Development and Validation of the Meiji Nutritional Profiling System (Meiji NPS) to Address Dietary Needs of Adults and Older Adults in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Scope and Principles of the Meiji NPS
2.2. Overview of Nutrients to Encourage/Limit and Food Groups to Encourage
2.3. Selection of Nutrients to Encourage
2.4. Selection of Food Groups to Encourage
2.5. Selection of Nutrients to Limit
2.6. Age-Appropriate RDVs
2.7. The Meiji NPS Algorithm
2.8. Nutrient Composition Database
2.9. Statistical Analysis
3. Results
3.1. The Meiji NPS for Adults and Older Adults
3.2. Convergent Validity between the Meiji NPS and NRF9.3
3.3. Convergent Validity of the Meiji NPS with HSR
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
The 0–100 Scaled Meiji NPS
| Items | For Adults | For Older Adults | ||
|---|---|---|---|---|
| r | p-Values | r | p-Values | |
| Total | 1.00 | <0.001 | 1.00 | <0.001 |
| Cereals | 1.00 | <0.001 | 1.00 | <0.001 |
| Potatoes and starches | 1.00 | <0.001 | 1.00 | <0.001 |
| Sugars and sweeteners | NA | NA | NA | NA |
| Pulses | 0.48 | <0.001 | 0.82 | <0.001 |
| Nuts and seeds | 0.46 | 0.0030 | NA | NA |
| Vegetables | 1.00 | <0.001 | 1.00 | <0.001 |
| Fruits | 1.00 | <0.001 | 1.00 | <0.001 |
| Mushrooms | 0.99 | <0.001 | 0.99 | <0.001 |
| Algae | 0.86 | <0.001 | 0.90 | <0.001 |
| Fish and seafood | 1.00 | <0.001 | 0.98 | <0.001 |
| Meat | 1.00 | <0.001 | 1.00 | <0.001 |
| Eggs | 1.00 | <0.001 | 0.99 | <0.001 |
| Milk and milk products | 1.00 | <0.001 | 1.00 | <0.001 |
| Fats and oils | 0.95 | 0.0513 | 1.00 | 0.0833 |
| Confectionaries | 1.00 | <0.001 | 1.00 | <0.001 |
| Beverages | 1.00 | <0.001 | 1.00 | <0.001 |
| Seasonings and spices | 0.97 | <0.001 | 0.95 | <0.001 |
References
- Drewnowski, A.; Nicklas, T.A.; O’Neil, C.E. The nutrient density approach to healthy eating: Challenges and opportunities. Public Health Nutr. 2014, 17, 2626–2636. [Google Scholar] [CrossRef]
- Drewnowski, A.; Fulgoni, V., 3rd. Nutrient profiling of foods: Creating a nutrient-rich food index. Nutr. Rev. 2008, 66, 23–39. [Google Scholar] [CrossRef]
- Drewnowski, A.; Fulgoni, V.L., 3rd. Nutrient density: Principles and evaluation tools. Am. J. Clin. Nutr. 2014, 99, 1223s–1228s. [Google Scholar] [CrossRef]
- Drewnowski, A. Uses of nutrient profiling to address public health needs: From regulation to reformulation. Proc. Nutr. Soc. 2017, 76, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Prentice, A. Sex differences in requirements for micronutrients across the lifecourse. Proc. Nutr. Soc. 2021, 80, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Dietary Reference Intakes for Japanese. 2020. Available online: https://www.mhlw.go.jp/content/001151422.pdf (accessed on 7 June 2023).
- WHO Regional Office for Europe. Use of Nutrient Profile Models for Nutrition and Health Policies: Meeting Report on the Use of Nutrient Models in the WHO Europian Region. Available online: https://www.who.int/europe/publications/i/item/WHO-EURO-2022-6201-45966-66383 (accessed on 2 February 2024).
- Dubois, P.; Albuquerque, P.; Allais, O.; Bonnet, C.; Bertail, P.; Combris, P.; Lahlou, S.; Rigal, N.; Ruffieux, B.; Chandon, P. Effects of front-of-pack labels on the nutritional quality of supermarket food purchases: Evidence from a large-scale randomized controlled trial. J. Acad. Mark. Sci. 2021, 49, 119–138. [Google Scholar] [CrossRef]
- Ahn, C.; Lee, C.G. Effect of NUTRI-SCORE labeling on sales of food items in stores at sports and non-sports facilities. Prev. Med. Rep. 2022, 29, 101919. [Google Scholar] [CrossRef] [PubMed]
- Egnell, M.; Boutron, I.; Péneau, S.; Ducrot, P.; Touvier, M.; Galan, P.; Fezeu, L.; Porcher, R.; Ravaud, P.; Hercberg, S.; et al. Impact of the Nutri-Score front-of-pack nutrition label on purchasing intentions of individuals with chronic diseases: Results of a randomised trial. BMJ Open 2022, 12, e058139. [Google Scholar] [CrossRef] [PubMed]
- About Health Star Ratings. Available online: http://healthstarrating.gov.au/internet/healthstarrating/publishing.nsf/Content/About-health-stars (accessed on 2 February 2024).
- Australia’s Health. 2014. Available online: https://www.aihw.gov.au/reports/australias-health/australias-health-2014/overview (accessed on 2 February 2024).
- Australian Dietary Guidelines. Available online: https://www.nhmrc.gov.au/adg (accessed on 2 February 2024).
- Nijman, C.A.; Zijp, I.M.; Sierksma, A.; Roodenburg, A.J.; Leenen, R.; van den Kerkhoff, C.; Weststrate, J.A.; Meijer, G.W. A method to improve the nutritional quality of foods and beverages based on dietary recommendations. Eur. J. Clin. Nutr. 2007, 61, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, U.; Charles, V.R.; Vlassopoulos, A.; Masset, G.; Spieldenner, J. Nutrient profiling for product reformulation: Public health impact and benefits for the consumer. Proc. Nutr. Soc. 2017, 76, 255–264. [Google Scholar] [CrossRef]
- Vlassopoulos, A.; Masset, G.; Charles, V.R.; Hoover, C.; Chesneau-Guillemont, C.; Leroy, F.; Lehmann, U.; Spieldenner, J.; Tee, E.S.; Gibney, M.; et al. A nutrient profiling system for the (re)formulation of a global food and beverage portfolio. Eur. J. Nutr. 2017, 56, 1105–1122. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.; Drewnowski, A.; Black, R.; Weststrate, J.A.; O’Shea, M. A Progressive nutrient profiling system to guide improvements in nutrient density of foods and beverages. Front. Nutr. 2021, 8, 774409. [Google Scholar] [CrossRef]
- Global Access to Nutrition Index 2021: Methodology. Available online: https://accesstonutrition.org/app/uploads/2020/06/Global-Index-2021-Methodology-FINAL.pdf (accessed on 2 February 2024).
- Nutrient Profiling: Report of a WHO/IASO Technical Meeting. Available online: https://www.yumpu.com/en/document/view/49710124/nutrient-profiling-world-health-organization (accessed on 2 February 2024).
- Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 2 February 2024).
- Nutrition Policy in Japan to Leave No One Behind. Available online: https://www.mhlw.go.jp/nutrition_policy/en (accessed on 2 February 2024).
- Hu, F.B. Diet strategies for promoting healthy aging and longevity: An epidemiological perspective. J. Intern. Med. 2023, 295, 508–531. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, T.; Tanemura, N.; Kimura, S.; Urushihara, H. Utility of a Specific Health Checkup database containing lifestyle behaviors and lifestyle diseases for employee health insurance in Japan. J. Epidemiol. 2020, 30, 57–66. [Google Scholar] [CrossRef]
- Kadoya, Y.; Hara, M.; Takahari, K.; Ishida, Y.; Tamaki, M. Disease control status and safety of telemedicine in patients with lifestyle diseases—A multicenter prospective observational study in Japan. Circ. Rep. 2020, 2, 351–356. [Google Scholar] [CrossRef]
- Katoch, O.R. Determinants of malnutrition among children: A systematic review. Nutrition 2022, 96, 111565. [Google Scholar] [CrossRef]
- Christian, P.; Smith, E.R. Adolescent undernutrition: Global burden, physiology, and nutritional risks. Ann. Nutr. Metab. 2018, 72, 316–328. [Google Scholar] [CrossRef]
- Wells, J.C.K.; Briend, A.; Boyd, E.M.; Berkely, J.A.; Hall, A.; Isanaka, S.; Webb, P.; Khara, T.; Dolan, C. Beyond wasted and stunted-a major shift to fight child undernutrition. Lancet Child. Adolesc. Health 2019, 3, 831–834. [Google Scholar] [CrossRef]
- Gallagher, P.G. Anemia in the pediatric patient. Blood 2022, 140, 571–593. [Google Scholar] [CrossRef]
- Summary of the Report of the Committee Meeting on the Promotion of a Healthy and Sustainable Food Environment. Available online: https://www.mhlw.go.jp/content/10900000/000836945.pdf (accessed on 2 February 2024).
- Strategic Initiative for a Healthy and Sustainable Food Environment (HSFE). Available online: https://sustainable-nutrition.mhlw.go.jp/en (accessed on 2 February 2024).
- Kurabayashi, T.; Nagai, K.; Morikawa, K.; Kamimura, N.; Yanase, T.; Hayashi, K. Prevalence of osteoporosis and osteopenia assessed by densitometry in Japanese puerperal women. J. Obstet. Gynaecol. Res. 2021, 47, 1388–1396. [Google Scholar] [CrossRef]
- Annual Report on the Ageing Society. Available online: https://www8.cao.go.jp/kourei/english/annualreport/index-wh.html (accessed on 2 February 2024).
- Chang, A.Y.; Skirbekk, V.F.; Tyrovolas, S.; Kassebaum, N.J.; Dieleman, J.L. Measuring population ageing: An analysis of the Global Burden of Disease Study 2017. Lancet Public Health 2019, 4, e159–e167. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Shaheen, M.; Grover, B. Nutrition and cognitive health: A life course approach. Front. Public Health 2023, 11, 1023907. [Google Scholar] [CrossRef] [PubMed]
- Azzolino, D.; Spolidoro, G.C.I.; Saporiti, E.; Luchetti, C.; Agostoni, C.; Cesari, M. Musculoskeletal changes across the lifespan: Nutrition and the life-course approach to prevention. Front. Med. 2021, 8, 697954. [Google Scholar] [CrossRef] [PubMed]
- Darnton-Hill, I.; Nishida, C.; James, W.P. A life course approach to diet, nutrition and the prevention of chronic diseases. Public Health Nutr. 2004, 7, 101–121. [Google Scholar] [CrossRef] [PubMed]
- Kac, G.; Pérez-Escamilla, R. Nutrition transition and obesity prevention through the life-course. Int. J. Obes. Suppl. 2013, 3, S6–S8. [Google Scholar] [CrossRef]
- Rodríguez-Mañas, L.; Murray, R.; Glencorse, C.; Sulo, S. Good nutrition across the lifespan is foundational for healthy aging and sustainable development. Front. Nutr. 2022, 9, 1113060. [Google Scholar] [CrossRef] [PubMed]
- Kashino, I.; Mizoue, T.; Yoshita, K.; Uenishi, K.; Hasegawa, Y.; Saito, H.; Aoyagi, S.; Kuranuki, S.; Nakamura, T. Evaluation of foods by using nutrient profile model. J. Jpn. Diet. Assoc. 2018, 61, 445–450. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Yoshizaki, T.; Tada, Y.; Okada, E.; Takebayashi, J.; Takimoto, H.; Ishimi, Y. A study on the characteristics of nutrient profile models in other countries for the development of a Japanese nutrient profile model. Jpn. J. Nutr. Diet. 2021, 79, 162–173. [Google Scholar] [CrossRef]
- Furuta, C.; Jinzu, H.; Cao, L.; Drewnowski, A.; Okabe, Y. Nutrient profiling of Japanese dishes: The development of a novel Ajinomoto Group Nutrient Profiling System. Front. Nutr. 2022, 9, 912148. [Google Scholar] [CrossRef]
- Oono, F.; Murakami, K.; Fujiwara, A.; Shinozaki, N.; Adachi, R.; Asakura, K.; Masayasu, S.; Sasaki, S. Development of a Diet Quality Score for Japanese and comparison with existing diet quality scores regarding inadequacy of nutrient intake. J. Nutr. 2023, 153, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, N.; Murakami, K.; Masayasu, S.; Sasaki, S. Usual nutrient intake distribution and prevalence of nutrient intake inadequacy among Japanese children and adults: A nationwide study based on 8-day dietary records. Nutrients 2023, 15, 5113. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.T.; Astrup, A.; Sjödin, A. Are dietary proteins the key to successful body weight management? A systematic review and meta-analysis of studies assessing body weight outcomes after interventions with increased dietary protein. Nutrients 2021, 13, 3193. [Google Scholar] [CrossRef] [PubMed]
- Huwiler, V.V.; Schönenberger, K.A.; Segesser von Brunegg, A.; Reber, E.; Mühlebach, S.; Stanga, Z.; Balmer, M.L. Prolonged isolated soluble dietary fibre supplementation in overweight and obese patients: A systematic review with meta-analysis of randomised controlled trials. Nutrients 2022, 14, 2627. [Google Scholar] [CrossRef] [PubMed]
- Jovanovski, E.; Mazhar, N.; Komishon, A.; Khayyat, R.; Li, D.; Blanco Mejia, S.; Khan, T.; Jenkins, A.L.; Smircic-Duvnjak, L.; Sievenpiper, J.L.; et al. Effect of viscous fiber supplementation on obesity indicators in individuals consuming calorie-restricted diets: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Nutr. 2021, 60, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemy, Z.; Rouhani, P.; Saneei, P. Dietary calcium intake in relation to type-2 diabetes and hyperglycemia in adults: A systematic review and dose-response meta-analysis of epidemiologic studies. Sci. Rep. 2022, 12, 1050. [Google Scholar] [CrossRef]
- Cormick, G.; Ciapponi, A.; Cafferata, M.L.; Cormick, M.S.; Belizán, J.M. Calcium supplementation for prevention of primary hypertension. Cochrane Database Syst. Rev. 2022, 1, Cd010037. [Google Scholar] [CrossRef]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Hirakawa, Y.; Doi, Y.; Hata, J.; Uchida, K.; Shirota, T.; Kitazono, T.; Kiyohara, Y. Self-reported dietary intake of potassium, calcium, and magnesium and risk of dementia in the Japanese: The Hisayama Study. J. Am. Geriatr. Soc. 2012, 60, 1515–1520. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, Y.; Zhang, D.; Liu, Y.; Xu, Z.; Gao, J.; Li, W.; Li, X. Effect of vitamin D supplementation on glycemic control in prediabetes: A meta-analysis. Nutrients 2021, 13, 4464. [Google Scholar] [CrossRef]
- Bahadorpour, S.; Hajhashemy, Z.; Saneei, P. Serum 25-hydroxyvitamin D levels and dyslipidemia: A systematic review and dose-response meta-analysis of epidemiologic studies. Nutr. Rev. 2022, 81, 1–25. [Google Scholar] [CrossRef]
- Mohammadi, S.; Hajhashemy, Z.; Saneei, P. Serum vitamin D levels in relation to type-2 diabetes and prediabetes in adults: A systematic review and dose-response meta-analysis of epidemiologic studies. Crit. Rev. Food Sci. Nutr. 2022, 62, 8178–8198. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Tokuda, Y. Differences and overlap between sarcopenia and physical frailty in older community-dwelling Japanese. Asia Pac. J. Clin. Nutr. 2019, 28, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, A.; Tsugawa, N.; Kondo, H.; Ao, M.; Fujiwara, H.; Hosokawa, N.; Matsumoto, S.; Tanaka, K.; Nakano, T. Associations between serum 25-hydroxyvitamin D(3) level and skeletal muscle mass and lower limb muscle strength in Japanese middle-aged subjects. Osteoporos. Sarcopenia 2017, 3, 53–58. [Google Scholar] [CrossRef]
- Mizuno, T.; Hosoyama, T.; Tomida, M.; Yamamoto, Y.; Nakamichi, Y.; Kato, S.; Kawai-Takaishi, M.; Ishizuka, S.; Nishita, Y.; Tange, C.; et al. Influence of vitamin D on sarcopenia pathophysiology: A longitudinal study in humans and basic research in knockout mice. J. Cachexia Sarcopenia Muscle 2022, 13, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Asakura, K.; Etoh, N.; Imamura, H.; Michikawa, T.; Nakamura, T.; Takeda, Y.; Mori, S.; Nishiwaki, Y. Vitamin D status in Japanese adults: Relationship of serum 25-hydroxyvitamin D with simultaneously measured dietary vitamin D intake and ultraviolet ray exposure. Nutrients 2020, 12, 743. [Google Scholar] [CrossRef]
- Weiler, H.A.; Sarafin, K.; Martineau, C.; Daoust, J.L.; Esslinger, K.; Greene-Finestone, L.S.; Loukine, L.; Dorais, V. Vitamin D status of people 3 to 79 years of age from the Canadian Health Measures Survey 2012–2019. J. Nutr. 2023, 153, 1150–1161. [Google Scholar] [CrossRef]
- Nasimi, N.; Sohrabi, Z.; Nunes, E.A.; Sadeghi, E.; Jamshidi, S.; Gholami, Z.; Akbarzadeh, M.; Faghih, S.; Akhlaghi, M.; Phillips, S.M. Whey protein supplementation with or without vitamin D on sarcopenia-related measures: A systematic review and meta-analysis. Adv. Nutr. 2023, 14, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Mesinovic, J.; Rodriguez, A.J.; Cervo, M.M.; Gandham, A.; Xu, C.L.H.; Glavas, C.; de Courten, B.; Zengin, A.; Ebeling, P.R.; Scott, D. Vitamin D supplementation and exercise for improving physical function, body composition and metabolic health in overweight or obese older adults with vitamin D deficiency: A pilot randomized, double-blind, placebo-controlled trial. Eur. J. Nutr. 2023, 62, 951–964. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, P.; Liu, P.; Hao, Q.; Chen, S.; Dong, B.; Wang, J. Association of vitamin D deficiency and frailty: A systematic review and meta-analysis. Maturitas 2016, 94, 70–76. [Google Scholar] [CrossRef]
- Jia, J.; Hu, J.; Huo, X.; Miao, R.; Zhang, Y.; Ma, F. Effects of vitamin D supplementation on cognitive function and blood Aβ-related biomarkers in older adults with Alzheimer’s disease: A randomised, double-blind, placebo-controlled trial. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1347–1352. [Google Scholar] [CrossRef]
- Sommer, I.; Griebler, U.; Kien, C.; Auer, S.; Klerings, I.; Hammer, R.; Holzer, P.; Gartlehner, G. Vitamin D deficiency as a risk factor for dementia: A systematic review and meta-analysis. BMC Geriatr. 2017, 17, 16. [Google Scholar] [CrossRef]
- Kalra, A.; Teixeira, A.L.; Diniz, B.S. Association of vitamin D levels with incident all-cause dementia in longitudinal observational studies: A systematic review and meta-analysis. J. Prev. Alzheimers Dis. 2020, 7, 14–20. [Google Scholar] [CrossRef]
- Jayedi, A.; Rashidy-Pour, A.; Shab-Bidar, S. Vitamin D status and risk of dementia and Alzheimer’s disease: A meta-analysis of dose-response (†). Nutr. Neurosci. 2019, 22, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Tateishi, Y.; Ichikawa, R.; Suzuki, K.; Kitahara, Y.; Someya, Y.; Tamura, Y. Effect of imbalance in dietary macronutrients on blood hemoglobin levels: A cross-sectional study in young underweight Japanese women. Front. Nutr. 2023, 10, 1121717. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, K.; Sato, H.; Kobae, K.; Yanagi, K.; Yamada, Y.; Ushiroda, C.; Hirano, K.; Ichimaru, S.; Seino, Y.; Ito, A.; et al. Young Japanese underweight women with “Cinderella weight” are prone to malnutrition, including vitamin deficiencies. Nutrients 2023, 15, 2216. [Google Scholar] [CrossRef] [PubMed]
- [List of Health Japan 21 Target Values] Kenkou Nihon 21 Mokuhyouchi Ichiran. Available online: https://www.mhlw.go.jp/www1/topics/kenko21_11/t2a.html (accessed on 2 February 2024). (In Japanese)
- Wakayama, R.; Takasugi, S.; Honda, K.; Kanaya, S. Application of a two-dimensional mapping-based visualization technique: Nutrient-value-based food grouping. Nutrients 2023, 15, 5006. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Zhang, Z.; Fullington, L.A.; Huang, T.T.; Kaliszewski, C.; Wei, J.; Zhao, L.; Huang, S.; Ellithorpe, A.; Wu, S.; et al. Dietary patterns and obesity in Chinese adults: A systematic review and meta-analysis. Nutrients 2022, 14, 4911. [Google Scholar] [CrossRef] [PubMed]
- Eslami, O.; Khorramrouz, F.; Sohouli, M.; Bagheri, N.; Shidfar, F.; Fernandez, M.L. Effect of nuts on components of metabolic syndrome in healthy adults with overweight/obesity: A systematic review and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Ge, L.; Johnston, B.C.; Shahinfar, H.; Safabakhsh, M.; Mohamadpur, S.; Ghorbaninejad, P.; Abyadeh, M.; Zeraattalab-Motlagh, S.; Soltani, S.; et al. Comparative effectiveness of single foods and food groups on body weight: A systematic review and network meta-analysis of 152 randomized controlled trials. Eur. J. Nutr. 2023, 62, 1153–1164. [Google Scholar] [CrossRef] [PubMed]
- Asbaghi, O.; Sadeghian, M.; Rahmani, S.; Mardani, M.; Khodadost, M.; Maleki, V.; Pirouzi, A.; Talebi, S.; Sadeghi, O. The effect of green coffee extract supplementation on anthropometric measures in adults: A comprehensive systematic review and dose-response meta-analysis of randomized clinical trials. Complement. Ther. Med. 2020, 51, 102424. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, R.; Mesas, A.E.; Garrido-Miguel, M.; Martínez-Ortega, I.A.; Jiménez-López, E.; Martínez-Vizcaíno, V. The relationship of tree nuts and peanuts with adiposity parameters: A systematic review and network meta-analysis. Nutrients 2021, 13, 2251. [Google Scholar] [CrossRef] [PubMed]
- Nishi, S.K.; Viguiliouk, E.; Blanco Mejia, S.; Kendall, C.W.C.; Bazinet, R.P.; Hanley, A.J.; Comelli, E.M.; Salas Salvadó, J.; Jenkins, D.J.A.; Sievenpiper, J.L. Are fatty nuts a weighty concern? A systematic review and meta-analysis and dose-response meta-regression of prospective cohorts and randomized controlled trials. Obes. Rev. 2021, 22, e13330. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, Y.; Liu, J.; Huang, Z.; Yang, X.; Qin, P.; Chen, C.; Luo, X.; Li, Y.; Wu, Y.; et al. Consumption of dairy products and the risk of overweight or obesity, hypertension, and type 2 diabetes mellitus: A dose-response meta-analysis and systematic review of cohort studies. Adv. Nutr. 2022, 13, 2165–2179. [Google Scholar] [CrossRef] [PubMed]
- Mullins, A.P.; Arjmandi, B.H. Health benefits of plant-based nutrition: Focus on beans in cardiometabolic diseases. Nutrients 2021, 13, 519. [Google Scholar] [CrossRef] [PubMed]
- Hirahatake, K.M.; Astrup, A.; Hill, J.O.; Slavin, J.L.; Allison, D.B.; Maki, K.C. Potential cardiometabolic health benefits of full-fat dairy: The evidence base. Adv. Nutr. 2020, 11, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Alissa, E.M.; Ferns, G.A. Dietary fruits and vegetables and cardiovascular diseases risk. Crit. Rev. Food Sci. Nutr. 2017, 57, 1950–1962. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.J.; Kim, M.H.; Choi, M.K. Dietary mineral intake from nuts and its relationship to hypertension among Korean adults. Biol. Trace Elem. Res. 2022, 200, 3519–3528. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Iqbal, K.; Andriolo, V.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of hypertension: A systematic review and dose-response meta-analysis of prospective studies. Adv. Nutr. 2017, 8, 793–803. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Zhou, A.; Cavadino, A.; Hyppönen, E. Evidence for a causal association between milk intake and cardiometabolic disease outcomes using a two-sample Mendelian Randomization analysis in up to 1,904,220 individuals. Int. J. Obes. 2021, 45, 1751–1762. [Google Scholar] [CrossRef]
- Wilunda, C.; Sawada, N.; Goto, A.; Yamaji, T.; Iwasaki, M.; Tsugane, S.; Noda, M. Soy food and isoflavones are not associated with changes in serum lipids and glycohemoglobin concentrations among Japanese adults: A cohort study. Eur. J. Nutr. 2020, 59, 2075–2087. [Google Scholar] [CrossRef]
- Wong, T.H.T.; Burlutsky, G.; Gopinath, B.; Flood, V.M.; Mitchell, P.; Louie, J.C.Y. The longitudinal association between coffee and tea consumption and the risk of metabolic syndrome and its component conditions in an older adult population. J. Nutr. Sci. 2022, 11, e79. [Google Scholar] [CrossRef]
- Wang, N.; Deng, Z.; Wen, L.; Ding, Y.; He, G. Relationships between maternal dietary patterns and blood lipid levels during pregnancy: A prospective cohort study in Shanghai, China. Int. J. Environ. Res. Public Health 2021, 18, 3701. [Google Scholar] [CrossRef]
- Buscemi, S.; Corleo, D.; Buscemi, C.; Randazzo, C.; Borzì, A.M.; Barile, A.M.; Rosafio, G.; Ciaccio, M.; Caldarella, R.; Meli, F.; et al. Influence of Habitual Dairy Food Intake on LDL Cholesterol in a Population-Based Cohort. Nutrients 2021, 13, 593. [Google Scholar] [CrossRef]
- Ushula, T.W.; Mamun, A.; Darssan, D.; Wang, W.Y.S.; Williams, G.M.; Whiting, S.J.; Najman, J.M. Dietary patterns and the risk of abnormal blood lipids among young adults: A prospective cohort study. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1165–1174. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H.; Rebholz, C.M.; Kim, J. Association between different types of plant-based diets and risk of dyslipidemia: A prospective cohort study. Nutrients 2021, 13, 220. [Google Scholar] [CrossRef]
- Kim, J.; Hoang, T.; Bu, S.Y.; Kim, J.M.; Choi, J.H.; Park, E.; Lee, S.M.; Park, E.; Min, J.Y.; Lee, I.S.; et al. Associations of dietary intake with cardiovascular disease, blood pressure, and lipid profile in the Korean population: A systematic review and meta-analysis. J. Lipid Atheroscler. 2020, 9, 205–229. [Google Scholar] [CrossRef]
- Xu, L.; Tian, Z.; Chen, H.; Zhao, Y.; Yang, Y. Anthocyanins, anthocyanin-rich berries, and cardiovascular risks: Systematic review and meta-analysis of 44 randomized controlled trials and 15 prospective cohort studies. Front. Nutr. 2021, 8, 747884. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.; Fanidi, A.; Bishop, T.R.P.; Sharp, S.J.; Imamura, F.; Dietrich, S.; Akbaraly, T.; Bes-Rastrollo, M.; Beulens, J.W.J.; Byberg, L.; et al. Associations of total legume, pulse, and soy consumption with incident type 2 diabetes: Federated meta-analysis of 27 studies from diverse world regions. J. Nutr. 2021, 151, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Japanese Food Standard Composition Table 2020 Edition (8th Edition). Available online: https://www.mext.go.jp/a_menu/syokuhinseibun/mext_01110.html (accessed on 2 February 2024). (In Japanese)
- McGlynn, N.D.; Khan, T.A.; Wang, L.; Zhang, R.; Chiavaroli, L.; Au-Yeung, F.; Lee, J.J.; Noronha, J.C.; Comelli, E.M.; Blanco Mejia, S.; et al. Association of low- and no-calorie sweetened beverages as a replacement for sugar-sweetened beverages with body weight and cardiometabolic risk: A systematic review and meta-analysis. JAMA Netw. Open 2022, 5, e222092. [Google Scholar] [CrossRef]
- Baba, A.; Sugawara, K. Drive for thinness in adolescent women. Jpn. J. Educ. Psychol. 2000, 48, 267–274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsumoto, M.; Tajima, R.; Fujiwara, A.; Yuan, X.; Okada, E.; Takimoto, H. Trends in food group intake according to body size among young Japanese women: The 2001–2019 National Health and Nutrition Survey. Nutrients 2022, 14, 4078. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Asakura, K.; Sasaki, S. Differential dietary habits among 570 young underweight Japanese women with and without a desire for thinness: A comparison with normal weight counterparts. Asia Pac. J. Clin. Nutr. 2016, 25, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, R.; Zhang, S.; Tange, C.; Nishita, Y.; Tomida, M.; Kinoshita, K.; Kato, Y.; Ando, F.; Shimokata, H.; Arai, H. Association of dietary intake with the transitions of frailty among Japanese community-dwelling older adults. J. Frailty Aging 2022, 11, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, R.; Tange, C.; Tomida, M.; Nishita, Y.; Kato, Y.; Yuki, A.; Ando, F.; Shimokata, H.; Arai, H. Dietary factors associated with the development of physical frailty in community-dwelling older adults. J. Nutr. Health Aging 2019, 23, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Jyväkorpi, S.K.; Urtamo, A.; Kivimäki, M.; Strandberg, T.E. Macronutrient composition and sarcopenia in the oldest-old men: The Helsinki Businessmen Study (HBS). Clin. Nutr. 2020, 39, 3839–3841. [Google Scholar] [CrossRef]
- Saturated Fatty Acid and Trans-Fatty Acid Intake for Adults and Children: WHO Guideline. Available online: https://www.who.int/publications/i/item/9789240073630 (accessed on 2 February 2024).
- Guideline: Sugars Intake for Adults and Children. Available online: https://www.who.int/publications/i/item/9789241549028 (accessed on 2 February 2024).
- Beal, T.; Ortenzi, F.; Fanzo, J. Estimated micronutrient shortfalls of the EAT-Lancet planetary health diet. Lancet Planet. Health 2023, 7, e233–e237. [Google Scholar] [CrossRef]
- Sasaki, S. [Consideration of Standards for a "Healthy Diet" from the Perspective of Ensuring the Nutritional Balance Necessary to Maintain and Improve Health] Kenkou no iji Zoushin ni Hitsuyou to Sareru Eiyou Baransu no Kakuho Kara Mita "Kenkou na Shokuji" no Kijun ni Tuite no Kentou; Ministry of Health, Labour and Welfare: Tokyo, Japan, 2014. (In Japanese)
- Fulgoni, V.L., 3rd; Keast, D.R.; Drewnowski, A. Development and validation of the nutrient-rich foods index: A tool to measure nutritional quality of foods. J. Nutr. 2009, 139, 1549–1554. [Google Scholar] [CrossRef]
- Drewnowski, A. The Nutrient Rich Foods Index helps to identify healthy, affordable foods. Am. J. Clin. Nutr. 2010, 91, 1095s–1101s. [Google Scholar] [CrossRef]
- Drewnowski, A.; Amanquah, D.; Gavin-Smith, B. Perspective: How to develop nutrient profiling models intended for global use: A manual. Adv. Nutr. 2021, 12, 609–620. [Google Scholar] [CrossRef]
- Drewnowski, A. Defining nutrient density: Development and validation of the nutrient rich foods index. J. Am. Coll. Nutr. 2009, 28, 421s–426s. [Google Scholar] [CrossRef]
- Zhai, J.; Ma, B.; Lyu, Q.; Guo, L.; Khatun, P.; Liang, R.; Cong, M.; Kong, Y. Validation of the nutrient-rich foods index estimated by 24-h dietary recall method among adults in Henan province of China. Public Health Nutr. 2022, 25, 1–9. [Google Scholar] [CrossRef]
- Murakami, K.; Livingstone, M.B.E.; Fujiwara, A.; Sasaki, S. Application of the Healthy Eating Index-2015 and the Nutrient-Rich Food Index 9.3 for assessing overall diet quality in the Japanese context: Different nutritional concerns from the US. PLoS ONE 2020, 15, e0228318. [Google Scholar] [CrossRef]
- National Health and Nutrition Survey. Available online: https://www.nibiohn.go.jp/eiken/kenkounippon21/en/eiyouchousa/ (accessed on 2 February 2024).
- Merchant, A.T.; Dehghan, M. Food composition database development for between country comparisons. Nutr. J. 2006, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Pennington, J.A.T. Food composition databases for bioactive food components. J. Food Compos. Anal. 2002, 15, 419–434. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Excel Calculator. Available online: http://www.healthstarrating.gov.au/internet/healthstarrating/publishing.nsf/Content/excel-calculator (accessed on 2 February 2024).
- Suzuki, T.; Kojima, N.; Osuka, Y.; Tokui, Y.; Takasugi, S.; Kawashima, A.; Yamaji, T.; Hosoi, E.; Won, C.W.; Kim, H. The effects of mold-fermented cheese on brain-derived neurotrophic factor in community-dwelling older Japanese women with mild cognitive impairment: A randomized, controlled, crossover trial. J. Am. Med. Dir. Assoc. 2019, 20, 1509–1514.e2. [Google Scholar] [CrossRef]
- Kim, H.; Osuka, Y.; Kojima, N.; Sasai, H.; Nakamura, K.; Oba, C.; Sasaki, M.; Suzuki, T. Inverse association between cheese consumption and lower cognitive function in Japanese community-dwelling older adults based on a cross-sectional study. Nutrients 2023, 15, 3181. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Triana, F.; Verdejo-Bravo, C.; Fernández-Pérez, C.; Martín-Sánchez, F.J. Effect of milk and other dairy products on the risk of frailty, sarcopenia, and cognitive performance decline in the elderly: A systematic review. Adv. Nutr. 2019, 10, S105–S119. [Google Scholar] [CrossRef]
- Huang, L.; Chen, H.; Gao, M.; Shen, J.; Tao, Y.; Huang, Y.; Lv, R.; Xie, R.; Lv, X.; Xu, X.; et al. Dietary factors in relation to the risk of cognitive impairment and physical frailty in Chinese older adults: A prospective cohort study. Eur. J. Nutr. 2024, 63, 267–277. [Google Scholar] [CrossRef]
- Duan, Y.; Qi, Q.; Cui, Y.; Yang, L.; Zhang, M.; Liu, H. Effects of dietary diversity on frailty in Chinese older adults: A 3-year cohort study. BMC Geriatr. 2023, 23, 141. [Google Scholar] [CrossRef]
- Sievenpiper, J.L. Low-carbohydrate diets and cardiometabolic health: The importance of carbohydrate quality over quantity. Nutr. Rev. 2020, 78, 69–77. [Google Scholar] [CrossRef]
- Miller, V.; Mente, A.; Dehghan, M.; Rangarajan, S.; Zhang, X.; Swaminathan, S.; Dagenais, G.; Gupta, R.; Mohan, V.; Lear, S.; et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): A prospective cohort study. Lancet 2017, 390, 2037–2049. [Google Scholar] [CrossRef]
- Sakurai, M.; Ishizaki, M.; Morikawa, Y.; Kido, T.; Naruse, Y.; Nakashima, Y.; Okamoto, C.; Nogawa, K.; Watanabe, Y.; Suwazono, Y.; et al. Frequency of consumption of balanced meals, bodyweight gain and incident risk of glucose intolerance in Japanese men and women: A cohort study. J. Diabetes Investig. 2021, 12, 763–770. [Google Scholar] [CrossRef]
- Kanehara, R.; Goto, A.; Sawada, N.; Mizoue, T.; Noda, M.; Hida, A.; Iwasaki, M.; Tsugane, S. Association between sugar and starch intakes and type 2 diabetes risk in middle-aged adults in a prospective cohort study. Eur. J. Clin. Nutr. 2022, 76, 746–755. [Google Scholar] [CrossRef]
- Lu, Y.; Sugawara, Y.; Matsuyama, S.; Fukao, A.; Tsuji, I. Association of dairy intake with all-cause, cancer, and cardiovascular disease mortality in Japanese adults: A 25-year population-based cohort. Eur. J. Nutr. 2022, 61, 1285–1297. [Google Scholar] [CrossRef]
- Okada, E.; Shirakawa, T.; Shivappa, N.; Wakai, K.; Suzuki, K.; Date, C.; Iso, H.; Hébert, J.R.; Tamakoshi, A. Dietary inflammatory index is associated with risk of all-cause and cardiovascular disease mortality but not with cancer mortality in middle-aged and older Japanese adults. J. Nutr. 2019, 149, 1451–1459. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, D.D.; Brader, L.; Bruun, J.M. Association between food, beverages and overweight/obesity in children and adolescents-a systematic review and meta-analysis of observational studies. Nutrients 2023, 15, 764. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Luo, M.; Li, Y.; Zheng, J.S.; Xiao, Q.; Luo, J. Fast-food restaurant, unhealthy eating, and childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2021, 22 (Suppl. 1), e12944. [Google Scholar] [CrossRef] [PubMed]
- Dallman, M.F.; Pecoraro, N.; Akana, S.F.; La Fleur, S.E.; Gomez, F.; Houshyar, H.; Bell, M.E.; Bhatnagar, S.; Laugero, K.D.; Manalo, S. Chronic stress and obesity: A new view of “comfort food”. Proc. Natl. Acad. Sci. USA 2003, 100, 11696–11701. [Google Scholar] [CrossRef] [PubMed]
- van Strien, T.; Gibson, E.L.; Baños, R.; Cebolla, A.; Winkens, L.H.H. Is comfort food actually comforting for emotional eaters? A (moderated) mediation analysis. Physiol. Behav. 2019, 211, 112671. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.Y.; Iso, H.; Yamagishi, K.; Sawada, N.; Tsugane, S. Chocolate consumption and risk of stroke among men and women: A large population-based, prospective cohort study. Atherosclerosis 2017, 260, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Allen, J. Gender differences in healthy and unhealthy food consumption and its relationship with depression in young adulthood. Community Ment. Health J. 2021, 57, 898–909. [Google Scholar] [CrossRef]
- Matsuyama, S.; Sawada, N.; Tomata, Y.; Zhang, S.; Goto, A.; Yamaji, T.; Iwasaki, M.; Inoue, M.; Tsuji, I.; Tsugane, S. Association between adherence to the Japanese diet and all-cause and cause-specific mortality: The Japan Public Health Center-based Prospective Study. Eur. J. Nutr. 2021, 60, 1327–1336. [Google Scholar] [CrossRef]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Doi, Y.; Uchida, K.; Shirota, T.; Yonemoto, K.; Kitazono, T.; Kiyohara, Y. Dietary patterns and risk of dementia in an elderly Japanese population: The Hisayama Study. Am. J. Clin. Nutr. 2013, 97, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Yoshida, D.; Ohara, T.; Hata, J.; Honda, T.; Hirakawa, Y.; Shibata, M.; Oishi, E.; Sakata, S.; Furuta, Y.; et al. Long-term association of vegetable and fruit intake with risk of dementia in Japanese older adults: The Hisayama study. BMC Geriatr. 2022, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Loveday, S.M. Protein digestion and absorption: The influence of food processing. Nutr. Res. Rev. 2023, 36, 544–559. [Google Scholar] [CrossRef] [PubMed]
- Sumi, K.; Tagawa, R.; Yamazaki, K.; Nakayama, K.; Ichimura, T.; Sanbongi, C.; Nakazato, K. Nutritional value of yogurt as a protein source: Digestibility/absorbability and effects on skeletal muscle. Nutrients 2023, 15, 4366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Geng, S.; Cheng, T.; Mao, K.; Chitrakar, B.; Gao, J.; Sang, Y. From the past to the future: Fermented milks and their health effects against human diseases. Food Front. 2023, 4, 1747–1777. [Google Scholar] [CrossRef]
- Mozaffarian, D.; El-Abbadi, N.H.; O’Hearn, M.; Erndt-Marino, J.; Masters, W.A.; Jacques, P.; Shi, P.; Blumberg, J.B.; Micha, R. Food Compass is a nutrient profiling system using expanded characteristics for assessing healthfulness of foods. Nat. Food 2021, 2, 809–818. [Google Scholar] [CrossRef]
- O’Hearn, M.; Erndt-Marino, J.; Gerber, S.; Lauren, B.N.; Economos, C.; Wong, J.B.; Blumberg, J.B.; Mozaffarian, D. Validation of Food Compass with a healthy diet, cardiometabolic health, and mortality among U.S. adults, 1999-2018. Nat. Commun. 2022, 13, 7066. [Google Scholar] [CrossRef]
- Drewnowski, A. Adjusting for protein quality by food source may affect nutrient density metrics. Nutr. Rev. 2021, 79, 1134–1144. [Google Scholar] [CrossRef] [PubMed]
- Hercberg, S.; Touvier, M.; Salas-Salvado, J. The Nutri-Score nutrition label. Int. J. Vitam. Nutr. Res. 2022, 92, 147–157. [Google Scholar] [CrossRef] [PubMed]
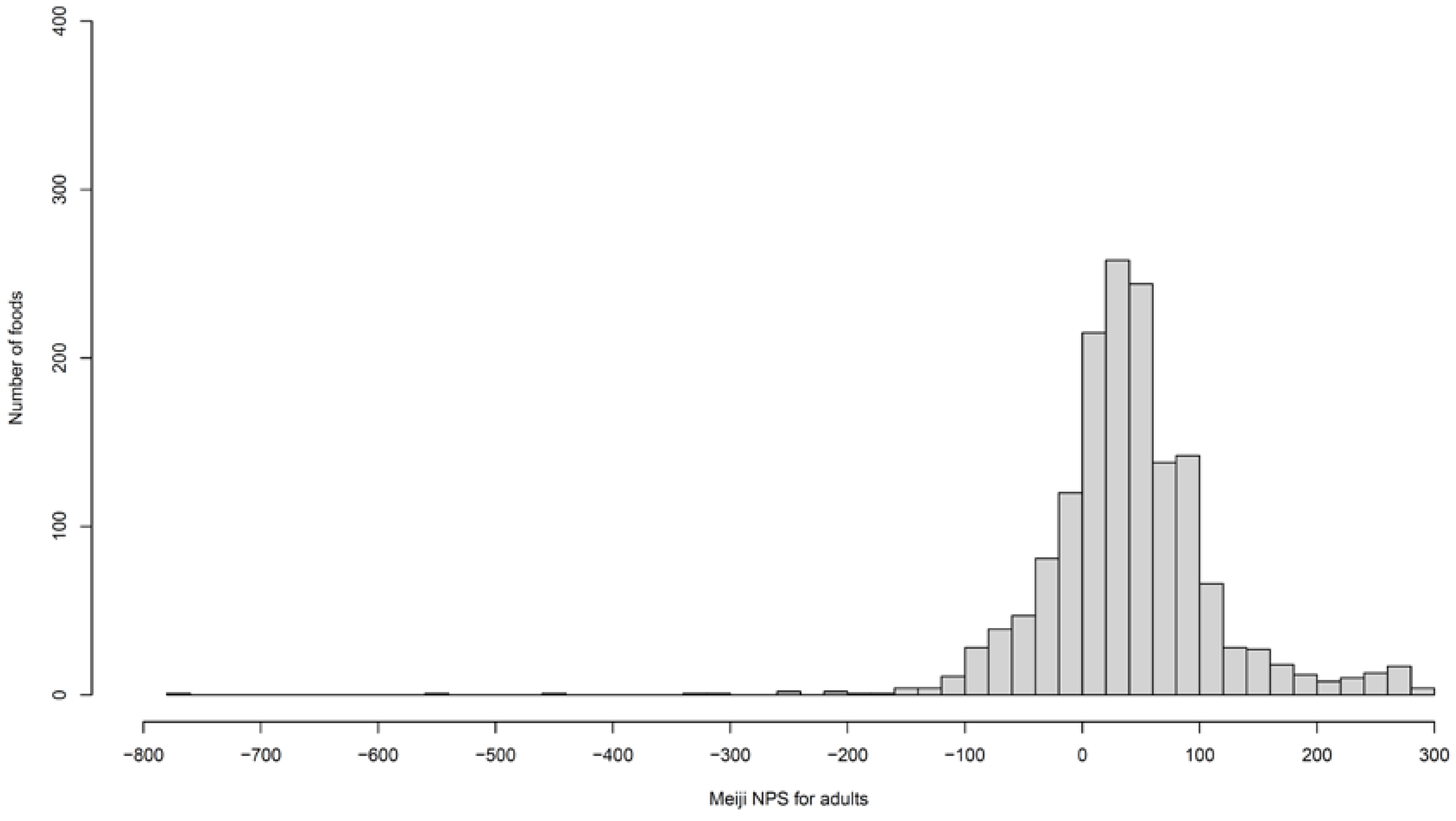
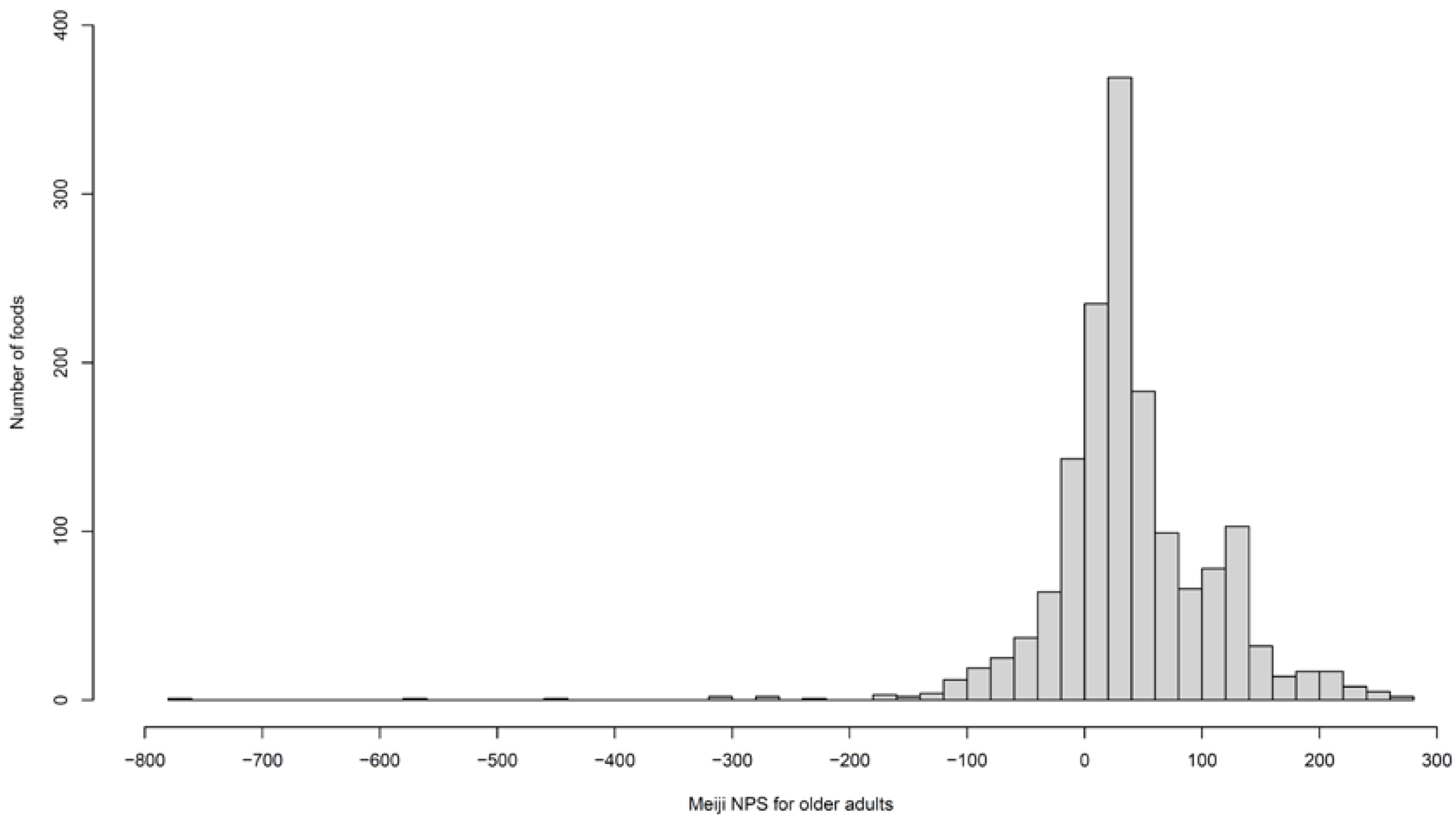
| Items | Meiji NPS for Adults | Meiji NPS for Older Adults | NRF9.3 | HSR |
|---|---|---|---|---|
| Nutrients to encourage | Protein Dietary fiber Calcium Iron Vitamin D | Protein Dietary fiber Calcium Vitamin D | Protein Dietary fiber Calcium Iron Potassium Magnesium Vitamin A Vitamin E Vitamin C | Protein Dietary fiber |
| Food groups to encourage | Fruits Vegetables Nuts Legumes Dairy | Fruits Vegetables Nuts Legumes Dairy | NA 1 | Fruits Vegetables Nuts Legumes |
| Nutrients to limit | Energy SFAs Sugar Salt equivalents 2 | Energy Sugar Salt equivalents 2 | SFAs Added sugar Sodium | Energy SFAs Total sugar Sodium |
| Items | For Adults | For Older Adults | |
|---|---|---|---|
| Nutrients to encourage | Protein | 65 g | 60 g |
| Dietary fiber | 21 g | 20 g | |
| Calcium | 1000 mg | 750 mg | |
| Iron | 12 mg | NA | |
| Vitamin D | 9.5 µg | 8.5 µg | |
| Nutrients to limit | Energy | 2800 kcal | 2400 kcal |
| SFAs | 31.1 g | NA | |
| Sugar | 70 g | 60 g | |
| Salt equivalents | 7.5 g | 7.5 g | |
| Food groups to encourage | Fruits | 200 g | 200 g |
| Vegetables | 350 g | 350 g | |
| Nuts | 75 g | 75 g | |
| Legumes | 100 g | 100 g | |
| Dairy | 130 g | 130 g | |
| Items | Meiji NPS for Adults | Meiji NPS for Older Adults | |||
|---|---|---|---|---|---|
| Cap | Percentage of RDV | Cap | Percentage of RDV | ||
| Nutrients to encourage | Protein | 65 g | 100% | 60 g | 100% |
| Dietary fiber | 21 g | 100% | 20 g | 100% | |
| Calcium | 423.9 mg | 42% | 389.4 mg | 52% | |
| Iron | 5.8 mg | 48% | NA | NA | |
| Vitamin D | 6.2 µg | 65% | 8.5 µg | 100% | |
| Nutrients to limit | Energy | NA | NA | NA | NA |
| SFAs | NA | NA | NA | NA | |
| Sugar | NA | NA | NA | NA | |
| Salt equivalents | NA | NA | NA | NA | |
| Food groups to encourage | Fruits | 200 g | 100% | 113 g | 57% |
| Vegetables | 157.7 g | 45% | 84.7 g | 24% | |
| Nuts | 75 g | 100% | 75 g | 100% | |
| Legumes | 90 g | 90% | 57 g | 57% | |
| Dairy | 108.5 g | 83% | 55 g | 42% | |
| Items | n | Mean | SD | Median | Max | Min | IQR |
|---|---|---|---|---|---|---|---|
| Pulses | 71 | 184.9 | 65.0 | 169.2 | 285.6 | 66.3 | 127.8 to 253.4 |
| Nuts and seeds | 40 | 163.5 | 60.6 | 147.3 | 292.4 | −9.1 | 129.5 to 196.9 |
| Algae | 14 | 121.1 | 102.5 | 152.1 | 265.6 | −73.9 | 79.0 to 176.0 |
| Mushrooms | 46 | 90.7 | 68.0 | 63.1 | 275.8 | −10.9 | 55.7 to 84.5 |
| Fish and seafood | 430 | 59.7 | 46.6 | 63.0 | 229.3 | −155.2 | 29.6 to 90.8 |
| Vegetables | 162 | 52.5 | 20.6 | 46.2 | 141.7 | 3.3 | 39.5 to 65.2 |
| Beverages | 10 | 44.4 | 102.8 | −3.4 | 251.7 | −6.6 | −6.4 to −0.7 |
| Milk and milk products | 46 | 40.9 | 69.4 | 67.6 | 186.4 | −140.1 | 15.8 to 83.1 |
| Eggs | 15 | 39.7 | 37.2 | 29.8 | 101.3 | −13.2 | 8.3 to 66.5 |
| Fruits | 71 | 31.4 | 34.1 | 40.2 | 77.7 | −165.6 | 31.2 to 46.4 |
| Potatoes and starches | 37 | 16.7 | 13.4 | 15.8 | 55.3 | −12.6 | 11.1 to 19.7 |
| Cereals | 156 | 11.8 | 34.6 | 7.0 | 133.1 | −100.3 | −0.2 to 21.4 |
| Meat | 303 | 5.3 | 40.6 | 13.7 | 108.0 | −119.3 | −15.3 to 33.7 |
| Confectionery | 98 | −34.6 | 31.0 | −29.6 | 43.5 | −152.2 | −52.6 to −12.9 |
| Seasonings and spices | 42 | −118.7 | 167.4 | −81.3 | 219.1 | −760.3 | −127.1 to −43.4 |
| Fats and oils | 4 | −130.4 | 73.5 | −134.8 | −49.2 | −202.9 | −186.8 to −78.5 |
| Sugars and sweeteners | 0 | NA | NA | NA | NA | NA | NA |
| Total | 1545 | 38.9 | 75.8 | 36.7 | 292.4 | −760.3 | 3.2 to 73.1 |
| Items | n | Mean | SD | Median | Max | Min | IQR |
|---|---|---|---|---|---|---|---|
| Nuts and seeds | 40 | 161.5 | 44.1 | 151.9 | 264.5 | 106.8 | 127.8 to 181.3 |
| Pulses | 71 | 132.5 | 55.8 | 125.7 | 220.3 | 15.2 | 90.7 to 186.2 |
| Mushrooms | 46 | 86.7 | 71.7 | 56.8 | 268.4 | −22.6 | 50.7 to 81.6 |
| Algae | 14 | 85.9 | 98.0 | 110.0 | 226.7 | −115.1 | 46.5 to 127.8 |
| Fish and seafood | 430 | 69.1 | 58.4 | 73.1 | 226.2 | −161.1 | 26.3 to 119.7 |
| Milk and milk products | 46 | 47.3 | 44.9 | 56.3 | 184.7 | −42.4 | 7.4 to 64.7 |
| Eggs | 15 | 47.0 | 48.1 | 28.4 | 130.5 | −19.0 | 9.4 to 84.2 |
| Vegetables | 162 | 43.5 | 17.3 | 39.6 | 100.8 | −6.2 | 32.1 to 53.8 |
| Beverages | 10 | 35.8 | 87.5 | −6.3 | 213.1 | −7.7 | −7.5 to −0.8 |
| Fruits | 71 | 25.2 | 38.9 | 36.1 | 78.8 | −173.0 | 26.6 to 43.2 |
| Meat | 303 | 17.3 | 19.7 | 23.3 | 77.0 | −76.9 | 5.7 to 30.3 |
| Potatoes and starches | 37 | 11.0 | 14.3 | 12.0 | 49.2 | −37.6 | 7.5 to 16.6 |
| Cereals | 156 | 7.5 | 27.6 | 3.7 | 94.5 | −106.8 | −1.0 to 17.6 |
| Fats and oils | 4 | −8.1 | 43.7 | −21.8 | 55.4 | −44.4 | −29.4 to −0.4 |
| Confectionery | 98 | −36.3 | 36.8 | −37.3 | 38.5 | −161.3 | −60.5 to −9.3 |
| Seasonings and spices | 42 | −126.1 | 166.1 | −92.1 | 180.3 | −770.2 | −131.1 to −45.3 |
| Sugars and sweeteners | 0 | NA | NA | NA | NA | NA | NA |
| Total | 1545 | 39.3 | 70.9 | 31.2 | 268.4 | −770.2 | 5.4 to 69.6 |
| Items | For Adults | For Older Adults | |||
|---|---|---|---|---|---|
| n | r | p-Values | r | p-Values | |
| Milk and milk products | 46 | 0.91 | <0.001 | 0.81 | <0.001 |
| Meat | 303 | 0.91 | <0.001 | 0.72 | <0.001 |
| Cereals | 156 | 0.91 | <0.001 | 0.89 | <0.001 |
| Beverages | 10 | 0.90 | <0.001 | 0.90 | <0.001 |
| Eggs | 15 | 0.84 | <0.001 | 0.79 | <0.001 |
| Fats and oils | 4 | 0.80 | 0.3333 | 0.40 | 0.7500 |
| Seasonings and spices | 42 | 0.79 | <0.001 | 0.78 | <0.001 |
| Confectionery | 98 | 0.75 | <0.001 | 0.82 | <0.001 |
| Fruits | 71 | 0.74 | <0.001 | 0.74 | <0.001 |
| Pulses | 71 | 0.68 | <0.001 | 0.68 | <0.001 |
| Nuts and seeds | 40 | 0.52 | <0.001 | 0.45 | 0.0043 |
| Potatoes and starches | 37 | 0.42 | 0.0110 | 0.49 | 0.0019 |
| Vegetables | 162 | 0.39 | <0.001 | 0.41 | <0.001 |
| Algae | 14 | 0.24 | 0.3998 | 0.20 | 0.4827 |
| Mushrooms | 46 | 0.14 | 0.3521 | 0.07 | 0.6666 |
| Fish and seafood | 430 | 0.07 | 0.1239 | −0.05 | 0.2625 |
| Sugars and sweeteners | 0 | NA | NA | NA | NA |
| Total | 1545 | 0.67 | <0.001 | 0.60 | <0.001 |
| Items | For Adults | For Older Adults | ||
|---|---|---|---|---|
| r | p-Values | r | p-Values | |
| Cereals | 0.89 | <0.001 | 0.84 | <0.001 |
| Meat | 0.89 | <0.001 | 0.83 | <0.001 |
| Beverages | 0.82 | 0.004 | 0.79 | 0.0068 |
| Potatoes and starches | 0.75 | <0.001 | 0.64 | <0.001 |
| Vegetables | 0.74 | <0.001 | 0.72 | <0.001 |
| Mushrooms | 0.74 | <0.001 | 0.75 | <0.001 |
| Seasonings and spices | 0.73 | <0.001 | 0.67 | <0.001 |
| Algae | 0.71 | <0.001 | 0.82 | <0.001 |
| Confectionery | 0.71 | <0.001 | 0.39 | <0.001 |
| Milk and milk products | 0.68 | <0.001 | 0.53 | <0.001 |
| Pulses | 0.60 | <0.001 | 0.75 | <0.001 |
| Fruits | 0.59 | <0.001 | 0.62 | <0.001 |
| Nuts and seeds | 0.60 | <0.001 | 0.75 | <0.001 |
| Fish and seafood | 0.35 | <0.001 | 0.32 | <0.001 |
| Eggs | −0.36 | 0.194 | −0.37 | 0.181 |
| Fats and oils | NA | NA | 0.11 | 0.895 |
| Sugars and sweeteners | NA | NA | NA | NA |
| Total | 0.64 | <0.001 | 0.61 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wakayama, R.; Drewnowski, A.; Horimoto, T.; Saito, Y.; Yu, T.; Suzuki, T.; Takasugi, S. Development and Validation of the Meiji Nutritional Profiling System (Meiji NPS) to Address Dietary Needs of Adults and Older Adults in Japan. Nutrients 2024, 16, 936. https://doi.org/10.3390/nu16070936
Wakayama R, Drewnowski A, Horimoto T, Saito Y, Yu T, Suzuki T, Takasugi S. Development and Validation of the Meiji Nutritional Profiling System (Meiji NPS) to Address Dietary Needs of Adults and Older Adults in Japan. Nutrients. 2024; 16(7):936. https://doi.org/10.3390/nu16070936
Chicago/Turabian StyleWakayama, Ryota, Adam Drewnowski, Tomohito Horimoto, Yoshie Saito, Tao Yu, Takao Suzuki, and Satoshi Takasugi. 2024. "Development and Validation of the Meiji Nutritional Profiling System (Meiji NPS) to Address Dietary Needs of Adults and Older Adults in Japan" Nutrients 16, no. 7: 936. https://doi.org/10.3390/nu16070936
APA StyleWakayama, R., Drewnowski, A., Horimoto, T., Saito, Y., Yu, T., Suzuki, T., & Takasugi, S. (2024). Development and Validation of the Meiji Nutritional Profiling System (Meiji NPS) to Address Dietary Needs of Adults and Older Adults in Japan. Nutrients, 16(7), 936. https://doi.org/10.3390/nu16070936






