Effects of sn-2 Palmitic Triacylglycerols and the Ratio of OPL to OPO in Human Milk Fat Substitute on Metabolic Regulation in Sprague-Dawley Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Animals and Treatment
2.3. Serum Collection
2.4. Serum Metabolomics Analysis
2.5. Serum Lipidomics Analysis
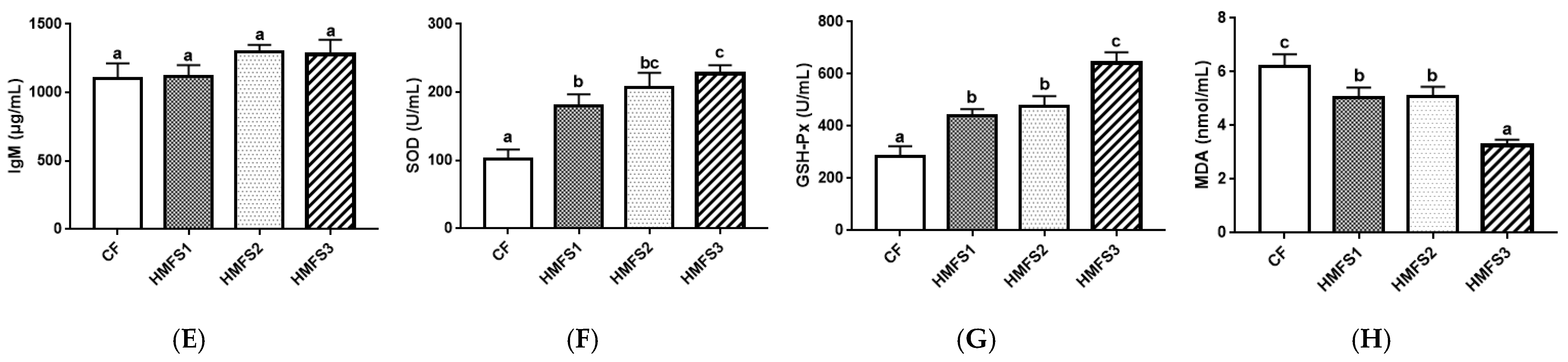
2.6. Serum Immunity-Related Parameter and Antioxidant Parameter Measurement
2.7. Statistical Analysis
3. Results and Discussion
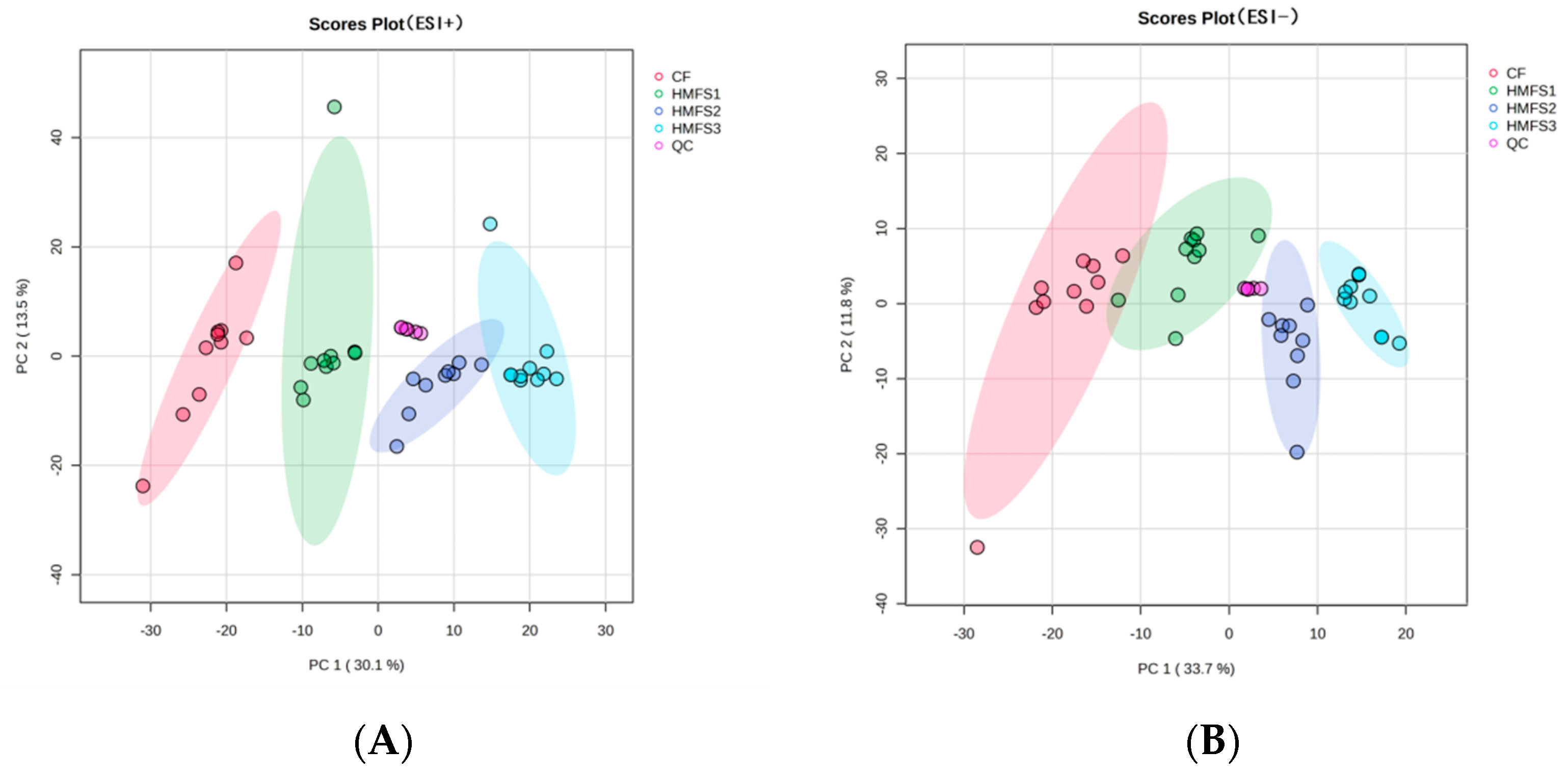
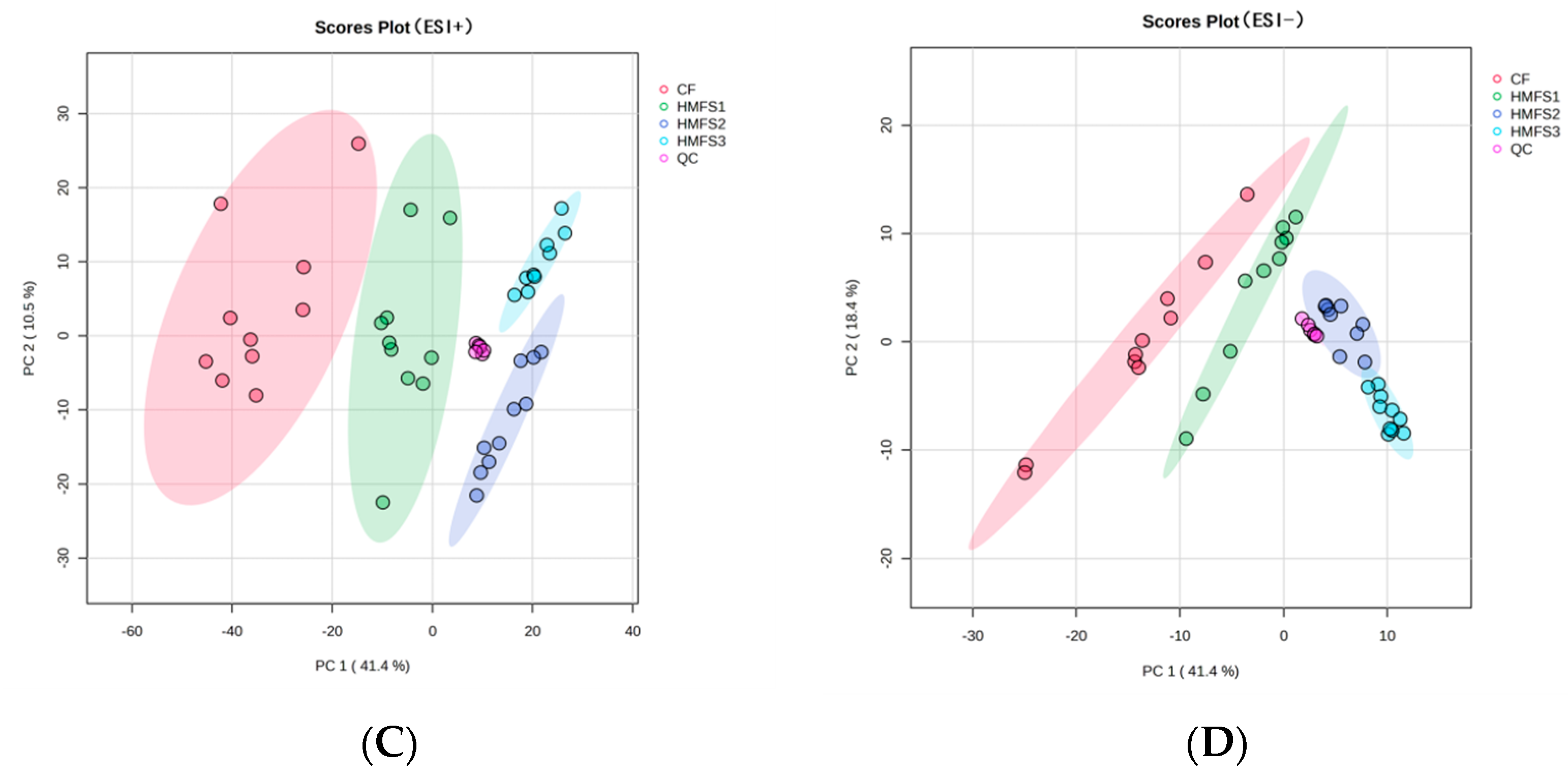
3.1. Metabolomics and Lipidomics Profiling
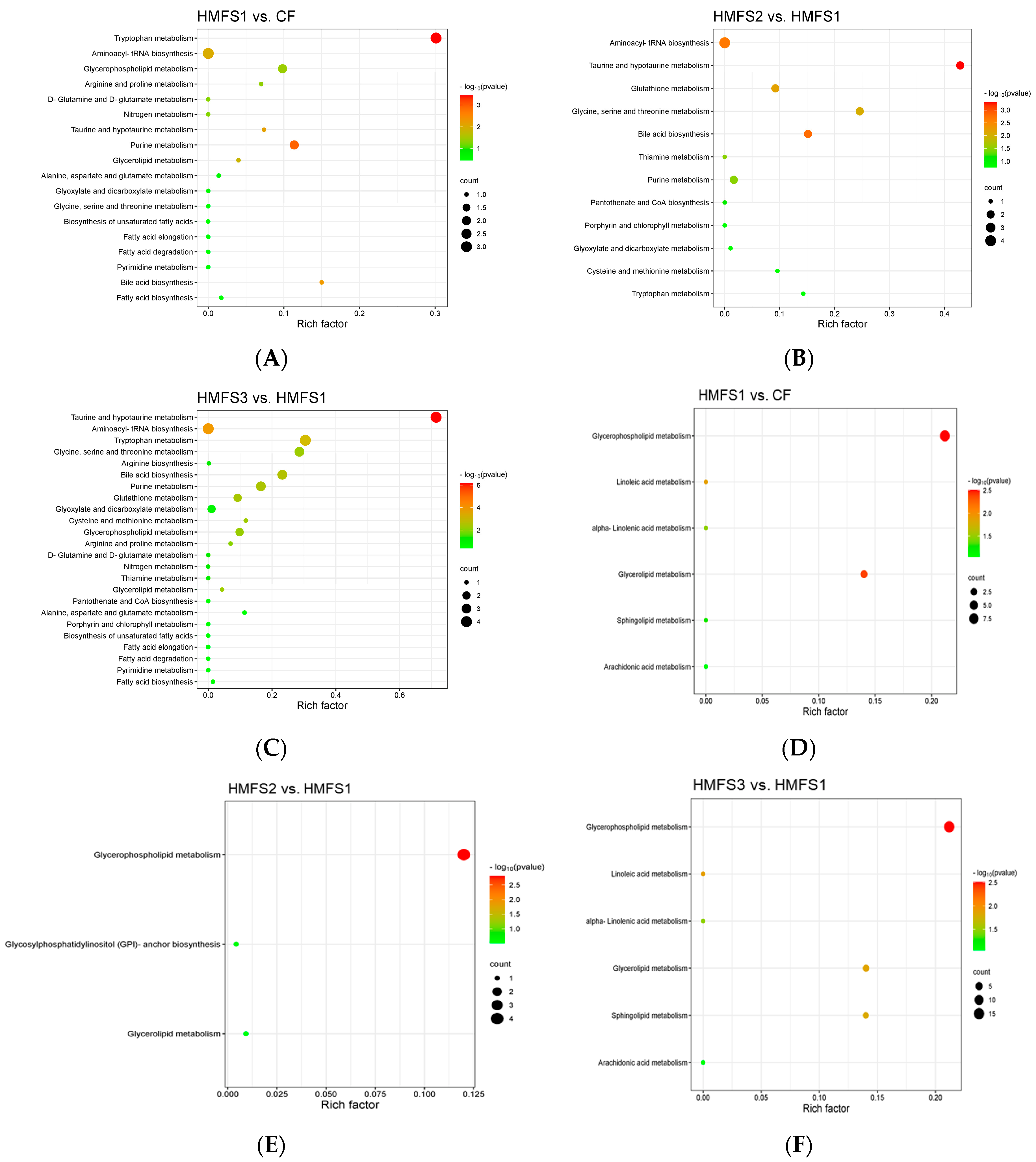
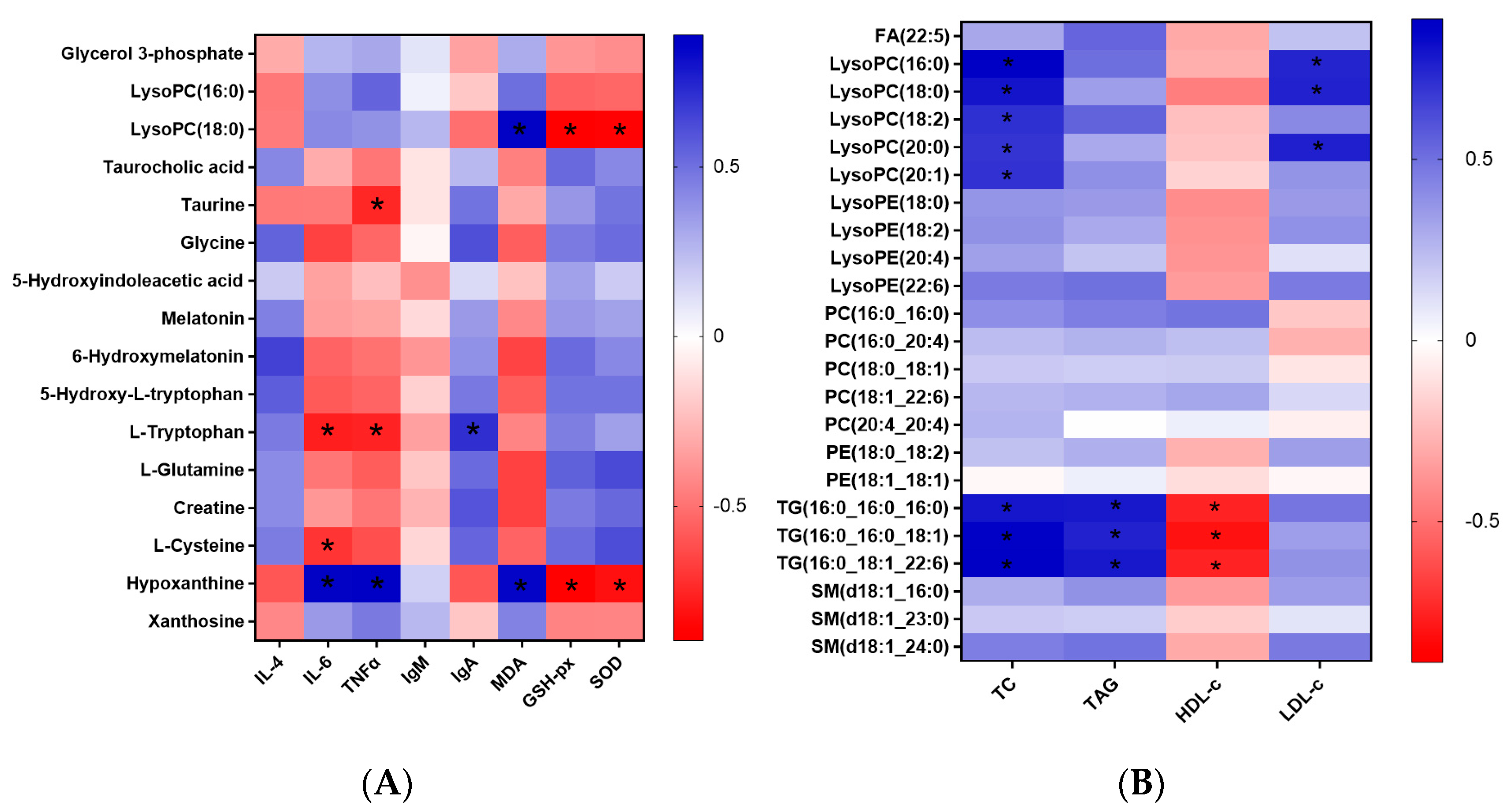
3.2. Metabolomics Analysis
3.3. Lipidomics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | Control fat |
| ELISA | Enzyme-linked Immunosorbent Assay |
| FA | Fatty acid |
| FC | Fold change |
| GSH-px | Glutathione peroxidase |
| HMDB ID | Human metabolome database identity document |
| HMFS | Human milk fat substitute |
| IgA | Immunoglobulin A |
| IgM | Immunoglobulin M |
| IL-1β | Interleukin-1β |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-10 | Interleukin-10 |
| LysoPC | Lysophosphatidyl choline |
| LysoPE | Lysophosphatidyl ethanolamine |
| MDA | Malondialdehyde |
| OPL | 1-Oleoyl-2-palmitoyl-3-linoleoylglycerol |
| OPO | 1,3-Dioleoyl-2-palmitoylglycerol |
| PA | Palmitic acid |
| PCA | Principal component analysis |
| PC | Phosphatidyl choline |
| PE | Phosphatidyl ethanolamine |
| QC | Quality control |
| RT | Retention time |
| SM | Sphingomyelin |
| SD | Sprague-Dawley |
| SOD | Superoxide dismutase |
| TAGs | Triacyclglycerols |
| TG | Triacylglycerol |
| TNF-α | Tumor necrosis factor-α |
| UHPLC-MS | Ultrahigh performance liquid chromatography-mass spectrometer |
| VIP | Variable importance in projection |
References
- Zou, X.; Ali, A.H.; Abed, S.M.; Zheng, G. Current knowledge of lipids in human milk and recent innovations in infant formulas. Curr. Opin. Food Sci. 2017, 16, 28–39. [Google Scholar] [CrossRef]
- Hageman, J.H.J.; Danielsen, M.; Nieuwenhuizen, A.G. Comparison of bovine milk fat and vegetable fat for infant formula: Implications for infant health. Int. Dairy J. 2019, 92, 37–49. [Google Scholar] [CrossRef]
- Ten-Domenech, I.; Beltran-Iturat, E.; Manuel Herrero-Martinez, J.; Simó-Alfonso, E. Triacylglycerol analysis in human milk and other mammalian species: Small-scale sample preparation, characterization, and statistical classification using HPLC-ELSD profiles. J. Agric. Food Chem. 2015, 63, 5761–5770. [Google Scholar] [CrossRef] [PubMed]
- Tu, A.Q.; Ma, Q.; Hua, B.; Du, Z. A comparative study of triacylglycerol composition in Chinese human milk within different lactation stages and imported infant formula by SFC coupled with Q-TOF-MS. Food Chem. 2017, 221, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Balter, A.; Vodsky, V.; Odetallh, Y. Chinese breast milk fat composition and its associated dietary factors: A pilot study on lactating mothers in Beijing. Front. Nutr. 2021, 8, 606950–606962. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zheng, S.S.; Lin, K.; Xu, X.; Lv, L.N. Effects of infant formula supplemented with prebiotics and OPO on infancy fecal microbiota: A pilot randomized clinical trial. Front. Cell. Infect. Microbiol. 2021, 11, 650407–650419. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, C.; Evivie, S.E.; Cao, T.; Wang, Z.; Huo, G. Infant formula supplemented with 1,3-olein-2-palmitin regulated the immunity, gut microbiota, and metabolites of mice colonized by feces from healthy infants. J. Dairy Sci. 2022, 105, 6405–6421. [Google Scholar] [CrossRef]
- Zhu, L.; Fang, S.Z.; Liu, W.W.; Zhang, H.; Zhang, Y.Q.; Xie, Z.H.; Yang, P.Y.; Wan, J.C.; Gao, B.Y.; Yu, L.L. The triacylglycerol structure and composition of a human milk fat substitute affect the absorption of fatty acids and calcium, lipid metabolism and bile acid metabolism in newly-weaned Sprague-Dawley rats. Food Funct. 2023, 14, 7574–7585. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.Y.; Zhang, H.; Wan, J.C.; Hu, J.Y.; Liu, J.C.; Wang, J.; Zhang, Y.Q.; Yu, L.L. Dietary sn-2 palmitic triacylglycerols reduced faecal lipids, calcium contents and altered lipid metabolism in Sprague-Dawley rats. Int. J. Food Sci. Nutr. 2019, 70, 474–483. [Google Scholar] [CrossRef]
- Guo, D.Y.; Li, F.; Zhao, J.X.; Zhang, H.; Liu, B.; Pan, J.C. Effect of an infant formula containing sn-2 palmitate on fecal microbiota and metabolome profiles of healthy term infants: A randomized, double-blind, parallel, controlled study. Food Funct. 2022, 13, 2003–2018. [Google Scholar] [CrossRef]
- Wei, T.; Tan, D.F.; Zhong, S.Y.; Zhang, H.; De, Z.Y.; Li, J. 1-Oleate-2-palmitate-3-linoleate glycerol improves lipid metabolism and gut microbiota and decreases the level of pro-inflammatory cytokines. Food Funct. 2023, 14, 5949–5961. [Google Scholar] [CrossRef] [PubMed]
- Bordon, J.; Aliberti, S.; Fernandez-Botran, R. Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int. J. Infect. Dis. 2013, 17, E76–E83. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Y.; Gao, Y.; Ju, Y.; Zhao, Y. The PRAK-NRF2 axis promotes the differentiation of Th17 cells by mediating the redox homeostasis and glycolysis. Proc. Natl. Acad. Sci. USA 2023, 120, 2212613120–2212613130. [Google Scholar] [CrossRef]
- Sims, N.R.; Anderson, M.F. Isolation of mitochondria from rat brain using Percoll density gradient centrifugation. Nat. Protoc. 2008, 3, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Du, A.; Fan, Z.; Shi, L. Novel top-down high-resolution mass spectrometry-based metabolomics and lipidomics reveal molecular change mechanism in A2 milk after CSN2 gene mutation. Food Chem. 2022, 391, 133270. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, J.; Xing, R.; Chen, Y. Integration of lipidomics and metabolomics for the authentication of camellia oil by ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry coupled with chemometrics. Food Chem. 2022, 373, 131534. [Google Scholar] [CrossRef]
- He, H.; An, F.; Huang, Q.; Kong, Y.; He, D.; Chen, L. Metabolic effect of AOS-iron in rats with iron deficiency anemia using LC-MS/MS based metabolomics. Food Res. Int. 2020, 130, 108913. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Y.Y.; Sun-Waterhouse, Y.; Zhao, Y. UHPLC-Q-Exactive Orbitrap MS/MS-based untargeted lipidomics reveals molecular mechanisms and metabolic pathways of lipid changes during golden pomfret (Trachinotus ovatus) fermentation. Food Chem. 2022, 396, 133676. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.H.; Wang, N.; Geng, F.; Ma, M. High-density lipoproteins from egg yolk’s effect on hyperlipidemia in a high-fat-diet obese mouse using lipidomic analysis. Food Biosci. 2020, 33, 100492. [Google Scholar] [CrossRef]
- Qu, P.; Rom, O.; Li, K.; Jia, L.; Gao, X. DT-109 ameliorates nonalcoholic steatohepatitis in nonhuman primates. Cell Metab. 2023, 35, 742–769. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, Y.; Li, Z.; Wang, X.; Qian, H. An integrated network pharmacology and metabolomics approach to reveal the immunomodulatory mechanism of Brassica rapa L. (Tibetan Turnip) in fatigue mice. Food Funct. 2022, 13, 11097–11110. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Fang, S.Z.; Zhang, H.; Sun, X.J.; Yang, P.Y.; Wan, J.C.; Zhang, Y.Q.; Lu, W.Y.; Yu, L.L. Total Sn-2 Palmitic Triacylglycerols and the Ratio of OPL to OPO in Human Milk Fat Substitute Modulated Bile Acid Metabolism and Intestinal Microbiota Composition in Rats. Nutrients 2023, 15, 4929. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Nie, M.; Pang, H.H.; Wang, B.H.; Hu, J.L.; Meng, X.J.; Li, K.; Ran, X.R.; Long, Q.X.; Deng, H.J. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat. Commun. 2021, 12, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.P.; Qiao, R.X.; Zhang, S.Y.; Guang, G. Metabolomic profiling reveals the intestinal toxicity of different length of microplastic fibers on zebrafish (Danio rerio). J. Hazard. Mater. 2021, 403, 123663. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Guan, X.; Yu, D.; Dou, T. Mogroside V reduce OVA-induced pulmonary inflammation based on lung and serum metabolomics. Phytomedicine 2021, 91, 153682–153693. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, F.; Lei, J.; Zhou, B. Gut microbial metabolite urolithin B attenuates intestinal immunity function in vivo in aging mice and in vitro in HT29 cells by regulating oxidative stress and inflammatory signalling. Food Funct. 2022, 13, 2372–2373. [Google Scholar] [CrossRef]
- Shi, J.; Qiu, H.; Xu, Q.; Ma, Y.; Ye, T.; Kuang, Z.; Qu, N. Integrated multi-omics analyses reveal effects of empagliflozin on intestinal homeostasis in high-fat-diet mice. iScience 2023, 26, 105816–105832. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.L.; Liu, B.S.; Sun, X.; Li, Z.; Chen, Y.; Guo, Z.; Liu, H.; Li, D.; Wang, C.; Zhu, D.; et al. Protective effects of alfalfa saponins on oxidative stress-induced apoptotic cells. Food Funct. 2020, 11, 8133–8140. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Zhong, R.; Zhang, S.F.; Wang, M.; Wen, X.; Yi, B. Hydroxytyrosol attenuates diquat-induced oxidative stress by activating Nrf2 pathway and modulating colonic microbiota in mice. J. Nutr. Biochem. 2023, 113, 109256–109266. [Google Scholar] [CrossRef]
- Zhan, G.; Long, M.; Shan, K.; Xie, C.; Yang, R.Q. Antioxidant effect of chrysanthemum morifolium (Chuju) extract on H2O2-treated LO2 cells as revealed by LC/MS-based metabolic profiling. Antioxidants 2022, 11, 1068. [Google Scholar] [CrossRef]
- Wei, B.; Peng, Z.; Zheng, W.; Yang, S.; Wu, M.; Liu, K. Probiotic-fermented tomato alleviates high-fat diet-induced obesity in mice: Insights from microbiome and metabolomics. Food Chem. 2024, 436, 137719–137731. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.L.; Lan, Y.Q.; Zhu, X.A.; Li, J.; Chen, T. Hepatic lipidomics analysis reveals the antiobesity and cholesterol-lowering effects of tangeretin in high-fat diet-fed rats. J. Agric. Food Chem. 2020, 68, 6142–6153. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Li, F.; Li, L.; Wang, Y.; Li, J.; Yang, Y.; Jiang, L.; Wang, L.; Qin, Y. Low-density lipoprotein receptor genotypes modify the sera metabolome of patients with homozygous familial hypercholesterolemia. iScience 2022, 25, 105334–105351. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yoo, H.J.; Lee, D. Oxidized LDL induces procoagulant profiles by increasing lysophosphatidylcholine levels, lysophosphatidylethanolamine levels, and Lp-PLA2 activity in borderline hypercholesterolemia. Nutr. Metab. Carbiovasc. Dis. 2020, 30, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Xing, M.; Chen, H.; Hu, J.; Nie, S. Metabolomics and lipidomics profiling reveals hypocholesterolemic and hypolipidemic effects of arabinoxylan on Type 2 diabetic rats. J. Agric. Food Chem. 2019, 67, 10614–10623. [Google Scholar] [CrossRef]
- Boren, J.; Taskinen, M.R.; Bjornson, E.; Packard, C.J. Metabolism of triglyceride-rich lipoproteins in health and dyslipidaemia. Nat. Rev. Cardiol. 2022, 19, 577–592. [Google Scholar] [CrossRef]

| Class | Ion Mode | Identity | HMDB ID | Observed m/z | RT (min) | Formula | Mass Error (ppm) | HMFS1 vs. CF | HMFS2 vs. HMFS1 | HMFS3 vs. HMFS1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VIP | FC | Trend | VIP | FC | Trend | VIP | FC | Trend | ||||||||
| Glycerophospholipids | − | Glycerol 3-phosphate | HMDB00126 | 171.0065 | 0.65 | C3H9O6P | 0.31 | 1.23 | 0.37 | ↓ ** | 0.91 | 0.97 | ↓ | 2.88 | 0.21 | ↓ ** |
| + | LysoPC (16:0) | HMDB10382 | 478.3291 | 7.50 | C24H50NO7P | −0.17 | 1.13 | 0.82 | ↓ | 0.88 | 0.92 | ↓ | 1.54 | 0.05 | ↓ ** | |
| + | LysoPC (18:0) | HMDB10384 | 524.3713 | 6.06 | C26H54NO7P | 0.53 | 1.24 | 0.45 | ↓ ** | 0.65 | 0.93 | ↓ | 1.83 | 0.33 | ↓ ** | |
| Bile acids | + | Taurocholic acid | HMDB00036 | 538.2805 | 5.07 | C26H45NO7S | −0.18 | 1.01 | 2.06 | ↑ * | 1.65 | 1.18 | ↑ | 1.91 | 3.08 | ↑ ** |
| Organosulfonic acids | + | Taurine | HMDB00251 | 126.0219 | 0.62 | C2H7NO3S | −0.24 | 1.18 | 1.29 | ↑ | 1.48 | 1.02 | ↑ | 2.11 | 2.94 | ↑ ** |
| − | Taurine | HMDB00251 | 124.0073 | 0.61 | C2H7NO3S | 0.03 | 1.33 | 1.11 | ↑ | 1.76 | 1.01 | ↑ | 1.98 | 3.16 | ↑ ** | |
| − | Glycine | HMDB00123 | 74.0246 | 0.65 | C2H5NO2 | −1.41 | 1.09 | 1.10 | ↑ | 1.84 | 5.57 | ↑ ** | 2.43 | 6.02 | ↑ ** | |
| Indoles and derivatives | + | 5-Hydroxyindoleacetic acid | HMDB00763 | 192.0656 | 3.13 | C10H9NO3 | 0.28 | 1.09 | 0.70 | ↓ | 0.93 | 1.63 | ↑ | 1.98 | 3.44 | ↑ ** |
| − | 5-Hydroxyindoleacetic acid | HMDB00763 | 190.0510 | 3.15 | C10H9NO3 | 0.16 | 1.18 | 0.86 | ↓ | 0.88 | 1.33 | ↑ | 1.69 | 3.01 | ↑ ** | |
| + | Melatonin | HMDB01389 | 255.1056 | 4.76 | C13H16N2O2 | −0.63 | 1.52 | 2.45 | ↑ | 1.63 | 1.51 | ↑ | 1.21 | 8.36 | ↑ ** | |
| − | 6-Hydroxymelatonin | HMDB04081 | 293.1142 | 4.09 | C13H16N2O3 | −0.19 | 1.98 | 2.79 | ↑ * | 0.82 | 1.06 | ↑ | 1.83 | 2.48 | ↑ ** | |
| + | 5-Hydroxy-L-tryptophan | HMDB00472 | 221.0920 | 2.74 | C11H12N2O3 | 0.19 | 1.09 | 7.61 | ↑ ** | 1.02 | 1.49 | ↑ | 0.44 | 1.08 | ↑ | |
| − | 5-Hydroxy-L-tryptophan | HMDB00472 | 219.0774 | 2.74 | C11H12N2O3 | −0.24 | 1.16 | 8.13 | ↑ ** | 1.29 | 1.27 | ↑ | 0.72 | 1.41 | ↑ | |
| + | L-Tryptophan | HMDB00929 | 205.0971 | 3.87 | C11H12N2O2 | −0.10 | 1.63 | 2.04 | ↑ ** | 1.26 | 2.54 | ↑ ** | 1.29 | 3.11 | ↑ ** | |
| − | L-Tryptophan | HMDB00929 | 203.0826 | 3.87 | C11H12N2O2 | 0.33 | 1.82 | 2.21 | ↑ ** | 1.04 | 2.15 | ↑ ** | 1.43 | 3.28 | ↑ ** | |
| Amino acids and derivatives | − | L-Glutamine | HMDB00641 | 145.0619 | 0.63 | C5H10N2O3 | 0.52 | 1.69 | 3.46 | ↑ ** | 1.77 | 1.00 | — | 2.05 | 3.15 | ↑ ** |
| − | Creatine | HMDB00064 | 130.0622 | 0.73 | C4H9N3O2 | 0.23 | 1.25 | 7.06 | ↑ ** | 0.88 | 1.10 | ↑ | 1.67 | 3.10 | ↑ ** | |
| − | L-Cysteine | HMDB00574 | 120.0125 | 1.00 | C3H7NO2S | 0.11 | 0.77 | 0.98 | ↓ | 2.00 | 4.25 | ↑ ** | 1.32 | 4.28 | ↑ ** | |
| Purines and purine derivatives | + | Hypoxanthine | HMDB00157 | 137.0458 | 3.54 | C5H4N4O | 0.55 | 0.80 | 0.60 | ↓ | 1.87 | 0.37 | ↓ ** | 1.74 | 0.18 | ↓ ** |
| + | Xanthosine | HMDB00299 | 285.0826 | 3.31 | C10H12N4O6 | −1.42 | 2.71 | 0.22 | ↓ ** | 1.76 | 0.22 | ↓ ** | 1.82 | 0.11 | ↓ ** | |
| − | Xanthosine | HMDB00299 | 283.0685 | 3.34 | C10H12N4O6 | −0.21 | 2.55 | 0.39 | ↓ ** | 1.98 | 0.37 | ↓ ** | 1.95 | 0.06 | ↓ ** | |
| Class | Ion Mode | Identity | HMDB ID | Observed m/z | RT (min) | Formula | Mass Error (ppm) | HMFS1 vs. CF | HMFS2 vs. HMFS1 | HMFS3 vs. HMFS1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VIP | FC | Trend | VIP | FC | Trend | VIP | FC | Trend | ||||||||
| Fatty acids | − | FA (22:5) | HMDB0247288 | 329.2486 | 3.94 | C22H34O2 | 0.01 | 1.84 | 0.42 | ↓ ** | 0.92 | 0.65 | ↓ | 1.63 | 0.43 | ↓ ** |
| Glycerophospholipids | + | LysoPC (16:0) | HMDB0010382 | 496.3398 | 2.15 | C24H50O7NP | 1.20 | 0.76 | 0.97 | ↓ | 0.85 | 0.93 | ↓ | 1.52 | 0.45 | ↓ ** |
| + | LysoPC (18:0) | HMDB0010384 | 524.3711 | 2.40 | C26H54O7NP | 0.75 | 1.59 | 0.45 | ↓ ** | 0.92 | 0.98 | ↓ | 1.81 | 0.31 | ↓ ** | |
| + | LysoPC (18:2) | HMDB0010386 | 520.3398 | 2.22 | C26H50O7NP | 0.37 | 1.51 | 0.35 | ↓ ** | 0.82 | 0.87 | ↓ | 1.15 | 0.45 | ↓ ** | |
| + | LysoPC (20:0) | HMDB0010390 | 552.4024 | 3.96 | C28H58O7NP | 0.68 | 0.91 | 1.00 | — | 1.14 | 0.38 | ↓ ** | 1.36 | 0.31 | ↓ ** | |
| + | LysoPC (20:1) | HMDB0010391 | 550.3867 | 3.36 | C28H56O7NP | 0.35 | 1.91 | 0.31 | ↓ ** | 0.75 | 0.82 | ↓ | 0.78 | 0.65 | ↓ | |
| − | LysoPE (18:0) | HMDB0011130 | 480.3096 | 3.67 | C23H48O7NP | 0.69 | 1.63 | 0.49 | ↓ ** | 0.87 | 1.00 | — | 1.03 | 0.45 | ↓ ** | |
| − | LysoPE (18:2) | HMDB0011507 | 476.2783 | 2.16 | C23H44O7NP | 0.47 | 1.81 | 0.38 | ↓ ** | 1.75 | 0.44 | ↓ ** | 1.38 | 0.22 | ↓ ** | |
| − | LysoPE (20:4) | HMDB0011487 | 500.2783 | 2.14 | C25H44O7NP | 1.32 | 1.59 | 0.33 | ↓ ** | 0.95 | 0.88 | ↓ | 1.55 | 0.41 | ↓ ** | |
| − | LysoPE (22:6) | HMDB0011496 | 524.2783 | 2.28 | C27H44O7NP | 0.25 | 1.21 | 0.28 | ↓ ** | 0.74 | 0.97 | ↓ | 1.98 | 0.34 | ↓ ** | |
| + | PC (16:0_16:0) | HMDB0000564 | 756.5514 | 7.87 | C40H80O8NP | 0.01 | 1.24 | 0.40 | ↓ ** | 0.85 | 0.82 | ↓ | 1.62 | 0.49 | ↓ ** | |
| + | PC (16:0_20:4) | HMDB0007982 | 804.5514 | 8.41 | C44H80O8NP | 1.05 | 0.76 | 0.75 | ↓ | 0.98 | 0.94 | ↓ | 1.54 | 0.42 | ↓ ** | |
| + | PC (18:0_18:1) | HMDB0008037 | 810.5983 | 10.97 | C44H86O8NP | 1.41 | 0.91 | 0.90 | ↓ | 0.94 | 0.93 | ↓ | 1.54 | 0.40 | ↓ ** | |
| + | PC (18:1_22:6) | HMDB0008090 | 854.5670 | 7.96 | C48H82O8NP | 0.01 | 1.24 | 0.48 | ↓ ** | 0.56 | 0.74 | ↓ | 1.39 | 0.40 | ↓ ** | |
| + | PC (20:4_20:4) | HMDB0008444 | 852.5514 | 6.72 | C48H80O8NP | 0.01 | 0.52 | 0.96 | ↓ | 0.73 | 0.64 | ↓ | 1.98 | 0.45 | ↓ ** | |
| − | PE (18:0_18:2) | HMDB0008994 | 742.5392 | 10.72 | C41H78O8NP | 1.29 | 0.32 | 0.97 | ↓ | 1.62 | 0.32 | ↓ ** | 1.32 | 0.31 | ↓ ** | |
| − | PE (18:1_18:1) | HMDB0009025 | 742.5392 | 7.33 | C41H78O8NP | 0.02 | 0.68 | 0.88 | ↓ | 1.61 | 0.45 | ↓ ** | 1.28 | 0.23 | ↓ ** | |
| Glycerolipids | + | TG (16:0_16:0_16:0) | HMDB0005356 | 829.7256 | 12.25 | C51H98O6 | 1.05 | 1.59 | 0.49 | ↓ ** | 1.52 | 0.07 | ↓ ** | 1.67 | 0.05 | ↓ ** |
| + | TG (16:0_16:0_18:1) | HMDB0005360 | 855.7412 | 14.75 | C53H100O6 | 1.51 | 1.38 | 0.44 | ↓ ** | 0.97 | 0.74 | ↓ | 1.18 | 0.24 | ↓ ** | |
| + | TG (16:0_18:1_22:6) | HMDB0044135 | 905.7593 | 12.24 | C59H100O6 | 0.17 | 0.94 | 0.81 | ↓ | 0.88 | 0.76 | ↓ | 1.28 | 0.36 | ↓ ** | |
| Sphingolipids | + | SM (d18:1_16:0) | HMDB0010169 | 725.5568 | 8.01 | C39H79O6N2P | 1.48 | 1.11 | 0.45 | ↓ ** | 0.98 | 0.83 | ↓ | 0.42 | 0.74 | ↓ |
| + | SM (d18:1_23:0) | HMDB0012105 | 823.6663 | 11.65 | C46H93O6N2P | 0.71 | 0.53 | 0.77 | ↓ | 0.93 | 0.82 | ↓ | 1.73 | 0.35 | ↓ ** | |
| + | SM (d18:1_24:0) | HMDB0011697 | 837.6820 | 11.83 | C47H95O6N2P | 1.50 | 0.96 | 0.88 | ↓ | 0.89 | 0.72 | ↓ | 1.92 | 0.48 | ↓ ** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Fang, S.; Zhang, Y.; Sun, X.; Yang, P.; Lu, W.; Yu, L. Effects of sn-2 Palmitic Triacylglycerols and the Ratio of OPL to OPO in Human Milk Fat Substitute on Metabolic Regulation in Sprague-Dawley Rats. Nutrients 2024, 16, 1299. https://doi.org/10.3390/nu16091299
Zhu L, Fang S, Zhang Y, Sun X, Yang P, Lu W, Yu L. Effects of sn-2 Palmitic Triacylglycerols and the Ratio of OPL to OPO in Human Milk Fat Substitute on Metabolic Regulation in Sprague-Dawley Rats. Nutrients. 2024; 16(9):1299. https://doi.org/10.3390/nu16091299
Chicago/Turabian StyleZhu, Lin, Shuaizhen Fang, Yaqiong Zhang, Xiangjun Sun, Puyu Yang, Weiying Lu, and Liangli Yu. 2024. "Effects of sn-2 Palmitic Triacylglycerols and the Ratio of OPL to OPO in Human Milk Fat Substitute on Metabolic Regulation in Sprague-Dawley Rats" Nutrients 16, no. 9: 1299. https://doi.org/10.3390/nu16091299
APA StyleZhu, L., Fang, S., Zhang, Y., Sun, X., Yang, P., Lu, W., & Yu, L. (2024). Effects of sn-2 Palmitic Triacylglycerols and the Ratio of OPL to OPO in Human Milk Fat Substitute on Metabolic Regulation in Sprague-Dawley Rats. Nutrients, 16(9), 1299. https://doi.org/10.3390/nu16091299






