Effects of Maca (Lepidium meyenii Walp.) on Physical Performance in Animals and Humans: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection Criteria
2.2. Search Strategy and Information Sources
2.3. Data Extraction
2.4. Risk of Publication Bias Across Studies
2.5. Assessment of Methodological Quality and Risk of Bias in Individual Studies
2.6. Statistical Analysis and Results Synthesis
3. Results
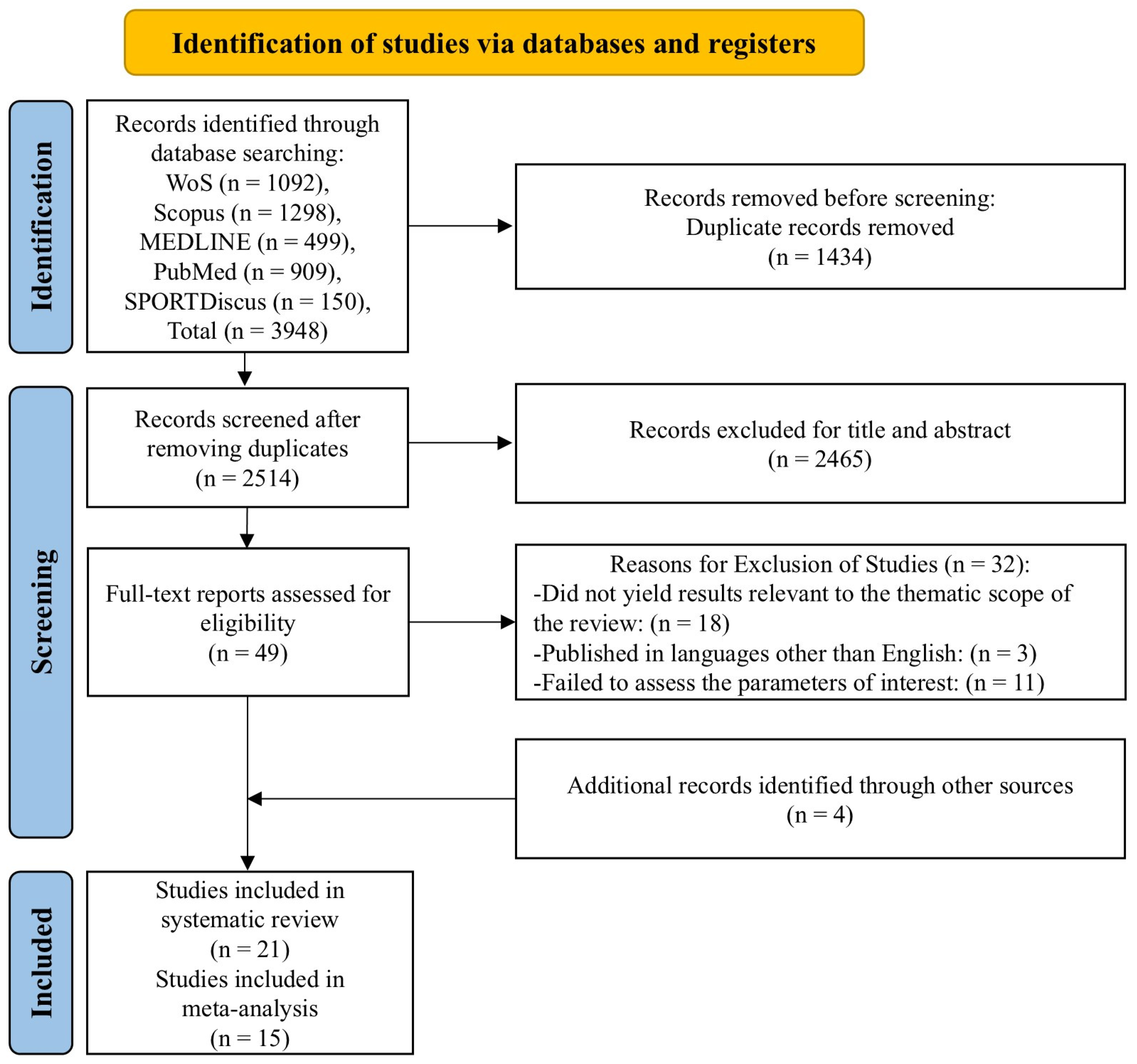
3.1. Study Selection
3.2. Assessment of Methodological Quality of Individual Studies
3.3. Meta-Analysis
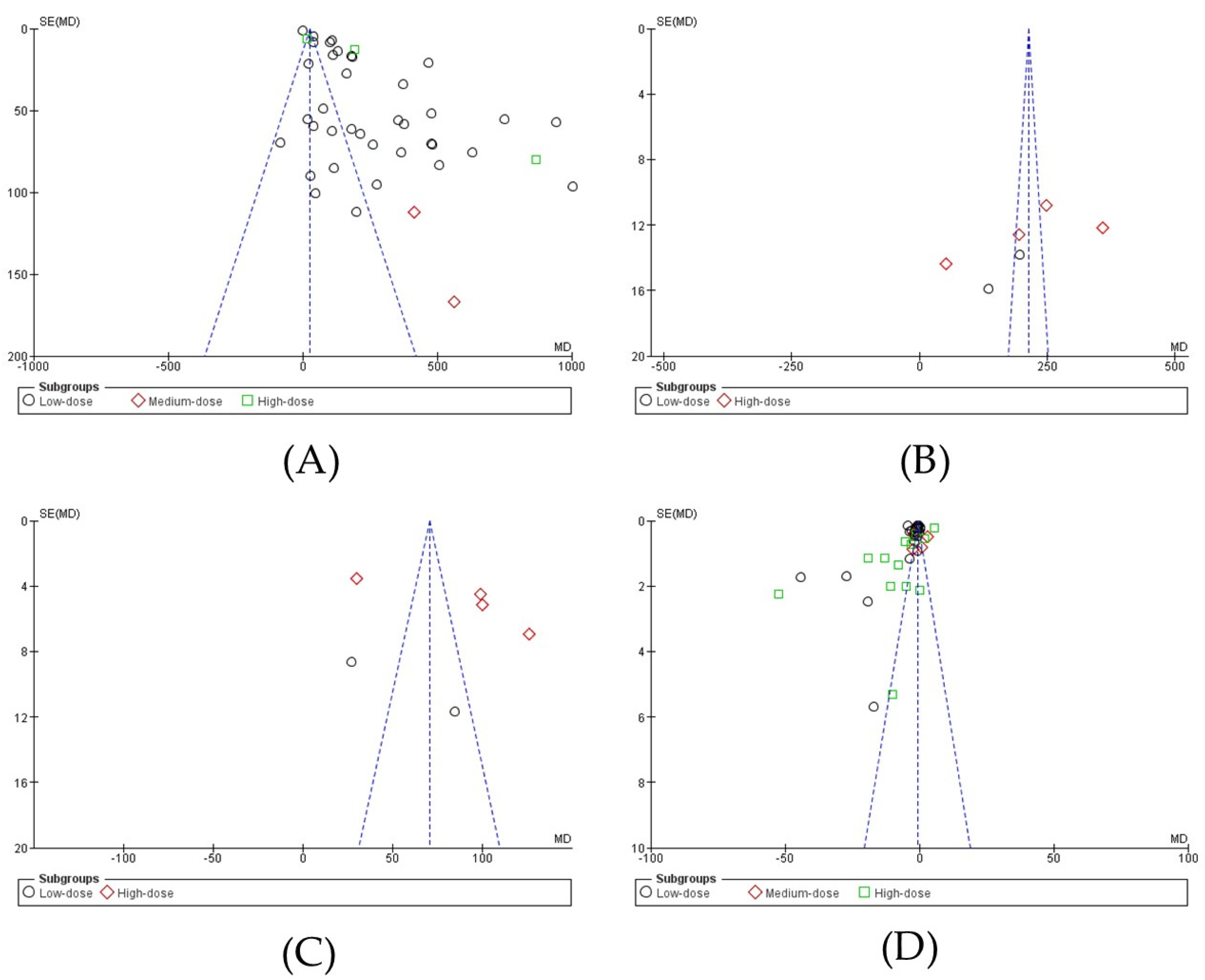
3.4. Publication Bias
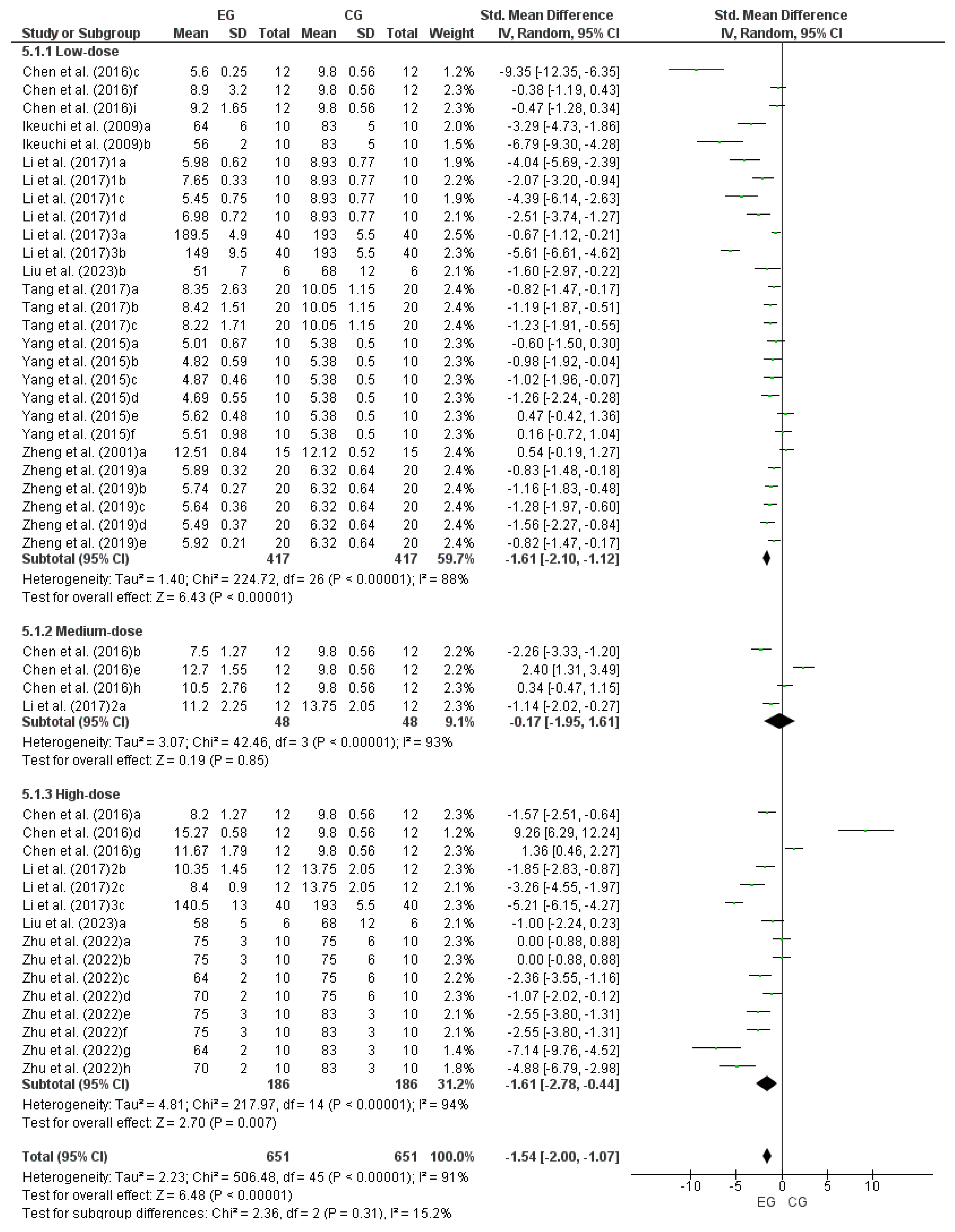
3.5. Effect of LmW on Forced Swimming Test
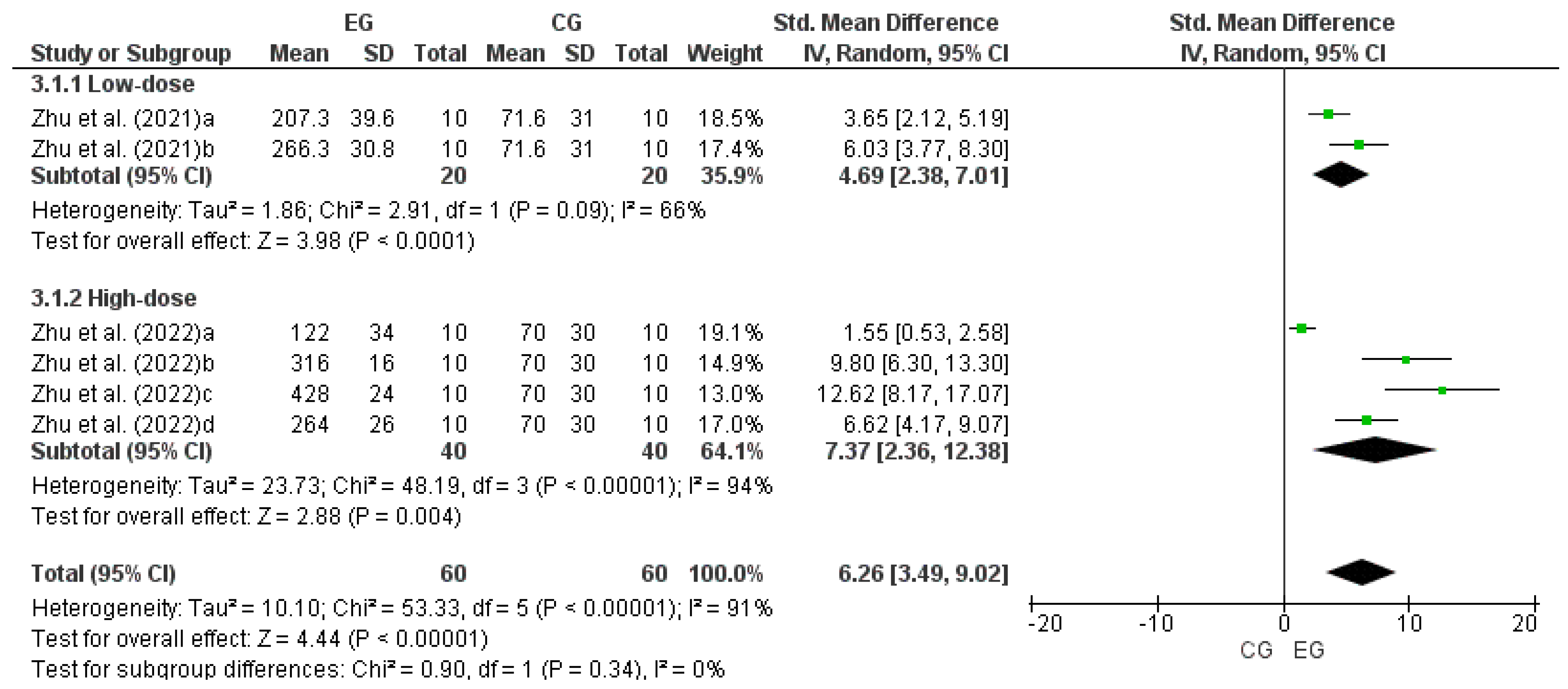
3.6. Effect of LmW on the Rota-Rod Test
3.7. Effect of LmW on the Grip Strength Test
3.8. Effect of LmW on Blood Lactic Acid
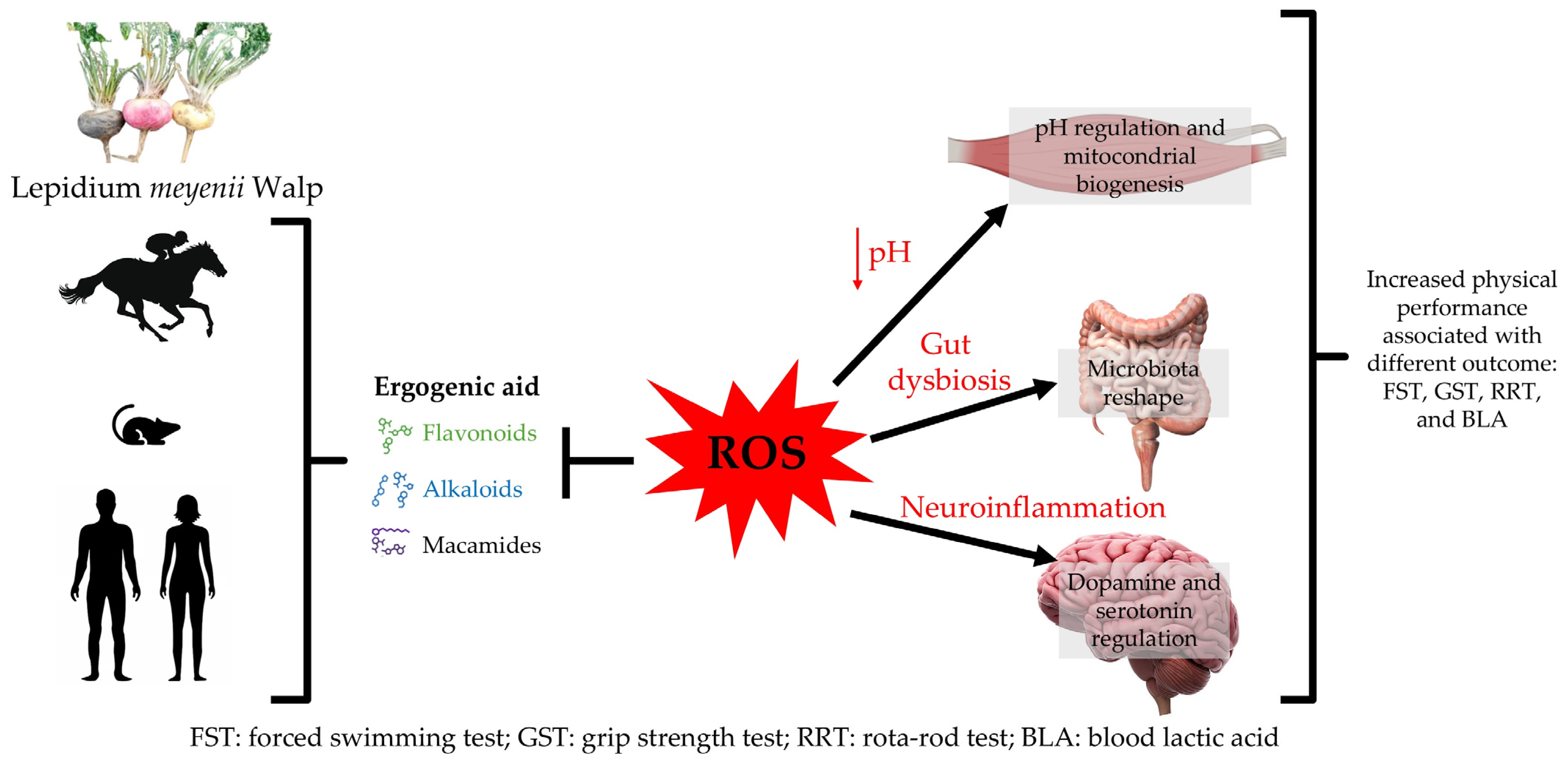
4. Discussion
4.1. Effect of LmW on Aerobic Test
4.2. Effect of LmW on Glycolytic Test
4.2.1. Effect on Muscle Lactic Acid Levels
4.2.2. Effect on Lactic Acid in Blood
4.3. Effect of LmW on Strength Test
4.4. Limitations
5. Conclusions
6. Future Lines of Research
Author Contributions
Funding
Conflicts of Interest
References
- Ikeuchi, S.; Minamida, M.; Nakamura, T.; Konishi, M.; Kamioka, H. Exploratory Systematic Review and Meta-Analysis of Panax Genus Plant Ingestion Evaluation in Exercise Endurance. Nutrients 2022, 14, 1185. [Google Scholar] [CrossRef]
- Amir, M.; Vohra, M.; Raj, R.G.; Osoro, I.; Sharma, A. Adaptogenic Herbs: A Natural Way to Improve Athletic Performance. Health Sci. Rev. 2023, 7, 100092. [Google Scholar] [CrossRef]
- Guo, S.; Rezaei, M.J. The Benefits of Ashwagandha (Withania somnifera) Supplements on Brain Function and Sports Performance. Front. Nutr. 2024, 11, 1439294. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Jagim, A.R.; Potter, G.D.M.; Garner, D.; Galpin, A.J. Rhodiola Rosea as an Adaptogen to Enhance Exercise Performance: A Review of the Literature. Br. J. Nutr. 2024, 131, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Cuenca, E.; Maté-Muñoz, J.L.; García-Fernández, P.; Serra-Paya, N.; Estevan, M.C.L.; Herreros, P.V.; Garnacho-Castaño, M.V. Effects of Beetroot Juice Supplementation on Cardiorespiratory Endurance in Athletes. A Systematic Review. Nutrients 2017, 9, 43. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F. Chemical Composition and Health Effects of Maca (Lepidium meyenii). Food Chem. 2019, 288, 422–443. [Google Scholar] [CrossRef]
- da Silva Leitão Peres, N.; Cabrera Parra Bortoluzzi, L.; Medeiros Marques, L.L.; Formigoni, M.; Fuchs, R.H.B.; Droval, A.A.; Reitz Cardoso, F.A. Medicinal Effects of Peruvian Maca (Lepidium meyenii): A Review. Food Funct. 2020, 11, 83–92. [Google Scholar] [CrossRef]
- Huerta Ojeda, Á.; Rodríguez Rojas, J.; Cuevas Guíñez, J.; Ciriza Velásquez, S.; Cancino-López, J.; Barahona-Fuentes, G.; Yeomans-Cabrera, M.-M.; Pavez, L.; Jorquera-Aguilera, C. The Effects of Maca (Lepidium meyenii Walp.) on Cellular Oxidative Stress: A Systematic Review and Meta-Analysis. Antioxidants 2024, 13, 1046. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, M.S.; Qu, F.; Lee, J.-W.; Kim, E. Maca (Lepidium meyenii Walp.) on Semen Quality Parameters: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2022, 13, 934740. [Google Scholar] [CrossRef]
- Choi, E.H.; Kang, J.I.; Cho, J.Y.; Lee, S.H.; Kim, T.S.; Yeo, I.H.; Chun, H.S. Supplementation of Standardized Lipid-Soluble Extract from Maca (Lepidium meyenii) Increases Swimming Endurance Capacity in Rats. J. Funct. Foods 2012, 4, 568–573. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Koyama, T.; Takei, S.; Kino, T.; Yazawa, K. Effects of Benzylglucosinolate on Endurance Capacity in Mice. J. Health Sci. 2009, 55, 178–182. [Google Scholar] [CrossRef][Green Version]
- Kreider, R.B.; Wilborn, C.D.; Taylor, L.; Campbell, B.; Almada, A.L.; Collins, R.; Cooke, M.; Earnest, C.P.; Greenwood, M.; Kalman, D.S.; et al. ISSN Exercise and Sport Nutrition Review: Research and Recommendations. J. Int. Soc. Sports Nutr. 2010, 7, 7. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jäger, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN Exercise & Sports Nutrition Review Update: Research & Recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Park, M.; Kim, B.; Kang, S. Effect of Black Maca Supplementation on Inflammatory Markers and Physical Fitness in Male Elite Athletes. Nutrients 2023, 15, 1618. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ma, L.-L.; Wang, Y.-Y.; Yang, Z.-H.; Huang, D.; Weng, H.; Zeng, X.-T. Methodological Quality (Risk of Bias) Assessment Tools for Primary and Secondary Medical Studies: What Are They and Which Is Better? Mil. Med. Res. 2020, 7, 2–11. [Google Scholar] [CrossRef]
- Hartling, L.; Milne, A.; Hamm, M.P.; Vandermeer, B.; Ansari, M.; Tsertsvadze, A.; Dryden, D.M. Testing the Newcastle Ottawa Scale Showed Low Reliability between Individual Reviewers. J. Clin. Epidemiol. 2013, 66, 982–993. [Google Scholar] [CrossRef]
- Chen, X.-F.; Liu, Y.-Y.; Cao, M.-J.; Zhang, L.-J.; Sun, L.-C.; Su, W.-J.; Liu, G.-M. Hypoxia Tolerance and Fatigue Relief Produced by Lepidium meyenii and Its Water-Soluble Polysaccharide in Mice. Food Sci. Technol. Res. 2016, 22, 611–621. [Google Scholar] [CrossRef][Green Version]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test, Graphical Test. BMJ 1998, 316, 629–634. [Google Scholar]
- Moskalewicz, A.; Oremus, M. No Clear Choice between Newcastle–Ottawa Scale and Appraisal Tool for Cross-Sectional Studies to Assess Methodological Quality in Cross-Sectional Studies of Health-Related Quality of Life and Breast Cancer. J. Clin. Epidemiol. 2020, 120, 94–103. [Google Scholar] [CrossRef]
- Sirriyeh, R.; Lawton, R.; Gardner, P.; Armitage, G. Reviewing Studies with Diverse Designs: The Development and Evaluation of a New Tool. J. Eval. Clin. Pract. 2012, 18, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V. Distribution Theory for Glass’s Estimator of Effect Size and Related Estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 2013. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Bilal, T.; Abas, I.; Korkmaz, S.; Ates, A.; Keser, O.; Kumas, C. Effects of Maca (Lepidium meyenii Walp.) Powder on Serum Indices and Metabolic Responses in Racehorses. J. Anim. Plant Sci. 2016, 26, 901–908. [Google Scholar]
- Li, J.; Sun, Q.; Meng, Q.; Wang, L.; Xiong, W.; Zhang, L. Anti-Fatigue Activity of Polysaccharide Fractions from Lepidium meyenii Walp. (Maca). Int. J. Biol. Macromol. 2017, 95, 1305–1311. [Google Scholar] [CrossRef]
- Li, R.-W.; He, J.-C.; Song, D.-H. Anti-Physical Fatigue Effect of Polysaccharides from Lepidium meyenii. Walp. and the Possible Mechanisms. Biomed. Res. 2017, 28, S433–S438. [Google Scholar]
- Li, J.; Chen, L.; Li, J.; Duan, Z.; Zhu, S.; Fan, L. The Composition Analysis of Maca (Lepidium meyenii Walp.) from Xinjiang and Its Antifatigue Activity. J. Food Qual. 2017, 2017, 2904951. [Google Scholar] [CrossRef]
- Li, Y.; Xin, Y.; Xu, F.; Zheng, M.; Xi, X.; Cui, X.; Cao, H.; Guo, H.; Han, C. Maca Polysaccharides: Extraction Optimization, Structural Features and Anti-Fatigue Activities. Int. J. Biol. Macromol. 2018, 115, 618–624. [Google Scholar] [CrossRef]
- Liu, T.; Peng, Z.; Lai, W.; Shao, Y.; Gao, Q.; He, M.; Zhou, W.; Guo, L.; Kang, J.; Jin, X.; et al. The Efficient Synthesis and Anti-Fatigue Activity Evaluation of Macamides: The Unique Bioactive Compounds in Maca. Molecules 2023, 28, 3943. [Google Scholar] [CrossRef]
- López-Fando, A.; Gómez-Serranillos, M.P.; Iglesias, I.; Lock, O.; Upamayta, U.P.; Carretero, M.E. Lepidium Peruvianum Chacon Restores Homeostasis Impaired by Restraint Stress. Phytother. Res. 2004, 18, 471–474. [Google Scholar] [CrossRef]
- Orhan, C.; Gencoglu, H.; Tuzcu, M.; Sahin, N.; Ojalvo, S.P.; Sylla, S.; Komorowski, J.R.; Sahin, K. Maca Could Improve Endurance Capacity Possibly by Increasing Mitochondrial Biogenesis Pathways and Antioxidant Response in Exercised Rats. J. Food Biochem. 2022, 46, e14159. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Jin, L.; Xie, L.; Huang, J.; Wang, N.; Chu, B.; Dai, X.; Liu, Y.; Wang, R.; Zhang, Y. Structural Characterization and Antifatigue Effect In Vivo of Maca (Lepidium meyenii Walp.) Polysaccharide. J. Food Sci. 2017, 82, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jin, W.; Lv, X.; Dai, P.; Ao, Y.; Wu, M.; Deng, W.; Yu, L. Effects of Macamides on Endurance Capacity and Anti-Fatigue Property in Prolonged Swimming Mice. Pharm. Biol. 2016, 54, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.L.; He, K.; Hwang, Z.Y.; Lu, Y.; Yan, S.J.; Kim, C.H.; Zheng, Q.Y. Effect of Aqueous Extract from Lepidium meyenii on Mouse Behavior in Forced Swimming Test. In Quality Management of Nutraceuticals; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2001; Volume 803, pp. 258–268. ISBN 9780841237735. [Google Scholar]
- Zheng, Y.; Zhang, W.-C.; Wu, Z.-Y.; Fu, C.-X.; Hui, A.-L.; Gao, H.; Chen, P.-P.; Du, B.; Zhang, H.-W. Two Macamide Extracts Relieve Physical Fatigue by Attenuating Muscle Damage in Mice. J. Sci. Food Agric. 2019, 99, 1405–1412. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, W.; Wang, N.; Jiang, W.; Cheng, Y.; Guo, Y.; Yao, W.; Hu, B.; Du, P.; Qian, H. Anti-Fatigue Effect of Lepidium meyenii Walp. (Maca) on Preventing Mitochondria-Mediated Muscle Damage and Oxidative Stress in Vivo and Vitro. Food Funct. 2021, 12, 3132–3141. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, R.; Hua, H.; Qian, H.; Du, P. Deciphering the Potential Role of Maca Compounds Prescription Influencing Gut Microbiota in the Management of Exercise-Induced Fatigue by Integrative Genomic Analysis. Front. Nutr. 2022, 9, 1004174. [Google Scholar] [CrossRef]
- Honma, A.; Fujiwara, Y.; Takei, S.; Kino, T. The Improvement of Daily Fatigue in Women Following the Intake of Maca (Lepidium meyenii) Extract Containing Benzyl Glucosinolate. Funct. Foods Health Dis. 2022, 12, 175–187. [Google Scholar] [CrossRef]
- Liu, M.-C.; Weng, P.-W.; Chien, Y.-H.; Wu, M.-H.; Hsu, W.-B.; Chen, S.-W.; Yang, M.-T. Effects of Lepidium meyenii (Maca) Extract Supplementation on Oxidative Stress, Muscle Damage, and Aerobic Capacity after Exhaustive Endurance Exercise. Isokinet. Exerc. Sci. 2024, 32, 349–357. [Google Scholar] [CrossRef]
- Stone, M.; Ibarra, A.; Roller, M.; Zangara, A.; Stevenson, E. A Pilot Investigation into the Effect of Maca Supplementation on Physical Activity and Sexual Desire in Sportsmen. J. Ethnopharmacol. 2009, 126, 574–576. [Google Scholar] [CrossRef]
- Ament, W.; Verkerke, G.J. Exercise and Fatigue. Sports Med. 2009, 39, 389–422. [Google Scholar] [CrossRef]
- Gan, Z.; Fu, T.; Kelly, D.P.; Vega, R.B. Skeletal Muscle Mitochondrial Remodeling in Exercise and Diseases. Cell Res. 2018, 28, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Gulick, T.; Cresci, S.; Caira, T.; Moore, D.D.; Kelly, D.P. The Peroxisome Proliferator-Activated Receptor Regulates Mitochondrial Fatty Acid Oxidative Enzyme Gene Expression. Proc. Natl. Acad. Sci. USA 1994, 91, 11012–11016. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.R.; Wilson, B.J.; Huss, J.M.; Kelly, D.P.; Alaynick, W.A.; Downes, M.; Evans, R.M.; Blanchette, M.; Giguère, V. Genome-Wide Orchestration of Cardiac Functions by the Orphan Nuclear Receptors ERRα and γ. Cell Metab. 2007, 5, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Li, H.; Xiang, J.; Yi, F.; Xu, L.; Jiang, B.; Xiao, P. Aqueous Extract of Black Maca Prevents Metabolism Disorder via Regulating the Glycolysis/Gluconeogenesis-TCA Cycle and PPARα Signaling Activation in Golden Hamsters Fed a High-Fat, High-Fructose Diet. Front. Pharmacol. 2018, 9, 333. [Google Scholar] [CrossRef]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.-Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional Co-Activator PGC-1α Drives the Formation of Slow-Twitch Muscle Fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef]
- Yi, D.; Yoshikawa, M.; Sugimoto, T.; Tomoo, K.; Okada, Y.; Hashimoto, T. Effects of Maca on Muscle Hypertrophy in C2C12 Skeletal Muscle Cells. Int. J. Mol. Sci. 2022, 23, 6825. [Google Scholar] [CrossRef]
- Chen, H.; Han, Y.; Jahan, I.; Wu, S.; Clark, B.C.; Wiseman, J.S. Extracts of Maca (Lepidium meyenii) Root Induce Increased Glucose Uptake by Inhibiting Mitochondrial Function in an Adipocyte Cell Line. J. Herb. Med. 2019, 17–18, 100282. [Google Scholar] [CrossRef]
- MacInnis, M.J.; Gibala, M.J. Physiological Adaptations to Interval Training and the Role of Exercise Intensity. J. Physiol. 2017, 595, 2915–2930. [Google Scholar] [CrossRef]
- Margolis, L.M.; Pasiakos, S.M. Optimizing Intramuscular Adaptations to Aerobic Exercise: Effects of Carbohydrate Restriction and Protein Supplementation on Mitochondrial Biogenesis. Adv. Nutr. 2013, 4, 657–664. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, R.; Hua, H.; Cheng, Y.; Guo, Y.; Qian, H.; Du, P. Network Pharmacology Exploration Reveals Gut Microbiota Modulation as a Common Therapeutic Mechanism for Anti-Fatigue Effect Treated with Maca Compounds Prescription. Nutrients 2022, 14, 1533. [Google Scholar] [CrossRef]
- Maes, M. Inflammatory and Oxidative and Nitrosative Stress Pathways Underpinning Chronic Fatigue, Somatization and Psychosomatic Symptoms. Curr. Opin. Psychiatry 2009, 22, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Proschinger, S.; Freese, J. Neuroimmunological and Neuroenergetic Aspects in Exercise-Induced Fatigue. Exerc. Immunol. Rev. 2019, 25, 8–19. [Google Scholar]
- Zhu, H.; Wang, R.; Hua, H.; Cheng, Y.; Guo, Y.; Qian, H.; Du, P. The Macamide Relieves Fatigue by Acting as Inhibitor of Inflammatory Response in Exercising Mice: From Central to Peripheral. Eur. J. Pharmacol. 2022, 917, 174758. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Ruiz, A.; Gonzales, C.; Villegas, L.; Cordova, A. Effect of Lepidium meyenii (Maca) Roots on Spermatogenesis of Male Rats. Asian J. Androl. 2001, 3, 231–233. [Google Scholar]
- Gonzales, G.F. Ethnobiology and Ethnopharmacology of Lepidium meyenii (Maca), a Plant from the Peruvian Highlands. Evid. Based Complement. Altern. Med. 2012, 2012, 193496. [Google Scholar] [CrossRef]
- Davies, K.J.A.; Quintanilha, A.T.; Brooks, G.A.; Packer, L. Free Radicals an Tissue Damage. Biochem. Biophys. Res. Commun. 1982, 107, 1198–1205. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Thomson, D.M. The Role of AMPK in the Regulation of Skeletal Muscle Size, Hypertrophy, and Regeneration. Int. J. Mol. Sci. 2018, 19, 3125. [Google Scholar] [CrossRef]
- O’Connell, M.D.L.; Wu, F.C.W. Androgen Effects on Skeletal Muscle: Implications for the Development and Management of Frailty. Asian J. Androl. 2014, 16, 203–212. [Google Scholar]
- Bamman, M.M.; Shipp, J.R.; Jiang, J.; Gower, B.A.; Hunter, G.R.; Goodman, A.; Mclafferty, C.L.; Urban, R.J.; McLafferty, C.L. Mechanical Load Increases Muscle IGF-I and Androgen Receptor MRNA Concentrations in Humans. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E383–E390. [Google Scholar] [CrossRef]
- Basualto-Alarcón, C.; Jorquera, G.; Altamirano, F.; Jaimovich, E.; Estrada, M. Testosterone Signals through MTOR and Androgen Receptor to Induce Muscle Hypertrophy. Med. Sci. Sports Exerc. 2013, 45, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Estrada, M.; Espinosa, A.; Müller, M.; Jaimovich, E. Testosterone Stimulates Intracellular Calcium Release and Mitogen-Activated Protein Kinases via a G Protein-Coupled Receptor in Skeletal Muscle Cells. Endocrinology 2003, 144, 3586–3597. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.; Ferrando, A.; Sheffield-Moore, M.; Urban, R. Testosterone and Muscle Protein Metabolism. Mayo Clin. Proc. 2000, 75, S55–S60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.Y.; Li, C.N.; Zhang, N.X.; Gao, X.C.; Shen, J.M.; Cheng, D.D.; Wang, Y.L.; Zhang, H.; Lv, J.W.; Sun, J.M. UPLC-QE-Orbitrap-Based Cell Metabolomics and Network Pharmacology to Reveal the Mechanism of N-Benzylhexadecanamide Isolated from Maca (Lepidium meyenii Walp.) against Testicular Dysfunction. Molecules 2023, 28, 4064. [Google Scholar] [CrossRef]

| Authors | Participants or Sample | Independent Variable | Dependent Variable | Supplementation Protocol | Results | Effect |
|---|---|---|---|---|---|---|
| Research in animals | ||||||
| Bilal et al. [26] | Racehorses: EG1 (n = 6) EG2 (n = 6) CG (n = 6) | EG: MPB CG: Basal diet | PO: LA | Maca root extract powder: EG1: basal diet + 50 g/day EG2: basal diet + 75 g/day CG: basal diet | LA (mmol/L): EG1 post-test = 2.27 vs. CG post-test = 1.56; p = 0.18 EG2 post-test = 2.05 vs. CG post-test = 1.56; p = 0.18 | LA (mmol/L): EG1 post-test vs. CG ↔ EG2 post-test vs. CG ↔ |
| Chen et al. [18] | Mice: EG1 (n = 12) EG2 (n = 12) EG3 (n = 12) EG4 (n = 12) EG5 (n = 12) EG6 (n = 12) EG7 (n = 12) EG8 (n = 12) EG9 (n = 12) CG (n = 12) | EG: MPB, ME and MWP CG: PL | PO: time (s) in FST, BLA | MPB: EG1 (high dose): 1.0 g/kg EG2 (medium dose): 0.5 g/kg EG3 (low dose): 0.1 g/kg ME: EG4 (high dose): 1.0 g/kg EG5 (medium dose): 0.5 g/kg EG6 (low dose): 0.1 g/kg MWP: EG7 (high dose): 1.0 g/kg EG8 (medium dose): 0.5 g/kg EG9 (low dose): 0.1 g/kg CG: distilled water | FST (s): MPB group: EG1 = 1970.4 ± 403.8 vs. CG = 843.6 ± 186; p < 0.01 EG2 = 1404 ± 546 vs. CG = 843.6 ± 186; p > 0.05 EG3 = 1350 ± 222 vs. CG = 843.6 ± 186; p > 0.05 ME group: EG4 = 2850 ± 1802.4 vs. CG = 843.6 ± 186; p > 0.05 EG5 = 2115 ± 843.6 vs. CG = 843.6 ± 186; p < 0.05 EG6 = 1846.8 ± 278.4 vs. CG =843.6 ± 186; p > 0.05 MWP group: EG7 = 2445.6 ± 478.2 vs. CG = 843.6 ± 186; p < 0.01 EG8 = 2215.2 ± 135.6 vs. CG = 843.6 ± 186; p < 0.05 EG9 = 2812.2 ± 907.8 vs. CG = 843.6 ± 186; p < 0.05 BLA (mmol/L): MPB groups: EG1 = 8.20 ± 1.27 vs. CG = 9.8 ± 0.56; p > 0.05 EG2 = 7.5 ± 1.27 vs. CG = 9.8 ± 0.56; p < 0.01 EG3 = 5.60 ± 0.25 vs. CG = 9.8 ± 0.56; p < 0.01 ME groups: EG4 = 15.27 ± 0.58 vs. CG = 9.8 ± 0.56; p > 0.05 EG5 = 12.70 ± 1.55 vs. CG = 9.8 ± 0.56; p > 0.05 EG6 = 8.90 ± 3.20 vs. CG = 9.8 ± 0.56; p > 0.05 MWP groups: EG7 = 11.67 ± 1.79 vs. CG = 9.8 ± 0.56; p > 0.05 EG8 = 10.5 ± 2.76 vs. CG = 9.8 ± 0.56; p > 0.05 EG9 = 9.2 ± 1.65 vs. CG = 9.8 ± 0.56; p > 0.05 | FST (s): MP group: EG1 vs. CG ↑ EG2 vs. CG ↔ EG3 vs. CG ↔ ME group: EG4 vs. CG ↔ EG5 vs. CG ↑ EG6 vs. CG ↔ MWP group: EG7 vs. CG ↑ EG8 vs. CG ↑ EG9 vs. CG ↑ BLA (mmol/L): MP groups: EG1 vs. CG ↔ EG2 vs. CG ↓ EG3 vs. CG ↓ MAE groups: EG4 vs. CG ↔ EG5 vs. CG ↔ EG6 vs. CG ↔ MWP groups: EG7 vs. CG ↔ EG8 vs. CG ↔ EG9 vs. CG ↔ |
| Choi et al. [10] | Mice: EG1 (n = 20) EG2 (n = 20) CG (n = 20) | EG1 and EG2: LME CG: PL | PO: time (s) in FST | Lipid soluble maca extract: EG1: 0.03 g/kg EG2: 0.1 g/kg CG: 10 mL/kg sterile water | FST (s): EG1 = 563 ± 58 vs. CG = 452 ± 46; p > 0.05 EG2 = 638 ± 62 vs. CG = 452 ± 46; p < 0.05 | FST (s): EG1 vs. CG ↔ EG2 vs. CG ↑ |
| Ikeuchi et al. [11] | Mice: EG1 (n = 10) EG2 (n = 10) CG (n = 10) | EG1 and EG2: Benzylglucosinolate CG: Distilled water | PO: time (s) in FST, LA | Benzylglucosinolate: EG1: 0.015 mg/kg EG2: 0.03 mg/kg CG: distilled water | FST (s): EG1 = 113 ± 26 vs. CG = 73 ± 7; p > 0.05 EG2 = 175 ± 27 vs. CG = 73 ± 7; p < 0.05 LA (mg/dL): EG1 = 64 ± 6 vs. CG = 83 ± 5; p < 0.01 EG1 = 56 ± 2 vs. CG = 83 ± 5; p < 0.01 | FST (s): EG1 vs. CG ↔ EG2 vs. CG ↑ LA (mg/dL): EG1 vs. CG ↓ EG2 vs. CG ↑ |
| Li et al. [27] (1) | Mice: EG1 (n = 10) EG2 (n = 10) EG3 (n = 10) EG4 (n = 10) CG (n = 10) | EG: MP CG: PL | PO: time (s) in FST, BLA | MP-1 EG1: 100 mg/kg EG2: 20 mg/kg MP-2 EG3: 100 mg/kg EG4: 20 mg/kg CG: saline solution | FST (s): EG1 = 672 ± 96 vs. CG = 300 ± 48; p < 0.05 EG2 = 462 ± 72 vs. CG = 300 ± 48; p < 0.05 EG3 = 765 ± 45 vs. CG = 300 ± 48; p < 0.05 EG4 = 480.6 ± 24 vs. CG = 300 ± 48; p < 0.05 BLA (mmol/L): EG1 = 5.98 ± 0.62 vs. CG = 8.93 ± 0.77; p < 0.05 EG2 = 7.65 ± 0.33 vs. CG = 8.93 ± 0.77; p < 0.05 EG3 = 5.45 ± 0.75 vs. CG = 8.93 ± 0.77; p < 0.05 EG4 = 6.98 ± 0.72 vs. CG = 8.93 ± 0.77; p < 0.05 | FST (s): EG1 vs. CG ↑ EG2 vs. CG ↑ EG3 vs. CG ↑ EG4 vs. CG ↑ BLA (mmol/L): EG1 vs. CG ↓ EG2 vs. CG ↓ EG3 vs. CG ↓ EG4 vs. CG ↓ |
| Li et al. [28] (2) | Mice: EG1 (n = 12) EG2 (n = 12) EG3 (n = 12) CG (n = 12) | EG: MP CG: PL | PO: time (s) in FST, BLA | MP EG1: 500 mg/kg EG2: 1000 mg/kg EG3: 2000 mg/kg CG: distilled water | FST (s): EG1 = 2118 ± 342.0 vs. CG = 1704 ± 180.6; p < 0.05 EG2 = 2568 ± 210.0 vs. CG = 1704 ± 180.6; p < 0.05 EG3 = 2958 ± 330.0 vs. CG = 1704 ± 180.6; p < 0.05 BLA (mmol/L): EG1 = 11.20 ± 2.25 vs. CG = 13.75 ± 2.05; p < 0.05 EG2 = 10.35 ± 1.45 vs. CG = 13.75 ± 2.05; p < 0.05 EG3 = 8.40 ± 0.90 vs. CG = 13.75 ± 2.05; p < 0.05 | FST (s): EG1 vs. CG ↑ EG2 vs. CG ↑ EG3 vs. CG ↑ BLA (mmol/L): EG1 vs. CG ↓ EG2 vs. CG ↓ EG3 vs. CG ↓ |
| Li et al. [29] (3) | Mice: EG1 (n = 40) EG2 (n = 40) EG3 (n = 40) CG (n = 40) | EG: Yellow maca root CG: PL | PO: time (s) in FST, BLA | Maca treatment: EG1: 40 mg/kg EG2: 400 mg/kg EG3: 1200 mg/kg CG: distilled water | FST (s): EG1 = 225 ± 19.8 vs. CG = 184.8 ± 24.6; p < 0.05 EG2 = 292.8 ± 37.8 vs. CG = 184.8 ± 24.6; p < 0.05 EG3 = 375 ± 76.8 vs. CG = 184.8 ± 24.6; p < 0.05 BLA (mmol/L): EG1 = 189.5 ± 4.9 vs. CG = 193 ± 5.5; p < 0.05 EG2 = 149.0 ± 9.5 vs. CG = 193 ± 5.5; p < 0.05 EG3 = 140.5 ± 13.0 vs. CG = 193 ± 5.5; p < 0.05 | FST (s): EG1 vs. CG ↑ EG2 vs. CG ↑ EG3 vs. CG ↑ BLA (mmol/L): EG1 vs. CG ↓ EG2 vs. CG ↓ EG3 vs. CG ↓ |
| Li et al. [30] | Mice: EG1 (n = 10) EG2 (n = 10) EG3 (n = 10) CG (n = 10) | EG: MP CG: PL | PO: time (s) in FST | MCP EG1: 150 mg/kg EG2: 300 mg/kg EG3: 600 mg/kg CG: distilled water | FST (s): EG1 = 3270 ± 438 vs. CG = 1596 ± 474; p < 0.01 EG2 = 2658 ± 1062 vs. CG = 1596 ± 474; p < 0.05 EG3 = 3108 ± 1464 vs. CG = 1596 ± 474; p < 0.01 | FST (s): EG1 vs. CG ↑ EG2 vs. CG ↑ EG3 vs. CG ↑ |
| Liu et al. [31] | Mice EG1 (n = 6) EG2 (n = 6) CG (n = 6) | EG: ME and NBH CG: PL | PO: time (s) in FST, BLA | ME EG1: 1000 mg/kg extract of maca NBH EG2: 10 mg/kg CG: distilled water | FST (s): EG1 = 102.5 ± 17.5 vs. CG = 88.5 ± 10.5; p > 0.05 EG2 = 217.0 ± 32.0 vs. CG = 88.5 ± 10.5; p < 0.05 BLA (mol/L) EG1 = 58 ± 5 vs. CG = 68 ± 12; p < 0.05 EG2 = 51 ± 7 vs. CG = 68 ± 12; p < 0.05 | FST (s): EG1 vs. CG ↔ EG1 vs. CG ↑ BLA (mol/L) EG1 vs. CG ↓ EG2 vs. CG ↓ |
| López-Fando et al. [32] | Mice: EG1 (n = 7) EG2 (n = 7) CG1 (n = 7) CG2 (n = 7) | EG1 and EG2: ME + stress by restraint in small flexible wire-mesh containers CG1 and CG2: PL + stress by restraint in small flexible wire-mesh containers | PO: time (s) in FST | ME EG1: 125 mg/kg EG2: 250 mg/kg CG1: 0.2 mL isotonic saline solution CG2: 0.2 mL isotonic saline solution without food and water | FST (s): EG1 = 132.6 ± 4.2 vs. CG1 = 132.6 ± 0.78; p > 0.05 EG1 = 132.6 ± 4.2 vs. CG2 = 169.8 ± 46.8; p < 0.05 EG2 = 192 ± 31.8 vs. CG1 = 132.6 ± 4.2; p < 0.05 EG2 = 192 ± 31.8 vs. CG2 = 169.8 ± 46.8; p > 0.05 | FST (s): EG1 vs. CG1 ↔ EG1 vs. CG2 ↓ EG2 vs. CG1 ↑ EG2 vs. CG2 ↔ |
| Orhan et al. [33] | Mice: EG1 (n = 7) EG2 (n = 7) CG1 (n = 7) | EG: MP and MP + FST CG: PL and PL + FST | PO: time in (s), FST | MP EG1: 40 mg/kg EG2: 40 mg/kg of MP + FST CG1: 1 mL of water | FST (min): EG1 = 16.10 ± 1.83; vs. CG1 = 0.00 ± 0.00; p > 0.05 EG2 = 11.15 ± 2.00; vs. CG1 = 0.00 ± 0.00; p < 0.01 | FST (min): EG1 vs. CG1 ↔ EG2 vs. CG1 ↑ |
| Tang et al. [34] | Mice: EG1 (n = 20) EG2 (n = 20) EG3 (n = 20) CG (n = 20) | EG: MP CG: PL | PO: time (s) in FST, LA | MP EG1: 100 mg/kg EG2: 50 mg/kg EG3: 25 mg/kg CG: distilled water | FST (s): EG1 = 10300.2 ± 4180.8 vs. CG = 2793.0 ± 1780.2; p < 0.01 EG2 = 6990.0 ± 3056.4 vs. CG = 2793.6 ± 1780.2; p < 0.01 EG3 = 2898.0 ± 2137.8 vs. CG = 2793.6 ± 1780.2; p < 0.05 LA (mmol/L): EG1 = 8.35 ± 2.63 vs. CG = 10.05 ± 1.15; p > 0.05 EG2 = 8.42 ± 1.51 vs. CG = 10.05 ± 1.15; p > 0.05 EG3 = 8.22 ± 1.71 vs. CG = 10.05 ± 1.15; p < 0.05 | FST (s): EG1 vs. CG ↑ EG2 vs. CG ↑ EG3 vs. CG ↑ LA (mmol/L): EG1 vs. CG ↔ EG2 vs. CG ↔ EG3 vs. CG ↓ |
| Yang et al. [35] | Mice: EG1 (n = 10) EG2 (n = 10) EG3 (n = 10) EG4 (n = 10) EG5 (n = 10) EG6 (n = 10) CG (n = 10) | EG: N-Benzyllinoleamide, N-benzyloleamide and N-benzylpalmitamide CG: PL | PO: time (s) in FST, LA | N- benzyllinoleamide EG1: 12 mg/kg EG2: 40 mg/kg N-benzyloleamide EG3: 12 mg/kg EG4: 40 mg/kg N-benzylpalmitamide EG5: 12 mg/kg EG6: 40 mg/kg CG: distilled water | FST (s): EG1 = 660 ± 300 vs. CG = 612 ± 108; p > 0.05 EG2 = 810 ± 336 vs. CG = 612 ± 108; p > 0.05 EG3 = 726 ± 246 vs. CG = 612 ± 108; p > 0.05 EG4 = 888 ± 282 vs. CG = 612 ± 108; p < 0.05 EG5 = 528 ± 192 vs. CG = 612 ± 108; p > 0.05 EG6 = 642 ± 264 vs. CG = 612 ± 108; p > 0.05 LA (mmol/L): EG1 = 5.01 ± 0.67 vs. CG = 5.38 ± 0.50; p > 0.05 EG2 = 4.82 ± 0.59 vs. CG = 5.38 ± 0.50; p < 0.05 EG3 = 4.87 ± 0.46 vs. CG = 5.38 ± 0.50; p < 0.05 EG4 = 4.69 ± 0.55 vs. CG = 5.38 ± 0.50; p < 0.05 EG5 = 5.62 ± 0.48 vs. CG = 5.38 ± 0.50; p > 0.05 EG6 = 5.51 ± 0.98 vs. CG = 5.38 ± 0.50; p > 0.05 | FST (s): EG1 vs. CG ↔ EG2 vs. CG ↔ EG3 vs. CG ↔ EG4 vs. CG ↑ EG5 vs. CG ↔ EG6 vs. CG ↔ LA (mmol/L): EG1 vs. CG ↔ EG2 vs. CG ↓ EG3 vs. CG ↓ EG4 vs. CG ↓ EG5 vs. CG ↔ EG6 vs. CG ↔ |
| Zheng et al. [36] | Mice: EG1 (n = 15) EG2 (n = 15 EG3 (n = 15) EG4 (n = 15) EG5 (n = 15) EG6 (n = 15) EG7 (n = 15) EG8 (n = 15) EG9 (n = 15) EG10 (n = 15) EG11 (n = 15) EG12 (n = 15) CG1 (n = 15) CG2 (n = 15) CG3 (n = 15) | EG: MacaForceTM AQ-1 CG: PL | PO: time in (s), FST | MacaForceTM AQ-1 7 days EG1: 4 mg/kg EG2: 10 mg/kg EG3: 20 mg/kg EG4: 40 mg/kg CG1: 10% ethanol/water solution 14 days EG5: 4 mg/kg EG6: 10 mg/kg EG7: 20 mg/kg EG8: 40 mg/kg CG2: 10% ethanol/water solution 21 days EG9: 4 mg/kg EG10: 10 mg/kg EG11: 20 mg/kg EG12: 40 mg/kg CG3: 10% ethanol/water solution | 7 days FST (s) EG1 = 646.8 ± 160.8 vs. CG1 = 628.2 ± 142.8; p > 0.05 EG2 = 668.4 ± 181.2 vs. CG1 = 628.2 ± 142.8; p > 0.05 EG3 = 810 ± 190.8 vs. CG1 = 628.2 ± 142.8; p < 0.05 EG4 = 1003.2 ± 176.4 vs. CG1 = 628.2 ± 142.8; p < 0.01 14 days FST (s) EG5 = 757.8 ± 118.8 vs. CG2 = 680.4 ± 147.6; p > 0.05 EG6 = 894 ± 201 vs. CG2 = 680.4 ± 147.6; p < 0.05 EG7 = 1045.2 ± 253.2 vs. CG2 = 680.4 ± 147.6; p < 0.05 EG8 = 1159.8 ± 231.6 vs. CG2 = 680.4 ± 147.6; p < 0.05 21 days FST (s) EG9 = 760.2 ± 176.4 vs. CG3 = 652.8 ± 165.6; p > 0.05 EG10 = 915 ± 219.6 vs. CG3 = 652.8 ± 165.6; p < 0.01 EG11 = 1130.4 ± 215.4 vs. CG3 = 652.8 ± 165.6; p < 0.01 EG12 = 1282.2 ± 241.2 vs. CG3 = 652.8 ± 165.6; p < 0.05 | 7 days FST (s) EG1 vs. CG1 ↔ EG2 vs. CG1 ↔ EG3 vs. CG1 ↑ EG4 vs. CG1 ↑ 14 days FST (s) EG5 vs. CG2 ↔ EG6 vs. CG2 ↑ EG7 vs. CG2 ↑ EG8 vs. CG2 ↑ 21 days FST (s) EG9 vs. CG3 ↔ EG10 vs. CG3 ↑ EG11 vs. CG3 ↑ EG12 vs. CG3 ↑ |
| Mice: EG (n = 15) CG (n = 15) | EG: MacaForceTM AQ-2 CG: PL | PO: time (s) in FST, LA | MacaForceTM AQ-2 EG: 40 mg/kg CG: 10% ethanol/water solution | FST (s) EG = 123.6 ± 3 vs. CG = 109.8 ± 2.4; p < 0.01 LA post 20 min rest (mmol/L) EG = 16.62 ± 0.67 vs. CG = 12.13 ± 0.52; p < 0.01 LA post 50 min rest (mmol/L) EG1 = 12.51 ± 0.84 vs. CG = 6.56 ± 0.35; p < 0.01 | FST (s) EG vs. CG ↑ LA post 20 min rest (mmol/L) EG vs. CG ↓ LA post 50 min rest (mmol/L) EG vs. CG ↓ | |
| Zheng et al. [37] | Mice: EG1 (n = 20) EG2 (n = 20) EG3 (n = 20) EG4 (n = 20) EG5 (n = 20) CG (n = 20) | EG: CME, PME, and maca tablet CG: PL | PO: time in (s), FST, BLA | CME EG1: 30 mg/kg EG2: 120 mg/kg PME EG3: 8 mg/kg EG4: 32 mg/kg Maca tablet EG5: 165 mg/kg CG: aqueous solution | FST (s): EG1 = 1330.8 ± 201.6 vs. CG = 975 ± 147.6; p > 0.05 EG2 = 1452 ± 177.6 vs. CG = 975 ± 147.6; p > 0.05 EG3 = 1725 ± 201 vs. CG = 975 ± 147.6; p < 0.05 EG4 = 1914 ± 208.8 vs. CG = 975 ± 147.6; p < 0.05 EG5 = 2303.4 ± 203.4 vs. CG = 975 ± 147.6; p < 0.05 BLA (mmol/L): EG1 = 5.89 ± 0.32 vs. CG = 6.32 ± 0.64; p < 0.05 EG2 = 5.74 ± 0.27 vs. CG = 6.32 ± 0.64; p < 0.05 EG3 = 5.64 ± 0.36 vs. CG = 6.32 ± 0.64; p < 0.05 EG4 = 5.49 ± 0.37 vs. CG = 6.32 ± 0.64; p < 0.05 EG5 = 5.92 ± 0.21 vs. CG = 6.32 ± 0.64; p < 0.05 | FST (s): EG1 vs. CG ↔ EG2 vs. CG ↔ EG3 vs. CG ↑ EG4 vs. CG ↑ EG5 vs. CG ↑ BLA (mmol/L): EG1 vs. CG ↓ EG2 vs. CG ↓ EG3 vs. CG ↓ EG5 vs. CG ↓ |
| Zhu et al. [38] | Mice: EG1 (n = 10) EG2 (n = 10) CG1 (n = 10) CG2 (n = 10) | EG: ME and caffeine CG: PL and PL + exercise | PO: time in (s), RRT, (gf); GST | ME: EG1: 10 mL/kg EG2: 10 mg/kg caffeine CG1: 10 mL/kg sterile water CG2: 10 mL/kg sterile water + exercise | GST (gf): EG1 = 185.83 ± 11.57 vs. CG = 158.46 ± 24.80; p < 0.05 EG2 = 242.93 ± 27.33 vs. CG = 158.46 ± 24.80; p < 0.01 RRT (min): EG1 = 207.3 ± 39.6 vs. CG = 71.6 ± 31.0; p < 0.01 EG2 = 266.3 ± 30.8 vs. CG = 71.6 ± 31.0; p < 0.01 | GST (gf): EG1 vs. CG ↑ EG2 vs. CG ↑ RRT (min): EG1 vs. CG ↑ EG2 vs. CG ↑ |
| Zhu et al. [39] | Mice EG1 (n = 10) EG2 (n = 10) EG3 (n = 10) EG4 (n = 10) CG (n = 10) | EG: MCP CG: PL | PO: time (s) in RRT, grams-force (gf); GST, BLA | MCP EG1: 1.0 g/kg MCP EG2: 2.0 g/kg MCP EG3: 4.0 g/kg MCP EG4: 10 mg/kg caffeine CG1: 1.0 g/kg sterile water CG2: 1.0 g/kg sterile water + Ex | RRT (s): EG1 = 122 ± 34 vs. CG1 = 70 ± 30; p < 0.05 EG2 = 316 ± 16 vs. CG1 = 70 ± 30; p < 0.01 EG3 = 428 ± 24 vs. CG1 = 70 ± 30; p < 0.01 EG4 = 264 ± 26 vs. CG1 = 70 ± 30; p < 0.01 GST (gf): EG1 = 179 ± 2 vs. CG1 = 149 ± 11; p < 0.01 EG2 = 248 ± 9 vs. CG1 = 149 ± 11; p < 0.01 EG3 = 275 ± 19 vs. CG1 = 149 ± 11; p < 0.01 EG4 = 249 ± 12 vs. CG1 = 149 ± 11; p < 0.01 BLA (μg/L): EG1 = 75 ± 3 vs. CG1 = 70 ± 2; p > 0.05 EG1 = 75 ± 3 vs. CG2 = 83 ± 3; p > 0.05 EG2 = 75 ± 3 vs. CG1 = 70 ± 2; p > 0.05 EG2 = 75 ± 3 vs. CG2 = 83 ± 3; p < 0.01 EG3 = 64 ± 2 vs. CG1 = 70 ± 2; p > 0.05 EG3 = 64 ± 2 vs. CG2 = 83 ± 3; p < 0.01 EG4 = 75 ± 6 vs. CG1 = 70 ± 2; p > 0.05 EG4 = 75 ± 6 vs. CG2 = 83 ± 3; p > 0.05 CG1 = 70 ± 2 vs. CG2 = 83 ± 3; p < 0.05 | RRT (s): EG1 vs. CG1 ↑ EG2 vs. CG1 ↑ EG3 vs. CG1 ↑ EG4 vs. CG1 ↑ GST (gf): EG1 vs. CG1 ↑ EG2 vs. CG1 ↑ EG3 vs. CG1 ↑ EG4 vs. CG1 ↑ BLA (μg/L): EG1 vs. CG1 ↔ EG1 vs. CG2 ↔ EG2 vs. CG1 ↔ EG2 vs. CG2 ↓ EG3 vs. CG1 ↔ EG3 vs. CG2 ↓ EG4 vs. CG1 ↔ EG4 vs. CG2 ↔ CG1 vs. CG2 ↔ |
| Research in humans | ||||||
| Honma et al. [40] | Adult women (n = 55) EG (n = 27) PL (n = 28) | EG: ME + benzylglucosinolate CG: PL | PO: VAS (cm) | ME + benzyl glucosinolate EG: 200 mg/kg CG: 200 mg/kg dextrine | VAS (cm): EG pre-test vs. post-test: 7.55 ± 0.65 vs. 5.20 ± 1.48; p < 0.01 CG pre-test vs. post-test: 7.52 ± 0.80 vs. 5.67 ± 1.74; p < 0.01 EG post-test vs. CG post-test: 5.20 ± 1.48 vs. 5.67 ± 1.74; p > 0.05 | VAS (cm): EG pre-test vs. test ↑ CG pre-test vs. post-test post-test vs. CG post-test ↔ |
| Lee et al. [14] | Male elite athletes (n = 44) SA, n = 15 RSA, n = 16 FSA, n = 13 | SA, RSA, and FSA: Black maca extract | PO: muscle strength (kg), muscle endurance (number of repetitions/1min), flexibility (cm), power (cm), agility (s), cardiovascular endurance (repetition) | Black maca extract SA: 5000 mg/kg RSA: 5000 mg/kg FSA: 5000 mg/kg | Left grip strength (kg): Pre SA = 43.8 ± 4.7 vs. post SA = 45.1 ± 4.0; p > 0.05 Pre RSA = 41.0 ± 5.9 vs. post RSA = 41.0 ± 5.0; p > 0,05 Pre FSA = 34.9 ± 7.5 vs. post FSA = 39.0 ± 9.9; p < 0.01 Right grip strength (kg) Pre SA = 46.0 ± 5.2 vs. post SA = 47.0 ± 6.3; p > 0.05 Pre RSA = 46.2 ± 5.8 vs. post RSA = 46.8 ± 5.4; p > 0,05 Pre FSA = 38.1 ± 8.1 vs. post FSA = 42.1 ± 9.8; p < 0.01 Sit-ups (rep): Pre SA = 40.2 ± 7.0 vs. post SA = 47.1 ± 7.7; p < 0.05 Pre RSA = 41.5 ± 9.3 vs. post RSA = 47.7 ± 8.0; p < 0,05 Pre FSA = 55.8 ± 11.2 vs. post FSA = 57.9 ± 8.7; p < 0.01 Sit-and-reach (cm): Pre SA = 8.53 ± 8.4 vs. post SA = 10.4 ± 7.2; p > 0.05 Pre RSA = 6.91 ± 11.4 vs. post RSA = 9.9 ± 11.1; p > 0,05 Pre FSA = 22.2 ± 9.5 vs. post FSA = 25.3 ± 8.1; p < 0.01 Long jump (cm): Pre SA = 210.5 ± 19.2 vs. post SA = 215.0 ± 15.1; p > 0.05 Pre RSA = 228.9 ± 14.0 vs. post RSA = 239.5 ± 12.8; p < 0.05 Pre FSA = 197.8 ± 33.5 vs. post FSA = 222.1 ± 32.5; p < 0.01 10 m shuttle run (s): Pre SA = 10.0 ± 0.6 vs. post SA = 10.5 ± 0.6; p < 0.05 Pre RSA = 9.1 ± 0.7 vs. post RSA = 9.5 ± 0.4; p < 0.05 Pre FSA = 10.0 ± 0.9 vs. post FSA = 11.0 ± 1.2; p < 0.01 20 m shuttle run (s): Pre SA = 33.3 ± 3.6 vs. post SA = 37.5 ± 8.0; p > 0.05 Pre RSA = 62.9 ± 9.4 vs. post RSA = 64.1 ± 12.6; p > 0.05 Pre FSA = 74.5 ± 19.3 vs. post FSA = 70.4 ± 17.1; p > 0.05 | Left grip strength (kg): Pre SA vs. post SA ↔ Pre RSA vs. post RSA ↔ Pre FSA vs. post FSA ↓ Right grip strength (kg) Pre SA vs. post SA ↔ Pre RSA vs. post RSA ↔ Pre FSA vs. post FSA ↓ Sit-ups (rep) Pre SA vs. post SA ↓ Pre RSA vs. RSA ↓ Pre FSA vs. post FSA ↓ Sit-and-reach (cm) Pre SA vs. post SASA ↔ Pre RSA vs. post RSA ↔Pre FSAA vs. post FSA ↓ Long jump (cm): Pre SA vs. post SA ↔ Pre RSA vs. post RSA ↓ Pre FSA vs. post FSA ↓ 10 m shuttle run (s) Pre SA vs. post SA ↓ Pre RSA vs. RSA ↓ Pre FSA vs. post FSA ↓ 20 m shuttle run (s): Pre SA vs. post SA ↔ Pre RSA vs. post RSA ↔ Pre RSA vs. post RSA ↔ |
| Liu et al. [41] | Healthy men EG (n = 9) CG (n = 11) | EG: Maca extract capsule CG: PL | PO: time (min) to exhaustion | Maca extract capsule: EG: 2 capsules of 2.25 g maca extract CG: 2 capsules of cornstarch | TTE (min) EG = 63.83 ± 3.44 vs. CG = 64.04 ± 2.76; p > 0.05 | TTE (min) EG vs. CG ↔ |
| Stone et al. [42] | Male cyclists: EG (n = 8) | EG: ME | PO: time (min); 40 km cycling time trial after 14 days of supplementation | ME EG: 2000 mg/kg | 40 km trial (min): Pre ME = 57.62 ± 3.14 vs. post ME = 56.56 ± 2.68; p < 0.01 | 40 km trial (min): Pre ME vs. post ME ↓ |
| Methodological Quality of Animal Studies (CAMARADES Scale) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | TOTAL |
| Bilal et al. [26] | * | 0 | 0 | 0 | 0 | * | * | 0 | * | * | 5 |
| Chen et al. [18] | * | * | * | 0 | 0 | * | * | 0 | * | * | 7 |
| Choi et al. [10] | * | * | * | 0 | 0 | * | * | 0 | * | * | 7 |
| Ikeuchi et al. [11] | * | * | 0 | 0 | 0 | * | 0 | 0 | * | * | 5 |
| Li et al. [27] (1) | * | * | * | 0 | 0 | * | * | 0 | * | * | 7 |
| Li et al. [28] (2) | * | * | * | 0 | 0 | * | * | 0 | * | * | 7 |
| Li et al. [29] (3) | * | * | * | 0 | 0 | * | * | 0 | * | * | 7 |
| Li et al. [30] | * | * | * | 0 | 0 | * | * | 0 | * | * | 7 |
| Liu et al. [31] | * | * | * | 0 | 0 | * | * | 0 | * | * | 7 |
| López-Fando et al. [32] | * | * | * | 0 | 0 | * | * | 0 | * | * | 7 |
| Orhan et al. [33] | * | * | * | 0 | 0 | * | * | * | * | * | 8 |
| Tang et al. [34] | * | * | * | 0 | 0 | * | * | 0 | * | * | 7 |
| Yang et al. [35] | * | * | * | 0 | 0 | * | * | 0 | * | * | 7 |
| Zheng et al. [36] | * | 0 | * | 0 | 0 | * | * | 0 | 0 | * | 5 |
| Zheng et al. [37] | * | * | 0 | 0 | 0 | * | * | 0 | * | * | 6 |
| Zhu et al. [38] | * | * | * | 0 | 0 | * | * | 0 | * | * | 7 |
| Zhu et al. [39] | * | * | * | 0 | 0 | * | * | 0 | * | * | 7 |
| Methodological quality of human studies (NOS) | |||||||||||
| Authors | (A) | (B) | (C) | TOTAL | |||||||
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | |||
| Honma et al. [40] | * | * | * | * | * | * | * | * | 0 | 8 | |
| Lee et al. [14] | * | * | * | * | * | 0 | * | 0 | 0 | 6 | |
| Liu et al. [41] | * | * | * | * | * | * | * | 0 | * | 8 | |
| Stone et al. [42] | * | * | * | * | * | 0 | * | 0 | 0 | 6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huerta Ojeda, Á.; Rodríguez Rojas, J.; Cancino-López, J.; Barahona-Fuentes, G.; Pavez, L.; Yeomans-Cabrera, M.-M.; Jorquera-Aguilera, C. Effects of Maca (Lepidium meyenii Walp.) on Physical Performance in Animals and Humans: A Systematic Review and Meta-Analysis. Nutrients 2025, 17, 107. https://doi.org/10.3390/nu17010107
Huerta Ojeda Á, Rodríguez Rojas J, Cancino-López J, Barahona-Fuentes G, Pavez L, Yeomans-Cabrera M-M, Jorquera-Aguilera C. Effects of Maca (Lepidium meyenii Walp.) on Physical Performance in Animals and Humans: A Systematic Review and Meta-Analysis. Nutrients. 2025; 17(1):107. https://doi.org/10.3390/nu17010107
Chicago/Turabian StyleHuerta Ojeda, Álvaro, Javiera Rodríguez Rojas, Jorge Cancino-López, Guillermo Barahona-Fuentes, Leonardo Pavez, María-Mercedes Yeomans-Cabrera, and Carlos Jorquera-Aguilera. 2025. "Effects of Maca (Lepidium meyenii Walp.) on Physical Performance in Animals and Humans: A Systematic Review and Meta-Analysis" Nutrients 17, no. 1: 107. https://doi.org/10.3390/nu17010107
APA StyleHuerta Ojeda, Á., Rodríguez Rojas, J., Cancino-López, J., Barahona-Fuentes, G., Pavez, L., Yeomans-Cabrera, M.-M., & Jorquera-Aguilera, C. (2025). Effects of Maca (Lepidium meyenii Walp.) on Physical Performance in Animals and Humans: A Systematic Review and Meta-Analysis. Nutrients, 17(1), 107. https://doi.org/10.3390/nu17010107