Nano-Selenium Alleviates Cd-Induced Chronic Colitis through Intestinal Flora
Abstract
1. Introduction
2. Materials and Methods
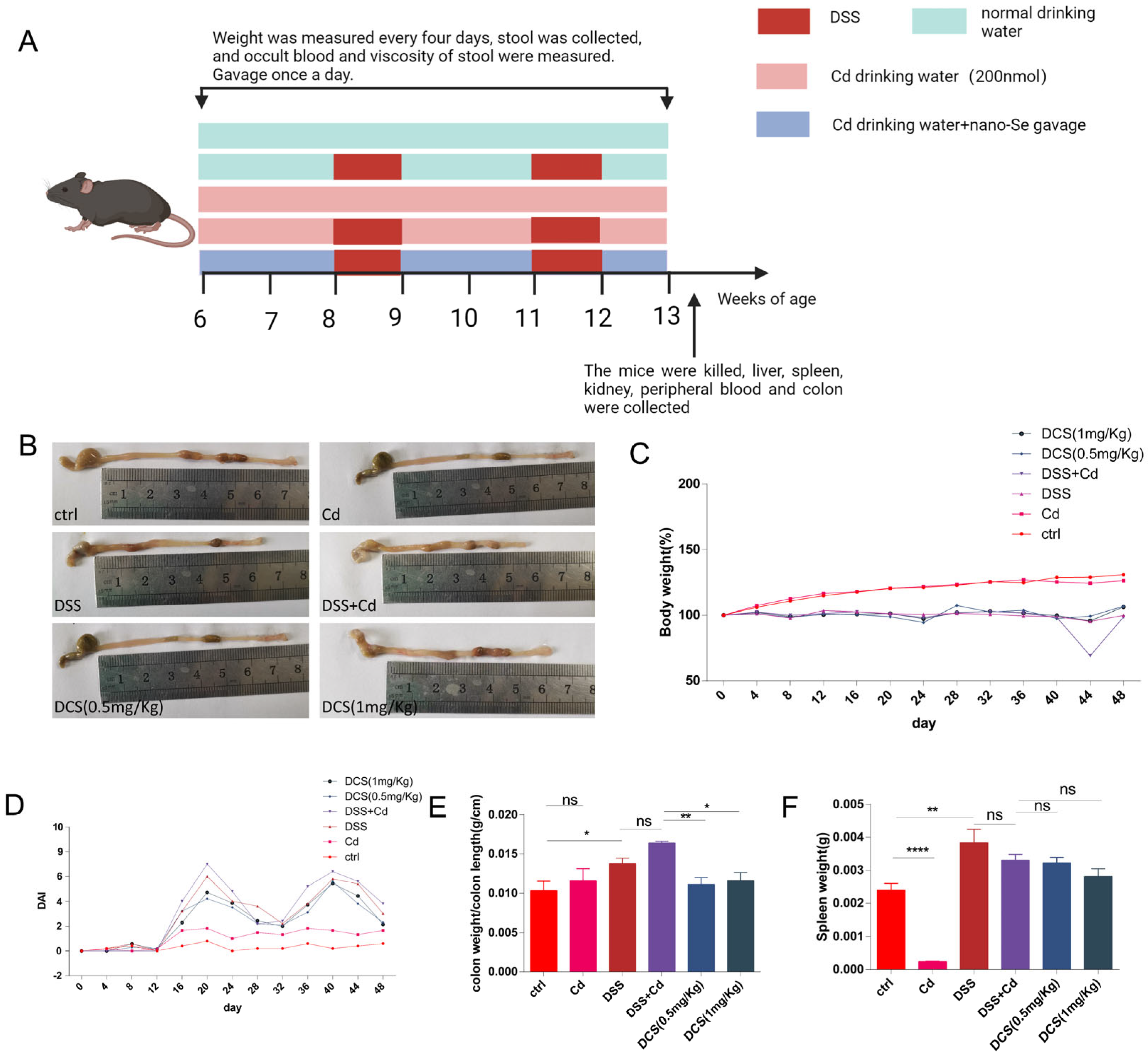
2.1. Animal Husbandry and Colitis Induction
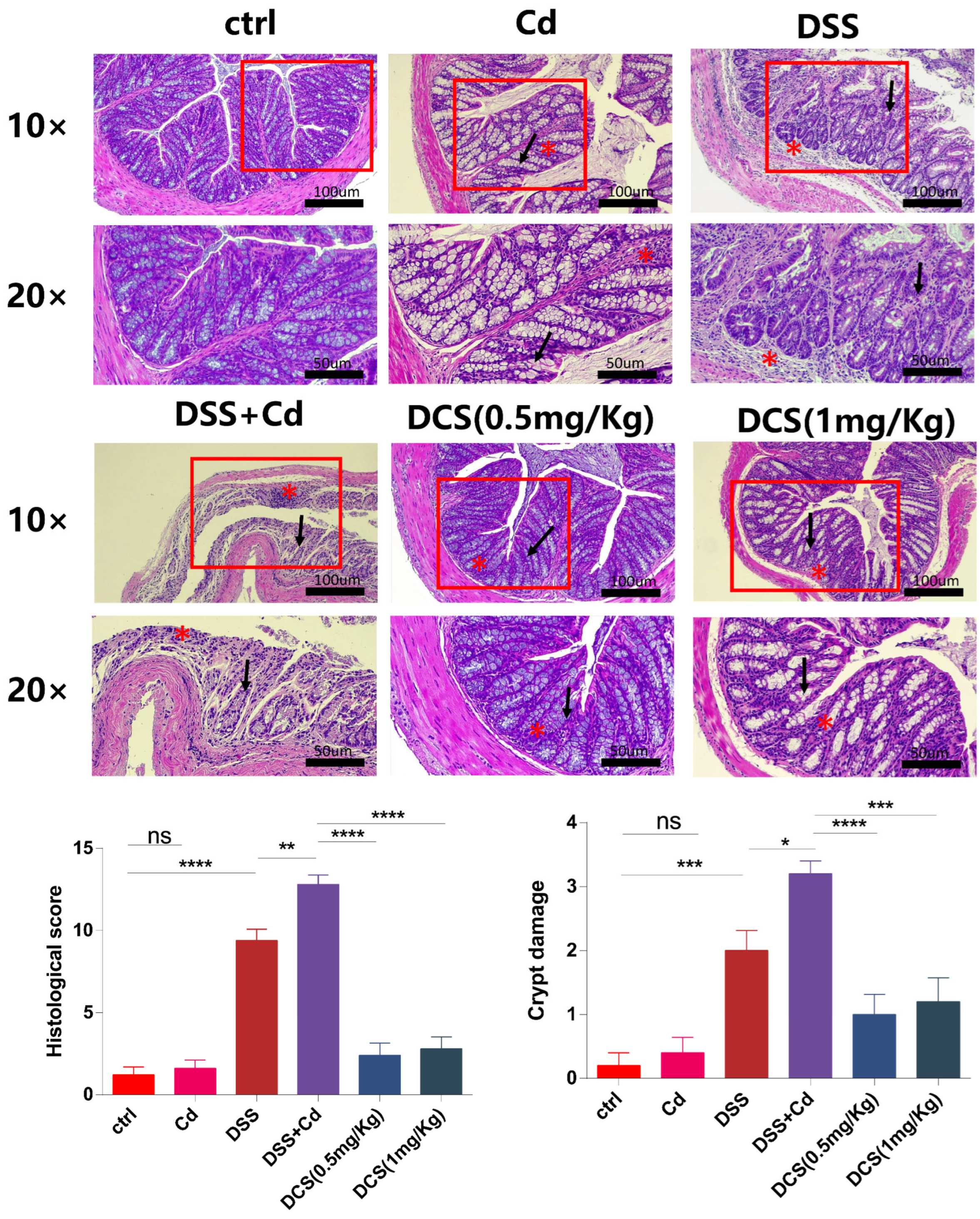
2.2. Histological Scoring and Periodic Acid-Schiff Staining
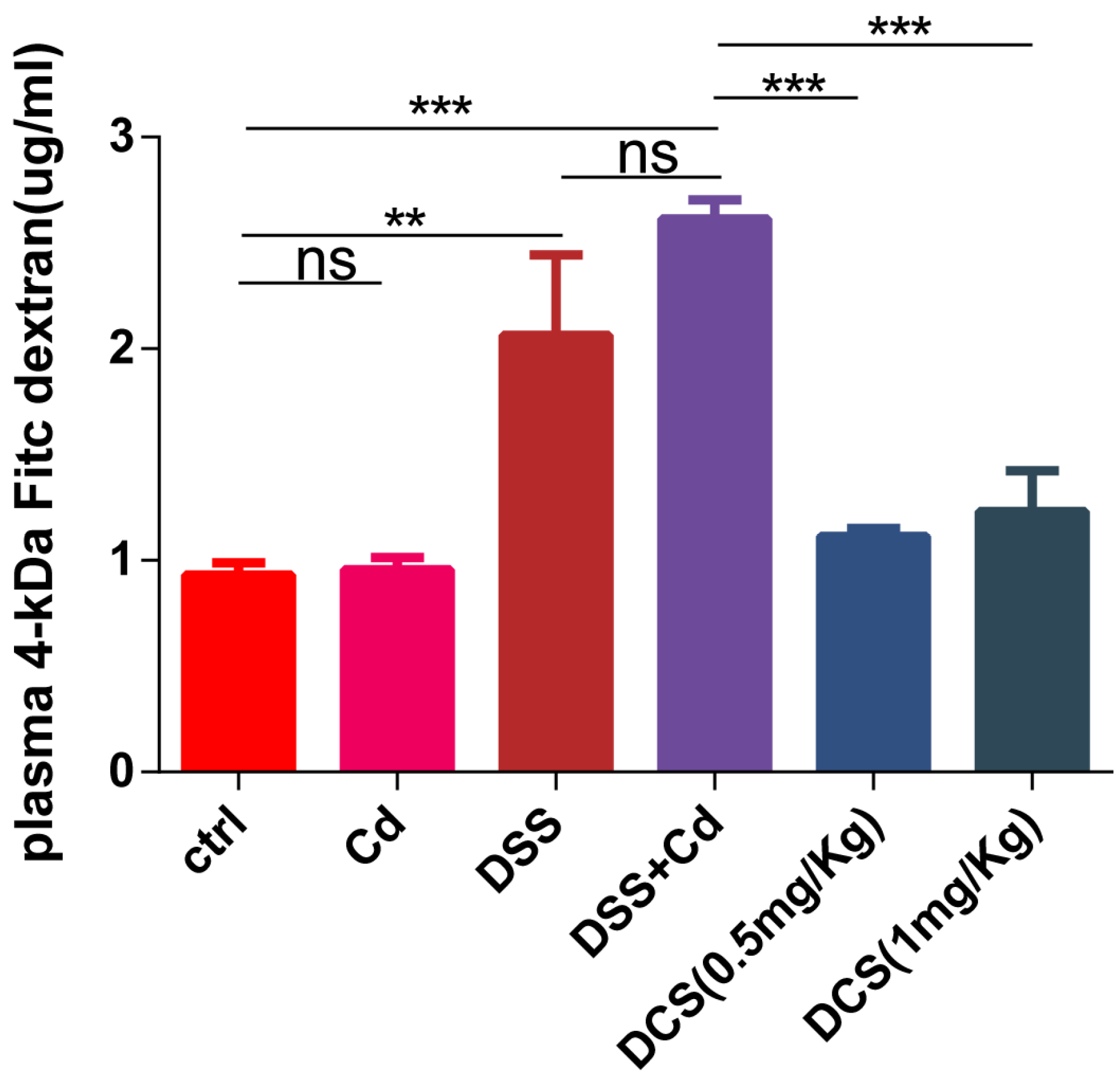
2.3. Analysis of Intestinal Permeability
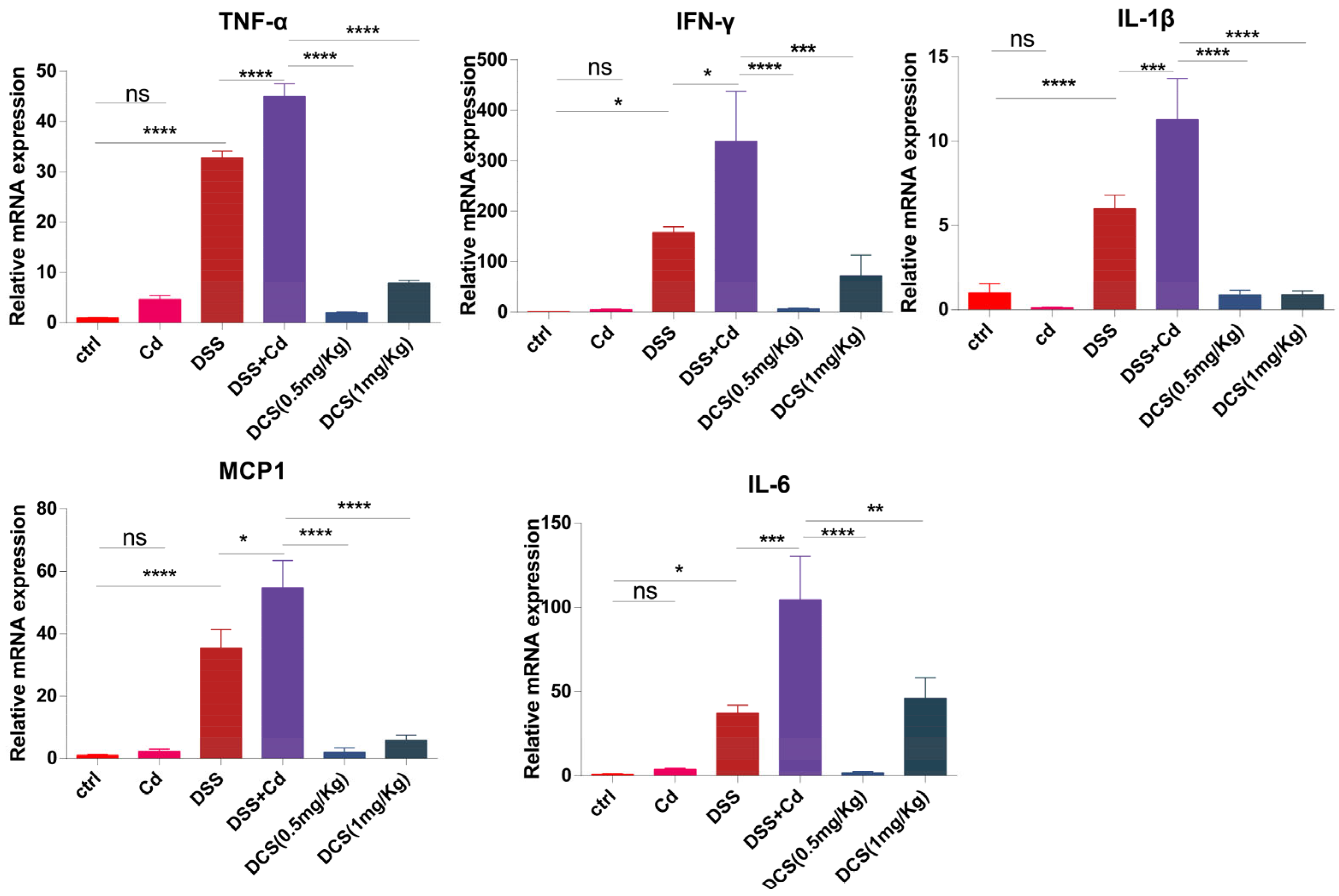
2.4. Quantitative Real-Time PCR
2.5. Serum ELISA
2.6. Short-Chain Fatty Acid (SCFA) Analysis
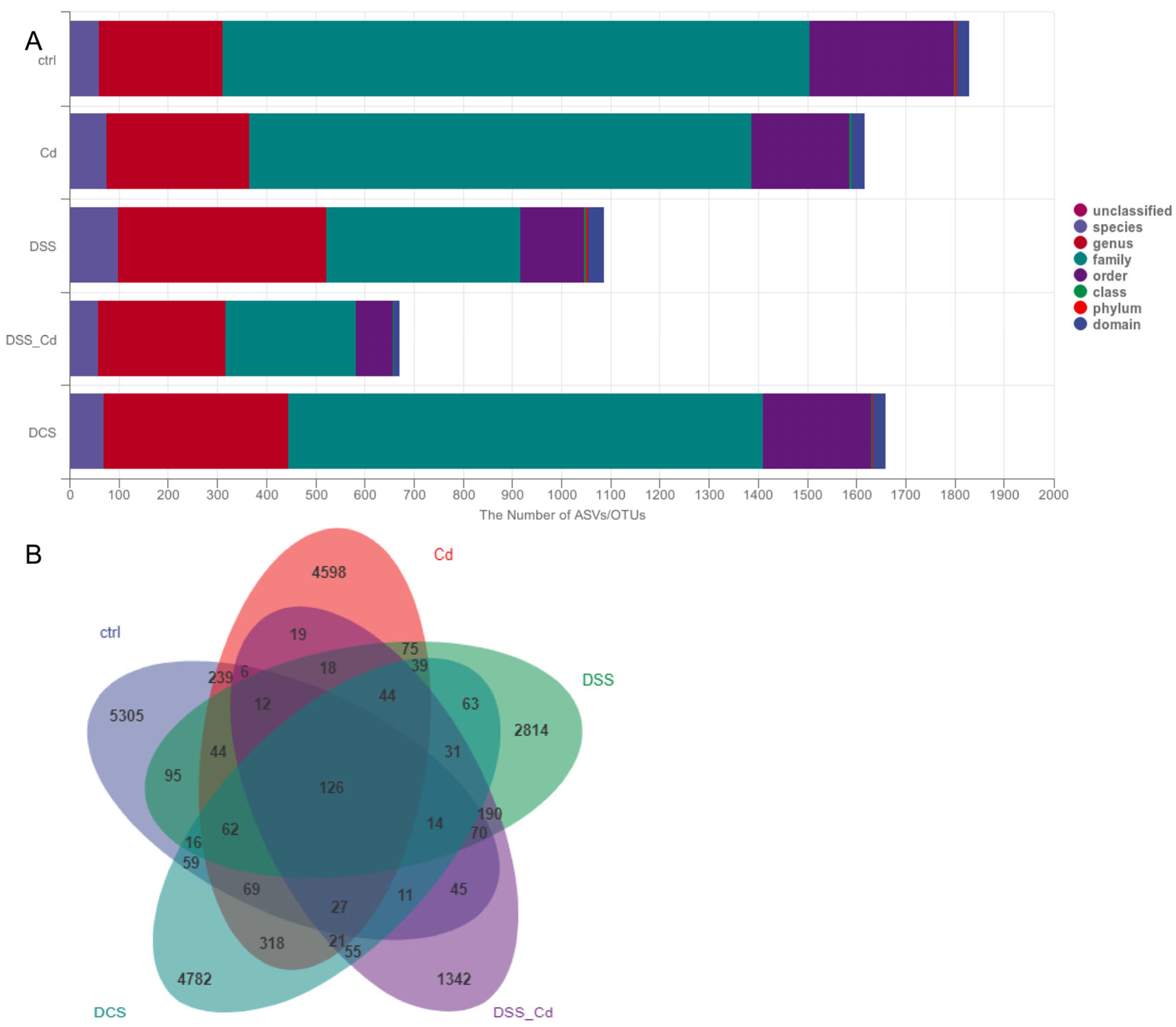
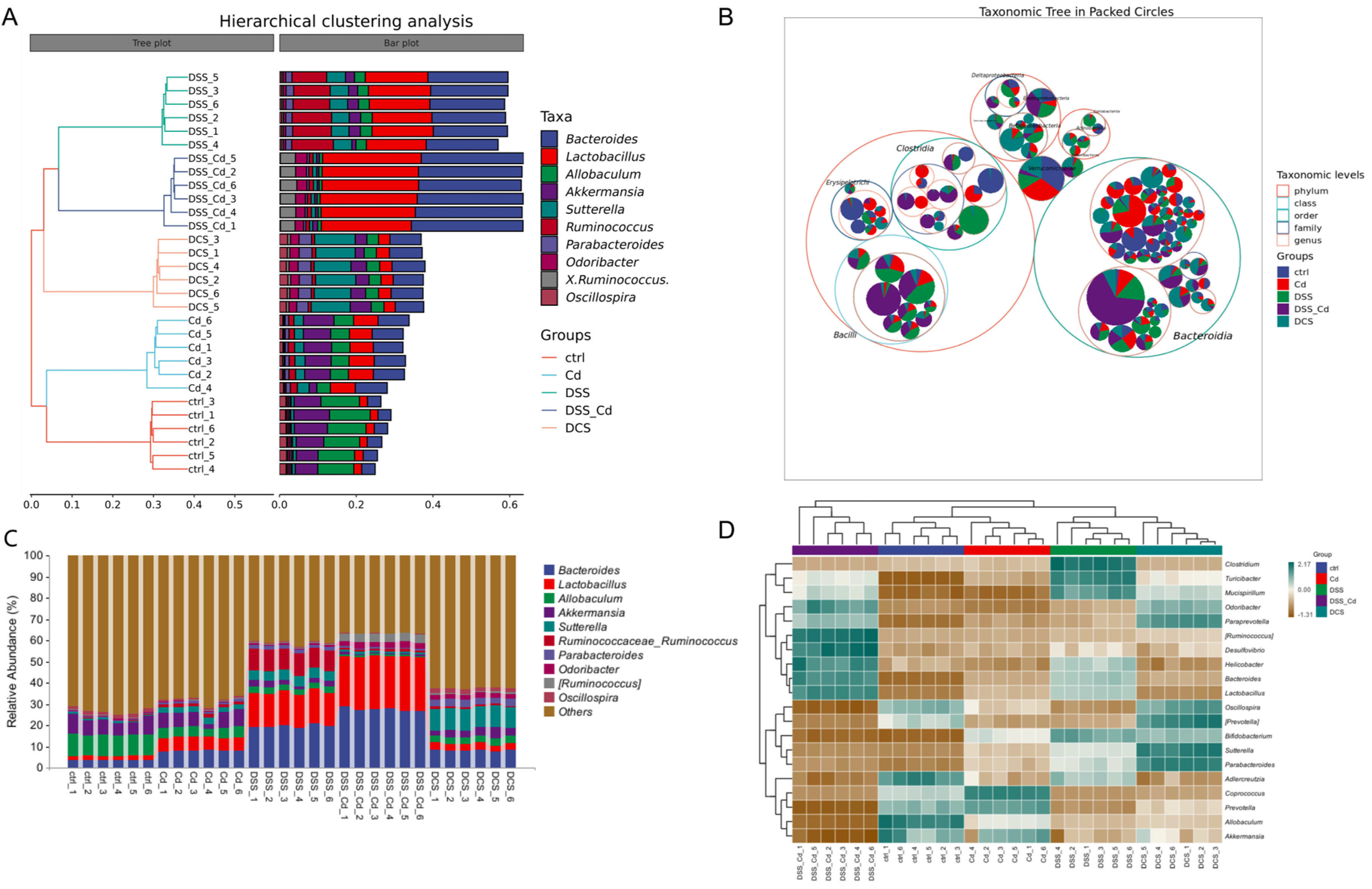
2.7. Gut Microbiota Analysis
2.8. Statistical Analysis
3. Results
3.1. Nano-Se Relieves the Chronic Colitis Induced by DSS in Mice
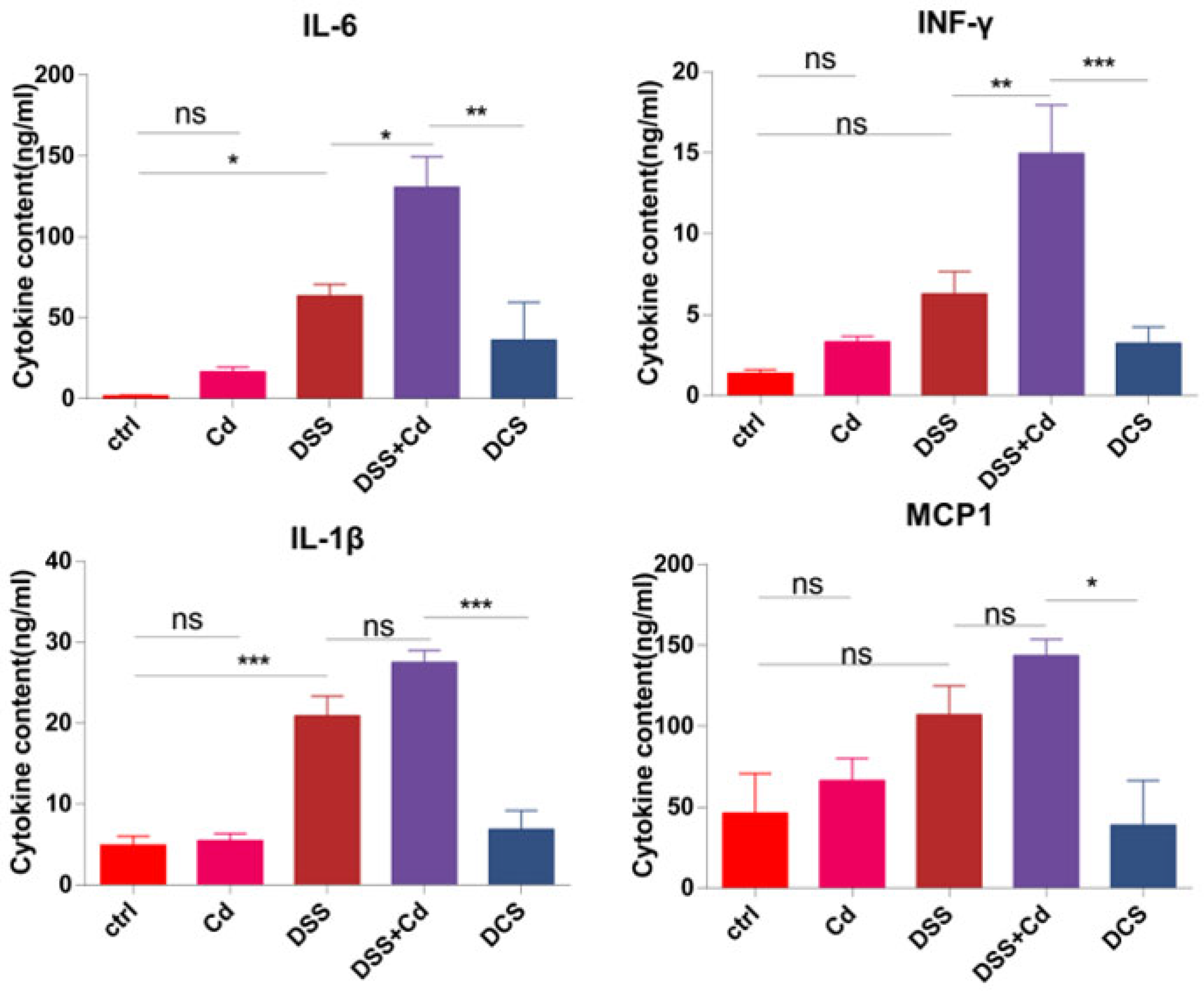
3.2. Nano-Se Alleviates Colonic Inflammatory Cytokines Expression
3.3. Nano-Se Protects the Intestinal Barrier
3.4. Nano-Selenium Relieves Colitis-Related Extraintestinal Inflammation
3.5. Effect of Nano-Selenium on SCFA Production
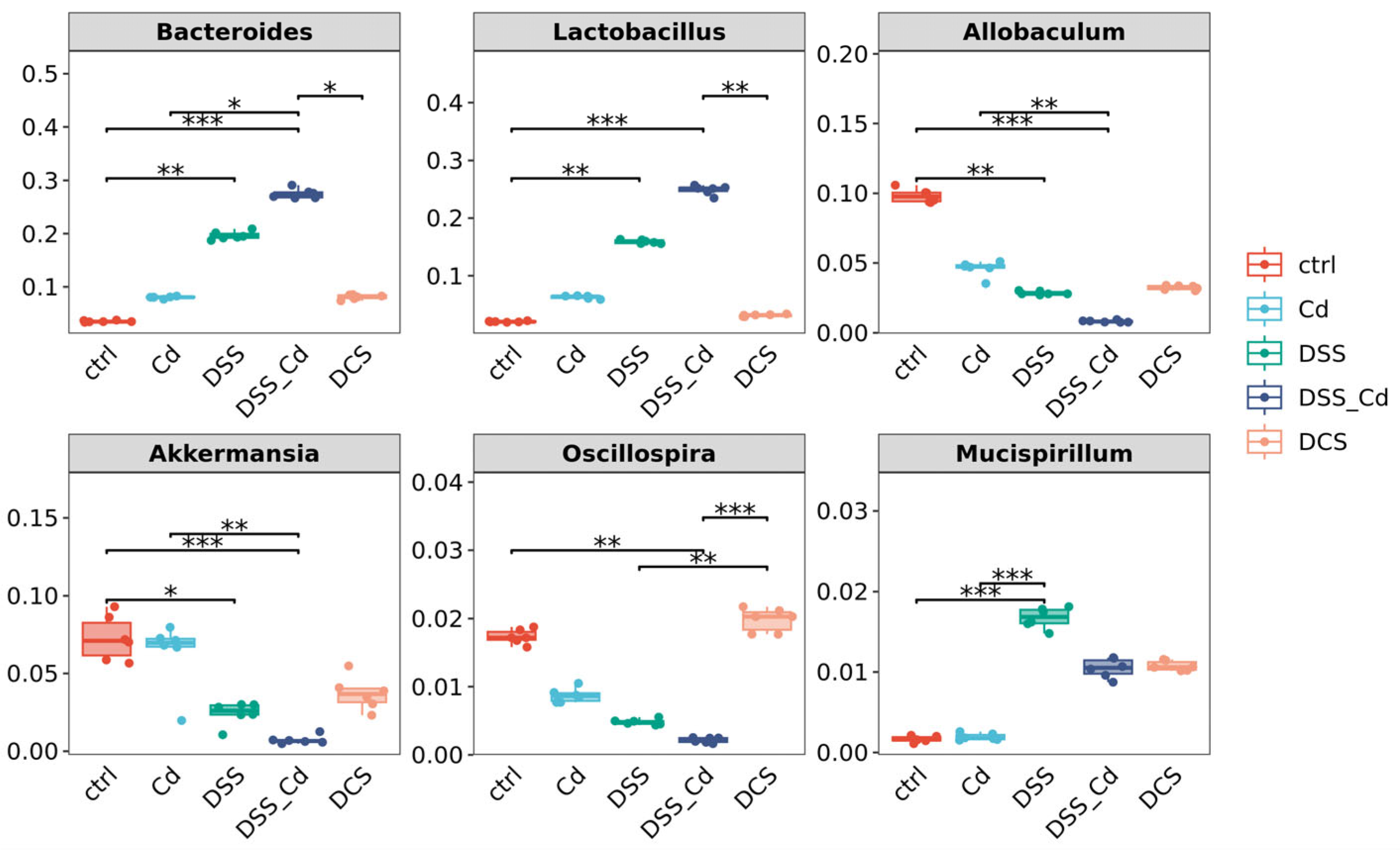
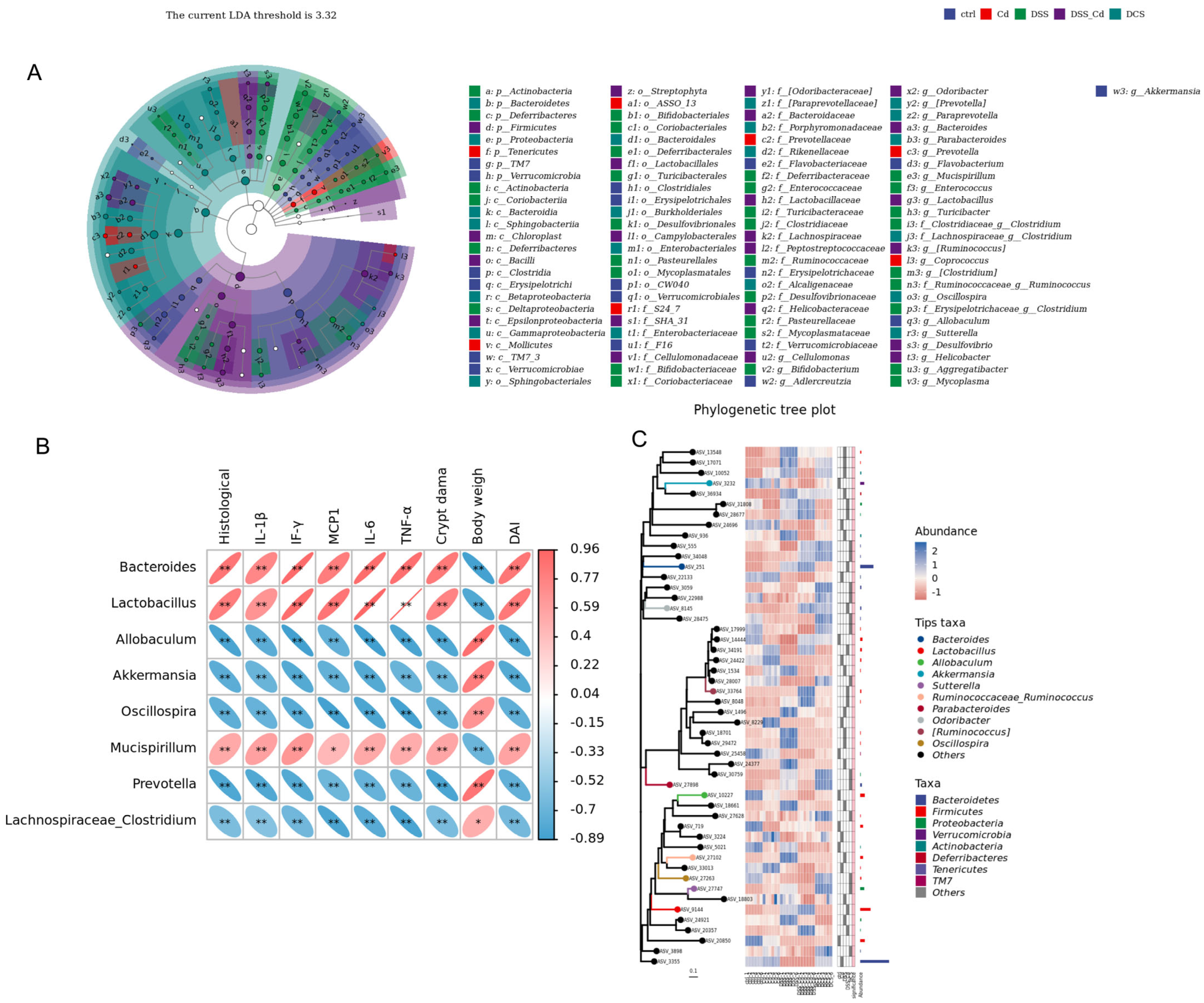
3.6. Nano-Se Improves DSS-Induced Changes in the Structure of Gut Microbiota
3.7. Conducting a Correlation Analysis to Examine the Relationship between Specific Species and Indications of Colitis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chakraborty, K.; Chatterjee, S.; Bhattacharyya, A. Modulation of phenotypic and functional maturation of murine bone-marrow-derived dendritic cells (BMDCs) induced by cadmium chloride. Int. Immunopharmacol. 2014, 20, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Khannazer, N.; Azizi, G.; Eslami, S.; Alhassan Mohammed, H.; Fayyaz, F.; Hosseinzadeh, R.; Usman, A.B.; Kamali, A.N.; Mohammadi, H.; Jadidi-Niaragh, F. The effects of cadmium exposure in the induction of inflammation. Immunopharmacol. Immunotoxicol. 2020, 42, 1–8. [Google Scholar] [CrossRef]
- Lener, M.R.; Reszka, E.; Marciniak, W.; Lesicka, M.; Baszuk, P.; Jabłońska, E.; Białkowska, K.; Muszyńska, M.; Pietrzak, S.; Derkacz, R. Blood cadmium levels as a marker for early lung cancer detection. J. Trace Elem. Med. Biol. 2021, 64, 126682. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C. Current health risk assessment practice for dietary cadmium: Data from different countries. Food Chem. Toxicol. 2017, 106, 430–445. [Google Scholar] [CrossRef]
- Ma, Y.; Su, Q.; Yue, C.; Zou, H.; Zhu, J.; Zhao, H.; Song, R.; Liu, Z. The effect of oxidative stress-induced autophagy by cadmium exposure in kidney, liver, and bone damage, and neurotoxicity. Int. J. Mol. Sci. 2022, 23, 13491. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A. Cadmium and cancer. In Cadmium: From Toxicity to Essentiality; Springer: Dordrecht, The Netherlands, 2013; pp. 491–507. [Google Scholar]
- McElroy, J.A.; Shafer, M.M.; Trentham-Dietz, A.; Hampton, J.M.; Newcomb, P.A. Cadmium exposure and breast cancer risk. J. Natl. Cancer Inst. 2006, 98, 869–873. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Filippini, T.; Ajsuvakova, O.P.; Skalnaya, M.G.; Aaseth, J.; Bjørklund, G.; Gatiatulina, E.R.; Popova, E.V.; Nemereshina, O.N.; Huang, P.-T. Cadmium and atherosclerosis: A review of toxicological mechanisms and a meta-analysis of epidemiologic studies. Environ. Res. 2018, 162, 240–260. [Google Scholar] [CrossRef]
- Combs, G.F., Jr.; Lü, J. Selenium as a cancer preventive agent. In Selenium: Its Molecular Biology and Role in Human Health; Springer: Berlin/Heidelberg, Germany, 2001; pp. 249–264. [Google Scholar]
- Arruebarrena, M.A.; Hawe, C.T.; Lee, Y.M.; Branco, R.C. Mechanisms of Cadmium Neurotoxicity. Int. J. Mol. Sci. 2023, 24, 16558. [Google Scholar] [CrossRef] [PubMed]
- Knoell, D.L.; Wyatt, T.A. The adverse impact of cadmium on immune function and lung host defense. Semin. Cell Dev. Biol. 2021, 115, 70–76. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Yao, W.; Ba, Q.; Wang, H. Effects of cadmium exposure on the immune system and immunoregulation. Front. Immunol. 2021, 12, 695484. [Google Scholar] [CrossRef]
- Adegoke, A.; Salami, A.; Olaleye, S. Cadmium exacerbates acetic acid induced experimental colitis in rats. Eur. Exp. Biol. 2017, 7, 27. [Google Scholar]
- Jiang, Z.; Mu, W.; Yang, Y.; Sun, M.; Liu, Y.; Gao, Z.; Li, J.; Gu, P.; Wang, H.; Lu, Y. Cadmium exacerbates dextran sulfate sodium-induced chronic colitis and impairs intestinal barrier. Sci. Total Environ. 2020, 744, 140844. [Google Scholar] [CrossRef]
- Toubhans, B.; Gazze, S.A.; Bissardon, C.; Bohic, S.; Gourlan, A.T.; Gonzalez, D.; Charlet, L.; Conlan, R.S.; Francis, L.W. Selenium nanoparticles trigger alterations in ovarian cancer cell biomechanics. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102258. [Google Scholar] [CrossRef] [PubMed]
- Sonkusre, P.; Cameotra, S.S. Biogenic selenium nanoparticles induce ROS-mediated necroptosis in PC-3 cancer cells through TNF activation. J. Nanobiotechnology 2017, 15, 43. [Google Scholar] [CrossRef]
- El-Borady, O.M.; Othman, M.S.; Atallah, H.H.; Moneim, A.E.A. Hypoglycemic potential of selenium nanoparticles capped with polyvinyl-pyrrolidone in streptozotocin-induced experimental diabetes in rats. Heliyon 2020, 6, e04045. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qiu, Q.; Zou, C.; Dou, L.; Liang, J. Regulation of hepatic carbohydrate metabolism by selenium during diabetes. Chem. -Biol. Interact. 2015, 232, 1–6. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, C.; Guo, J.; Song, Y. Selenium status and cardiovascular diseases: Meta-analysis of prospective observational studies and randomized controlled trials. Eur. J. Clin. Nutr. 2016, 70, 162–169. [Google Scholar] [CrossRef]
- Gîlcă-Blanariu, G.-E.; Diaconescu, S.; Ciocoiu, M.; Ștefănescu, G. New insights into the role of trace elements in IBD. BioMed Res. Int. 2018, 2018, 1813047. [Google Scholar] [CrossRef]
- Short, S.P.; Pilat, J.M.; Williams, C.S. Roles for selenium and selenoprotein P in the development, progression, and prevention of intestinal disease. Free. Radic. Biol. Med. 2018, 127, 26–35. [Google Scholar] [CrossRef]
- Kaushal, N.; Kudva, A.K.; Patterson, A.D.; Chiaro, C.; Kennett, M.J.; Desai, D.; Amin, S.; Carlson, B.A.; Cantorna, M.T.; Prabhu, K.S. Crucial role of macrophage selenoproteins in experimental colitis. J. Immunol. 2014, 193, 3683–3692. [Google Scholar] [CrossRef]
- Rayman, M.P.; Winther, K.H.; Pastor-Barriuso, R.; Cold, F.; Thvilum, M.; Stranges, S.; Guallar, E.; Cold, S. Effect of long-term selenium supplementation on mortality: Results from a multiple-dose, randomised controlled trial. Free. Radic. Biol. Med. 2018, 127, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.K.; Campa, A.; Lai, S.; Martinez, S.S.; Tsalaile, L.; Burns, P.; Farahani, M.; Li, Y.; Van Widenfelt, E.; Page, J.B. Effect of micronutrient supplementation on disease progression in asymptomatic, antiretroviral-naive, HIV-infected adults in Botswana: A randomized clinical trial. Jama 2013, 310, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, B.E.; Klaus, J.R.; Llabre, M.M.; Gonzalez, A.; Lawrence, P.J.; Maher, K.J.; Greeson, J.M.; Baum, M.K.; Shor-Posner, G.; Skyler, J.S. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: A randomized controlled trial. Arch. Intern. Med. 2007, 167, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Błażejak, S. Current knowledge on the importance of selenium in food for living organisms: A review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. The role of selenium in arsenic and cadmium toxicity: An updated review of scientific literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Barceló, J.; Poschenrieder, C. Hyperaccumulation of trace elements: From uptake and tolerance mechanisms to litter decomposition; selenium as an example. Plant Soil 2011, 341, 31–35. [Google Scholar] [CrossRef]
- Liu, S.; Yu, H.; Li, P.; Wang, C.; Liu, G.; Zhang, X.; Zhang, C.; Qi, M.; Ji, H. Dietary nano-selenium alleviated intestinal damage of juvenile grass carp (Ctenopharyngodon idella) induced by high-fat diet: Insight from intestinal morphology, tight junction, inflammation, anti-oxidization and intestinal microbiota. Anim. Nutr. 2022, 8, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Guo, Q.; Huang, J.; Wang, Z.; Chen, Y.; Dong, Y. Eucommia ulmoides polysaccharide modified nano-selenium effectively alleviated DSS-induced colitis through enhancing intestinal mucosal barrier function and antioxidant capacity. J. Nanobiotechnology 2023, 21, 222. [Google Scholar] [CrossRef] [PubMed]
- Tarmizi, A.A.A.; Adam, S.H.; Ramli, N.N.N.; Abd, N.A. The Ameliorative Effects of Selenium Nanoparticles (SeNPs) on Diabetic Rat Model: A Narrative Review. Sains Malays. 2023, 52, 2037–2053. [Google Scholar] [CrossRef]
- BenithaJ, G.; Gheena, S.; Ramani, P.; Kumar, R.; Ramalingam, K.; Ramasubramaniam, A. Anticancer activity of green synthesized selenium nanoparticles from garcinia mangostana crude extract against MCF-7 breast cancer cells. J. Popul. Ther. Clin. Pharmacol. 2023, 30, 74–82. [Google Scholar]
- Martínez-Esquivias, F.; Perez-Larios, A.; Guzmán-Flores, J.M. Effect of Administration of Selenium Nanoparticles Synthesized Using Onion Extract on Biochemical and Inflammatory Parameters in Mice Fed with High-Fructose Diet: In Vivo and In Silico Analysis. Biol. Trace Elem. Res. 2024, 202, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Guo, K.; Zhang, C.; Talukder, M.; Lv, M.-W.; Li, J.-Y.; Li, J.-L. Comparison of nanoparticle-selenium, selenium-enriched yeast and sodium selenite on the alleviation of cadmium-induced inflammation via NF-kB/IκB pathway in heart. Sci. Total Environ. 2021, 773, 145442. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.-X.; Chang, B.; Zhu, J.-F.; Yang, F.-L.; Li, Y.; Jiang, X.-F.; Wang, D.-N.; Lu, C.-L.; Sun, X. Sodium selenite ameliorates dextran sulfate sodium-induced chronic colitis in mice by decreasing Th1, Th17, and γδT and increasing CD4 (+) CD25 (+) regulatory T-cell responses. World J. Gastroenterol. 2017, 23, 3850. [Google Scholar] [CrossRef] [PubMed]
- Bär, F.; Bochmann, W.; Widok, A.; Von Medem, K.; Pagel, R.; Hirose, M.; Yu, X.; Kalies, K.; König, P.; Böhm, R. Mitochondrial gene polymorphisms that protect mice from colitis. Gastroenterology 2013, 145, 1055–1063.e1053. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, X.-S. Hydrogen sulfide from a NaHS source attenuates dextran sulfate sodium (DSS)-induced inflammation via inhibiting nuclear factor-κB. J. Zhejiang Univ. Sci. B 2016, 17, 209. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, X.; Xia, S.; Chen, C.; Chen, X.; Zhang, Y.; Farag, M.A.; Xiao, J.; Nie, S. Celery soluble dietary fiber antagonizes flavonoids ameliorative effect on dextran-sodium-sulfate-induced colitis in mice. J. Adv. Res. 2023, 52, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Teng, P.-Y.; Kim, W.K.; Applegate, T.J. Assay considerations for fluorescein isothiocyanate-dextran (FITC-d): An indicator of intestinal permeability in broiler chickens. Poult. Sci. 2021, 100, 101202. [Google Scholar] [CrossRef]
- Kim, K.-S.; Lee, Y.; Chae, W.; Cho, J.-Y. An Improved Method to Quantify Short-Chain Fatty Acids in Biological Samples Using Gas Chromatography—Mass Spectrometry. Metabolites 2022, 12, 525. [Google Scholar] [CrossRef] [PubMed]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef]
- Wang, P.; Chen, H.; Kopittke, P.M.; Zhao, F.-J. Cadmium contamination in agricultural soils of China and the impact on food safety. Environ. Pollut. 2019, 249, 1038–1048. [Google Scholar] [CrossRef]
- Guo, K.; Ge, J.; Zhang, C.; Lv, M.-W.; Zhang, Q.; Talukder, M.; Li, J.-L. Cadmium induced cardiac inflammation in chicken (Gallus gallus) via modulating cytochrome P450 systems and Nrf2 mediated antioxidant defense. Chemosphere 2020, 249, 125858. [Google Scholar] [CrossRef] [PubMed]
- Newairy, A.; El-Sharaky, A.; Badreldeen, M.; Eweda, S.; Sheweita, S. The hepatoprotective effects of selenium against cadmium toxicity in rats. Toxicology 2007, 242, 23–30. [Google Scholar] [CrossRef]
- Hiller, F.; Oldorff, L.; Besselt, K.; Kipp, A.P. Differential acute effects of selenomethionine and sodium selenite on the severity of colitis. Nutrients 2015, 7, 2687–2706. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Mayer, L. The Immune Response in Inflammatory Bowel Disease. Off. J. Am. Coll. Gastroenterol. Assoc. Can. De Gastroenterol. 2007, 102, 2058–2069. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Terciolo, C.; Bracarense, A.P.F.; Payros, D.; Pinton, P.; Oswald, I.P. In vitro and in vivo effects of a mycotoxin, deoxynivalenol, and a trace metal, cadmium, alone or in a mixture on the intestinal barrier. Environ. Int. 2019, 132, 105082. [Google Scholar] [CrossRef] [PubMed]
- Shigetomi, K.; Ikenouchi, J. Regulation of the epithelial barrier by post-translational modifications of tight junction membrane proteins. J. Biochem. 2018, 163, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.J.; Tepass, U. Adherens junctions: From molecules to morphogenesis. Nat. Rev. Mol. Cell Biol. 2010, 11, 502–514. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Liu, K.; Shen, J. Exposing to cadmium stress cause profound toxic effect on microbiota of the mice intestinal tract. PLoS ONE 2014, 9, e85323. [Google Scholar] [CrossRef]
- Kim, E.; Xu, X.; Steiner, H.; Ahmer, B.; Cormet-Boyaka, E.; Boyaka, P. Chronic ingestion of low doses of cadmium alters the gut microbiome and immune homeostasis to enhance allergic sensitization (MUC9P. 743). J. Immunol. 2015, 194, 205–207. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Cao, S.; Wang, L.; Wang, D.; Yang, H.; Feng, Y.; Wang, S.; Li, L. Caffeic acid ameliorates colitis in association with increased Akkermansia population in the gut microbiota of mice. Oncotarget 2016, 7, 31790. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, Y.; Chen, S.; Zeng, Y.; Fu, X.; Chen, T.; Luo, S.; Zhang, X. The role of the probiotic Akkermansia muciniphila in brain functions: Insights underpinning therapeutic potential. Crit. Rev. Microbiol. 2023, 49, 151–176. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Wen, S.; Long-Kun, D.; Man, Y.; Chang, S.; Min, Z.; Shuang-Yu, L.; Xin, Q.; Jie, M.; Liang, W. Three important short-chain fatty acids (SCFAs) attenuate the inflammatory response induced by 5-FU and maintain the integrity of intestinal mucosal tight junction. BMC Immunol. 2022, 23, 19. [Google Scholar] [CrossRef] [PubMed]



| Score | Body Weight Loss | Fecal Consistency | Fecal Occult Blood |
|---|---|---|---|
| 0 | 0 | Negative | Negative |
| 1 | 1–5% | Soft stool | Light blue |
| 2 | 5–10% | Mucoid stool | Blue |
| 3 | 10–20% | Watery stool | Dark blue |
| 4 | >20% | Gross blood |
| Score | Severity of Inflammation | Depth of Injury | Crypt Damage | Percentage of the Involved Area |
|---|---|---|---|---|
| 0 | None | None | None | |
| 1 | Slight | Mucosal | Basal 1/3 damaged | 1–10% |
| 2 | Moderate | Mucosal and submucosal | Basal 2/3 damaged | 10–25% |
| 3 | Severe | Transmural | Only surface epithelium intact | 25–50% |
| 4 | Entire crypt and epithelium lost | 50–100% |
| Gene | Sense | Anti-Sense |
|---|---|---|
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| TNF-α | GCCTCCTCACCCACCACCATCA | CCAAGTAGACCTGCCCAGACT |
| IL-6 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA |
| MCP1 | TTAAAAACCTGGATCGGAACCAA | GCATTAGCTTCAGATTTACGGGT |
| IFN-γ | ATGAACGCTACACACTGCATC | CCATCCTTTTGCCAGTTCCTC |
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Guo, S.; Gong, P.; Ba, Q.; Yao, W. Nano-Selenium Alleviates Cd-Induced Chronic Colitis through Intestinal Flora. Nutrients 2024, 16, 1330. https://doi.org/10.3390/nu16091330
Zhou C, Guo S, Gong P, Ba Q, Yao W. Nano-Selenium Alleviates Cd-Induced Chronic Colitis through Intestinal Flora. Nutrients. 2024; 16(9):1330. https://doi.org/10.3390/nu16091330
Chicago/Turabian StyleZhou, Chengdong, Shengliang Guo, Pin Gong, Qian Ba, and Wenbo Yao. 2024. "Nano-Selenium Alleviates Cd-Induced Chronic Colitis through Intestinal Flora" Nutrients 16, no. 9: 1330. https://doi.org/10.3390/nu16091330
APA StyleZhou, C., Guo, S., Gong, P., Ba, Q., & Yao, W. (2024). Nano-Selenium Alleviates Cd-Induced Chronic Colitis through Intestinal Flora. Nutrients, 16(9), 1330. https://doi.org/10.3390/nu16091330






