Vitamin D and LC-PUFA and the Presence of Fetal Heart Defects—A Further Part of a Case-Control Study
Abstract
1. Introduction
1.1. What’s New?
- This study provides new data on the intake and supplementation of vitamin D as well as long-chain fatty acids in the context of the occurrence of fetal heart defects, as there are few studies analyzing both the intake and supplementation of these ingredients before and during pregnancy in the context of congenital heart defects.
- Compared to the study group with fetal heart defects, women from the control group featured a significantly higher average intake of vitamin D and omega-3 acids together with additional supplementation. Additional supplementation of these ingredients in the diet may prove helpful and reduce the risk of occurrence of CHD in the children, which was not demonstrated in this study, however, further studies on a larger population group taking into account these ingredients would undoubtedly be needed.
1.2. What Are the Clinical Implications?
- It is worth indicating preconceptional supplementation of vitamin D and DHA in the recommendations for women preparing for conception and during pregnancy in order to protect their fetuses against CHD. Such supplementation may prove to be a kind of a protection against the occurrence of heart defects in children, however, insufficient intake of these important ingredients is observed.
- It is also worth educating women of reproductive age and healthcare workers to indicate and promote the necessary information on the benefits of a well-balanced diet and/or additional supplementation.
- In order to prevent congenital heart defects in the fetus, the participation of a dietician in preconception counseling, including medical, gynecological, family doctor’s offices should be considered for providing health education for future parents. Training and online counseling should be delivered for public health specialists and for those who want to have a child by providing reliable and up-to-date information on nutritional recommendations
1.3. The Aim of the Study
2. Material and Methods
2.1. Study Group
2.2. Statistical Analysis
3. Results
4. Discussion
4.1. Vitamin D—Intake and Supplementation vs. CHD
4.2. n-3 LC-PUFA (Including DHA)—Intake and Supplementation vs. CHD
5. Strengths and Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Chen, S.; Zühlke, L.; Black, G.C.; Choy, M.-K.; Li, N.; Keavney, B.D. Global birth prevalence of congenital heart defects 1970–2017: Updated systematic review and meta-analysis of 260 studies. Int. J. Epidemiol. 2019, 48, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Mires, S.; Caputo, M.; Overton, T.; Skerritt, C. Maternal micronutrient deficiency and congenital heart disease risk: A systematic review of observational studies. Birth Defects Res. 2022, 114, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.C.; Thorsteinsdottir, F.; Christesen, H.T.; Hjortdal, V.E.; Heitmann, B.L.; Specht, I.O.; Händel, M.N. Vitamin D Supplementation and Vitamin D Status during Pregnancy and the Risk of Congenital Anomalies—A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2125. [Google Scholar] [CrossRef] [PubMed]
- Amza, M.; Hamoud, B.H.; Sima, R.-M.; Dinu, M.-D.; Gorecki, G.-P.; Popescu, M.; Gică, N.; Poenaru, M.-O.; Pleș, L. Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA)—Should They Be Mandatory Supplements in Pregnancy? Biomedicines 2024, 12, 1471. [Google Scholar] [CrossRef]
- Liu, W.; Ren, L.; Fang, F.; Chen, R. Maternal pre-pregnancy overweight or obesity and risk of birth defects in offspring: Population-based cohort study. Acta Obstet. Gynecol. Scand. 2024, 103, 862–872. [Google Scholar] [CrossRef]
- Adams, B.J.; Kirby, K.J.; Sorensen, C.J.; Pollard, L.E.; Audhya, T. Evidence based recommendations for an optimal prenatal supplement for women in the US: Vitamins and related nutrients. Matern. Health Neonatol. Perinatol. 2022, 8, 4. [Google Scholar] [CrossRef]
- Wendołowicz, A.; Stefańska, E.; Ostrowska, L. Żywienie kobiet w okresie ciąży [Nutrition for Pregnant Women]. Med. Ogólna Nauk. Zdrowiu 2014, 21, 341–345. [Google Scholar] [CrossRef]
- Grzelak, T.; Suliga, K.; Sperling, M.; Pelczyńska, M.; Czyżewska, K. Ocena stosowania suplementów diety wśród kobiet ciężarnych lub planujących ciążę [Assessment of the use of dietary supplements among pregnant women or those planning a pregnancy]. Forum Zaburzeń Metab. 2016, 7, 8–15. [Google Scholar]
- Aparicio, E.; Martín-Grau, C.; Hernández-Martinez, C.; Voltas, N.; Canals, J.; Arija, V. Changes in fatty acid levels (saturated, monounsaturated and polyunsaturated) during pregnancy. BMC Pregnancy Childbirth 2021, 21, 778. [Google Scholar] [CrossRef]
- Bałtuć, K. The influence of the vitamin d during pregnancy and in maternal and child health. Pol. Przegląd Nauk. Zdrowiu 2019, 4, 363–368. [Google Scholar] [CrossRef]
- Mokhtar, A.W.; Fawzy, A.; Allam, M.R.; Amer, M.R.; Hamed, S.M. Maternal vitamin D level and vitamin D receptor gene polymorphism as a risk factor for congenital heart diseases in offspring; An Egyptian case-control study. Genes Dis. 2019, 6, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, P.; Frank, G.; Cianci, R.; Dominici, F.; Mappa, I.; Rizzo, G.; De Santis, G.L.; Bigioni, G.; Di Renzo, L. Fish Consumption and DHA Supplementation during Pregnancy: Study of Gestational and Neonatal Outcomes. Nutrients 2024, 16, 3051. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E.J.; Birch, C.S.; Bonwick, G.A.; English, A.; Metcalfe, P.; Li, W. Optimal omegas—Barriers and novel methods to narrow omega-3 gaps. A narrative review. Front. Nutr. 2024, 11, 1325099. [Google Scholar] [CrossRef] [PubMed]
- Sley, E.G.; Rosen, E.M.; van ‘t Erve, T.J.; Sathyanarayana, S.; Barrett, E.S.; Nguyen, R.H.; Bush, N.R.; Milne, G.L.; Swan, S.H.; Ferguson, K.K. Omega-3 fatty acid supplement use and oxidative stress levels in pregnancy. PLoS ONE 2020, 15, e0240244. [Google Scholar] [CrossRef] [PubMed]
- Koster, M.P.H.; van Duijn, L.; Krul-Poel, Y.; Laven, J.; Helbing, W.; Simsek, S.; Steegers-Theunissen, R. A Compromised Maternal Vitamin D Status is Associated With Congenital Heart Defects in Offspring. Early Hum. Dev. 2018, 117, 50–56. [Google Scholar] [CrossRef]
- Kolmaga, A.; Trafalska, E.; Gaszyńska, E.; Murlewska, J.; Witkowski, S.; Sylwestrzak, O.; Sokołowski, Ł.; Respondek-Liberska, M.; Strzelecka, I. Folic Acid and Selected Risk Factors for Fetal Heart Defects—Preliminary Study Results. Nutrients 2024, 16, 3024. [Google Scholar] [CrossRef]
- Wajszczyk, B.; Chwojnowska, Z.; Nasiadko, D.; Rybaczuk, M. Instrukcja Korzystania Z Programu Dieta 6.0 Do Planowania I Bieżącej Oceny Żywienia Indywidualnego; National Institute of Public Health—National Institute of Hygiene: Warsaw, Poland, 2021. [Google Scholar]
- Stoś, K.; Głowala, A.; Ziółkowska, I. Normy a suplementacja [Norms and supplementation]. In Normy Żywienia Dla Populacji Polski I Ich Zastosowanie [Nutrition Standards for the Polish Population and Their Use]; Jarosz, M., Rychlik, E., Stoś, K., Charzewska, J., Eds.; Narodowy Instytut Zdrowia Publicznego—Państwowy Zakład Higieny: Warszawa, Poland, 2020; pp. 414–463. [Google Scholar]
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Cuartas, S.; Torre, M. Metabolism and importance of polyunsaturated fatty acids in pregnancy and lactation. Rev. Cuba. Pediatría 2021, 93, e1194. [Google Scholar]
- Zimmer, M.; Sieroszewski, P.; Oszukowski, P.; Huras, H.; Fuchs, T.; Pawlosek, A. Polish Society of Gynecologists and Obstetricians recommendations on supplementation in pregnancy. Ginekol. Pol. 2020, 91, 644–653. [Google Scholar] [CrossRef]
- Çobanoğullari, H.; Ergoren, M.C.; Dundar, M.; Bertelli, M.; Tulay, P. Periconceptional Mediterranean diet during pregnancy on children’s health. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E65–E73. [Google Scholar] [CrossRef]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019. [Google Scholar] [CrossRef]
- Sosnowska, C. Wytyczne dotyczące profilaktyki i leczenia niedoboru witaminy D—Aktualizacja z 2023 r. w Polsce [Guidelines for Preventing and Treating Vitamin D Deficiency—Update as of 2023 in Poland]. Stand. Med. Pediatr. 2023, 20, 365–374. [Google Scholar]
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; Van Goudoever, J.B.; De Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. Nutrition during pregnancy, lactation, and early childhood and its implications for maternal and long-term child health: The EarlyNutrition Project recommendations. Ann. Nutr. Metab. 2019, 74, 93–106. [Google Scholar] [CrossRef]
- Wierzejska, R. Oszacowanie spożycia witaminy D i jej źródła w diecie kobiet ciężarnych [Estimation of vitamin D intake and its sources in the diet of pregnant women]. Zyw. Człowieka Metab. 2018, 45, 172–180. [Google Scholar]
- Agustina, R.; Rianda, D.; Lasepa, W.; Birahmatika, F.S.; Stajic, V.; Mufida, R. Nutrient intakes of pregnant and lactating women in Indonesia and Malaysia: Systematic review and meta-analysis. Front. Nutr. 2023, 10, 1030343. [Google Scholar] [CrossRef]
- Kocyłowski, R.; Lewicka, I.; Grzesiak, M.; Gaj, Z.; Sobańska, A.; Poznaniak, J.; von Kaisenberg, C.; Suliburska, J. Assessment of dietary intake and mineral status in pregnant women. Arch. Gynecol. Obstet. 2018, 297, 1433–1440. [Google Scholar] [CrossRef]
- Bärebring, L.; Amberntsson, A.; Winkvist, A.; Augustin, H. Validation of Dietary Vitamin D Intake from Two Food Frequency Questionnaires, Using Food Records and the Biomarker 25-Hydroxyvitamin D among Pregnant Women. Nutrients 2018, 10, 745. [Google Scholar] [CrossRef]
- Jun, S.; Gahche, J.J.; Potischman, N.; Dwyer, J.T.D.; Guenther, P.M.P.; Sauder, K.A.; Bailey, R.L.P. Dietary supplement use and its micronutrient contribution during pregnancy and lactation in the United States Shinyoung. Obstet. Gynecol. 2020, 135, 623–633. [Google Scholar] [CrossRef]
- Dilli, D.; Doğan, N.N.; Örün, U.A.; Koç, M.; Zenciroğlu, A.; Karademir, S.; Akduman, H. Maternal and neonatal micronutrient levels in newborns with CHD. Cardiol. Young 2018, 28, 523–529. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Wei, L.; Zhang, H.; Zhang, J.; Zhou, X.; Zhu, S.; Du, Y.; Su, R.; Fang, C.; et al. DHA supplementation and pregnancy complications. J. Transl. Med. 2023, 21, 394. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Lyden, E.; Anderson-Berry, A.; Hanson, C. Omega-3 Fatty Acid Intake of Pregnant Women and Women of Childbearing Age in the United States: Potential for Deficiency? Nutrients 2017, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Grot, M.; Grabowska, K.; Białek-Dratwa, A.; Grajek, M. Pregnant women’s diets and fatty acid intake—A study of the frequency of intake of products that are sources of fatty acids among women in the third trimester of pregnancy. J. Educ. Health Sport 2022, 12, 579–595. [Google Scholar] [CrossRef]
- Nikolajeva, K.; Aizbalte, O.; Rezgale, R.; Cauce, V.; Zacs, D.; Meija, L. The Intake of Omega-3 Fatty Acids, the Omega-3 Index in Pregnant Women, and Their Correlations with Gestational Length and Newborn Birth Weight. Nutrients 2024, 16, 2150. [Google Scholar] [CrossRef] [PubMed]
- Masina, M.; Medithi, S.; Muley, A. Impact of maternal essential fatty acid intake on the birth weight of infants. J. Mother Child 2023, 27, 147–157. [Google Scholar] [CrossRef]
- Wiesner, A.; Paśko, P. Stosowanie suplementów u kobiet ciężarnych w świetle najnowszych rekomendacji Polskiego Towarzystwa Ginekologów i Położników [The use of supplements in pregnant women in the light of the latest recommendations of the Polish Society of Gynecologists and Obstetricians]. Farm Pol. 2021, 77, 40–47. [Google Scholar] [CrossRef]
- Roke, K.; Rattner, J.; Brauer, P.; Mutch, D.M. Awareness of Omega-3 fatty acids and possible health effects among young adults. Can. J. Diet. Pract. Res. 2018, 79, 106–112. [Google Scholar] [CrossRef]
- Wierzejska, R.; Jarosz, M.; Wojda, B.; Siuba-Strzelińska, M. Dietetyczne spożycie DHA w czasie ciąży: Znaczna różnica między rzeczywistym spożyciem a aktualnymi zaleceniami żywieniowymi [Dietary intake of DHA during pregnancy: Significant difference between actual intake and current dietary recommendations]. Rocz. Panstw. Zakl. Hig. 2018, 69, 381–386. [Google Scholar] [CrossRef]
- Capra, L.; Tezza, G.; Mazzei, F.; Boner, A.L. The origins of health and disease: The influence of maternal diseases and lifestyle during. Ital. J. Pediatr. 2013, 39, 7. Available online: http://www.ijponline.net/content/39/1/7 (accessed on 12 September 2024). [CrossRef]
- Obermann-Borst, S.A.; Vujkovic, M.; de Vries, J.H.; Wildhagen, M.F.; Looman, C.W.; de Jonge, R.; Steegers, E.A.P.; Steegers-Theunissen, R.P.M. A maternal dietary pattern characterised by fish and seafood in association with the risk of congenital heart defects in the offspring. BJOG 2011, 118, 1205–1215. [Google Scholar] [CrossRef]
- Gallo, L.A.; Steane, S.E.; Young, S.L.; de Jersey, S.; Schoenaker, D.A.J.M.; Borg, D.J.; Lockett, J.; Collins, C.E.; Perkins, A.V.; Kumar, S.; et al. Dietary supplements, guideline alignment and biochemical nutrient status in pregnancy: Findings from the Queensland Family Cohort pilot study. Matern. Child Nutr. 2024, 20, e13589. [Google Scholar] [CrossRef]
- Lowensohn, R.I.; Stadler, D.D.; Naze, C. Current Concepts of Maternal Nutrition. Obstet. Gynecol. Surv. 2016, 71, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Ługowska, K.; Kolanowski, W. The Nutritional Behaviour of Pregnant Women in Poland. Int. J. Environ. Res. Public Health 2019, 16, 4357. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.G.; de Sousa, J.M.; Assunção, D.G.F.; de Araujo, E.K.S.; Bezerra, D.S.; Dametto, J.F.d.S.; Ribeiro, K.D.d.S. Impacts of Consumption of Ultra-Processed Foods on the Maternal-Child Health: A Systematic Review. Front. Nutr. 2022, 9, 821657. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cheng, Y.; Zeng, L.; Dang, S.; Yan, H. Maternal Diet Diversity During Pregnancy and Congenital Heart Defects: A Case-Control Study. Eur. J. Clin. Nutr. 2021, 75, 355–363. [Google Scholar] [CrossRef]
- Zakaria, H.; Abusanana, S.; Mussa, B.M.; Al Dhaheri, A.S.; Stojanovska, L.; Mohamad, M.N.; Saleh, S.T.; Ali, H.I.; Ismail, L.C. The Role of Lifestyle Interventions in the Prevention and Treatment of Gestational Diabetes Mellitus. Medicina 2023, 59, 287. [Google Scholar] [CrossRef]
- Cheng, Z.; Gu, R.; Lian, Z.; Gu, H.F. Evaluation of the association between maternal folic acid supplementation and the risk of congenital heart disease: A systematic review and meta-analysis. Nutr. J. 2022, 21, 20. [Google Scholar] [CrossRef]

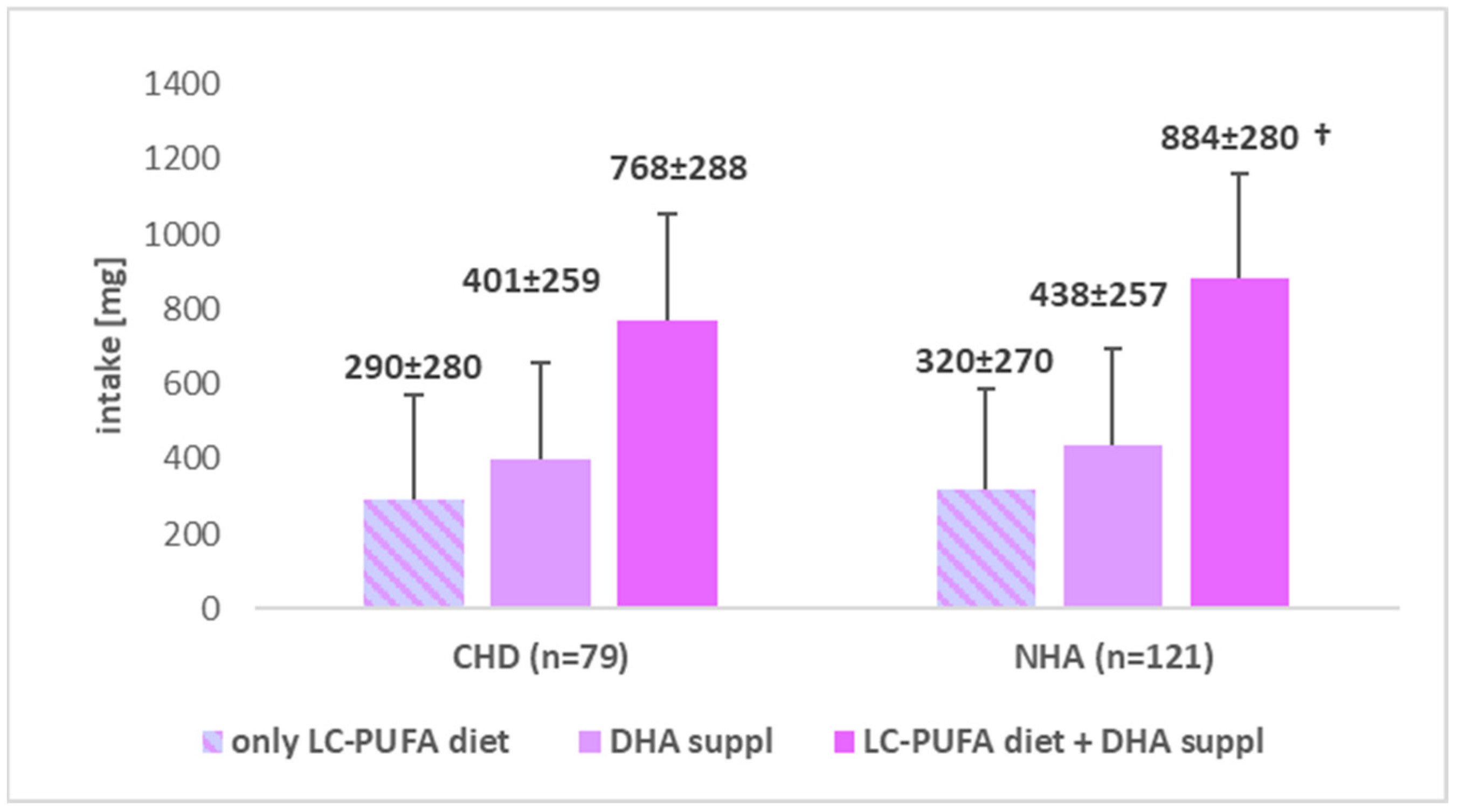
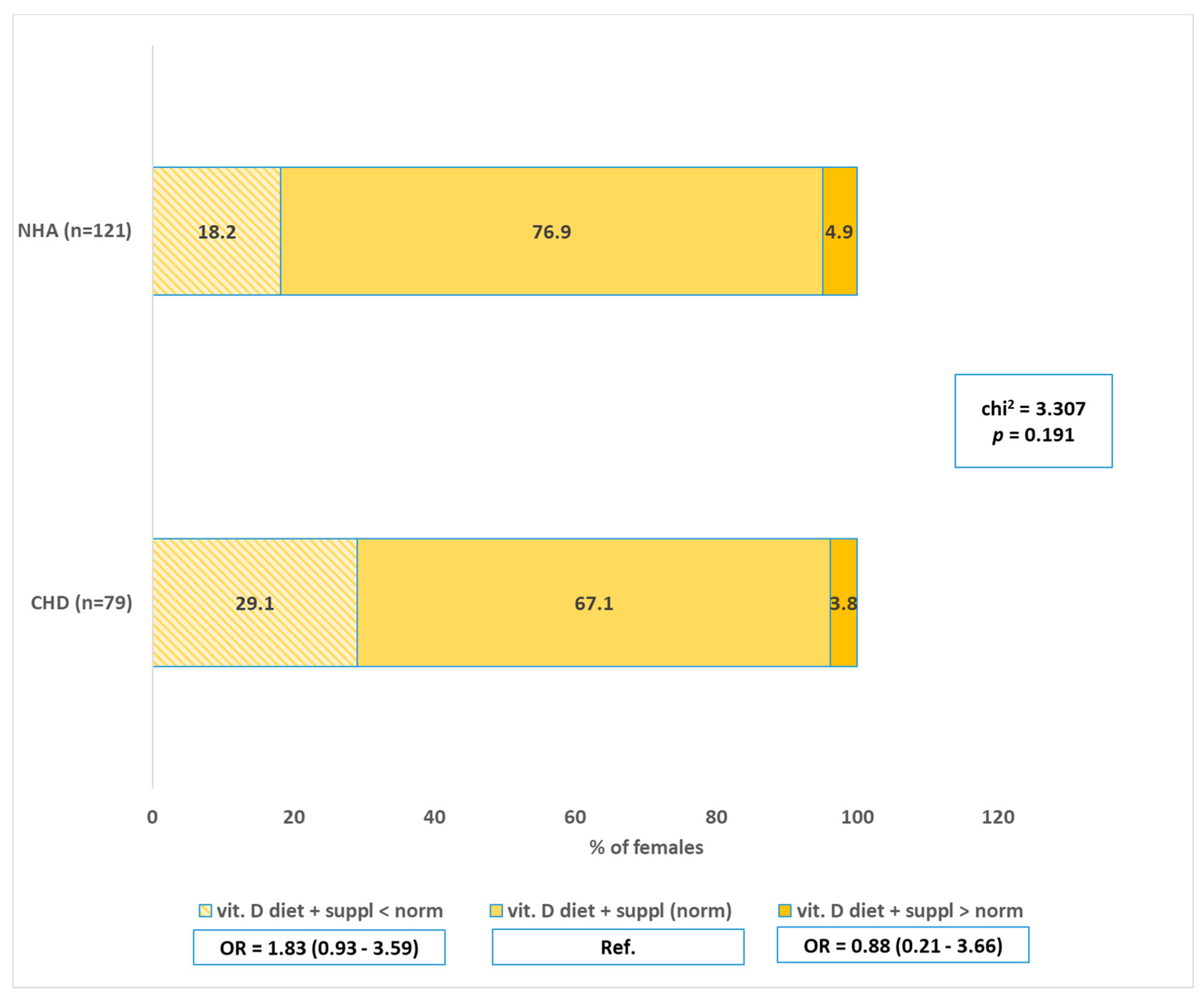
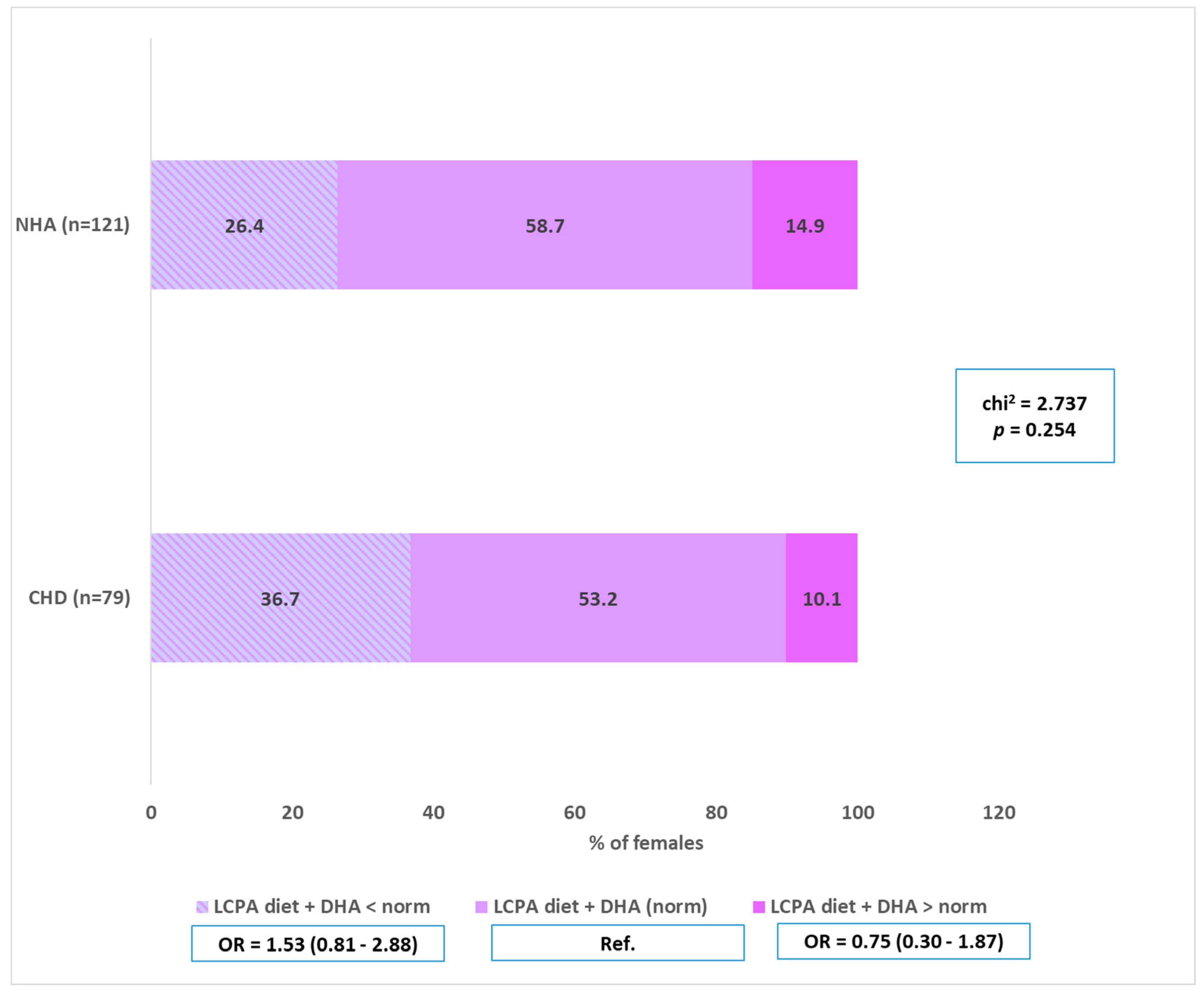
| Variables | CHD (A) (n = 79) | NHA (B) (n = 121) | |||
|---|---|---|---|---|---|
| N | % | N | % | OR (95%CI) | |
| Vitamin D—diet | |||||
| Implementation below the norm | 78 | 98.73 | 120 | 99.17 | |
| Implementation normal | 1 | 1.27 | 0 | 0 | Ref. |
| Implementation above the norm | 0 | 0 | 1 | 0.83 | |
| chi2 = 2.185; p = 0.335 | |||||
| Vitamin D—implementation of the norm before pregnancy = diet + supplementation | |||||
| Supplementation before pregnancy and implementation of the norm | 14 | 17.7 | 24 | 19.8 | Ref. |
| No supplementation before pregnancy and not meeting the norm | 65 | 82.3 | 97 | 80.2 | 1.15 (0.55–2.39) |
| chi2 = 0.139; p = 0.709 | |||||
| Vitamin D-supplementation | |||||
| Supplementation before pregnancy | 14 | 18.42 | 24 | 20.69 | Ref. |
| Supplementation from 1 to 8 weeks of pregnancy | 34 | 44.74 | 58 | 50.00 | 1.00 (0.41–2.47) |
| Supplementation above the 8th week of pregnancy | 9 | 11.84 | 19 | 16.38 | 0.81 (0.29–2.39) |
| No supplementation | 19 | 25.00 | 15 | 12.93 | 2.17 (0.80–5.90) |
| chi2 = 5.469; p = 0.141 | |||||
| Variables | CHD (A) (n = 79) | NHA (B) (n = 121) | |||
|---|---|---|---|---|---|
| N | % | N | % | OR (95%CI) | |
| LC-PUFA—diet | |||||
| Implementation below the norm | 39 | 49.37 | 51 | 42.15 | 0.87 (0.38–1.96) |
| Implementation normal | 15 | 18.99 | 17 | 14.05 | Ref. |
| Implementation above the norm | 25 | 31.65 | 53 | 43.80 | 0.53 (0.23–1.25) |
| chi2 = 3.093; p = 0.213 | |||||
| LC-PUFA—implementation of the norm before pregnancy = diet + supplementation | |||||
| Supplementation before pregnancy and implementation of the norm | 3 | 3.8 | 5 | 4.1 | Ref. |
| No supplementation before pregnancy and not meeting the norm | 76 | 96.2 | 116 | 95.9 | 0.91 (0.21–3.92) |
| chi2 = 0.014; p = 0.906 | |||||
| DHA-supplementation | |||||
| Supplementation before pregnancy | 5 | 6.49 | 5 | 4.24 | Ref. |
| Supplementation from 1 to 8 weeks of pregnancy | 23 | 29.87 | 34 | 28.81 | 0.68 (0.17–2.63) |
| Supplementation above the 8th week of pregnancy | 21 | 27.27 | 41 | 34.75 | 0.51 (0.13–1.99) |
| No supplementation | 28 | 36.36 | 38 | 32.20 | 0.74 (0.19–2.81) |
| chi2 = 2.276; p = 0.517 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolmaga, A.; Trafalska, E.; Gaszyńska, E.; Gawron-Skarbek, A.; Witkowski, S.; Murlewska, J.; Respondek-Liberska, M.; Strzelecka, I. Vitamin D and LC-PUFA and the Presence of Fetal Heart Defects—A Further Part of a Case-Control Study. Nutrients 2025, 17, 18. https://doi.org/10.3390/nu17010018
Kolmaga A, Trafalska E, Gaszyńska E, Gawron-Skarbek A, Witkowski S, Murlewska J, Respondek-Liberska M, Strzelecka I. Vitamin D and LC-PUFA and the Presence of Fetal Heart Defects—A Further Part of a Case-Control Study. Nutrients. 2025; 17(1):18. https://doi.org/10.3390/nu17010018
Chicago/Turabian StyleKolmaga, Agnieszka, Elżbieta Trafalska, Ewelina Gaszyńska, Anna Gawron-Skarbek, Sławomir Witkowski, Julia Murlewska, Maria Respondek-Liberska, and Iwona Strzelecka. 2025. "Vitamin D and LC-PUFA and the Presence of Fetal Heart Defects—A Further Part of a Case-Control Study" Nutrients 17, no. 1: 18. https://doi.org/10.3390/nu17010018
APA StyleKolmaga, A., Trafalska, E., Gaszyńska, E., Gawron-Skarbek, A., Witkowski, S., Murlewska, J., Respondek-Liberska, M., & Strzelecka, I. (2025). Vitamin D and LC-PUFA and the Presence of Fetal Heart Defects—A Further Part of a Case-Control Study. Nutrients, 17(1), 18. https://doi.org/10.3390/nu17010018








