Abstract
Chlorogenic acid (CGA) is a type of polyphenol compound found in rich concentrations in many plants such as green coffee beans. As an active natural substance, CGA exerts diverse therapeutic effects in response to a variety of pathological challenges, particularly conditions associated with chronic metabolic diseases and age-related disorders. It shows multidimensional functions, including neuroprotection for neurodegenerative disorders and diabetic peripheral neuropathy, anti-inflammation, anti-oxidation, anti-pathogens, mitigation of cardiovascular disorders, skin diseases, diabetes mellitus, liver and kidney injuries, and anti-tumor activities. Mechanistically, its integrative functions act through the modulation of anti-inflammation/oxidation and metabolic homeostasis. It can thwart inflammatory constituents at multiple levels such as curtailing NF-kB pathways to neutralize primitive inflammatory factors, hindering inflammatory propagation, and alleviating inflammation-related tissue injury. It concurrently raises pivotal antioxidants by activating the Nrf2 pathway, thus scavenging excessive cellular free radicals. It elevates AMPK pathways for the maintenance and restoration of metabolic homeostasis of glucose and lipids. Additionally, CGA shows functions of neuromodulation by targeting neuroreceptors and ion channels. In this review, we systematically recapitulate CGA’s pharmacological activities, medicinal properties, and mechanistic actions as a potential therapeutic agent. Further studies for defining its specific targeting molecules, improving its bioavailability, and validating its clinical efficacy are required to corroborate the therapeutic effects of CGA.
1. Introduction
Chlorogenic acid (CGA) family members are abundant dietary phenolic acid compounds in plants, conjugating the hydroxy group of quinic acid and the carboxyl group of caffeic acid as the parent structure. CGA family includes (1) 1L-(−)-quinic acid, (2) caffeic acid (CA), (3) ferulic acid, and (4) the p-coumaric acid (p-CoQA) group including p-CoQAs, caffeoylquinic acids (CQAs), and feruloylquinic acids (FQAs) [1,2,3,4]. The CGA family has shown multiple protective effects on mitigating many chronic inflammatory and age-related disorders through exerting the central actions of anti-inflammation, antioxidation, and metabolic homeostasis modulation [1,2,3,4].
CGA has limited bioavailability in plant foods due to the esterification with cell wall components such as proteins, lignin, and cellulose [5]; thus, appropriate food processing is needed to facilitate release [6]. CGAs are enriched in green coffee bean extract (GCE), which may comprise 54% of its contents [7]. Particularly, 5-CQA and 3-CQA present about 35−40% and 10−15% among CGA components, respectively [8]. The roasting process leads to a dramatic decrease in the total amount of CGAs and changes in CGA compositions with main contents of 3,4-di-CQA, 5-CQA, 4-CQA, and 3-CQA [9]. One-third of CGA is metabolized quickly after direct absorption in the upper gastrointestinal tract upon oral administration [3,10]. The esterase secreted by the intestine microbes (such as Lactobacillus gasseri, Bifidobacterium lactis, and Escherichia coli) can hydrolyze the remaining CGAs and release CGA and quinic acid to be absorbed in the intestines [11,12].
CGA exhibits a good safety profile, which has not shown any obvious adverse effect and toxicity to normal cells or tissues, and is well-tolerated by humans [13,14]. In an acute toxicity experiment, no side effects are observed in mice for two weeks upon an intake of CGA-enriched GCE (1 g/kg) [15]. A single dose of GCE (2 g/kg) (containing 50% CGA) in rats does not cause any type of toxicity. Rats with an intake of CGA (250, 500, and 1000 mg/kg) show no adverse effects in three months [16]. Cautiously, a high-dosage consumption of CGA (2 g/day) or black tea (4 g/4 L/day) four times in 7 days can moderately increase plasma homocysteine levels by 12% or 11% in humans, respectively [17].
In this review, the literature search was conducted between 2005 and 2024 in the database of PubMed for articles related to the subjects using the specific keywords of “chlorogenic acid” and (“inflammation” or “oxidation”). Inclusion criteria included full-text publications in English. Exclusion criteria included preprints and extracts without mention of CGA as the active ingredient in the abstract. The publications in this search result served as core literature for this review.
2. Functional Hubs of CGA’s Pharmacological Effects
2.1. Anti-Inflammation and Anti-Oxidation (Figure 1A)
Multidimensional effects of CGA in multiorgan are exerted through or related to its anti-inflammation and anti-oxidation properties. CGA has shown compelling immunomodulatory effects for mitigating pathological developments related to inflammatory response and/or oxidative stress [18]. The mechanisms underlying the anti-inflammation properties of CGA are multi-dimensional. First, it attenuates pathogen-activated nuclear factor-κB (NF-κB), c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinases (ERK), and p38-mitogen-activated protein kinase (MAPK) signaling pathways [19,20,21,22,23] (Figure 2A). Second, it inhibits the synthesis of many pro-inflammatory factors such as tumor necrosis factor-α (TNF-α), interleukin 1 beta (IL-1β), IL-6, interferon-γ, monocyte chemotactic protein-1, and macrophage inflammatory protein-1α during the inflammatory response [24,25,26] (Figure 2A). CGA could suppress TNF-α-induced inflammatory and oxidative stress in the pre-adipocyte 3T3-L1 cell line [27]. In lipopolysaccharide (LPS)-treated RAW264.7 cells, CGA inhibits cyclooxygenase-2 (COX-2) upregulation and suppresses the release of PGE2.19. Third, it reduces Toll-like receptor (TLR) activity and modulates the release of cytokine and chemokine, thus suppressing sepsis-induced pathologies [28,29,30,31]. CGA could counteract LPS-induced inflammation and oxidation by activation of the CD36/AMPK/PGC-1alpha pathway in RAW264.7 macrophages [32]. Intraperitoneal administration of CGA decreases neutrophilic infiltration by counteracting LPS-induced TLR-4, TNF-α, and NF-κB signaling in mouse liver [28]. CGA inhibits the systemic accumulation of high-mobility group box 1 (HMGB-1) and prevents sepsis-induced mortality [29,33]. The antioxidative activities of CGA are related to the activation of nuclear factor erythroid 2-related factor 2 (Nrf2)-dependent or -independent pathways as well as its anti-inflammatory properties [20,34,35,36,37] (Figure 2B). CGA effectively eliminates free radicals and inhibits oxidative injuries and apoptosis in multi-tissues by suppressing caspases’ activities [20,34].
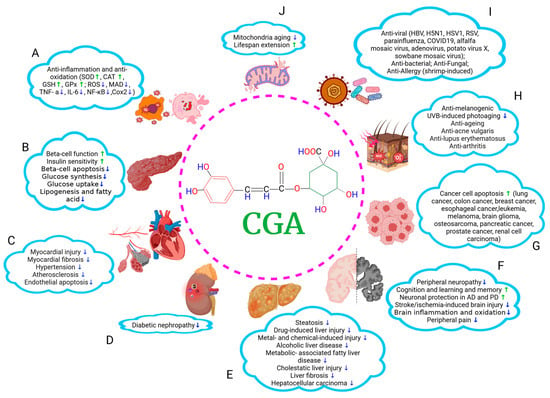
Figure 1.
A summary of therapeutic effects of CGA on multiorgan. CGA shows various beneficial roles in many pathological conditions. It can (A) mitigate inflammatory response and oxidative stress; (B) modulate glucose and lipid homeostasis and alleviate DMs; (C–E) protect cardiovascular system, kidneys, and liver; (F) facilitate the recovery from neurological impairments such as neurodegenerative disorders and diabetic peripheral neuropathy; (G) inhibit tumor cell proliferation and migration; (H) ameliorate skin pathologies; (I) execute anti-pathogen effects, and (J) exert antiaging effects. ↑, increasing; ↓, decreasing. The graph was created with Biorender.com.

Figure 2.
The potential mechanistic actions of CGA on multi-targets. CGA can potentially (A) target NF-kB, MPAKs, and JAK pathways to mitigate inflammation; (B) activate Nrf2-dependent and independent pathways to execute antioxidation function; (C) regulate lipid metabolism through increasing lipolysis and fatty acid oxidation and suppressing synthesis of cholesterol and fatty acids; (D) modulate glucose metabolism through increasing glycolysis and suppressing glucose uptake and glucose synthesis; and (E) exhibit neuromodulation through targeting multiple neuroreceptors and ion channels. The graph was created with Biorender.com.
2.2. Glucose and Lipid Metabolic Homeostasis Modulation (Figure 1B)
CGAs can facilitate the maintenance of metabolic homeostasis of glucose and lipids [38]. One mechanism involves the modulation of activities of AMP-activated protein kinase (AMPK) and ERK1/2 [4] (Figure 2C,D). AMPK is a master energy sensor that regulates cellular glucose and lipid metabolism. These functions directly underlie the CGA effects on the mitigation of chronic metabolic-associated syndromes such as obesity, diabetes mellitus (DM), and their complications. CGA or CGA-containing extracts can inhibit pancreatic lipase activity [39]. CGA exhibits inhibitory effects on the function of many lipid metabolic enzymes including fatty acid synthase, HMG-CoA reductase, and cholesterol acyltransferase in mice fed on a high-fat diet (HFD) [40] (Figure 2C). CGA can upregulate AMPK and carnitine palmitoyltransferase I (CPT-1) and inhibit acetyl-CoA carboxylase (ACC), thus reducing hepatic and blood levels of triglyceride (TG) and free fatty acids (FFA) in HFD rats [41] (Figure 2C). CGA can facilitate cholesterol elimination by modulating homeostasis of bilirubin and bile acids via farnesoid X receptor (FXR) and peroxisome proliferator-activated receptor (PPAR) gamma coactivator 1-alpha (PGC-1α) or fibroblast growth factor (FGF) 15 pathways [42,43].
CGA can attenuate glucose absorption. CGA can decrease sodium–glucose co-transporter 1 (SGLT-1), thus reducing glucose uptake and causing reduced glucose-dependent insulinotropic polypeptide (GIP) release and altered gut microbiota profile [44]. CGA activates AMPK pathways and suppresses HFD-induced upregulation of SGLT-1, glucose transporter type 2 (GLUT-2), and proglucagon (Plg), leading to an increase in the translocation of GLUT4 to plasma membranes and inhibition of liver glucose production [45,46] (Figure 2D). Activation of AMPK may also be prompted by caffeic acid, a metabolite of CGA, for modulating glucose transport [47].
CGA can reduce glucose release. CGA can inhibit glucose-6-phosphatase (G6Pase), the enzyme converting glycogen to glucose [44]. CGA inhibits the expression and activity of hepatic α-glucosidase and G6Pase, reduces the hydrolysis of hepatic glycogen, and activates AMPK pathways, resulting in attenuation of hepatic steatosis and improvements of metabolic indexes including fasting serum glucose (FSG) level, glucose tolerance, glucose uptake, insulin sensitivity, and lipid profiles [38,48,49] (Figure 2D).
CGA can modulate plasma levels of glucose and lipids. In HFD golden hamsters or rats, CGA upregulates hepatic PPAR-α levels, increases the activity of hepatic lipase (HL), decreases hepatic levels of TG and FFA and fasting serum levels of TG, FFA, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), FSG, and insulin (FSI), as well as attenuates the activity of lipoprotein lipase (LPL) in skeletal muscle [50,51]. CGA-containing GCEs (100 mg/kg, 6 weeks) can decrease blood glucose levels, body weight, and fat mass in mice fed on an HFD [52]. CGA (oral gavage 80 mg/kg/day, 12 weeks) in db/db mice can lower FSG, adiponectin, and TG, and increase muscle glycogen via up-regulating hepatic PPAR-α and inhibiting G6Pase expression [53]. Post-meal CGA treatment (60 min) decreases the level of blood sugar compared to the placebo in rats [54].
2.3. Human Subject Studies
In a randomized crossover study, healthy postmenopausal women (BMI 25–40, n = 16) with consumption of the bioactive yogurt containing curcumin and CGA showed significantly lower plasma levels of TNFα compared to the placebo group and the baseline [55]. In an acute pilot study, healthy subjects (n = 31) were given a single dose of a polyphenol-rich beverage (PRB) or placebo. The plasma levels of 8-iso-PGF2-alpha and advanced oxidation protein products were decreased, and hydroxyl radical antioxidant capacity at one-hour post intake of PRB was increased compared to the baseline [56].
In a cohort of 15 patients with impaired glucose tolerance (IGT), CGA (400 mg three times per day for 3 months) decreased FSG, insulinogenic index, body weight, body mass, waist circumference, TG, TC, LDL-c, and very low-density lipoprotein levels, with an upregulated Matsuda index [57]. In a randomized, double-blind controlled trial, participants (n = 65) were given an 8-week cooked ham enriched with a pool of antioxidants (including 22.5 mg CGA/100 g cooked ham) or received a placebo. Subjects with intervention showed significantly lower levels of ox-LDL, malondialdehyde (MDA), TC, high-sensitive C-reactive protein (hs-CRP), and IL-6 [58]. In a cohort of overweight dyslipidemic subjects (n = 90), a nutraceutical (containing bergamot, phytosterols, vitamin C, and CGA) or placebo was administered for 8 weeks. The subjects with the treatment showed improved lipid and glucose metabolism, which were associated with reduced levels of TG, LDL-c, non-HDL-c, the ratio of leptin/adiponectin, hs-CRP, and TNFα [59]. In a randomized, cross-over, controlled study, hypercholesterolemic subjects (n = 27) were administered soluble green/roasted (35:65) coffee or placebo for 8 weeks. The subjects showed lower lipid parameters (TC, TG, LDL-c, VLDL-c), MDA, and protein carbonyl group oxidation, systolic and diastolic blood pressures (SBP, DBP), heart rate, and body weight compared with the baselines [60]. Habitual coffee intake decreased serum levels of IL-18 and 8-isoprostane but increased adiponectin and HDL-c in healthy subjects (n = 47) [61]. In a study, healthy, overweight subjects (n = 142, BMI ≥25 to <30 kg/m2) were given a high-CGA (369 mg CGA/serving) or control (35 mg CGA/serving) coffee for 12 weeks. subjects with an intake of high-CGA coffee showed significant improvements in lowering the visceral fat area (VFA), total abdominal fat area (TFA), BMI, and waist circumference compared to those in the control group [62]. In a cohort of 21 patients with metabolic syndrome, CGA-containing GCE (400 mg twice per day for 2 months) showed a decrease in levels of FSG, insulin resistance, weight, and BMI in patients [8]. In a study of healthy Japanese women (n = 57), plasma CGA showed a negative association with FSG, glycated hemoglobin, and CRP [63].
CGAs showed diverse effects on neuroprotection for neurodegenerative disorders and diabetic peripheral neuropathy, mitigation of cardiovascular disorders, skin diseases, diabetic mellitus, liver and kidney injuries, and anti-tumor activities (Figure 1). Mechanistically, their anti-inflammation and anti-oxidation properties and metabolic modulations underlie these pharmacological activities for protection against cell injuries, restoration of cellular function, and maintenance of physiological and metabolic homeostasis (Figure 2), which is discussed across various tissues and disorders in this review.
3. Cardiovascular Protective Effect
CGA can exert protective roles at multiple levels in various cardiovascular complications, including mitigating hypotension, improving endothelial cell function, alleviating atherosclerosis, and ameliorating cardiomyopathy.
3.1. Hypotensive Effects (Figure 1C)
CGA can function as a hypotensive agent to lower blood pressure in a dose-dependent manner in spontaneously hypertensive rats (SHRs) [64,65]. Mechanistically, CGA-mediated vasodilation can occur through suppressing the activity of NADPH oxidase (NOX), inhibiting the generation of radical oxygen species (ROS), and increasing nitric oxide (NO), thus mitigating endothelial dysfunction in SHRs [64]. Particularly, the nitric oxide synthase (NOS), COX, and endothelium-derived hyperpolarizing factor (EDHF) pathways are involved in CGA-mediated vasodilation [66]. Furthermore, HPT-related pathogenic factors, including angiotensin-converting enzyme (ACE), arginase, and cholinesterase, are suppressed in cyclosporine-induced HPT rats upon administration of CGA for one week, suggesting the hypotensive and cardioprotective effects of CGAs [67]. Collectively, CGAs can ameliorate HPT by enhancing vasodilation, mitigating endothelial dysfunction, and reducing vascular remodeling triggered by hypoxia. The underlying mechanisms are related to an elevation of EDRFs and associated enzymes (NO, PGI2, Ach, NOS, and COX), suppression of oxidations (ROS and NOX) and vasoconstrictors (arginase, ANG II, cholinesterase, and ACE), decrease in levels of hypoxia-inducible factor 1α (HIF-1α) and phosphorylated c-Src, and an enhancement of Shc/Grb2/ERK1/2 signaling [2,64,66,67].
3.2. Effects of Endothelial Protections and Anti-Atherosclerosis (Figure 1C)
CGA can inhibit the oxidation of LDL and the subsequent endothelial damage caused by oxidized LDL (ox-LDL). CGAs can lower blood lipid levels [68,69,70]. CGA inhibits Cu2+-induced LDL oxidation [71,72]. Paraoxonase 1 (PON1) is an esterase that inhibits the formation of oxidized lipoproteins (ox-LDL and ox-HDL) [73]. CGA can protect PON1 from inactivation, thus suppressing the generation of ox-LDL [74]. CGA upregulates sirtuin 1 (SIRT1) and AMPK/PGC-1 activity, thus protecting mitochondrial function and suppressing ox-LDL-caused endothelial injuries [75]. CGA suppresses the levels of transient receptor potential canonical channel 1 (TRPC1) and decreases ROS and Ca2+, thus mitigating lysophosphatidylcholine (LPC)-induced endothelial injuries [69,76]. CGA protects endothelial cells by reducing ROS, xanthine oxidase-1, and HOCl-induced oxidative damage, enhancing superoxide dismutase (SOD), and producing NO and heme oxygenase (HO)-1 [77,78]. CGA modulates mtROS/JNK/NF-κB signaling, thus inhibiting inflammation and regulating mitochondrial bioenergetics in hearts. Moreover, CGA can regulate ankyrin-B levels as a cardiomyocytic defender [2].
CGAs can alleviate atherosclerosis by inhibiting endothelial damage, platelet–leukocyte interactions, and the levels of adhesion molecules, as well as upregulating prometabolic and antiplatelet pathways [2]. CGA inhibits adhesion molecules in early atherosclerosis, including IL-1β and TNFα-induced vascular cell adhesion molecule-1, intercellular cell adhesion molecule-1, and endothelial selectin; it also blocks α-glucosidase activities in human endothelial cells [79,80,81]. CGA suppresses hypoxia-induced HIF-1α-VEGF signaling, thus blocking angiogenesis and mitigating atherosclerosis [82]. Furthermore, CGA can inhibit VEGF-induced endothelial proliferation and migration by modulating VEGFR2, ERK ½, and protein kinase B (Akt) signaling [83]. CGA prevents platelet aggregation in the atherothrombotic process via the A2A receptor/adenylate cyclase (AC)/cAMP/protein kinase A (PKA) pathway [84,85]. CGA (400 mg/kg/day) can decrease the lesional areas of atherosclerosis in ApoE−/− mice by activating the PPARγ–liver X receptor α (LXRα)–ATP-binding cassette transporter A1 (ABCA1) signaling [86,87].
3.3. Cardioprotective Effects (Figure 1C)
Myocardial injuries can be triggered by TNF-α signaling which is activated by MAPKs such as p38 and JNK/SAPK and NF-κB pathways [88,89]. CGAs can regulate NF-κB and PPARα pathways, lower HIF-1α expression, and suppress cardiac apoptotic signaling, thus executing beneficial effects against cardiac hypertrophy and heart failure (HF) [2]. In a transverse aortic constriction (TAC)-induced HF mouse model and a TNF-α-induced pluripotent stem cell-derived cardiomyocyte injury model, CGA can inhibit NF-κB and JNK pathways, exhibiting cardioprotection [90]. CGA inhibits cardiomyocytic hypertrophy by upregulating IκBα and suppressing NF-κB to be translocated into the nucleus [91]. CGA-enriched chrysanthemum extract (CME) prevents myocardial hypertrophy by targeting HIF-1α and PPARα pathways in rats with renal hypertension and H9C2 cells with stimulation of ANG II-hypoxia [92]. CGAs have been shown to effectively protect from peroxidation of heart membranes and cardiac mitochondria [93,94,95]. Moreover, 3,5-di-CQA shows inhibition of myocardial injuries through increasing the activity of phosphatidylinositol 3-kinase (PI3K)/Akt in tert-butyl hydroperoxide (TBHP)-treated H9C2 cells [96].
CGAs can mitigate the inflammations, oxidations, defects of mitochondrial respiration, lysosomal dysfunction, and apoptosis, showing alleviative roles in multiple myocardial infarction (MI) models, including the animal models induced by ISO, left anterior descending coronary artery (LAD), and carbon tetrachloride (CCl4), as well as LAD-induced myocardial ischemia/reperfusion (I/R) senescence-accelerated prone 8 (SAMP8) mouse [97,98,99,100,101]. CGA-enriched extracts from Erigeron multiradiatus (Lindl.) Benth. inhibit NF-κB and JNK activation and suppress myocardial leukocyte infiltration and inflammatory response, thus alleviating acute MI in rats after a single administration intravenously (10, 20, and 40 mg/kg) [102].
3.4. Human Subject Studies for Cardiovascular Protection
CGA could lower SBP and DBP in patients with mild hypertension [7,14,103,104]. For example, Kozuma et al. showed that daily oral ingestion of GCE (93 or 185 mg for 4 weeks) could lead to a reduction of 4.7 and 5.6 mmHg in levels of systolic blood pressure (SBP) and a decrease of 3.3 and 3.9 mmHg in levels of diastolic blood pressure (DBP), respectively, in patients with hypertension [7]. In a randomized trial in Japanese patients with mild essential hypertension (HPT), CGA (140 mg/day) for 12 weeks could lower 10 mmHg of SBP and 6 mmHg of DBP [14]. Mild HPT patients taking CGA (228 mg/day for 1 month) showed a reduction of 3.3 and 2.8 mmHg in levels of SBP and DBP, respectively [104]. Ferulic acid is considered one of the active substances of CGA for producing a strong hypotensive effect via muscarinic acetylcholine receptors after short- and long-term ingestions [64,65]. In a clinical trial of patients with borderline or stage 1 hypertension (n = 37), a single intake of coffee with a high content of CGAs and low content of hydroxyhydroquinone (HHQ) significantly improved postprandial flow-mediated vasodilation and decreased circulating 8-isoprostane levels, which was effective for improving postprandial endothelial dysfunction [105]. A separate study showed that healthy male adults with ingestion of CGA without HHQ for four weeks could significantly increase postprandial fat oxidation and the ratio of postprandial biological antioxidant potential (BAP) to the derivatives of reactive oxygen metabolites (d-ROMs) compared to those with an intake of CGA with HHQ [106]. CGAs can incorporate specific phenolic acids into LDL particles to lower the risk of their oxidations in human subjects [70,71,107].
In a randomized controlled trial, healthy adults with an 8-week consumption of CGA-enriched coffee beverages showed a significant decrease in levels of twelve urine oxylipins compared to the baseline. Oxylipins are generated during foam cell formation in atherogenesis and thus are biomarkers for CVDs [108]. In a cohort of healthy subjects (n = 25), the impact of consumption of coffee containing 787 mg or 407 mg CGAs on CVD risk markers such as oxysterols and FFAs was assessed. Subjects with an intake of coffee showed a decrease in oxysterols and FFAs and an increase in cholesteryl esters. While subjects in the placebo group showed an elevation of oxysterols and FFAs and a reduction in cholesteryl esters [109]. Healthy subjects with consumption of decaffeinated GCE (CGAs accounting for about 51.2% constituents) showed an acute improvement in flow-mediated dilation (%FMD) of the brachial artery [110]. A study from two randomized trials with healthy male subjects (n = 15) showed that coffee intake could acutely improve human vascular function, likely through 5-CQA and its physiological metabolites [111]. A higher response of FMD induced by CGA-rich coffee was also reported in a study with 12 healthy subjects [112]. Healthy adults with an intake of a coffee berry beverage (containing 440 mg chlorogenic acid) could increase subjective energic arousal and hemodynamic responses from cerebral blood flow compared with the baseline [113]. In a placebo-controlled double-blind pilot study with healthy Japanese men (n = 16), subjects with the intake of GCE showed significantly greater changes in cardio-ankle vascular index (CAVI) (e.g., increasing FMD and decreasing sympathetic nervous activity) than those in the placebo group [114]. In a randomized, double-blind, placebo-controlled study, subjects (n = 50, BMI ≥ 25 to <30 kg/m2) were given a nutraceutical containing CGA and luteolin extracts for 6 months. Participants in the treatment group showed significantly decreased body weight, glycemic and lipid parameters (TC, TG, LDL-c) as well as improved hepatic functionality, carotid-media thickness (CIMT), and endothelial function compared to the subjects in the placebo group [115]. In a separate study of subjects with metabolic syndrome (n = 50), a 6-month intake of the same nutraceutical significantly improved hepatic and cardio-metabolic parameters in the patients [116].
4. Mitigative Effects on Diabetes Mellitus (DM)
CGA has shown its functions in protecting β cells from apoptosis, improving β cell function, facilitating glycemic control, and mitigating DM complications.
4.1. Protective Effects on β Cells (Figure 1B)
CGA can competitively reduce α-amylase activity [81,117]. CGA shows inhibition on porcine pancreatic α-amylase (PPA), PPA-I, and PPA-II [118]. CGA can enhance insulin secretion in β cells and Langerhans from rat islets [119,120]. CGA can reduce obesity-related insulin resistance in mice fed on HFD or high-fat milk, spontaneously obese mice, or rats fed on HFD [121,122,123]. One mechanism underlying CGA’s effects on decreasing insulin resistance and increasing insulin sensitivity is related to antioxidative stress. CGA reduces levels of lipid hydrogen peroxide and increases plasma antioxidants such as glutathione (GSH), vitamin C, vitamin E, and ceruloplasmin in DM model rats [124]. CGA scavenges thiobarbituric acid reactive substances and hydroperoxide through upregulation of SOD, catalase (CAT), glutathione peroxidase (GSH-Px), and glutathione S-transferase (GST) in the liver and kidney [125]. CGA suppresses inflammatory response by downregulation of F4/80+ and CD68+ macrophages in the liver and white adipose tissues [121]. CGA increases GSH and GSH-Px and reduces ROS, thus protecting β cells from exposure to streptozotocin (STZ) [120]. In STZ-induced DM rats, CGA (5 mg/kg/day, 45 days) in combination with tetrahydrocurcumin (80 mg/kg/day, 45 days) can mitigate the STZ-induced aberrances of enzymes related to gluconeogenesis (G6Pase and fructose-1,6-bisphosphatase) and glycolysis (glucokinase and hexokinase), thus lowering the levels of blood glucose and glycosylated hemoglobin (HbA(1C)) and elevating the levels of insulin, C-peptide, hemoglobin, and glycogen [126].
4.2. Mitigative Effects on DM Complications (Figure 1D)
CGA reduces glomerular hypertrophy and proliferation and mesangial cell expansions, decreases kidney malondialdehyde (MDA) levels, increases antioxidants (such as SOD, CAT, and GSH-Px), and reduces factors associated with oxidation and inflammation (such as IL-6, TNF-α, COX-2, and IL-1β) in the kidney of a diabetic nephropathy rat model [127,128]. CGA-containing extracts suppress vascular proliferation in kidneys induced by STZ and decrease serum VEGF levels induced by HIF-1α in DM mice [129,130]. In a diabetic retinopathy rat model, CGA shows restoration of the impaired tight junction protein occludin, mitigation of aberrant retinal vascular permeability, and protection of the integrity of the blood–retinal barrier [131]. In DM mice, CGA alleviates diabetic peripheral neuropathy (DPN)-induced auditory dysfunction by functional restoration of cochlear hair cells and protection of the external auditory canal [132]. CGA can relieve DM-induced neuropathic pain [133].
4.3. Human Subject Studies for Glycemic Control
CGA can attenuate FSG and insulin production in patients [57]. In a randomized, double-blind, placebo-controlled crossover study to evaluate acute response, a one-time intake of green tea catechins (GTC) together with coffee CGA significantly increased GLP-1 and decreased blood sugar levels and GIP secretion in healthy subjects compared with the placebo group after consumption of a 75 g glucose load [134]. This data was echoed by a related study showing that a three-week intake of GTC + CGA-enriched beverages exhibited similar beneficial effects in postprandial glycemic control and diabetic prevention [135]. In a cohort of subjects with prediabetic impaired fasting glucose (IFG), CGA-rich Cynarascolymus (Cs) extracts (n = 27) or placebo (n = 27) were administered. The subjects in the treatment group showed significant improvements in glycemic control, insulin sensitivity, and many metabolic parameters (TC, LDL-c, HDL-c, TG, ApoA, ApoB, and glycated hemoglobin) [136]. In a randomized clinical trial in patients with metabolic syndrome, participants with an intake of GCE (400 mg, twice per day, 8 weeks) significantly decreased SBP, FBS, homoeostatic model of assessment of insulin resistance, waist circumference, and appetite scores in comparison to those in the placebo group [8].
5. Hepatoprotection
CGA can mediate hepatoprotective roles in various pathological conditions of the liver via antioxidant and anti-inflammatory features [4]. (1) It can inhibit TLR4-mediated activation of NF-κB, thus suppressing pro-inflammatory responses; (2) it can activate the AMPK pathway to modulate metabolic homeostasis; (3) it can increase the activity of the Nrf2 pathway, thus exerting antioxidant effects; and (4) it can inhibit caspases’ activation to suppress hepatic apoptosis induced by chemicals or toxins.
5.1. Hepatoprotection from Metal-, Chemical-, Drug-, and Toxin-Induced Liver Injury (Figure 1E)
CGA can activate Nrf2 and inhibit the TLR4/NF-κB signaling cascade, reduce activities of serum liver enzymes, oxidation, and inflammation, and alleviate liver injuries caused by the following metals and chemicals: sodium arsenite [137], lead (Pb) [138], cadmium (Cd) [139], aluminum chloride [140], polychlorinated biphenyls [141], TAA [142], carbon tetrachloride (CCl4) [143], D-gal [144], L-carnitine [145], lipopolysaccharide (LPS) [146,147], palmitic acid [148], and aflatoxin B1 [149].
CGA mitigates acetaminophen-induced hepatic injuries by inhibiting apoptosis and oxidation, ameliorating liver inflammation, activating Nrf2, promoting mitophagy, and suppressing activities of metabolic enzymes such as cytochrome P450 (CYP) [20,24,150,151,152,153,154]. CGA can ameliorate hepatotoxicity triggered by many other drugs including tamoxifen, methotrexate, triptolide, and monocrotaline [155,156,157,158].
CGA can attenuate alcohol-induced pathologies such as steatosis, apoptosis, and fibrosis by regulating CYP2E1/Nrf2 and TLR4/NF-κB [159], scavenging mitochondrial and intracellular ROS [160], and facilitating n-butyric acid generation for homeostatic regulation of the gut–liver axis [161].
5.2. Mitigative Effects on Metabolic-Associated Fatty Liver Disease (MAFLD) (Figure 1E)
CGA inhibits HMG-CoA reductase, thus reducing the quantity of palmitic acid, oleic acid, or linoleic acid-induced large lipid droplets in the hepatic cell line HepG2 [162,163]. CGA attenuates MAFLD in HFD mice by increasing the production of glucagon-like peptide-1, reducing ER stress, suppressing mucosa barrier injury in the intestine, and inhibiting JNK signaling, as a result of autophagic suppression and insulin-resistant mitigation [123,164,165].
CGA in combination with metformin [166] or geniposide [167,168,169,170] improves MAFLD through multiple mechanisms. CGA combined with telmisartan improves rat MAFLD caused by high fructose, possibly through suppressing sphingosine kinase 1 (SPHK-1)/sphingosine-1-phosphate/TLR4 pathways [171]. Lipid metabolism is modulated by CGA in combination with caffeine via the AMPKα-LXRα pathway in HFD-fed mice [172]. CGA alleviates liver inflammation during non-alcoholic steatohepatitis (NASH) progression by blocking the LPS-TLR4-MyD88 signaling pathway via direct binding to MyD88 and by activation of Nrf2/PPARα signaling [173].
In an α-naphthylisothiocyanate-induced mouse model with cholestatic liver injury, CGA suppresses cell death and neutrophilic and monocytic infiltration and reverses dysregulated hepatocyte transporters and enzymes related to synthesis, uptake, metabolism, and efflux of bile acids [43,174]. In a rat model of hepatic ischemia/reperfusion injury, CGA attenuated liver damage by suppressing HMGB1/TLR-4/NF-κB signaling and mitochondria-mediated apoptosis [175].
5.3. Mitigative Effects on Liver Fibrosis and Hepatocellular Carcinoma (HCC) (Figure 1E)
CGA attenuates Schistosoma japonicum cercaria-induced hepatic fibrosis in animals, partially through regulating IL-13/miR-21/Smad7 [176]. CGA suppresses CCl4-induced liver fibrosis by suppressing miR-21/TGF-β1/Smad7 signaling or inhibiting TLR4/NF-κB signaling and stimulating the Nrf2 pathway [22,177,178]. In a methionine and choline deficiency diet (MCDD)-caused nonalcoholic steatohepatitis (NASH) model, CGA increases the biogenetics of mitochondria and suppresses the generation of extracellular matrix triggered by HMGB1 in liver endothelial cells, thus attenuating liver fibrosis [179].
CGA suppresses HepG2 growth and HCC formation via inhibition of ERK1/2, matrix metalloproteinase (MMP)-2/9, and DNA methyltransferase 1 [180,181]. CGA in combination with protocatechuic acid forces HepG2 cells to enter apoptosis [182]. CGA in combination with caffeine and trigonelline can inhibit the tumorigenesis related to diethylnitrosamine (DEN)/CCl4-caused liver fibrosis [183]. CGA can restore the disorganized gut microbiota and aberrant metabolites in DEN/CCl4-caused HCC in animals [184].
5.4. Human Subject Studies for Hepatic Protection
In a clinical study with subjects with NDFLD in type 2 DM, neither CGA nor caffeine showed significant effects on improving stiffness of the liver and other hepatic outcomes. The TC was lower in the caffeine group and insulin was higher in the CGA plus caffeine group than in the placebo group, respectively [185]. In a randomized controlled clinical trial with HCC patients (n = 291) transcatheter arterial chemoembolization (TACE) therapy was administered, with or without FZJDXJ, a Chinese medicine formulation, for 48 weeks. The active ingredients of FZJDXJ included formononetin, CGA, caffeic acid, luteolin, gallic acid, diosgenin, ergosterol endoperoxide, and lupeol, which might potentially target AKT/CyclinD1/p21/p27 pathways. In addition, molecular docking showed that CGA and gallic acid could effectively interact with the phosphorylation site Thr308 of AKT1. FZJDXJ and TACE treatment significantly prolonged one-year overall survival (OS) and progression-free survival (PFS) of patients compared with TACE treatment alone [186].
6. Neuroprotection
CGA has shown diverse neuroprotective effects on various neuropathological conditions which may be exerted through inhibition of neuroinflammation, reduction in ROS production, prevention of oxidation, and suppression of neuronal apoptosis [187,188,189,190].
6.1. Protective Effects against Neuronal Injury (Figure 1F)
CGA inhibits H2O2-induced apoptosis by blocking pro-apoptotic factors caspase-3 and pro-poly (ADP-ribose) polymerase (PARP) and upregulating anti-apoptotic factors Bcl-2 and Bcl-X(L) in neuronal cells and PC12 cells [34,191]. CGA can reduce overactive microglia-induced neuroinflammation in the cortex. CGA suppresses TNF-α secretion and NO generation in LPS-stimulated primary microglia, increasing the survival of dopaminergic neurons [192]. CGA counteracts the TNFα-activated NF-kB pathway in an immortalized human oligodendrocyte cell line M03-13 by suppressing intracellular superoxide ions, mitochondrial ROS, and protein levels of NADPH oxidases (NOXs)/dual oxidase 2 (DUOX2) [193]. CGA protects cerebellar granule cells from NO-caused death in vitro [194]. CGA protects rat cortical neurons against glutamate-induced neurotoxicity and oxidation [195] and prevents AMPA-induced neurotoxicity in oligodendrocytes derived from the optic nerve through suppression of PKC and caspase-dependent signaling [196].
CGA and its metabolites are thought to be able to pass the blood–brain barrier (BBB) and execute their impacts on the nervous system [197,198,199,200]. CGA attenuates methotrexate-induced oxidative damage in rat cerebellum [201]. CGA inhibits cadmium-induced rat brain damage via suppressing lipid peroxidation, increasing antioxidant activity, and attenuating mitochondrial dysfunction and DNA breakdown [202]. It has demonstrated its protective effects against scopolamine-induced amnesia in mice [203,204], alcohol-induced neuronal injury in neonates [205], pilocarpine-induced oxidative stress [206], 3-nitropropionic acid-caused neurotoxicity and genotoxicity [207], kainic acid-induced cytotoxicity and learning and memory loss in mice [208], and L-buthionine-(S, R)-sulfoximine-caused oxidation in mouse forebrain [209].
6.2. Mitigative Effects on Alzheimer’s Disease (AD) (Figure 1F)
CGA or extracts containing CGA can inhibit Aβ aggregation-caused cellular injury in SH-SY5Y cells, a neuroblastoma cell line, and PC12 cells [210,211,212,213]. It suppresses the Aβ1–42 self-induced aggregation in PC12 cells [213]. In Aβ-treated hippocampal neurons, CGA increases survival and decreases apoptosis via decreasing activities of lactate dehydrogenase (LDH) and the levels of MDA and raising the levels of SOD and GSH-Px [214]. CGA facilitates Aβ clearance and cognitive improvement by enhancing the expression of hippocampal LDL receptor-related protein 1 and restoring perivascular deposition of aquaporin 4 [215].
CGA prevents Aβ deposition and neuronal loss and ameliorates learning and memory deterioration in APP/PS2 mice [216]. CGA restores spatial learning and memory in SAMP8 mice, a mouse model showing plaques with Aβ depositions and age-related cognitive defects [217]. CGA inhibits acetylcholinesterase (AChE) activity in rat brains, suggesting its beneficial effect against cognitive impairment [218,219]. Molecular docking simulations suggest that CGA can bind towards AChE [220]. CGA inhibits AChE, decreases the hippocampal and frontal cortical levels of MDA, and improves the deteriorated short-term or working memory and defective cognition induced by scopolamine, a muscarinic receptor antagonist [203].
6.3. Mitigative Effects on Parkinson’s Disease (PD) (Figure 1F)
CGA has demonstrated preventative effects against PD. CGA improves the decrease in α-synuclein-induced cell viability and blocks the interplay between oxidized dopamine and α-synuclein [221]. CGA attenuates the 6-OHDA-caused apoptosis of SH-SY5Y cells [222,223]. CGA combined with caffeic acid prevents rotenone-caused Parkinsonian pathology in nigral dopaminergic and intestinal enteric neurons [224]. CGA enhances the expression of tyrosine hydroxylase and anti-inflammatory cytokine IL-10 and reduces the drug-induced neuroinflammatory factors such as IL-1β, TNF-α, and NF-κB in substantia nigra [192,225]. CGA inhibits the activation of pro-apoptotic proteins including Bax and caspase-3 and elevates the levels of anti-apoptotic factors such as Bcl-2 [226].
6.4. Effects on Ischemia-Induced Brain Injury (Figure 1F)
CGA protects against injury caused by cerebral ischemia/reperfusion [227]. It can decrease mortality [228], increase neurological deficit scores [228,229], mitigate sensory–motor functional deficits [198], attenuate infarct volume [198,228,229,230], reduce neuronal loss [231,232,233], suppress brain edema [198,229,230], decrease BBB injury [198,230], and ameliorate ischemia-induced cognitive deficits [229,232,233]. The mechanisms underlying CGA-mediated protection from ischemia-induced brain injury are as follows: (1) It upregulates the activity of SOD2 and GSH and suppresses ROS generation, LDH secretion, and MDA elevation through the Nrf2 pathways [229,232,233]; (2) It decreases the ischemia-induced pro-inflammatory factors such as TNF-α and IL-2 but increases anti-inflammatory cytokines such as IL-4 and IL-13 [230,233]; (3) It inhibits apoptotic markers such as caspase-3 and increases anti-apoptotic factors such as Bcl2 in ischemia [229,230,232]; (4) It facilitates the expression of neurotrophins such as BDNF and NGF for neuronal repair in response to cerebral ischemia/reperfusion [228,229]; (5) It decreases the expression of metalloproteinases such as MMP-2 and MMP-9 for protection of BBB integrity in the cerebral ischemia brain [198]; and (6) It increases endothelial marker CD31 but decreases endothelin-1 to improve from vascular damage [232].
6.5. Effects on Cognitive Function (Figure 1F)
CGA protects against anxiolytic and depressive processes in a mouse model of anxiety [234]. CGA improves cognitive impairments in sleep-deprived mice via immunomodulatory effects and gut microbial metabolic modulation. The potential contributive mechanism was Nrf2/PPAR activation [235]. CGA attenuates the polarization of macrophages and alleviates cognitive impairments in an LPS-induced neuroinflammation mouse model by targeting the TNFα signaling pathway [236]. CGA improves memory dysfunction and attenuates frontal cortex inflammation in diabetic rats [237]. Dried loquat fruit extract containing CGA improves corticosterone-induced depression-like behaviors in mice [238].
6.6. Modulation of Neuropathic Pain (Figure 1F)
Neuropathic pain is related to immunomodulation and inflammatory response [18]. CGA shows antinociceptive efficacies in pains related to tonic and inflammations and chronic neuropathy [133,239,240,241], which may be a result of CGA’s anti-inflammatory activities on suppression of peripheral release of many pro-inflammatory factors, including TNF-α, NO, and ILs [239,242,243]. Oxidative stress involves all stages of neuropathy and its related pain since free radicals are key mediators causing peripheral nerve injury [244,245]. Data have demonstrated that ROS is a crucial contributor to the development of neuropathic and inflammatory pain [246,247,248,249,250], which can be attenuated by various phenolic antioxidants [251,252,253]. CGA has strong antioxidant activities for scavenging free radicals such as ROS [254]. It is reasonable to posit that CGA can reduce neuropathic pain by scavenging ROS.
CGA-enriched herb extracts execute antinociceptive actions in various animal models [255,256]. Acidosis-induced and trigeminal nociceptive pain can be reduced by CGA [257,258]. CGA can suppress the inflammatory cascade and decrease mechanical and cold hyperalgesia in the rat model of chronic constrictive nerve injury (CCI) [240,241]. The underlying mechanism is probably realized through facilitating the activation of gamma-aminobutyric acid A (GABAA) receptors in the spinal cord, a major inhibitory neuronal transmission for pain modulation [259,260] (Figure 2E). However, CGA seems ineffective in mitigating acute pain [133].
CGA may directly act on ion channels related to neuropathic pain for its mitigative effects. For example, voltage-gated potassium channel subfamily A member 4 (Kv1.4), which is specifically expressed in nociceptive sensory neurons in small diameters (Aδ and C fibers), is involved in neuropathic pain when its function is suppressed [261,262]. Kv activities are upregulated by CGA in trigeminal ganglions on the basal level and PGE2-induced inflammations [263,264], leading to an attenuation of neuronal excitability-related pain induction [264,265,266,267] (Figure 2E). Furthermore, CGA can suppress acid-sensing ion channels in sensory ganglions [257,268], presenting another potential peripheral antinociceptive pathway.
6.7. Human Subject Studies for Neuroprotection
Several studies show that regularly prolonged intake of CGA has positive effects on cognitive function in humans [269,270,271]. In a cohort of healthy subjects with self-description of memory decline (n = 38, 50–69 years old), individuals were given a CGA-enriched beverage or placebo for 4 months. The data showed that CGA improved some categories of cognitions (such as attention shifting, function of execution, and motor and psychomotor speed) and increased plasma levels of early cognitive impairment biomarkers such as apolipoprotein A1 and transthyretin [270]. In another cohort of the elderly with subjective memory complaints (n = 8), subjects were administered CGA (330 mg) for 6 months, and similar improvements were observed including memory for composition and verb use, cognition of flexibility, function of execution and attention, and motor speed. Furthermore, there were reductions in the plasma levels of Aβ42 and Aβ42/Aβ40 and an increase in the plasma level of dehydroepiandrosterone sulfate [271]. In a recent randomized controlled trial on 34 individuals with mild cognitive impairment who were administered two periods of CGA (554 mg of CGA or placebo, twice/day) for 3 months with a monthly interval, data showed improvements in cognitive functions, especially attention and executive function [269]. In a randomized, double-blind, placebo-controlled crossover study, healthy humans with consumption of CGA-enriched coffee berry extracts increased arousal, but limited cognitive effects were observed [272]. Ingestion of CGA (600 mg) over 5 days in healthy subjects (n = 9) shortened sleep latency without effects on sleep architecture, enhanced parasympathetic activity, and increased fat oxidation during sleep [273].
7. Anticancer Effect
CGA has the role of an anticancer agent in various types of cancer cells by arresting cell proliferation, promoting apoptosis, and facilitating intracellular DNA impairment [13] (Figure 1G).
7.1. Breast Cancer
CGA exhibits cytotoxicity in breast cancer cell lines such as MCF-7 with an IC50 of 127 µM, resulting in DNA injury, cell cycle stall, and apoptosis [274,275]. A possible mechanism is that CGA can bind to PKC in the cytosol and translocate it to the plasma membrane, thus disturbing the cell cycle, arresting cells at the G1, and reducing cells in the S phase [274]. CGA shows cytotoxicity on breast cancer cell lines such as MDA-MB-231, MDA-MB-453, and 4T1 in dose- and time-dependent manners through downregulation of NF-κB pathway [276]. It also modulates the epithelial–mesenchymal transition (EMT) process of breast cancer cells by downregulation of N-cadherin and upregulation of E-cadherin [276]. In a breast cancer cell-bearing BALB/c mouse model, CGA suppresses tumor growth by increasing the expression of p53, Bax, and the ratio of Bax/Bcl-2 [276,277].
7.2. Colorectal Cancer
CGA can stall the cells in the S phase and cause DNA injury in human colon cancer cell lines such as HCT116 and HT29 by increasing ROS production, upregulation of phosphorylated p53, HO-1, and Nrf2 [278]. CGA activates the mitochondrial apoptotic pathway in cancer cells by showing DNA breakdown, cleavage of pro-caspase-9 and PARP-1, and upregulation of Bax and the Bax/Bcl-2 ratio [279]. CGA and its metabolites can increase the levels of pro-caspase-3 and activated caspase-3 in human colon cancer cell lines such as Caco-2 [280]. CGA combined with lactoferrin arrests SW480 cells at the G0/G1 phase and decreases cell viability [281].
7.3. Esophageal Cancer
Evidence reveals that CGA can suppress proliferation and colony formation on many esophageal cancer cell lines such as KYSE30/70/140/150/180/510 [282]. In esophageal cancer cell line-bearing non-obese diabetic (NOD)/severe combined immunodeficiency disease (SCID) mouse models, CGA (50 mg/kg) inhibits the propagation and size of the tumor and reduces esophageal hyperplasia, thus extending mouse lifespan. CGA decreases expressions of survivin and SOX2 in esophageal squamous carcinoma [282].
7.4. Leukemia
CGA (10–25 µg/mL) causes the apoptosis of Bcr-Abl+ leukemia cell lines by an increase in intracellular H2O2, O2− and levels of caspases, as well as PARP degradation and suppression of p-STAT-5 and p-CrkL [283]. Similar results have been reported in U937 and HL-60 leukemia cells. CGA (50–200 µM) facilitates cancer cell death through the induction of ROS and activation of caspase-dependent signaling, leading to reduction in membrane potentials of mitochondria, DNA damage, and apoptosis [284,285].
7.5. Lung Cancer
CGA (2–50 µM) can suppress the progression of human lung cancer cell line A549 by increasing the levels of annexin-V, Bax, and CASP3, activating p38 and Jun, and decreasing Bcl-2 and tumor stem cell markers including NANOG, POU5F1, and SOX2, indicating multiple kinase pathways and ROS signaling underlying CGA-mediated anti-lung cancer activity [286]. This finding has been echoed by in vivo experiments using an A549-bearing nude mouse (BALB/c) model, in which CGA (120 mg/kg) reduces their tumor mass and size by binding with annexin A2 and inhibiting the expression of NF-κB downstream antiapoptotic genes, thus suppressing cancer cell growth and migration [287].
7.6. Melanoma
CGA (1–1.5 mM) reduces the growth of melanoma C32 cells by increasing the expression of antioxidant molecules such as SOD and GSH-Px, thus decreasing oxidation [288]. CGA prevents B16F10 melanoma cell proliferation by facilitating the tumor-associated macrophage (TAM) polarization from M2 to M1. CGA with an anti-PD1 antibody can decrease the CD4+ Foxp3+ T cell ratio and increase the CD8+ T cell ratio, leading to an enhancement of immunotherapeutic activity in vivo [289].
7.7. Brain Glioma
CGA (0.5–5 µM) downregulates the macrophagic STAT−1 and STAT-6, leading to the apoptosis and proliferation stall of glioma cells (U87) [290]. In G422 cancer cell-bearing mice, CGA (20 or 40 mg/kg) decreases tumor mass by increasing M1 TAM and suppressing M2 TAM [291]. In glioma C6 cell-bearing Kunming mice, CGA treatment reduces tumor area and prolongs the median survival time of mice [292]. CGA (200 µM) can show neuroprotection of the bortezomib-caused neurite injury and loss of cell volume, which is also confirmed in neuroblastoma SH-SY5Y and rat dPC-12 cells [293].
7.8. Osteosarcoma
CGA reduces the proliferation of osteosarcoma cell lines such as U2OS, MG-63, and Saos-2 by increasing the activity of caspase-3, caspase-7, and PARP, and inducing apoptosis through the blockage of the STAT3/Snail pathway [294,295]. CGA in combination with doxorubicin suppresses cellular metabolic activity, colony formation, and cell growth of U2OS and MG-63 cells by upregulating caspase-3 and PARP and suppressing the p44/42 MAPK pathway, thus inducing apoptosis [296].
7.9. Pancreatic Cancer
CGA (100–300 µM) can stall cells at the G2/M phase and suppress cell proliferation and colony formation of pancreatic carcinoma cells (PANC-1), which can be synergically enhanced in combination with thermal cycling hyperthermia (TC-HT) (10 cycles) with or without a low-intensity pulsed electric field (LIPEF) [297,298]. The underlined mechanism involves CGA-mediated excessive ROS production, causing mitochondrial dysfunction, leading to increases in cleaved levels of caspase-3, caspase-9, PARP, and Bax/Bcl-2 ratio [297,298]. These data are further validated by in vivo experiments showing that CGA can reduce tumor growth and volume in pancreatic cancer cell-bearing nude mice by modifying cancer cell metabolism through decreasing levels of cyclin D1, c-Myc, and cyclin-dependent kinase-2 (CDK-2), interrupting mitochondrial respiration, and suppressing aerobic glycolysis [299].
7.10. Prostate Cancer
CGA arrests cells at the phase of G1 and inhibits cell viability of prostate cancer cell DU145 by suppressing the levels of HIF-1α and SPHK-1, PCNA, cyclin-D, CDK-4, p-Akt, p-GSK-3β, and VEGF [300].
7.11. Renal Cell Carcinoma (RCC)
CGA (IC50 40 µM) selectively suppresses cell proliferation and colony formation of human RCC A498 cells but without effects on human embryonic kidney (HEK293) cells through upregulation of cleaved levels of caspase-3, caspase-9, and PARP and the ratio of Bax/Bcl-2 and inhibition of the PI3K/Akt/mTOR pathway [301].
7.12. Human Subject Studies for Cancer Management
In an open-label, dose-escalation phase I trial on patients with recurrent high-grade glioma after standard-of-care treatments (n = 26), CGA was intramuscularly injected into patients once daily for 28 days. The median OS after CGA treatment was 11.3 months, which showed a prolonged trend as compared with the median OS (5.7 to 7.5 months) for patients in similar stages under standard-of-care therapeutics [302].
8. Skin Protection
CGA has shown diverse dermal protective roles in various skin conditions such as anti-UV-induced photoaging, promoting skin slap survival, improving skin barrier function, mitigating systemic lupus erythematosus (SLE)-like symptoms, and suppressing melanogenesis.
8.1. Dermal Protection against Skin Pathologies (Figure 1H)
(1) CGA shows anti-inflammatory and antiaging effects by inhibiting UVA-activated TGF/Smad2/3 signaling, decreasing ROS, pro-inflammatory factors IL-1β and TNF-a, reducing apoptosis and necrosis, attenuating DNA damage, promoting cell repair, and increasing synthesis of collagens in dermal fibroblasts [303,304]; CGA ameliorates deoxynivalenol-induced dermal injury by activating Nrf2 and inhibiting MAPK/NF-kb/NLRP3 pathways [305]; (2) CGA promotes skin flap survival in rats by downregulating MDA and NO, upregulating GSH and SOD, and elevating VEGF expression and capillary density, leading to blood perfusion [306]; (3) CGA restores the epidermal skin barrier by upregulation of filaggrin, involucrin, and envoplakin and induction of diverse responses of cytokines in epidermal keratinocytes [307]; (4) CGA has anti-acne vulgaris effects. CGA rescues P. acnes-induced skin lesions in ears including redness, swelling, and erythema, downregulates the levels of pro-inflammatory factors by suppressing NF-κB signaling, and inhibits lipogenesis by attenuating AKT/mTOR/SREBP signaling [308]; and (5) CGA relieves SLE-like skin lesions. CGA down-regulates IL-17 levels, mitigates SLE-caused injuries in the skin and mucous membranes, and improves arthritis-like syndromes in MRL/lpr mice [309]; (6) CGA-containing hydrogel promotes the formation of microvessels from HUVEC cells and proliferation of HaCAT cells. In a skin-wound rat model, CGA hydrogel facilitates the wound-healing process by modulating macrophage polarization, alleviating the production of pro-inflammatory cytokines, enhancing collagen deposition, and increasing the expression of CD31 and VEGF [310].
8.2. Anti-Melanogenesis Effects (Figure 1H)
In melanoma B16 cells, CGA likely acts on melanin as a substrate, but its metabolites may inhibit melanogenesis by suppressing tyrosinase activity [311]. CGA and caffeic acid derivatives inhibit melanocyte-stimulating hormone (α-MSH)-induced melanogenesis [312,313,314]. CGA binds to tyrosinase. The molecular docking simulation of CGA on tyrosinase shows the binding energy of −4.59 kcal/mol through interactions with ARG 321 and ARG 374 residues of tyrosinase. Therefore, CGA has the potential as an anti-hyperpigmentation agent through the inhibition of tyrosinase [315].
8.3. Human Subject Studies for Skin Protection
In a randomized, double-blind, controlled clinical study, subjects (n = 46) were administered jujube syrup containing gallic acid (1140 ± 17.65 μg/mL) and CGA (1520 ± 25.77 μg/mL) or placebo (23 in each group) twice a day for 8 weeks. The number of facial pigment spots and pigmented areas and percentages were significantly lower in the participants taking jujube syrup than in those in the placebo group [316]. In a double-blind, placebo-controlled study, female subjects with mildly xerotic skin (n = 49) were given a beverage containing coffee polyphenols (CPPs) (270 mg/100 mL/day) or placebo for 8 weeks. The intake of CPPs improved skin barrier and microcirculatory functions by lowering skin dryness, transepidermal water loss, and skin surface pH, increasing free fatty acids and lactic acid in the stratum corneum, and promoting skin blood flow [317].
10. Extending Lifespan in Worms
CGA reduces the generation of ROS in worms and increases their lifespan through the DAF-16/FOXO and Nrf2/SKN-1 signaling axis under normal conditions or in challenge to oxidation [331]. CGA can prolong about 20.1% of C. elegans’ lifespan by attenuating the age-associated decrease in body mobility and enhancing stress challenge via DAF-16-regulated insulin/IGF-1 signaling [332]. CGA prolongs about 24% and 9% of the lifespans of DAF-16a- and DAF-16f-rescued worms, respectively, through the activation of Nrf2/SKN-1 [333] (Figure 1J).
11. Other Protective Roles of CGA
11.1. Lung Protective Effects
CGA counteracts paraquat-induced oxidative, fibrotic, and inflammatory injuries to the lungs in rats [334]. KAT2A is the crucial regulatory gene for the expression of pro-inflammatory factors. CGA acts as a KAT2A inhibitor, attenuating the acute lung inflammation and improving the impaired respiratory function in a mouse model of LPS-induced acute lung injury [335]. In LPS and polyinosinic:polycytidylic acid (POLY I:C)-induced acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) models, CGA counteracts the inflammatory and oxidative stress in human airway epithelial cells and in BALB/c mice through targeting the TLR4/TLR3/NLRP3 inflammasome axis [336].
11.2. Intestinal Protective Effects
CGA alleviates intestinal inflammation and injury in broilers induced by necrotic enteritis challenge through suppressing the mtDNA-cGAS-STING signaling pathway [337]. In a rat model of post-infectious irritable bowel syndrome (PI-IBS), rectal application of CGA ameliorated PI-IBS-related pathologies, probably by increasing glycine levels and modulating gut microbial-released extracellular vesicles [338].
11.3. Ovarian Protective Effects
CGA significantly counteracts oxidative stress, pro-inflammatory, and pro-apoptotic markers in cisplatin (CDDP)-induced ovarian damage in rats [339]. CGA mitigates symptoms in patients with polycystic ovarian syndrome (PCOS) and improves follicular development, hormone status, and oxidative stress in PCOS rats, likely through modulating HIF-1alpha signaling [340].
11.4. Human Subject Studies for Menopausal Symptom Management
In a randomized, placebo-controlled, double-blind, parallel-group trial with healthy women (n = 82), the effects of CGAs on menopausal symptoms were examined. The subjects were administered CGAs (270 mg) or the placebo for 4 weeks. CGAs significantly decreased the modified Kupperman index of menopausal symptoms and reduced the number of hot flushes, the severity of hot flushes during sleep, and the severity of daytime sweats compared to the placebo group. No adverse effects were observed in the CGAs group [341].
12. Summary
CGA shows diverse pharmacological effects and acts through multidimensional scientific domains. The mechanistic hubs underlie its integrative functions of anti-inflammation, antioxidation, and modulation of metabolic homeostasis (Figure 1, Table 1). First, CGA can curtail NF-κB, JAK, and MAPK pathways, stalling the production of predominant pro-inflammatory factors including TNF-α, NO, COX-2, PGE2, and ILs. It can contain and thwart inflammatory pathway constituents at multiple levels by counteracting primordial inflammatory factors, attenuating inflammatory propagation, and impeding inflammation-related tissue injury. Second, CGA concurrently elevates multiple pivotal antioxidant factors such as HO-1 and NOQ-1 via Nrf2-dependent or independent pathways, leading to scavenging excessive cellular free radicals. Third, it can regulate and help maintain the metabolic homeostasis of lipids and glucose through the activation of the AMPK pathway, modulating glucose release and absorption and lipid synthesis. Fourth, CGA exhibits neuromodulation by targeting multiple neuroreceptors and channels such as GABA receptors, potassium channels, and acid-sensing ion channels, achieving antinociceptive effects (Figure 2, Table 1).

Table 1.
A summary of the potential mechanisms underlying CGA’s pharmacological activities and related experimental models.
Though our current understanding of CGA has been substantially expanded in contemporary years, there are many important scientific gaps yet to be addressed in future studies. (1) The specific molecular targets of CGA on NF-κB, MAPK, and Nrf2 pathways remain elusive. Molecular docking modeling can provide an insightful lead to the direct interaction of CGA and targeting molecules, followed by functional characterizations. Furthermore, an in-depth analysis including next-generation sequencing and multiome approaches at tissue and single-cell levels should be applied to reveal a systemic and comprehensive picture of CGA’s biological effects at transcriptional, translational, epigenetic, and intermolecular levels. (2) The pharmacokinetic data of CGA is inadequate due to its limited bioavailability. Upon oral ingestion, a significant portion of CGA remains in the colon and becomes metabolized and absorbed into circulation. Therefore, the observed pharmacological effects are likely to result from CGA and its bioactive metabolites, which further impedes the mechanistic interpretation of the data. Furthermore, efforts to modify the structure of CGA or develop novel and effective drug delivery systems such as liposomes, micelles, and nanoparticles for CGA are ongoing and need further validation for bioavailability, tissue distribution, and efficacy. (3) Clinical studies are required to translate CGA efficacy from bench to bedside for patients. Most current studies are performed on in vitro or in vivo models, which may not truthfully recapitulate the pathological conditions in real patients. Moreover, supraphysiological concentrations of CGA are used in many studies, which may lead to a misinterpretation of the value of CGA effects. (4) There has been a rising popularity of green coffee bean powder recently. The consumption of CGA-enriched natural products such as GCE, fruits, and vegetables at a dose equivalent to daily intake may empower a versatile way to extend its health benefits to the general population without any safety concerns. The development of novel approaches for raw material processing to preserve the CGA and other bioactive substances and improve their release and absorption upon ingestion is a task for the dietary supplementary industry.
In summary, recent advances in our understanding of CGAs have supported its therapeutic potential in many disorders. It is necessary to propel properly designed clinical trials and prospective studies to further elucidate and validate its efficacy in clinics.
Author Contributions
Conceptualization, W.T.; writing—original draft preparation, V.N., E.G.T. and W.T.; writing—review and editing, T.C. and W.T.; visualization, D.M.; supervision, W.T.; project administration, V.N.; funding acquisition, T.C. and W.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (R01AR073172 and 1R21AR083066 to W.T.) and Department of Defense (HT9425-23-10008 to W.T.), and National Heart, Lung, and Blood Institute (R01HL160541 to T.C.) and National Center for Advancing Translational Science (R03TR004450 to T.C.).
Acknowledgments
We are very grateful to Li Meng for his critical review comments.
Conflicts of Interest
EGT is an employee of TritaliMed, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Clifford, M.N.; Jaganath, I.B.; Ludwig, I.A.; Crozier, A. Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability and bioactivity. Nat. Prod. Rep. 2017, 34, 1391–1421. [Google Scholar] [CrossRef]
- Li, L.; Su, C.; Chen, X.; Wang, Q.; Jiao, W.; Luo, H.; Tang, J.; Wang, W.; Li, S.; Guo, S. Chlorogenic Acids in Cardiovascular Disease: A Review of Dietary Consumption, Pharmacology, and Pharmacokinetics. J. Agric. Food Chem. 2020, 68, 6464–6484. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Steiling, H.; Williamson, G.; Crozier, A. Bioavailability of chlorogenic acids following acute ingestion of coffee by humans with an ileostomy. Arch. Biochem. Biophys. 2010, 501, 98–105. [Google Scholar] [CrossRef]
- Xue, H.; Wei, M.; Ji, L. Chlorogenic acids: A pharmacological systematic review on their hepatoprotective effects. Phytomedicine 2023, 118, 154961. [Google Scholar] [CrossRef]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.; Liu, R.H. Processed sweet corn has higher antioxidant activity. J. Agric. Food Chem. 2002, 50, 4959–4964. [Google Scholar] [CrossRef] [PubMed]
- Kozuma, K.; Tsuchiya, S.; Kohori, J.; Hase, T.; Tokimitsu, I. Antihypertensive effect of green coffee bean extract on mildly hypertensive subjects. Hypertens. Res. 2005, 28, 711–718. [Google Scholar] [CrossRef]
- Roshan, H.; Nikpayam, O.; Sedaghat, M.; Sohrab, G. Effects of green coffee extract supplementation on anthropometric indices, glycaemic control, blood pressure, lipid profile, insulin resistance and appetite in patients with the metabolic syndrome: A randomised clinical trial. Br. J. Nutr. 2018, 119, 250–258. [Google Scholar] [CrossRef]
- Jeon, J.-S.; Kim, H.-T.; Jeong, I.-H.; Hong, S.-R.; Oh, M.-S.; Yoon, M.-H.; Shim, J.-H.; Jeong, J.H.; El-Aty, A.A. Contents of chlorogenic acids and caffeine in various coffee-related products. J. Adv. Res. 2019, 17, 85–94. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Paz de Pena, M.; Concepcion, C.; Alan, C. Catabolism of coffee chlorogenic acids by human colonic microbiota. Biofactors 2013, 39, 623–632. [Google Scholar] [CrossRef]
- Couteau, D.; McCartney, A.L.; Gibson, G.R.; Williamson, G.; Faulds, C.B. Isolation and characterization of human colonic bacteria able to hydrolyse chlorogenic acid. J. Appl. Microbiol. 2001, 90, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Atanasov, A.G.; Li, Y.; Kumar, N.; Bishayee, A. Chlorogenic acid for cancer prevention and therapy: Current status on efficacy and mechanisms of action. Pharmacol. Res. 2022, 186, 106505. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Arai, Y.; Mitsui, Y.; Kusaura, T.; Okawa, W.; Kajihara, Y.; Saito, I. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin. Exp. Hypertens. 2006, 28, 439–449. [Google Scholar] [CrossRef]
- Faria, W.C.S.; da Silva, A.A.; Veggi, N.; Kawashita, N.H.; Lemes, S.A.D.F.; de Barros, W.M.; Cardoso, E.D.C.; Converti, A.; Moura, W.d.M.; Bragagnolo, N. Acute and subacute oral toxicity assessment of dry encapsulated and non-encapsulated green coffee fruit extracts. J. Food Drug Anal. 2020, 28, 337–355. [Google Scholar] [PubMed]
- Venkatakrishna, K.; Sudeep, H.V.; Shyamprasad, K. Acute and sub-chronic toxicity evaluation of a standardized green coffee bean extract (CGA-7) in Wistar albino rats. SAGE Open Med. 2021, 9, 2050312120984885. [Google Scholar]
- Olthof, M.R.; Hollman, P.C.; Zock, P.L.; Katan, M.B. Consumption of high doses of chlorogenic acid, present in coffee, or of black tea increases plasma total homocysteine concentrations in humans. Am. J. Clin. Nutr. 2001, 73, 532–538. [Google Scholar] [CrossRef]
- Bagdas, D.; Gul, Z.; Meade, J.A.; Cam, B.; Cinkilic, N.; Gurun, M.S. Pharmacologic Overview of Chlorogenic Acid and its Metabolites in Chronic Pain and Inflammation. Curr. Neuropharmacol. 2020, 18, 216–228. [Google Scholar] [CrossRef]
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264.7 cells through suppressing NF-kappaB and JNK/AP-1 activation. Int. Immunopharmacol. 2009, 9, 1042–1048. [Google Scholar] [CrossRef]
- Ji, L.; Jiang, P.; Lu, B.; Sheng, Y.; Wang, X.; Wang, Z. Chlorogenic acid, a dietary polyphenol, protects acetaminophen-induced liver injury and its mechanism. J. Nutr. Biochem. 2013, 24, 1911–1919. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.-W.; Park, Y.; Lee, H.-J.; Kim, K.-W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Shi, H.; Dong, L.; Jiang, J.; Zhao, J.; Zhao, G.; Dang, X.; Lu, X.; Jia, M. Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology 2013, 303, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Domitrovic, R.; Jakovac, H.; Romic, Z.; Rahelic, D.; Tadic, Z. Antifibrotic activity of Taraxacum officinale root in carbon tetrachloride-induced liver damage in mice. J. Ethnopharmacol. 2010, 130, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Sheng, Y.; Lu, B.; Ji, L. The therapeutic detoxification of chlorogenic acid against acetaminophen-induced liver injury by ameliorating hepatic inflammation. Chem. Biol. Interact. 2015, 238, 93–101. [Google Scholar] [CrossRef]
- Kang, T.Y.; Yang, H.R.; Zhang, J.; Li, D.; Lin, J.; Wang, L.; Xu, X. The studies of chlorogenic Acid antitumor mechanism by gene chip detection: The immune pathway gene expression. J. Anal. Methods Chem. 2013, 2013, 617243. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, T. The polyphenol chlorogenic acid inhibits staphylococcal exotoxin-induced inflammatory cytokines and chemokines. Immunopharmacol. Immunotoxicol. 2002, 24, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Goya, L.; Sánchez-Medina, A.; Redondo-Puente, M.; Dupak, R.; Bravo, L.; Sarriá, B. Main Colonic Metabolites from Coffee Chlorogenic Acid May Counteract Tumor Necrosis Factor-alpha-Induced Inflammation and Oxidative Stress in 3T3-L1 Cells. Molecules 2023, 29, 88. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, J.; Yu, X.; Tao, W.; Jiang, F.; Yin, Z.; Liu, C. Protective effects of chlorogenic acid on acute hepatotoxicity induced by lipopolysaccharide in mice. Inflamm. Res. 2010, 59, 871–877. [Google Scholar] [CrossRef]
- Lee, C.H.; Yoon, S.J.; Lee, S.M. Chlorogenic acid attenuates high mobility group box 1 (HMGB1) and enhances host defense mechanisms in murine sepsis. Mol. Med. 2013, 18, 1437–1448. [Google Scholar] [CrossRef]
- Chen, J.; Xie, H.; Chen, D.; Yu, B.; Mao, X.; Zheng, P.; Yu, J.; Luo, Y.; Luo, J.; He, J. Chlorogenic Acid Improves Intestinal Development via Suppressing Mucosa Inflammation and Cell Apoptosis in Weaned Pigs. ACS Omega 2018, 3, 2211–2219. [Google Scholar] [CrossRef]
- Yun, N.; Kang, J.-W.; Lee, S.-M. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: Molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012, 23, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Zhang, Z.; Liu, J.; Chen, L.; Tian, Y.; Xu, W.; Zeng, T.; Wu, W.; Lu, L. Chlorogenic Acid Alleviates LPS-Induced Inflammation and Oxidative Stress by Modulating CD36/AMPK/PGC-1alpha in RAW264.7 Macrophages. Int. J. Mol. Sci. 2023, 24, 13516. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Baek, S.-I.; Yun, J.; Lee, S.; Yoon, D.Y.; Jung, J.-K.; Jung, S.-H.; Hwang, B.Y.; Hong, J.T.; Han, S.-B.; et al. IRAK4 as a molecular target in the amelioration of innate immunity-related endotoxic shock and acute liver injury by chlorogenic acid. J. Immunol. 2015, 194, 1122–1130. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Shim, J.; Kim, H.W.; Kim, J.; Jang, Y.J.; Yang, H.; Park, J.; Choi, S.H.; Yoon, J.H.; et al. Caffeinated coffee, decaffeinated coffee, and the phenolic phytochemical chlorogenic acid up-regulate NQO1 expression and prevent H2O2-induced apoptosis in primary cortical neurons. Neurochem. Int. 2012, 60, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Gul, N.Y.; Topal, A.; Tas, S.; Ozyigit, M.O.; Cinkilic, N.; Gul, Z.; Etoz, B.C.; Ziyanok, S.; Inan, S.; et al. Pharmacologic overview of systemic chlorogenic acid therapy on experimental wound healing. Naunyn. Schmiedebergs Arch. Pharmacol. 2014, 387, 1101–1116. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Shibata, H.; Sakamoto, Y.; Oka, M.; Kono, Y. Natural antioxidant, chlorogenic acid, protects against DNA breakage caused by monochloramine. Biosci. Biotechnol. Biochem. 1999, 63, 1295–1297. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, X.; Guo, K.; Zhou, F.; Yang, H. Use of Chlorogenic Acid against Diabetes Mellitus and Its Complications. J. Immunol. Res. 2020, 2020, 9680508. [Google Scholar] [CrossRef]
- Li, W.N.; Han, Y.D.; Liu, Y.H.; Chen, Y.; Xiao, Y. Effects of Chlorogenic acid extract from leaves of Eucommia ulmoides on key enzyme activities in lipid metabolism. Tradit. Chin. Drug Res. Clin. Pharmacol. 2012, 23, 4. [Google Scholar]
- Cho, A.-S.; Jeon, S.-M.; Kim, M.-J.; Yeo, J.; Seo, K.-I.; Choi, M.-S.; Lee, M.-K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef]
- Sudeep, H.V.; Venkatakrishna, K.; Patel, D.; Shyamprasad, K. Biomechanism of chlorogenic acid complex mediated plasma free fatty acid metabolism in rat liver. BMC Complement Altern. Med. 2016, 16, 274. [Google Scholar]
- Ye, X.; Li, J.; Gao, Z.; Wang, D.; Wang, H.; Wu, J. Chlorogenic Acid Inhibits Lipid Deposition by Regulating the Enterohepatic FXR-FGF15 Pathway. Biomed. Res. Int. 2022, 2022, 4919153. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, L.; Cao, F.; Liu, P.; Bao, H.; Yan, Y.; Dong, X.; Wang, D.; Wang, Z.; Gong, P. Modulation of transport and metabolism of bile acids and bilirubin by chlorogenic acid against hepatotoxicity and cholestasis in bile duct ligation rats: Involvement of SIRT1-mediated deacetylation of FXR and PGC-1alpha. J. Hepatobiliary Pancreat Sci. 2018, 25, 195–205. [Google Scholar] [CrossRef]
- Tunnicliffe, J.M.; Cowan, T.; Shearer, J. Chapter 86—Chlorogenic Acid in Whole Body and Tissue-Specific Glucose Regulation. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Ong, K.W.; Hsu, A.; Tan, B.K. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: A contributor to the beneficial effects of coffee on diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.J.; Zhu, Q.; Zhong, Y.L.; Xu, S.H.; Wang, Z. Chlorogenic Acid Maintains Glucose Homeostasis through Modulating the Expression of SGLT-1, GLUT-2, and PLG in Different Intestinal Segments of Sprague-Dawley Rats Fed a High-Fat Diet. Biomed. Environ. Sci. 2015, 28, 894–903. [Google Scholar] [PubMed]
- Tsuda, S.; Egawa, T.; Ma, X.; Oshima, R.; Kurogi, E.; Hayashi, T. Coffee polyphenol caffeic acid but not chlorogenic acid increases 5′AMP-activated protein kinase and insulin-independent glucose transport in rat skeletal muscle. J. Nutr. Biochem. 2012, 23, 1403–1409. [Google Scholar] [CrossRef]
- Ong, K.W.; Hsu, A.; Tan, B.K. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by ampk activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Henry-Vitrac, C.; Ibarra, A.; Roller, M.; Merillon, J.M.; Vitrac, X. Contribution of chlorogenic acids to the inhibition of human hepatic glucose-6-phosphatase activity in vitro by Svetol, a standardized decaffeinated green coffee extract. J. Agric. Food Chem. 2010, 58, 4141–4144. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Chang, C.Q.; Ma, F.Y.; Yu, C.L. Modulating effects of chlorogenic acid on lipids and glucose metabolism and expression of hepatic peroxisome proliferator-activated receptor-alpha in golden hamsters fed on high fat diet. Biomed. Environ. Sci. 2009, 22, 122–129. [Google Scholar] [CrossRef]
- Wan, C.; Wong, C.N.; Pin, W.; Wong, M.H.; Kwok, C.; Chan, R.Y.; Yu, P.H.; Chan, S. Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-alpha in hypercholesterolemic rats induced with a high-cholesterol diet. Phytother. Res. 2013, 27, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-K.; Park, S.-B.; Lee, D.-R.; Lee, H.J.; Jin, Y.-Y.; Yang, S.H.; Suh, J.-W. Green coffee bean extract improves obesity by decreasing body fat in high-fat diet-induced obese mice. Asian Pac. J. Trop. Med. 2016, 9, 635–643. [Google Scholar] [CrossRef]
- Zhang, L.T.; Chang, C.Q.; Liu, Y.; Chen, Z.M. Effect of chlorogenic acid on disordered glucose and lipid metabolism in db/db mice and its mechanism. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2011, 33, 281–286. [Google Scholar]
- Tunnicliffe, J.M.; Eller, L.K.; Reimer, R.A.; Hittel, D.S.; Shearer, J. Chlorogenic acid differentially affects postprandial glucose and glucose-dependent insulinotropic polypeptide response in rats. Appl. Physiol. Nutr. Metab. 2011, 36, 650–659. [Google Scholar] [CrossRef]
- Nasef, N.A.; Thota, R.N.; Mutukumira, A.N.; Rutherfurd-Markwick, K.; Dickens, M.; Gopal, P.; Singh, H.; Garg, M.L. Bioactive Yoghurt Containing Curcumin and Chlorogenic Acid Reduces Inflammation in Postmenopausal Women. Nutrients 2022, 14, 4619. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.V.; Rodriguez, L.C.; Hammond, L.; DiSilvestro, R.; Hunter, J.M.; Pietrzkowski, Z. Acute reduction of serum 8-iso-PGF2-alpha and advanced oxidation protein products in vivo by a polyphenol-rich beverage; a pilot clinical study with phytochemical and in vitro antioxidant characterization. Nutr. J. 2011, 10, 67. [Google Scholar] [CrossRef]
- Zuniga, L.Y.; Aceves-de la Mora, M.C.A.; Gonzalez-Ortiz, M.; Ramos-Nunez, J.L.; Martinez-Abundis, E. Effect of Chlorogenic Acid Administration on Glycemic Control, Insulin Secretion, and Insulin Sensitivity in Patients with Impaired Glucose Tolerance. J. Med. Food 2018, 21, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Victoria-Montesinos, D.; Arcusa, R.; García-Muñoz, A.M.; Pérez-Piñero, S.; Sánchez-Macarro, M.; Avellaneda, A.; López-Román, F. Effects of the Consumption of Low-Fat Cooked Ham with Reduced Salt Enriched with Antioxidants on the Improvement of Cardiovascular Health: A Randomized Clinical Trial. Nutrients 2021, 13, 1480. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Three-arm, placebo-controlled, randomized clinical trial evaluating the metabolic effect of a combined nutraceutical containing a bergamot standardized flavonoid extract in dyslipidemic overweight subjects. Phytother. Res. 2019, 33, 2094–2101. [Google Scholar] [CrossRef]
- Martinez-Lopez, S.; Sarria, B.; Mateos, R.; Bravo-Clemente, L. Moderate consumption of a soluble green/roasted coffee rich in caffeoylquinic acids reduces cardiovascular risk markers: Results from a randomized, cross-over, controlled trial in healthy and hypercholesterolemic subjects. Eur. J. Nutr. 2019, 58, 865–878. [Google Scholar] [CrossRef]
- Kempf, K.; Herder, C.; Erlund, I.; Kolb, H.; Martin, S.; Carstensen, M.; Koenig, W.; Sundvall, J.; Bidel, S.; Kuha, S.; et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am. J. Clin. Nutr. 2010, 91, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Kobayashi, S.; Yamaguchi, T.; Hibi, M.; Fukuhara, I.; Osaki, N. Coffee Abundant in Chlorogenic Acids Reduces Abdominal Fat in Overweight Adults: A Randomized, Double-Blind, Controlled Trial. Nutrients 2019, 11, 1617. [Google Scholar] [CrossRef]
- Lee, A.H.; Tan, L.; Hiramatsu, N.; Ishisaka, A.; Alfonso, H.; Tanaka, A.; Uemura, N.; Fujiwara, Y.; Takechi, R. Plasma concentrations of coffee polyphenols and plasma biomarkers of diabetes risk in healthy Japanese women. Nutr. Diabetes 2016, 6, e212. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yamamoto, N.; Jokura, H.; Yamamoto, M.; Fujii, A.; Tokimitsu, I.; Saito, I. Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Kagawa, D.; Ochiai, R.; Tokimitsu, I.; Saito, I. Green coffee bean extract and its metabolites have a hypotensive effect in spontaneously hypertensive rats. Hypertens. Res. 2002, 25, 99–107. [Google Scholar] [CrossRef]
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994, 74, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, R.; Wang, L.; Yan, R.; Hou, R.; Gao, S.; Yang, B. Effects of an aqueous extract of Crataegus pinnatifida Bge. var. major N.E.Br. fruit on experimental atherosclerosis in rats. J. Ethnopharmacol. 2013, 148, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Balzan, S.; Hernandes, A.; Reichert, C.L.; Donaduzzi, C.; Pires, V.A.; Junior, A.G.; Junior, E.L. Lipid-lowering effects of standardized extracts of Ilex paraguariensis in high-fat-diet rats. Fitoterapia 2013, 86, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Yukawa, G.S.; Mune, M.; Otani, H.; Tone, Y.; Liang, X.-M.; Iwahashi, H.; Sakamoto, W. Effects of coffee consumption on oxidative susceptibility of low-density lipoproteins and serum lipid levels in humans. Biochemistry 2004, 69, 70–74. [Google Scholar] [CrossRef]
- Nardini, M.; D’Aquino, M.; Tomassi, G.; Gentili, V.; Di Felice, M.; Scaccini, C. Inhibition of human low-density lipoprotein oxidation by caffeic acid and other hydroxycinnamic acid derivatives. Free Radic. Biol. Med. 1995, 19, 541–552. [Google Scholar] [CrossRef]
- Roland, A.; Patterson, R.A.; Leake, D.S. Measurement of copper-binding sites on low density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 594–602. [Google Scholar] [CrossRef][Green Version]
- Aviram, M. HDL—Associated paraoxonase 1 (PON1) and dietary antioxidants attenuate lipoprotein oxidation, macrophage foam cells formation and atherosclerosis development. Pathophysiol. Haemost Thromb. 2006, 35, 146–151. [Google Scholar] [CrossRef]
- Gugliucci, A.; Bastos, D.H. Chlorogenic acid protects paraoxonase 1 activity in high density lipoprotein from inactivation caused by physiological concentrations of hypochlorite. Fitoterapia 2009, 80, 138–142. [Google Scholar] [CrossRef]
- Tsai, K.L.; Hung, C.H.; Chan, S.H.; Hsieh, P.L.; Ou, H.C.; Cheng, Y.H.; Chu, P.M. Chlorogenic Acid Protects Against oxLDL-Induced Oxidative Damage and Mitochondrial Dysfunction by Modulating SIRT1 in Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, e1700928. [Google Scholar] [CrossRef]
- Jung, H.J.; Im, S.S.; Song, D.K.; Bae, J.H. Effects of chlorogenic acid on intracellular calcium regulation in lysophosphatidylcholine-treated endothelial cells. BMB Rep. 2017, 50, 323–328. [Google Scholar] [CrossRef]
- Huang, W.; Fu, L.; Li, C.; Xu, L.; Zhang, L.; Zhang, W. Quercetin, Hyperin, and Chlorogenic Acid Improve Endothelial Function by Antioxidant, Antiinflammatory, and ACE Inhibitory Effects. J. Food Sci. 2017, 82, 1239–1246. [Google Scholar] [CrossRef]
- Jiang, R.; Hodgson, J.M.; Mas, E.; Croft, K.D.; Ward, N.C. Chlorogenic acid improves ex vivo vessel function and protects endothelial cells against HOCl-induced oxidative damage, via increased production of nitric oxide and induction of Hmox-1. J. Nutr. Biochem. 2016, 27, 53–60. [Google Scholar] [CrossRef]
- Hebeda, C.B.; Bolonheis, S.M.; Nakasato, A.; Belinati, K.; Souza, P.D.; Gouvea, D.R.; Lopes, N.P.; Farsky, S.H. Effects of chlorogenic acid on neutrophil locomotion functions in response to inflammatory stimulus. J. Ethnopharmacol. 2011, 135, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Chen, C.H.; Lee, M.F.; Chang, T.; Yu, Y.M. Chlorogenic acid attenuates adhesion molecules upregulation in IL-1beta-treated endothelial cells. Eur. J. Nutr. 2010, 49, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic. Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Hwang, S.J.; Park, J.H.; Lee, H.J. Chlorogenic acid inhibits hypoxia-induced angiogenesis via down-regulation of the HIF-1alpha/AKT pathway. Cell Oncol. 2015, 38, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Hu, J.; Zhou, X.; Cheung, P.C. Inhibition of vascular endothelial growth factor-induced angiogenesis by chlorogenic acid via targeting the vascular endothelial growth factor receptor 2-mediated signaling pathway. J. Funct. Foods 2017, 32, 285–295. [Google Scholar] [CrossRef]
- Fuentes, E.; Caballero, J.; Alarcon, M.; Rojas, A.; Palomo, I. Chlorogenic acid inhibits human platelet activation and thrombus formation. PLoS ONE 2014, 9, e90699. [Google Scholar] [CrossRef]
- Cho, H.J.; Kang, H.J.; Kim, Y.J.; Lee, D.H.; Kwon, H.W.; Kim, Y.Y.; Park, H.J. Inhibition of platelet aggregation by chlorogenic acid via cAMP and cGMP-dependent manner. Blood Coagul. Fibrinolysis. 2012, 23, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Moon, J.; Cho, Y.; Chung, J.H.; Shin, M.J. Quercetin up-regulates expressions of peroxisome proliferator-activated receptor gamma, liver X receptor alpha, and ATP binding cassette transporter A1 genes and increases cholesterol efflux in human macrophage cell line. Nutr. Res. 2013, 33, 136–143. [Google Scholar] [CrossRef]
- Wu, C.; Luan, H.; Zhang, X.; Wang, S.; Zhang, X.; Sun, X.; Guo, P. Chlorogenic acid protects against atherosclerosis in ApoE−/− mice and promotes cholesterol efflux from RAW264.7 macrophages. PLoS ONE 2014, 9, e95452. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-S.; Kang, J.C.; Jang, Y.C.; Park, J.S.; Jang, S.Y.; Kim, D.-E.; Kim, B.; Shin, H.-S. Cardioprotective effects of rhamnetin in H9c2 cardiomyoblast cells under H2O2-induced apoptosis. J. Ethnopharmacol. 2014, 153, 552–560. [Google Scholar] [CrossRef]
- Gordon, J.W.; Shaw, J.A.; Kirshenbaum, L.A. Multiple facets of NF-kappaB in the heart: To be or not to NF-kappaB. Circ. Res. 2011, 108, 1122–1132. [Google Scholar] [CrossRef]
- Tian, L.; Su, C.P.; Wang, Q.; Wu, F.J.; Bai, R.; Zhang, H.M.; Liu, J.Y.; Lu, W.J.; Wang, W.; Lan, F.; et al. Chlorogenic acid: A potent molecule that protects cardiomyocytes from TNF-alpha-induced injury via inhibiting NF-kappaB and JNK signals. J. Cell. Mol. Med. 2019, 23, 4666–4678. [Google Scholar] [CrossRef]
- Li, Y.; Shen, D.; Tang, X.; Li, X.; Wo, D.; Yan, H.; Song, R.; Feng, J.; Li, P.; Zhang, J.; et al. Chlorogenic acid prevents isoproterenol-induced hypertrophy in neonatal rat myocytes. Toxicol. Lett. 2014, 226, 257–263. [Google Scholar] [CrossRef]
- Gao, T.; Zhu, Z.Y.; Zhou, X.; Xie, M.L. Chrysanthemum morifolium extract improves hypertension-induced cardiac hypertrophy in rats by reduction of blood pressure and inhibition of myocardial hypoxia inducible factor-1alpha expression. Pharm. Biol. 2016, 54, 2895–2900. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.-W.; Li, J.-L.; Guo, B.-B.; Fan, H.-M.; Zhao, W.-M.; Wang, H.-Y. Chlorogenic acid analogues from Gynura nepalensis protect H9c2 cardiomyoblasts against H2O2-induced apoptosis. Acta Pharmacol. Sin. 2016, 37, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Chlopcikova, S.; Psotová, J.; Miketová, P.; Soušek, J.; Lichnovský, V.; Šimánek, V. Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes. Part II. caffeic, chlorogenic and rosmarinic acids. Phytother. Res. 2004, 18, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, G.; Liu, H.; Hou, Y.L. Protective effect of bioactive compounds from Lonicera japonica Thunb. against H2O2-induced cytotoxicity using neonatal rat cardiomyocytes. Iran J. Basic. Med. Sci. 2016, 19, 97–105. [Google Scholar]
- Bi, Y.-M.; Wu, Y.-T.; Chen, L.; Tan, Z.-B.; Fan, H.-J.; Xie, L.-P.; Zhang, W.-T.; Chen, H.-M.; Li, J.; Liu, B.; et al. 3,5-Dicaffeoylquinic acid protects H9C2 cells against oxidative stress-induced apoptosis via activation of the PI3K/Akt signaling pathway. Food Nutr. Res. 2018, 62, 1423. [Google Scholar] [CrossRef]
- Li, Y.; Ren, X.; Lio, C.; Sun, W.; Lai, K.; Liu, Y.; Zhang, Z.; Liang, J.; Zhou, H.; Liu, L.; et al. A chlorogenic acid-phospholipid complex ameliorates post-myocardial infarction inflammatory response mediated by mitochondrial reactive oxygen species in SAMP8 mice. Pharmacol. Res. 2018, 130, 110–122. [Google Scholar] [CrossRef]
- Akila, P.; Asaikumar, L.; Vennila, L. Chlorogenic acid ameliorates isoproterenol-induced myocardial injury in rats by stabilizing mitochondrial and lysosomal enzymes. Biomed. Pharmacother. 2017, 85, 582–591. [Google Scholar] [CrossRef]
- Akila, P.; Vennila, L. Chlorogenic acid a dietary polyphenol attenuates isoproterenol induced myocardial oxidative stress in rat myocardium: An in vivo study. Biomed. Pharmacother. 2016, 84, 208–214. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Al-Rasheed, N.M.; Faddah, L.; Mohamed, A.M.; Mohammad, R.A.; Al-Amin, M. Potential impact of silymarin in combination with chlorogenic acid and/or melatonin in combating cardiomyopathy induced by carbon tetrachloride. Saudi. J. Biol. Sci. 2014, 21, 265–274. [Google Scholar] [CrossRef]
- Kanno, Y.; Watanabe, R.; Zempo, H.; Ogawa, M.; Suzuki, J.-I.; Isobe, M. Chlorogenic acid attenuates ventricular remodeling after myocardial infarction in mice. Int. Heart J. 2013, 54, 176–180. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Ren, X.; Zhou, H.; Wang, K.; Zhang, H.; Luo, P. Caffeoylquinic Acid Derivatives Extract of Erigeron multiradiatus Alleviated Acute Myocardial Ischemia Reperfusion Injury in Rats through Inhibiting NF-KappaB and JNK Activations. Mediat. Inflamm. 2016, 2016, 7961940. [Google Scholar] [CrossRef]
- Ochiai, R.; Chikama, A.; Kataoka, K.; Tokimitsu, I.; Maekawa, Y.; Ohishi, M.; Rakugi, H.; Mikami, H. Effects of hydroxyhydroquinone-reduced coffee on vasoreactivity and blood pressure. Hypertens. Res. 2009, 32, 969–974. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Chikama, A.; Mori, K.; Watanabe, T.; Shioya, Y.; Katsuragi, Y.; Tokimitsu, I. Hydroxyhydroquinone-free coffee: A double-blind, randomized controlled dose-response study of blood pressure. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 408–414. [Google Scholar] [CrossRef]
- Kajikawa, M.; Maruhashi, T.; Hidaka, T.; Nakano, Y.; Kurisu, S.; Matsumoto, T.; Iwamoto, Y.; Kishimoto, S.; Matsui, S.; Aibara, Y.; et al. Coffee with a high content of chlorogenic acids and low content of hydroxyhydroquinone improves postprandial endothelial dysfunction in patients with borderline and stage 1 hypertension. Eur. J. Nutr. 2019, 58, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Katada, S.; Watanabe, T.; Mizuno, T.; Kobayashi, S.; Takeshita, M.; Osaki, N.; Kobayashi, S.; Katsuragi, Y. Effects of Chlorogenic Acid-Enriched and Hydroxyhydroquinone-Reduced Coffee on Postprandial Fat Oxidation and Antioxidative Capacity in Healthy Men: A Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. Nutrients 2018, 10, 525. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Nardini, M.; Belelli, F.; Scaccini, C. Coffee drinking induces incorporation of phenolic acids into LDL and increases the resistance of LDL to ex vivo oxidation in humans. Am. J. Clin. Nutr. 2007, 86, 604–609. [Google Scholar] [CrossRef]
- Lara-Guzmán, O.J.; Medina, S.; Álvarez, R.; Oger, C.; Durand, T.; Galano, J.-M.; Zuluaga, N.; Gil-Izquierdo, A.; Muñoz-Durango, K. Oxylipin regulation by phenolic compounds from coffee beverage: Positive outcomes from a randomized controlled trial in healthy adults and macrophage derived foam cells. Free Radic. Biol. Med. 2020, 160, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Lara-Guzman, O.J.; Alvarez, R.; Munoz-Durango, K. Changes in the plasma lipidome of healthy subjects after coffee consumption reveal potential cardiovascular benefits: A randomized controlled trial. Free Radic. Biol. Med. 2021, 176, 345–355. [Google Scholar] [CrossRef]
- Naylor, L.H.; Zimmermann, D.; Guitard-Uldry, M.; Poquet, L.; Lévêques, A.; Eriksen, B.; Rhlid, R.B.; Galaffu, N.; D’urzo, C.; De Castro, A.; et al. Acute dose-response effect of coffee-derived chlorogenic acids on the human vasculature in healthy volunteers: A randomized controlled trial. Am. J. Clin. Nutr. 2021, 113, 370–379. [Google Scholar] [CrossRef]
- Mills, C.E.; Flury, A.; Marmet, C.; Poquet, L.; Rimoldi, S.F.; Sartori, C.; Rexhaj, E.; Brenner, R.; Allemann, Y.; Zimmermann, D.; et al. Mediation of coffee-induced improvements in human vascular function by chlorogenic acids and its metabolites: Two randomized, controlled, crossover intervention trials. Clin. Nutr. 2017, 36, 1520–1529. [Google Scholar] [CrossRef]
- Boon, E.A.J.; Croft, K.D.; Shinde, S.; Hodgson, J.M.; Ward, N.C. The acute effect of coffee on endothelial function and glucose metabolism following a glucose load in healthy human volunteers. Food Funct. 2017, 8, 3366–3373. [Google Scholar] [CrossRef] [PubMed]
- Jackson, P.A.; Wightman, E.L.; Veasey, R.; Forster, J.; Khan, J.; Saunders, C.; Mitchell, S.; Haskell-Ramsay, C.F.; Kennedy, D.O. A Randomized, Crossover Study of the Acute Cognitive and Cerebral Blood Flow Effects of Phenolic, Nitrate and Botanical Beverages in Young, Healthy Humans. Nutrients 2020, 12, 2254. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Nomura, T.; Jokura, H.; Kitamura, N.; Saiki, A.; Fujii, A. Chlorogenic acid-enriched green coffee bean extract affects arterial stiffness assessed by the cardio-ankle vascular index in healthy men: A pilot study. Int. J. Food Sci. Nutr. 2019, 70, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Terzo, S.; Amato, A.; Magán-Fernández, A.; Castellino, G.; Calvi, P.; Chianetta, R.; Giglio, R.V.; Patti, A.M.; Nikolic, D.; Firenze, A.; et al. A Nutraceutical Containing Chlorogenic Acid and Luteolin Improves Cardiometabolic Parameters in Subjects with Pre-Obesity: A 6-Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2023, 15, 462. [Google Scholar] [CrossRef] [PubMed]
- Castellino, G.; Nikolic, D.; Magán-Fernández, A.; Malfa, G.A.; Chianetta, R.; Patti, A.M.; Amato, A.; Montalto, G.; Toth, P.P.; Banach, M.; et al. Altilix® Supplement Containing Chlorogenic Acid and Luteolin Improved Hepatic and Cardiometabolic Parameters in Subjects with Metabolic Syndrome: A 6 Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2580. [Google Scholar] [CrossRef]
- Pérez-Nájera, V.C.; Gutiérrez-Uribe, J.A.; Antunes-Ricardo, M.; Hidalgo-Figueroa, S.; Del-Toro-Sánchez, C.L.; Salazar-Olivo, L.A.; Lugo-Cervantes, E. Smilax aristolochiifolia Root Extract and Its Compounds Chlorogenic Acid and Astilbin Inhibit the Activity of alpha-Amylase and alpha-Glucosidase Enzymes. Evid. Based Complement. Alternat. Med. 2018, 2018, 6247306. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Kinetic analysis and mechanism on the inhibition of chlorogenic acid and its components against porcine pancreas alpha-amylase isozymes I and II. J. Agric. Food Chem. 2009, 57, 9218–9225. [Google Scholar] [CrossRef]
- Tousch, D.; Lajoix, A.-D.; Hosy, E.; Azay-Milhau, J.; Ferrare, K.; Jahannault, C.; Cros, G.; Petit, P. Chicoric acid, a new compound able to enhance insulin release and glucose uptake. Biochem. Biophys. Res. Commun. 2008, 377, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Gomez, B.; Ramos, S.; Goya, L.; Mesa, M.D.; del Castillo, M.D.; Martín, M. Coffee silverskin extract improves glucose-stimulated insulin secretion and protects against streptozotocin-induced damage in pancreatic INS-1E beta cells. Food Res. Int. 2016, 89, 1015–1022. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, M.; Liu, D. Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in mice. Pharm. Res. 2015, 32, 1200–1209. [Google Scholar] [CrossRef]
- Song, S.J.; Choi, S.; Park, T. Decaffeinated green coffee bean extract attenuates diet-induced obesity and insulin resistance in mice. Evid. Based Complement. Alternat Med. 2014, 2014, 718379. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Gao, Y.-Q.; Zhang, Y.; Wang, H.; Liu, G.-S.; Lei, J.-Y. Chlorogenic acid alleviates autophagy and insulin resistance by suppressing JNK pathway in a rat model of nonalcoholic fatty liver disease. J. Biosci. 2018, 43, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Karthikesan, K.; Pari, L.; Menon, V.P. Protective effect of tetrahydrocurcumin and chlorogenic acid against streptozotocin–nicotinamide generated oxidative stress induced diabetes. J. Funct. Foods 2010, 2, 134–142. [Google Scholar] [CrossRef]
- Pari, L.; Karthikesan, K.; Menon, V.P. Comparative and combined effect of chlorogenic acid and tetrahydrocurcumin on antioxidant disparities in chemical induced experimental diabetes. Mol. Cell. Biochem. 2010, 341, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Karthikesan, K.; Pari, L.; Menon, V.P. Combined treatment of tetrahydrocurcumin and chlorogenic acid exerts potential antihyperglycemic effect on streptozotocin-nicotinamide-induced diabetic rats. Gen. Physiol. Biophys. 2010, 29, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-kB pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef]
- Ye, H.-Y.; Li, Z.-Y.; Zheng, Y.; Chen, Y.; Zhou, Z.-H.; Jin, J. The attenuation of chlorogenic acid on oxidative stress for renal injury in streptozotocin-induced diabetic nephropathy rats. Arch. Pharm. Res. 2016, 39, 989–997. [Google Scholar] [CrossRef]
- Mei, X.; Zhou, L.; Zhang, T.; Lu, B.; Sheng, Y.; Ji, L. Chlorogenic acid attenuates diabetic retinopathy by reducing VEGF expression and inhibiting VEGF-mediated retinal neoangiogenesis. Vascul. Pharmacol. 2018, 101, 29–37. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, T.; Lu, B.; Yu, Z.; Mei, X.; Abulizi, P.; Ji, L. Lonicerae Japonicae Flos attenuates diabetic retinopathy by inhibiting retinal angiogenesis. J. Ethnopharmacol. 2016, 189, 117–125. [Google Scholar] [CrossRef]
- Shin, J.Y.; Sohn, J.; Park, K.H. Chlorogenic acid decreases retinal vascular hyperpermeability in diabetic rat model. J. Korean Med. Sci. 2013, 28, 608–613. [Google Scholar] [CrossRef]
- Hong, B.N.; Nam, Y.H.; Woo, S.H.; Kang, T.H. Chlorogenic acid rescues sensorineural auditory function in a diabetic animal model. Neurosci. Lett. 2017, 640, 64–69. [Google Scholar] [CrossRef]
- Bagdas, D.; Ozboluk, H.Y.; Cinkilic, N.; Gurun, M.S. Antinociceptive effect of chlorogenic acid in rats with painful diabetic neuropathy. J. Med. Food 2014, 17, 730–732. [Google Scholar] [CrossRef] [PubMed]
- Yanagimoto, A.; Matsui, Y.; Yamaguchi, T.; Saito, S.; Hanada, R.; Hibi, M. Acute Dose-Response Effectiveness of Combined Catechins and Chlorogenic Acids on Postprandial Glycemic Responses in Healthy Men: Results from Two Randomized Studies. Nutrients 2023, 15, 777. [Google Scholar] [CrossRef] [PubMed]
- Yanagimoto, A.; Matsui, Y.; Yamaguchi, T.; Hibi, M.; Kobayashi, S.; Osaki, N. Effects of Ingesting Both Catechins and Chlorogenic Acids on Glucose, Incretin, and Insulin Sensitivity in Healthy Men: A Randomized, Double-Blinded, Placebo-Controlled Crossover Trial. Nutrients 2022, 14, 5063. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Riva, A.; Petrangolini, G.; Allegrini, P.; Bernardinelli, L.; Fazia, T.; Peroni, G.; Gasparri, C.; Nichetti, M.; Faliva, M.A.; et al. The Metabolic Effects of Cynara Supplementation in Overweight and Obese Class I Subjects with Newly Detected Impaired Fasting Glycemia: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial. Nutrients 2020, 12, 3298. [Google Scholar] [CrossRef] [PubMed]
- Dkhil, M.A.; Moneim, A.E.; Bauomy, A.A.; Khalil, M.; Al-Shaebi, E.M.; Al-Quraishy, S. Chlorogenic acid prevents hepatotoxicity in arsenic-treated mice: Role of oxidative stress and apoptosis. Mol. Biol. Rep. 2020, 47, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Li, H.; Zhou, J.; Wang, S. Chlorogenic acid relieves lead-induced cognitive impairments and hepato-renal damage via regulating the dysbiosis of the gut microbiota in mice. Food Funct. 2019, 10, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, X.; Liu, Y.; Wang, S.; Cheng, D. Protection Mechanisms Underlying Oral Administration of Chlorogenic Acid against Cadmium-Induced Hepatorenal Injury Related to Regulating Intestinal Flora Balance. J. Agric. Food Chem. 2021, 69, 1675–1683. [Google Scholar] [CrossRef]
- Cheng, D.; Zhang, X.; Xu, L.; Li, X.; Hou, L.; Wang, C. Protective and prophylactic effects of chlorogenic acid on aluminum-induced acute hepatotoxicity and hematotoxicity in mice. Chem. Biol. Interact. 2017, 273, 125–132. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Yin, W.; Shan, W.; Dai, J.; Yang, Y.; Li, L. Combination of chlorogenic acid and salvianolic acid B protects against polychlorinated biphenyls-induced oxidative stress through Nrf2. Environ. Toxicol. Pharmacol. 2016, 46, 255–263. [Google Scholar] [CrossRef]
- Hussein, R.M.; Sawy, D.M.; Kandeil, M.A.; Farghaly, H.S. Chlorogenic acid, quercetin, coenzyme Q10 and silymarin modulate Keap1-Nrf2/heme oxygenase-1 signaling in thioacetamide-induced acute liver toxicity. Life Sci. 2021, 277, 119460. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Shi, H.; Wang, Y.; Liu, X.; Cheng, Y.; Li, H.; Zhao, H.; Wang, S.; Dong, L. Activation of Nrf2 pathway and inhibition of NLRP3 inflammasome activation contribute to the protective effect of chlorogenic acid on acute liver injury. Int. Immunopharmacol. 2018, 54, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yu, Y.-H.; Wang, S.-T.; Ren, J.; Camer, D.; Hua, Y.-Z.; Zhang, Q.; Huang, J.; Xue, D.-L.; Zhang, X.-F.; et al. Chlorogenic acid protects D-galactose-induced liver and kidney injury via antioxidation and anti-inflammation effects in mice. Pharm. Biol. 2016, 54, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, L.; Chen, R.; Zhao, Y.; Ren, D.; Yang, X. Chlorogenic acid inhibits trimethylamine-N-oxide formation and remodels intestinal microbiota to alleviate liver dysfunction in high L-carnitine feeding mice. Food Funct. 2021, 12, 10500–10511. [Google Scholar] [CrossRef]
- Zhou, Y.; Ruan, Z.; Wen, Y.; Yang, Y.; Mi, S.; Zhou, L.; Wu, X.; Ding, S.; Deng, Z.; Wu, G.; et al. Chlorogenic acid from honeysuckle improves hepatic lipid dysregulation and modulates hepatic fatty acid composition in rats with chronic endotoxin infusion. J. Clin. Biochem. Nutr. 2016, 58, 146–155. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhou, Y.; Ruan, Z.; Zhou, L.; Shu, X.; Sun, X.; Mi, S.; Yang, Y.; Yin, Y. Chlorogenic acid ameliorates endotoxin-induced liver injury by promoting mitochondrial oxidative phosphorylation. Biochem. Biophys. Res. Commun. 2016, 469, 1083–1089. [Google Scholar] [CrossRef]
- Yang, L.; Wei, J.; Sheng, F.; Li, P. Attenuation of Palmitic Acid-Induced Lipotoxicity by Chlorogenic Acid through Activation of SIRT1 in Hepatocytes. Mol. Nutr. Food Res. 2019, 63, e1801432. [Google Scholar] [CrossRef]
- Cheng, K.; Niu, J.; Zhang, J.; Qiao, Y.; Dong, G.; Guo, R.; Zheng, X.; Song, Z.; Huang, J.; Wang, J.; et al. Hepatoprotective effects of chlorogenic acid on mice exposed to aflatoxin B1: Modulation of oxidative stress and inflammation. Toxicon 2023, 231, 107177. [Google Scholar] [CrossRef]
- Pang, C.; Sheng, Y.C.; Jiang, P.; Wei, H.; Ji, L.L. Chlorogenic acid prevents acetaminophen-induced liver injury: The involvement of CYP450 metabolic enzymes and some antioxidant signals. J. Zhejiang Univ. Sci. B 2015, 16, 602–610. [Google Scholar] [CrossRef]
- Wei, M.; Zheng, Z.; Shi, L.; Jin, Y.; Ji, L. Natural Polyphenol Chlorogenic Acid Protects Against Acetaminophen-Induced Hepatotoxicity by Activating ERK/Nrf2 Antioxidative Pathway. Toxicol. Sci. 2018, 162, 99–112. [Google Scholar] [CrossRef]
- Hu, B.; Li, J.; Gong, D.; Dai, Y.; Wang, P.; Wan, L.; Xu, S. Long-Term Consumption of Food-Derived Chlorogenic Acid Protects Mice against Acetaminophen-Induced Hepatotoxicity via Promoting PINK1-Dependent Mitophagy and Inhibiting Apoptosis. Toxics 2022, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Guo, Q.; Wei, M.; Huang, Z.; Shi, L.; Sheng, Y.; Ji, L. Chlorogenic acid alleviates acetaminophen-induced liver injury in mice via regulating Nrf2-mediated HSP60-initiated liver inflammation. Eur. J. Pharmacol. 2020, 883, 173286. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Gu, X.; Li, H.; Zheng, Z.; Qiu, Z.; Sheng, Y.; Lu, B.; Wang, Z.; Ji, L. EGR1 is crucial for the chlorogenic acid-provided promotion on liver regeneration and repair after APAP-induced liver injury. Cell Biol. Toxicol. 2023, 39, 2685–2707. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Olusola, J.K.; Arunsi, U.O.; Oyelere, A.K. Chlorogenic acid abates oxido-inflammatory and apoptotic responses in the liver and kidney of Tamoxifen-treated rats. Toxicol. Res. 2021, 10, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Rashid, S.; Nafees, S.; Hasan, S.K.; Shahid, A.; Majed, F.; Sultana, S. Protective effect of Chlorogenic acid against methotrexate induced oxidative stress, inflammation and apoptosis in rat liver: An experimental approach. Chem. Biol. Interact. 2017, 272, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-M.; Chen, R.-X.; Zhang, L.-L.; Ding, N.-N.; Liu, C.; Cui, Y.; Cheng, Y.-X. In vivo protective effects of chlorogenic acid against triptolide-induced hepatotoxicity and its mechanism. Pharm. Biol. 2018, 56, 626–631. [Google Scholar] [CrossRef]
- Zheng, Z.; Shi, L.; Sheng, Y.; Zhang, J.; Lu, B.; Ji, L. Chlorogenic acid suppresses monocrotaline-induced sinusoidal obstruction syndrome: The potential contribution of NFkappaB, Egr1, Nrf2, MAPKs and PI3K signals. Environ. Toxicol. Pharmacol. 2016, 46, 80–89. [Google Scholar] [CrossRef]
- Buko, V.; Zavodnik, I.; Budryn, G.; Zakłos-Szyda, M.; Belonovskaya, E.; Kirko, S.; Żyżelewicz, D.; Zakrzeska, A.; Bakunovich, A.; Rusin, V.; et al. Chlorogenic Acid Protects against Advanced Alcoholic Steatohepatitis in Rats via Modulation of Redox Homeostasis, Inflammation, and Lipogenesis. Nutrients 2021, 13, 4155. [Google Scholar] [CrossRef]
- Kim, H.; Pan, J.H.; Kim, S.H.; Lee, J.H.; Park, J.W. Chlorogenic acid ameliorates alcohol-induced liver injuries through scavenging reactive oxygen species. Biochimie 2018, 150, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jiang, W.; Liu, C.; Wang, C.; Hu, B.; Guo, Y.; Cheng, Y.; Qian, H. Ameliorative effects of chlorogenic acid on alcoholic liver injury in mice via gut microbiota informatics. Eur. J. Pharmacol. 2022, 928, 175096. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Xiao, Y.; Lin, Y.; Mo, Z.; Chen, Y.; Peng, X.; Xiang, C.; Li, Y.; Li, W. Chlorogenic acid-enriched extract from Eucommia ulmoides leaves inhibits hepatic lipid accumulation through regulation of cholesterol metabolism in HepG2 cells. Pharm. Biol. 2016, 54, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Tsukui, T.; Chen, Z.; Fuda, H.; Furukawa, T.; Oura, K.; Sakurai, T.; Hui, S.-P.; Chiba, H. Novel Fluorescence-Based Method To Characterize the Antioxidative Effects of Food Metabolites on Lipid Droplets in Cultured Hepatocytes. J. Agric. Food Chem. 2019, 67, 9934–9941. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kim, Y.; Lee, E.S.; Huh, J.H.; Chung, C.H. Caffeic acid ameliorates hepatic steatosis and reduces ER stress in high fat diet-induced obese mice by regulating autophagy. Nutrition 2018, 55–56, 63–70. [Google Scholar] [CrossRef]
- Shi, A.; Li, T.; Zheng, Y.; Song, Y.; Wang, H.; Wang, N.; Dong, L.; Shi, H. Chlorogenic Acid Improves NAFLD by Regulating gut Microbiota and GLP-1. Front. Pharmacol. 2021, 12, 693048. [Google Scholar] [CrossRef] [PubMed]
- Zamani-Garmsiri, F.; Ghasempour, G.; Aliabadi, M.; Hashemnia, S.M.R.; Emamgholipour, S.; Meshkani, R. Combination of metformin and chlorogenic acid attenuates hepatic steatosis and inflammation in high-fat diet fed mice. IUBMB Life 2021, 73, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xin, X.; Liu, Q.; Tian, H.-J.; Peng, J.-H.; Zhao, Y.; Hu, Y.-Y.; Feng, Q. Geniposide and Chlorogenic Acid Combination Improves Non-Alcoholic Fatty Liver Disease Involving the Potent Suppression of Elevated Hepatic SCD-1. Front. Pharmacol. 2021, 12, 653641. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Tian, H.J.; Fang, Y.; Hu, Y.Y.; Peng, J.H. Amelioration of Non-Alcoholic Steatohepatitis by Atractylodes macrocephala Polysaccharide, Chlorogenic Acid, and Geniposide Combination Is Associated With Reducing Endotoxin Gut Leakage. Front. Cell. Infect. Microbiol. 2022, 12, 827516. [Google Scholar] [CrossRef]
- Xin, X.; Jin, Y.; Wang, X.; Cai, B.; An, Z.; Hu, Y.-Y.; Feng, Q. A Combination of Geniposide and Chlorogenic Acid Combination Ameliorates Nonalcoholic Steatohepatitis in Mice by Inhibiting Kupffer Cell Activation. Biomed. Res. Int. 2021, 2021, 6615881. [Google Scholar] [CrossRef]
- Peng, J.-H.; Leng, J.; Tian, H.-J.; Yang, T.; Fang, Y.; Feng, Q.; Zhao, Y.; Hu, Y.-Y. Geniposide and Chlorogenic Acid Combination Ameliorates Non-alcoholic Steatohepatitis Involving the Protection on the Gut Barrier Function in Mouse Induced by High-Fat Diet. Front. Pharmacol. 2018, 9, 1399. [Google Scholar] [CrossRef]
- Alqarni, I.; Bassiouni, Y.A.; Badr, A.M.; Ali, R.A. Telmisartan and/or chlorogenic acid attenuates fructose-induced non-alcoholic fatty liver disease in rats: Implications of cross-talk between angiotensin, the sphingosine kinase/sphingoine-1-phosphate pathway, and TLR4 receptors. Biochem. Pharmacol. 2019, 164, 252–262. [Google Scholar] [CrossRef]
- Xu, M.; Yang, L.; Zhu, Y.; Liao, M.; Chu, L.; Li, X.; Lin, L.; Zheng, G. Collaborative effects of chlorogenic acid and caffeine on lipid metabolism via the AMPKalpha-LXRalpha/SREBP-1c pathway in high-fat diet-induced obese mice. Food Funct. 2019, 10, 7489–7497. [Google Scholar] [CrossRef]
- Gu, X.; Wei, M.; Hu, F.; Ouyang, H.; Huang, Z.; Lu, B.; Ji, L. Chlorogenic acid ameliorated non-alcoholic steatohepatitis via alleviating hepatic inflammation initiated by LPS/TLR4/MyD88 signaling pathway. Chem. Biol. Interact. 2023, 376, 110461. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Luo, M.; Yang, J.; Cheng, Y.; Huang, J.; Lu, C.; Song, D.; Ye, M.; Dai, M.; Gonzalez, F.J. Chlorogenic acid inhibits cholestatic liver injury induced by alpha-naphthylisothiocyanate: Involvement of STAT3 and NFkappaB signalling regulation. J. Pharm. Pharmacol. 2016, 68, 1203–1213. [Google Scholar] [CrossRef]
- Li, K.; Feng, Z.; Wang, L.; Ma, X.; Wang, L.; Liu, K.; Geng, X.; Peng, C. Chlorogenic Acid Alleviates Hepatic Ischemia-Reperfusion Injury by Inhibiting Oxidative Stress, Inflammation, and Mitochondria-Mediated Apoptosis In Vivo and In Vitro. Inflammation 2023, 46, 1061–1076. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, F.; Xue, J.; Zhou, X.; Luo, L.; Ma, Q.; Chen, Y.F.; Zhang, J.; Zhang, S.L.; Zhao, L. Antischistosomiasis Liver Fibrosis Effects of Chlorogenic Acid through IL-13/miR-21/Smad7 Signaling Interactions In Vivo and In Vitro. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Luo, L.; Ma, Q.; Zhao, L. Chlorogenic Acid Inhibits Liver Fibrosis by Blocking the miR-21-Regulated TGF-beta1/Smad7 Signaling Pathway In Vitro and In Vivo. Front. Pharmacol. 2017, 8, 929. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shi, A.; Dong, L.; Lu, X.; Wang, Y.; Zhao, J.; Dai, F.; Guo, X. Chlorogenic acid protects against liver fibrosis in vivo and in vitro through inhibition of oxidative stress. Clin. Nutr. 2016, 35, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Ouyang, H.; Guo, Q.; Wei, M.; Lu, B.; Kai, G.; Ji, L. Chlorogenic acid alleviated liver fibrosis in methionine and choline deficient diet-induced nonalcoholic steatohepatitis in mice and its mechanism. J. Nutr. Biochem. 2022, 106, 109020. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, N.; Hou, N.; Dong, L.; Li, J. Chlorogenic acid inhibits hepatocellular carcinoma in vitro and in vivo. J. Nutr. Biochem. 2017, 46, 68–73. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Y.; Li, Y.; Hu, Y.; Zhang, Q.; Huang, Y.; Shi, K.; Ran, C.; Hou, J.; Zhou, G.; et al. Chlorogenic Acid Decreases Malignant Characteristics of Hepatocellular Carcinoma Cells by Inhibiting DNMT1 Expression. Front. Pharmacol. 2020, 11, 867. [Google Scholar] [CrossRef]
- Buskaran, K.; Hussein, M.Z.; Moklas, M.A.M.; Masarudin, M.J.; Fakurazi, S. Graphene Oxide Loaded with Protocatechuic Acid and Chlorogenic Acid Dual Drug Nanodelivery System for Human Hepatocellular Carcinoma Therapeutic Application. Int. J. Mol. Sci. 2021, 22, 5786. [Google Scholar] [CrossRef]
- Romualdo, G.R.; Prata, G.B.; da Silva, T.C.; Evangelista, A.F.; Reis, R.M.; Vinken, M.; Moreno, F.S.; Cogliati, B.; Barbisan, L.F. The combination of coffee compounds attenuates early fibrosis-associated hepatocarcinogenesis in mice: Involvement of miRNA profile modulation. J. Nutr. Biochem. 2020, 85, 108479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, D.; Qiao, S.; Wu, X.; Cao, S.; Wang, L.; Su, X.; Li, L. Metabolic and microbial signatures in rat hepatocellular carcinoma treated with caffeic acid and chlorogenic acid. Sci. Rep. 2017, 7, 4508. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Samadi, M.; Qorbani, M.; Merat, S.; Adibi, H.; Poustchi, H.; Hekmatdoost, A. Effects of supplementation with main coffee components including caffeine and/or chlorogenic acid on hepatic, metabolic, and inflammatory indices in patients with non-alcoholic fatty liver disease and type 2 diabetes: A randomized, double-blind, placebo-controlled, clinical trial. Nutr. J. 2021, 20, 35. [Google Scholar]
- Yang, X.; Feng, Y.; Liu, Y.; Ye, X.; Ji, X.; Sun, L.; Gao, F.; Zhang, Q.; Li, Y.; Zhu, B. Fuzheng Jiedu Xiaoji formulation inhibits hepatocellular carcinoma progression in patients by targeting the AKT/CyclinD1/p21/p27 pathway. Phytomedicine 2021, 87, 153575. [Google Scholar] [CrossRef]
- Yao, J.; Peng, S.; Xu, J.; Fang, J. Reversing ROS-mediated neurotoxicity by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway. Biofactors 2019, 45, 616–626. [Google Scholar] [CrossRef]
- Wang, X.; Fan, X.; Yuan, S.; Jiao, W.; Liu, B.; Cao, J.; Jiang, W. Chlorogenic acid protects against aluminium-induced cytotoxicity through chelation and antioxidant actions in primary hippocampal neuronal cells. Food Funct. 2017, 8, 2924–2934. [Google Scholar] [CrossRef]
- Gul, Z.; Demircan, C.; Bagdas, D.; Buyukuysal, R.L. Protective Effects of Chlorogenic Acid and its Metabolites on Hydrogen Peroxide-Induced Alterations in Rat Brain Slices: A Comparative Study with Resveratrol. Neurochem. Res. 2016, 41, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Mira, A.; Yamashita, S.; Katakura, Y.; Shimizu, K. In vitro neuroprotective activities of compounds from Angelica shikokiana Makino. Molecules 2015, 20, 4813–4832. [Google Scholar] [CrossRef]
- Cho, E.S.; Jang, Y.J.; Hwang, M.K.; Kang, N.J.; Lee, K.W.; Lee, H.J. Attenuation of oxidative neuronal cell death by coffee phenolic phytochemicals. Mutat. Res. 2009, 661, 18–24. [Google Scholar] [CrossRef]
- Shen, W.; Qi, R.; Zhang, J.; Wang, Z.; Wang, H.; Hu, C.; Zhao, Y.; Bie, M.; Wang, Y.; Fu, Y.; et al. Chlorogenic acid inhibits LPS-induced microglial activation and improves survival of dopaminergic neurons. Brain Res. Bull. 2012, 88, 487–494. [Google Scholar] [CrossRef]
- La Rosa, G.; Sozio, C.; Pipicelli, L.; Raia, M.; Palmiero, A.; Santillo, M.; Damiano, S. Antioxidant, Anti-Inflammatory and Pro-Differentiative Effects of Chlorogenic Acid on M03-13 Human Oligodendrocyte-like Cells. Int. J. Mol. Sci. 2023, 24, 16731. [Google Scholar] [CrossRef]
- Taram, F.; Winter, A.N.; Linseman, D.A. Neuroprotection comparison of chlorogenic acid and its metabolites against mechanistically distinct cell death-inducing agents in cultured cerebellar granule neurons. Brain Res. 2016, 1648, 69–80. [Google Scholar] [CrossRef]
- Rebai, O.; Belkhir, M.; Sanchez-Gomez, M.V.; Matute, C.; Fattouch, S.; Amri, M. Differential Molecular Targets for Neuroprotective Effect of Chlorogenic Acid and its Related Compounds Against Glutamate Induced Excitotoxicity and Oxidative Stress in Rat Cortical Neurons. Neurochem. Res. 2017, 42, 3559–3572. [Google Scholar] [CrossRef] [PubMed]
- Rebai, O.; Amri, M. Chlorogenic Acid Prevents AMPA-Mediated Excitotoxicity in Optic Nerve Oligodendrocytes Through a PKC and Caspase-Dependent Pathways. Neurotox. Res. 2018, 34, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Camfield, D.A.; Silber, B.Y.; Scholey, A.B.; Nolidin, K.; Goh, A.; Stough, C. A randomised placebo-controlled trial to differentiate the acute cognitive and mood effects of chlorogenic acid from decaffeinated coffee. PLoS ONE 2013, 8, e82897. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, J.-S.; Jang, H.-J.; Kim, S.-M.; Chang, M.S.; Park, S.H.; Kim, K.S.; Bae, J.; Park, J.-W.; Lee, B.; et al. Chlorogenic acid ameliorates brain damage and edema by inhibiting matrix metalloproteinase-2 and 9 in a rat model of focal cerebral ischemia. Eur. J. Pharmacol. 2012, 689, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Cropley, V.; Croft, R.; Silber, B.; Neale, C.; Scholey, A.; Stough, C.; Schmitt, J. Does coffee enriched with chlorogenic acids improve mood and cognition after acute administration in healthy elderly? A pilot study. Psychopharmacology 2012, 219, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, R.; Ito, H.; Iguchi, A.; Shinomiya, K.; Kamei, C.; Hatano, T.; Yoshida, T. Effects of chlorogenic acid and its metabolites on spontaneous locomotor activity in mice. Biosci. Biotechnol. Biochem. 2006, 70, 2560–2563. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vardi, N.; Parlakpinar, H.; Ates, B. Beneficial effects of chlorogenic acid on methotrexate-induced cerebellar Purkinje cell damage in rats. J. Chem. Neuroanat. 2012, 43, 43–47. [Google Scholar] [CrossRef]
- Hao, M.L.; Pan, N.; Zhang, Q.H.; Wang, X.H. Therapeutic efficacy of chlorogenic acid on cadmium-induced oxidative neuropathy in a murine model. Exp. Ther. Med. 2015, 9, 1887–1894. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Lee, H.-K.; Kim, J.-A.; Hong, S.-I.; Kim, H.-C.; Jo, T.-H.; Park, Y.-I.; Lee, C.-K.; Kim, Y.-B.; Lee, S.-Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef]
- Nazir, N.; Zahoor, M.; Nisar, M.; Karim, N.; Latif, A.; Ahmad, S.; Uddin, Z. Evaluation of neuroprotective and anti-amnesic effects of Elaeagnus umbellata Thunb. On scopolamine-induced memory impairment in mice. BMC Complement Med. Ther. 2020, 20, 143. [Google Scholar] [CrossRef]
- Guo, Z.; Li, J. Chlorogenic Acid Prevents Alcohol-induced Brain Damage in Neonatal Rat. Transl. Neurosci. 2017, 8, 176–181. [Google Scholar] [CrossRef][Green Version]
- Aseervatham, G.S.B.; Suryakala, U.; Doulethunisha; Sundaram, S.; Bose, P.C.; Sivasudha, T. Expression pattern of NMDA receptors reveals antiepileptic potential of apigenin 8-C-glucoside and chlorogenic acid in pilocarpine induced epileptic mice. Biomed. Pharmacother. 2016, 82, 54–64. [Google Scholar] [CrossRef]
- Alarcón-Herrera, N.; Flores-Maya, S.; Bellido, B.; García-Bores, A.M.; Mendoza, E.; Ávila-Acevedo, G.; Hernández-Echeagaray, E. Protective effects of chlorogenic acid in 3-nitropropionic acid induced toxicity and genotoxicity. Food Chem. Toxicol. 2017, 109, 1018–1025. [Google Scholar] [CrossRef]
- Tu, Q.; Tang, X.; Hu, Z. Chlorogenic acid protection of neuronal nitric oxide synthase-positive neurons in the hippocampus of mice with impaired learning and memory. Neural. Regen. Res. 2008, 3, 4. [Google Scholar]
- Nakajima, Y.; Shimazawa, M.; Mishima, S.; Hara, H. Water extract of propolis and its main constituents, caffeoylquinic acid derivatives, exert neuroprotective effects via antioxidant actions. Life Sci. 2007, 80, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Ciaramelli, C.; Palmioli, A.; De Luigi, A.; Colombo, L.; Sala, G.; Riva, C.; Zoia, C.P.; Salmona, M.; Airoldi, C. NMR-driven identification of anti-amyloidogenic compounds in green and roasted coffee extracts. Food Chem. 2018, 252, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.F.; Jeon, Y.; Sung, Y.W.; Lee, J.H.; Jeong, H.; Kim, Y.M.; Yun, H.S.; Chin, Y.W.; Jeon, S.; Cho, K.S.; et al. Nardostachys jatamansi Ethanol Extract Ameliorates Abeta42 Cytotoxicity. Biol. Pharm. Bull. 2018, 41, 470–477. [Google Scholar] [CrossRef]
- Gao, L.; Li, X.; Meng, S.; Ma, T.; Wan, L.; Xu, S. Chlorogenic Acid Alleviates Abeta(25–35)-Induced Autophagy and Cognitive Impairment via the mTOR/TFEB Signaling Pathway. Drug Des. Devel. Ther. 2020, 14, 1705–1716. [Google Scholar] [CrossRef]
- Wei, M.; Chen, L.; Liu, J.; Zhao, J.; Liu, W.; Feng, F. Protective effects of a Chotosan Fraction and its active components on beta-amyloid-induced neurotoxicity. Neurosci. Lett. 2016, 617, 143–149. [Google Scholar] [CrossRef]
- Shi, M.; Sun, F.; Wang, Y.; Kang, J.; Zhang, S.; Li, H. CGA restrains the apoptosis of Abeta(25–35)-induced hippocampal neurons. Int. J. Neurosci. 2020, 130, 700–707. [Google Scholar] [CrossRef]
- Ishida, K.; Misawa, K.; Nishimura, H.; Hirata, T.; Yamamoto, M.; Ota, N. 5-Caffeoylquinic Acid Ameliorates Cognitive Decline and Reduces Abeta Deposition by Modulating Abeta Clearance Pathways in APP/PS2 Transgenic Mice. Nutrients 2020, 12, 494. [Google Scholar] [CrossRef]
- Ishida, K.; Yamamoto, M.; Misawa, K.; Nishimura, H.; Misawa, K.; Ota, N.; Shimotoyodome, A. Coffee polyphenols prevent cognitive dysfunction and suppress amyloid beta plaques in APP/PS2 transgenic mouse. Neurosci. Res. 2020, 154, 35–44. [Google Scholar] [CrossRef]
- Han, J.; Miyamae, Y.; Shigemori, H.; Isoda, H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience 2010, 169, 1039–1045. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef]
- Orhan, I.; Sener, B.; Choudhary, M.I.; Khalid, A. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J. Ethnopharmacol. 2004, 91, 57–60. [Google Scholar] [CrossRef]
- Yadav, E.; Singh, D.; Debnath, B.; Rathee, P.; Yadav, P.; Verma, A. Molecular Docking and Cognitive Impairment Attenuating Effect of Phenolic Compound Rich Fraction of Trianthema portulacastrum in Scopolamine Induced Alzheimer’s Disease Like Condition. Neurochem. Res. 2019, 44, 1665–1677. [Google Scholar] [CrossRef]
- Teraoka, M.; Nakaso, K.; Kusumoto, C.; Katano, S.; Tajima, N.; Yamashita, A.; Zushi, T.; Ito, S.; Matsura, T. Cytoprotective effect of chlorogenic acid against alpha-synuclein-related toxicity in catecholaminergic PC12 cells. J. Clin. Biochem. Nutr. 2012, 51, 122–127. [Google Scholar] [CrossRef]
- Shan, S.; Tian, L.; Fang, R. Chlorogenic Acid Exerts Beneficial Effects in 6-Hydroxydopamine-Induced Neurotoxicity by Inhibition of Endoplasmic Reticulum Stress. Med. Sci. Monit. 2019, 25, 453–459. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Ma, S.-X.; Hong, S.-I.; Kim, S.Y.; Lee, S.-Y.; Jang, C.-G. Eucommia ulmoides Oliv. bark. attenuates 6-hydroxydopamine-induced neuronal cell death through inhibition of oxidative stress in SH-SY5Y cells. J. Ethnopharmacol. 2014, 152, 173–182. [Google Scholar] [CrossRef]
- Miyazaki, I.; Isooka, N.; Wada, K.; Kikuoka, R.; Kitamura, Y.; Asanuma, M. Effects of Enteric Environmental Modification by Coffee Components on Neurodegeneration in Rotenone-Treated Mice. Cells 2019, 8, 221. [Google Scholar] [CrossRef]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Kumar, G.; Gedda, M.R.; Tiwari, N.; Patnaik, R.; Singh, R.K.; Singh, S.P. Effect of Chlorogenic Acid Supplementation in MPTP-Intoxicated Mouse. Front. Pharmacol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Rathore, A.S.; Dilnashin, H.; Singh, R.; Singh, S.P. Neuroprotective Effect of Chlorogenic Acid on Mitochondrial Dysfunction-Mediated Apoptotic Death of DA Neurons in a Parkinsonian Mouse Model. Oxid. Med. Cell. Longev. 2020, 2020, 6571484. [Google Scholar] [CrossRef]
- Socala, K.; Szopa, A.; Serefko, A.; Poleszak, E.; Wlaz, P. Neuroprotective Effects of Coffee Bioactive Compounds: A Review. Int. J. Mol. Sci. 2020, 22, 107. [Google Scholar] [CrossRef]
- Miao, M.; Cao, L.; Li, R.; Fang, X.; Miao, Y. Protective effect of chlorogenic acid on the focal cerebral ischemia reperfusion rat models. Saudi. Pharm. J. 2017, 25, 556–563. [Google Scholar] [CrossRef]
- Liu, D.; Wang, H.; Zhang, Y.; Zhang, Z. Protective Effects of Chlorogenic Acid on Cerebral Ischemia/Reperfusion Injury Rats by Regulating Oxidative Stress-Related Nrf2 Pathway. Drug Des. Devel Ther. 2020, 14, 51–60. [Google Scholar] [CrossRef]
- Kumar, G.; Mukherjee, S.; Paliwal, P.; Singh, S.S.; Birla, H.; Singh, S.P.; Krishnamurthy, S.; Patnaik, R. Neuroprotective effect of chlorogenic acid in global cerebral ischemia-reperfusion rat model. Naunyn. Schmiedebergs Arch. Pharmacol. 2019, 392, 1293–1309. [Google Scholar] [CrossRef]
- Ahn, E.H.; Kim, D.W.; Shin, M.J.; Kwon, S.W.; Kim, Y.N.; Kim, D.-S.; Lim, S.S.; Kim, J.; Park, J.; Eum, W.S.; et al. Chlorogenic Acid Improves Neuroprotective Effect of PEP-1-Ribosomal Protein S3 Against Ischemic Insult. Exp. Neurobiol. 2011, 20, 169–175. [Google Scholar] [CrossRef]
- Hermawati, E.; Arfian, N.; Mustofa, M.; Partadiredja, G. Chlorogenic acid ameliorates memory loss and hippocampal cell death after transient global ischemia. Eur. J. Neurosci. 2020, 51, 651–669. [Google Scholar] [CrossRef]
- Lee, T.-K.; Kang, I.-J.; Kim, B.; Sim, H.J.; Kim, D.W.; Ahn, J.H.; Lee, J.-C.; Ryoo, S.; Shin, M.C.; Cho, J.H.; et al. Experimental Pretreatment with Chlorogenic Acid Prevents Transient Ischemia-Induced Cognitive Decline and Neuronal Damage in the Hippocampus through Anti-Oxidative and Anti-Inflammatory Effects. Molecules 2020, 25, 3578. [Google Scholar] [CrossRef]
- Bouayed, J.; Rammal, H.; Dicko, A.; Younos, C.; Soulimani, R. Chlorogenic acid, a polyphenol from Prunus domestica (Mirabelle), with coupled anxiolytic and antioxidant effects. J. Neurol. Sci. 2007, 262, 77–84. [Google Scholar] [CrossRef]
- Zhu, H.; Shen, F.; Wang, X.; Qian, H.; Liu, Y. Chlorogenic acid improves the cognitive deficits of sleep-deprived mice via regulation of immunity function and intestinal flora. Phytomedicine 2024, 123, 155194. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Su, X.; Kang, Y.; Si, J.; Wang, L.; Li, X.; Ma, K. Effect and mechanism of chlorogenic acid on cognitive dysfunction in mice by lipopolysaccharide-induced neuroinflammation. Front. Immunol. 2023, 14, 1178188. [Google Scholar] [CrossRef]
- Munawaroh, F.; Arfian, N.; Saputri, L.; Kencana, S.M.S.; Sari, D.C.R. Chlorogenic acid may improve memory function and decrease inflamation of frontal lobe in diabetic rat. Med. J. Malays. 2023, 78, 476–483. [Google Scholar]
- Lim, D.W.; Yoo, G.; Lee, C. Dried Loquat Fruit Extract Containing Chlorogenic Acid Prevents Depressive-like Behaviors Induced by Repeated Corticosteroid Injections in Mice. Molecules 2023, 28, 5612. [Google Scholar] [CrossRef]
- dos Santos, M.D.; Almeida, M.C.; Lopes, N.P.; de Souza, G.E. Evaluation of the anti-inflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef]
- Hara, K.; Haranishi, Y.; Kataoka, K.; Takahashi, Y.; Terada, T.; Nakamura, M.; Sata, T. Chlorogenic acid administered intrathecally alleviates mechanical and cold hyperalgesia in a rat neuropathic pain model. Eur. J. Pharmacol. 2014, 723, 459–464. [Google Scholar] [CrossRef]
- Bagdas, D.; Cinkilic, N.; Ozboluk, H.Y.; Ozyigit, M.O.; Gurun, M.S. Antihyperalgesic activity of chlorogenic acid in experimental neuropathic pain. J. Nat. Med. 2013, 67, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Satti, N.K.; Sharma, P.; Sharma, V.K.; Suri, K.A.; Bani, S. Differential effects of chlorogenic acid on various immunological parameters relevant to rheumatoid arthritis. Phytother. Res. 2012, 26, 1156–1165. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Yang, T.; Ye, Y.; Shan, J.; Yin, Z.; Luo, L. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. Injury 2010, 41, 746–752. [Google Scholar] [CrossRef]
- Carrasco, C.; Naziroglu, M.; Rodriguez, A.B.; Pariente, J.A. Neuropathic Pain: Delving into the Oxidative Origin and the Possible Implication of Transient Receptor Potential Channels. Front. Physiol. 2018, 9, 95. [Google Scholar] [CrossRef]
- Duggett, N.A.; Griffiths, L.A.; McKenna, O.E.; de Santis, V.; Yongsanguanchai, N.; Mokori, E.B.; Flatters, S.J. Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience 2016, 333, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Moalem, G.; Tracey, D.J. Immune and inflammatory mechanisms in neuropathic pain. Brain Res. Rev. 2006, 51, 240–264. [Google Scholar] [CrossRef] [PubMed]
- Yowtak, J.; Lee, K.Y.; Kim, H.Y.; Wang, J.; Chung, K.; Chung, J.M. Reactive oxygen species contribute to neuropathic pain by reducing spinal GABA release. Pain 2011, 152, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Kim, H.K.; Mo Chung, J.; Chung, K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain 2007, 131, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Gao, X.; Chung, J.M.; Chung, K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci. Lett. 2006, 391, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Park, S.K.; Zhou, J.-L.; Taglialatela, G.; Chung, K.; Coggeshall, R.E.; Chung, J.M. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain 2004, 111, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, A.; Shanbhag, R.; Chamallamudi, M.R.; Singh, V.P.; Mudgal, J. Ameliorative effect of caffeic acid against inflammatory pain in rodents. Eur. J. Pharmacol. 2011, 666, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Gao, Y.; Zhong, X.; Xu, C.; Li, G.; Liu, S.; Lin, J.; Li, X.; Zhang, Y.; Liu, H.; et al. Effect of sodium ferulate on the hyperalgesia mediated by P2X3 receptor in the neuropathic pain rats. Brain Res. 2010, 1313, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Xu, C.; Liang, S.; Gao, Y.; Li, G.; Wei, J.; Wan, F.; Liu, S.; Lin, J. Role of sodium ferulate in the nociceptive sensory facilitation of neuropathic pain injury mediated by P2X3 receptor. Neurochem. Int. 2008, 53, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Hamann, F.R.; Brusco, I.; Severo, G.d.C.; de Carvalho, L.M.; Faccin, H.; Gobo, L.; Oliveira, S.M.; Rubin, M.A. Mansoa alliacea extract presents antinociceptive effect in a chronic inflammatory pain model in mice through opioid mechanisms. Neurochem. Int. 2019, 122, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Yonathan, M.; Asres, K.; Assefa, A.; Bucar, F. In vivo anti-inflammatory and anti-nociceptive activities of Cheilanthes farinosa. J. Ethnopharmacol. 2006, 108, 462–470. [Google Scholar] [CrossRef]
- Qu, Z.W.; Liu, T.T.; Qiu, C.Y.; Li, J.D.; Hu, W.P. Inhibition of acid-sensing ion channels by chlorogenic acid in rat dorsal root ganglion neurons. Neurosci. Lett. 2014, 567, 35–39. [Google Scholar] [CrossRef]
- Mahomoodally, M.F. Traditional medicines in Africa: An appraisal of ten potent african medicinal plants. Evid. Based Complement Alternat. Med. 2013, 2013, 617459. [Google Scholar] [CrossRef]
- Malan, T.P.; Mata, H.P.; Porreca, F. Spinal GABA(A) and GABA(B) receptor pharmacology in a rat model of neuropathic pain. Anesthesiology 2002, 96, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Yaksh, T.L. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain 1997, 70, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Gamper, N. Potassium channels in peripheral pain pathways: Expression, function and therapeutic potential. Curr. Neuropharmacol. 2013, 11, 621–640. [Google Scholar] [CrossRef] [PubMed]
- Pearce, R.J.; Duchen, M.R. Differential expression of membrane currents in dissociated mouse primary sensory neurons. Neuroscience 1994, 63, 1041–1056. [Google Scholar] [CrossRef]
- Rasband, M.N.; Park, E.W.; Vanderah, T.W.; Lai, J.; Porreca, F.; Trimmer, J.S. Distinct potassium channels on pain-sensing neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 13373–13378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Lu, X.-W.; Song, N.; Kou, L.; Wu, M.-K.; Liu, F.; Wang, H.; Shen, J.-F. Chlorogenic acid alters the voltage-gated potassium channel currents of trigeminal ganglion neurons. Int. J. Oral Sci. 2014, 6, 233–240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Harriott, A.M.; Gold, M.S. Contribution of primary afferent channels to neuropathic pain. Curr. Pain Headache Rep. 2009, 13, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Birinyi-Strachan, L.C.; Gunning, S.J.; Lewis, R.J.; Nicholson, G.M. Block of voltage-gated potassium channels by Pacific ciguatoxin-1 contributes to increased neuronal excitability in rat sensory neurons. Toxicol. Appl. Pharmacol. 2005, 204, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Everill, B.; Kocsis, J.D. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J. Neurophysiol. 1999, 82, 700–708. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kakita, K.; Tsubouchi, H.; Adachi, M.; Takehana, S.; Shimazu, Y.; Takeda, M. Local subcutaneous injection of chlorogenic acid inhibits the nociceptive trigeminal spinal nucleus caudalis neurons in rats. Neurosci. Res. 2018, 134, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, R.; Saitou, K.; Suzukamo, C.; Osaki, N.; Asada, T. Effect of Chlorogenic Acids on Cognitive Function in Mild Cognitive Impairment: A Randomized Controlled Crossover Trial. J. Alzheimers Dis. 2019, 72, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Saitou, K.; Ochiai, R.; Kozuma, K.; Sato, H.; Koikeda, T.; Osaki, N.; Katsuragi, Y. Effect of Chlorogenic Acids on Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 1337. [Google Scholar] [CrossRef]
- Kato, M.; Ochiai, R.; Kozuma, K.; Sato, H.; Katsuragi, Y. Effect of Chlorogenic Acid Intake on Cognitive Function in the Elderly: A Pilot Study. Evid. Based Complement. Alternat. Med. 2018, 2018, 8608497. [Google Scholar] [CrossRef]
- Jackson, P.A.; Haskell-Ramsay, C.; Forster, J.; Khan, J.; Veasey, R.; Kennedy, D.O.; Wilson, A.R.; Saunders, C.; Wightman, E.L. Acute cognitive performance and mood effects of coffee berry and apple extracts: A randomised, double blind, placebo controlled crossover study in healthy humans. Nutr. Neurosci. 2022, 25, 2335–2343. [Google Scholar] [CrossRef]
- Park, I.; Ochiai, R.; Ogata, H.; Kayaba, M.; Hari, S.; Hibi, M.; Katsuragi, Y.; Satoh, M.; Tokuyama, K. Effects of subacute ingestion of chlorogenic acids on sleep architecture and energy metabolism through activity of the autonomic nervous system: A randomised, placebo-controlled, double-blinded cross-over trial. Br. J. Nutr. 2017, 117, 979–984. [Google Scholar] [CrossRef]
- Deka, S.J.; Gorai, S.; Manna, D.; Trivedi, V. Evidence of PKC Binding and Translocation to Explain the Anticancer Mechanism of Chlorogenic Acid in Breast Cancer Cells. Curr. Mol. Med. 2017, 17, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Suberu, J.O.; Romero-Canelon, I.; Sullivan, N.; Lapkin, A.A.; Barker, G.C. Comparative cytotoxicity of artemisinin and cisplatin and their interactions with chlorogenic acids in MCF7 breast cancer cells. ChemMedChem 2014, 9, 2791–2797. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Wang, S.; Zhang, Q.; Zhao, J.; Song, L. Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-kappaB signaling pathway. Oncol. Rep. 2021, 45, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Changizi, Z.; Moslehi, A.; Rohani, A.H.; Eidi, A. Chlorogenic acid induces 4T1 breast cancer tumor’s apoptosis via p53, Bax, Bcl-2, and caspase-3 signaling pathways in BALB/c mice. J. Biochem. Mol. Toxicol. 2021, 35, e22642. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Liu, N.; Han, J.; Yan, Y.; Li, J. Chlorogenic acid induces reactive oxygen species generation and inhibits the viability of human colon cancer cells. Anticancer Drugs 2017, 28, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Gouthamchandra, K.; Sudeep, H.V.; Venkatesh, B.J.; Prasad, K.S. Chlorogenic acid complex (CGA7), standardized extract from green coffee beans exerts anticancer effects against cultured human colon cancer HCT-116 cells. Food Sci. Hum. Wellness 2017, 6, 147–153. [Google Scholar] [CrossRef]
- Sadeghi Ekbatan, S.; Li, X.Q.; Ghorbani, M.; Azadi, B.; Kubow, S. Chlorogenic Acid and Its Microbial Metabolites Exert Anti-Proliferative Effects, S-Phase Cell-Cycle Arrest and Apoptosis in Human Colon Cancer Caco-2 Cells. Int. J. Mol. Sci. 2018, 19, 723. [Google Scholar] [CrossRef] [PubMed]
- Luque-Badillo, A.C.; Hernandez-Tapia, G.; Ramirez-Castillo, D.A.; Espinoza-Serrano, D.; Cortes-Limon, A.M.; Cortes-Gallardo, J.P.; Jacobo-Velázquez, D.A.; Martinez-Fierro, M.L.; Rios-Ibarra, C.P. Gold nanoparticles enhance microRNA 31 detection in colon cancer cells after inhibition with chlorogenic acid. Oncol. Lett. 2021, 22, 742. [Google Scholar] [CrossRef]
- Zhan, Y.; Li, R.; Feng, C.; Li, X.; Huang, S.; Wang, L.; Liu, Z.; Jiang, J.; Han, Y. Chlorogenic acid inhibits esophageal squamous cell carcinoma growth in vitro and in vivo by downregulating the expression of BMI1 and SOX2. Biomed. Pharmacother. 2020, 121, 109602. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, S.; Mandal, L.; Pal, B.C.; Bagchi, J.; Biswas, N.; Chaudhuri, J.; Chowdhury, A.A.; Manna, A.; Chaudhuri, U.; Konar, A.; et al. Involvement of ROS in chlorogenic acid-induced apoptosis of Bcr-Abl+ CML cells. Biochem. Pharmacol. 2010, 80, 1662–1675. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhou, C.Y.; Qiu, C.H.; Lu, X.M.; Wang, Y.T. Chlorogenic acid induced apoptosis and inhibition of proliferation in human acute promyelocytic leukemia HL-60 cells. Mol. Med. Rep. 2013, 8, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-S.; Liu, C.-W.; Ma, Y.-S.; Weng, S.-W.; Tang, N.-Y.; Wu, S.-H.; Ji, B.-C.; Ma, C.-Y.; Ko, Y.-C.; Funayama, S.; et al. Chlorogenic acid induces apoptotic cell death in U937 leukemia cells through caspase- and mitochondria-dependent pathways. Vivo Novemb. 2012, 26, 971–978. [Google Scholar]
- Yamagata, K.; Izawa, Y.; Onodera, D.; Tagami, M. Chlorogenic acid regulates apoptosis and stem cell marker-related gene expression in A549 human lung cancer cells. Mol. Cell. Biochem. 2018, 441, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Du, H.; Chen, P. Chlorogenic acid inhibits the proliferation of human lung cancer A549 cell lines by targeting annexin A2 in vitro and in vivo. Biomed. Pharmacother. 2020, 131, 110673. [Google Scholar] [CrossRef]
- Kimsa-Dudek, M.; Krawczyk, A.; Synowiec-Wojtarowicz, A.; Dudek, S.; Pawlowska-Goral, K. The impact of the co-exposure of melanoma cells to chlorogenic acid and a moderate-strength static magnetic field. J. Food Biochem. 2020, 44, e13512. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, S.; Yin, P.; Zhang, S.; Xu, J.; Zhang, Q.; Shi, S.; Zhang, T. Combination immunotherapy of chlorogenic acid liposomes modified with sialic acid and PD-1 blockers effectively enhances the anti-tumor immune response and therapeutic effects. Drug Deliv. 2021, 28, 1849–1860. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar]
- Xue, N.; Zhou, Q.; Ji, M.; Jin, J.; Lai, F.; Chen, J.; Zhang, M.; Jia, J.; Yang, H.; Zhang, J.; et al. Chlorogenic acid inhibits glioblastoma growth through repolarizating macrophage from M2 to M1 phenotype. Sci. Rep. 2017, 7, 39011. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, D.; Gong, T.; He, Q.; Guo, C.; Zhang, P.; Song, X.; Ruan, J.; Gong, T. Chlorogenic acid sustained-release gel for treatment of glioma and hepatocellular carcinoma. Eur. J. Pharm. Biopharm. 2021, 166, 103–110. [Google Scholar] [CrossRef]
- Matsuda, R.; Sakagami, H.; Amano, S.; Iijima, Y.; Sano, M.; Uesawa, Y.; Tamura, N.; Oishi, Y.; Takeshima, H. Inhibition of Neurotoxicity/Anticancer Activity of Bortezomib by Caffeic Acid and Chlorogenic Acid. Anticancer Res. 2022, 42, 781–790. [Google Scholar] [CrossRef]
- Sapio, L.; Salzillo, A.; Illiano, M.; Ragone, A.; Spina, A.; Chiosi, E.; Pacifico, S.; Catauro, M.; Naviglio, S. Chlorogenic acid activates ERK1/2 and inhibits proliferation of osteosarcoma cells. J. Cell. Physiol. 2020, 235, 3741–3752. [Google Scholar] [CrossRef]
- Zhang, F.; Yin, G.; Han, X.; Jiang, X.; Bao, Z. Chlorogenic acid inhibits osteosarcoma carcinogenesis via suppressing the STAT3/Snail pathway. J. Cell. Biochem. 2019, 120, 10342–10350. [Google Scholar] [CrossRef]
- Salzillo, A.; Ragone, A.; Spina, A.; Naviglio, S.; Sapio, L. Chlorogenic Acid Enhances Doxorubicin-Mediated Cytotoxic Effect in Osteosarcoma Cells. Int. J. Mol. Sci. 2021, 22, 8586. [Google Scholar] [CrossRef]
- Lu, C.H.; Kuo, Y.Y.; Lin, G.B.; Chen, W.T.; Chao, C.Y. Application of non-invasive low-intensity pulsed electric field with thermal cycling-hyperthermia for synergistically enhanced anticancer effect of chlorogenic acid on PANC-1 cells. PLoS ONE 2020, 15, e0222126. [Google Scholar] [CrossRef]
- Lu, C.H.; Chen, W.T.; Hsieh, C.H.; Kuo, Y.Y.; Chao, C.Y. Thermal cycling-hyperthermia in combination with polyphenols, epigallocatechin gallate and chlorogenic acid, exerts synergistic anticancer effect against human pancreatic cancer PANC-1 cells. PLoS ONE 2019, 14, e0217676. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Said, A.M.; Huang, H.; Papa, A.P.D.; Jin, G.; Wu, S.; Ma, N.; Lan, L.; Shangguan, F.; Zhang, Q. Chlorogenic acid depresses cellular bioenergetics to suppress pancreatic carcinoma through modulating c-Myc-TFR1 axis. Phytother. Res. 2021, 35, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Lee, S.O.; Kim, K.R.; Lee, H.J. Sphingosine Kinase-1 Involves the Inhibitory Action of HIF-1alpha by Chlorogenic Acid in Hypoxic DU145 Cells. Int. J. Mol. Sci. 2017, 18, 325. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Xie, Z.; Rao, J.; Xu, G.; Huang, K.; Li, W.; Yin, Z. Chlorogenic acid inhibits proliferation and induces apoptosis in A498 human kidney cancer cells via inactivating PI3K/Akt/mTOR signalling pathway. J. Pharm. Pharmacol. 2019, 71, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Li, S.; Kang, X.; Deng, J.; Yang, H.; Chen, F.; Jiang, J.; Zhang, J.; Li, W. Phase I study of chlorogenic acid injection for recurrent high-grade glioma with long-term follow-up. Cancer Biol. Med. 2023, 20, 465–476. [Google Scholar] [CrossRef]
- Girsang, E.; Ginting, C.N.; Lister, I.N.E.; Gunawan, K.Y.; Widowati, W. Anti-inflammatory and antiaging properties of chlorogenic acid on UV-induced fibroblast cell. PeerJ 2021, 9, e11419. [Google Scholar] [CrossRef] [PubMed]
- Xue, N.; Liu, Y.; Jin, J.; Ji, M.; Chen, X. Chlorogenic Acid Prevents UVA-Induced Skin Photoaging through Regulating Collagen Metabolism and Apoptosis in Human Dermal Fibroblasts. Int. J. Mol. Sci. 2022, 23, 6941. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, Z.; Wu, N.; Li, J.; Xi, N.; Xu, M.; Wu, F.; Fu, Q.; Yan, G.; Liu, Y.; et al. Chlorogenic acid attenuates deoxynivalenol-induced apoptosis and pyroptosis in human keratinocytes via activating Nrf2/HO-1 and inhibiting MAPK/NF-kappaB/NLRP3 pathways. Biomed. Pharmacother. 2024, 170, 116003. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Etoz, B.C.; Ozturkoglu, S.I.; Cinkilic, N.; Ozyigit, M.O.; Gul, Z.; Buyukcoskun, N.I.; Ozluk, K.; Gurun, M.S. Effects of systemic chlorogenic acid on random-pattern dorsal skin flap survival in diabetic rats. Biol. Pharm. Bull. 2014, 37, 361–370. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, K.H.; Do, H.K.; Kim, D.Y.; Kim, W. Impact of chlorogenic acid on modulation of significant genes in dermal fibroblasts and epidermal keratinocytes. Biochem. Biophys. Res. Commun. 2021, 583, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; He, W.; Li, X.; Ji, X.; Liu, J. Anti-acne vulgaris effects of chlorogenic acid by anti-inflammatory activity and lipogenesis inhibition. Exp. Dermatol. 2021, 30, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, X.; You, S.; Hao, M.; Li, J.; Chen, X.; Jin, J. Chlorogenic Acid Relieves the Lupus Erythematosus-like Skin Lesions and Arthritis in MRL/lpr Mice. Pharmaceuticals 2022, 15, 1372. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, L.; Hou, Y.; He, W.; Wang, X.; Zhang, D.; Hu, J. Self-assembly of chlorogenic acid into hydrogel for accelerating wound healing. Colloids Surf B Biointerfaces 2023, 228, 113440. [Google Scholar] [CrossRef]
- Li, H.R.; Habasi, M.; Xie, L.Z.; Aisa, H.A. Effect of chlorogenic acid on melanogenesis of B16 melanoma cells. Molecules 2014, 19, 12940–12948. [Google Scholar] [CrossRef]
- Sim, J.; Lanka, S.; Jo, J.-W.; Chaudhary, C.L.; Vishwanath, M.; Jung, C.-H.; Lee, Y.-H.; Kim, E.-Y.; Kim, Y.-S.; Hyun, S.-S.; et al. Inhibitory Effect of Chlorogenic Acid Analogues Comprising Pyridine and Pyrimidine on alpha-MSH-Stimulated Melanogenesis and Stability of Acyl Analogues in Methanol. Pharmaceuticals 2021, 14, 1176. [Google Scholar] [CrossRef]
- Jo, H.; Zhou, Y.; Viji, M.; Choi, M.; Lim, J.Y.; Sim, J.; Rhee, J.; Kim, Y.; Seo, S.Y.; Kim, W.J.; et al. Synthesis, biological evaluation, and metabolic stability of chlorogenic acid derivatives possessing thiazole as potent inhibitors of alpha-MSH-stimulated melanogenesis. Bioorg. Med. Chem. Lett. 2017, 27, 4854–4857. [Google Scholar] [CrossRef]
- Natarajan, K.; Singh, S.; Burke, T.R.; Grunberger, D., Jr.; Aggarwal, B.B. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc. Natl. Acad. Sci. USA 1996, 93, 9090–9095. [Google Scholar] [CrossRef]
- Yudantara, I.M.A.; Cahyani, N.K.N.; Saputra, M.A.W.; Dewi, N.K.D.P. Chlorogenic acid and kojic acid as anti-hyperpigmentation: In silico study. Pharm. Rep. 2022, 1, 23. [Google Scholar] [CrossRef]
- Aafi, E.; Shams Ardakani, M.R.; Ahmad Nasrollahi, S.; Mirabzadeh Ardakani, M.; Samadi, A.; Hajimahmoodi, M.; Naeimifar, A.; Pourjabbar, Z.; Amiri, F.; Firooz, A. Brightening effect of Ziziphus jujuba (jujube) fruit extract on facial skin: A randomized, double-blind, clinical study. Dermatol. Ther. 2022, 35, e15535. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, S.; Haramizu, S.; Sasaoka, S.; Yasuda, Y.; Tsujimura, H.; Murase, T. Coffee polyphenols extracted from green coffee beans improve skin properties and microcirculatory function. Biosci. Biotechnol. Biochem. 2017, 81, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, J. Control of hepatitis B in China: Prevention and treatment. Expert Rev. Anti. Infect. Ther. 2011, 9, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Tang, W.; Xu, Y. Chapter 68—Anti-Hepatitis B Virus Activity of Chlorogenic Acid and Its Related Compounds. In Coffee in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Leung, W.W.-M.; Ho, S.C.; Chan, H.L.Y.; Wong, V.; Yeo, W.; Mok, T.S.K. Moderate coffee consumption reduces the risk of hepatocellular carcinoma in hepatitis B chronic carriers: A case-control study. J. Epidemiol. Community Health 2011, 65, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.F.; Shi, L.P.; Ren, Y.D.; Liu, Q.F.; Liu, H.F.; Zhang, R.J.; Li, Z.; Zhu, F.H.; He, P.L.; Tang, W. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antiviral. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Namba, T.; Matsuse, T. A historical study of coffee in Japanese and Asian countries: Focusing the medicinal uses in Asian traditional medicines. Yakushigaku Zasshi 2002, 37, 65–75. [Google Scholar]
- Shek, F.W.; Benyon, R.C. How can transforming growth factor beta be targeted usefully to combat liver fibrosis? Eur. J. Gastroenterol. Hepatol. 2004, 16, 123–126. [Google Scholar] [CrossRef]
- MacDougall, J.R.; Matrisian, L.M. Contributions of tumor and stromal matrix metalloproteinases to tumor progression, invasion and metastasis. Cancer Metastasis Rev. 1995, 14, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Lee, Y.C.; Kim, C.H. Hepatitis B viral HBx induces matrix metalloproteinase-9 gene expression through activation of ERK and PI-3K/AKT pathways: Involvement of invasive potential. FASEB J. 2004, 18, 1123–1125. [Google Scholar] [CrossRef] [PubMed]
- Saksena, K.N.; Mink, G.I. The effects of oxidized phenolic compounds on the infectivity of four “stable” viruses. Virology 1970, 40, 540–546. [Google Scholar] [CrossRef]
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C.C. Antiviral activity of Plantago major extracts and related compounds in vitro. Antiviral. Res. 2002, 55, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Ke, C.; Tang, W.; Yang, X.; Tang, C.; Qin, G.; Xu, R.; Li, T.; Chen, X.; Zuo, J.; et al. Isolation of chlorogenic acids and their derivatives from Stemona japonica by preparative HPLC and evaluation of their anti-AIV (H5N1) activity in vitro. Phytochem. Anal. 2007, 18, 213–218. [Google Scholar] [CrossRef]
- Ozcelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef]
- Yun, Z.; Zou, Z.; Sun, S.; Che, H. Chlorogenic acid improves food allergy through the AMPK/ACC/CPT-1 pathway. J. Food Biochem. 2022, 46, e14505. [Google Scholar] [CrossRef]
- Cho, M.; Kim, Y.; You, S.; Hwang, D.Y.; Jang, M. Chlorogenic Acid of Cirsium japonicum Resists Oxidative Stress Caused by Aging and Prolongs Healthspan via SKN-1/Nrf2 and DAF-16/FOXO in Caenorhabditis elegans. Metabolites 2023, 13, 224. [Google Scholar] [CrossRef]
- Zheng, S.Q.; Huang, X.B.; Xing, T.K.; Ding, A.J.; Wu, G.S.; Luo, H.R. Chlorogenic Acid Extends the Lifespan of Caenorhabditis elegans via Insulin/IGF-1 Signaling Pathway. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 464–472. [Google Scholar]
- Siswanto, F.M.; Sakuma, R.; Oguro, A.; Imaoka, S. Chlorogenic Acid Activates Nrf2/SKN-1 and Prolongs the Lifespan of Caenorhabditis elegans via the Akt-FOXO3/DAF16a-DDB1 Pathway and Activation of DAF16f. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1503–1516. [Google Scholar] [CrossRef]
- Larki-Harchegani, A.; Fayazbakhsh, F.; Nourian, A.; Nili-Ahmadabadi, A. Chlorogenic acid protective effects on paraquat-induced pulmonary oxidative damage and fibrosis in rats. J. Biochem. Mol. Toxicol. 2023, 37, e23352. [Google Scholar] [CrossRef]
- Lv, B.; Guo, J.; Du, Y.; Chen, Y.; Zhao, X.; Yu, B.; Liu, J.; Cui, T.; Mao, H.; Wang, X.; et al. Chlorogenic acid reduces inflammation by inhibiting the elevated expression of KAT2A to ameliorate lipopolysaccharide-induced acute lung injury. Br. J. Pharmacol. 2023, 180, 2156–2171. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Saha, P.; Syamprasad, N.P.; Panda, S.R.; Rajdev, B.; Jannu, A.K.; Sharma, P.; Naidu, V.G.M. Targeting TLR4/3 using chlorogenic acid ameliorates LPS+POLY I:C-induced acute respiratory distress syndrome via alleviating oxidative stress-mediated NLRP3/NF-kappaB axis. Clin. Sci. 2023, 137, 785–805. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Chen, P.; Wang, Y.; Xu, L.; Zhang, K.; Zhao, J.; Liu, H. Chlorogenic acid protects against intestinal inflammation and injury by inactivating the mtDNA-cGAS-STING signaling pathway in broilers under necrotic enteritis challenge. Poult. Sci. 2024, 103, 103274. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhong, Y.; Zhang, W.; Wang, Z.; Xiao, H.; Zhang, W.; Xie, J.; Peng, X.; Luo, J.; Xu, W. Chlorogenic Acid Ameliorates Post-Infectious Irritable Bowel Syndrome by Regulating Extracellular Vesicles of Gut Microbes. Adv. Sci. 2023, 10, e2302798. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Mentese, A.; Livaoglu, A.; Alemdar, N.; Aliyazicioglu, Y.; Demir, S. Chlorogenic acid attenuates cisplatin-induced ovarian injury in rats. Drug Chem. Toxicol. 2024, 47, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shi, C.; Wang, Z. Therapeutic Effects and Molecular Mechanism of Chlorogenic Acid on Polycystic Ovarian Syndrome: Role of HIF-1alpha. Nutrients 2023, 15, 2833. [Google Scholar] [CrossRef] [PubMed]
- Enokuchi, Y.; Suzuki, A.; Yamaguchi, T.; Ochiai, R.; Terauchi, M.; Kataoka, K. Effects of Chlorogenic Acids on Menopausal Symptoms in Healthy Women: A Randomized, Placebo-Controlled, Double-Blind, Parallel-Group Trial. Nutrients 2020, 12, 3757. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).