Therapeutic Implications of Phenolic Acids for Ameliorating Inflammatory Bowel Disease
Abstract
1. Introduction
2. Chemical Structures and Primary Dietary Sources of Phenolic Acids
3. Effect of Phenolic Acids on Intestinal Mucosal Barrier
4. Effect of Phenolic Acids on Oxidative Stress
5. Effect of Phenolic Acids on Immune System
6. Effect of Phenolic Acids on Gut Microbes
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kaplan, G.G.; Windsor, J.W. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 56–66. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Han, X.; Jiang, H.; Zhang, L.; Hu, J.; Shi, L.; Li, J. Long-term trends in the burden of inflammatory bowel disease in China over three decades: A joinpoint regression and age-period-cohort analysis based on GBD 2019. Front. Public Health 2022, 10, 994619. [Google Scholar] [CrossRef]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef]
- Borren, N.Z.; van der Woude, C.J.; Ananthakrishnan, A.N. Fatigue in IBD: Epidemiology, pathophysiology and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 247–259. [Google Scholar] [CrossRef]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef]
- Levine, A.; Sigall Boneh, R.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef]
- Vinayagam, R.; Jayachandran, M.; Xu, B. Antidiabetic Effects of Simple Phenolic Acids: A Comprehensive Review. Phytother. Res. 2016, 30, 184–199. [Google Scholar] [CrossRef]
- Wood, A.; Baxter, G.; Thies, F.; Kyle, J.; Duthie, G. A systematic review of salicylates in foods: Estimated daily intake of a Scottish population. Mol. Nutr. Food Res. 2011, 55 (Suppl. 1), S7–S14. [Google Scholar] [CrossRef]
- Gao, Y.; Tian, R.; Liu, H.; Xue, H.; Zhang, R.; Han, S.; Ji, L.; Huang, W.; Zhan, J.; You, Y. Research progress on intervention effect and mechanism of protocatechuic acid on nonalcoholic fatty liver disease. Crit. Rev. Food Sci. Nutr. 2022, 62, 9053–9075. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.X.; Guo, S.D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Chaudhary, A.; Jaswal, V.S.; Choudhary, S.; Sonika; Sharma, A.; Beniwal, V.; Tuli, H.S.; Sharma, S. Ferulic Acid: A Promising Therapeutic Phytochemical and Recent Patents Advances. Recent. Pat. Inflamm. Allergy Drug Discov. 2019, 13, 115–123. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Xu, X.; Luo, A.; Lu, X.; Liu, M.; Wang, H.; Song, H.; Wei, C.; Wang, Y.; Duan, X. p-Hydroxybenzoic acid alleviates inflammatory responses and intestinal mucosal damage in DSS-induced colitis by activating ERβ signaling. J. Funct. Foods 2021, 87, 104835. [Google Scholar] [CrossRef]
- Han, X.; Li, M.; Sun, L.; Liu, X.; Yin, Y.; Hao, J.; Zhang, W. p-Hydroxybenzoic Acid Ameliorates Colitis by Improving the Mucosal Barrier in a Gut Microbiota-Dependent Manner. Nutrients 2022, 14, 5383. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X.; Zhou, F.; Xiao, S.; Zhong, L.; Hu, S.; Zhou, Z.; Li, L.; Tan, Y. Protocatechuic Acid Alleviates Dextran-Sulfate-Sodium-Induced Ulcerative Colitis in Mice via the Regulation of Intestinal Flora and Ferroptosis. Molecules 2023, 28, 3775. [Google Scholar] [CrossRef]
- Crespo, I.; San-Miguel, B.; Mauriz, J.L.; Ortiz de Urbina, J.J.; Almar, M.; Tunon, M.J.; Gonzalez-Gallego, J. Protective Effect of Protocatechuic Acid on TNBS-Induced Colitis in Mice Is Associated with Modulation of the SphK/S1P Signaling Pathway. Nutrients 2017, 9, 288. [Google Scholar] [CrossRef]
- Farombi, E.O.; Adedara, I.A.; Awoyemi, O.V.; Njoku, C.R.; Micah, G.O.; Esogwa, C.U.; Owumi, S.E.; Olopade, J.O. Dietary protocatechuic acid ameliorates dextran sulphate sodium-induced ulcerative colitis and hepatotoxicity in rats. Food Funct. 2016, 7, 913–921. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Mohebali, N.; Norhaizan, M.E.; Looi, C.Y. Gallic acid attenuates dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Des. Dev. Ther. 2015, 9, 3923–3934. [Google Scholar] [CrossRef]
- Yu, T.-Y.; Feng, Y.-M.; Kong, W.-S.; Li, S.-N.; Sun, X.-J.; Zhou, G.; Xie, R.-F.; Zhou, X. Gallic acid ameliorates dextran sulfate sodium-induced ulcerative colitis in mice via inhibiting NLRP3 inflammasome. Front. Pharmacol. 2023, 14, 1095721. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Mohebali, N.; Esa, N.M.; Looi, C.Y.; Ismail, S.; Saadatdoust, Z. Gallic acid suppresses inflammation in dextran sodium sulfate-induced colitis in mice: Possible mechanisms. Int. Immunopharmacol. 2015, 28, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Gu, P.; Shen, H. Gallic acid improved inflammation via NF-kappaB pathway in TNBS-induced ulcerative colitis. Int. Immunopharmacol. 2019, 67, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liu, T.; Meng, P.; Luo, Y.; Zhu, S.; Wang, Y.; Ma, M.; Han, J.; Zhou, J.; Su, X. Gallic acid ameliorates colitis by trapping deleterious metabolite ammonia and improving gut microbiota dysbiosis. Mbio 2024, 15, e02752-23. [Google Scholar] [CrossRef]
- Hu, R.; Wu, S.; Li, B.; Tan, J.; Yan, J.; Wang, Y.; Tang, Z.; Liu, M.; Fu, C.; Zhang, H.; et al. Dietary ferulic acid and vanillic acid on inflammation, gut barrier function and growth performance in lipopolysaccharide-challenged piglets. Anim. Nutr. 2022, 8, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Kim, M.-C.; Um, J.-Y.; Hong, S.-H. The Beneficial Effect of Vanillic Acid on Ulcerative Colitis. Molecules 2010, 15, 7208–7217. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Xiao, J. Protective effects of syringic acid on inflammation, apoptosis and intestinal barrier function in Caco-2 cells following oxygen-glucose deprivation/reoxygenation-induced injury. Exp. Ther. Med. 2022, 23, 66. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhu, S.; Niu, Z.; Yin, Y. The protective effect of syringic acid on dextran sulfate sodium-induced experimental colitis in BALB/c mice. Drug Dev. Res. 2019, 80, 731–740. [Google Scholar] [CrossRef]
- Luo, Q.; Gong, P.; Shi, R.; Chen, W.; Wang, C.; Zhang, C.; Wu, Z. Syringic Acid Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice by Modulating Gut Microbiota. J. Agric. Food Chem. 2023, 71, 8458–8470. [Google Scholar] [CrossRef]
- Ekhtiar, M.; Ghasemi-Dehnoo, M.; Mirzaei, Y.; Azadegan-Dehkordi, F.; Amini-Khoei, H.; Lorigooini, Z.; Samiei-Sefat, A.; Bagheri, N. The coumaric acid and syringic acid ameliorate acetic acid-induced ulcerative colitis in rats via modulator of Nrf2/HO-1 and pro-inflammatory cytokines. Int. Immunopharmacol. 2023, 120, 110309. [Google Scholar] [CrossRef]
- Habboby, M.; Munaf Hashim, A. Evaluation of Anti-inflammatory Effects of Cinnamic Acid Against Dextran Sodium Sulfate Induced Ulcerative Colitis in Male Mice. Iraqi J. Pharm. Sci. 2023, 32, 33–40. [Google Scholar] [CrossRef]
- Kang, C.; Kim, J.; Ju, S.; Cho, H.; Kim, H.Y.; Yoon, I.-S.; Yoo, J.-W.; Jung, Y. Colon-Targeted Trans-Cinnamic Acid Ameliorates Rat Colitis by Activating GPR109A. Pharmaceutics 2023, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Liu, M.; Lu, Q.; Fan, C.; Lu, H.; Feng, C.; Yang, X.; Li, H.; Tang, W. Blockade of TLRs-triggered macrophage activation by caffeic acid exerted protective effects on experimental ulcerative colitis. Cell. Immunol. 2021, 365, 104364. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Zhong, R.; Wang, M.; Zhou, Y.; Chen, Y.; Yi, B.; Hou, F.; Liu, L.; Zhao, Y.; Chen, L.; et al. Caffeic Acid Supplement Alleviates Colonic Inflammation and Oxidative Stress Potentially Through Improved Gut Microbiota Community in Mice. Front. Microbiol. 2021, 12, 784211. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Qian, H.; Zhang, D.; Jiang, Z. Ferulic acid relieved ulcerative colitis by inhibiting the TXNIP/NLRP3 pathway in rats. Cell Biol. Int. 2022, 47, 417–427. [Google Scholar] [CrossRef]
- Sadar, S.S.; Vyawahare, N.S.; Bodhankar, S.L. Ferulic Acid Ameliorates Tnbs-Induced Ulcerative Colitis Through Modulation of Cytokines, Oxidative Stress, Inos, Cox-2, and Apoptosis in Laboratory Rats. Excli J. 2016, 15, 482–499. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y. Anti-inflammatory effects of sinapic acid on 2,4,6-trinitrobenzenesulfonic acid-induced colitis in mice. Arch. Pharm. Res. 2018, 41, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Wang, C.Q.; Zeng, Z.; Ren, Y.; Li, D.Y.; Song, J.L. Ameliorative Effect of Sinapic Acid on Dextran Sodium Sulfate- (DSS-) Induced Ulcerative Colitis in Kunming (KM) Mice. Oxidative Med. Cell. Longev. 2020, 2020, 8393504. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kim, S.; So, B.R.; Kim, Y.; Kim, C.-K.; Lee, J.J.; Jung, S.K. Sinapic acid alleviates inflammatory bowel disease (IBD) through localization of tight junction proteins by direct binding to TAK1 and improves intestinal microbiota. Front. Pharmacol. 2023, 14, 1217111. [Google Scholar] [CrossRef]
- Lan, H.; Zhang, L.Y.; He, W.; Li, W.Y.; Zeng, Z.; Qian, B.; Wang, C.; Song, J.L. Sinapic Acid Alleviated Inflammation-Induced Intestinal Epithelial Barrier Dysfunction in Lipopolysaccharide- (LPS-) Treated Caco-2 Cells. Mediat. Inflamm. 2021, 2021, 5514075. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Cao, S.; Cromie, M.; Shen, Y.; Feng, Y.; Yang, H.; Li, L. Chlorogenic Acid Ameliorates Experimental Colitis by Promoting Growth of Akkermansia in Mice. Nutrients 2017, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Zatorski, H.; Salaga, M.; Zielinska, M.; Piechota-Polanczyk, A.; Owczarek, K.; Kordek, R.; Lewandowska, U.; Chen, C.; Fichna, J. Experimental colitis in mice is attenuated by topical administration of chlorogenic acid. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, C.; Yu, L.; Sheng, T.; Wu, Z.; Wang, X.; Zhang, D.; Lin, Y.; Gong, Y. Chlorogenic Acid Attenuates Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice through MAPK/ERK/JNK Pathway. Biomed. Res. Int. 2019, 2019, 6769789. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Chlorogenic Acid Protects Against Indomethacin-Induced Inflammation and Mucosa Damage by Decreasing Bacteroides-Derived LPS. Front. Immunol. 2020, 11, 1125. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Dehnoo, M.; Amini-Khoei, H.; Lorigooini, Z.; Ashrafi-Dehkordi, K.; Rafieian-Kopaei, M. Coumaric acid ameliorates experimental colitis in rats through attenuation of oxidative stress, inflammatory response and apoptosis. Inflammopharmacology 2022, 30, 2359–2371. [Google Scholar] [CrossRef]
- Ghasemi-Dehnoo, M.; Amini-Khoei, H.; Lorigooini, Z.; AnjomShoa, M.; Rafieian-Kopaei, M. Ferulic acid ameliorates ulcerative colitis in a rat model via the inhibition of two LPS-TLR4-NF-κB and NF-κB-INOS-NO signaling pathways and thus alleviating the inflammatory, oxidative and apoptotic conditions in the colon tissue. Inflammopharmacology 2023, 31, 2587–2597. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Raish, M.; Ahmad, A.; Bin Jardan, Y.A.; Ansari, M.A.; Ahad, A.; Alkharfy, K.M.; Alaofi, A.L.; Al-Jenoobi, F.I. Sinapic Acid Ameliorates Acetic Acid-Induced Ulcerative Colitis in Rats by Suppressing Inflammation, Oxidative Stress, and Apoptosis. Molecules 2022, 27, 4139. [Google Scholar] [CrossRef] [PubMed]
- Hossen, I.; Hua, W.; Ting, L.; Mehmood, A.; Jingyi, S.; Duoxia, X.; Yanping, C.; Hongqing, W.; Zhipeng, G.; Kaiqi, Z.; et al. Phytochemicals and inflammatory bowel disease: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1321–1345. [Google Scholar] [CrossRef]
- Zielinska, D.; Zielinski, H.; Laparra-Llopis, J.M.; Szawara-Nowak, D.; Honke, J.; Gimenez-Bastida, J.A. Caffeic Acid Modulates Processes Associated with Intestinal Inflammation. Nutrients 2021, 13, 554. [Google Scholar] [CrossRef]
- Lee, Y.M.; Shin, D.W.; Lim, B.O. Chlorogenic Acid Improves Symptoms of Inflammatory Bowel Disease in Interleukin-10 Knockout Mice. J. Med. Food 2020, 23, 1043–1053. [Google Scholar] [CrossRef]
- Shin, H.S.; Satsu, H.; Bae, M.J.; Zhao, Z.; Ogiwara, H.; Totsuka, M.; Shimizu, M. Anti-inflammatory effect of chlorogenic acid on the IL-8 production in Caco-2 cells and the dextran sulphate sodium-induced colitis symptoms in C57BL/6 mice. Food Chem. 2015, 168, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhang, D.; Wan, X.; Bai, Y.; Yuan, C.; Wang, T.; Yuan, D.; Zhang, C.; Liu, C. Chlorogenic Acid Suppresses miR-155 and Ameliorates Ulcerative Colitis through the NF-kappaB/NLRP3 Inflammasome Pathway. Mol. Nutr. Food Res. 2020, 64, e2000452. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wu, Y.; Lu, D.; Pang, J.; Hu, J.; Zhang, X.; Wang, Z.; Zhang, G.; Wang, J. Polyphenol-rich diet mediates interplay between macrophage-neutrophil and gut microbiota to alleviate intestinal inflammation. Cell Death Dis. 2023, 14, 656. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Ishimoto, T.; Fu, L.; Zhang, J.; Zhang, Z.; Liu, Y. The Gut Microbiota in Inflammatory Bowel Disease. Front. Cell. Infect. Microbiol. 2022, 12, 733992. [Google Scholar] [CrossRef]
- Zhang, P.; Jiao, H.; Wang, C.; Lin, Y.; You, S. Chlorogenic Acid Ameliorates Colitis and Alters Colonic Microbiota in a Mouse Model of Dextran Sulfate Sodium-Induced Colitis. Front. Physiol. 2019, 10, 325. [Google Scholar] [CrossRef]
| Phenolic Compounds | Substituent Group | |
|---|---|---|
Benzoic acid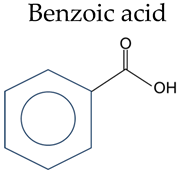 | Salicylic acid | 2-OH |
| 3-Hydroxybenzoic acid | 3-OH | |
| 4-Hydroxybenzoic acid | 4-OH | |
| 2,3-Dihydroxybenzoic | 2,3-OH | |
| Gentisic acid | 2,5-OH | |
| Protocatechuic acid | 3,4-OH | |
| Gallic acid | 3,4,5-OH | |
| Vanillic acid | 4-OH, 3-OCH3 | |
| Isovanillic Acid | 3-OH, 4-OCH3 | |
| Syringic | 4-OH, 3,5-OCH3 | |
Cinnamic acid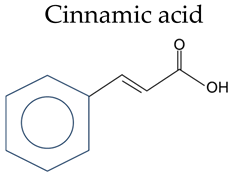 | Cinnamic acid | / |
| o-Coumaric acid | 2-OH | |
| p-Coumaric acid | 4-OH | |
| Caffeic acid | 3,4-OH | |
| Ferulic Acid | 4-OH, 3-OCH3 | |
| Sinapic acid | 4-OH, 3,5-OCH3 | |
| Chlorogenic acid | 3,4-OH, 1-Quinic |
| Phenolic Acid Compounds | Model | Dose | Effect | Reference |
|---|---|---|---|---|
| 4-Hydroxybenzoic acid | 2.5% DSS treated mice for 7 days | 10–40 mg/kg and 100 mg/kg, orally | reduced inflammatory cytokines; improved mucosal barrier | Xu X [15], Han X [16] |
| 10 ng/mL TNF-α treated Caco-2 cells | 3, 10 and 30 μM | decreased the expression of proinflammatory cytokines; increased the expression of tight junction proteins | Xu X [15] | |
| Protocatechuic Acid | 3.5% DSS treated C57BL/6 mice for 7 days | 5, 10, and 20 mg/kg, orally | reduced inflammatory factors; increased occluding protein expression | Yang X [17] |
| 20 mg/mL of TNBS-treated BALB/c mice through the catheter | 30 and 60 mg/kg, intraperitoneally | prevented the macroscopic damage and the increase in myeloperoxidase activity; increased expression of antioxidant enzymes; reduced expression of proinflammatory cytokines | Crespo I [18] | |
| 5% DSS treated rat for 5 days | 10 mg/kg, orally | prevented the increase in proinflammatory cytokines in the plasma; suppressed the DSS-mediated elevation in colonic myeloperoxidase activity | Farombi EO [19] | |
| Gallic acid | 2.5% DSS treated BALB/c mice for 7 days | 10 mg/kg, orally | reduced inflammation; improved oxidative stress; upregulated the expression of Nrf2 and its downstream targets | Pandurangan AK [20] |
| 2.5% DSS treated BALB/c mice for 7 days | 10 mg/kg, orally | reduced the expression of inflammatory mediators; suppressed p65-NF-κB and IL-6/p-STAT3 activation | Pandurangan AK [21] | |
| TNBS treated BALB/c mice through the catheter. | 20, 40, 60 mg/kg, orally | reduced inflammation; suppressed NF-κB | Zhu L [22] | |
| 10 ng/mL IL-1β treated HIEC-6 cells | 20, 40, 60 mg/kg | inhibited apoptosis | Zhu L [22] | |
| 3.5% DSS treated BALB/c mice for 7 days | 40, 80, 120 mg/kg, orally | reduced inflammation; downregulated the expressions of NLRP3 | Yu T-Y [23] | |
| 2.5% DSS treated C56B/6L mice for 10 days | 200 mg/kg, orally | trapped deleterious metabolite ammonia; improved gut microbiota dysbiosis | Peng J [24] | |
| Vanillic acid | 10 mg/kg LPS-treated weaned piglet | 4000 mg/kg in diet | decreased serum levels of proinflammatory factor; enhanced the expression of tight junction protein; modulated gut microbiota | Hu R [25] |
| 5% DSS treated BALB/c mice for 7 days | 200 mg/kg, orally | relieved colitis; reduced IL-6 | Kim S-J [26] | |
| Syringic Acid | OGD/R-stimulated cell injury in Caco-2 cell | 0.1, 1.0 and 10.0 μM | inhibited intestinal barrier disruption; ameliorated apoptosis; attenuated oxidant stress; suppressed the release of inflammatory cytokines | Xiang S [27] |
| 3.5% DSS treated BALB/c mice for 7 days | 25 mg/kg, orally | suppressed proinflammatory cytokine; prevented DSS-induced colon damage; reduced the activity of MPO | Fang W [28] | |
| 2.5% DSS treated C56BL/6 mice for 7 days | 50 mg/kg, orally | regulated oxidative stress; alleviated inflammatory response; relieved proptosis | Luo Q [29] | |
| 0.8 mL of 7% acetic acid was instilled into the rat colon through the cannula | 10, 25, and 50 mg/kg | decreased the mean macroscopic ulcer score; increased HO-1, Nrf2, and NQO1 mRNA expression; decreased the tissue levels of TNF-α and IL-1β | Ekhtiar M [30] | |
| Cinnamic acid | 2.5% DSS treated albino mice for 7 days | 25 and 50 mg/kg, orally | reduced the levels of TNF-α and IL-6 | Habboby M [31] |
| 0.4 mL 120 mg/mL DNBS was instilled into the rat colon through the cannula | 30 mg/kg, orally | activated GPR109A in the inflamed colon | Kang C [32] | |
| Coumaric acid | 0.8 mL 7% acetic acid was rectally injected into rats | 50, 100, and 150 mg/kg, orally | improved oxidative stress; improved the inflammation | Ghasemi-Dehnoo M [33] |
| 0.8 mL 7% acetic acid was rectally injected into rats | 100 and 150 mg/kg | decreased the mean macroscopic ulcer score; increased HO-1, Nrf2, and NQO1 mRNA expression; decreased the tissue levels of TNF-α and IL-1β | Ekhtiar M [30] | |
| Caffeic acid | 3.5% DSS treated C57BL mice t for 7 days | 50 mg/kg, orally | suppressed the production of inflammatory cytokines; interfered with the infiltration and function of mononuclear macrophages | Xiang C [34] |
| 3.5% DSS treated ICR mice for 7 days | 250 mg/kg, orally | decreased proinflammatory cytokines; increased the level of IL-10; altered the gut microbiome composition | Wan F [35] | |
| 1 ng/mL IL-1β treated CCD-18Co cells | 10 and 50 μM | reduced the biosynthesis of IL-8 and MCP1, | Zielinska D [36] | |
| Ferulic acid | 0.8 mL 7% acetic acid was rectally injected into rats | 20, 40, and 60 mg/kg, orally | inhibited inflammatory, apoptotic, and production of MDA and NO; increased the activity of antioxidant factors | Ghasemi-Dehnoo M [37] |
| 1% DSS treated C57BL mice t for 16 days | 50 mg/kg, orally | improved histopathologic score and MPO activity | Islam MS [38] | |
| 100 mg/kg TNBS was rectally injected into rats | 20 and 40 mg/kg, orally | suppression of oxidative stress, apoptosis, production of proinflammatory cytokines, and inhibition of COX-2 synthesis | Sadar SS [39] | |
| 100 mg/kg TNBS was rectally injected into rats | 10, 20 and 250 mg/kg, orally | inhibited the inflammatory injury of endothelial cells; | Yu S [40] | |
| 10 ng/mL TNF-α treated HIMECs | 125, 250, 500 μM | reduced the expression of inflammatory factors; improved cell viability | Yu S [40] | |
| Sinapic acid | 30 mg/kg TNBS was rectally injected into BABL/c mice | 10, 30, and 100 mg/kg, orally | improved the macroscopic changes of colonic damage; improved the changes in expression of biochemical mediators of inflammation | Lee JY [41] |
| 20 μg/mL LPS and 20 ng/mL TNF-α treated Caco-2 cells | 12.5, 25 and 50 μM | suppressed impairment of intestinal permeability and cellular reorganization of tight junction proteins | Jang S [42] | |
| 2% DSS treated C57BL mice for 7 days | 2 and 10 mg/kg, orally | alleviated DSS-induced IBD; modified gut microbiota | Jang S [42] | |
| 20 μg/mL LPS-treated Caco-2 cells | 5, 10 and 15 μM | reduced the expression of proinflammatory cytokines; improved tight junction mRNA levels | Lan H [43] | |
| 2 mL 4% acetic acid was rectally injected into rats | 40 mg/kg, orally | suppressed inflammation, oxidative stress, and apoptosis | Shahid M [44] | |
| 2% DSS treated Kunming mice for 7 days | 10 and 50 mg/kg, orally | attenuated intestinal permeability; reduced inflammatory; attenuated oxidative damage; reduced the activation of the NLRP3 inflammasome | Qian B [45] | |
| Chlorogenic acid | 2.5% DSS treated C57BL/6 mice for 8 days | 1 mM, orally | suppressed inflammation; modified gut microbiota; promoted the growth of Akkermansia | Zhang Z [46] |
| BALB/c mice intracolonic administration of 4 mg in 0.1 mL of 30% ethanol TNBS | 20 mg/kg, orally | anti-inflammatory; decreased neutrophil infiltration and suppression of NF-κB-dependent pathways. | Zatorski H [47] | |
| 5% DSS treated C57BL/6 mice for 7 days | 30, 60, and 120 mg/kg, orally | reduced mucosal damage; inhibited colonic mucosal inflammation; improved colitis through MAPK/ERK/JNK signaling pathway | Gao W [48] | |
| 2 mM H2O2 and 10 ng/mL of TNF-α treated Caco-2 cells | 0.5, 1 and 2 mM | reduced IL-8 secretion | Shin HS [49] | |
| 3% DSS treated C57BL/6 mice for 8 days | 1 mM, orally | reduced proinflammatory cytokines | Shin HS [49] | |
| 2.5% DSS treated C57BL/6 mice for 5 days | 2% in diet | decreased the production of proinflammatory cytokines; and restored intestinal microbial diversity. | Zhang P [50] | |
| 5 mg/kg indomethacin treated C57BL/6 mice for 5 days | 50 mg/kg, orally | prevented inflammation; prevented intestinal barrier dysfunction; decreased Bacteroides-derived LPS | Yan Y [51] | |
| IL-10 KO mice | 1 mg/kg, orally | increased the ratio of CD4+/CD8+ T cell subsets; prevented inflammation | Lee YM [52] | |
| 3% DSS treated BALB/c mice for 7 days | 20 and 40 mg/kg, orally | prevented inflammation | Zeng J [53] | |
| 0.5 ug/mL LPS and 2 nM ATP induced Raw264.7 cells | 15.63, 31.25, 62.5, 125 and 250 μM | improved the cellular vitality | Zeng J [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Han, X. Therapeutic Implications of Phenolic Acids for Ameliorating Inflammatory Bowel Disease. Nutrients 2024, 16, 1347. https://doi.org/10.3390/nu16091347
Lu Y, Han X. Therapeutic Implications of Phenolic Acids for Ameliorating Inflammatory Bowel Disease. Nutrients. 2024; 16(9):1347. https://doi.org/10.3390/nu16091347
Chicago/Turabian StyleLu, Yanan, and Xue Han. 2024. "Therapeutic Implications of Phenolic Acids for Ameliorating Inflammatory Bowel Disease" Nutrients 16, no. 9: 1347. https://doi.org/10.3390/nu16091347
APA StyleLu, Y., & Han, X. (2024). Therapeutic Implications of Phenolic Acids for Ameliorating Inflammatory Bowel Disease. Nutrients, 16(9), 1347. https://doi.org/10.3390/nu16091347






