Vitamin D Supplementation in Critically Ill—Narrative Review
Abstract
1. Introduction
2. Methods
2.1. Study Eligibility
2.2. Literature Search and Selection
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
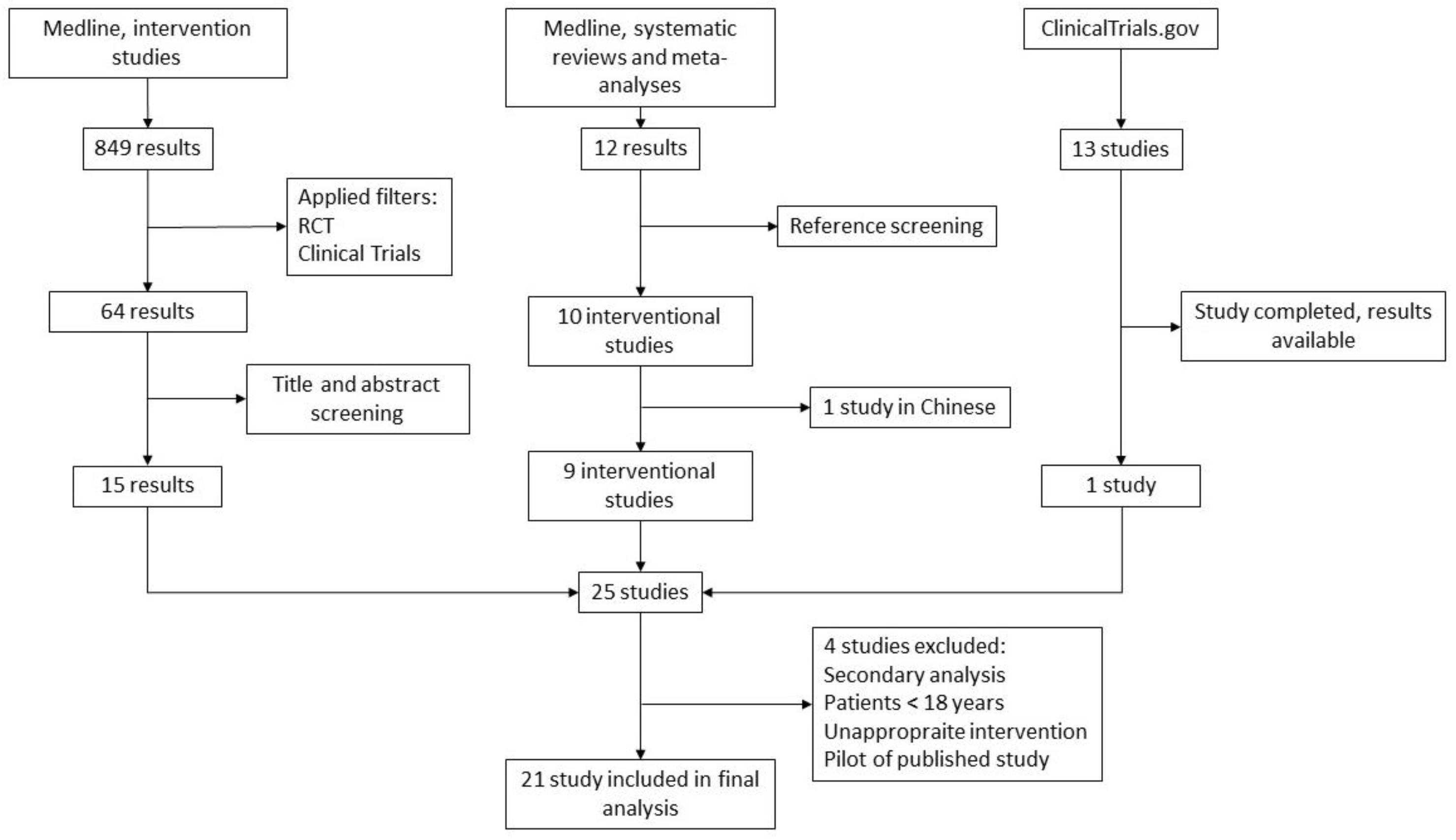
3.1. Literature Search
3.2. Study Characteristics
3.3. Outcomes
3.4. Data Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, P.; Eisman, J.A.; Center, J.R. Vitamin D deficiency in critically ill patients. N. Engl. J. Med. 2009, 360, 1912–1914. [Google Scholar] [CrossRef] [PubMed]
- Zajic, P.; Amrein, K. Vitamin D deficiency in the ICU: A systematic review. Minerva Endocrinol. 2014, 39, 275–287. [Google Scholar]
- Braun, A.; Chang, D.; Mahadevappa, K.; Gibbons, F.K.; Liu, Y.; Giovannucci, E.; Christopher, K.B. Association of low serum 25-hydroxyvitamin D levels and mortality in the critically ill. Crit. Care Med. 2011, 39, 671–677. [Google Scholar] [CrossRef]
- Perron, R.M.; Lee, P. Efficacy of high-dose vitamin D supplementation in the critically ill patients. Inflamm. Allergy Drug Targets 2013, 12, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Lee, P. Vitamin D metabolism and deficiency in critical illness. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 769–781. [Google Scholar] [CrossRef]
- Braun, A.B.; Litonjua, A.A.; Moromizato, T.; Gibbons, F.K.; Giovannucci, E.; Christopher, K.B. Association of low serum 25-hydroxyvitamin D levels and acute kidney injury in the critically ill. Crit. Care Med. 2012, 40, 3170–3179. [Google Scholar] [CrossRef]
- De Haan, K.; Groeneveld, A.B.; de Geus, H.R.; Egal, M.; Struijs, A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: Systematic review and meta-analysis. Crit. Care 2014, 18, 660. [Google Scholar] [CrossRef]
- Parekh, D.; Thickett, D.R.; Turner, A.M. Vitamin D deficiency and acute lung injury. Inflamm. Allergy Drug Targets 2013, 12, 253–261. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F.; Luo, B.A.; Qin, L.L. The association between vitamin D deficiency and community-acquired pneumonia: A meta-analysis of observational studies. Medicine 2019, 98, e17252. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Abioye, A.I.; Bromage, S.; Fawzi, W. Effect of micronutrient supplements on influenza and other respiratory tract infections among adults: A systematic review and meta-analysis. BMJ Glob. Health 2021, 6, e003176. [Google Scholar] [CrossRef]
- Hasanloei, M.A.V.; Rahimlou, M.; Eivazloo, A.; Sane, S.; Ayremlou, P.; Hashemi, R. Effect of Oral Versus Intramuscular Vitamin D Replacement on Oxidative Stress and Outcomes in Traumatic Mechanical Ventilated Patients Admitted to Intensive Care Unit. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2020, 35, 548–558. [Google Scholar] [CrossRef]
- Fabbri, A.; Infante, M.; Ricordi, C. Editorial—Vitamin D status: A key modulator of innate immunity and natural defense from acute viral respiratory infections. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4048–4052. [Google Scholar] [CrossRef]
- Orford, N.; Cattigan, C.; Brennan, S.L.; Kotowicz, M.; Pasco, J.; Cooper, D.J. The association between critical illness and changes in bone turnover in adults: A systematic review. Osteoporos. Int. 2014, 25, 2335–2346. [Google Scholar] [CrossRef] [PubMed]
- Orford, N.R.; Bailey, M.; Bellomo, R.; Pasco, J.A.; Cattigan, C.; Elderkin, T.; Brennan-Olsen, S.L.; Cooper, D.J.; Kotowicz, M.A. The association of time and medications with changes in bone mineral density in the 2 years after critical illness. Crit. Care 2017, 21, 69. [Google Scholar] [CrossRef]
- Parekh, D.; Patel, J.M.; Scott, A.; Lax, S.; Dancer, R.C.; D’Souza, V.; Greenwood, H.; Fraser, W.D.; Gao, F.; Sapey, E.; et al. Vitamin D Deficiency in Human and Murine Sepsis. Crit. Care Med. 2017, 45, 282–289. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and immune function: An overview. Proc. Nutr. Soc. 2012, 71, 50–61. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef]
- Lugg, S.T.; Howells, P.A.; Thickett, D.R. Optimal Vitamin D Supplementation Levels for Cardiovascular Disease Protection. Dis. Markers 2015, 2015, 864370. [Google Scholar] [CrossRef] [PubMed]
- Dancer, R.C.; Parekh, D.; Lax, S.; D’Souza, V.; Zheng, S.; Bassford, C.R.; Park, D.; Bartis, D.G.; Mahida, R.; Turner, A.M.; et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015, 70, 617–624. [Google Scholar] [CrossRef]
- Amrein, K.; Hoffmann, M.; Lobmeyr, E.; Martucci, G. Vitamin D in critical care: Where are we now and what is next? Curr. Opin. Crit. Care 2021, 27, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Langlois, P.L.; D’Aragon, F.; Manzanares, W. Vitamin D in the ICU: More sun for critically ill adult patients? Nutrition 2019, 61, 173–178. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Sahraian, M.A.; Jamalimoghadamsiahkali, S.; Asadi, A.; Zarei, A.; Zendehdel, A.; Varzandi, T.; Mohammadnabi, S.; Alijani, N.; Karimi, M.; et al. Treatment With 25-Hydroxyvitamin D3 (Calcifediol) Is Associated With a Reduction in the Blood Neutrophil-to-Lymphocyte Ratio Marker of Disease Severity in Hospitalized Patients With COVID-19: A Pilot Multicenter, Randomized, Placebo-Controlled, Double-Blinded Clinical Trial. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2021, 27, 1242–1251. [Google Scholar] [CrossRef]
- Pal, R.; Banerjee, M. Spurious undermining of the adjuvant role of vitamin D in COVID-19. Diabetes Metab. Syndr. 2021, 15, 102230. [Google Scholar] [CrossRef]
- Pal, R.; Banerjee, M.; Bhadada, S.K.; Shetty, A.J.; Singh, B.; Vyas, A. Vitamin D supplementation and clinical outcomes in COVID-19: A systematic review and meta-analysis. J. Endocrinol. Investig. 2021, 45, 53–68. [Google Scholar] [CrossRef]
- Christopher, K.B. Vitamin D supplementation in the ICU patient. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 187–192. [Google Scholar] [CrossRef]
- Lan, S.H.; Lai, C.C.; Chang, S.P.; Lu, L.C.; Hung, S.H.; Lin, W.T. Vitamin D supplementation and the outcomes of critically ill adult patients: A systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2020, 10, 14261. [Google Scholar] [CrossRef] [PubMed]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Putzu, A.; Belletti, A.; Cassina, T.; Clivio, S.; Monti, G.; Zangrillo, A.; Landoni, G. Vitamin D and outcomes in adult critically ill patients. A systematic review and meta-analysis of randomized trials. J. Crit. Care 2017, 38, 109–114. [Google Scholar] [CrossRef]
- Stroehlein, J.K.; Wallqvist, J.; Iannizzi, C.; Mikolajewska, A.; Metzendorf, M.I.; Benstoem, C.; Meybohm, P.; Becker, M.; Skoetz, N.; Stegemann, M.; et al. Vitamin D supplementation for the treatment of COVID-19: A living systematic review. Cochrane Database Syst. Rev. 2021, 5, CD015043. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Holick, M.F. The IOM D-lemma. Public Health Nutr. 2011, 14, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Jirak, P.; Larbig, R.; Shomanova, Z.; Fröb, E.J.; Dankl, D.; Torgersen, C.; Frank, N.; Mahringer, M.; Butkiene, D.; Haake, H.; et al. Myocardial injury in severe COVID-19 is similar to pneumonias of other origin: Results from a multicentre study. ESC Heart Fail. 2021, 8, 37–46. [Google Scholar] [CrossRef]
- Menger, J.; Lee, Z.Y.; Notz, Q.; Wallqvist, J.; Hasan, M.S.; Elke, G.; Dworschak, M.; Meybohm, P.; Heyland, D.K.; Stoppe, C. Administration of vitamin D and its metabolites in critically ill adult patients: An updated systematic review with meta-analysis of randomized controlled trials. Crit. Care 2022, 26, 268. [Google Scholar] [CrossRef]
- Langlois, P.L.; Szwec, C.; D’Aragon, F.; Heyland, D.K.; Manzanares, W. Vitamin D supplementation in the critically ill: A systematic review and meta-analysis. Clin. Nutr. 2018, 37, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Soni, K.D.; Trikha, A. Does Vitamin D Improve All-cause Mortality in Critically Ill Adults? An Updated Systematic Review and Meta-analysis of Randomized Controlled Trials. Indian J. Crit. Care Med. 2022, 26, 853–862. [Google Scholar] [CrossRef]
- Peng, L.; Li, L.; Wang, P.; Chong, W.; Li, Y.; Zha, X.; Deng, H.; Fan, H.; Zhang, Y. Association between Vitamin D supplementation and mortality in critically ill patients: A systematic review and meta-analysis of randomized clinical trials. PLoS ONE 2020, 15, e0243768. [Google Scholar] [CrossRef]
- Gao, Z.; Xie, J.; Li, C.; Liu, L.; Yang, Y. High Dose Vitamin D3 Supplementation Is Not Associated with Lower Mortality in Critically Ill Patients: A Meta-Analysis of Randomized Control Trials. Front. Nutr. 2022, 9, 762316. [Google Scholar] [CrossRef]
- Cao, M.; He, C.; Gong, M.; Wu, S.; He, J. The effects of vitamin D on all-cause mortality in different diseases: An evidence-map and umbrella review of 116 randomized controlled trials. Front. Nutr. 2023, 10, 1132528. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Mei, Y.; Zhang, K.; Xu, X. The Effect of Vitamin D Supplementation on Clinical Outcomes for Critically Ill Patients: A Systemic Review and Meta-Analysis of Randomized Clinical Trials. Front. Nutr. 2021, 8, 664940. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Ginde, A.A.; Brown, S.M.; Baughman, A.; Collar, E.M.; Ely, E.W.; Gong, M.N.; Hope, A.A.; Hou, P.C.; Hough, C.L.; et al. Effect of Early High-Dose Vitamin D3 Repletion on Cognitive Outcomes in Critically Ill Adults. Chest 2021, 160, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, A.; Choudhary, A.; Sharma, S.; Khurana, L.; Sharma, N.; Kumar, V.; Bisht, A. Neuroprotective Role of Oral Vitamin D Supplementation on Consciousness and Inflammatory Biomarkers in Determining Severity Outcome in Acute Traumatic Brain Injury Patients: A Double-Blind Randomized Clinical Trial. Clin. Drug Investig. 2020, 40, 327–334. [Google Scholar] [CrossRef]
- Han, J.E.; Jones, J.L.; Tangpricha, V.; Brown, M.A.; Brown, L.A.S.; Hao, L.; Hebbar, G.; Lee, M.J.; Liu, S.; Ziegler, T.R.; et al. High Dose Vitamin D Administration in Ventilated Intensive Care Unit Patients: A Pilot Double Blind Randomized Controlled Trial. J. Clin. Transl. Endocrinol. 2016, 4, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, G.; Van Roosbroeck, D.; Vanhove, P.; Wouters, P.J.; De Pourcq, L.; Bouillon, R. Bone turnover in prolonged critical illness: Effect of vitamin D. J. Clin. Endocrinol. Metab. 2003, 88, 4623–4632. [Google Scholar] [CrossRef] [PubMed]
- Ginde, A.A.; Brower, R.G.; Caterino, J.M.; Finck, L.; Banner-Goodspeed, V.M.; Grissom, C.K.; Hayden, D.; Hough, C.L.; Hyzy, R.C.; Khan, A.; et al. Early High-Dose Vitamin D(3) for Critically Ill, Vitamin D-Deficient Patients. N. Engl. J. Med. 2019, 381, 2529–2540. [Google Scholar] [CrossRef] [PubMed]
- Bychinin, M.V.; Klypa, T.V.; Mandel, I.A.; Yusubalieva, G.M.; Baklaushev, V.P.; Kolyshkina, N.A.; Troitsky, A.V. Effect of vitamin D3 supplementation on cellular immunity and inflammatory markers in COVID-19 patients admitted to the ICU. Sci. Rep. 2022, 12, 18604. [Google Scholar] [CrossRef]
- Amrein, K.; Schnedl, C.; Holl, A.; Riedl, R.; Christopher, K.B.; Pachler, C.; Urbanic Purkart, T.; Waltensdorfer, A.; Munch, A.; Warnkross, H.; et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: The VITdAL-ICU randomized clinical trial. JAMA 2014, 312, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.E.; Raed, A.; Donnino, M.W.; Ginde, A.A.; Waikar, S.S. Randomized controlled trial of calcitriol in severe sepsis. Am. J. Respir. Crit. Care Med. 2014, 190, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Miroliaee, A.E.; Salamzadeh, J.; Shokouhi, S.; Fatemi, A.; Ardehali, S.H.; Hajiesmaeili, M.R.; Sahraei, Z. Effect of Vitamin D Supplementation on Procalcitonin as Prognostic Biomarker in Patients with Ventilator Associated Pneumonia Complicated with Vitamin D Deficiency. Iran. J. Pharm. Res. 2017, 16, 1254–1263. [Google Scholar] [PubMed]
- Miri, M.; Kouchek, M.; Rahat Dahmardeh, A.; Sistanizad, M. Effect of High-Dose Vitamin D on Duration of Mechanical Ventilation in ICU Patients. Iran. J. Pharm. Res. 2019, 18, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Subramaniam, R.; Baidya, D.K.; Aggarwal, P.; Wig, N. Effect of Early Administration of Vitamin D on Clinical Outcome in Critically Ill Sepsis Patients: A Randomized Placebo-controlled Trial. Indian J. Crit. Care Med. 2021, 25, 1147–1154. [Google Scholar] [CrossRef]
- Yousefian, M.; Sadegi, S.; Sakaki, M. Vitamin D supplements’ effect on expediting the weaning process in patients with the stroke. Electron. J. Gen. Med. 2019, 16, em133. [Google Scholar] [CrossRef]
- Dickerson, R.N.; Berry, S.C.; Ziebarth, J.D.; Swanson, J.M.; Maish, G.O., 3rd; Minard, G.; Brown, R.O. Dose-response effect of ergocalciferol therapy on serum 25-hydroxyvitamin D concentration during critical illness. Nutrition 2015, 31, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Sistanizad, M.; Kouchek, M.; Miri, M.; Salarian, S.; Shojaei, S.; Moeini Vasegh, F.; Seifi Kafshgari, H.; Qobadighadikolaei, R. High dose vitamin D improves total serum antioxidant capacity and ICU outcome in critically ill patients—A randomized, double-blind clinical trial. Eur. J. Integr. Med. 2021, 42, 101271. [Google Scholar] [CrossRef]
- Karsy, M.; Guan, J.; Eli, I.; Brock, A.A.; Menacho, S.T.; Park, M.S. The effect of supplementation of vitamin D in neurocritical care patients: RandomizEd Clinical TrIal oF hYpovitaminosis D (RECTIFY). J. Neurosurg. 2019, 133, 1103–1112. [Google Scholar] [CrossRef]
- Amrein, K.; Sourij, H.; Wagner, G.; Holl, A.; Pieber, T.R.; Smolle, K.H.; Stojakovic, T.; Schnedl, C.; Dobnig, H. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: A randomized, double-blind, placebo-controlled pilot study. Crit. Care 2011, 15, R104. [Google Scholar] [CrossRef]
- Alizadeh, N.; Khalili, H.; Mohammadi, M.; Abdollahi, A.; Ala, S. Effect of vitamin D on stress-induced hyperglycaemia and insulin resistance in critically ill patients. Int. J. Clin. Pract. 2016, 70, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.A.; De Pascale, G.; Needleman, J.S.; Nakazawa, H.; Kaneki, M.; Bajwa, E.K.; Camargo, C.A., Jr.; Bhan, I. Effect of Cholecalciferol Supplementation on Vitamin D Status and Cathelicidin Levels in Sepsis: A Randomized, Placebo-Controlled Trial. Crit. Care Med. 2015, 43, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Jones, J.L.; Han, J.E.; Alvarez, J.A.; Sloan, J.H.; Konrad, R.J.; Zughaier, S.M.; Martin, G.S.; Ziegler, T.R.; Tangpricha, V. High-Dose Vitamin D(3) Administration Is Associated with Increases in Hemoglobin Concentrations in Mechanically Ventilated Critically Ill Adults: A Pilot Double-Blind, Randomized, Placebo-Controlled Trial. J. Parenter. Enter. Nutr. 2018, 42, 87–94. [Google Scholar] [CrossRef]
- Han, J.E.; Alvarez, J.A.; Jones, J.L.; Tangpricha, V.; Brown, M.A.; Hao, L.; Brown, L.A.S.; Martin, G.S.; Ziegler, T.R. Impact of high-dose vitamin D(3) on plasma free 25-hydroxyvitamin D concentrations and antimicrobial peptides in critically ill mechanically ventilated adults. Nutrition 2017, 38, 102–108. [Google Scholar] [CrossRef]
- Nair, P.; Venkatesh, B.; Lee, P.; Kerr, S.; Hoechter, D.J.; Dimeski, G.; Grice, J.; Myburgh, J.; Center, J.R. A Randomized Study of a Single Dose of Intramuscular Cholecalciferol in Critically Ill Adults. Crit. Care Med. 2015, 43, 2313–2320. [Google Scholar] [CrossRef]
- Han, J.E.; Alvarez, J.A.; Staitieh, B.; Tangpricha, V.; Hao, L.; Ziegler, T.R.; Martin, G.S.; Brown, L.A.S. Oxidative stress in critically ill ventilated adults: Effects of vitamin D(3) and associations with alveolar macrophage function. Eur. J. Clin. Nutr. 2018, 72, 744–751. [Google Scholar] [CrossRef]
- Ingels, C.; Vanhorebeek, I.; Van Cromphaut, S.; Wouters, P.J.; Derese, I.; Dehouwer, A.; Møller, H.J.; Hansen, T.K.; Billen, J.; Mathieu, C.; et al. Effect of Intravenous 25OHD Supplementation on Bone Turnover and Inflammation in Prolonged Critically Ill Patients. Horm. Metab. Res. 2020, 52, 168–178. [Google Scholar] [CrossRef]
- Vranešić Bender, D.; Giljević, Z.; Kušec, V.; Laktašic Žerjavić, N.; Bošnjak Pašic, M.; Vrdoljak, E.; Lkinas Kelećić, D.; Reiner, Ž.; Anić, B.; Krznarić, Ž. Guidelines for the prevention, detection and therapy of vitamin D deficiency in adults. Liječ. Vjesn. 2016, 138, 121–132. [Google Scholar]
- Schleicher, R.L.; Sternberg, M.R.; Looker, A.C.; Yetley, E.A.; Lacher, D.A.; Sempos, C.T.; Taylor, C.L.; Durazo-Arvizu, R.A.; Maw, K.L.; Chaudhary-Webb, M.; et al. National Estimates of Serum Total 25-Hydroxyvitamin D and Metabolite Concentrations Measured by Liquid Chromatography-Tandem Mass Spectrometry in the US Population during 2007–2010. J. Nutr. 2016, 146, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Dowling, K.G.; Skrabakova, Z.; Gonzalez-Gross, M.; Valtuena, J.; De Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Molgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef]
- Amrein, K.; Zajic, P.; Schnedl, C.; Waltensdorfer, A.; Fruhwald, S.; Holl, A.; Purkart, T.; Wunsch, G.; Valentin, T.; Grisold, A.; et al. Vitamin D status and its association with season, hospital and sepsis mortality in critical illness. Crit. Care 2014, 18, R47. [Google Scholar] [CrossRef]
- Schoenmakers, I.; Fraser, W.D.; Forbes, A. Vitamin D and acute and severe illness—A mechanistic and pharmacokinetic perspective. Nutr. Res. Rev. 2023, 36, 23–38. [Google Scholar] [CrossRef]
- Vieth, R. Vitamin D toxicity, policy, and science. J. Bone Miner. Res. 2007, 22 (Suppl. S2), V64–V68. [Google Scholar] [CrossRef]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Pludowski, P. Supplementing Vitamin D in Different Patient Groups to Reduce Deficiency. Nutrients 2023, 15, 3725. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, A.P.; Atallah, A.N.; Aldrighi, J.M.; Pires, A.L.R.; Dos Santos Puga, M.E.; Pinto, A. Insufficient evidence for vitamin D use in COVID-19: A rapid systematic review. Int. J. Clin. Pract. 2021, 75, e14649. [Google Scholar] [CrossRef] [PubMed]
- Guven, M.; Gultekin, H. The effect of high-dose parenteral vitamin D3 on COVID-19-related inhospital mortality in critical COVID-19 patients during intensive care unit admission: An observational cohort study. Eur. J. Clin. Nutr. 2021, 75, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Saxena, D.; Mavalankar, D. Vitamin D supplementation, COVID-19 and disease severity: A meta-analysis. QJM Mon. J. Assoc. Physicians 2021, 114, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Camargo, C.A., Jr.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J. Clin. Endocrinol. Metab. 2013, 98, 4619–4628. [Google Scholar] [CrossRef]
- Adams, J.S.; Clemens, T.L.; Parrish, J.A.; Holick, M.F. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. N. Engl. J. Med. 1982, 306, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.W.; Paris, P.W.; Clemens, T.L.; Nolan, J.; Holick, M.F. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am. J. Clin. Nutr. 1985, 42, 644–649. [Google Scholar] [CrossRef]
- Heaney, R.P.; Recker, R.R.; Grote, J.; Horst, R.L.; Armas, L.A. Vitamin D(3) is more potent than vitamin D(2) in humans. J. Clin. Endocrinol. Metab. 2011, 96, E447–E452. [Google Scholar] [CrossRef]
- Matsuoka, L.Y.; Wortsman, J.; Hollis, B.W. Suntanning and cutaneous synthesis of vitamin D3. J. Lab. Clin. Med. 1990, 116, 87–90. [Google Scholar]
- Hollis, B.W.; Johnson, D.; Hulsey, T.C.; Ebeling, M.; Wagner, C.L. Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. J. Bone Miner. Res. 2011, 26, 2341–2357. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Bassatne, A.; Basbous, M.; Chakhtoura, M.; El Zein, O.; Rahme, M.; El-Hajj Fuleihan, G. The link between COVID-19 and VItamin D (VIVID): A systematic review and meta-analysis. Metab. Clin. Exp. 2021, 119, 154753. [Google Scholar] [CrossRef] [PubMed]
- Varikasuvu, S.R.; Thangappazham, B.; Vykunta, A.; Duggina, P.; Manne, M.; Raj, H.; Aloori, S. COVID-19 and vitamin D (Co-VIVID study): A systematic review and meta-analysis of randomized controlled trials. Expert Rev. Anti-Infect. Ther. 2022, 20, 907–913. [Google Scholar] [CrossRef]

| Author, Year | N Participants Included in Study | N Participants Included in Analysis | Participants | Vitamin D Status as Inclusion Criteria | Mean Vitamin D Values (nmol/L) | Intervention | Comparator | Main Outcome | Other Outcomes | Signifficance of the Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Ginde, 2019 [48] | 1360 | 1059 | Adult ICU patients (33.7% mechanically ventilated) | <50 nmol/L | Intervention: 28 ± 12; Control: 27 ± 12 | Cholecalciferol, EN; 1 × 540,000 IU | Placebo | 90-day mortality | Hospital LoS; Ventilator free days | No significant difference in primary or secondary outcomes |
| Bychinin, 2022 [49] | 110 | 56 | Adult patients, COVID-19 (33.6% mechanically ventilated) | <75 nmol/L | Intervention: 24 (IQR 14–52.5); Control: 27.5 (IQR 21.5–37.5) | Cholecalciferol, EN; 60,000 IU weekly + 5000 IU daily/5 weeks | Placebo | Cell immunity and inflammatory markers on the 7th day after the intervention | Mortality; ICU LoS; Hospital LoS; Duration of respiratory support | Higher values of NK and NKT cells in the intervention group; Shorter ICU and hospital Los and lower number of days on mechanical ventilation in the control group |
| Hasanloei, 2019 [13] | 80 | 72 | Adult trauma patients, mechanically ventilated | 25–75 nmol/L | Intervention: 42.7 ± 11.3; 46.6 ± 8.2; Control: 42.5 ± 8.1 | Cholecalciferol; 50,000 IU PO/6 days 300,000 IU IM | Placebo | Duration of respiratory support | Mortality; ICU LoS | Less days on respiratory support in both intervention groups; No difference in mortality and shorter ICU LoS in both intervention groups |
| Amrein, 2014 [50] | 492 | 475 | Adult ICU patients (64% mechanically ventilated) | <50 nmol/L | Intervention: 32.5 ± 10; Control: 32.7 ± 10.7 | Cholecalciferol, EN; 540,000 IU bolus + 90,000 IU monthly/5 months | Placebo | Hospital LoS | Hospital mortality; 6-months mortality; ICU LoS; Hospital LoS; Duration of respiratory support | No significant difference in primary or secondary outcomes |
| Leaf, 2014 [51] | 67 | 67 | Adult patients with sepsis (70% mechanically ventilated) | Not measured | Not reported | Calcitriol, IV; 2 mcg | Placebo | Immune and inflammatory markers | 28-day mortality; ICU LoS; Hospital LoS; Duration of respiratory support | No significant difference in primary or secondary outcomes |
| Miroliaee, 2017 [52] | 51 | 46 | Adult patients with VAP | <75 nmol/L | Intervention: 42.8 ± 15.3; Control: 48.7 ± 11.5 | Cholecalciferol, IM; 300,000 IU | Placebo | Inflammatory markers on day 7 | 28-day mortality | Lower IL-6 levels in the intervention group; no difference in CRP levels and lower 28-day mortality in the intervention group |
| Miri, 2018 [53] | 40 | 40 | Adult ICU patients, mechanically ventilated | <70 nmol/L | Intervention: 21.1 ± 17; Control: 28.4 ± 45.6 | Cholecalciferol, IM; 300,000 IU | Placebo | Duration of respiratory support | 28-day mortality; ICU LoS; Hospital LoS | No difference in the primary outcome; lower mortality in intervention group and no difference in ICU LoS |
| Bhattacharyya, 2021 [54] | 63 | 63 | Adult patients with sepsis | Not reported | Intervention 30.1 ± 15.5; Control: 38.7 ± 26.5 | Cholecalciferol, EN; 540,000 IU | Placebo | ICU LoS | 90-day mortality; Hospital LoS; Duration of respiratory support | No significant difference in primary or secondary outcomes |
| Yousefian, 2019 [55] | 99 | 99 | Adult patients with CVI, mechanically ventilated | Patients with normal and those with low vitamin D levels | Intervention: 22.1 ± 5.7; 26.3 ± 12; Control: 70 ± 25 | Cholecalciferol, EN; 300,000 IU 3× weekly + calcium 500 mg | Placebo, patients with vitamin D > 50 nmol/L | Duration of respiratory support | Shorter duration of respiratory support in the control group | |
| Dickerson, 2015 [56] | 65 | 65 | Adult trauma patients | <50 nmol/L | Intervention: 36 ± 6 40 ± 7 37 ± 6 | Ergocalciferol, EN; 50,000 IU 1×, 2× or 3× weekly | / | Vitamin D levels after two weeks of supplementation | ICU LoS; | Higher number of patients reached normal vitamin D levels in group 3 |
| Sistanizad, 2021 [57] | 36 | 30 | Adult patients, mechanically ventilated | Not reported | Intervention: 18 ± 5.8; Control: 13.1 ± 1.8 | Cholecalciferol, IM; 300,000 IU | Placebo | Antioxidative capacity | 28-day mortality; ICU LoS; Duration of respiratory support | Increased antioxidative capacity in the intervention group; lower mortality and ICU Los and respiratory support in the intervention group |
| Karsy, 2022 [58] | 274 | 267 | Adult patients, head trauma, vitamin D deficiency | <50 nmol/L | / | Cholecalciferol, EN; 540,000 IU | Placebo | Hospital LoS | 6-month mortality; ICU LoS | No significant difference in primary or secondary outcomes |
| Leaf, 2022 [51] | 150 | 150 | Adult patients, High risk of AKI (83% mechanically ventilated) | Not reported | Intervention: 47 (IQR 28–65) 43.2 (IQR 26.2–71) Control: 36.2 (IQR 24.2–57.5) | Calcifediol, PO, 400 mcg × 1 + 200 mcg/4 days; Calcitriol, PO, 4 mcg/5 days | Placebo | 7-day mortality; RRT after 7 days | 28-day mortality; ICU LoS; Hospital LoS | No significant difference in primary or secondary outcomes |
| Amrein, 2011 [59] | 25 | 25 | Adult patients, vitamin D deficiency (84% mechanically ventilated) | <50 nmol/L | Intervention: 32.7; Control: 32.2 | Cholecalciferol, EN; 540,000 IU | Placebo | Number of patients with vitamin D levels > 50 nmol/L within 7 days | 28-day mortality; ICU LoS; Hospital LoS; Duration of respiratory support | Higher vitamin D levels in the intervention group; no difference in secondary outcomes |
| Alizadeh, 2016 [60] | 59 | 50 | Adult surgical patients with glucose intolerance and low vitamin D levels | <75 nmol/L | Intervention: 30.2 ± 12.8; Control: 27 ± 15.7 | Cholecalciferol, IM; 600,000 IU | Placebo | Blood glucose levels and metabolic markers on day 7 | Higher levels of adiponectin in the intervention group and no difference in other outcomes | |
| Quraishi, 2015 [61] | 30 | 30 | Adult patients with sepsis | Not reported | Intervention: 37.5 (IQR 30–50); 42.5 (IQR 32.5–62.5); Control: 47.5 (IQR 32.5–55) | Cholecalciferol, EN; 200,000 IU 400,000 IU | Placebo | Vitamin D levels on the 3rd, 5th and 7th day after the intervention | 30-day mortality; ICU LoS; Hospital LoS | Higher levels of vitamin D in both intervention groups; shorter hospital LoS for both intervention groups and no difference in ICU LoS and mortality |
| Smith, 2018 [62] | 30 | 30 | Adult patients, mechanically ventilated | Not reported | Intervention: 58 ± 19.5 50 ± 18.2 Control: 53.75 ± 30.5 | Cholecalciferol, EN; 50,000 IU/5 days (total 250,000 IU) 100,000 IU/5 days (total 500,000 IU) | Placebo | Hepcidin and hemoglobin levels 4 weeks after the intervention | Higher levels of haemoglobin and lower levels of hepcidin in the second intervention group | |
| Han, 2018 [65] | 30 | 30 | Adult patients, mechanically ventilated | Not reported | Intervention: 58 ± 19.5 50 ± 18.25 Control: 53.75 ± 30.5 | Cholecalciferol, EN; 50,000 IU/5 days (total 250,000 IU) 100,000 IU/5 days (total 500,000 IU) | Placebo | Antioxidative capacity | Decreased levels of GSSG in intervention group and no difference in other outcomes | |
| Ingels, 2020 [66] | 24 | 24 | Adult patients, ICU LoS > 10 dana | Not reported | Intervention: 23 (IQR 18–32.7); Control: 17 (IQR 12.7–25.5) | Cholecalciferol, IV; 200 mcg bolus + 15 mcg daily/10 days | Placebo | Inflammatory markers and bone metabolism markers | No significant difference in the reported outcomes | |
| Nair, 2015 [64] | 50 | 50 | Adult patients, SIRS | Regardless of vitamin D levels | Intervention: 52 (IQR 40–67) 42 (IQR 32–62) | Cholecalciferol, IM; 150,000 IU 300,000 IU | No control group | Vitamin D levels increase on days 1, 7 and 14 | 14-day mortality; ICU LoS; Hospital LoS | Increase in vitamin D levels in the intervention group; no difference in secondary outcomes |
| Han, 2016 [63] | 31 | 30 | Adult patients, duration of mechanical ventilation > 72 h, ICU LoS > 96 h | Not reported | Intervention: 58 ± 19.5 50 ± 18.2 Control: 53.7 ± 30.5 | Cholecalciferol, EN; 50,000 IU/5 days (total 250,000 IU) 100,000 IU/5 days (total 500,000 IU) | Placebo | Free vitamin D levels on day 7 after the intervention | No significant difference in reported outcomes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saric, L.; Domazet Bugarin, J.; Dosenovic, S. Vitamin D Supplementation in Critically Ill—Narrative Review. Nutrients 2025, 17, 156. https://doi.org/10.3390/nu17010156
Saric L, Domazet Bugarin J, Dosenovic S. Vitamin D Supplementation in Critically Ill—Narrative Review. Nutrients. 2025; 17(1):156. https://doi.org/10.3390/nu17010156
Chicago/Turabian StyleSaric, Lenko, Josipa Domazet Bugarin, and Svjetlana Dosenovic. 2025. "Vitamin D Supplementation in Critically Ill—Narrative Review" Nutrients 17, no. 1: 156. https://doi.org/10.3390/nu17010156
APA StyleSaric, L., Domazet Bugarin, J., & Dosenovic, S. (2025). Vitamin D Supplementation in Critically Ill—Narrative Review. Nutrients, 17(1), 156. https://doi.org/10.3390/nu17010156






