Abstract
Prostate cancer, accounting for 375,304 deaths in 2020, is the second most prevalent cancer in men worldwide. While many treatments exist for prostate cancer, novel therapeutic agents with higher efficacy are needed to target aggressive and hormone-resistant forms of prostate cancer, while sparing healthy cells. Plant-derived chemotherapy drugs such as docetaxel and paclitaxel have been established to treat cancers including prostate cancer. Carnosic acid (CA), a phenolic diterpene found in the herb rosemary (Rosmarinus officinalis) has been shown to have anticancer properties but its effects in prostate cancer and its mechanisms of action have not been examined. CA dose-dependently inhibited PC-3 and LNCaP prostate cancer cell survival and proliferation (IC50: 64, 21 µM, respectively). Furthermore, CA decreased phosphorylation/activation of Akt, mTOR, and p70 S6K. A notable increase in phosphorylation/activation of AMP-activated kinase (AMPK), acetyl-CoA carboxylase (ACC) and its upstream regulator sestrin-2 was seen with CA treatment. Our data indicate that CA inhibits AKT-mTORC1-p70S6K and activates Sestrin-2-AMPK signaling leading to a decrease in survival and proliferation. The use of inhibitors and small RNA interference (siRNA) approaches should be employed, in future studies, to elucidate the mechanisms involved in carnosic acid’s inhibitory effects of prostate cancer.
1. Introduction
Prostate cancer affects millions of people and is the second leading cause of death in men accounting for 375,304 deaths in 2020 worldwide [1,2,3]. The risk of developing prostate cancer increases with age, with more than 85% of those diagnosed being older than 60 years of age [1,3,4]. Prostate cancer is strongly associated with alterations in oncogenes and/or tumor suppressor genes [5,6]. These aberrations result in changes in gene transcription and translation of proteins involved in proliferation and cell death [7,8].
Accumulation of DNA mutations as well as epigenetic alternations in proteins, lead to uncontrolled cell proliferation and evasion of apoptosis which give rise to tumor formation and cancer [9]. Cellular signaling cascades, such as the Ras-mitogen activated protein kinase (MAPK) and the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mechanistic target of rapamycin (mTOR)/mTORC1 complex are overactivated, contributing to increased protein synthesis, proliferation, and cell survival [10,11,12,13]. These signaling cascades are activated by growth factor receptor such as epidermal growth factor receptor (EGFR) signaling [14].
Androgens play a large role in the development as well as progression of prostate cancer. Prostate epithelial cells express high levels of androgen receptors (AR) [15], with a higher expression being in the luminal epithelial compared to the basal epithelial cells [16]. AR contains three main functional binding domains: the N-terminal transcriptional regulation domain, the DNA binding domain (DBD) and the ligand binding domain [17,18]. Androgens such as testosterone are the main ligands that bind to the ligand binding domain of ARs and form dimers in the nucleus, which bind to androgen response elements (AREs) in AR-regulated genes and upregulate their transcription leading to cell growth and proliferation, cell cycle progression, and protein synthesis [18,19,20,21,22].
Activated Akt leads to the downstream activation of the mammalian target of rapamycin (mTOR) and p70 S6 kinase (p70 S6K) [23,24], resulting in increased protein synthesis and proliferation. Deletions in phosphatase and tensin homolog (PTEN), the negative regulator of PI3K, and overactivation of Akt drives prostate cancer [25], while targeting this cascade may provide treatment benefits [26,27,28]. Other genetic aberrations seen in prostate cancer include loss of RB1 and p53, N-Myc overexpression, AR, c-MYC and FOXA1 overexpression [22,29]. These alterations lead to loss of basal cell layer, disruption of normal tissue architecture, detectable levels of prostate specific antigen (PSA) in the bloodstream, and prostate cell proliferation [15].
Adenosine monophosphate-activated protein kinase (AMPK) is a 62 kDa heterotrimeric protein with anticancer effects [30,31,32,33]. Its activation leads to the inhibition of the mTORC1 complex [34]. This is achieved through the phosphorylation of raptor, mTOR’s binding partner, at Ser722 and Ser792 [35]. AMPK activation causes the phosphorylation/inhibition of acetyl-CoA carboxylase (ACC) and 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoA reductase) leading to impairment of fatty acid and sterol synthesis, respectively [36,37]. Liver kinase B1 (LKB1), Ca2+/calmodulin (CaM)-dependent protein kinase kinases (CaMKK) and transforming growth factor beta-(TGF-β)-activated kinase 1 (TAK1) are responsible for AMPK activation by phosphorylating the Thr172 residue [38,39,40,41]. Sestrin-2 has been implicated in AMPK activation by directly interacting with the α subunit [42,43], leading to mTOR inhibition [44].
Plant-derived chemicals serve as valuable sources for cancer treatment [45]. Paclitaxel and docetaxel, potent drugs used in the treatment of various cancers including prostate cancer, were originally isolated from the bark of the Pacific yew tree (Taxus brevifolia) and the needles of the European yew tree (Taxus baccata), respectively [46,47]. These taxanes act by hindering mitosis through microtubule binding, leading to inhibition of depolymerization and formation of mitotic spindles [48,49]. However, due to an increase in cancer cases and the growing resistance to current chemotherapy drugs, alternative treatment strategies are required.
In the past few years our lab has focused on finding novel plant-derived chemicals with anticancer potential. We observed promising effects against prostate cancer using the polyphenol resveratrol that were associated with inhibition of the Akt-mTOR and activation of the AMPK pathways [50]. More recently we examined the effects of rosemary extract (RE) [51,52,53,54,55], and RE polyphenols [56,57]. RE elicited inhibitory effects in prostate [54], breast [53] and lung cancer cells [55]. Recently, we discovered that the diterpene carnosic acid (CA), contained in RE, has significant inhibitory effect on lung cancer cell survival and proliferation [57]. Nevertheless, limited studies have examined the effects of CA in prostate cancer. Treatment of PC-3 and DU-145 androgen-insensitive prostate cancer cells with CA (6.25–100 µg/mL, 18.8–301 µM) resulted in a decrease in cell viability [58] and proliferation and induced both extrinsic and intrinsic apoptotic pathways in PC-3 cells [59]. Treatment of LNCaP, 22Rv1, PC-3 and DU145 cells with CA decreased cell viability, proliferation and induced apoptosis [60] and caused cell cycle arrest [61].
Although these limited studies have previously demonstrated the anticancer potential of CA, its effects on signaling cascades and, thus, its mechanism of action are currently unknown. In the present study, we investigated the effects of CA on prostate cancer cells. We hypothesized that CA would inhibit prostate cancer cell survival and proliferation while increasing AMPK signaling.
2. Materials and Methods
2.1. Materials
Human PC-3, LNCaP prostate cancer and PNT1A prostate epithelial cells were obtained from America Type Culture Collection (ATCC). Cell culture (RPMI) media, fetal bovine serum (FBS), trypsin, and antibiotic were from GIBCO (Burlington, ON, Canada). Antibodies that recognized total or phosphorylated (AMPK: cat. No. 5831, pAMPK (Thr172): cat. No. 2535, ACC cat. No. 3676S, pACC (Ser79) cat. No. 11881S, Akt cat. No. 9272S, pAkt (Ser473) cat. No. 9271S, mTOR Cat. No. 2972S, pmTOR Ser2448 Cat. No. 2971S, Raptor Cat. No. 2280S, pRaptor Ser792 Cat. No. 2083S, and p70 S6K Cat. No. 9202S, pp70 S6K Thr389 Cat. No. 9205S, Sestrin-2 cat. No. 8487, vinculin Cat. No. 4650S and β-actin cat. No. 4967 were purchased from Cell Signaling Technology via New England Biolabs (Mississauga, ON, Canada). Bovine serum albumin, dimethyl sulfoxide (DMSO), methylene blue and crystal violet stain, and docetaxel were purchased from Millipore Sigma (Oakville, ON, Canada). Carnosic acid was purchased from MedChemExpress in Monmouth Junction, NJ, USA.
2.2. Cell Culture and Treatment
PC-3, LNCaP and PNT1A cells were grown in RPMI media supplemented with 10% (v/v) FBS, and 1% (v/v) antibiotic-antimycotic solution in a humidified atmosphere of 37 °C at 5% CO2. Initial stock solutions of CA (100 mM) and DTX (10 mM) were prepared in DMSO followed by working solutions using cell culture media. The time of exposure and concentration of carnosic acid (CA), and docetaxel (DTX) are indicated in each figure.
2.3. Clonogenic Survival Assay
Clonogenic survival assays were performed as previously described [51]. Cells were seeded at a density of 1000 cells/well in a 6-well plate and were allowed to adhere overnight. Cells were incubated with indicated concentrations of CA for 7 days. Following 7 days of treatment, cells were washed twice and stained with 0.05% (w/v) methylene blue dye. The next day, the cells were counted and colonies with >50 cells were recorded.
2.4. Cell Proliferation Assay
The crystal violet cell proliferation assay was performed as described previously [62]. Cells were seeded (4000 cells/well) in sextuplicate in 96-well plates and treated with the indicated concentration of CA for 24 or 72 h. Cells were fixed with 10% formalin and stained using 0.5% crystal violet stain following a 24 or 72 h treatment. The plate was allowed to dry, and cells were solubilized, and the absorbance was measured at 570 nm KC4 plate reader (Bio-Tek, Winooski, VT, USA). The data are expressed as percent of control.
2.5. Immunoblotting
Immunoblotting was performed as previously described [62]. Cells were seeded and allowed to grow to 90% confluence. Following their treatment cells were washed with ice-cold PBS and then lysed with ice-cold lysis buffer. Lysates were collected and 5% β-mercaptoethanol containing SDS buffer was added and boiled for 5 min. 20 µg protein samples were separated using SDS-PAGE, transferred on PVDF membrane, and incubated with primary antibody buffer overnight at 4 °C and the following day the membranes were incubated with horse radish peroxidase (HRP)-linked IgG anti-rabbit secondary antibody for 1 h at room temperature before being visualized (using BioRad chemidoc imager). Signals were detected using Bio-Rad Clarity Western ECL Solution. Densitometric analysis was performed using Image J 1.54g software and expressed relative to the control group.
2.6. Statistical Analysis
All results are expressed as the mean of several individual experiments ± standard error of the mean (SEM). Significance testing was done using Graphpad Prism 9 software to perform analysis of variance (ANOVA). Significant ANOVA results were followed by Dunnett’s post hoc test. p-values less than 0.05 were considered significant.
3. Results
3.1. Carnosic Acid Inhibits Prostate Cancer Cell Survival
PC-3 prostate cancer cells were treated with 5, 10, 15, 30, or 60 µM of CA and the ability of cells to survive and form colonies was examined by performing a clonogenic survival assay [53]. Carnosic acid dose-dependently decreased cell survival. A significant (p < 0.0001) decrease in cell survival was seen at a concentration as low as 5 µM and complete inhibition of cell survival was seen at and above 30 µM (CA 5 µM: 51.6 ± 5.5%, p < 0.0001; CA 10 µM: 21.3 ± 4.9%, p < 0.0001; CA 15 µM: 11.6 ± 4.5% of control, p < 0.0001; Figure 1B). In addition, we used LNCaP prostate cancer cells and treated them with 2.5, 5, 10, 15, or 20 µM of CA. We found a dose-dependent decrease in cell survival with significant inhibition seen at a concentration as low as 2.5 µM and complete inhibition seen at 15 µM. (CA 2.5 µM: 61.9 ± 1.9%, p < 0.0001; CA 5 µM: 24.2 ± 1.9%, p < 0.0001; CA 10 µM: 2.8 ± 1.0% of control, p < 0.0001; Figure 1D).
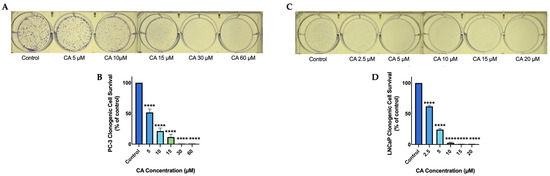
Figure 1.
CA dose-dependently Inhibits prostate cancer cell survival. PC-3 (A,B) and LNCaP (C,D) prostate cancer cells were treated without (Control) or with the indicated concentrations of carnosic acid (CA) for 7 days followed by staining with methylene blue and colony counting Representative images were taken using a 12 MP camera (A,C). Data are the mean ± SEM of 3–4 independent experiments. **** p < 0.0001.
3.2. Carnosic Acid Inhibits Prostate Cancer Cell Proliferation
Cells were treated with 5, 10, 20, 40, 60, 80, 100, 125, or 150 µM of CA and proliferation was assessed using the crystal violet assay. Following treatment with CA; PC-3, and LNCaP cell proliferation decreased in a dose-dependent manner with a calculated IC50 value of 64 and 21 µM, respectively. For PC-3 cells, CA concentrations ranging from 100–150 µM resulted in maximum inhibition of proliferation (CA 40 µM: 76.8 ± 6.7%, p < 0.001; CA 60 µM: 62.0 ± 5.1%, p < 0.0001; CA 80 µM: 32.2 ± 3.1%, p < 0.0001; CA 100 µM: 17.35 ± 1.9%, p < 0.0001; CA 125 µM: 13.9 ± 0.8%, p < 0.0001; CA 150 µM: 14.6 ± 1.0% of control, p < 0.0001; Figure 2A (blue trendline)). For LNCaP cells, CA concentrations ranging from 80–150 µM resulted in maximum inhibition of proliferation (CA 10 µM: 76.7 ± 4.4%, p < 0.01; CA 20 µM: 53.8 ± 6.1%, p < 0.001; CA 40 µM: 25.7 ± 2.3%, p < 0.001; CA 60 µM: 15.3 ± 2.1%, p < 0.0001; CA 80 µM: 9.8 ± 3.4%, p < 0.0001; CA 100 µM: 8.9 ± 3.6%, p < 0.0001; CA 125 µM: 9.4 ± 3.6%, p < 0.0001; CA 150 µM: 9.2 ± 3.7% of control, p < 0.0001; Figure 2A (red trendline)).
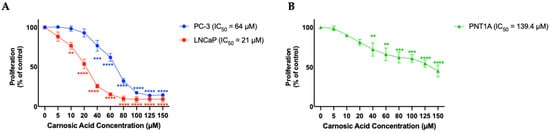
Figure 2.
Effects of CA on Prostate Cancer cells (A) and PNT1A epithelial cells (B). PC-3 and LNCaP prostate cancer cells (A) and PNT1A prostate epithelial cells (B) were treated without (Control) or with the indicated concentrations of carnosic acid (CA) followed by fixing with 10% formalin and stained with 0.5% crystal violet dye. Crystal violet was solubilized, and absorbance was read at 570 nm. The data are the mean ± SEM of 4–6 independent experiments.** p < 0.01, *** p < 0.001, **** p < 0.0001.
PNT1A cells, which represent normal prostate epithelium were treated with the same concentrations of CA for 24 h and found an inhibitory effect with an IC50 of 139.4 µM (Figure 2B), a much higher concentration compared to the IC50 for PC-3 and LNCaP prostate cancer cells.
3.3. Carnosic Acid Inhibits Akt Signaling
Once it was identified that survival and proliferation of PC-3 prostate cancer cells was inhibited by CA, Akt signalling was investigated since it plays a major role in protein synthesis, cell proliferation and survival [63]. Treatment of PC-3 cells with CA resulted in decreased phosphorylation of Akt Ser473 residue, an established marker of its activation [64]. A significant inhibition was seen after both 24 and 48 h of CA treatment (CA 24 h: 32.9 ± 16.9%, p < 0.05; CA 48 h: 5.5 ± 3.3% of control, p <0.001; Figure 3). Interestingly, treatment with docetaxel (DTX) (10 nM, 48 h) an established chemotherapeutic drug had no significant effect of Akt phosphorylation/activation.
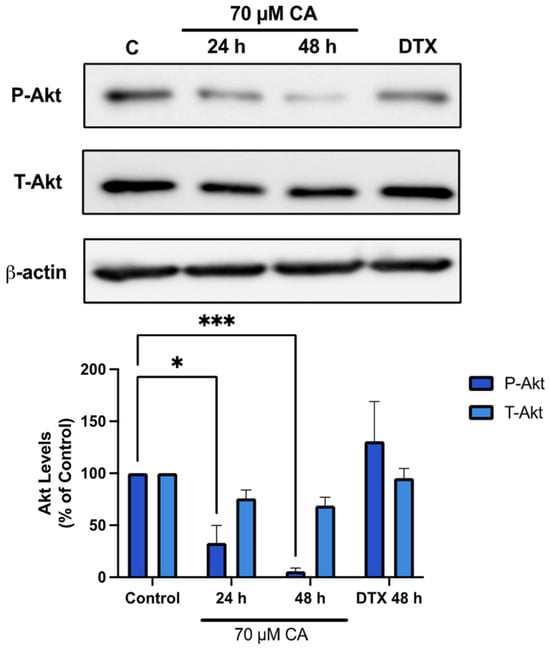
Figure 3.
Carnosic acid inhibits Akt. PC-3 cells were treated without (Control) or with the indicated concentrations of CA or DTX for 24 or 48 h followed by whole cell lysate preparation and total protein yield assessment. Lysates (20 µg of protein) were resolved by SDS-PAGE and immunoblotted with specific antibodies against total or phosphorylated Akt (Ser473) or β-actin. Arbitrary units were used to express densitometry of bands using ImageJ software and the data are expressed as percent of control. The data are the mean ± SEM of 4–6 independent experiments. * p < 0.05, *** p < 0.001.
3.4. Carnosic Acid Inhibits mTORC1-p70 S6K Signaling
mTOR and p70 S6K the downstream targets of Akt were also examined as they are key players in protein synthesis and cell proliferation signalling. Cells treated with CA showed a significant decrease in mTOR phosphorylation/activation (CA 24 h: 45.5 ± 10.3%, p < 0.01; CA 48 h: 32.0 ± 7.8% of control, p < 0.001; Figure 4A). The mTORC1 complex consists of mTOR and the regulatory protein, raptor, allowing it to interact with downstream targets [65]. Cells treated with CA showed a significant increase in raptor phosphorylation (CA 24 h: 617.7 ± 146.5%, p < 0.01; CA 48 h: 544.0 ± 106.2% of control, p < 0.01; Figure 4B). A decrease in p70 S6K phosphorylation/activation was also seen (CA 24 h: 24.9 ± 23.3%, p < 0.01; CA 48 h: 2.1 ± 1.2% of control, p < 0.001; Figure 4C).
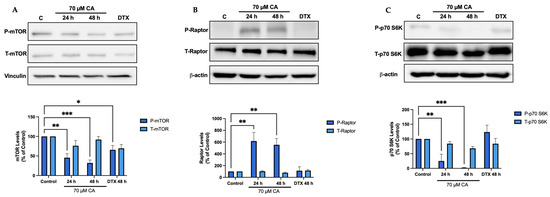
Figure 4.
Carnosic acid inhibits mTOR-p70 S6K signaling. (A) mTOR phosphorylation. (B) raptor phosphorylation. (C) p70 S6K phosphorylation. PC-3 cells were treated without (Control) or with the indicated concentrations of CA or DTX for 24 or 48 h followed by whole cell lysate preparation and total protein yield assessment. Lysates (20 µg of protein) were resolved by SDS-PAGE and immunoblotted with specific antibodies against total or phosphorylated mTOR (Ser2448), raptor (Ser972), p70 S6K (Thr389), vinculin or β-actin. Arbitrary units were used to express densitometry of bands using ImageJ software and the data are expressed as percent of control. The data are the mean ± SEM of 2–6 independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001.
3.5. Carnosic Acid Activates AMPK Signaling
Previous studies by our group found a robust phosphorylation/activation of AMPK by CA in muscle [66], fat [67] and lung cancer cells [57] and since AMPK plays a key role in energy homeostasis [68] and its activation may lead to anticancer effects [69,70], we examined the effects of CA on prostate cancer cell AMPK and its downstream target ACC. Treatment of PC-3 cells with CA significantly increased AMPK phosphorylation/activation (CA 24 h: 816.2 ± 167.0%, p < 0.01; CA 48 h: 1997.9 ± 314.9% of control, p < 0.0001; Figure 5A) and phosphorylation of ACC (CA 24 h: 806.7 ± 217.8%, p < 0.01; CA 48 h: 936.3 ± 269.3% of control, p < 0.001; Figure 5B).
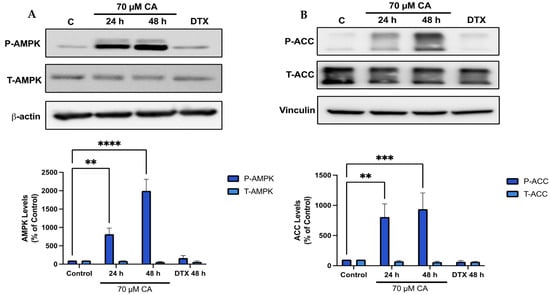
Figure 5.
Carnosic acid activates AMPK. PC-3 cells were treated without (Control) or with the indicated concentrations of CA or DTX for 24 or 48 h followed by whole cell lysate preparation and total protein yield assessment. Lysates (20 µg of protein) were resolved by SDS-PAGE and immunoblotted with specific antibodies against total or phosphorylated AMPK (Thr172), ACC (Ser79), β-actin or vinculin. (A) AMPK phosphorylation/activation. (B) ACC phosphorylation/inhibition. Arbitrary units were used to express densitometry of bands using ImageJ software and the data are expressed as percent of control. The data are the mean ± SEM of 4–6 independent experiments. ** p < 0.01, *** p < 0.001, **** p < 0.0001.
3.6. Carnosic Acid Activates Upstream Regulators of AMPK Signaling
In an attempt to understand the mechanism of AMPK activation by CA, we examined sestrin-2 which is known to have a direct effect of activating AMPK [71,72]. We found a significant increase in sestrin-2 levels with CA (CA 24 h: 162.7 ± 9.2%, p < 0.05; CA 48 h: 236.3 ± 38.27% of control, p < 0.001; Figure 6), whereas treatment with DTX had no effect on in Sestrin-2 levels.
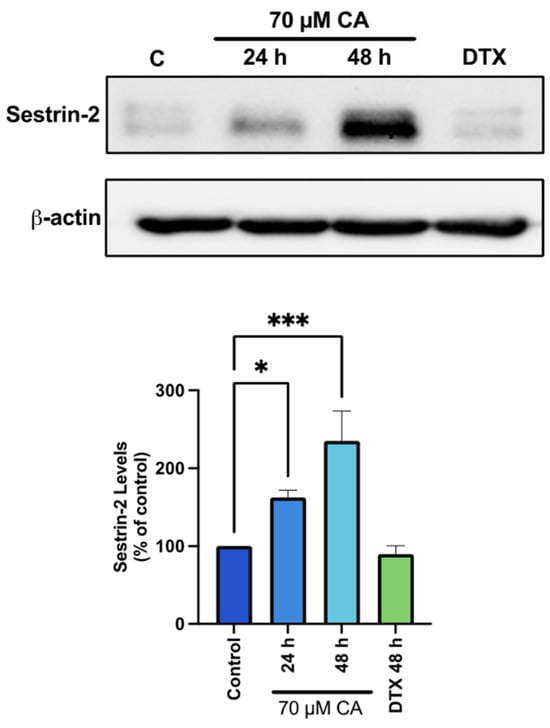
Figure 6.
Activation of sestrin-2 by carnosic acid. PC-3 cells were treated without (Control) or with the indicated concentrations of CA or DTX for 24 or 48 h followed by whole cell lysate preparation and total protein yield assessment. Lysates (20 µg of protein) were resolved by SDS-PAGE and immunoblotted with specific antibody against total Sestrin-2 or β-actin. Arbitrary units were used to express densitometry of bands using ImageJ software and the data are expressed as percent of control. The data are the mean ± SEM of 3–5 independent experiments. * p < 0.05, *** p < 0.001.
3.7. AMPK Activation; Akt and mTOR Inhibition Mimic the Effects of Carnosic Acid
We used the established AMPK activator, 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) (500 µM), the Akt inhibitor, wortmannin (1 µM), and the mTOR inhibitor, rapamycin (200 nM) and examined and compared their effects to the effects of CA treatment. We found significant inhibition of PC-3 prostate cancer cell proliferation with these treatments that were comparable to those achieved with CA treatment (Figure 7).
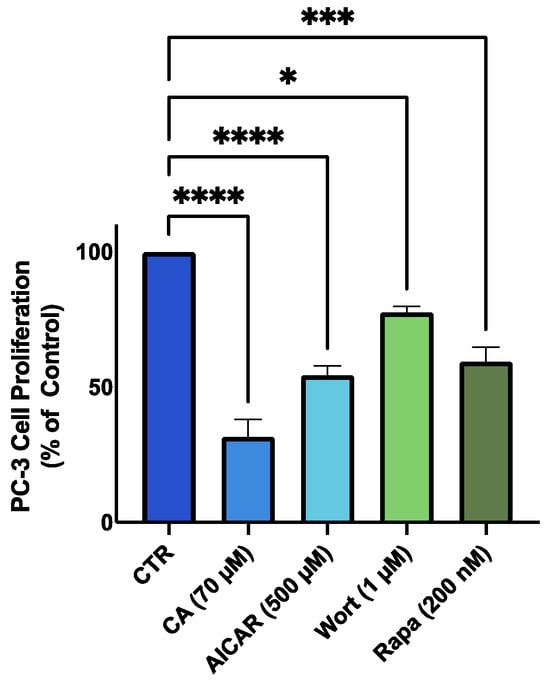
Figure 7.
Effects of CA, AICAR, wortmannin and rapamycin on PC-3 prostate cancer cells. PC-3 cells were treated without (Control) or with 70 µM of carnosic acid (CA), 500 µM 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) (AMPK activator), 1 µM wortmannin (Wort) (Akt inhibitor), or 200 nM rapamycin (Rapa) (mTOR inhibitor) for 48 h followed by fixing with 10% formalin and stained with 0.5% crystal violet dye. Crystal violet was solubilized, and absorbance was read at 570 nm. The data are the mean ± SEM of 2–3 independent experiments. * p < 0.05, *** p < 0.001, **** p < 0.0001.
4. Discussion
In previous studies from our lab we found that rosemary extract (RE) inhibited survival and proliferation of prostate [54], breast [53] and lung cancer cells [55]. In recent studies we focused on the diterpene CA, contained in RE and found significant inhibition of lung cancer cell survival and proliferation [57]. In the present study, we examined the effects of CA on prostate cancer cells. Our data show that treatment of PC-3 prostate cancer cells with CA resulted in inhibition of survival and proliferation. These data are in agreement with the findings of other studies by Yesil-Celiktas et al. [58], Kar et al. [59], Petiwala et al. [60], and Ossikbayeva et al. [61] showing a significant decrease in proliferation and survival of PC-3 and DU-145 prostate cancer cells with CA treatment.
Additionally, we examined the androgen sensitive, LNCaP prostate cancer cells and found significant inhibitory effects in agreement with findings by Petiwala et al. [60], while LNCaP cells lack the PTEN tumor suppressor, they are androgen responsive and therefore susceptible to cancer treatments such as androgen deprivation therapy [73]. Here we show that LNCaP cells are more susceptible to CA treatment with a lower IC50 compared to PC-3 cells. Petiwala et al. [60] found that treatment of LNCaP cells with CA resulted in significant androgen receptor (AR) degradation, and therefore this inhibitory effect on AR signaling may explain why LNCaP cells are more suspectable to CA treatment compared to the androgen-independent PC-3 cells. On the other hand, PNT1A cells, which have no known mutations and represent normal prostate epithelium were seen to have an IC50 2-fold and 6-fold higher than PC-3 and LNCaP cells, respectively. These data indicate that CA concentrations that cause major inhibitory effects in prostate cancer cells have little toxicity to normal cells. Although, in vivo animal studies are required to examine the effects of CA in normal/healthy tissues, the present data suggest that CA may preferentially target cancer cells while sparing normal/healthy cells.
The PC-3 prostate cancer cells are androgen independent, lack the tumor suppressor, p53 [74], and the tumor suppressor (PTEN) leading to over activation of the PI3K-Akt cascade resulting in enhanced cell survival and proliferation [75,76,77]. Our studies show a decrease in phosphorylation/activation of Akt in PC-3 cells with CA treatment. Our findings are in agreement with the data by Kar et al. [59], who found inhibition of Akt phosphorylation, paired with increased phosphatase 2A (PP2A) activity in PC-3 cells treated with CA [59]. Whether the inhibition of Akt, seen in our study, is due to increased PP2A activity remains to be examined in future experiments. Similar to our data, carnosic acid has been shown to inhibit Akt phosphorylation/activation in hepatoma [78], lung cancer [79] and gastric cancer cells [80]. Studies have shown that Akt is elevated in approximately 70–100% of advanced cases of prostate cancer [81,82] and therefore chemicals such as CA that target Akt may hold a significant therapeutic potential.
Activated Akt leads to downstream phosphorylation and activation of mTOR and p p70 S6K resulting in increased protein synthesis and cell proliferation [83]. Our study is the first to show a decrease in phosphorylation/activation of mTOR and p70 S6K with CA treatment in PC-3 prostate cancer cells. While no other studies have examined the effects of CA treatment on prostate cancer cell mTOR and p70 S6K, CA inhibited mTOR in hepatoma [78], lung cancer [79] and gastric cancer cells [80]. In PC-3 prostate cancer cells mTOR and p70 S6K was inhibited with RE [58] and carnosol (COH) [84] treatment.
Furthermore, in the present study, we saw a robust phosphorylation of AMPK on Thr172, an established marker of its activation [38] with CA treatment. Although, we did not measure AMPK activity directly, the robust phosphorylation of acetyl-CoA carboxylase (ACC), a downstream target of AMPK, confirms AMPK activation. Phosphorylation of ACC by AMPK inhibits its activity resulting in inhibition of fatty acid synthesis and promotion of fatty acid oxidation [36,37]. Fatty acid synthesis is very important in sustaining cancer cell bioenergetic requirements and proliferation, and based on evidence of the key role of ACC in the regulation of fatty acid synthesis, and its overexpression in cancer cells, ACC has emerged as an attractive target for cancer treatment [85,86,87]. The inhibition of ACC, seen in our study, may contribute to inhibition of prostate cancer cell proliferation and survival.
As mentioned above, activation of Akt leads to activation of mTOR-p70 S6K signaling whereas inhibition of Akt leads to the inhibition of mTORC1-p70 S6K [23,24]. On the other hand, activation of AMPK phosphorylates/inhibits raptor, the regulatory protein associated with mTOR in the mTORC1 complex, resulting in mTOR inhibition [88]. In the present study, we found increased phosphorylation of raptor suggesting inhibition of the mTORC1 complex with CA treatment. Therefore, the inhibition of mTORC1-p70 S6K signaling, seen with CA treatment in the present study, could be due to both inhibition of Akt and activation of AMPK. These data are in agreement with previous studies, that found significant increase in AMPK phosphorylation/activation associated with significant phosphorylation of raptor and inhibition of p70 S6K signaling in H1299 lung cancer cells with RE treatment [55].
To elucidate how CA causes phosphorylation/activation of AMPK, we examined its upstream regulator, sestrin-2. Sestrin-2 acts as a scaffold to facilitate the interaction between AMPK and its upstream kinase and tumor suppressor LKB1 [89,90,91]. This is the first study to show that CA treatment increases the levels of sestrin-2 in PC-3 prostate cancer cells. However, these data are well-aligned with our previous findings in lung cancer cells showing increased expression of sestrin-2 with CA treatment [57]. One study has shown that overexpression of sestrin-2 in human prostate cancer PC-3 cells significantly reduced their proliferation and sensitized them to radiation treatment [92]. It should be noted that sestrin-2 expression varies among different types of cancer and both a tumor suppressor and a tumor promoter role has been indicated in different studies [93]. Sestrin-2 expression could be induced by DNA damage in a p53-dependent manner or by increased ROS levels in a p53-independent mechanism [44,94]. PC-3 cells lack the tumor suppressor p53 and have low basal levels of sestrin-2. It is possible that CA exhibits antitumor effects in PC-3 cells (lacking p53 expression) by increasing sestrin-2 levels independent of p53, similar to the effects seen in H1299 lung cancer cells [57] and to the effects seen with quercetin treatment of HCT116 and HT-29 colon cancer cells [95]. Treatment of HCT116 and HT-29 colon cancer cells with quercetin increased sestrin-2 expression and induced apoptosis by a mechanism that was p53-independent but involved increased ROS levels [95].
AMPK may be activated directly by sestrin-2 [71,72], or by sestrin-2-LKB1 dependent mechanism [89]. LKB1 levels are significantly lower in prostate cancer tissue compared to healthy prostate tissue, and PC-3 prostate cancer cells express low levels of LKB1 [96]. Although we did not examine the effects of CA on LKB1, it is possible that CA increases LKB1 levels and/or activity as seen in lung cancer cells [57]. AMPK activation is implicated in inhibition of protein synthesis [35], induction of autophagy [97] and may regulate apoptosis [98] overall leading to anticancer effects [30,31,32,33].
The use of the known AMPK activator, AICAR resulted in a significant decrease in PC-3 cell proliferation in agreement with studies by others showing inhibition of prostate cancer cell proliferation by AICAR [99,100]. Furthermore, the use of wortmannin and rapamycin resulted in similar inhibition of prostate cancer proliferation as CA. It has been shown that inhibitors of Akt and mTOR significantly reduce proliferation of prostate cancer cells [101,102,103]. Currently Akt (MK2206) and mTOR (Temsirolimus) inhibitors are being evaluated in clinical trials for patients with various forms of cancer including prostate cancer (NCT01480154, 00919035). Our findings show that CA is able to inhibit both Akt and mTOR and have effects similar to clinically evaluated inhibitors of these pathways, providing strong rationale for continuing the examination of the effects of CA in prostate cancer.
Overall, our data show a significant decrease in prostate cancer cell survival and proliferation in association with inhibition of Akt-mTOR-p70 S6K and activation of sestrin-2-AMPK signaling with CA treatment (Figure 8).
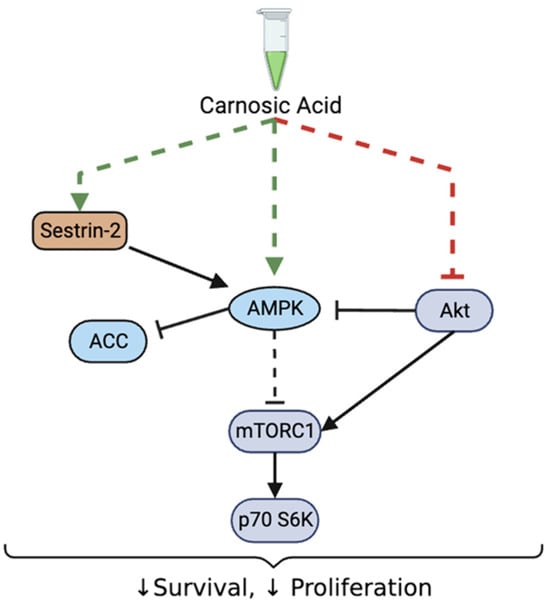
Figure 8.
Summary of the effects of CA on prostate cancer cells. Carnosic acid decreased survival and proliferation of prostate cancer cells. These effects were associated with decreased Akt, mTOR and p70 S6K, increased total sestrin-2 levels and increased phosphorylation of raptor, AMPK, and ACC.
5. Conclusions
In the present study, prostate cancer cells treated with CA showed a significant decrease in survival and proliferation associated with decreased levels of phosphorylated/activated Akt and increased levels of phosphorylated/activated AMPK. Downstream of AMPK, CA inhibited mTORC1-p70 S6K and ACC signaling. Future studies should aim to examine the effects of CA in vivo utilizing animals xenografted with prostate cancer cells.
Author Contributions
Conceptualization, M.N. and E.T.; methodology, M.N.; software, M.N.; formal analysis, M.N.; investigation, M.N.; resources, E.T.; data curation, M.N. and E.T.; writing—original draft preparation, M.N.; writing—review and editing, M.N., N.S.K.S., V.A.F. and E.T.; supervision, E.T.; project administration, E.T.; funding acquisition, E.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the Canadian Prostate Cancer Fight Foundation/Ride For Dad (PCFF/RFD) grant. M.N. was supported by an Ontario Graduate Scholarship (OGS).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; La Vecchia, C. Epidemiology and Prevention of Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.D. Epidemiology of Prostate Cancer. Urology 2003, 62, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Ahmadiyeh, N.; Pomerantz, M.M.; Grisanzio, C.; Herman, P.; Jia, L.; Almendro, V.; He, H.H.; Brown, M.; Liu, X.S.; Davis, M.; et al. 8q24 Prostate, Breast, and Colon Cancer Risk Loci Show Tissue-Specific Long-Range Interaction with MYC. Proc. Natl. Acad. Sci. USA 2010, 107, 9742–9746. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Eeles, R. Germline Genetic Profiling in Prostate Cancer: Latest Developments and Potential Clinical Applications. Future Sci. OA 2015, 2, FSO87. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Van Allen, E.M.; Wu, Y.-M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.-M.; Montgomery, B.; Taplin, M.-E.; Pritchard, C.C.; Attard, G.; et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Abeshouse, A.; Ahn, J.; Akbani, R.; Ally, A.; Amin, S.; Andry, C.D.; Annala, M.; Aprikian, A.; Armenia, J.; Arora, A.; et al. The Molecular Taxonomy of Primary Prostate Cancer. Cell 2015, 163, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Horn, G.; Moulton, K.; Oza, A.; Byler, S.; Kokolus, S.; Longacre, M. Cancer Development, Progression, and Therapy: An Epigenetic Overview. Int. J. Mol. Sci. 2013, 14, 21087–21113. [Google Scholar] [CrossRef]
- Liao, R.S.; Ma, S.; Miao, L.; Li, R.; Yin, Y.; Raj, G.V. Androgen Receptor-Mediated Non-Genomic Regulation of Prostate Cancer Cell Proliferation. Transl. Androl. Urol. 2013, 2, 187. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the Phosphoinositide 3-Kinase Pathway in Cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Khan, K.H.; Yap, T.A.; Yan, L.; Cunningham, D. Targeting the PI3K-AKT-mTOR Signaling Network in Cancer. Chin. J. Cancer 2013, 32, 253–265. [Google Scholar] [CrossRef]
- Nitulescu, G.M.; Van De Venter, M.; Nitulescu, G.; Ungurianu, A.; Juzenas, P.; Peng, Q.; Olaru, O.T.; Grădinaru, D.; Tsatsakis, A.; Tsoukalas, D.; et al. The Akt Pathway in Oncology Therapy and beyond (Review). Int. J. Oncol. 2018, 53, 2319–2331. [Google Scholar] [CrossRef]
- Porta, C.; Paglino, C.; Mosca, A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front. Oncol. 2014, 4, 64. [Google Scholar] [CrossRef]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate Cancer. Nat. Rev. Dis. Primer 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Labbé, D.P.; Brown, M. Transcriptional Regulation in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a030437. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Saltzman, A.; Yeh, S.; Young, W.; Keller, E.; Lee, H.J.; Wang, C.; Mizokami, A. Androgen Receptor: An Overview. Crit. Rev. Eukaryot. Gene Expr. 1995, 5, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar] [PubMed]
- Eder, I.E.; Culig, Z.; Putz, T.; Nessler-Menardi, C.; Bartsch, G.; Klocker, H. Molecular Biology of the Androgen Receptor: From Molecular Understanding to the Clinic. Eur. Urol. 2001, 40, 241–251. [Google Scholar] [CrossRef]
- Modi, P.K.; Faiena, I.; Kim, I.Y. Chapter 3—Androgen Receptor. In Prostate Cancer, 2nd ed.; Mydlo, J.H., Godec, C.J., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 21–28. ISBN 978-0-12-800077-9. [Google Scholar]
- Tyagi, V.; Scordo, M.; Yoon, R.S.; Liporace, F.A.; Greene, L.W. Revisiting the Role of Testosterone: Are We Missing Something? Rev. Urol. 2017, 19, 16–24. [Google Scholar] [CrossRef]
- Fujita, K.; Nonomura, N. Role of Androgen Receptor in Prostate Cancer: A Review. World J. Mens Health 2019, 37, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Guertin, D.A.; Sabatini, D.M. Defining the Role of mTOR in Cancer. Cancer Cell 2007, 12, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Pópulo, H.; Lopes, J.M.; Soares, P. The mTOR Signalling Pathway in Human Cancer. Int. J. Mol. Sci. 2012, 13, 1886–1918. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Huang, J. Phosphatidylinositol 3-Kinase-AKT-Mammalian Target of Rapamycin Pathway Is Essential for Neuroendocrine Differentiation of Prostate Cancer. J. Biol. Chem. 2007, 282, 3571–3583. [Google Scholar] [CrossRef] [PubMed]
- Toren, P.; Zoubeidi, A. Targeting the PI3K/Akt Pathway in Prostate Cancer: Challenges and Opportunities (Review). Int. J. Oncol. 2014, 45, 1793–1801. [Google Scholar] [CrossRef]
- Roudsari, N.M.; Lashgari, N.-A.; Momtaz, S.; Abaft, S.; Jamali, F.; Safaiepour, P.; Narimisa, K.; Jackson, G.; Bishayee, A.; Rezaei, N.; et al. Inhibitors of the PI3K/Akt/mTOR Pathway in Prostate Cancer Chemoprevention and Intervention. Pharmaceutics 2021, 13, 1195. [Google Scholar] [CrossRef] [PubMed]
- Shorning, B.Y.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.M.; Bieberich, C.J.; Dang, C.V.; Nelson, W.G.; Yegnasubramanian, S.; De Marzo, A.M. MYC and Prostate Cancer. Genes Cancer 2010, 1, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Saud, S.M.; Young, M.R.; Chen, G.; Hua, B. Targeting AMPK for Cancer Prevention and Treatment. Oncotarget 2015, 6, 7365–7378. [Google Scholar] [CrossRef]
- Carling, D. AMPK Signalling in Health and Disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef]
- Umezawa, S.; Higurashi, T.; Nakajima, A. AMPK: Therapeutic Target for Diabetes and Cancer Prevention. Curr. Pharm. Des. 2017, 23, 3629–3644. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Hardie, D.G. New Insights into Activation and Function of the AMPK. Nat. Rev. Mol. Cell Biol. 2023, 24, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Carling, D. The AMP-Activated Protein Kinase Cascade—A Unifying System for Energy Control. Trends Biochem. Sci. 2004, 29, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK Phosphorylation of Raptor Mediates a Metabolic Checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Carling, D.; Zammit, V.A.; Hardie, D.G. A Common Bicyclic Protein Kinase Cascade Inactivates the Regulatory Enzymes of Fatty Acid and Cholesterol Biosynthesis. FEBS Lett. 1987, 223, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Goldstein, J.L.; Brown, M.S. Replacement of Serine-871 of Hamster 3-Hydroxy-3-Methylglutaryl-CoA Reductase Prevents Phosphorylation by AMP-Activated Kinase and Blocks Inhibition of Sterol Synthesis Induced by ATP Depletion. Proc. Natl. Acad. Sci. USA 1993, 90, 9261–9265. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Davison, M.; Woods, A.; Davies, S.P.; Beri, R.K.; Carling, D.; Hardie, D.G. Characterization of the AMP-Activated Protein Kinase Kinase from Rat Liver and Identification of Threonine 172 as the Major Site at Which It Phosphorylates AMP-Activated Protein Kinase. J. Biol. Chem. 1996, 271, 27879–27887. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Martín, G.; Høyer-Hansen, M.; García-García, C.; Fumarola, C.; Farkas, T.; López-Rivas, A.; Jäättelä, M. TAK1 Activates AMPK-Dependent Cytoprotective Autophagy in TRAIL-Treated Epithelial Cells. EMBO J. 2009, 28, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Johnstone, S.R.; Dickerson, K.; Leiper, F.C.; Fryer, L.G.D.; Neumann, D.; Schlattner, U.; Wallimann, T.; Carlson, M.; Carling, D. LKB1 Is the Upstream Kinase in the AMP-Activated Protein Kinase Cascade. Curr. Biol. 2003, 13, 2004–2008. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, D.; Dyck, J.R.B.; Li, Y.; Zhang, H.; Morishima, M.; Mann, D.L.; Taffet, G.E.; Baldini, A.; Khoury, D.S.; et al. A Pivotal Role for Endogenous TGF-Beta-Activated Kinase-1 in the LKB1/AMP-Activated Protein Kinase Energy-Sensor Pathway. Proc. Natl. Acad. Sci. USA 2006, 103, 17378–17383. [Google Scholar] [CrossRef]
- Gong, L.; Wang, Z.; Wang, Z.; Zhang, Z. Sestrin2 as a Potential Target for Regulating Metabolic-Related Diseases. Front. Endocrinol. 2021, 12, 751020. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, S.; Jayachandran, I.; Balasubramanyam, M.; Mohan, V.; Venkatesan, B.; Manickam, N. Sestrin2 Regulates Monocyte Activation through AMPK-mTOR Nexus under High-Glucose and Dyslipidemic Conditions. J. Cell. Biochem. 2019, 120, 8201–8213. [Google Scholar] [CrossRef] [PubMed]
- Budanov, A.V.; Karin, M. p53 Target Genes Sestrin1 and Sestrin2 Connect Genotoxic Stress and mTOR Signaling. Cell 2008, 134, 451–460, Erratum in Cell 2009, 136, 378. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wightman, E.L. Herbal Extracts and Phytochemicals: Plant Secondary Metabolites and the Enhancement of Human Brain Function. Adv. Nutr. 2011, 2, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.A. How Taxol/Paclitaxel Kills Cancer Cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Van Oosterom, A.T.; Schrijvers, D. Docetaxel (Taxotere), a Review of Preclinical and Clinical Experience. Part II: Clinical Experience. Anticancer. Drugs 1995, 6, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Abal, M.; Andreu, J.M.; Barasoain, I. Taxanes: Microtubule and Centrosome Targets, and Cell Cycle Dependent Mechanisms of Action. Curr. Cancer Drug Targets 2003, 3, 193–203. [Google Scholar] [CrossRef]
- Pienta, K.J. Preclinical Mechanisms of Action of Docetaxel and Docetaxel Combinations in Prostate Cancer. Semin. Oncol. 2001, 28, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Rashid, A.; Liu, C.; Sanli, T.; Tsiani, E.; Singh, G.; Bristow, R.G.; Dayes, I.; Lukka, H.; Wright, J.; Tsakiridis, T. Resveratrol Enhances Prostate Cancer Cell Response to Ionizing Radiation. Modulation of the AMPK, Akt and mTOR Pathways. Radiat. Oncol. 2011, 6, 144. [Google Scholar] [CrossRef]
- Moore, J.; Megaly, M.; MacNeil, A.J.; Klentrou, P.; Tsiani, E. Rosemary Extract Reduces Akt/mTOR/p70S6K Activation and Inhibits Proliferation and Survival of A549 Human Lung Cancer Cells. Biomed. Pharmacother. 2016, 83, 725–732. [Google Scholar] [CrossRef]
- Moore, J.; Yousef, M.; Tsiani, E. Anticancer Effects of Rosemary (Rosmarinus officinalis L.) Extract and Rosemary Extract Polyphenols. Nutrients 2016, 8, 731. [Google Scholar] [CrossRef] [PubMed]
- Jaglanian, A.; Tsiani, E. Rosemary Extract Inhibits Proliferation, Survival, Akt, and mTOR Signaling in Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 810. [Google Scholar] [CrossRef] [PubMed]
- Jaglanian, A.; Termini, D.; Tsiani, E. Rosemary (Rosmarinus officinalis L.) Extract Inhibits Prostate Cancer Cell Proliferation and Survival by Targeting Akt and mTOR. Biomed. Pharmacother. 2020, 131, 110717. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.J.; Moore, J.; Song, J.; Tsiani, E.L. Inhibition of Non-Small Cell Lung Cancer Proliferation and Survival by Rosemary Extract Is Associated with Activation of ERK and AMPK. Life 2021, 12, 52. [Google Scholar] [CrossRef]
- O’Neill, E.J.; Hartogh, D.J.D.; Azizi, K.; Tsiani, E. Anticancer Properties of Carnosol: A Summary of in Vitro and In Vivo Evidence. Antioxidants 2020, 9, 961. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.J.; Sze, N.S.K.; MacPherson, R.E.K.; Tsiani, E. Carnosic Acid against Lung Cancer: Induction of Autophagy and Activation of Sestrin-2/LKB1/AMPK Signalling. Int. J. Mol. Sci. 2024, 25, 1950. [Google Scholar] [CrossRef] [PubMed]
- Yesil-Celiktas, O.; Sevimli, C.; Bedir, E.; Vardar-Sukan, F. Inhibitory Effects of Rosemary Extracts, Carnosic Acid and Rosmarinic Acid on the Growth of Various Human Cancer Cell Lines. Plant Foods Hum. Nutr. Dordr. Neth. 2010, 65, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Palit, S.; Ball, W.B.; Das, P.K. Carnosic Acid Modulates Akt/IKK/NF-κB Signaling by PP2A and Induces Intrinsic and Extrinsic Pathway Mediated Apoptosis in Human Prostate Carcinoma PC-3 Cells. Apoptosis 2012, 17, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Petiwala, S.M.; Li, G.; Bosland, M.C.; Lantvit, D.D.; Petukhov, P.A.; Johnson, J.J. Carnosic Acid Promotes Degradation of the Androgen Receptor and Is Regulated by the Unfolded Protein Response Pathway in Vitro and in Vivo. Carcinogenesis 2016, 37, 827–838. [Google Scholar] [CrossRef]
- Ossikbayeva, S.; Khanin, M.; Sharoni, Y.; Trachtenberg, A.; Tuleukhanov, S.; Sensenig, R.; Rom, S.; Danilenko, M.; Orynbayeva, Z. Curcumin and Carnosic Acid Cooperate to Inhibit Proliferation and Alter Mitochondrial Function of Metastatic Prostate Cancer Cells. Antioxidants 2021, 10, 1591. [Google Scholar] [CrossRef]
- Moore, J.; Pickering, G.; Gaudette, N.J.; Tsiani, E. Resveratrol-Fortification of Red Wine Does Not Provide Greater Inhibition of Human Lung Cancer Cell Survival Compared to Non-Fortified Wine. J. Mol. Biochem. 2015, 4, 52–62. [Google Scholar]
- Shaw, R.J.; Cantley, L.C. Ras, PI(3)K and mTOR Signalling Controls Tumour Cell Growth. Nature 2006, 441, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and Regulation of Akt/PKB by the Rictor-mTOR Complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Hara, K.; Maruki, Y.; Long, X.; Yoshino, K.; Oshiro, N.; Hidayat, S.; Tokunaga, C.; Avruch, J.; Yonezawa, K. Raptor, a Binding Partner of Target of Rapamycin (TOR), Mediates TOR Action. Cell 2002, 110, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Naimi, M.; Vlavcheski, F.; Murphy, B.; Hudlicky, T.; Tsiani, E. Carnosic Acid as a Component of Rosemary Extract Stimulates Skeletal Muscle Cell Glucose Uptake via AMPK Activation. Clin. Exp. Pharmacol. Physiol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Vlavcheski, F.; Giacca, A.; MacPherson, R.E.K.; Tsiani, E. Carnosic Acid Attenuates the Free Fatty Acid-Induced Insulin Resistance in Muscle Cells and Adipocytes. Cells 2022, 11, 167. [Google Scholar] [CrossRef]
- Hardie, D.G. The AMP-Activated Protein Kinase Pathway—New Players Upstream and Downstream. J. Cell Sci. 2004, 117, 5479–5487. [Google Scholar] [CrossRef] [PubMed]
- Shackelford, D.B.; Shaw, R.J. The LKB1-AMPK Pathway: Metabolism and Growth Control in Tumour Suppression. Nat. Rev. Cancer 2009, 9, 563–575. [Google Scholar] [CrossRef]
- Khan, A.S.; Frigo, D.E. A Spatiotemporal Hypothesis for the Regulation, Role, and Targeting of AMPK in Prostate Cancer. Nat. Rev. Urol. 2017, 14, 164–180. [Google Scholar] [CrossRef]
- Kim, G.T.; Lee, S.H.; Kim, Y.M. Quercetin Regulates Sestrin 2-AMPK-mTOR Signaling Pathway and Induces Apoptosis via Increased Intracellular ROS in HCT116 Colon Cancer Cells. J. Cancer Prev. 2013, 18, 264–270. [Google Scholar] [CrossRef]
- Seo, K.; Ki, S.H.; Park, E.Y.; Shin, S.M. 5-Fluorouracil Inhibits Cell Migration by Induction of Sestrin2 in Colon Cancer Cells. Arch. Pharm. Res. 2017, 40, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.G.; Bort, A.; Vara-Ciruelos, D.; Díaz-Laviada, I. Androgen Deprivation Induces Reprogramming of Prostate Cancer Cells to Stem-Like Cells. Cells 2020, 9, 1441. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Crumbaker, M.; Khoja, L.; Joshua, A.M. AR Signaling and the PI3K Pathway in Prostate Cancer. Cancers 2017, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.K.; Sellers, W.R. Akt-Regulated Pathways in Prostate Cancer. Oncogene 2005, 24, 7465–7474. [Google Scholar] [CrossRef] [PubMed]
- Keniry, M.; Parsons, R. The Role of PTEN Signaling Perturbations in Cancer and in Targeted Therapy. Oncogene 2008, 27, 5477–5485. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Liu, H.; Yao, Y.; Geng, L.; Zhang, X.; Jiang, L.; Shi, B.; Yang, F. Carnosic Acid Induces Autophagic Cell Death through Inhibition of the Akt/mTOR Pathway in Human Hepatoma Cells. J. Appl. Toxicol. 2015, 35, 485–492. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, J.; Fan, Y.; Li, Y. Antiproliferative Activity of Carnosic Acid Is Mediated via Inhibition of Cell Migration and Invasion, and Suppression of Phosphatidylinositol 3-Kinases (PI3K)/AKT/Mammalian Target of Rapamycin (mTOR) Signaling Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 7864–7871. [Google Scholar] [CrossRef]
- El-Huneidi, W.; Bajbouj, K.; Muhammad, J.S.; Vinod, A.; Shafarin, J.; Khoder, G.; Saleh, M.A.; Taneera, J.; Abu-Gharbieh, E. Carnosic Acid Induces Apoptosis and Inhibits Akt/mTOR Signaling in Human Gastric Cancer Cell Lines. Pharmaceuticals 2021, 14, 230. [Google Scholar] [CrossRef]
- Sun, M.; Wang, G.; Paciga, J.E.; Feldman, R.I.; Yuan, Z.-Q.; Ma, X.-L.; Shelley, S.A.; Jove, R.; Tsichlis, P.N.; Nicosia, S.V.; et al. AKT1/PKBα Kinase Is Frequently Elevated in Human Cancers and Its Constitutive Activation Is Required for Oncogenic Transformation in NIH3T3 Cells. Am. J. Pathol. 2001, 159, 431–437. [Google Scholar] [CrossRef]
- Carver, B.S.; Chapinski, C.; Wongvipat, J.; Hieronymus, H.; Chen, Y.; Chandarlapaty, S.; Arora, V.K.; Le, C.; Koutcher, J.; Scher, H.; et al. Reciprocal Feedback Regulation of PI3K and Androgen Receptor Signaling in PTEN-Deficient Prostate Cancer. Cancer Cell 2011, 19, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Guan, K.-L. mTOR as a Central Hub of Nutrient Signalling and Cell Growth. Nat. Cell Biol. 2019, 21, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J.; Syed, D.N.; Heren, C.R.; Suh, Y.; Adhami, V.M.; Mukhtar, H. Carnosol, a Dietary Diterpene, Displays Growth Inhibitory Effects in Human Prostate Cancer PC3 Cells Leading to G2-Phase Cell Cycle Arrest and Targets the 5’-AMP-Activated Protein Kinase (AMPK) Pathway. Pharm. Res. 2008, 25, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Nie, Q.; Wang, Z.; Di, Y.; Chen, X.; Ren, K. Targeting Acetyl-CoA Carboxylase 1 for Cancer Therapy. Front. Pharmacol. 2023, 14, 1129010. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, J.; Zhang, N.; Yang, Q.; Jin, Y.; Wang, Y. The Acetyl-CoA Carboxylase Enzyme: A Target for Cancer Therapy? Expert Rev. Anticancer Ther. 2015, 15, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, T. Recent Development in Acetyl-CoA Carboxylase Inhibitors and Their Potential as Novel Drugs. Future Med. Chem. 2020, 12, 533–561. [Google Scholar] [CrossRef]
- Agarwal, S.; Bell, C.M.; Rothbart, S.B.; Moran, R.G. AMP-Activated Protein Kinase (AMPK) Control of mTORC1 Is P53- and TSC2-Independent in Pemetrexed-Treated Carcinoma Cells. J. Biol. Chem. 2015, 290, 27473–27486. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.; Chen, L.; Wang, J.; Zhang, M.; Yang, H.; Ma, Y.; Budanov, A.; Lee, J.H.; Karin, M.; Li, J. Sestrin2 Promotes LKB1-Mediated AMPK Activation in the Ischemic Heart. FASEB J. 2015, 29, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Shao, H.; Liu, S.; Niu, Y.; Fu, L. Globular Adiponectin Ameliorates Insulin Resistance in Skeletal Muscle by Enhancing the LKB1-Mediated AMPK Activation via SESN2. Sports Med. Health Sci. 2023, 5, 34–41. [Google Scholar] [CrossRef]
- Quan, N.; Sun, W.; Wang, L.; Chen, X.; Bogan, J.S.; Zhou, X.; Cates, C.; Liu, Q.; Zheng, Y.; Li, J. Sestrin2 Prevents Age-Related Intolerance to Ischemia and Reperfusion Injury by Modulating Substrate Metabolism. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 4153–4167. [Google Scholar] [CrossRef]
- Fu, H.; Song, W.; Wang, Y.; Deng, W.; Tang, T.; Fan, W.; Qu, S. Radiosensitizing Effects of Sestrin2 in PC3 Prostate Cancer Cells. Iran. J. Basic Med. Sci. 2018, 21, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Luo, M.; Zhang, J.; Han, F.; Hou, N.; Pan, R.; Sun, X. A Paradoxical Role for Sestrin 2 Protein in Tumor Suppression and Tumorigenesis. Cancer Cell Int. 2021, 21, 606. [Google Scholar] [CrossRef]
- Ding, B.; Haidurov, A.; Chawla, A.; Parmigiani, A.; van de Kamp, G.; Dalina, A.; Yuan, F.; Lee, J.H.; Chumakov, P.M.; Grossman, S.R.; et al. P53-Inducible SESTRINs Might Play Opposite Roles in the Regulation of Early and Late Stages of Lung Carcinogenesis. Oncotarget 2019, 10, 6997–7009. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.T.; Lee, S.H.; Kim, J.I.; Kim, Y.M. Quercetin Regulates the Sestrin 2-AMPK-P38 MAPK Signaling Pathway and Induces Apoptosis by Increasing the Generation of Intracellular ROS in a P53-Independent Manner. Int. J. Mol. Med. 2014, 33, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Cai, F.; Liu, X.; Guo, L. LKB1 Suppresses Proliferation and Invasion of Prostate Cancer through Hedgehog Signaling Pathway. Int. J. Clin. Exp. Pathol. 2014, 7, 8480–8488. [Google Scholar]
- Di Bartolomeo, S.; Corazzari, M.; Nazio, F.; Oliverio, S.; Lisi, G.; Antonioli, M.; Pagliarini, V.; Matteoni, S.; Fuoco, C.; Giunta, L.; et al. The Dynamic Interaction of AMBRA1 with the Dynein Motor Complex Regulates Mammalian Autophagy. J. Cell Biol. 2010, 191, 155–168. [Google Scholar] [CrossRef]
- Grenier, A.; Poulain, L.; Mondesir, J.; Jacquel, A.; Bosc, C.; Stuani, L.; Mouche, S.; Larrue, C.; Sahal, A.; Birsen, R.; et al. AMPK-PERK Axis Represses Oxidative Metabolism and Enhances Apoptotic Priming of Mitochondria in Acute Myeloid Leukemia. Cell Rep. 2022, 38, 110197. [Google Scholar] [CrossRef]
- Su, C.-C.; Hsieh, K.-L.; Liu, P.-L.; Yeh, H.-C.; Huang, S.-P.; Fang, S.-H.; Cheng, W.-C.; Huang, K.-H.; Chiu, F.-Y.; Lin, I.-L.; et al. AICAR Induces Apoptosis and Inhibits Migration and Invasion in Prostate Cancer Cells Through an AMPK/mTOR-Dependent Pathway. Int. J. Mol. Sci. 2019, 20, 1647. [Google Scholar] [CrossRef]
- Sauer, H.; Engel, S.; Milosevic, N.; Sharifpanah, F.; Wartenberg, M. Activation of AMP-Kinase by AICAR Induces Apoptosis of DU-145 Prostate Cancer Cells through Generation of Reactive Oxygen Species and Activation of c-Jun N-Terminal Kinase. Int. J. Oncol. 2012, 40, 501–508. [Google Scholar] [CrossRef]
- Ihara, M.; Shichijo, K.; Takeshita, S.; Kudo, T. Wortmannin, a Specific Inhibitor of Phosphatidylinositol-3-Kinase, Induces Accumulation of DNA Double-Strand Breaks. J. Radiat. Res. 2020, 61, 171–176. [Google Scholar] [CrossRef]
- Cleary, J.M.; Shapiro, G.I. Development of Phosphoinositide-3 Kinase Pathway Inhibitors for Advanced Cancer. Curr. Oncol. Rep. 2010, 12, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Easton, J.B.; Houghton, P.J. mTOR and Cancer Therapy. Oncogene 2006, 25, 6436–6446. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).