Comparisons of Post-Load Glucose at Different Time Points for Identifying High Risks of MASLD Progression
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Clinical and Laboratory Assessments
2.3. Liver Steatosis Assessments
2.4. Assessment of Hepatic and Extrahepatic Complications
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
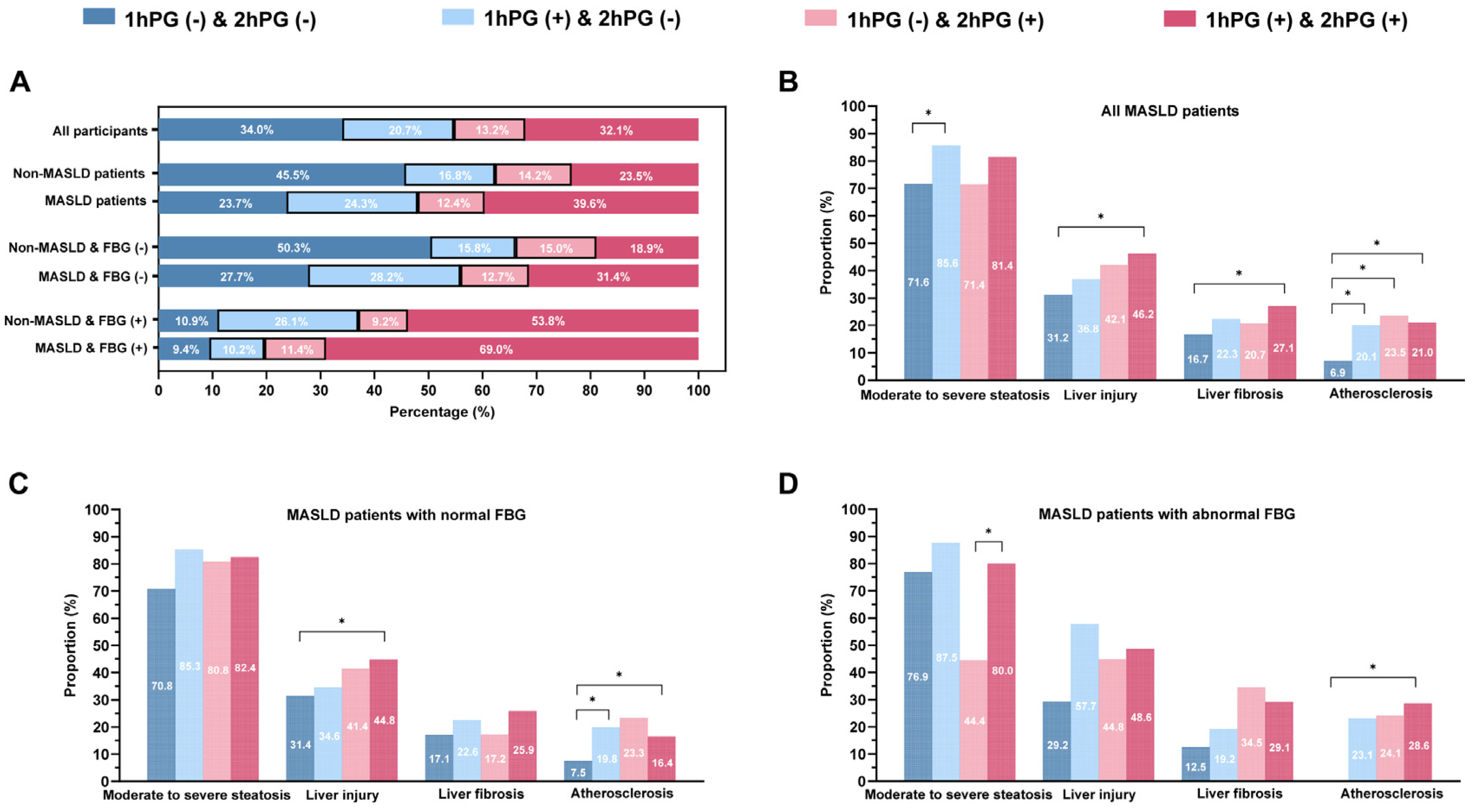
3.2. Clinical Characteristics of MASLD Patients Subgrouped by 1hPG and 2hPG
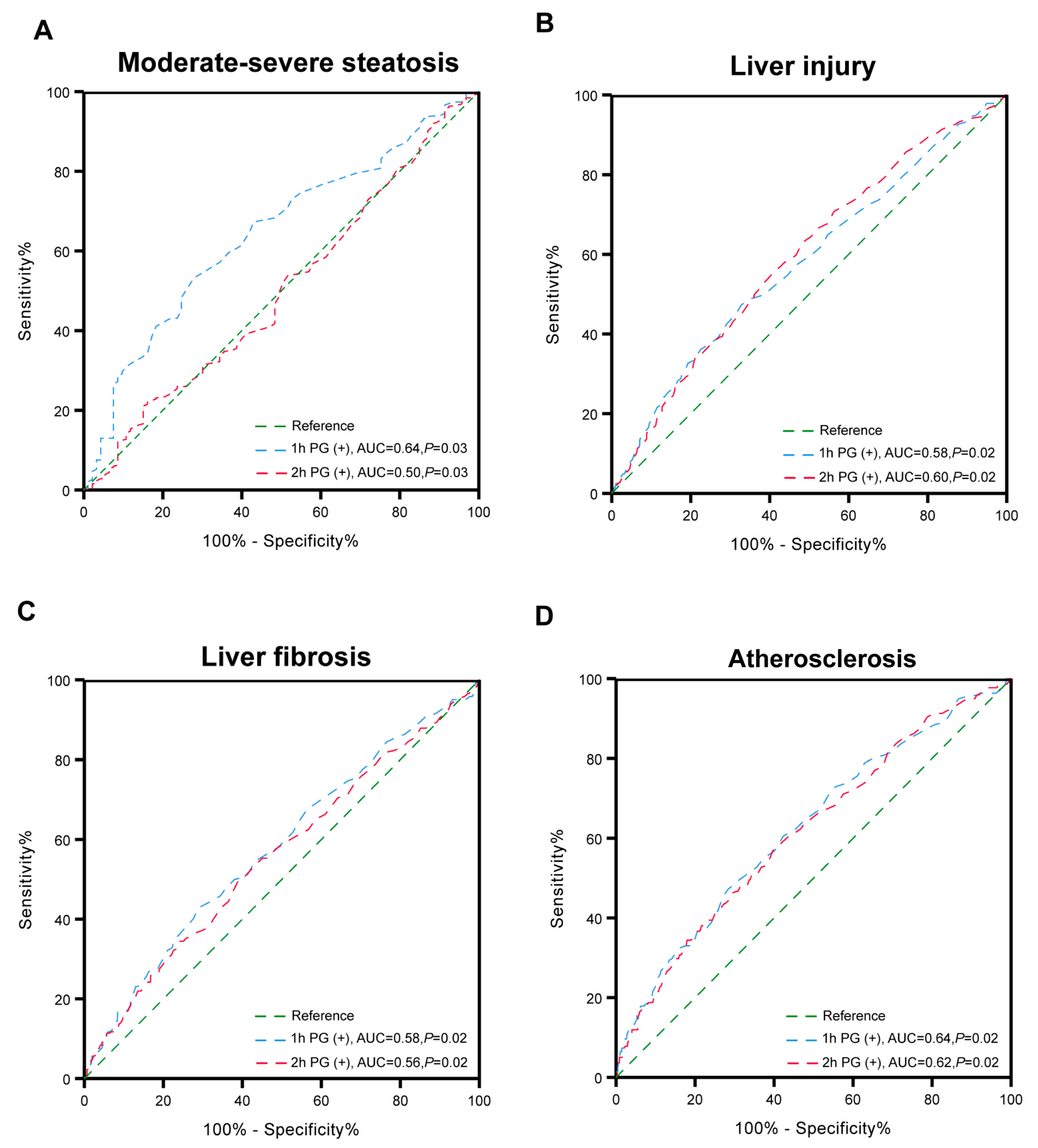
3.3. Associations of Glucose Status with Clinical Outcomes
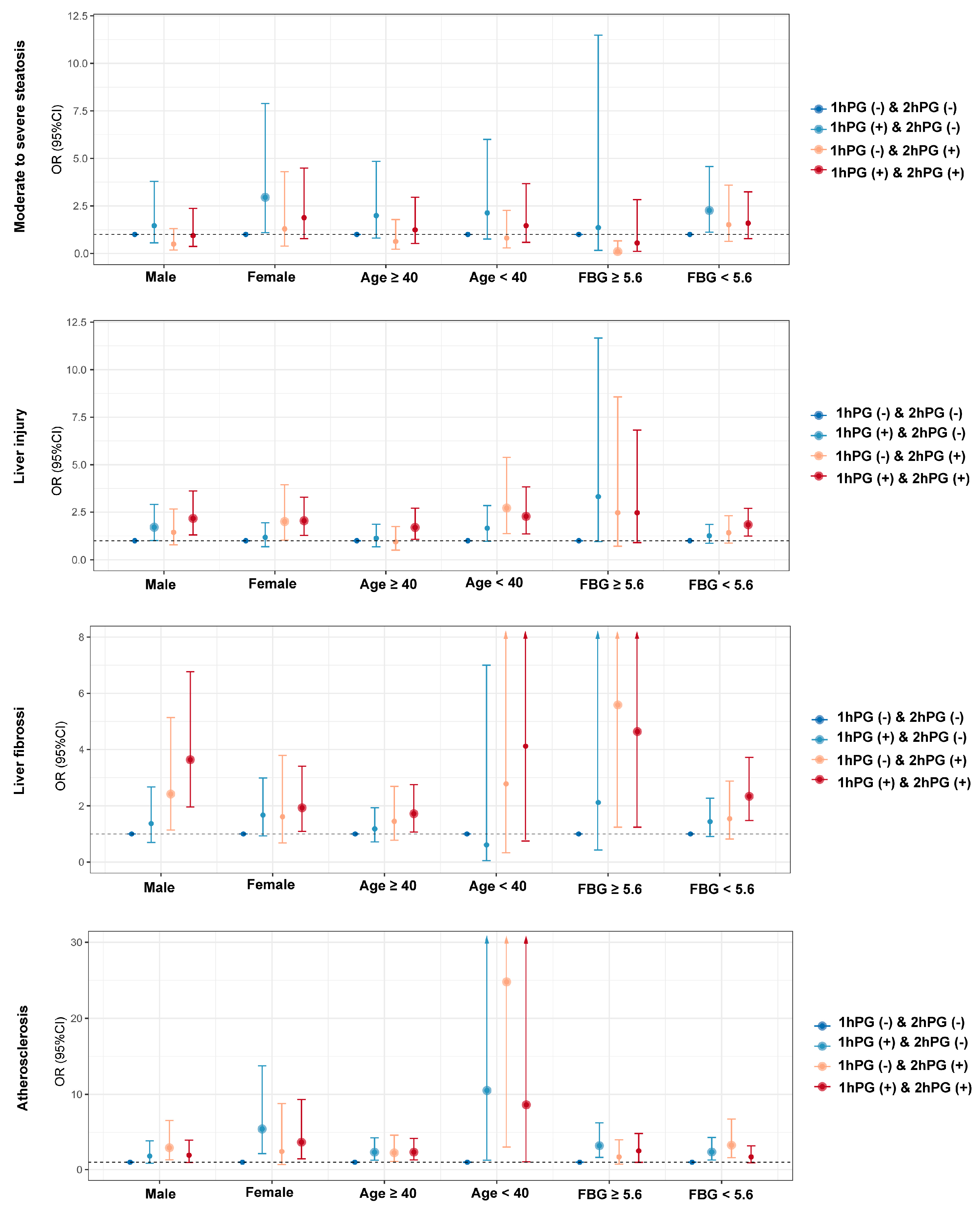
3.4. The Relationship of Glucose Status and Clinical Outcome Among Different Subgroups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Hagström, H.; Vessby, J.; Ekstedt, M.; Shang, Y. 99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical. J. Hepatol. 2024, 80, e76–e77. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Rojas, A.D.C.; Zuarth-Vázquez, J.M.; Uribe, M.; Barbero-Becerra, V.J. Insulin resistance and Metabolic dysfunction-associated steatotic liver disease (MASLD): Pathways of action of hypoglycemic agents. Ann. Hepatol. 2024, 29, 101182. [Google Scholar] [CrossRef]
- Sawada, K.; Chung, H.; Softic, S.; Moreno-Fernandez, M.E.; Divanovic, S. The bidirectional immune crosstalk in metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2023, 35, 1852–1871. [Google Scholar] [CrossRef] [PubMed]
- Tincopa, M.A.; Anstee, Q.M.; Loomba, R. New and emerging treatments for metabolic dysfunction-associated steatohepatitis. Cell Metab. 2024, 36, 912–926. [Google Scholar] [CrossRef]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.Y.; Zheng, M.H. Current status and future trends of the global burden of MASLD. Trends Endocrinol. Metab. 2024, 35, 697–707. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Global variation in diabetes diagnosis and prevalence based on fasting glucose and hemoglobin A1c. Nat. Med. 2023, 29, 2885–2901. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, F.; Mancuso, E.; Mazza, E.; Mannino, G.C.; Fiorentino, T.V.; Arturi, F.; Succurro, E.; Perticone, M.; Sciacqua, A.; Montalcini, T.; et al. One-hour post-load glucose levels are associated with hepatic steatosis assessed by transient elastography. Diabetes Obes. Metab. 2024, 26, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, R.; Fiorentino, T.V.; Marini, M.A.; Sesti, G.; Bergman, M. One-hour post-load glucose is associated with severity of hepatic fibrosis risk. Diabetes Res. Clin. Pract. 2022, 189, 109977. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Liao, W.C.; Fang, Y.J.; Chen, S.J.; Gau, S.Y. Differences in Major Adverse Cardiovascular Events of Nonalcoholic Fatty Liver Disease by Race and Ethnicity. Clin. Mol. Hepatol. 2024, 30, 978–981. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO); European Association for the Study of the Liver (EASL). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Ye, J.; Zhuang, X.; Li, X.; Gong, X.; Sun, Y.; Wang, W.; Feng, S.; Wu, T.; Zhong, B. Novel metabolic classification for extrahepatic complication of metabolic associated fatty liver disease: A data-driven cluster analysis with international validation. Metabolism 2022, 136, 155294. [Google Scholar] [CrossRef]
- Kaul, S. Evidence for the Universal Blood Pressure Goal of <130/80 mm Hg Is Strong. Hypertension 2020, 76, 1391–1399. [Google Scholar] [PubMed]
- Colagiuri, S. Definition and Classification of Diabetes and Prediabetes and Emerging Data on Phenotypes. Endocrinol. Metab. Clin. N. Am. 2021, 50, 319–336. [Google Scholar] [CrossRef]
- Burger, P.M.; Koudstaal, S.; Dorresteijn, J.A.N.; Savarese, G.; van der Meer, M.G.; de Borst, G.J.; Mosterd, A.; Visseren, F.L.J.; on behalf of the UCC-SMART Study Group. Metabolic syndrome and risk of incident heart failure in non-diabetic patients with established cardiovascular disease. Int. J. Cardiol. 2023, 379, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.C.; Matthews, D.R.; Hermans, M.P. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998, 21, 2191–2192. [Google Scholar] [CrossRef]
- Bergman, M.; Manco, M.; Satman, I.; Chan, J.; Schmidt, M.I.; Sesti, G.; Fiorentino, T.V.; Abdul-Ghani, M.; Jagannathan, R.; Aravindakshan, P.K.T.; et al. International Diabetes Federation Position Statement on the 1-hour post-load plasma glucose for the diagnosis of intermediate hyperglycaemia and type 2 diabetes. Diabetes Res. Clin. Pract. 2024, 209, 111589. [Google Scholar] [CrossRef]
- Burger, P.M.; Koudstaal, S.; Dorresteijn, J.A.N.; Savarese, G.; van der Meer, M.G.; de Borst, G.J.; Mosterd, A.; Visseren, F.L.J.; UCC-SMART Study Group. Quantitative ultrasound approaches for diagnosis and monitoring hepatic steatosis in nonalcoholic fatty liver disease. Theranostics 2020, 10, 4277–4289. [Google Scholar]
- Boyce, C.J.; Pickhardt, P.J.; Kim, D.H.; Taylor, A.J.; Winter, T.C.; Bruce, R.J.; Lindstrom, M.J.; Hinshaw, J.L. Hepatic steatosis (fatty liver disease) in asymptomatic adults identified by unenhanced low-dose CT. AJR Am. J. Roentgenol. 2010, 194, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Starekova, J.; Hernando, D.; Pickhardt, P.J.; Reeder, S.B. Quantification of Liver Fat Content with CT and MRI: State of the Art. Radiology 2021, 301, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Vieira Barbosa, J.; Milligan, S.; Frick, A.; Broestl, J.; Younossi, Z.; Afdhal, N.H.; Lai, M. Fibrosis-4 Index as an Independent Predictor of Mortality and Liver-Related Outcomes in NAFLD. Hepatol. Commun. 2021, 6, 765–779. [Google Scholar] [CrossRef]
- Channon, K.M.; Newby, D.E.; Nicol, E.D.; Deanfield, J. Cardiovascular computed tomography imaging for coronary artery disease risk: Plaque, flow and fat. Heart 2022, 108, 1510–1515. [Google Scholar] [CrossRef]
- Della Pepa, G.; Lupoli, R.; Masulli, M.; Boccia, R.; De Angelis, R.; Gianfrancesco, S.; Piccolo, R.; Rainone, C.; Rivellese, A.A.; Annuzzi, G.; et al. Blood glucose control and metabolic dysfunction-associated steatotic liver disease in people with type 1 diabetes. J. Endocrinol. Investig. 2024, 47, 2371–2378. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, A.L.; Tavaglione, F.; Romeo, S.; Charlton, M. Endocrine aspects of metabolic dysfunction-associated steatotic liver disease (MASLD): Beyond insulin resistance. J. Hepatol. 2023, 79, 1524–1541. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, V.; Maia, I.; Neves, J.S.; Salazar, D.; Ferreira, M.J.; Mendonça, F.; Silva, M.M.; Borges-Canha, M.; Viana, S.; Costa, C.; et al. Oral glucose tolerance testing at 1 h and 2 h: Relationship with glucose and cardiometabolic parameters and agreement for pre-diabetes diagnosis in patients with morbid obesity. Diabetol. Metab. Syndr. 2022, 14, 91. [Google Scholar] [CrossRef] [PubMed]
- Pareek, M.; Bhatt, D.L.; Nielsen, M.L.; Jagannathan, R.; Eriksson, K.F.; Nilsson, P.M.; Bergman, M.; Olsen, M.H. Enhanced Predictive Capability of a 1-hour Oral Glucose Tolerance Test: A Prospective Population-Based Cohort Study. Diabetes Care 2018, 41, 171–177. [Google Scholar] [CrossRef]
- Kılınç, S.; Demirbaş, T.; Atay, E.; Ceran, Ö.; Atay, Z. Elevated 1-h post-load plasma glucose levels in normal glucose tolerance children with obesity is associated with early carotid atherosclerosis. Obes. Res. Clin. Pract. 2020, 14, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Brannick, B.; Dagogo-Jack, S. Prediabetes and Cardiovascular Disease: Pathophysiology and Interventions for Prevention and Risk Reduction. Endocrinol. Metab. Clin. N. Am. 2018, 47, 33–50. [Google Scholar] [CrossRef]
- Succurro, E.; Marini, M.A.; Arturi, F.; Grembiale, A.; Lugarà, M.; Andreozzi, F.; Sciacqua, A.; Lauro, R.; Hribal, M.L.; Perticone, F.; et al. Elevated one-hour post-load plasma glucose levels identifies subjects with normal glucose tolerance but early carotid atherosclerosis. Atherosclerosis 2009, 207, 245–249. [Google Scholar] [CrossRef]
- George, K.; Alberti, M.M. Impaired glucose tolerance: What are the clinical implications? Diabetes Res. Clin. Pract. 1998, 40, S3–S8. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, Z.; Yu, X.; Zeng, T.; Xu, M.; Xu, Y.; Hu, R.; Chen, G.; Su, Q.; Mu, Y.; et al. Age-specific modifiable risk factor profiles for cardiovascular disease and all-cause mortality: A nationwide, population-based, prospective cohort study. Lancet Reg. Health-West. Pac. 2021, 17, 100277. [Google Scholar] [CrossRef]
- Barr, E.L.; Boyko, E.J.; Zimmet, P.Z.; Wolfe, R.; Tonkin, A.M.; Shaw, J.E. Continuous relationships between non-diabetic hyperglycemia and both cardiovascular disease and all-cause mortality: The Australian Diabetes, Obesity, and Lifestyle (AusDiab) study. Diabetologia 2009, 52, 415–424. [Google Scholar] [CrossRef]
- DECODE Study Group; the European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: Comparison of fasting and 2-hour diagnostic criteria. Arch. Intern. Med. 2001, 161, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Meigs, J.B.; Nathan, D.M.; D’Agostino, R.B., Sr.; Wilson, P.W. Framingham Offspring Study. Fasting and post challenge glycemia and cardiovascular disease risk: The Framingham Offspring Study. Diabetes Care 2002, 25, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.R.L.; Leite, N.C.; Moram, C.B.M.; Salles, G.F. Long-term visit-to-visit glycemic variability as predictor of micro- and macrovascular complications in patients with type 2 diabetes: The Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovasc. Diabetol. 2018, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, T.; Sueta, D.; Tabata, N.; Takashio, S.; Yamamoto, E.; Izumiya, Y.; Tsujita, K.; Kojima, S.; Kaikita, K.; Matsui, K.; et al. Effects of the Mean Amplitude of Glycemic Excursions and Vascular Endothelial Dysfunction on Cardiovascular Events in Nondiabetic Patients With Coronary Artery Disease. J. Am. Heart Assoc. 2017, 6, e004841. [Google Scholar] [CrossRef]
- Marfella, R.; Verrazzo, G.; Acampora, R.; La Marca, C.; Giunta, R.; Lucarelli, C.; Paolisso, G.; Ceriello, A.; Giugliano, D. Glutathione reverses systemic hemodynamic changes by acute hyperglycemia in healthy subjects. Am. J. Physiol. 1995, 268, E1167–E1173. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Kawamori, R.; Toyofuku, Y.; Kitahara, Y.; Sato, F.; Shimizu, T.; Miura, K.; Mine, T.; Tanaka, Y.; Mitsumata, M.; et al. Repetitive fluctuations in blood glucose enhance monocyte adhesion to the endothelium of rat thoracic aorta. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2275–2280. [Google Scholar] [CrossRef]
- Peng, M.; He, S.; Wang, J.; An, Y.; Qian, X.; Zhang, B.; Zhang, L.; Chen, B.; Yang, Z.; Li, G.; et al. Efficacy of 1-hour Postload Plasma Glucose as a Suitable Measurement in Predicting Type 2 Diabetes and Diabetes-Related Complications: A Post Hoc Analysis of the 30-Year Follow-up of the Da Qing IGT and Diabetes Study. Diabetes Obes. Metab. 2024, 26, 2329–2338. [Google Scholar] [CrossRef]
- Cefalo, C.M.A.; Riccio, A.; Fiorentino, T.V.; Succurro, E.; Mannino, G.C.; Perticone, M.; Sciacqua, A.; Andreozzi, F.; Sesti, G. Pathophysiological characteristics of subjects with intermediate hyperglycemia and type 2 diabetes identified by 1-hour plasma glucose during an oral glucose tolerance test. Diabetes Res. Clin. Pract. 2024, 217, 111856. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, G.; De Bacquer, D.; Gyberg, V.; De Backer, G.; Kotseva, K.; Mellbin, L.G.; Risebrink, R.; Tuomilehto, J.; Wood, D.; Rydén, L. Saving time by replacing the standardised two-hour oral glucose tolerance test with a one-hour test: Validation of a new screening algorithm in patients with coronary artery disease from the ESC-EORP EUROASPIRE V registry. Diabetes Res. Clin. Pract. 2022, 183, 109156. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, P.; Weiskirchen, R. Cellular and Molecular Mechanisms of Insulin Resistance. Curr. Tissue Microenviron. Rep. 2024, 5, 79–90. [Google Scholar] [CrossRef]
- Muzurović, E.; Mikhailidis, D.P.; Mantzoros, C. Non-alcoholic fatty liver disease, insulin resistance, metabolic syndrome and their association with vascular risk. Metabolism 2021, 119, 154770. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.R.; Brancati, F.L.; Yeh, H.-C. Trends in the Prevalence of Type 2 Diabetes in Asians Versus Whites: Results from the United States National Health Interview Survey, 1997–2008. Diabetes Care 2011, 34, 353–357. [Google Scholar] [CrossRef]
- Bae, J.S.; Lee, D.H.; Suh, K.S.; Kim, H.; Lee, K.B.; Lee, J.Y.; Han, J.K. Noninvasive assessment of hepatic steatosis using a pathologic reference standard: Comparison of CT, MRI, and US-based techniques. Ultrasonography 2022, 41, 344–354. [Google Scholar] [CrossRef]
| Characteristic | Total (n = 2214) | Non-MASLD (n = 1049) | MASLD (n = 1165) | p |
|---|---|---|---|---|
| Age (years) | 45.66 ± 15.21 | 45.54 ± 15.84 | 45.76 ± 14.62 | 0.74 |
| Male, n (%) | 1048 (47.3%) | 440 (41.9%) | 608 (52.2%) | <0.001 |
| BMI (kg/m2) | 25.14 ± 4.91 | 23.18 ± 3.84 | 26.90 ± 5.09 | <0.001 |
| WC (cm) | 88.38 ± 12.18 | 83.91 ± 10.22 | 92.40 ± 12.41 | <0.001 |
| WHR | 0.91 ± 0.07 | 0.89 ± 0.07 | 0.92 ± 0.06 | <0.001 |
| Hypertension, n (%) | 1025 (46.3%) | 396 (37.8%) | 629 (54.0%) | <0.001 |
| T2DM, n (%) | 384 (17.3%) | 118 (11.2%) | 266 (19.4%) | <0.001 |
| FBG (mmol/L) | 4.80 (4.50, 5.30) | 4.70 (4.40, 5.10) | 5.00 (4.60, 5.50) | <0.001 |
| 1hPG (mmol/L) | 8.70 (7.70, 10.00) | 8.30 (7.30, 9.40) | 9.10 (8.00, 10.70) | <0.001 |
| 2hPG (mmol/L) | 7.50 (6.20, 9.70) | 7.00 (5.80, 8.70) | 7.90 (6.60, 10.60) | <0.001 |
| FINS (μU/mL) | 7.44 (4.79, 10.42) | 5.74 (4.04, 8.83) | 8.98 (5.75, 12.31) | <0.001 |
| HOMA-IR | 1.61 (1.00, 2.37) | 1.22 (0.82, 1.87) | 2.00 (1.25, 2.81) | <0.001 |
| HOMA-β (%) | 105.7 (69.23, 164.95) | 95.87 (63.64, 149.67) | 113.20 (75.27, 178.46) | <0.001 |
| HBA1c (%) | 5.73 (5.30, 5.90) | 5.73 (5.30, 5.86) | 5.73 (5.36, 5.90) | 0.048 |
| CHOL (mmol/L) | 4.93 ± 1.18 | 4.80 ± 1.19 | 5.05 ± 1.17 | <0.001 |
| TG (mmol/L) | 1.32 (0.95, 1.84) | 1.12 (0.83, 1.59) | 1.50 (1.11, 2.17) | <0.001 |
| HDL-C (mmol/L) | 1.22 ± 0.34 | 1.27 ± 0.36 | 1.17 ± 0.31 | <0.001 |
| LDL-C (mmol/L) | 3.08 ± 0.85 | 2.96 ± 0.84 | 3.20 ± 0.85 | <0.001 |
| UA (μmol/L) | 382.71 ± 114.08 | 354.09 ± 104.41 | 408.48 ± 116.29 | <0.001 |
| ALT (U/L) | 21.00 (14.00, 32.00) | 17.00 (12.00, 26.00) | 25.00 (17.00, 39.00) | <0.001 |
| AST (U/L) | 21.00 (17.00, 27.00) | 20.00 (17.00, 25.00) | 22.00 (18.00, 29.00) | <0.001 |
| ALP (U/L) | 72.00 (61.00, 87.00) | 71.00 (60.00, 86.00) | 74.00 (63.00, 88.00) | 0.007 |
| ALB(g/L) | 40.90 (38.30, 43.40) | 40.20 (37.70, 43.00) | 41.30 (39.00, 43.90) | <0.001 |
| Platelet (109/L) | 252.03 ± 70.74 | 247.99 ± 70.16 | 255.66 ± 71.09 | 0.10 |
| FIB-4 index | 0.85 (0.57, 1.27) | 0.82 (0.53, 1.20) | 0.88 (0.58, 1.30) | 0.004 |
| CACS category | <0.001 | |||
| 0, n (%) | 1208 (54.6%) | 665 (63.4%) | 543 (46.6%) | |
| 1–99, n (%) | 758 (34.2%) | 339 (32.3%) | 419 (36.0%) | |
| 100–399, n (%) | 248 (11.2%) | 43 (4.3%) | 183 (17.4%) | |
| >400, n (%) | 0 (0.0%) | 2 (0.2%) | 20 (1.7%) |
| Characteristics | 1hPG (−) and 2hPG (−) (n = 276) | 1hPG (+) and 2hPG (−) (n = 283) | 1hPG (−) and 2hPG (+) (n = 145) | 1hPG (+) and 2hPG (+) (n = 461) | p |
|---|---|---|---|---|---|
| Age (years) | 41.31 ± 15.68 | 45.54 ± 15.15 **a | 46.06 ± 14.61 *a | 48.47 ± 12.91 ***a | <0.001 |
| Male, n (%) | 116 (42.0%) | 153 (54.1%) *a | 91 (62.8%) *a | 248 (53.8%) *a | <0.001 |
| BMI (kg/m2) | 25.64 ± 5.50 | 25.88 ± 5.85 | 28.10 ± 4.26 ***a ***b | 27.88 ± 4.33 ***a ***b | <0.001 |
| WC (cm) | 89.29 ± 13.96 | 90.40 ± 13.51 | 95.40 ± 10.71 ***a ***b | 94.53 ± 10.46 ***a ***b | <0.001 |
| WHR | 0.91 ± 0.14 | 0.91 ± 0.06 | 0.93 ± 0.06 ***a **b | 0.94 ± 0.11 ***a ***b | <0.001 |
| Hypertension, n (%) | 118 (42.8%) | 118 (41.7%) | 93 (64.1%) *a *b | 300 (65.1%) *a *b | <0.001 |
| T2DM, n (%) | 0 (0.0%) | 0 (0.0%) | 34 (23.5%) ***a ***b | 232 (50.3%) ***a ***b **c | <0.001 |
| FBG (mmol/L) | 4.60 (4.30, 4.90) | 4.90 (4.60, 5.30) ***a | 4.90 (4.60, 5.40) ***a | 5.30 (4.90, 5.90) ***a ***b ***c | <0.001 |
| 1hPG (mmol/L) | 7.50 (6.70, 8.00) | 9.40 (9.00, 10.65) ***a | 7.90 (7.50, 8.20) ***b | 10.50 (9.40, 13.00) ***a ***b ***c | <0.001 |
| 2hPG (mmol/L) | 6.40 (5.50, 7.10) | 6.80 (6.10, 7.20) | 9.60 (8.50, 10.90) ***a ***b | 11.00 (9.10, 13.40) ***a ***b *c | <0.001 |
| FINS (μU/mL) | 7.47 (4.59, 11.23) | 8.65 (5.07, 11.91) | 9.53 (6.92, 15.35) ***a ***b | 8.98 (7.15, 13.00) ***a ***b | <0.001 |
| HOMA-IR | 1.60 (0.94, 2.26) | 1.83 (1.06, 2.63) | 2.16 (1.45, 3.17) ***a **b | 2.27 (1.68, 3.19) ***a ***b | <0.001 |
| HOMA-β (%) | 94.53 (65.12, 146.34) | 105.80 (68.67, 167.76) | 106.36 (69.72, 171.16) | 113.33 (74.00, 179.57) ***a | <0.001 |
| HBA1c (%) | 5.50 (5.20, 5.73) | 5.50 (5.30, 5.77) | 5.73 (5.50, 6.10) ***a ***b | 5.86 (5.60, 6.30) ***a ***b | <0.001 |
| CHOL (mmol/L) | 5.03 ± 1.12 | 4.99 ± 1.09 | 5.13 ± 1.25 | 5.08 ± 1.22 | 0.60 |
| TG (mmol/L) | 1.27 (0.95, 1.69) | 1.32 (0.98, 1.86) | 1.87 (1.31, 2.56) ***a ***b | 1.66 (1.23, 2.38) ***a ***b | <0.001 |
| HDL-C (mmol/L) | 1.26 ± 0.34 | 1.21 ± 0.32 | 1.06 ± 0.26 ***a ***b | 1.12 ± 0.27 ***a ***b | <0.001 |
| LDL-C (mmol/L) | 3.16 ± 0.80 | 3.16 ± 0.86 | 3.25 ± 0.83 | 3.23 ± 0.88 | 0.45 |
| UA (μmol/L) | 385.22 ± 115.29 | 396.71 ± 117.97 | 440.12 ± 121.55 ***a **b | 419.68 ± 110.76 ***a *b | <0.001 |
| ALT (U/L) | 21.00 (14.00, 32.00) | 23.00 (17.00, 38.50) *a | 27.00 (18.00, 40.00) **a | 28.00 (20.00, 41.00) ***a *b | <0.001 |
| AST (U/L) | 21.00 (17.00, 25.00) | 22.00 (18.00, 29.00) *a | 22.00 (18.00, 31.00) | 24.00 (19.00, 31.00) ***a | <0.001 |
| ALP (U/L) | 74.00 (60.00, 88.00) | 72.00 (62.00, 86.00) | 74.00 (64.00, 87.00) | 74.00 (63.00, 89.00) | 0.40 |
| ALB (g/L) | 41.10 (38.60, 43.42) | 41.50 (39.10, 43.65) | 41.60 (38.80, 44.00) | 41.20 (39.00, 44.00) | 0.61 |
| CT value (HU) | 33.00 (31.00, 41.50) | 32.50 (28.62, 35.25) | 32.50 (30.00, 42.25) | 32.25 (24.00, 34.38) | 0.11 |
| Platelet (109/L) | 253.23 ± 70.64 | 253.23 ± 70.64 | 253.23 ± 70.64 | 253.23 ± 70.64 | 0.26 |
| FIB-4 index | 0.73 (0.46, 1.06) | 0.85 (0.56, 1.23) *a | 0.79 (0.49, 1.09) | 0.87 (0.61, 1.32) ***a *c | <0.001 |
| CACS category > 100, n (%) | 19 (6.9%) | 57 (20.1%) *a | 34 (23.5%) *a | 97 (21.0%) *a | <0.001 |
| Characteristics | 1hPG (−) and 2hPG (−) (n = 276) | 1hPG (+) and 2hPG (−) (n = 283) | 1hPG (−) and 2hPG (+) (n = 145) | 1hPG (+) and 2hPG (+) (n = 461) | p for Trend |
|---|---|---|---|---|---|
| Moderate–severe steatosis | |||||
| Crude | Reference | 2.36 (1.25–4.48) | 0.99 (0.51–1.93) | 1.74 (0.99–3.08) | 0.28 |
| Model 1 | Reference | 2.12 (1.11–4.07) | 0.84 (0.42–1.67) | 1.47 (0.81–2.68) | 0.63 |
| Model 2 | Reference | 2.19 (1.13–4.25) | 0.80 (0.39–1.63) | 1.44 (0.78–2.64) | 0.72 |
| Liver injury | |||||
| Crude | Reference | 1.28 (0.90–1.82) | 1.60 (1.06–2.43) | 1.90 (1.39–2.60) | <0.001 |
| Model 1 | Reference | 1.42 (0.99–2.04) | 1.67 (1.08–2.58) | 2.13 (1.52–2.99) | <0.001 |
| Model 2 | Reference | 1.40 (0.97–2.02) | 1.55 (0.99–2.43) | 2.03 (1.44–2.86) | <0.001 |
| Liver fibrosis | |||||
| Crude | Reference | 1.43 (0.94–2.18) | 1.30 (0.78–2.18) | 1.86 (1.28–2.71) | 0.002 |
| Model 1 | Reference | 1.44 (0.93–2.22) | 1.69 (0.99–2.89) | 2.43 (1.63–3.63) | <0.001 |
| Model 2 | Reference | 1.49 (0.96–2.32) | 1.99 (1.15–3.45) | 2.72 (1.79–4.11) | <0.001 |
| CVD | |||||
| Crude | Reference | 3.41 (1.97–5.91) | 4.14 (2.26–7.58) | 3.60 (2.15–6.04) | <0.001 |
| Model 1 | Reference | 2.73 (1.53–4.88) | 2.85 (1.49–5.44) | 2.31 (1.34–4.01) | 0.049 |
| Model 2 | Reference | 2.77 (1.55–4.96) | 2.98 (1.54–5.78) | 2.41 (1.38–4.21) | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, L.; Luo, L.; Sun, Y.; Wang, W.; Dong, Z.; Cao, X.; Ye, J.; Zhong, B. Comparisons of Post-Load Glucose at Different Time Points for Identifying High Risks of MASLD Progression. Nutrients 2025, 17, 152. https://doi.org/10.3390/nu17010152
Teng L, Luo L, Sun Y, Wang W, Dong Z, Cao X, Ye J, Zhong B. Comparisons of Post-Load Glucose at Different Time Points for Identifying High Risks of MASLD Progression. Nutrients. 2025; 17(1):152. https://doi.org/10.3390/nu17010152
Chicago/Turabian StyleTeng, Long, Ling Luo, Yanhong Sun, Wei Wang, Zhi Dong, Xiaopei Cao, Junzhao Ye, and Bihui Zhong. 2025. "Comparisons of Post-Load Glucose at Different Time Points for Identifying High Risks of MASLD Progression" Nutrients 17, no. 1: 152. https://doi.org/10.3390/nu17010152
APA StyleTeng, L., Luo, L., Sun, Y., Wang, W., Dong, Z., Cao, X., Ye, J., & Zhong, B. (2025). Comparisons of Post-Load Glucose at Different Time Points for Identifying High Risks of MASLD Progression. Nutrients, 17(1), 152. https://doi.org/10.3390/nu17010152





