Effects of Different Types of Intermittent Fasting Interventions on Metabolic Health in Healthy Individuals (EDIF): A Randomised Trial with a Controlled-Run in Phase
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
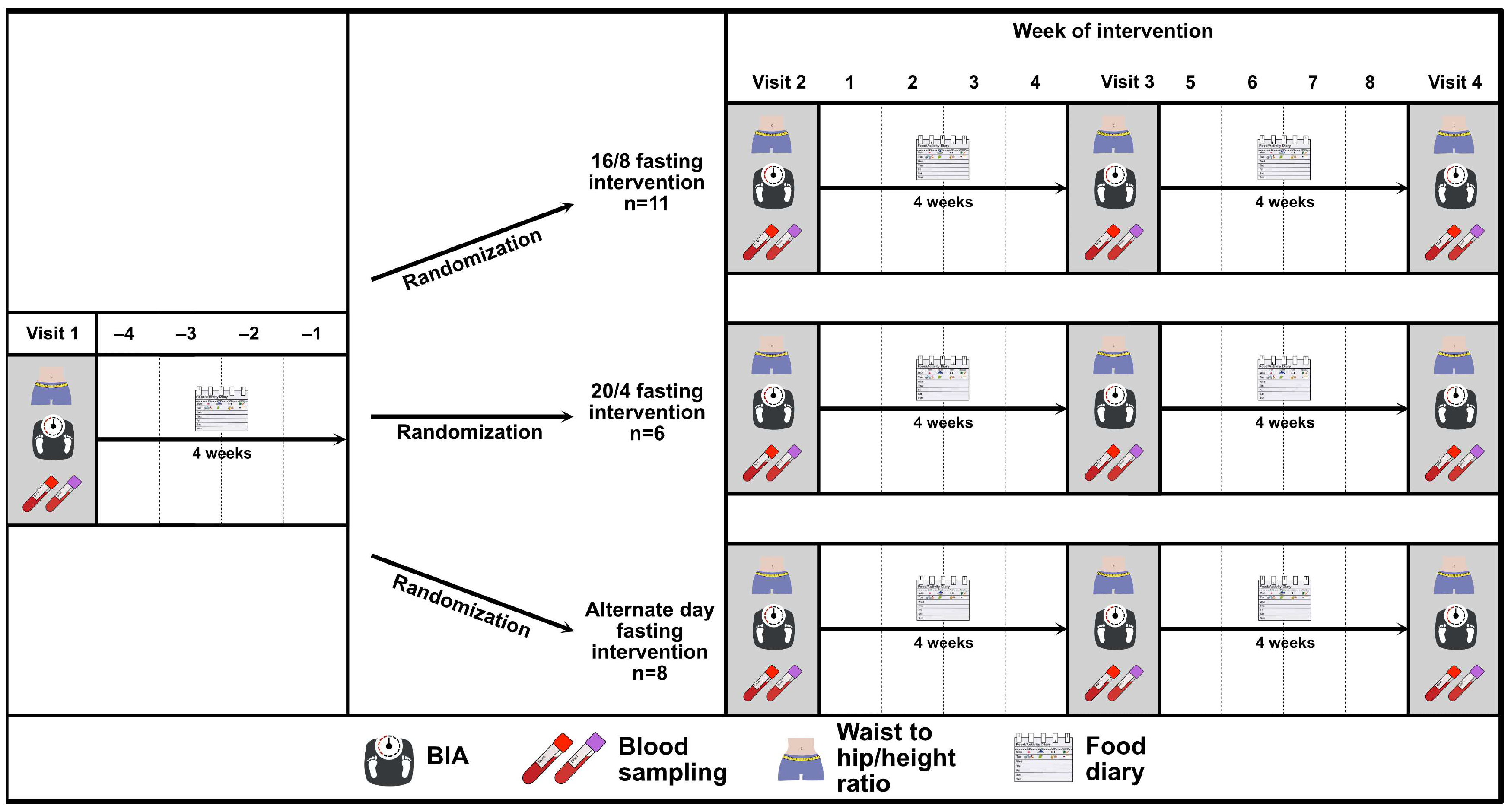
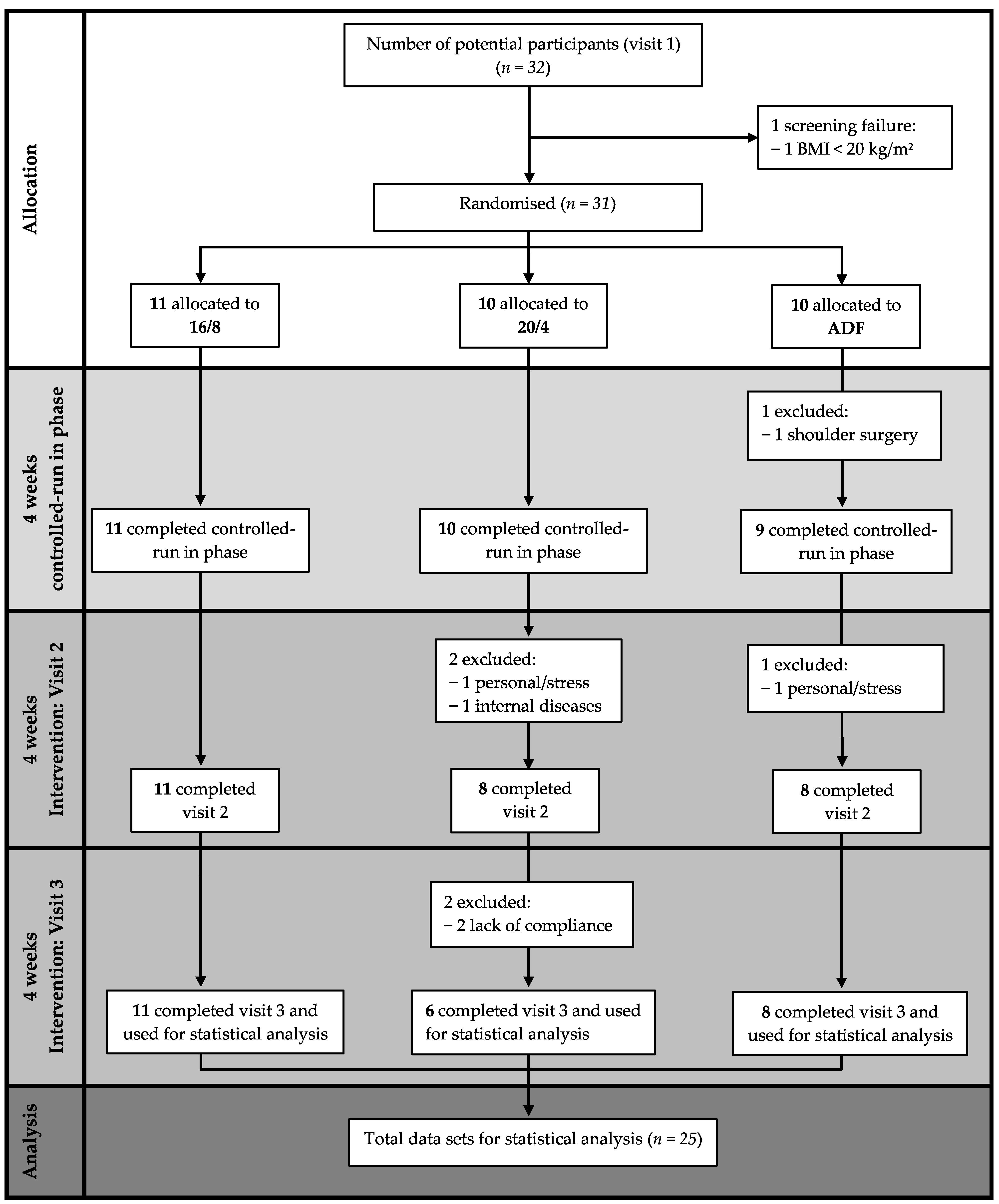
2.2. Study Design
2.3. Analytical Procedures
2.3.1. Anthropometry and Body Composition
2.3.2. Venous Blood Sampling and Storage
2.3.3. Glucose Assessment
- Oral Glucose Tolerance Test (OGTT)
- Continuous Glucose Monitoring (CGM)
2.3.4. Nutritional Tracking
2.4. Definition of the Intermittent Fasting Protocols
2.4.1. The 16/8 Intermittent Fasting (16/8) Protocol
2.4.2. The 20/4 Intermittent Fasting (20/4) Protocol
2.4.3. Alternate Day Fasting (ADF) Protocol
2.5. Statistical Analysis
3. Results
3.1. Anthropometry
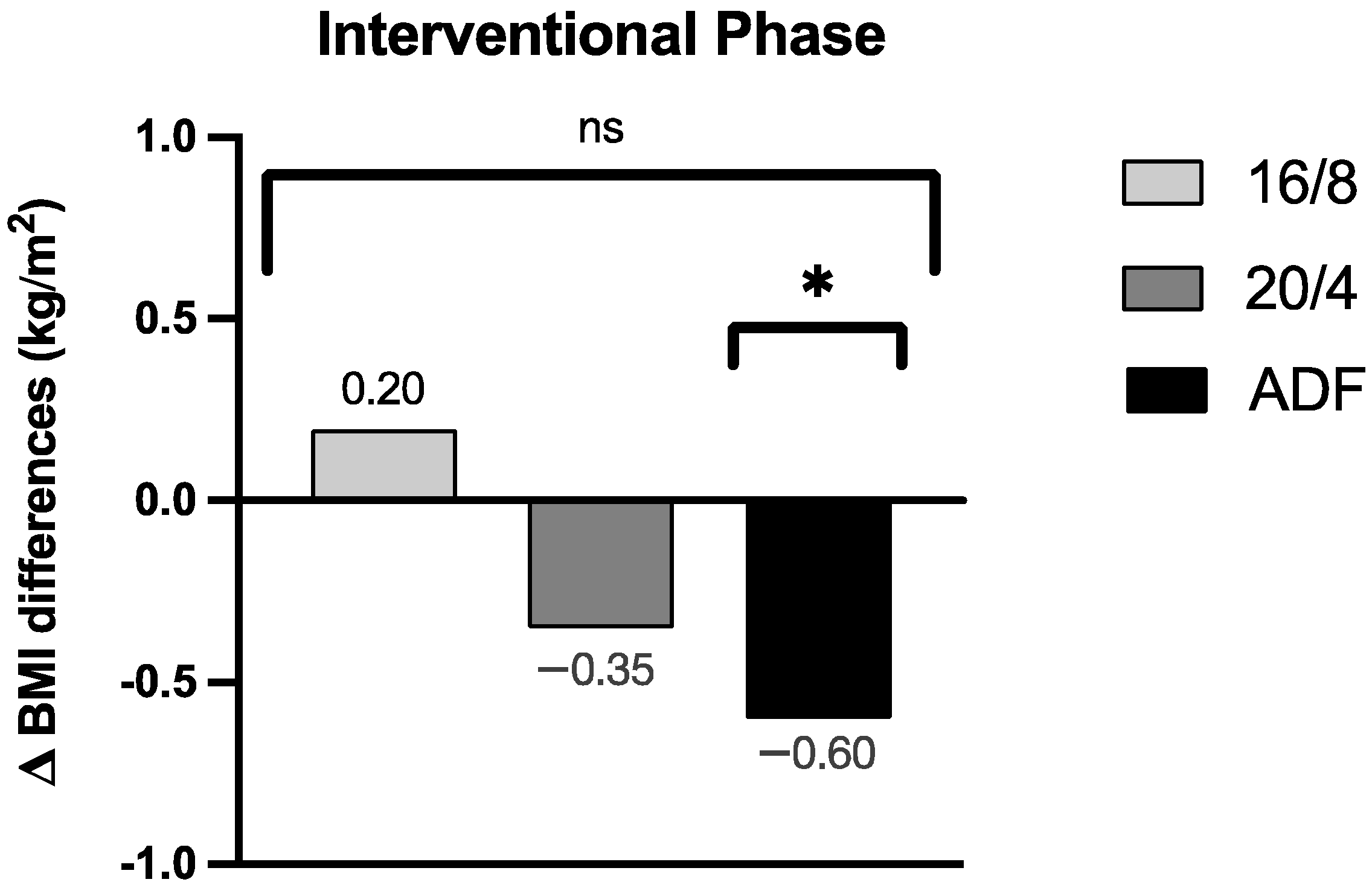
3.1.1. Body Mass Index
3.1.2. Bodyweight
3.1.3. Waist-to-Hip-Ratio
3.2. Body Composition
3.2.1. Body Fat Mass and Body Fat in Percentage
3.2.2. Fat-Free Mass
3.2.3. Skeletal Muscle Mass
3.3. Adherence and Food Diary
3.4. Metabolic and Inflammatory Markers
3.4.1. C-Reactive Protein (CRP)
3.4.2. Blood Cholesterol
3.4.3. Triglycerides
3.4.4. Ketone Bodies
3.4.5. Blood Count
3.5. Glycaemia
3.5.1. CGM-Derived Glycaemia
3.5.2. Glycaemia during OGTT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameter | Cohort | Δ Differences during Interventional Phase | p-Value (Respective Cohort) | p-Value (Group Effect) |
|---|---|---|---|---|
| Leukocytes (g/L) | 16/8 | Δ −0.05 ± 1.23 | 0.90 | 0.41 |
| 20/4 | Δ −0.88 ± 1.60 | 0.23 | ||
| ADF | Δ −0.70 ± 0.38 | 0.001 ** | ||
| Erythrocytes (g/L) | 16/8 | Δ −0.05 ± 0.20 | 0.44 | 0.28 |
| 20/4 | Δ −0.25 ± 0.52 | 0.30 | ||
| ADF | Δ −0.03 ± 0.17 | 0.68 | ||
| Haemoglobin (g/dL) | 16/8 | Δ −0.08 ± 0.51 | 0.64 | 0.61 |
| 20/4 | Δ −0.24 ± 1.50 | 0.254 | ||
| ADF | Δ −0.06 ± 0.38 | 0.66 | ||
| Haematocrit (%) | 16/8 | Δ −0.20 ± 1.81 | 0.74 | 0.45 |
| 20/4 | Δ −2.67 ± 3.98 | 0.16 | ||
| ADF | Δ −0.50 ± 1.41 | 0.35 | ||
| MCV (fl) | 16/8 | Δ −0.10 ± 1.37 | 0.82 | 0.09 |
| 20/4 | Δ 0.50 ± 2.59 | 0.66 | ||
| ADF | Δ −0.63 ± 2.13 | 0.43 | ||
| MCH (pg) | 16/8 | Δ −0.10 ± 0.57 | 0.59 | 0.29 |
| 20/4 | Δ −0.17 ± 0.75 | 0.61 | ||
| ADF | Δ −0.13 ± 0.64 | 0.60 | ||
| MCHC (g/dL) | 16/8 | Δ −0.10 ± 0.57 | 0.59 | 0.66 |
| 20/4 | Δ −0.50 ± 1.05 | 0.30 | ||
| ADF | Δ 0.38 ± 0.74 | 0.20 | ||
| RDW (%) | 16/8 | Δ −0.12 ± 0.43 | 0.40 | 0.29 |
| 20/4 | Δ 0.27 ± 0.73 | 0.41 | ||
| ADF | Δ −0.28 ± 0.41 | 0.10 | ||
| Thrombocytes (g/L) | 16/8 | Δ 3.10 ± 22.30 | 0.67 | 0.08 |
| 20/4 | Δ 1.67 ± 33.00 | 0.91 | ||
| ADF | Δ −14.13 ± 26.42 | 0.17 | ||
| MPV (fl) | 16/8 | Δ −0.20 ± 0.42 | 0.17 | 0.13 |
| 20/4 | Δ −0.17 ± 0.41 | 0.36 | ||
| ADF | Δ 0.25 ± 0.46 | 0.17 | ||
| Neutrophile granulocytes (g/L) | 16/8 | Δ −0.02 ± 1.06 | 0.95 | 0.25 |
| 20/4 | Δ −0.72 ± 1.49 | 0.29 | ||
| ADF | Δ −0.50 ± 0.45 | 0.02 * | ||
| Immature granulocytes (g/L) | 16/8 | Δ −0.001 ± 0.01 | 0.76 | 0.30 |
| 20/4 | Δ 0.00 ± 0.02 | >0.99 | ||
| ADF | Δ −0.01 ± 0.01 | 0.10 | ||
| Eosinophile granulocytes (g/L) | 16/8 | Δ 0.03 ± 0.07 | 0.25 | 0.10 |
| 20/4 | Δ −0.04 ± 0.03 | 0.02 * | ||
| ADF | Δ −0.04 ± 0.05 | 0.07 | ||
| Basophile granulocytes (g/L) | 16/8 | Δ 0.00 ± 0.01 | >0.99 | 0.23 |
| 20/4 | Δ −0.01 ± 0.01 | 0.03 * | ||
| ADF | Δ −0.01 ± 0.01 | 0.17 | ||
| Monocytes (g/L) | 16/8 | Δ −0.06 ± 0.18 | 0.35 | 0.34 |
| 20/4 | Δ −0.07 ± 0.07 | 0.045 * | ||
| ADF | Δ −0.05 ± 0.12 | 0.27 | ||
| Lymphocytes (g/L) | 16/8 | Δ 0.01 ± 0.33 | 0.96 | 0.11 |
| 20/4 | Δ −0.05 ± 0.15 | 0.41 | ||
| ADF | Δ −0.10 ± 0.47 | 0.57 |
References
- World Obesity Atlas 2023|World Obesity Federation. Available online: https://www.worldobesity.org/resources/resource-library/world-obesity-atlas-2023 (accessed on 18 September 2023).
- World Health Organization Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 24 March 2023).
- St-Onge, M.P.; Ard, J.; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; Varady, K. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement from the American Heart Association. Circulation 2017, 135, e96–e121. [Google Scholar] [CrossRef] [PubMed]
- Welton, S.; Minty, R.; O’Driscoll, T.; Willms, H.; Poirier, D.; Madden, S.; Kelly, L. Intermittent Fasting and Weight Loss: Systematic Review. Can. Fam. Physician 2020, 66, 117. [Google Scholar] [PubMed]
- Byrne, N.M.; Sainsbury, A.; King, N.A.; Hills, A.P.; Wood, R.E. Intermittent Energy Restriction Improves Weight Loss Efficiency in Obese Men: The MATADOR Study. Int. J. Obes. 2018, 42, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Heilbronn, L.K.; Smith, S.R.; Martin, C.K.; Anton, S.D.; Ravussin, E. Alternate-Day Fasting in Nonobese Subjects: Effects on Body Weight, Body Composition, and Energy Metabolism. Am. J. Clin. Nutr. 2005, 81, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Stekovic, S.; Hofer, S.J.; Tripolt, N.; Aon, M.A.; Royer, P.; Pein, L.; Stadler, J.T.; Pendl, T.; Prietl, B.; Url, J.; et al. Alternate Day Fasting Improves Physiological and Molecular Markers of Aging in Healthy, Non-Obese Humans. Cell Metab. 2019, 30, 462–476.e6. [Google Scholar] [CrossRef] [PubMed]
- Streja, D.A.; Marliss, E.B.; Steiner, G. The Effects of Prolonged Fasting on Plasma Triglyceride Kinetics in Man. Metabolism 1977, 26, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Crupi, A.N.; Haase, J.; Brandhorst, S.; Longo, V.D. Periodic and Intermittent Fasting in Diabetes and Cardiovascular Disease. Curr. Diab. Rep. 2020, 20, 83. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, A.; Tripolt, N.J.; Pferschy, P.N.; Kojzar, H.; Aziz, F.; Muller, A.; Schauer, M.; Oulhaj, A.; Aberer, F.; Sourij, C.; et al. Efficacy and Safety of Intermittent Fasting in People with Insulin-Treated Type 2 Diabetes (INTERFAST-2)—A Randomized Controlled Trial. Diabetes Care 2023, 46, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Allaf, M.; Elghazaly, H.; Mohamed, O.G.; Fareen, M.F.K.; Zaman, S.; Salmasi, A.M.; Tsilidis, K.; Dehghan, A. Intermittent Fasting for the Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2021, 2021, CD013496. [Google Scholar] [CrossRef]
- Kesztyüs, D.; Cermak, P.; Gulich, M.; Kesztyüs, T. Adherence to Time-Restricted Feeding and Impact on Abdominal Obesity in Primary Care Patients: Results of a Pilot Study in a Pre–Post Design. Nutrients 2019, 11, 2854. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A. Intermittent versus Daily Calorie Restriction: Which Diet Regimen Is More Effective for Weight Loss? Obes. Rev. 2011, 12, e593–e601. [Google Scholar] [CrossRef] [PubMed]
- Antoni, R.; Robertson, T.M.; Robertson, M.D.; Johnston, J.D. A Pilot Feasibility Study Exploring the Effects of a Moderate Time-Restricted Feeding Intervention on Energy Intake, Adiposity and Metabolic Physiology in Free-Living Human Subjects. J. Nutr. Sci. 2018, 7, e22. [Google Scholar] [CrossRef]
- Harriss, D.J.; Macsween, A.; Atkinson, G. Ethical Standards in Sport and Exercise Science Research: 2020 Update. Int. J. Sports Med. 2019, 40, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Northcote, R.J.; Canning, G.P.; Ballantyne, D. Electrocardiographic Findings in Male Veteran Endurance Athletes. Br. Heart J. 1989, 61, 155. [Google Scholar] [CrossRef] [PubMed]
- Rijnbeek, P.R.; Van Herpen, G.; Bots, M.L.; Man, S.; Verweij, N.; Hofman, A.; Hillege, H.; Numans, M.E.; Swenne, C.A.; Witteman, J.C.M.; et al. Normal Values of the Electrocardiogram for Ages 16–90 Years. J. Electrocardiol. 2014, 47, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, G.C.; Plous, S. Research Randomizer (Version 4.0) [Computer Software]. Available online: https://www.randomizer.org/about/ (accessed on 14 June 2022).
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Moser, O.; Tripolt, N.; Pferschy, P.; Obermayer, A.; Kojzar, H.; Mueller, A.; Yildirim, H.; Sourij, C.; Eckstein, M.; Sourij, H. Performance of the Intermittently Scanned Continuous Glucose Monitoring (IsCGM) System during a High Oral Glucose Challenge in Adults with Type 1 Diabetes—A Prospective Secondary Outcome Analysis. Biosensors 2021, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Schierbauer, J.R.; Günther, S.; Haupt, S.; Zimmer, R.T.; Zunner, B.E.M.; Zimmermann, P.; Wachsmuth, N.B.; Eckstein, M.L.; Aberer, F.; Sourij, H.; et al. Accuracy of Real Time Continuous Glucose Monitoring during Different Liquid Solution Challenges in Healthy Adults: A Randomized Controlled Cross-Over Trial. Sensors 2022, 22, 3104. [Google Scholar] [CrossRef]
- Battelino, T.; Danne, T.; Bergenstal, R.M.; Amiel, S.A.; Beck, R.; Biester, T.; Bosi, E.; Buckingham, B.A.; Cefalu, W.T.; Close, K.L.; et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care 2019, 42, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Baum Martinez, I.; Peters, B.; Schwarz, J.; Schuppelius, B.; Steckhan, N.; Koppold-Liebscher, D.A.; Michalsen, A.; Pivovarova-Ramich, O. Validation of a Smartphone Application for the Assessment of Dietary Compliance in an Intermittent Fasting Trial. Nutrients 2022, 14, 3697. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Hong, N.; Kim, K.W.; Cho, S.J.; Lee, M.; Lee, Y.H.; Lee, Y.H.; Kang, E.S.; Cha, B.S.; Lee, B.W. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 1645. [Google Scholar] [CrossRef] [PubMed]
- Tripepi, G.; Chesnaye, N.C.; Dekker, F.W.; Zoccali, C.; Jager, K.J. Intention to Treat and per Protocol Analysis in Clinical Trials. Nephrology 2020, 25, 513–517. [Google Scholar] [CrossRef]
- Cai, H.; Qin, Y.L.; Shi, Z.Y.; Chen, J.H.; Zeng, M.J.; Zhou, W.; Chen, R.Q.; Chen, Z.Y. Effects of Alternate-Day Fasting on Body Weight and Dyslipidaemia in Patients with Non-Alcoholic Fatty Liver Disease: A Randomised Controlled Trial. BMC Gastroenterol. 2019, 19, 219. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; McStay, M.; Gabel, K.; Varady, K.A. Time Restricted Eating for the Prevention of Type 2 Diabetes. J. Physiol. 2022, 600, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.S.; Manoogian, E.N.C.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S.; et al. Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans Who Are Overweight: A Feasibility Study. Obesity 2020, 28, 860–869. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Maranhão Pureza, I.R.; da Silva Junior, A.E.; Silva Praxedes, D.R.; Lessa Vasconcelos, L.G.; de Lima Macena, M.; Vieira de Melo, I.S.; de Menezes Toledo Florêncio, T.M.; Bueno, N.B. Effects of Time-Restricted Feeding on Body Weight, Body Composition and Vital Signs in Low-Income Women with Obesity: A 12-Month Randomized Clinical Trial. Clin. Nutr. 2021, 40, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men with Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of Eight Weeks of Time-Restricted Feeding (16/8) on Basal Metabolism, Maximal Strength, Body Composition, Inflammation, and Cardiovascular Risk Factors in Resistance-Trained Males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Arguin, H.; Dionne, I.J.; Sénéchal, M.; Bouchard, D.R.; Carpentier, A.C.; Ardilouze, J.L.; Tremblay, A.; Leblanc, C.; Brochu, M. Short- and Long-Term Effects of Continuous versus Intermittent Restrictive Diet Approaches on Body Composition and the Metabolic Profile in Overweight and Obese Postmenopausal Women: A Pilot Study. Menopause 2012, 19, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Clifton, P.M.; Keogh, J.B. Effect of Intermittent Compared with Continuous Energy Restricted Diet on Glycemic Control in Patients with Type 2 Diabetes: A Randomized Noninferiority Trial. JAMA Netw Open 2018, 1, e180756. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, S.R.; Halset, E.H.; Gåsbakk, S.; Rehfeld, J.F.; Kulseng, B.; Truby, H.; Martins, C. Compensatory Mechanisms Activated with Intermittent Energy Restriction: A Randomized Control Trial. Clin. Nutr. 2018, 37, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Seimon, R.V.; Roekenes, J.A.; Zibellini, J.; Zhu, B.; Gibson, A.A.; Hills, A.P.; Wood, R.E.; King, N.A.; Byrne, N.M.; Sainsbury, A. Do Intermittent Diets Provide Physiological Benefits over Continuous Diets for Weight Loss? A Systematic Review of Clinical Trials. Mol. Cell Endocrinol. 2015, 418 Pt 2, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.A. Intermittent Fasting and Time-Restricted Eating Role in Dietary Interventions and Precision Nutrition. Front. Public Health 2022, 10, 1017254. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G.; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, I.; Evangelista, A.; Ponzo, V.; Ciccone, G.; Soldati, L.; Santarpia, L.; Contaldo, F.; Pasanisi, F.; Ghigo, E.; Bo, S. Intermittent versus Continuous Energy Restriction on Weight Loss and Cardiometabolic Outcomes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Transl. Med. 2018, 16, 371. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L. Interventions to Promote Cardiometabolic Health and Slow Cardiovascular Ageing. Nat. Rev. Cardiol. 2018, 15, 566–577. [Google Scholar] [CrossRef]
- Chen, D.; Fulcher, J.; Scott, E.S.; Jenkins, A.J. Precision Medicine Approaches for Management of Type 2 Diabetes. In Precision Medicine in Diabetes: A Multidisciplinary Approach to an Emerging Paradigm; Springer: Cham, Switzerland, 2022; pp. 1–52. [Google Scholar] [CrossRef]
- Shazman, S. Understanding Type 2 Diabetes Mellitus Risk Parameters through Intermittent Fasting: A Machine Learning Approach. Nutrients 2023, 15, 3926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, C.; Wang, H.; Ma, Z.; Liu, D.; Guan, X.; Liu, Y.; Fu, Y.; Cui, M.; Dong, J. Intermittent Fasting versus Continuous Calorie Restriction: Which Is Better for Weight Loss? Nutrients 2022, 14, 1781. [Google Scholar] [CrossRef] [PubMed]
- Krause, L.; Lampert, T. Relation between Overweight/Obesity and Self-Rated Health among Adolescents in Germany. Do Socio-Economic Status and Type of School Have an Impact on That Relation? Int. J. Environ. Res. Public Health 2015, 12, 2262–2276. [Google Scholar] [CrossRef] [PubMed]
- Haftenberger, M.; Mensink, G.B.M.; Herzog, B.; Kluttig, A.; Greiser, K.H.; Merz, B.; Nöthlings, U.; Schlesinger, S.; Vogt, S.; Thorand, B.; et al. Changes in Body Weight and Obesity Status in German Adults: Results of Seven Population-Based Prospective Studies. Eur. J. Clin. Nutr. 2015, 70, 300–305. [Google Scholar] [CrossRef]
| Parameter | Overall (n = 25) | 16/8 (n = 11) | 20/4 (n = 6) | ADF (n = 8) | p-Values |
|---|---|---|---|---|---|
| Age (y) | 25.9 ± 3.1 | 26.3 ± 4.1 | 25.8 ± 2.1 | 25.4 ± 2.1 | 0.94 |
| Bodyweight (kg) | 78.0 ± 14.1 | 79.4 ± 18.4 | 72.5 ± 10.4 | 76.8 ± 9.2 | 0.77 |
| Height (cm) | 177.0 ± 8.5 | 177.8 ± 11.3 | 176.0 ± 6.5 | 176.4 ± 6.4 | 0.80 |
| BMI (kg/m2) | 24.8 ± 3.4 | 25.0 ± 4.5 | 23.2 ± 2.2 | 24.7 ± 2.4 | 0.90 |
| Waist-to-hip-Ratio | 0.91 ± 0.01 | 0.89 ± 0.03 | 0.88 ± 0.06 | 0.86 ± 0.03 | 0.56 |
| Parameter | Cohort | Δ Differences during Interventional Phase | p-Value (Respective Cohort) | p-Value (Group Effect) |
|---|---|---|---|---|
| Carbohydrates (kcal) | 16/8 | Δ −96.85 ± 193.7 | 0.12 | 0.41 |
| 20/4 | Δ −141.0 ± 156.4 | 0.04 * | ||
| ADF | Δ −256.2 ± 249.1 | 0.04 * | ||
| Proteins (kcal) | 16/8 | Δ −10.16 ± 58.37 | 0.60 | 0.22 |
| 20/4 | Δ 25.67 ± 42.27 | 0.16 | ||
| ADF | Δ −115.7 ± 49.82 | 0.001 * | ||
| Fats (kcal) | 16/8 | Δ −60.96 ± 189.2 | 0.34 | 0.16 |
| 20/4 | Δ 10.11 ± 126.6 | 0.83 | ||
| ADF | Δ −154.4 ± 105.0 | 0.008 * |
| Glycaemic Range | 16/8 (n = 11) | 20/4 (n = 6) | ADF (n = 8) | p-Value (Group Effect) |
|---|---|---|---|---|
| TAR (%) | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0.99 |
| TIR (%) | 98 ± 1 | 98 ± 1 | 97 ± 3 | 0.85 |
| TBR (%) | 1 ± 0 | 2 ± 1 | 2 ± 2 | >0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herz, D.; Karl, S.; Weiß, J.; Zimmermann, P.; Haupt, S.; Zimmer, R.T.; Schierbauer, J.; Wachsmuth, N.B.; Erlmann, M.P.; Niedrist, T.; et al. Effects of Different Types of Intermittent Fasting Interventions on Metabolic Health in Healthy Individuals (EDIF): A Randomised Trial with a Controlled-Run in Phase. Nutrients 2024, 16, 1114. https://doi.org/10.3390/nu16081114
Herz D, Karl S, Weiß J, Zimmermann P, Haupt S, Zimmer RT, Schierbauer J, Wachsmuth NB, Erlmann MP, Niedrist T, et al. Effects of Different Types of Intermittent Fasting Interventions on Metabolic Health in Healthy Individuals (EDIF): A Randomised Trial with a Controlled-Run in Phase. Nutrients. 2024; 16(8):1114. https://doi.org/10.3390/nu16081114
Chicago/Turabian StyleHerz, Daniel, Sebastian Karl, Johannes Weiß, Paul Zimmermann, Sandra Haupt, Rebecca Tanja Zimmer, Janis Schierbauer, Nadine Bianca Wachsmuth, Maximilian Paul Erlmann, Tobias Niedrist, and et al. 2024. "Effects of Different Types of Intermittent Fasting Interventions on Metabolic Health in Healthy Individuals (EDIF): A Randomised Trial with a Controlled-Run in Phase" Nutrients 16, no. 8: 1114. https://doi.org/10.3390/nu16081114
APA StyleHerz, D., Karl, S., Weiß, J., Zimmermann, P., Haupt, S., Zimmer, R. T., Schierbauer, J., Wachsmuth, N. B., Erlmann, M. P., Niedrist, T., Khoramipour, K., Voit, T., Rilstone, S., Sourij, H., & Moser, O. (2024). Effects of Different Types of Intermittent Fasting Interventions on Metabolic Health in Healthy Individuals (EDIF): A Randomised Trial with a Controlled-Run in Phase. Nutrients, 16(8), 1114. https://doi.org/10.3390/nu16081114







