Effects of Taraxaci Herba (Dandelion) on Testosterone Propionate-Induced Benign Prostatic Hyperplasia in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of TH Extract
2.2. Animals
2.3. TP-Induced Benign Prostatic Hyperplasia Rat Model
2.4. Administration of TH Extract and Positive Drug
2.5. Histology
2.6. Determination of Serum Testosterone and DHT Concentrations
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
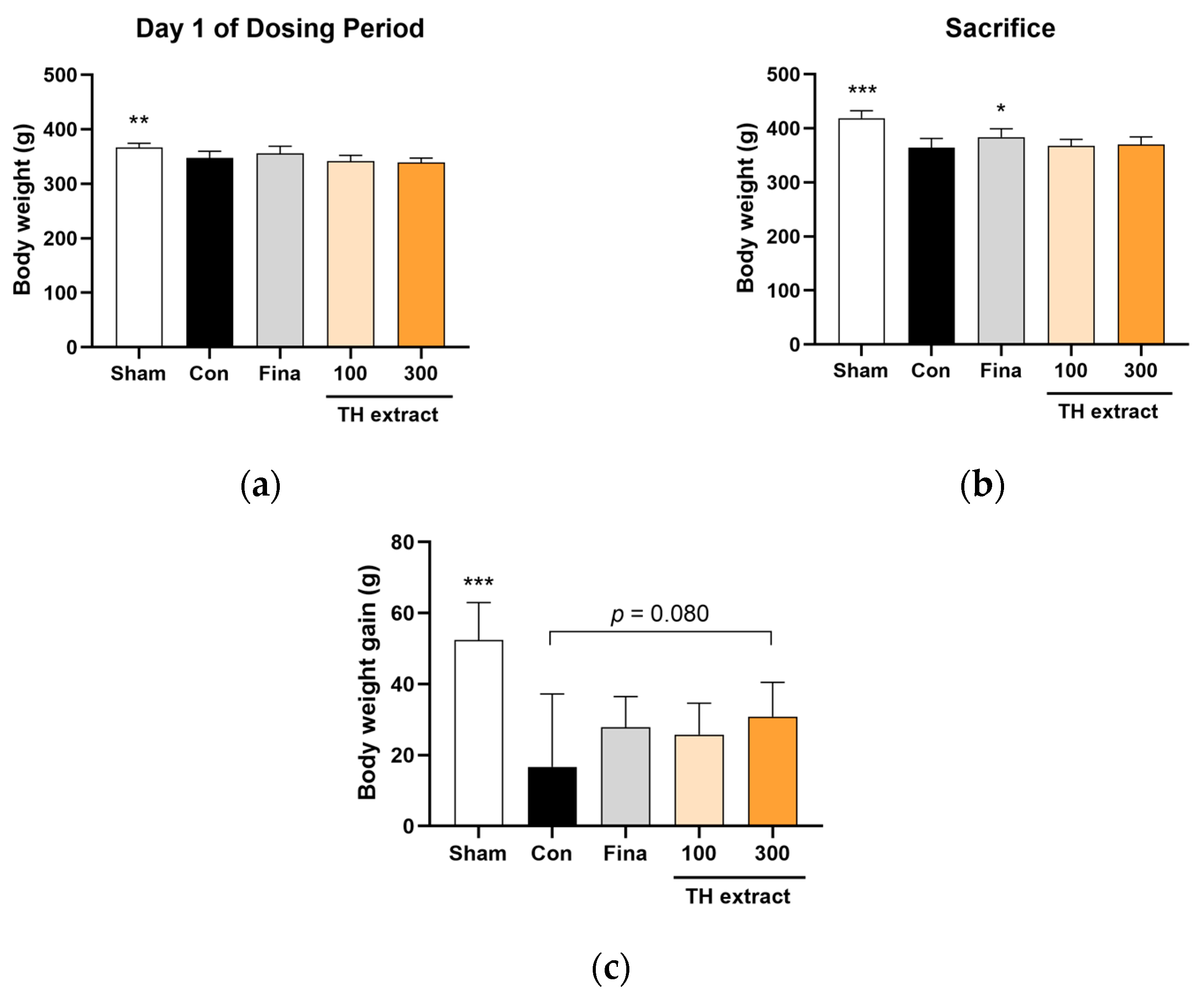
3.1. Body Weight Changes
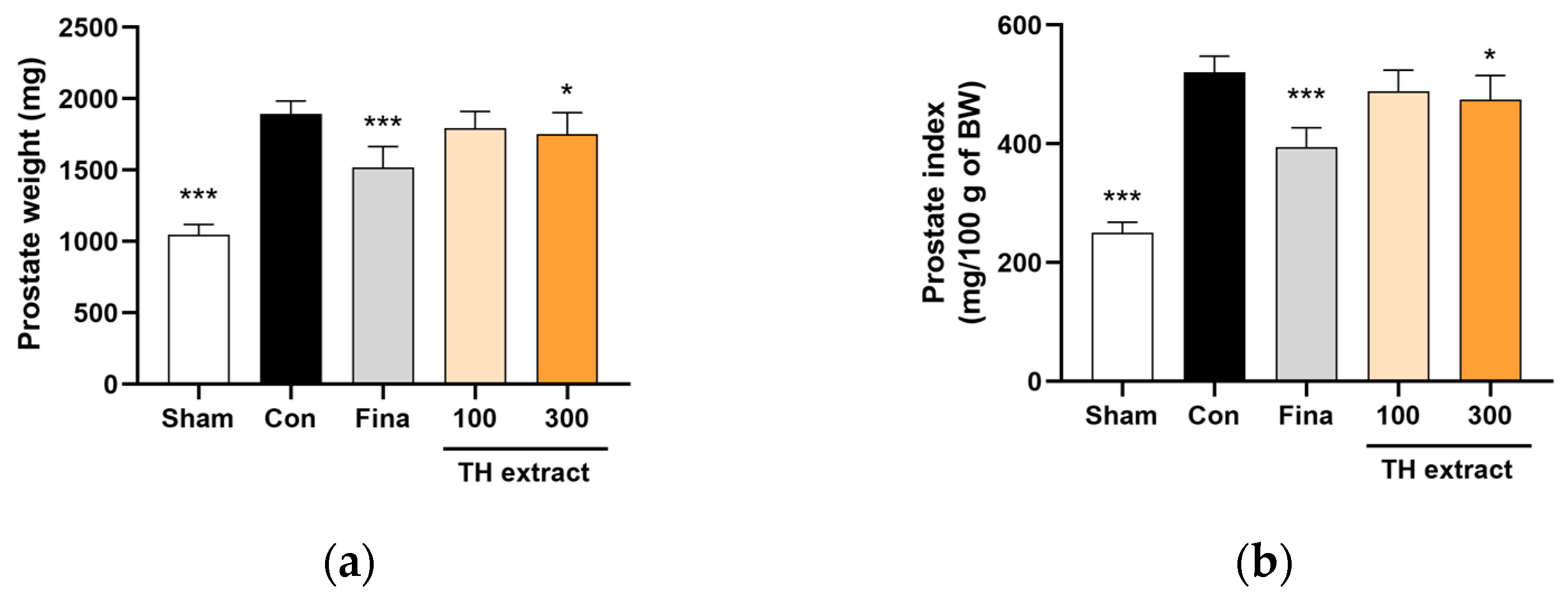
3.2. Prostate Weight and Prostate Index
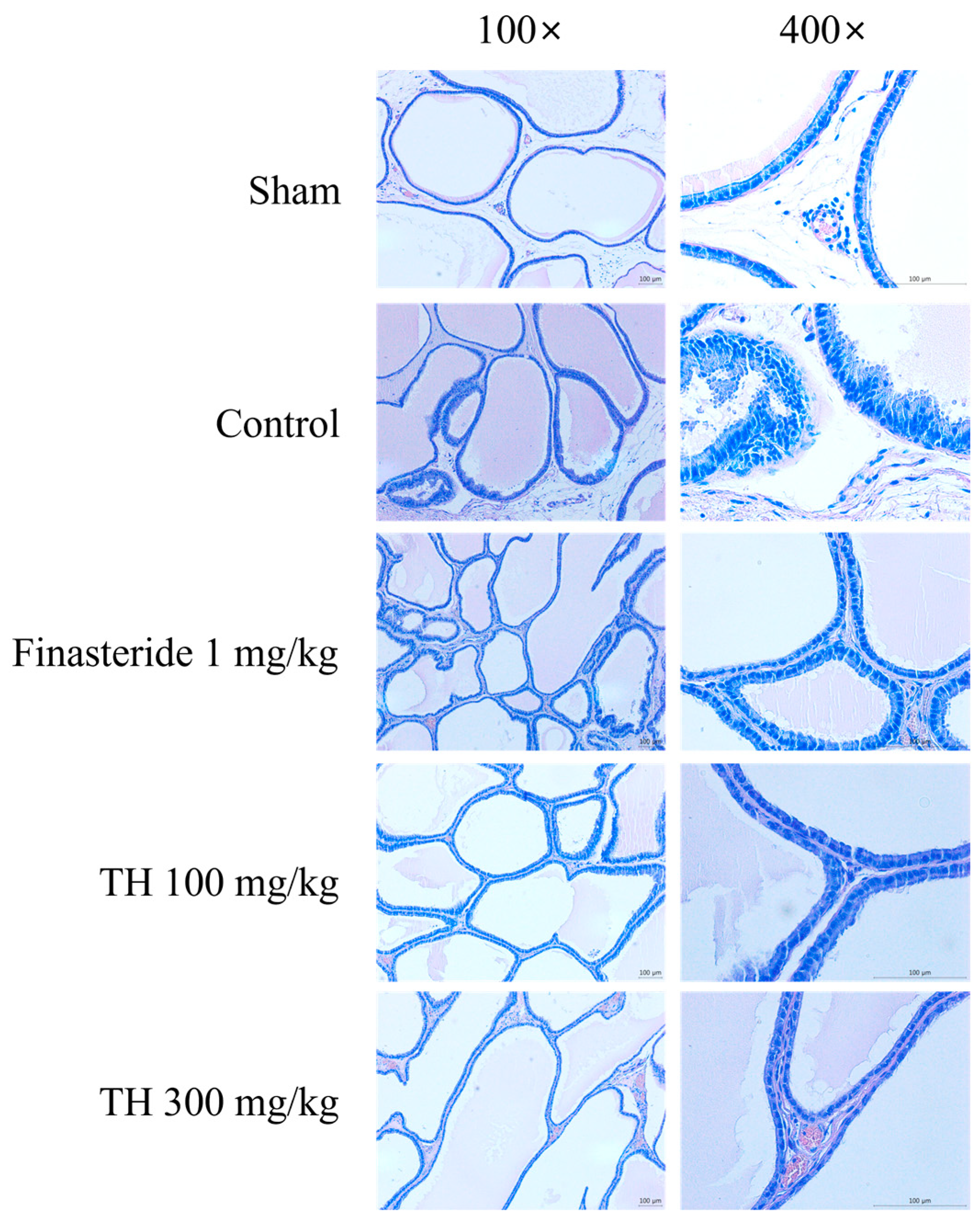
3.3. H&E-Stained Prostate Tissues
3.4. Histological Scores
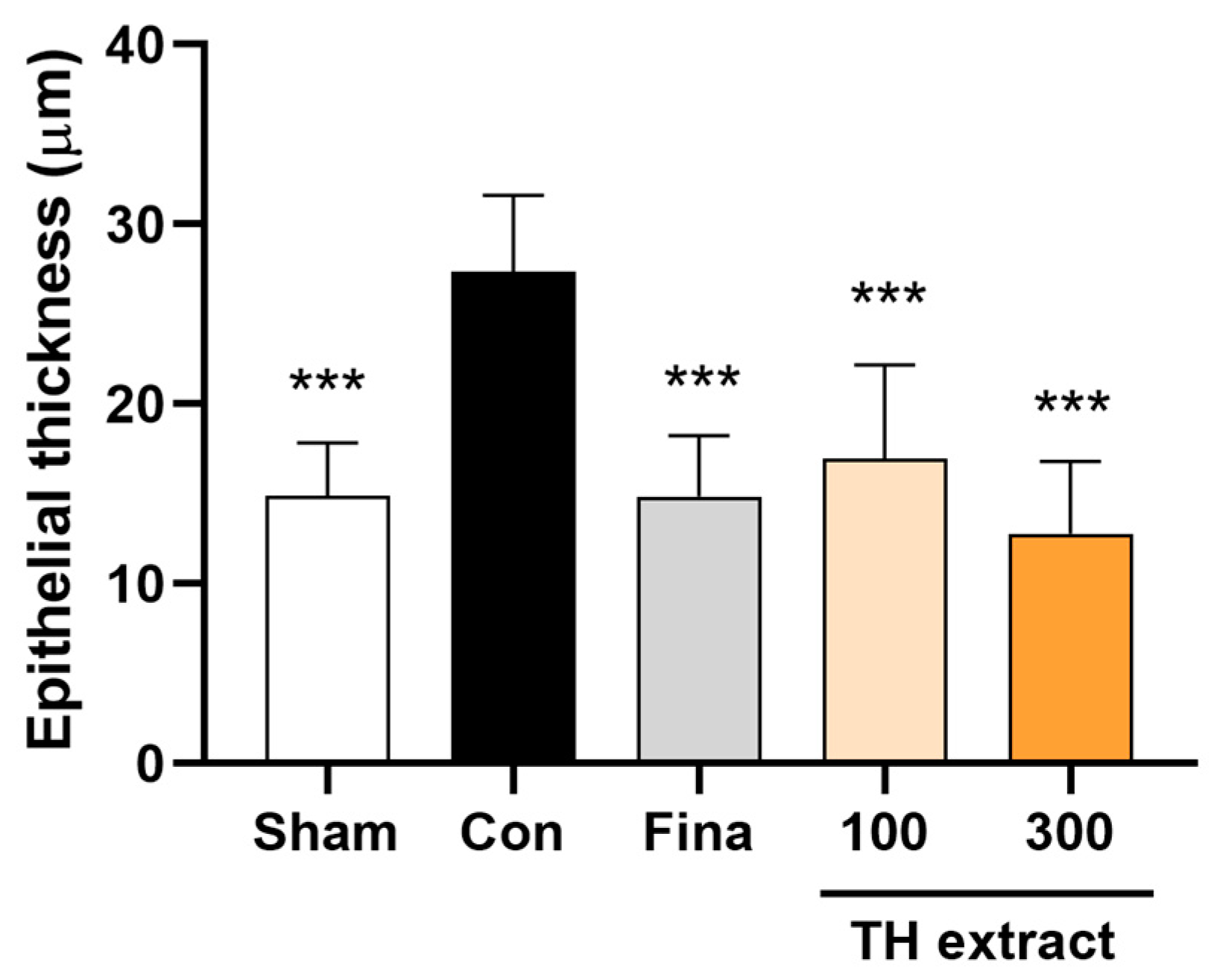
3.5. Epithelial Thickness of the Prostate
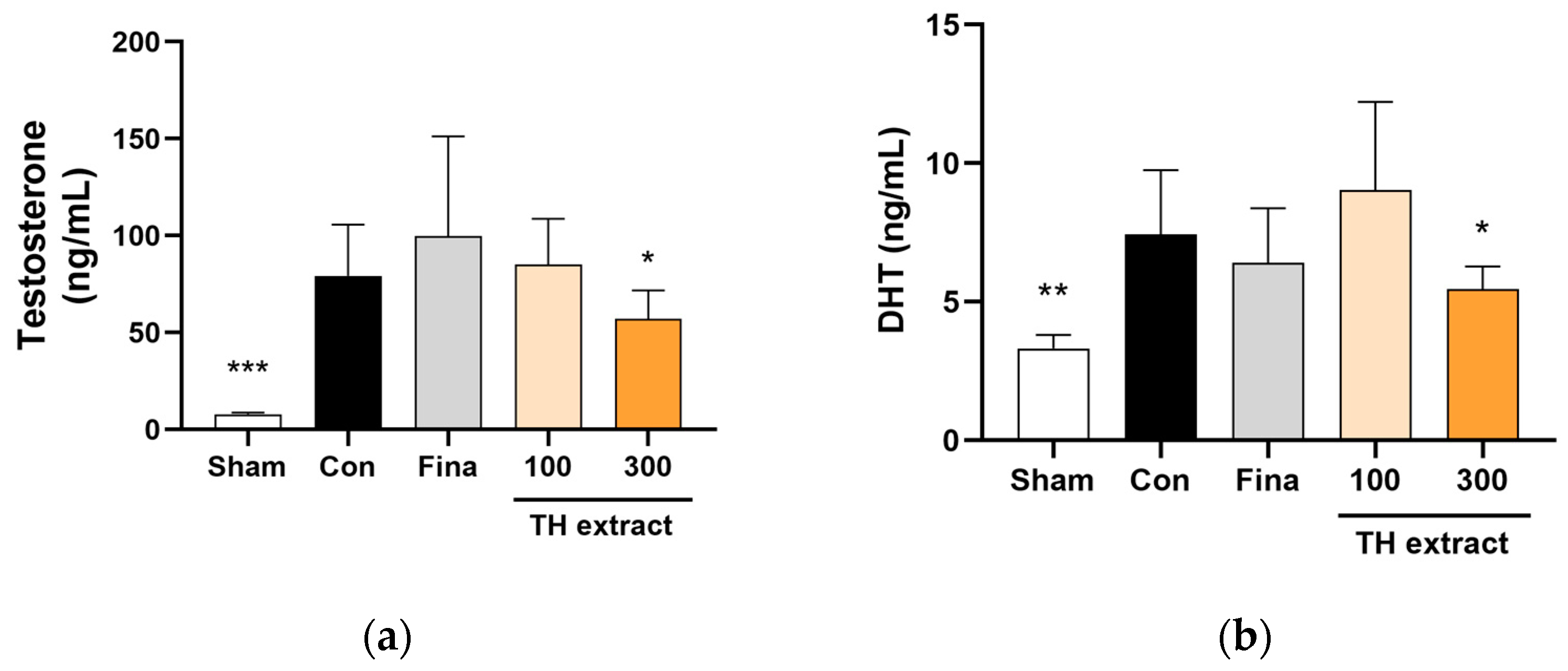
3.6. Serum Testosterone and DHT Concentrations
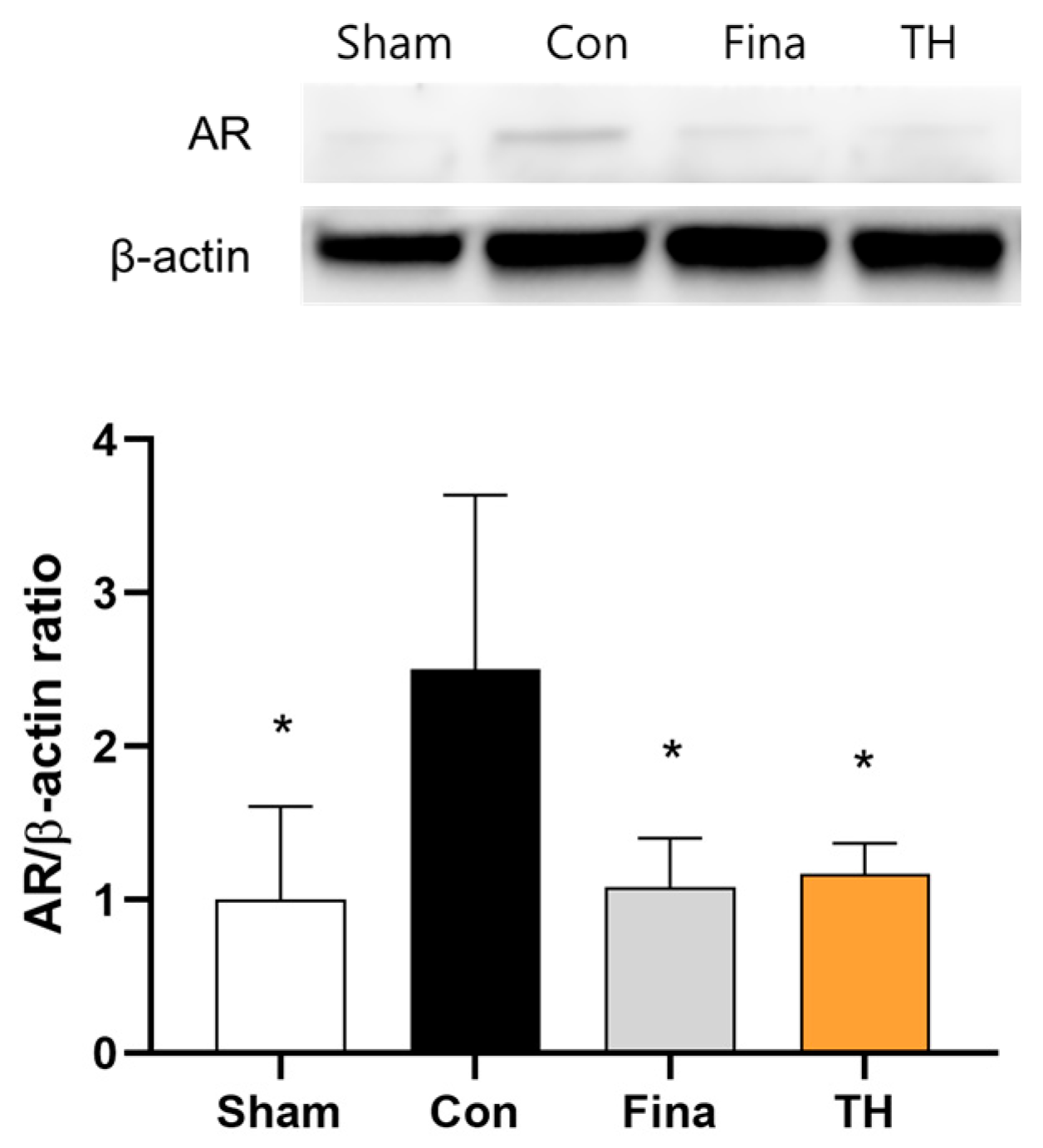
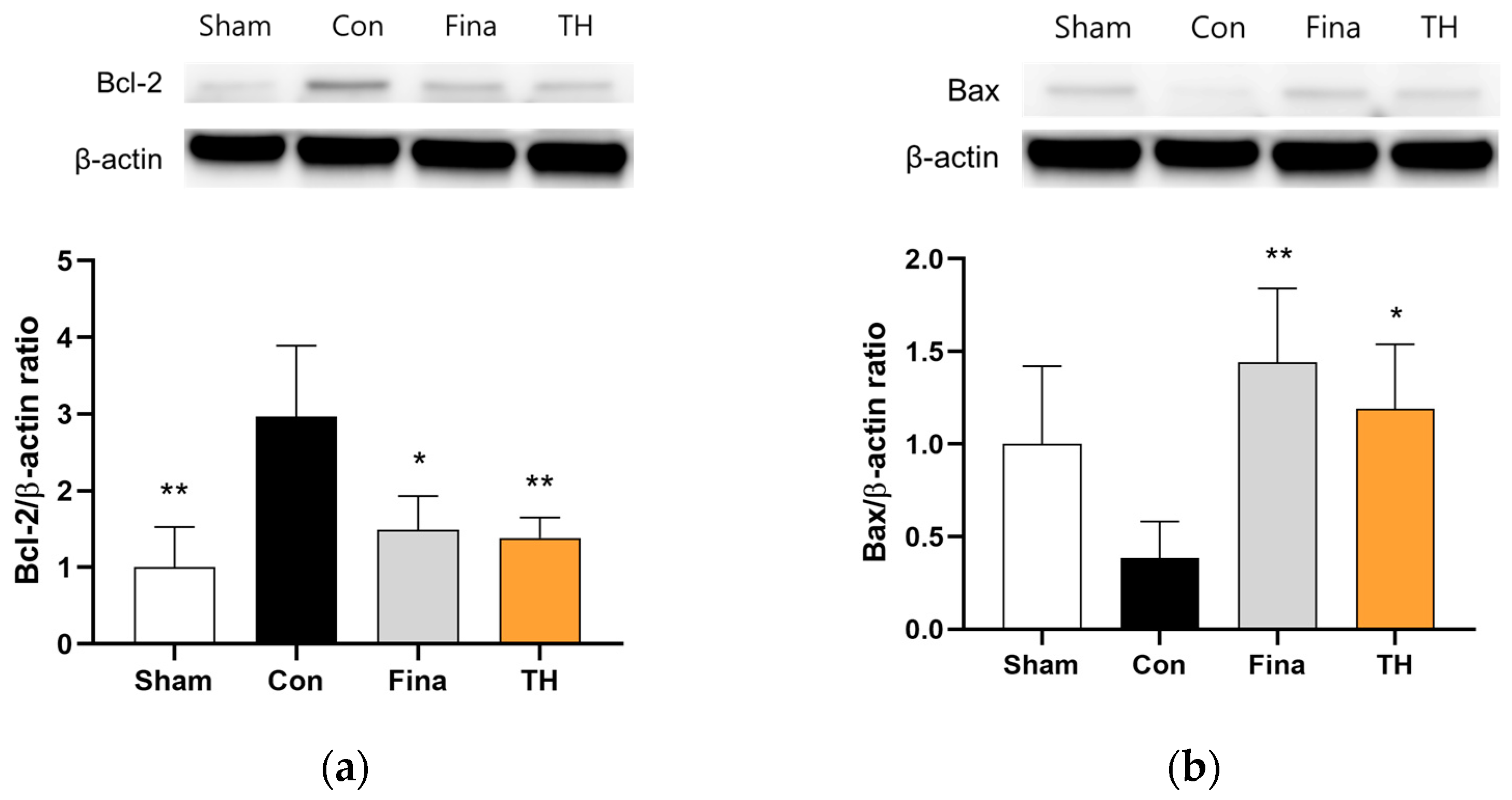
3.7. Protein Expressions of AR and Apoptosis-Related Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barry, M.J.; Fowler, F.J., Jr.; O’Leary, M.P.; Bruskewitz, R.C.; Holtgrewe, H.L.; Mebust, W.K.; Cockett, A.T. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J. Urol. 1992, 148, 1549 1557; discussion 1564. [Google Scholar] [CrossRef]
- Egan, K.B. The Epidemiology of Benign Prostatic Hyperplasia Associated with Lower Urinary Tract Symptoms: Prevalence and Incident Rates. Urol. Clin. N. Am. 2016, 43, 289–297. [Google Scholar] [CrossRef]
- Madersbacher, S.; Sampson, N.; Culig, Z. Pathophysiology of Benign Prostatic Hyperplasia and Benign Prostatic Enlargement: A Mini-Review. Gerontology 2019, 65, 458–464. [Google Scholar] [CrossRef]
- Roehrborn, C.G. Pathology of benign prostatic hyperplasia. Int. J. Impot. Res. 2008, 20 (Suppl. S3), S11–S18. [Google Scholar] [CrossRef]
- Nicholson, T.M.; Ricke, W.A. Androgens and estrogens in benign prostatic hyperplasia: Past, present and future. Differentiation 2011, 82, 184–199. [Google Scholar] [CrossRef]
- Vickman, R.E.; Franco, O.E.; Moline, D.C.; Vander Griend, D.J.; Thumbikat, P.; Hayward, S.W. The role of the androgen receptor in prostate development and benign prostatic hyperplasia: A review. Asian J. Urol. 2020, 7, 191–202. [Google Scholar] [CrossRef]
- Andriole, G.; Bruchovsky, N.; Chung, L.W.; Matsumoto, A.M.; Rittmaster, R.; Roehrborn, C.; Russell, D.; Tindall, D. Dihydrotestosterone and the prostate: The scientific rationale for 5alpha-reductase inhibitors in the treatment of benign prostatic hyperplasia. J. Urol. 2004, 172, 1399–1403. [Google Scholar] [CrossRef]
- Gao, W.; Bohl, C.E.; Dalton, J.T. Chemistry and structural biology of androgen receptor. Chem. Rev. 2005, 105, 3352–3370. [Google Scholar] [CrossRef]
- Sandhu, J.S.; Bixler, B.R.; Dahm, P.; Goueli, R.; Kirkby, E.; Stoffel, J.T.; Wilt, T.J. Management of Lower Urinary Tract Symptoms Attributed to Benign Prostatic Hyperplasia (BPH): AUA Guideline Amendment 2023. J. Urol. 2024, 211, 11–19. [Google Scholar] [CrossRef]
- Zlotta, A.R.; Teillac, P.; Raynaud, J.P.; Schulman, C.C. Evaluation of male sexual function in patients with Lower Urinary Tract Symptoms (LUTS) associated with Benign Prostatic Hyperplasia (BPH) treated with a phytotherapeutic agent (Permixon), Tamsulosin or Finasteride. Eur. Urol. 2005, 48, 269–276. [Google Scholar] [CrossRef]
- Bullock, T.L.; Andriole, G.L., Jr. Emerging drug therapies for benign prostatic hyperplasia. Expert. Opin. Emerg. Drugs 2006, 11, 111–123. [Google Scholar] [CrossRef]
- McConnell, J.D.; Bruskewitz, R.; Walsh, P.; Andriole, G.; Lieber, M.; Holtgrewe, H.L.; Albertsen, P.; Roehrborn, C.G.; Nickel, J.C.; Wang, D.Z.; et al. The effect of finasteride on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. Finasteride Long-Term Efficacy and Safety Study Group. N. Engl. J. Med. 1998, 338, 557–563. [Google Scholar] [CrossRef]
- Hirshburg, J.M.; Kelsey, P.A.; Therrien, C.A.; Gavino, A.C.; Reichenberg, J.S. Adverse Effects and Safety of 5-alpha Reductase Inhibitors (Finasteride, Dutasteride): A Systematic Review. J. Clin. Aesthet. Dermatol. 2016, 9, 56–62. [Google Scholar] [PubMed]
- Leisegang, K.; Jimenez, M.; Durairajanayagam, D.; Finelli, R.; Majzoub, A.; Henkel, R.; Agarwal, A. A systematic review of herbal medicine in the clinical treatment of benign prostatic hyperplasia. Phytomed. Plus 2022, 2, 100153. [Google Scholar] [CrossRef]
- Jang, M.H.; Ahn, T.W. Inhibitory effects of Taraxacum mongolicum with phreatic water on melanin synthesis. Integr. Med. Res. 2015, 4, 76–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schutz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef]
- Di Napoli, A.; Zucchetti, P. A comprehensive review of the benefits of Taraxacum officinale on human health. Bull. Natl. Res. Cent. 2021, 45, 110. [Google Scholar] [CrossRef]
- Amritpal Singh, A.S.; Samir Malhotra, S.M.; Ravi Subban, R.S. Dandelion (Taraxacum officinale)-hepatoprotective herb with therapeutic potential. Pharmacogn. Rev. 2008, 2, 163–167. [Google Scholar]
- Mir, M.A.; Sawhney, S.; Jassal, M. Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. Wudpecker J. Pharm. Pharmocol. 2013, 2, 1–5. [Google Scholar]
- Fan, M.; Zhang, X.; Song, H.; Zhang, Y. Dandelion (Taraxacum Genus): A Review of Chemical Constituents and Pharmacological Effects. Molecules 2023, 28, 5022. [Google Scholar] [CrossRef]
- Lee, M.-H.; Song, S.-H.; Ham, I.-H.; Bu, Y.-M.; Kim, H.-C.; Choi, H.-Y. Anti-inflammatory effect and contents from the aerial part and root of the various Taraxacum spp. distributed in Korea. Korea J. Herbol. 2010, 25, 77–84. [Google Scholar]
- Park, J.H.; Seo, B.I. A philological study on poisoning and side effect of Taraxci Herba. J. Jeahan Orient. Med. Acad. 2011, 9, 19–24. [Google Scholar]
- McVary, K.T. Clinical evaluation of benign prostatic hyperplasia. Rev. Urol. 2003, 5 (Suppl. S4), S3–S11. [Google Scholar] [PubMed]
- Lim, S.; Kim, H.K.; Lee, W.; Kim, S. Botanical preparation HX109 inhibits macrophage-mediated activation of prostate epithelial cells through the CCL4-STAT3 pathway: Implication for the mechanism underlying HX109 suppression of prostate hyperplasia. Heliyon 2020, 6, e04267. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Kim, E.K.; Fan, M.; Tang, Y.; Hwang, Y.J.; Sung, S.H. Effect of Paecilomyces tenuipes Extract on Testosterone-Induced Benign Prostatic Hyperplasia in Sprague-Dawley Rats. Int. J. Environ. Res. Public Health 2019, 16, 3764. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.D.; Thanh Ha, T.N.; Duy, T.N.; Hoang, N.N.; PhamTien, D.; Thai, H.P.; Thi, H.N.; Thi Lan, P.D.; Quoc, B.P.; Ivkin, D.Y.; et al. Linh Phu Khang Tue Tinh inhibited prostate proliferation in rats induced benign prostatic hyperplasia by testosterone propionate. J. Ethnopharmacol. 2021, 279, 114388. [Google Scholar] [CrossRef] [PubMed]
- Brown, C. Intra-abdominal castration in the rat. Lab. Anim. 2008, 37, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMC Vet. Res. 2020, 16, 242. [Google Scholar] [CrossRef]
- WHO. WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 1999; Volume 3, pp. 328–337. [Google Scholar]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Kania-Dobrowolska, M.; Baraniak, J. Dandelion (Taraxacum officinale L.) as a Source of Biologically Active Compounds Supporting the Therapy of Co-Existing Diseases in Metabolic Syndrome. Foods 2022, 11, 2858. [Google Scholar] [CrossRef]
- Aremu, O.O.; Oyedeji, A.O.; Oyedeji, O.O.; Nkeh-Chungag, B.N.; Rusike, C.R.S. In Vitro and In Vivo Antioxidant Properties of Taraxacum officinale in Nomega-Nitro-l-Arginine Methyl Ester (L-NAME)-Induced Hypertensive Rats. Antioxidants 2019, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Scolnik, M.D.; Servadio, C.; Abramovici, A. Comparative study of experimentally induced benign and atypical hyperplasia in the ventral prostate of different rat strains. J. Androl. 1994, 15, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Golomb, E.; Kruglikova, A.; Dvir, D.; Parnes, N.; Abramovici, A. Induction of atypical prostatic hyperplasia in rats by sympathomimetic stimulation. Prostate 1998, 34, 214–221. [Google Scholar] [CrossRef]
- Kochakian, C.D.; Endahl, B.R. Changes in body weight of normal and castrated rats by different doses of testosterone propionate. Proc. Soc. Exp. Biol. Med. 1959, 100, 520–522. [Google Scholar] [CrossRef]
- Sorokina, I.V.; Zhukova, N.A.; Meshkova, Y.V.; Baev, D.S.; Tolstikova, T.G.; Bakarev, M.A.; Lushnikova, E.L. Modeling of Benign Prostatic Hyperplasia in Rats with a High Dose of Testosterone. Bull. Exp. Biol. Med. 2022, 173, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.; Tang, J.; Yin, G.; Long, Z.; He, L.; Zhou, C.; Luo, L.; Qi, L.; Wang, L. Animal models of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2021, 24, 49–57. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, L.; Salari, K.; Bechis, S.K.; Ge, R.; Wu, S.; Rassoulian, C.; Pham, J.; Wu, C.L.; Tabatabaei, S.; et al. Androgenic to oestrogenic switch in the human adult prostate gland is regulated by epigenetic silencing of steroid 5alpha-reductase 2. J. Pathol. 2017, 243, 457–467. [Google Scholar] [CrossRef]
- Pais, P. Potency of a novel saw palmetto ethanol extract, SPET-085, for inhibition of 5alpha-reductase II. Adv. Ther. 2010, 27, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.-H.; Chiu, P.-C.; Chang, J.-C.; Lin, S.-M.; Li, Y.-J.; Lo, D.-Y.; Lai, L.-R.; Wu, S.-C.; Chiou, R.Y.Y. Inhibition of testosterone-mediated benign prostatic enlargement of orchiectomized Sprague-Dawley rats by diets supplemented with bio-elicited peanut sprout powder (BPSP) and three new BPSP-extracted natural compounds identified. J. Funct. Foods 2021, 79, 104383. [Google Scholar] [CrossRef]
- Shin, I.S.; Lee, M.Y.; Jung, D.Y.; Seo, C.S.; Ha, H.K.; Shin, H.K. Ursolic acid reduces prostate size and dihydrotestosterone level in a rat model of benign prostatic hyperplasia. Food Chem. Toxicol. 2012, 50, 884–888. [Google Scholar] [CrossRef]
- Shin, S.J.; Lee, K.H.; Chung, K.S.; Cheon, S.Y.; An, H.J. The traditional Korean herbal medicine Ga-Gam-Nai-Go-Hyan suppresses testosterone-induced benign prostatic hyperplasia by regulating inflammatory responses and apoptosis. Exp. Ther. Med. 2017, 13, 1025–1031. [Google Scholar] [CrossRef][Green Version]
- Kemppainen, J.A.; Lane, M.V.; Sar, M.; Wilson, E.M. Androgen receptor phosphorylation, turnover, nuclear transport, and transcriptional activation. Specificity for steroids and antihormones. J. Biol. Chem. 1992, 267, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Seo, C.S.; Wijerathne, C.U.; Jeong, H.Y.; Moon, O.S.; Seo, Y.W.; Won, Y.S.; Son, H.Y.; Lim, J.H.; Kwun, H.J. Effect of Veratrum maackii on Testosterone Propionate-Induced Benign Prostatic Hyperplasia in Rats. Biol. Pharm. Bull. 2019, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.H.; Li, H.Y.; Chung, S.D.; Chiang, H.S.; Yu, H.J. Significant association between serum dihydrotestosterone level and prostate volume among Taiwanese men aged 40–79 years. Aging Male 2012, 15, 28–33. [Google Scholar] [CrossRef]
- Izumi, K.; Mizokami, A.; Lin, W.J.; Lai, K.P.; Chang, C. Androgen receptor roles in the development of benign prostate hyperplasia. Am. J. Pathol. 2013, 182, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, T.M.; Sehgal, P.D.; Drew, S.A.; Huang, W.; Ricke, W.A. Sex steroid receptor expression and localization in benign prostatic hyperplasia varies with tissue compartment. Differentiation 2013, 85, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Torrealba, N.; Rodriguez-Berriguete, G.; Vera, R.; Fraile, B.; Olmedilla, G.; Martinez-Onsurbe, P.; Sanchez-Chapado, M.; Paniagua, R.; Royuela, M. Homeostasis: Apoptosis and cell cycle in normal and pathological prostate. Aging Male 2020, 23, 335–345. [Google Scholar] [CrossRef]
- Minutoli, L.; Rinaldi, M.; Marini, H.; Irrera, N.; Crea, G.; Lorenzini, C.; Puzzolo, D.; Valenti, A.; Pisani, A.; Adamo, E.B.; et al. Apoptotic Pathways Linked to Endocrine System as Potential Therapeutic Targets for Benign Prostatic Hyperplasia. Int. J. Mol. Sci. 2016, 17, 1311. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, S.; Kim, S.; Kim, J.H.; Bae, B.S.; Koo, G.B.; So, S.H.; Lee, J.; Lee, Y.H. Effects of red ginseng oil(KGC11o) on testosterone-propionate-induced benign prostatic hyperplasia. J. Ginseng Res. 2022, 46, 473–480. [Google Scholar] [CrossRef]
- Hu, C. Taraxacum: Phytochemistry and health benefits. Chin. Herbal. Med. 2018, 10, 353–361. [Google Scholar] [CrossRef]
- Mo, S. Effect of increasing nitric oxide and dihydrotestosterone by Taraxacum coreanum extract. J. Appl. Biol. Chem. 2019, 62, 305–313. [Google Scholar] [CrossRef]
- Amory, J.K.; Wang, C.; Swerdloff, R.S.; Anawalt, B.D.; Matsumoto, A.M.; Bremner, W.J.; Walker, S.E.; Haberer, L.J.; Clark, R.V. The effect of 5alpha-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J. Clin. Endocrinol. Metab. 2007, 92, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
| Variable (Score Values) | Sham | Control | Finasteride | TH 100 | TH 300 |
|---|---|---|---|---|---|
| Low-Power Magnification | |||||
| Luminal shape regular (1), villous (3), papillary (4), cribriform (5) | 1.33 (0.82) 1 | 2.11 (1.05) | 1.25 (0.71) | 1.89 (1.05) | 1.56 (1.13) |
| Acinar shape tubular (1), branched (3), irregular (5) | 1.00 (0.00) | 2.33 (2.00) | 1.00 (0.00) | 1.33 (1.00) | 1.22 (0.67) |
| Interacinar space large or moderate (1); back-to-back glands (5) | 1.00 (0.00) | 2.33 (2.00) | 1.00 (0.00) | 2.67 (2.00) | 1.22 (0.67) |
| Stroma fine (1), abundant (3), fibrosis/severe smooth muscle hyperplasia (5) | 2.33 (1.03) | 2.78 (1.56) | 2.50 (1.41) | 2.11 (1.45) | 2.11 (1.05) |
| High-Power Magnification | |||||
| Epithelial shape flattened (1), cuboidal (1), cylindrical (3), hexagonal (5) | 1.67 (1.03) | 2.33 (1.00) | 1.50 (0.93) | 1.67 (1.00) | 1.22 (0.67) |
| Number of layers mono (1), oligo 2–4 (3), pluri, >5 (5) | 1.67 (1.03) | 2.56 (0.88) | 1.00 (0.00) ** | 1.67 (1.00) | 1.22 (0.67) ** |
| If >1, then add focal (3), diffuse (5) | 1.00 (1.55) | 2.33 (1.32) | 0.00 (0.00) ** | 1.33 (1.58) | 0.33 (1.00) ** |
| Alignment polar (1), apolar (3) | 1.00 (0.00) * | 2.11 (1.05) | 1.00 (0.00) * | 1.67 (1.00) | 1.22 (0.67) |
| If there is piling up of epithelial cells, add (3) | 2.00 (1.55) | 3.00 (0.00) | 1.50 (1.60) | 2.00 (1.50) | 1.33 (1.58) * |
| If there is budding out of epithelial cells into stroma, add (5) | 0.00 (0.00) * | 3.89 (2.20) | 1.88 (2.59) | 2.22 (2.64) | 2.78 (2.64) |
| If periacinar clusters of epithelial cells are found, add (3) | 0.50 (1.22) | 1.00 (1.50) | 0.38 (1.06) | 1.00 (1.50) | 0.00 (0.00) |
| If isolated clusters of epithelial cells are found outside acini, add (5) | 0.83 (2.04) * | 3.33 (2.50) | 0.00 (0.00) *** | 0.00 (0.00) *** | 0.56 (1.67) ** |
| Lesion distribution (for apolar or budding out cells, no lesion = 0): unilobar: isolated (2): multiple (6). Bilobar: isolated (4); multiple (8) | 2.00 (2.19) | 5.33 (0.94) | 1.50 (2.07) * | 3.56 (3.13) | 2.67 (3.46) |
| Nuclear shape round, regular (1), irregular (5) | 1.00 (0.00) ** | 3.67 (0.67) | 1.00 (0.00) ** | 2.33 (2.00) | 1.89 (1.76) |
| Nuclear size small (2), large (2), small and large in the same acinus (4) | 2.00 (0.00) ** | 3.33 (0.33) | 2.00 (0.00) ** | 2.67 (1.00) | 2.22 (0.67) * |
| Mitoses per field absent (0); isolated, 1–2 (2); abundant, 3–5 (5); excessive, >5 (10) | 0.33 (0.82) ** | 2.78 (0.60) | 1.00 (1.07) * | 0.67 (1.00) ** | 0.56 (1.67) ** |
| Basement membrane intact (1); interrupted (5) | 1.00 (0.00) ** | 4.56 (0.44) | 2.00 (1.85) * | 2.78 (2.11) | 2.33 (2.00) * |
| Thin (1); thick (5) | 1.00 (0.00) ** | 4.11 (0.59) | 1.50 (1.41) ** | 2.78 (2.11) | 1.89 (1.76) * |
| Total score (arbitrary units) | 21.67 (8.36) *** | 53.89 (5.23) | 22.00 (6.95) *** | 34.33 (17.70) * | 26.33 (14.31) *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.M.; Lee, S.M.; Song, J. Effects of Taraxaci Herba (Dandelion) on Testosterone Propionate-Induced Benign Prostatic Hyperplasia in Rats. Nutrients 2024, 16, 1189. https://doi.org/10.3390/nu16081189
Lee SM, Lee SM, Song J. Effects of Taraxaci Herba (Dandelion) on Testosterone Propionate-Induced Benign Prostatic Hyperplasia in Rats. Nutrients. 2024; 16(8):1189. https://doi.org/10.3390/nu16081189
Chicago/Turabian StyleLee, Seong Min, Sang Mok Lee, and Jungbin Song. 2024. "Effects of Taraxaci Herba (Dandelion) on Testosterone Propionate-Induced Benign Prostatic Hyperplasia in Rats" Nutrients 16, no. 8: 1189. https://doi.org/10.3390/nu16081189
APA StyleLee, S. M., Lee, S. M., & Song, J. (2024). Effects of Taraxaci Herba (Dandelion) on Testosterone Propionate-Induced Benign Prostatic Hyperplasia in Rats. Nutrients, 16(8), 1189. https://doi.org/10.3390/nu16081189





