Adding a Leafy Vegetable Fraction to Diets Decreases the Risk of Red Meat Mortality in MASLD Subjects: Results from the MICOL Cohort
Abstract
1. Introduction
2. Materials and Methods
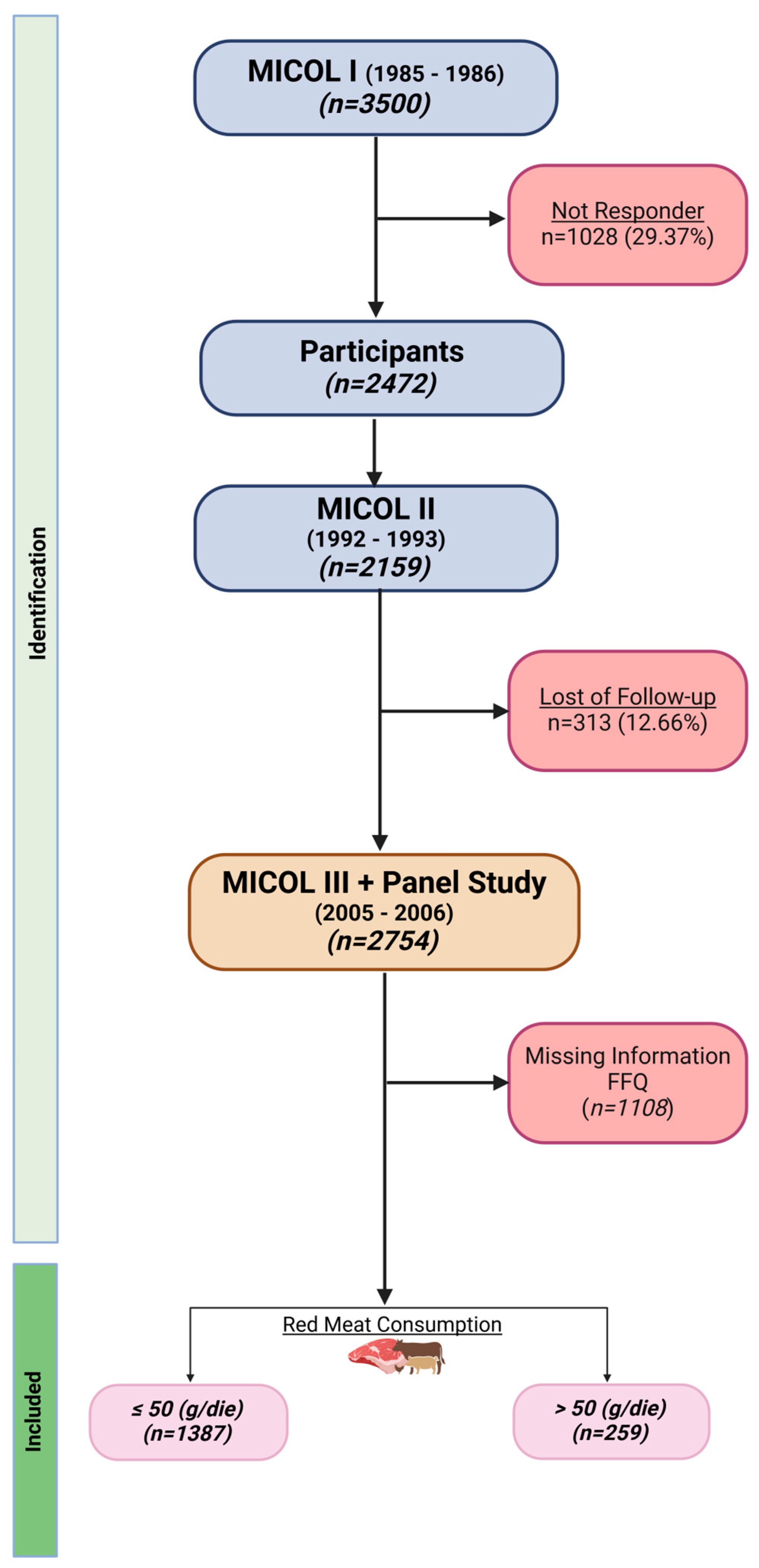
2.1. Study Design and Population
2.2. Lifestyle, Clinical, and Dietary Assessment
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Strengths and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marmot, M.; Atinmo, T.; Byers, T.; Chen, J.; Hirohata, T.; Jackson, A.; James, W.; Kolonel, L.; Kumanyika, S.; Leitzmann, C.; et al. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; World Cancer Research Fund/American Institute for Cancer Research: Washington, DC, USA, 2007. [Google Scholar]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2020, 150, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K.; Internationl Agency for Research on Cancer Monograph Working Group. Benbrahim–Tallaa. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef] [PubMed]
- Turesky, R.J. Mechanistic Evidence for Red Meat and Processed Meat Intake and Cancer Risk: A Follow-up on the International Agency for Research on Cancer Evaluation of 2015. Chimia 2018, 72, 718–724. [Google Scholar] [CrossRef]
- El-Bayoumy, K.; Chae, Y.H.; Upadhyaya, P.; Rivenson, A.; Kuertzke, C.; Reddy, B.; Hecht, S.S. Comparative tumorigenicity of benzo[a]pyrene, 1-nitropyrene and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine administered by gavage to female CD rats. Carcinogenesis 1995, 16, 431–434. [Google Scholar] [CrossRef]
- Gammon, M.D.; Santella, R.M.; Neugut, A.I.; Eng, S.M.; Teitelbaum, S.L.; Paykin, A.; Levin, B.; Terry, M.B.; Young, T.L.; Wang, L.W.; et al. Environmental toxins and breast cancer on Long Island. I. Polycyclic aromatic hydrocarbon DNA adducts. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 677–685. [Google Scholar]
- Shirai, T.; Tamano, S.; Sano, M.; Masui, T.; Hasegawa, R.; Ito, N. Carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in rats: Dose-response studies. Princess Takamatsu Symp. 1995, 23, 232–239. [Google Scholar] [PubMed]
- Sinha, R.; Gustafson, D.R.; Kulldorff, M.; Wen, W.Q.; Cerhan, J.R.; Zheng, W. 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, a carcinogen in high-temperature-cooked meat, and breast cancer risk. J. Natl. Cancer Inst. 2000, 92, 1352–1354. [Google Scholar] [CrossRef]
- Tricker, A.R. N-nitroso compounds and man: Sources of exposure, endogenous formation and occurrence in body fluids. Eur. J. Cancer Prev. 1997, 6, 226–268. [Google Scholar] [CrossRef]
- Sugimura, T.; Wakabayashi, K.; Makagama, H.; Nagao, M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004, 95, 290–299. [Google Scholar] [CrossRef]
- Felton, J.S.; Jagerstad, M.; Knize, M.G.; Skog, K.; Wakabayashi, K. Food Borne Carcinogens: Heterocyclic Amines; Wiley: New York, NY, USA, 2002; p. 392. [Google Scholar]
- Philips, D.H. Polycyclic aromatic hydrocarbons in the diet. Mutat. Res. 1999, 443, 139–147. [Google Scholar] [CrossRef]
- Sancar, A.; Reardon, J.T. Nucleotide excision repair in, E. coli and man. Adv. Protein Chem. 2004, 69, 43–71. [Google Scholar] [PubMed]
- Delaney, J.C.; Essigmann, J.M. Biological properties of single chemical-DNA adducts: A twenty year perspective. Chem. Res. Toxicol. 2008, 21, 232–252. [Google Scholar] [CrossRef] [PubMed]
- Joosen, A.M.C.; Kughnle, G.G.C.; Aspinall, S.M.; Barrow, T.M.; Lecommandeiur, E.; Azqueta, A.; Collins, A.R.; Bingham, S.A. Effect of processed and red meat on endogenous nitrosation and DNA damage. Carcinogenesis 2009, 30, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Bastide, N.M.; Chenni, F.; Audebert, M.; Santarelli, R.L.; Taché, S.; Naud, N.; Baradat, M.; Jouanin, I.; Surya, R.; Hobbs, D.A.; et al. A central role for heme iron in colon carcinogenesis associated with red meat intake. Cancer Res. 2015, 75, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Le Dieci Raccomandazioni del Fondo Mondiale per la Ricerca sul Cancro. SmartFood. IEO. Available online: https://smartfood.ieo.it/media/2039/wcrf-raccomandazioni-documento-smartfood.pdf (accessed on 10 March 2024).
- Klurfeld, D.M. Research gaps in evaluating the relationship of meat and health. Meat Sci. 2015, 109, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Doll, R.; Peto, R. The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. J. Natl. Cancer Inst. 1981, 66, 1191–1308. [Google Scholar] [CrossRef] [PubMed]
- Bøhn, S.K.; Myrstad, M.C.; Thoresen, M.; Holden, M.; Karlsen, A.; Tunheim, S.H.; Erlund, I.; Svendsen, M.; Seljeflot, I.; Moskaug, J.O.; et al. Blood cell gene expression associated with cellular stress defense is modulated by antioxidant-rich food in a randomised controlled clinical trial of male smokers. BMC Med. 2010, 16, 54. [Google Scholar] [CrossRef]
- Macready, A.L.; George, T.W.; Chong, M.F.; Alimbetov, S.D.; Jin, Y.; Vidal, A.; Spencer, J.P.E.; Kennedy, O.B.; Tuohy, K.M.M.; Minihane, A.M.; et al. Flavonoid-rich fruit and vegetables improve microvascular reactivity and inflammatory status in men at risk of cardiovascular disease—FLAVURS: A randomized controlled trial. Am. J. Clin. Mutr. 2014, 99, 479–489. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Potte, J.D. Vegetables, fruit, and cancer. II. Mechanisms. Cancer Causes Control 1991, 2, 427–442. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; De Michieli, F.; Cassader, M.; Rizzetto, M.; Durazzo, M.; Fagà, E.; Silli, B.; Pagano, G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003, 37, 909–916. [Google Scholar] [CrossRef]
- Iannone, V.; Babu, A.F.; Lok, J.; Gómez-Gallego, C.; D’Auria, G.; Vazquez-Uribe, R.; Vaaben, T.H.; Bongers, M.; Mikkonen, S.; Vaittinen, M.; et al. Changes in liver metabolic pathways demonstrate efficacy of the combined dietary and microbial therapeutic intervention in MASLD mouse model. Mol. Metab. 2023, 78, 101823. [Google Scholar] [CrossRef]
- Salehi-Sahlabadi, A.; Teymoori, F.; Ahmadirad, H.; Mokhtari, E.; Azadi, M.; Seraj, S.S.; Hekmatddost, A. Nutrient patterns and non-alcoholic fatty liver disease in Iranian Adul: A case-control study. Front. Nutr. 2022, 9, 977403. [Google Scholar] [CrossRef] [PubMed]
- Chiloiro, M.; Caruso, M.G.; Cisternino, A.M.; Inguaggiato, R.; Reddavide, R.; Bonfiglio, C.; Guerra, V.; Notarnicola, M.; De Michele, G.; Correale, M.; et al. Ultrasound evaluation and correlates of fatty liver disease: A population study in a Mediterranean area. Metab. Syndr. Relat. Disord. 2013, 11, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Sever, P. New hypertension guidelines from the National Institute for Health and Clinical Excellence and the British Hypertension Society. J. Renin Angiotensin. Aldosterone Syst. 2006, 7, 61–63. [Google Scholar] [CrossRef]
- Leoci, C.C.S.; Guerra, V.; Cisternino, A.M.; Misciagna, G. Reliability and validity of a self administrered semi-quantitative food frequency questionnaire. Giorn. Italy Nutr. Clin. Prev. 1993, 71, 1269–1324. [Google Scholar]
- Castelló, A.; Pollán, M.; Buijsse, B.; Ruiz, A.; Casas, A.M.; Baena-Cañada, J.M.; Lope, V.; Antolín, S.; Ramos, M.; Muñoz, M.; et al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: Case-control EpiGEICAM study. Br. J. Cancer. 2014, 111, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Tammi, R.; Kaartinen, N.E.; Harald, K.; Maukonen, M.; Tapanainen, H.; Smith-Warner, S.A.; Albanes, D.; Eriksson, J.G.; Jousilahti, P.; Koskinen, S.; et al. Partial substitution of red meat or processed meat with plant-based foods and the risk of colorectal cancer. Eur. J. Epidemiol. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Sesti, G.; Fiorentino, T.V.; Hribal, M.L.; Sciacqua, A.; Perticone, F. Association of hepatic insulin resistance indexes to nonalcoholic fatty liver disease and related biomarkers. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1182–1187. [Google Scholar] [CrossRef]
- Montonen, J.; Boeing, H.; Fritsche, A.; Schleicher, E.; Joost, H.G.; Schulze, M.B.; Steffen, A.; Pischon, T. Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. Eur. J. Nutr. 2013, 52, 337–345. [Google Scholar] [CrossRef]
- Samuel, V.T.; Liu, Z.X.; Qu, X.; Elder, B.D.; Bilz, S.; Befroy, D.; Romanelli, A.J.; Shulman, G. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 2004, 279, 32345–32353. [Google Scholar] [CrossRef]
- Fan, N.; Peng, L.; Xia, Z.; Zhang, L.; Song, Z.; Wang, Y.; Peng, Y. Triglycerides to high-density lipoprotein cholesterol ratio as a surrogate for nonalcoholic fatty liver disease: A cross-sectional study. Lipids Health Dis. 2019, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Isakov, N.F.; Webb, M.; Orenstein, D.; Shibolet, O.; Kariv, R. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J. Hepatol. 2018, 68, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Rahimi-Sakak, F.; Maroofi, M.; Emamat, H.; Hekmatdoost, A. Red and Processed Meat Intake in Relation to Non-Alcoholic Fatty Liver Disease Risk: Results from a Case-Control Study. Clin. Nutr. Res. 2022, 11, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Ye, M.; Zhang, S.; Zhang, Q.; Meng, G.; Liu, L.; Wu, H.; Gu, Y.; Wang, Y.; et al. Does a high intake of green leafy vegetables protect from NAFLD? Evidence from a large population study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1691–1701. [Google Scholar] [CrossRef]
- Guo, W.; Ge, X.; Lu, J.; Xu, X.; Gao, J.; Wang, Q.; Song, C.; Zhang, Q.; Yu, C. Diet and Risk of Non-Alcoholic Fatty Liver Disease, Cirrhosis, and Liver Cancer: A Large Prospective Cohort Study in UK Biobank. Nutrients 2022, 14, 5335. [Google Scholar] [CrossRef] [PubMed]
- Boeing, H.; Bechtold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.M.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Chen, G.Y. Flavonoids and Colorectal Cancer Prevention. Antioxidants 2018, 7, 187. [Google Scholar] [CrossRef]
- Snowdon, D.A. Animal product consumption and mortality because of all causes combined, coronary heart disease, stroke, diabetes, and cancer in Seventh-day Adventists. Am. J. Clin. Nutr. 1988, 48, 739–748. [Google Scholar] [CrossRef]
- Song, Y.; Manson, J.E.; Buring, J.E.; Liu, S. A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: The women’s health study. Diabetes Care 2004, 27, 2108–2115. [Google Scholar] [CrossRef]
- Sinha, R.; Cross, A.J.; Graubard, B.I.; Leitzmann, M.F.; Schatzkin, A. Meat intake and mortality: A prospective study of over half a million people. Arch. Intern. Med. 2009, 169, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Red meat consumption and mortality: Results from 2 prospective cohort studies. Arch. Intern. Med. 2012, 172, 555–563. [Google Scholar] [PubMed]
- Hebels, D.G.A.; Sveje, K.M.; de Kok, M.; van Herwijnen, M.H.M.; Kuhnle, G.G.C.; Engels, L.G.J.B.; Vleugels-Simon, C.B.E.M.; Mares, W.G.N.; Pierik, M.; Masclee, A.A.M.; et al. Red meat intake-induced increases in fecal water genotoxicity correlate with pro-carcinogenic gene expression changes in the human colon. Food Chem. Toxicol. 2012, 50, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Chan, A.T. Environmental Factors, Gut Microbiota, and Colorectal Cancer Prevention. Clin. Gastroenetrol. Hepatol. 2019, 17, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.J.; Sinha, R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ. Mol. Mutagen. 2004, 44, 44–55. [Google Scholar] [CrossRef]
- Song, M.; Garrett, W.S.; Chan, A.T. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015, 148, 1244–1260. [Google Scholar] [CrossRef] [PubMed]
- Würtz, A.M.; Jakobsen, M.U.; Bertoia, M.L.; Hou, T.; Schmidt, E.B.; Willett, W.C.; Overvad, K.; Sun, Q.; Manson, J.E.; Hu, F.B.; et al. Replacing the consumption of red meat with other major dietary protein sources and risk of type 2 diabetes mellitus: A prospective cohort study. Am. J. Clin. Nutr. 2021, 113, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Scwedhelm, C.; Hoffmann, G.; Knüppel, S.; Preterre, A.L.; Iqbal, K.; Bechthold, A.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; et al. Food groups and risk of colorectal cancer. Int. J. Cancer. 2018, 142, 1748–1758. [Google Scholar] [CrossRef] [PubMed]
- McPherson-Kay, R. Fiber, stool bulk, and bile acid output: Implications for colon cancer risk. Prev. Med. 1987, 16, 540–544. [Google Scholar] [CrossRef]
- Derry, M.M.; Raina, K.; Agarwal, C.; Agarwal, R. Identifying molecular targets of lifestyle modifications in colon cancer prevention. Front. Oncol. 2013, 3, 119. [Google Scholar] [CrossRef]
- Zampa, A.; Silvi, S.; Fabiani, R.; Morozzi, G.; Orpianesi, C.; Cresci, A. Effects of different digestible carbohydrates on bile acid metabolism and SCFA production by human gut micro-flora grown in an in vitro semi-continuous culture. Anaerobe 2004, 10, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.; Borowicki, A.; Scharlau, D.; Schettler, A.; Scheu, K.; Obst, U.; Glei, M. Effects of synbiotic fermentation products on primary chemoprevention in human colon cells. J. Nutr. Biochem. 2012, 23, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Samraj, A.N.; Pearce, O.M.T.; Läubli, H.; Crittenden, A.N.; Bergfeld, A.K.; Banda, K.; Gregg, C.J.; Bingman, A.E.; Secrest, P.; Diaz, S.L.; et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc. Natl. Acad. Sci. USA 2015, 112, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Tappel, A. Heme of consumed red meat can act as a catalyst of oxidative damage and could initiate colon, breast and prostate cancers, heart disease and other diseases. Med. Hypotheses 2007, 68, 562–564. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; Continuous Update Project Expert Report; World Cancer Research Fund/American Institute for Cancer Research: Washington, DC, USA, 2018. [Google Scholar]


| Parameters * | Total Cohort (n = 1646) | Red Meat (g/die) | p ^ | |
|---|---|---|---|---|
| ≤50 (n = 1387) | >50 (n = 259) | |||
| Gender (M) (%) | 912 (55.41) | 713 (51.41) | 199 (76.83) | <0.001 ѱ |
| Age (yrs) | 65.39 ± 9.47 | 66.06 ± 9.43 | 61.84 ± 8.88 | <0.0001 |
| Degree of Education (%) | 0.28 ѱ | |||
| No | 987 (60.48) | 826 (59.99) | 161 (63.14) | |
| Elementary School | 292 (17.89) | 242 (17.57) | 50 (19.61) | |
| Secondary School | 205 (12.56) | 176 (12.78) | 29 (11.37) | |
| High School | 64 (3.92) | 59 (4.29) | 5 (1.96) | |
| Short Degree | 84 (5.15) | 74 (5.37) | 10 (3.92) | |
| Smoke (Yes) (%) | 238 (14.49) | 180 (13.02) | 58 (22.39) | <0.001 ѱ |
| BMI (Kg/m2) | 29.80 ± 9.90 | 29.69 ± 10.47 | 30.38 ± 5.93 | 0.09 |
| Systolic Pressure (mmHg) | 138.67 ± 84.73 | 139.12 ± 82.62 | 136.30 ± 95.37 | <0.0001 |
| Diastolic Pressure (mmHg) | 84.09 ± 88.29 | 83.71 ± 86.08 | 86.12 ± 99.44 | 0.68 |
| Diabetes (Yes) (%) | 237 (14.42) | 206 (14.87) | 31 (11.97) | 0.22 ѱ |
| Hypertension (Yes) (%) | 808 (49.27) | 707 (51.16) | 101 (39.15) | <0.001 ѱ |
| Blood Parameters | ||||
| Glucose (mg/dL) | 118.47 ± 245.85 | 119.34 ± 267.44 | 113.80 ± 33.21 | 0.08 |
| Cholesterol (mg/mL) | 205.94 ± 244.73 | 206.61 ± 266.02 | 202.36 ± 41.07 | 0.62 |
| HDL (mg/dL) | 63.18 ± 346.92 | 58.60 ± 267.44 | 87.71 ± 618.38 | 0.01 |
| LDL (mg/dL) | 157.12 ± 596.61 | 142.47 ± 460.47 | 235.55 ± 1059.51 | 0.89 |
| Triglycerides (mg/dL) | 151.49 ± 357.46 | 142.39 ± 279.00 | 200.22 ± 627.46 | 0.002 |
| Fatty Acids (mg/dL) | 683.86 ± 2149.95 | 679.54 ± 2411.83 | 707.01 ± 2467.59 | 0.30 |
| Total Bilirubin (mg/dL) | 134.52 ± 1148.48 | 159.48 ± 1249.61 | 0.87 ± 0.29 | 0.67 |
| GOT (U/L) | 638.96 ± 2419.08 | 640.41 ± 2421.92 | 631.17 ± 2408.48 | 0.46 |
| GGT (U/I) | 97.39 ± 883.95 | 82.94 ± 801.90 | 174.75 ± 1233.01 | 0.0002 |
| SGPT (U/L) | 24.13 ± 246.67 | 25.12 ± 268.64 | 18.83 ± 14.17 | 0.0002 |
| Leafy Vegetables (g/die) (%) | 0.01 ѱ | |||
| ≤28.49 | 832 (50.55) | 720 (51.91) | 112 (43.24) | |
| >28.49 | 814 (49.45) | 667 (48.09) | 147 (56.76) | |
| Parameters | HR | se (HR) | p | 95% (C.I.) |
|---|---|---|---|---|
| Red Meat (g/die) | ||||
| ≤50 [Ref.] | - | - | - | - |
| >50 | 2.02 | 0.41 | 0.001 | 1.36 to 3.01 |
| Red Meat # Leafy Vegetables (g/die) | ||||
| ≤50 & ≤28.49 [Ref.] | - | - | - | - |
| ≤50 & >28.49 | 1.06 | 0.18 | 0.73 | 0.75 to 1.49 |
| >50 & ≤28.49 | 2.36 | 0.64 | 0.002 | 1.38 to 4.01 |
| >50 & >28.49 | 1.85 | 0.52 | 0.03 | 1.06 to 3.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donghia, R.; Tatoli, R.; Campanella, A.; Cuccaro, F.; Bonfiglio, C.; Giannelli, G. Adding a Leafy Vegetable Fraction to Diets Decreases the Risk of Red Meat Mortality in MASLD Subjects: Results from the MICOL Cohort. Nutrients 2024, 16, 1207. https://doi.org/10.3390/nu16081207
Donghia R, Tatoli R, Campanella A, Cuccaro F, Bonfiglio C, Giannelli G. Adding a Leafy Vegetable Fraction to Diets Decreases the Risk of Red Meat Mortality in MASLD Subjects: Results from the MICOL Cohort. Nutrients. 2024; 16(8):1207. https://doi.org/10.3390/nu16081207
Chicago/Turabian StyleDonghia, Rossella, Rossella Tatoli, Angelo Campanella, Francesco Cuccaro, Caterina Bonfiglio, and Gianluigi Giannelli. 2024. "Adding a Leafy Vegetable Fraction to Diets Decreases the Risk of Red Meat Mortality in MASLD Subjects: Results from the MICOL Cohort" Nutrients 16, no. 8: 1207. https://doi.org/10.3390/nu16081207
APA StyleDonghia, R., Tatoli, R., Campanella, A., Cuccaro, F., Bonfiglio, C., & Giannelli, G. (2024). Adding a Leafy Vegetable Fraction to Diets Decreases the Risk of Red Meat Mortality in MASLD Subjects: Results from the MICOL Cohort. Nutrients, 16(8), 1207. https://doi.org/10.3390/nu16081207







