins: Lessons learned from the bench. Childs Nerv. Syst. 2011, 27, 27–33. [Google Scholar] [CrossRef]
- Beard, R.S., Jr.; Reynolds, J.J.; Bearden, S.E. Hyperhomocysteinemia increases permeability of the blood-brain barrier by NMDA receptor-dependent regulation of adherens and tight junctions. Blood 2011, 118, 2007–2014. [Google Scholar] [CrossRef]
- Schenkelaars, N.; van Rossem, L.; Willemsen, S.P.; Faas, M.M.; Schoenmakers, S.; Steegers-Theunissen, R.P.M. The intake of ultra-processed foods and homocysteine levels in women with(out) overweight and obesity: The Rotterdam Periconceptional Cohort. Eur. J. Nutr. 2024, 63, 1257–1269. [Google Scholar] [CrossRef]
- Foscolou, A.; Rallidis, L.S.; Tsirebolos, G.; Critselis, E.; Katsimardos, A.; Drosatos, A.; Chrysohoou, C.; Tousoulis, D.; Pitsavos, C.; Panagiotakos, D.B. The association between homocysteine levels, Mediterranean diet and cardiovascular disease: A case-control study. Int. J. Food Sci. Nutr. 2019, 70, 603–611. [Google Scholar] [CrossRef]
- Mehndiratta, P.; Chapman Smith, S.; Worrall, B.B. Etiologic stroke subtypes: Updated definition and efficient workup strategies. Curr. Treat Options Cardiovasc. Med. 2015, 17, 357. [Google Scholar] [CrossRef]
- Soto, A.; Guillen-Grima, F.; Morales, G.; Munoz, S.; Aguinaga-Ontoso, I.; Fuentes-Aspe, R. Prevalence and incidence of ictus in Europe: Systematic review and meta-analysis. An. Sist. Sanit. Navar. 2022, 45, e0979. [Google Scholar] [CrossRef]
- Collaborators, G.B.D.S. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Sarmah, D.; Mounica, L.; Kaur, H.; Kesharwani, R.; Verma, G.; Veeresh, P.; Kotian, V.; Kalia, K.; Borah, A.; et al. Cell Death Pathways in Ischemic Stroke and Targeted Pharmacotherapy. Transl. Stroke Res. 2020, 11, 1185–1202. [Google Scholar] [CrossRef]
- Rajashekar, D.; Liang, J.W. Intracerebral Hemorrhage. In StatPerls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hankey, G.J.; Eikelboom, J.W. Homocysteine and stroke. Lancet 2005, 365, 194–196. [Google Scholar] [CrossRef]
- Perry, I.J. Homocysteine and risk of stroke. J. Cardiovasc. Risk 1999, 6, 235–240. [Google Scholar] [CrossRef]
- Gonzalez-Lamuno, D.; Arrieta-Blanco, F.J.; Fuentes, E.D.; Forga-Visa, M.T.; Morales-Conejo, M.; Pena-Quintana, L.; Vitoria-Minana, I. Hyperhomocysteinemia in Adult Patients: A Treatable Metabolic Condition. Nutrients 2023, 16, 135. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, X.; He, M.; Qin, X.; Tang, G.; Huo, Y.; Li, J.; Fu, J.; Huang, X.; Cheng, X.; et al. Homocysteine and Stroke Risk: Modifying Effect of Methylenetetrahydrofolate Reductase C677T Polymorphism and Folic Acid Intervention. Stroke 2017, 48, 1183–1190. [Google Scholar] [CrossRef]
- Holmes, M.V.; Newcombe, P.; Hubacek, J.A.; Sofat, R.; Ricketts, S.L.; Cooper, J.; Breteler, M.M.; Bautista, L.E.; Sharma, P.; Whittaker, J.C.; et al. Effect modification by population dietary folate on the association between MTHFR genotype, homocysteine, and stroke risk: A meta-analysis of genetic studies and randomised trials. Lancet 2011, 378, 584–594. [Google Scholar] [CrossRef]
- Markus, H.S.; de Leeuw, F.E. Cerebral small vessel disease: Recent advances and future directions. Int. J. Stroke 2023, 18, 4–14. [Google Scholar] [CrossRef]
- Cao, Y.; Su, N.; Zhang, D.; Zhou, L.; Yao, M.; Zhang, S.; Cui, L.; Zhu, Y.; Ni, J. Correlation between total homocysteine and cerebral small vessel disease: A Mendelian randomization study. Eur. J. Neurol. 2021, 28, 1931–1938. [Google Scholar] [CrossRef]
- Teng, Z.; Feng, J.; Liu, R.; Ji, Y.; Xu, J.; Jiang, X.; Chen, H.; Dong, Y.; Meng, N.; Xiao, Y.; et al. Cerebral small vessel disease mediates the association between homocysteine and cognitive function. Front Aging Neurosci. 2022, 14, 868777. [Google Scholar] [CrossRef] [PubMed]
- Kloppenborg, R.P.; Nederkoorn, P.J.; van der Graaf, Y.; Geerlings, M.I. Homocysteine and cerebral small vessel disease in patients with symptomatic atherosclerotic disease. The SMART-MR study. Atherosclerosis 2011, 216, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K.; Tanaka, M.; Okazaki, S.; Yagita, Y.; Sakaguchi, M.; Mochizuki, H.; Kitagawa, K. Increased Total Homocysteine Levels Predict the Risk of Incident Dementia Independent of Cerebral Small-Vessel Diseases and Vascular Risk Factors. J. Alzheimers Dis. 2016, 49, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.W.; Kwon, H.M.; Jeong, H.Y.; Park, J.H.; Kwon, H.; Jeong, S.M. Serum homocysteine level is related to cerebral small vessel disease in a healthy population. Neurology 2019, 92, e317–e325. [Google Scholar] [CrossRef]
- Zhang, P.; Xie, X.; Zhang, Y. Associations between homocysteine, vitamin B12, and folate and the risk of all-cause mortality in American adults with stroke. Front. Nutr. 2023, 10, 1279207. [Google Scholar] [CrossRef]
- Hankey, G.J.; Eikelboom, J.W. Homocysteine and vascular disease. Lancet 1999, 354, 407–413. [Google Scholar] [CrossRef]
- Christen, W.G.; Ajani, U.A.; Glynn, R.J.; Hennekens, C.H. Blood levels of homocysteine and increased risks of cardiovascular disease: Causal or casual? Arch. Intern Med. 2000, 160, 422–434. [Google Scholar] [CrossRef]
- Meleady, R.; Graham, I. Plasma homocysteine as a cardiovascular risk factor: Causal, consequential, or of no consequence? Nutr. Rev. 1999, 57, 299–305. [Google Scholar] [CrossRef]
- Ntaios, G. Homocysteine, B Vitamins, and Cardiovascular Risk. In Foods and Dietary Supplements in the Prevention and Treatment of Disease in Older Adults; Academic Press: Cambridge, MA, USA, 2015; pp. 309–318. [Google Scholar]
- Davson, H.; Hollingsworth, G.; Segal, M.B. The mechanism of drainage of the cerebrospinal fluid. Brain 1970, 93, 665–678. [Google Scholar] [CrossRef]
- Wallenstein, M.B.; McKhann, G.M. 2nd. Salomon Hakim and the discovery of normal-pressure hydrocephalus. Neurosurgery 2010, 67, 155–159. [Google Scholar] [CrossRef]
- Pyykko, O.T.; Nerg, O.; Niskasaari, H.M.; Niskasaari, T.; Koivisto, A.M.; Hiltunen, M.; Pihlajamaki, J.; Rauramaa, T.; Kojoukhova, M.; Alafuzoff, I.; et al. Incidence, Comorbidities, and Mortality in Idiopathic Normal Pressure Hydrocephalus. World Neurosurg. 2018, 112, e624–e631. [Google Scholar] [CrossRef] [PubMed]
- Skalicky, P.; Mladek, A.; Vlasak, A.; De Lacy, P.; Benes, V.; Bradac, O. Normal pressure hydrocephalus-an overview of pathophysiological mechanisms and diagnostic procedures. Neurosurg. Rev. 2020, 43, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Vanneste, J.A. Diagnosis and management of normal-pressure hydrocephalus. J. Neurol. 2000, 247, 5–14. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Hu, F.; Ding, J.; Wang, X. Pathogenesis and pathophysiology of idiopathic normal pressure hydrocephalus. CNS Neurosci. Ther. 2020, 26, 1230–1240. [Google Scholar] [CrossRef]
- Ye, S.; Feng, K.; Li, Y.; Liu, S.; Wu, Q.; Feng, J.; Liao, X.; Jiang, C.; Liang, B.; Yuan, L.; et al. High homocysteine is associated with idiopathic normal pressure hydrocephalus in deep perforating arteriopathy: A cross-sectional study. BMC Geriatr. 2023, 23, 382. [Google Scholar]
- Bateman, G.A. Vascular compliance in normal pressure hydrocephalus. AJNR Am. J. Neuroradiol. 2000, 21, 1574–1585. [Google Scholar]
- Guillotin, S.; Vallet, A.; Lorthois, S.; Cestac, P.; Schmidt, E.; Delcourt, N. Association Between Homocysteine, Frailty and Biomechanical Response of the CNS in NPH-Suspected Patients. J. Gerontol. 2022, 77, 1335–1343. [Google Scholar] [CrossRef]
- Sosvorova, L.; Bestak, J.; Bicikova, M.; Mohapl, M.; Hill, M.; Kubatova, J.; Hampl, R. Determination of homocysteine in cerebrospinal fluid as an indicator for surgery treatment in patients with hydrocefalus. Physiol. Res. 2014, 63, 521–527. [Google Scholar] [CrossRef]
- Sosvorova, L.; Mohapl, M.; Hill, M.; Starka, L.; Bicikova, M.; Vitku, J.; Kanceva, R.; Bestak, J.; Hampl, R. Steroid hormones and homocysteine in the outcome of patients with normal pressure hydrocephalus. Physiol. Res. 2015, 64 (Suppl. 2), S227–S236. [Google Scholar] [CrossRef]
- Cains, S.; Shepherd, A.; Nabiuni, M.; Owen-Lynch, P.J.; Miyan, J. Addressing a folate imbalance in fetal cerebrospinal fluid can decrease the incidence of congenital hydrocephalus. J. Neuropathol. Exp. Neurol. 2009, 68, 404–416. [Google Scholar] [CrossRef]
- Wyse, A.T.S.; Bobermin, L.D.; Dos Santos, T.M.; Quincozes-Santos, A. Homocysteine and Gliotoxicity. Neurotox. Res. 2021, 39, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.K.; Kalani, A.; Givvimani, S.; Sathnur, P.B.; Tyagi, S.C.; Tyagi, N. Hydrogen sulfide attenuates neurodegeneration and neurovascular dysfunction induced by intracerebral-administered homocysteine in mice. Neuroscience 2013, 252, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Singh, N. Homocysteine excess: Delineating the possible mechanism of neurotoxicity and depression. Fundam. Clin. Pharmacol. 2015, 29, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, D.E.; Wang, X.L.; Adachi, T.; Hara, H.; Duarte, N.; Green, K.; Wilcken, B. Relationship between homocysteine and superoxide dismutase in homocystinuria: Possible relevance to cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1199–1202. [Google Scholar] [CrossRef]
- Vitvitsky, V.; Thomas, M.; Ghorpade, A.; Gendelman, H.E.; Banerjee, R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J. Biol. Chem. 2006, 281, 35785–35793. [Google Scholar] [CrossRef]
- Siow, Y.L.; Au-Yeung, K.K.; Woo, C.W.; O, K. Homocysteine stimulates phosphorylation of NADPH oxidase p47phox and p67phox subunits in monocytes via protein kinase Cbeta activation. Biochem. J. 2006, 398, 73–82. [Google Scholar] [CrossRef]
- Lehotsky, J.; Tothova, B.; Kovalska, M.; Dobrota, D.; Benova, A.; Kalenska, D.; Kaplan, P. Role of Homocysteine in the Ischemic Stroke and Development of Ischemic Tolerance. Front. Neurosci. 2016, 10, 538. [Google Scholar] [CrossRef]
- Chen, S.; Dong, Z.; Cheng, M.; Zhao, Y.; Wang, M.; Sai, N.; Wang, X.; Liu, H.; Huang, G.; Zhang, X. Homocysteine exaggerates microglia activation and neuroinflammation through microglia localized STAT3 overactivation following ischemic stroke. J. Neuroinflammation 2017, 14, 187. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, X.; Wang, H. Hyperhomocysteinemia and Endothelial Dysfunction. Curr. Hypertens Rev. 2009, 5, 158–165. [Google Scholar] [CrossRef]
- Sachdev, P. Homocysteine and neuropsychiatric disorders. Braz. J. Psychiatry 2004, 26, 50–56. [Google Scholar] [CrossRef]
- Paul, R.; Dutta, A.; Phukan, B.C.; Mazumder, M.K.; Justin-Thenmozhi, A.; Manivasagam, T.; Bhattacharya, P.; Borah, A. Accumulation of Cholesterol and Homocysteine in the Nigrostriatal Pathway of Brain Contributes to the Dopaminergic Neurodegeneration in Mice. Neuroscience 2018, 388, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Canteli, M.; Iadecola, C. Alzheimer’s Disease and Vascular Aging: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Paul, G.; Elabi, O.F. Microvascular Changes in Parkinson’s Disease- Focus on the Neurovascular Unit. Front Aging Neurosci. 2022, 14, 853372. [Google Scholar] [CrossRef] [PubMed]
- Ahlskog, J.E. Levodopa, homocysteine and Parkinson’s disease: What’s the problem? Park. Relat. Disord. 2023, 109, 105357. [Google Scholar] [CrossRef] [PubMed]
- Stanger, O.; Fowler, B.; Piertzik, K.; Huemer, M.; Haschke-Becher, E.; Semmler, A.; Lorenzl, S.; Linnebank, M. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: Review and treatment recommendations. Expert Rev. Neurother. 2009, 9, 1393–1412. [Google Scholar] [CrossRef]
- Lentz, S.R. Homocysteine and vascular dysfunction. Life Sci. 1997, 61, 1205–1215. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Das, U.N.; Stefanadis, C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J. Am. Coll. Cardiol. 2004, 44, 152–158. [Google Scholar] [CrossRef]
- Kataria, N.; Yadav, P.; Kumar, R.; Kumar, N.; Singh, M.; Kant, R.; Kalyani, V. Effect of Vitamin B6, B9, and B12 Supplementation on Homocysteine Level and Cardiovascular Outcomes in Stroke Patients: A Meta-Analysis of Randomized Controlled Trials. Cureus 2021, 13, e14958. [Google Scholar] [CrossRef]
- Liu, S.; West, R.; Randell, E.; Longerich, L.; O’Connor, K.S.; Scott, H.; Crowley, M.; Lam, A.; Prabhakaran, V.; McCourt, C. A comprehensive evaluation of food fortification with folic acid for the primary prevention of neural tube defects. BMC Pregnancy Childbirth 2004, 4, 20. [Google Scholar] [CrossRef]
- Collaboration, H.L.T. Lowering blood homocysteine with folic acid based supplements: Meta-analysis of randomised trials. Homocysteine Lowering Trialists’ Collaboration. BMJ 1998, 316, 894–898. [Google Scholar] [CrossRef]
- Katsiki, N.; Perez-Martinez, P.; Mikhailidis, D.P. Homocysteine and Non-Cardiac Vascular Disease. Curr. Pharm. Des. 2017, 23, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Ford, T.C.; Downey, L.A.; Simpson, T.; McPhee, G.; Oliver, C.; Stough, C. The Effect of a High-Dose Vitamin B Multivitamin Supplement on the Relationship between Brain Metabolism and Blood Biomarkers of Oxidative Stress: A Randomized Control Trial. Nutrients 2018, 10, 1860. [Google Scholar] [CrossRef] [PubMed]
- Jacques, P.F.; Selhub, J.; Bostom, A.G.; Wilson, P.W.; Rosenberg, I.H. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N. Engl. J. Med. 1999, 340, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Botto, L.D.; Erickson, J.D.; Berry, R.J.; Sambell, C.; Johansen, H.; Friedman, J.M. Improvement in stroke mortality in Canada and the United States, 1990 to 2002. Circulation 2006, 113, 1335–1343. [Google Scholar] [CrossRef]
- Toole, J.F.; Malinow, M.R.; Chambless, L.E.; Spence, J.D.; Pettigrew, L.C.; Howard, V.J.; Stampfer, M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: The Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA 2004, 291, 565–575. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Y.; Wang, Y.; Li, L.; Liao, Y.; Zhang, Y.; Yu, D. The effect of folic acid in patients with cardiovascular disease: A systematic review and meta-analysis. Medicine 2019, 98, e17095. [Google Scholar] [CrossRef]
- Heinz, J.; Kropf, S.; Luley, C.; Dierkes, J. Homocysteine as a risk factor for cardiovascular disease in patients treated by dialysis: A meta-analysis. Am. J. Kidney Dis. 2009, 54, 478–489. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, T.; Wan, Z.; Lu, Q.; Zhang, X.; Qiu, Z.; Li, L.; Zhu, K.; Liu, L.; Pan, A.; et al. Associations of Serum Folate and Vitamin B12 Levels With Cardiovascular Disease Mortality Among Patients With Type 2 Diabetes. JAMA Netw. Open 2022, 5, e2146124. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Q.; Gong, C.X. Hydrocephalus presented as the prominent symptom of severe 5,10-methylenetetrahydrofolate reductase deficiency in an infant: A case report. Med. Int. 2022, 2, 12. [Google Scholar] [CrossRef]
- Ingrid Goh, Y.; Bollano, E.; Einarson, T.R.; Koren, G. Prenatal multivitamin supplementation and rates of congenital anomalies: A meta-analysis. J Obstet. Gynaecol. Can. 2006, 28, 680–689. [Google Scholar] [CrossRef]
- Deakova, Z.; Orszaghova, Z.; Andrezalova, L.; Slezak, P.; Lehotay, J.; Muchova, J.; Burki, C.; Durackova, Z. Influence of oak wood polyphenols on cysteine, homocysteine and glutathione total levels and PON1 activities in human adult volunteers—A pilot study. Gen. Physiol. Biophys. 2015, 34, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, L.; Si, X.; Tian, J.L.; Zhang, Y.; Gui, H.L.; Li, B.; Tan, D.H. Current progress on the mechanisms of hyperhomocysteinemia-induced vascular injury and use of natural polyphenol compounds. Eur. J. Pharmacol. 2021, 905, 174168. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Lista, J.; Alcala-Diaz, J.F.; Torres-Pena, J.D.; Quintana-Navarro, G.M.; Fuentes, F.; Garcia-Rios, A.; Ortiz-Morales, A.M.; Gonzalez-Requero, A.I.; Perez-Caballero, A.I.; Yubero-Serrano, E.M.; et al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): A randomised controlled trial. Lancet 2022, 399, 1876–1885. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef]
- Lee, J.; Mitchell, A.E. Pharmacokinetics of quercetin absorption from apples and onions in healthy humans. J. Agric. Food Chem. 2012, 60, 3874–3881. [Google Scholar] [CrossRef]
- Somerset, S.M.; Johannot, L. Dietary flavonoid sources in Australian adults. Nutr. Cancer 2008, 60, 442–449. [Google Scholar] [CrossRef]
- Farhan, M. Green Tea Catechins: Nature’s Way of Preventing and Treating Cancer. Int. J. Mol. Sci. 2022, 23, 10713. [Google Scholar] [CrossRef]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial Properties of Green Tea Catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef]
- Castaldo, L.; Narvaez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Minno, G.D.; Ritieni, A. Red Wine Consumption and Cardiovascular Health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef]
- Tabeshpour, J.; Mehri, S.; Shaebani Behbahani, F.; Hosseinzadeh, H. Protective effects of Vitis vinifera (grapes) and one of its biologically active constituents, resveratrol, against natural and chemical toxicities: A comprehensive review. Phytother. Res. 2018, 32, 2164–2190. [Google Scholar] [CrossRef]
- Kocaadam, B.; Sanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive Compounds of Strawberry and Blueberry and Their Potential Health Effects Based on Human Intervention Studies: A Brief Overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Salis, C.; Papageorgiou, L.; Papakonstantinou, E.; Hagidimitriou, M.; Vlachakis, D. Olive Oil Polyphenols in Neurodegenerative Pathologies. Adv. Exp. Med. Biol. 2020, 1195, 77–91. [Google Scholar]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and Dark Chocolate Polyphenols: From Biology to Clinical Applications. Front Immunol. 2017, 8, 677. [Google Scholar] [CrossRef]
- Ciaramelli, C.; Palmioli, A.; Angotti, I.; Colombo, L.; De Luigi, A.; Sala, G.; Salmona, M.; Airoldi, C. NMR-Driven Identification of Cinnamon Bud and Bark Components With Anti-Abeta Activity. Front. Chem. 2022, 10, 896253. [Google Scholar] [CrossRef]
- Najar, A.M.; Romero-Bernal, M.; Del Rio, C.; Montaner, J. A Review on Polyphenols in Salicornia ramosissima with Special Emphasis on Their Beneficial Effects on Brain Ischemia. Nutrients 2023, 15, 793. [Google Scholar] [CrossRef]
- Li, H.Z.; Liu, K.G.; Zeng, N.X.; Wu, X.F.; Lu, W.J.; Xu, H.F.; Yan, C.; Wu, L.L. Luteolin Enhances Choroid Plexus 5-MTHF Brain Transport to Promote Hippocampal Neurogenesis in LOD Rats. Front. Pharmacol. 2022, 13, 826568. [Google Scholar] [CrossRef]
- Meng, B.; Gao, W.; Wei, J.; Pu, L.; Tang, Z.; Guo, C. Quercetin Increases Hepatic Homocysteine Remethylation and Transsulfuration in Rats Fed a Methionine-Enriched Diet. Biomed. Res. Int. 2015, 2015, 815210. [Google Scholar] [CrossRef]
- Turovskaya, M.V.; Gaidin, S.G.; Mal’tseva, V.N.; Zinchenko, V.P.; Turovsky, E.A. Taxifolin protects neurons against ischemic injury in vitro via the activation of antioxidant systems and signal transduction pathways of GABAergic neurons. Mol. Cell. Neurosci. 2019, 96, 10–24. [Google Scholar] [CrossRef]
- Liang, G.; Shi, B.; Luo, W.; Yang, J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav. Brain Funct. 2015, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Y.Z.; Ding, Y.H.; Wang, J.; Geng, J.; Yang, H.; Ren, J.; Tang, J.Y.; Gao, J. Neuroprotective effects of gallic acid against hypoxia/reoxygenation-induced mitochondrial dysfunctions in vitro and cerebral ischemia/reperfusion injury in vivo. Brain Res. 2014, 1589, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Racek, J.; Rusnakova, H.; Trefil, L.; Siala, K.K. The influence of folate and antioxidants on homocysteine levels and oxidative stress in patients with hyperlipidemia and hyperhomocysteinemia. Physiol. Res. 2005, 54, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Celik, N.; Vurmaz, A.; Kahraman, A. Protective effect of quercetin on homocysteine-induced oxidative stress. Nutrition 2017, 33, 291–296. [Google Scholar] [CrossRef]
- Koz, S.T.; Etem, E.O.; Baydas, G.; Yuce, H.; Ozercan, H.I.; Kuloglu, T.; Koz, S.; Etem, A.; Demir, N. Effects of resveratrol on blood homocysteine level, on homocysteine induced oxidative stress, apoptosis and cognitive dysfunctions in rats. Brain Res. 2012, 1484, 29–38. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Yu, B.; Liang, H.; Mao, S.; Hu, X.; Feng, Y.; Xu, J.; Chu, L. Quercetin improves cerebral ischemia/reperfusion injury by promoting microglia/macrophages M2 polarization via regulating PI3K/Akt/NF-kappaB signaling pathway. Biomed. Pharmacother. 2023, 168, 115653. [Google Scholar] [CrossRef]
- Zeini, S.; Davoodian, N.; Kazemi, H.; Shareghi Brojeni, M.; Ghani, E.; Arab Firouzjaei, M.; Atashabparvar, A. Resveratrol prevents cognitive impairment and hippocampal inflammatory response induced by lipopolysaccharide in a mouse model of chronic neuroinflammation. Physiol. Behav. 2024, 278, 114508. [Google Scholar] [CrossRef]
- Schroecksnadel, K.; Winkler, C.; Wirleitner, B.; Schennach, H.; Weiss, G.; Fuchs, D. Anti-inflammatory compound resveratrol suppresses homocysteine formation in stimulated human peripheral blood mononuclear cells in vitro. Clin. Chem. Lab. Med. 2005, 43, 1084–1088. [Google Scholar] [CrossRef]
- Manna, C.; Napoli, D.; Cacciapuoti, G.; Porcelli, M.; Zappia, V. Olive oil phenolic compounds inhibit homocysteine-induced endothelial cell adhesion regardless of their different antioxidant activity. J. Agric. Food Chem. 2009, 57, 3478–3482. [Google Scholar] [CrossRef]
- Zhao, H.P.; Feng, J.; Sun, K.; Liu, Y.Y.; Wei, X.H.; Fan, J.Y.; Huang, P.; Mao, X.W.; Zhou, Z.; Wang, C.S.; et al. Caffeic acid inhibits acute hyperhomocysteinemia-induced leukocyte rolling and adhesion in mouse cerebral venules. Microcirculation 2012, 19, 233–244. [Google Scholar] [CrossRef]
- Zhao, X.; Hui, Q.C.; Xu, R.; Gao, N.; Cao, P. Resveratrol: A new approach to ameliorate hyperhomocysteinaemia-induced renal dysfunction. Exp. Ther. Med. 2022, 24, 510. [Google Scholar] [CrossRef] [PubMed]
- Nájar, A.M.; Pérez-Sánchez, S.; del Río, C.; Domínguez, C.; Azcárate, C.L.; de Torres, R.; Lamana-Vallverdú, M.; Romero-Bernal, M.; González-Díaz, A.; Cádiz-Gurrea, M.d.l.L.; et al. Dietary supplementation with polyphenol-rich Salicornia ramosissima extracts: Assessing safety, efficacy, and impact on cardiovascular health biomarkers in healthy volunteers. J. Funct. Foods. 2024, 122, 106539. [Google Scholar] [CrossRef]
- Portincasa, P.; Calamita, G. Phytocompounds modulating Aquaporins: Clinical benefits are anticipated. Food Chem. 2019, 274, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Park, H.; Jeong, M.J.; Kang, T.C. Epigallocatechin-3-Gallate and PEDF 335 Peptide, 67LR Activators, Attenuate Vasogenic Edema, and Astroglial Degeneration Following Status Epilepticus. Antioxidants 2020, 9, 854. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, S. Protective mechanisms of tea polyphenols regulating the PI3K/Akt pathway on early brain injury after subarachnoid hemorrhage in rats. Cell. Mol. Biol. 2023, 69, 76–82. [Google Scholar]
- Liu, X.; Wang, Z.; Wang, P.; Yu, B.; Liu, Y.; Xue, Y. Green tea polyphenols alleviate early BBB damage during experimental focal cerebral ischemia through regulating tight junctions and PKCalpha signaling. BMC Complement. Altern. Med. 2013, 13, 187. [Google Scholar] [CrossRef]
- Catalao, C.H.; Correa, D.A.; Saito, S.T.; Lopes Lda, S. Camellia sinensis neuroprotective role in experimentally induced hydrocephalus in Wistar rats. Childs Nerv. Syst. 2014, 30, 591–597. [Google Scholar] [CrossRef]
- Etus, V.; Altug, T.; Belce, A.; Ceylan, S. Green tea polyphenol (-)-epigallocatechin gallate prevents oxidative damage on periventricular white matter of infantile rats with hydrocephalus. Tohoku J. Exp. Med. 2003, 200, 203–209. [Google Scholar] [CrossRef]
- Laird, M.D.; Sukumari-Ramesh, S.; Swift, A.E.; Meiler, S.E.; Vender, J.R.; Dhandapani, K.M. Curcumin attenuates cerebral edema following traumatic brain injury in mice: A possible role for aquaporin-4? J. Neurochem. 2010, 113, 637–648. [Google Scholar] [CrossRef]
- Jin, Z.; Ke, J.; Guo, P.; Wang, Y.; Wu, H. Quercetin improves blood-brain barrier dysfunction in rats with cerebral ischemia reperfusion via Wnt signaling pathway. Am. J. Transl. Res. 2019, 11, 4683–4695. [Google Scholar]
- Jin, W.; Ying, G.; ShaoYue, Z. Vanillic Acid Improve Neural Function after Focal Cerebral Ischemia-reperfusion Rats. Int. J. Pharmacol. 2018, 14, 488–494. [Google Scholar]
- Wang, J.; Mao, J.; Wang, R.; Li, S.; Wu, B.; Yuan, Y. Kaempferol Protects Against Cerebral Ischemia Reperfusion Injury Through Intervening Oxidative and Inflammatory Stress Induced Apoptosis. Front Pharmacol. 2020, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zhang, R.; Li, Y.; Li, Y.; Yang, Z.; Yang, H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 2017, 40, 1444–1456. [Google Scholar] [CrossRef]
- Shah, M.A.; Kang, J.B.; Park, D.J.; Kim, M.O.; Koh, P.O. Chlorogenic acid alleviates neurobehavioral disorders and brain damage in focal ischemia animal models. Neurosci. Lett. 2021, 760, 136085. [Google Scholar] [CrossRef]
- Bayes, J.; Bedaso, A.; Peng, W.; Adams, J.; Sibbritt, D. The effect of polyphenols in post stroke adults: A systematic review of randomised controlled trials. Clin. Nutr. ESPEN 2023, 54, 113–121. [Google Scholar] [CrossRef]
- Garbagnati, F.; Cairella, G.; De Martino, A.; Multari, M.; Scognamiglio, U.; Venturiero, V.; Paolucci, S. Is antioxidant and n-3 supplementation able to improve functional status in poststroke patients? Results from the Nutristroke Trial. Cerebrovasc. Dis. 2009, 27, 375–383. [Google Scholar] [CrossRef]
- Nájar, A.M.; Lopez-Azcarate, C.; Domínguez Ruiz, C.; Núñez-Jurado, D.; de Torres, R.; López, R.; Camino-Moya, M.; Magni, E.; Montero-Ramirez, E.; Bocero, A.; et al. Evaluating the Clinical Impact of a Polyphenol-Rich Extract from Salicornia ramosissima on Patients with Transient Ischemic Attack and Minor Stroke. Nutrients 2024, 16, 4307. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Salguero, C.; Romero-Bernal, M.; González-Díaz, Á.; Doush, E.S.; del Río, C.; Echevarría, M.; Montaner, J. Hyperhomocysteinemia: Underlying Links to Stroke and Hydrocephalus, with a Focus on Polyphenol-Based Therapeutic Approaches. Nutrients 2025, 17, 40. https://doi.org/10.3390/nu17010040
Ortiz-Salguero C, Romero-Bernal M, González-Díaz Á, Doush ES, del Río C, Echevarría M, Montaner J. Hyperhomocysteinemia: Underlying Links to Stroke and Hydrocephalus, with a Focus on Polyphenol-Based Therapeutic Approaches. Nutrients. 2025; 17(1):40. https://doi.org/10.3390/nu17010040
Chicago/Turabian StyleOrtiz-Salguero, Carmen, Marina Romero-Bernal, Ángela González-Díaz, Elaheh Sobh Doush, Carmen del Río, Miriam Echevarría, and Joan Montaner. 2025. "Hyperhomocysteinemia: Underlying Links to Stroke and Hydrocephalus, with a Focus on Polyphenol-Based Therapeutic Approaches" Nutrients 17, no. 1: 40. https://doi.org/10.3390/nu17010040
APA StyleOrtiz-Salguero, C., Romero-Bernal, M., González-Díaz, Á., Doush, E. S., del Río, C., Echevarría, M., & Montaner, J. (2025). Hyperhomocysteinemia: Underlying Links to Stroke and Hydrocephalus, with a Focus on Polyphenol-Based Therapeutic Approaches. Nutrients, 17(1), 40. https://doi.org/10.3390/nu17010040





