Homicide or Happiness: Did Folate Fortification and Public Health Campaigns Influence Homicide Rates and the Great American Crime Decline?
Abstract
1. Introduction
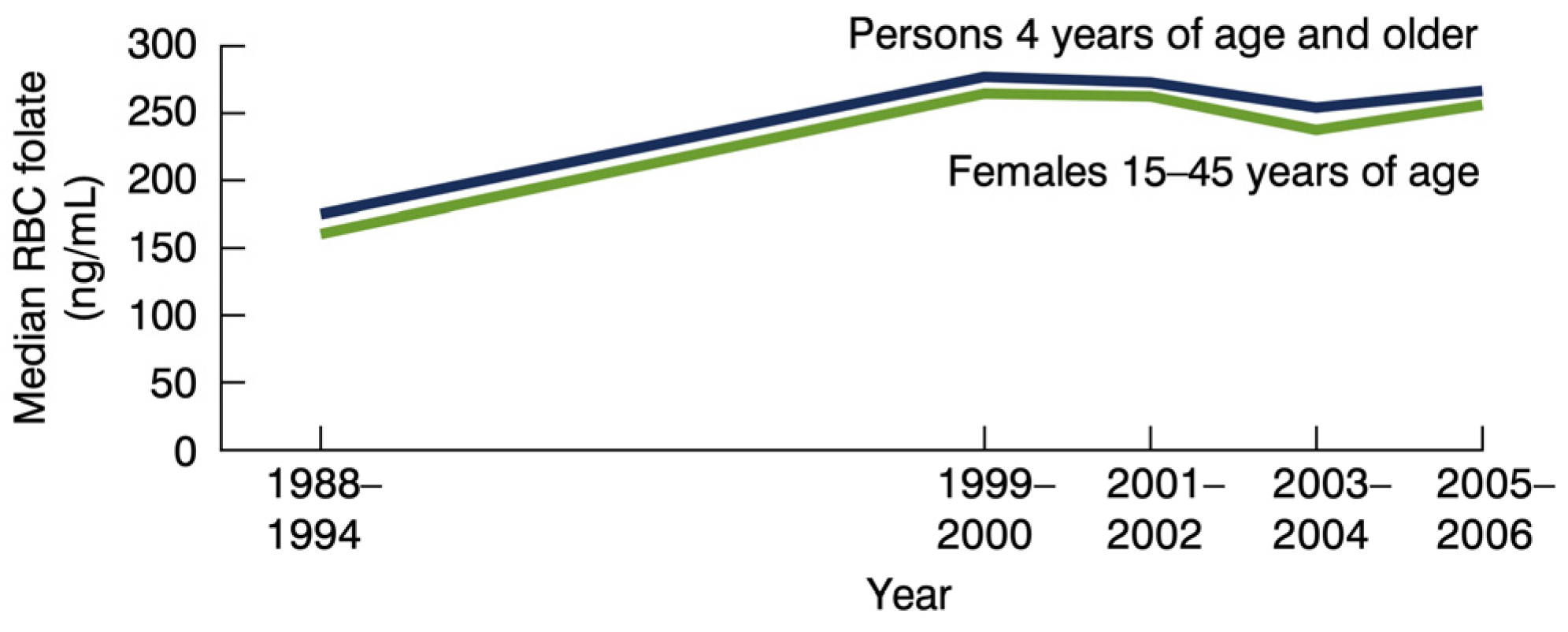
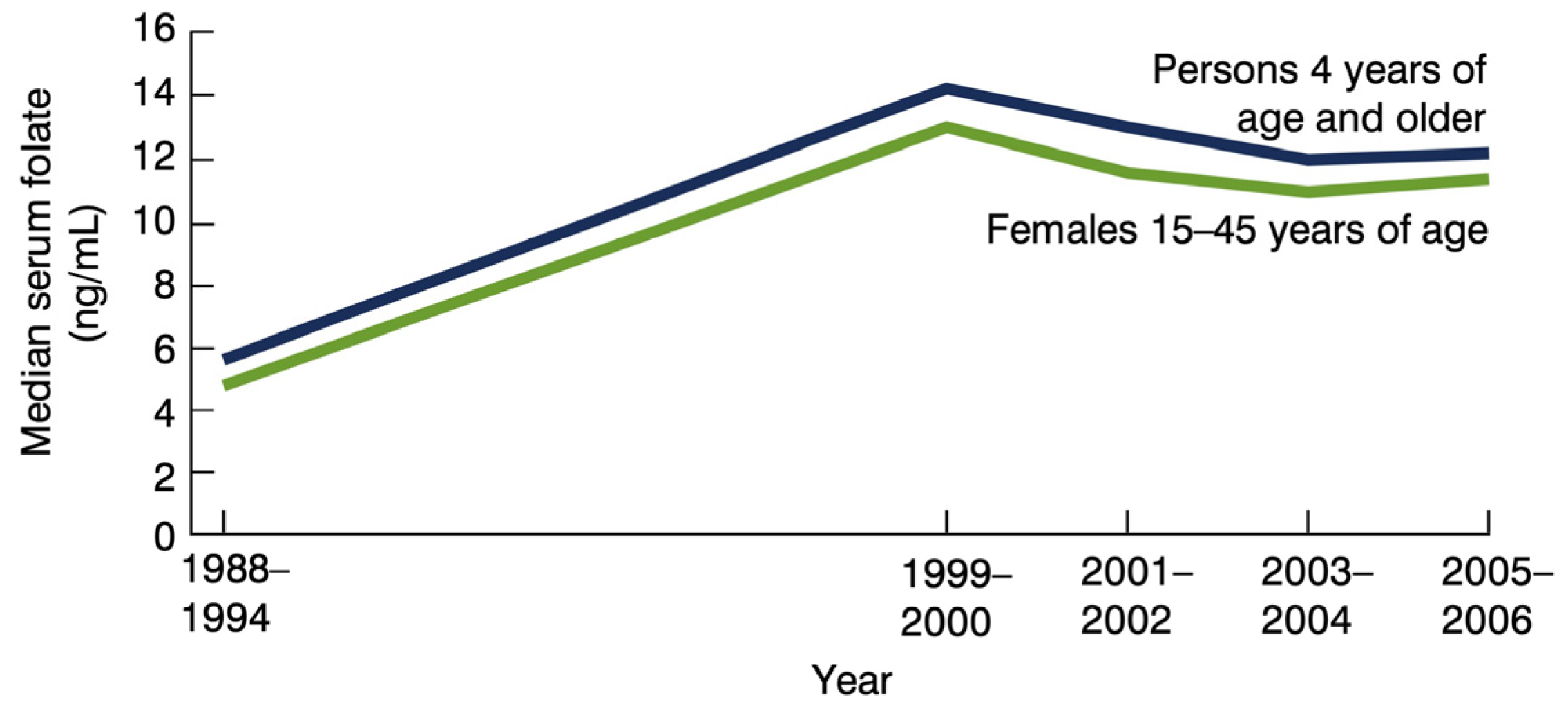
2. Folic Acid Recommendations
3. Folate, Brain, and Behavior
4. Gut-Brain-Microbiome Links
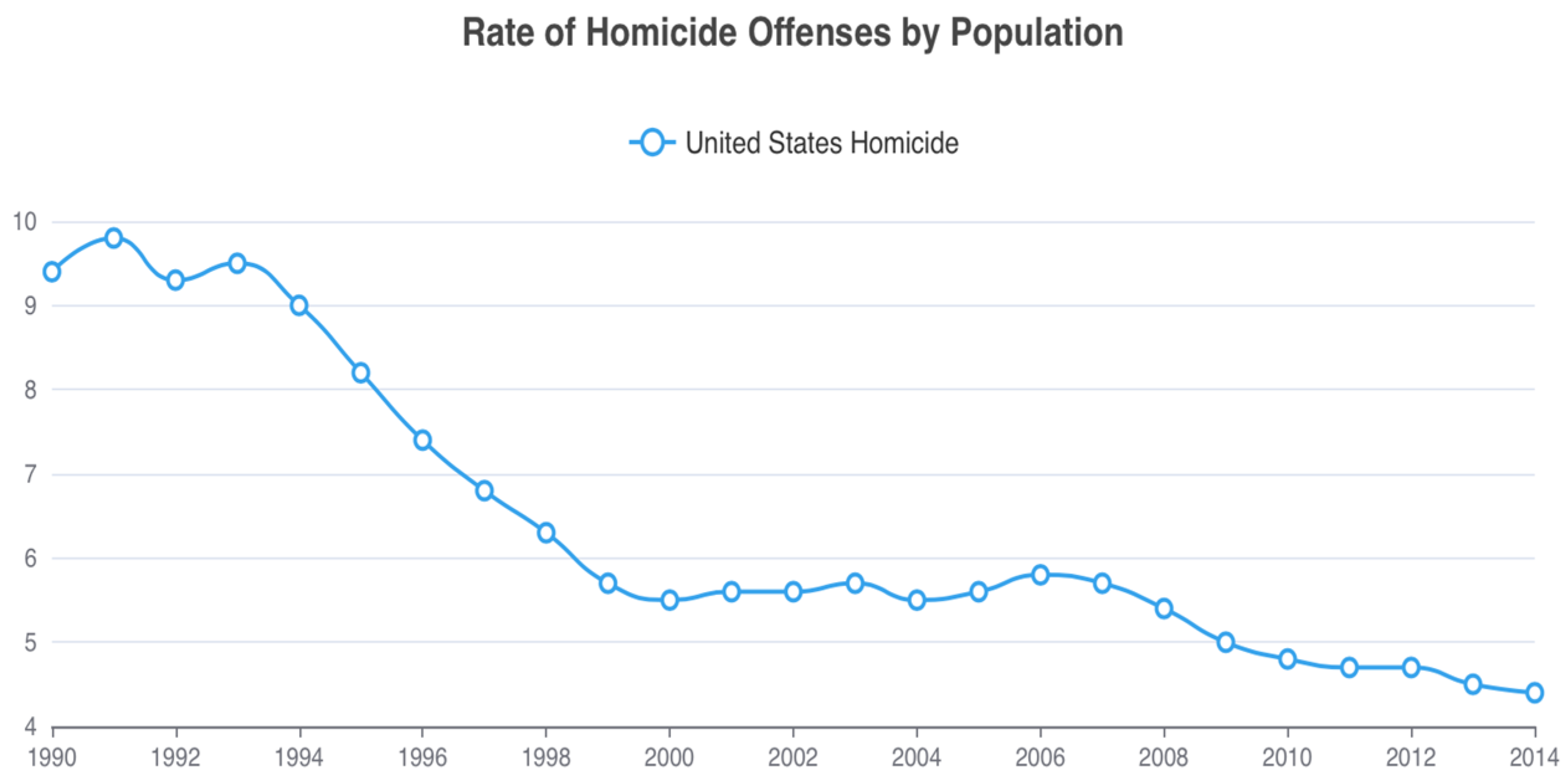
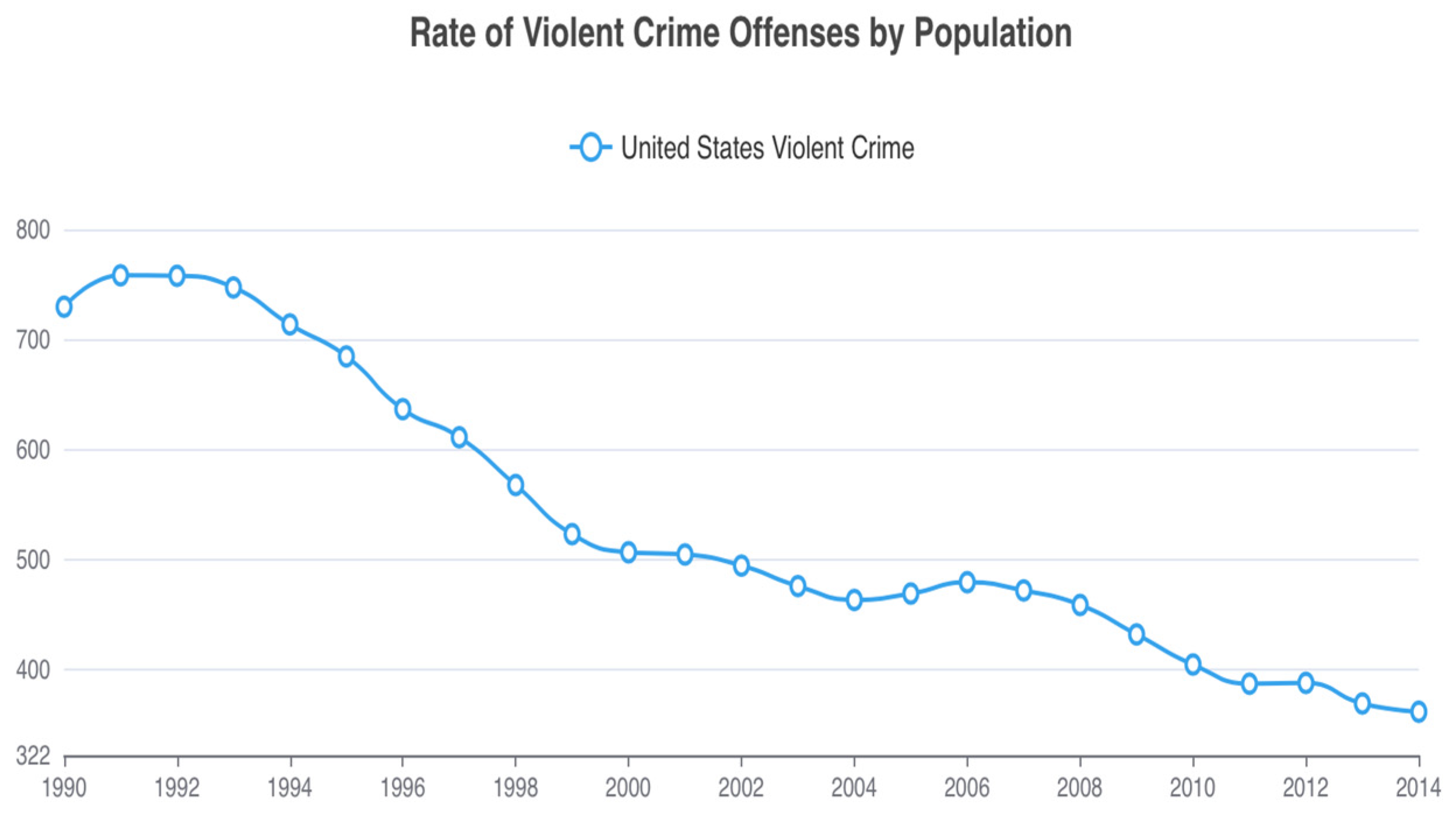
5. Violent Suicide and Homicide—Complexity
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kris-Etherton, P.M.; Petersen, K.S.; Hibbeln, J.R.; Hurley, D.; Kolick, V.; Peoples, S.; Rodriguez, N.; Woodward-Lopez, G. Nutrition and behavioral health disorders: Depression and anxiety. Nutr. Rev. 2021, 79, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, S.; Morkl, S.; Muller-Stierlin, A.S. Nutritional psychiatry in the treatment of psychotic disorders: Current hypotheses and research challenges. Brain Behav. Immun. Health 2020, 5, 100070. [Google Scholar] [CrossRef] [PubMed]
- Tcherni-Buzzeo, M. Dietary interventions, the gut microbiome, and aggressive behavior: Review of research evidence and potential next steps. Aggress. Behav. 2023, 49, 15–32. [Google Scholar] [CrossRef] [PubMed]
- Choy, O. Nutritional factors associated with aggression. Front. Psychiatry 2023, 14, 1176061. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Schweren, L.J.S.; Larsson, H.; Li, L.; Du Rietz, E.; Haavik, J.; Lifelines Cohort, S.; Grimstvedt Kvalvik, L.; Solberg, B.S.; Klungsoyr, K.; et al. Do Poor Diet and Lifestyle Behaviors Modify the Genetic Susceptibility to Impulsivity in the General Population? Nutrients 2023, 15, 1625. [Google Scholar] [CrossRef] [PubMed]
- Zaalberg, A. The effects of nutrients and neurotoxicants on aggressive behavior. J. Crim. Just. 2019, 65, 101592. [Google Scholar] [CrossRef]
- Faraone, S.V.; Banaschewski, T.; Coghill, D.; Zheng, Y.; Biederman, J.; Bellgrove, M.A.; Newcorn, J.H.; Gignac, M.; Al Saud, N.M.; Manor, I.; et al. The World Federation of ADHD International Consensus Statement: 208 Evidence-based conclusions about the disorder. Neurosci. Biobehav. Rev. 2021, 128, 789–818. [Google Scholar] [CrossRef]
- Prescott, S.L.; Logan, A.C.; D’Adamo, C.R.; Holton, K.F.; Lowry, C.A.; Marks, J.; Moodie, R.; Polland, B. Nutritional Criminology: Why the Emerging Research on Ultra- Processed Food Matters to Health and Justice. Int. J. Environ. Res. Public Health 2024, 21, 120. [Google Scholar] [CrossRef]
- Robinson, M. Eating ourselves to death: How food is a drug and what food abuse costs. Drug Sci. Policy Law 2022, 8, 20503245221112577. [Google Scholar] [CrossRef]
- Hibbeln, J.R. From homicide to happiness—A commentary on omega-3 fatty acids in human society. Cleave Award Lecture. Nutr. Health 2007, 19, 9–19. [Google Scholar] [CrossRef]
- Grenell, R.G.; Gabay, S. (Eds.) Biological Foundations of Psychiatry; Raven Press: New York, NY, USA, 1976. [Google Scholar]
- Liwinski, T.; Lang, U.E. Folate and Its Significance in Depressive Disorders and Suicidality: A Comprehensive Narrative Review. Nutrients 2023, 15, 3859. [Google Scholar] [CrossRef] [PubMed]
- Falade, J.; Onaolapo, A.Y.; Onaolapo, O.J. The Role of Folate-supplementation in Depression: A Narrative Review. Curr. Psychopharmacol. 2021, 10, 115–122. [Google Scholar] [CrossRef]
- Zimring, F.E. The Great American Crime Decline; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Levitt, S.D. Understanding why crime fell in the 1990s: Four factors that explain the decline and six that do not. J. Econ. Perspect. 2004, 18, 163–190. [Google Scholar] [CrossRef]
- Rosenfeld, R. The case of the unsolved crime decline. Sci. Am. 2004, 290, 82–89. [Google Scholar] [CrossRef]
- Parker, K.F. Unequal Crime Decline; New York University Press: New York, NY, USA, 2008. [Google Scholar]
- McKeown, R.E.; Cuffe, S.P.; Schulz, R.M. US suicide rates by age group, 1970-2002: An examination of recent trends. Am. J. Public Health 2006, 96, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Wald, N.J.; Polani, P.E. Neural-tube defects and vitamins: The need for a randomized clinical trial. Br. J. Obstet. Gynaecol. 1984, 91, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Milunsky, A.; Jick, H.; Jick, S.S.; Bruell, C.L.; MacLaughlin, D.S.; Rothman, K.J.; Willett, W. Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects. JAMA 1989, 262, 2847–2852. [Google Scholar] [CrossRef] [PubMed]
- Kolata, G. Sharp Cut in Serious Birth Defect Is Tied to Vitamins in Pregnancy. The New York Times, 24 November 1989; p. A-1. [Google Scholar]
- Anon. Early Multivitamin Use Found to Reduce Risk of Birth Defect. The Atlanta Journal Constitution, 24 November 1989; p. 1. [Google Scholar]
- The Associated Press. Taking B vitamin prenatally can reduce spina bifida risk. Waco Tribune-Herald, 15 April 1990; p. 32. [Google Scholar]
- MacCorquodale, D.W. Nutrition knowledge equals food for thought. Richmond Times-Dispatch, 7 February 1990; p. 6. [Google Scholar]
- Centers for Disease Control. Recommendations for the Use of Folic Acid to Reduce the Number of Cases of Spina Bifida and Other Neural Tube Defects. MMWR Recomm Rep. 1992, 41, 1–7. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00019479.htm (accessed on 15 February 2024).
- McDowell, M.A.; Lacher, D.A.; Pfeiffer, C.M.; Mulinare, J.; Picciano, M.F.; Rader, J.I.; Yetley, E.A.; Kennedy-Stephenson, J.; Johnson, C.L. Blood Folate Levels: The Latest NHANES Results; NCHS Data Briefs, No. 6; National Center for Health Statistics, Centers for Disease Control: Hyattsville, MD, USA, 2008. Available online: https://www.cdc.gov/nchs/products/databriefs/db06.htm#:~:text=Median%20RBC%20folate%20levels%20of%20persons%204%20years%20of%20age,%2D1994%20and%201999%2D2000 (accessed on 15 February 2024).
- Senti, F.R.; Pilch, S.M. Analysis of folate data from the second National Health and Nutrition Examination Survey (NHANES II). J. Nutr. 1985, 115, 1398–1402. [Google Scholar] [CrossRef]
- Moss, A.J. Use of Vitamin and Mineral Supplements in the United States: Current Users, Types of Products, and Nutrients; US Department of Health and Human Services, Public Health Service, Centers for Disease Control, National Center for Health Statistics: Hyattsville, MD, USA, 1989. [Google Scholar]
- Ervin, R.B.; Wright, J.D.; Kennedy-Stephenson, J. Use of dietary supplements in the United States, 1988–1994. Natl. Cent. Health Stat. Vital Health Stat. 1999, 11, 244. [Google Scholar]
- Mishra, S.; Gahche, J.J.; Ogden, C.L.; Dimeler, M.; Potischman, N.; Ahluwalia, N. Dietary Supplement Use in the United States: National Health and Nutrition Examination Survey, 2017–March 2020. National Health Statistics Reports; No 183; National Center for Health Statistics: Hyattsville, MD, USA, 2023. [Google Scholar] [CrossRef]
- Bowman, L. Folic Acid Ordered Added to Foods; The San Francisco Examiner: San Francisco, CA, USA, 1996; p. 2. [Google Scholar]
- Logan, A.C.; Nicholson, J.J.; Schoenthaler, S.J.; Prescott, S.L. Neurolaw: Revisiting Huberty v. McDonald’s through the Lens of Nutritional Criminology and Food Crime. Laws 2024, 13, 17. [Google Scholar] [CrossRef]
- Clarke, A.G.; Prescott, F. Studies in Vitamin B Deficiency. Br. Med. J. 1943, 2, 503–504. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Watson, G.; Comrey, A.L. Nutritional replacement for mental illness. J. Psychol. 1954, 38, 251–264. [Google Scholar] [CrossRef]
- Young, S.N.; Ghadirian, A.M. Folic-Acid and Psychopathology. Prog. Neuro-Psychopharmacol. 1989, 13, 841–863. [Google Scholar] [CrossRef] [PubMed]
- Kariks, J.; Perry, S.W. Folic-Acid Deficiency in Psychiatric Patients. Med. J. Aust. 1970, 1, 1192–1195. [Google Scholar] [CrossRef] [PubMed]
- Crellin, R.; Bottiglieri, T.; Reynolds, E.H. Folates and Psychiatric-Disorders—Clinical Potential. Drugs 1993, 45, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Walder, K.R.; Berk, M.; Marx, W.; Walker, A.J.; Maes, M.; Puri, B.K. The interplay between oxidative stress and bioenergetic failure in neuropsychiatric illnesses: Can we explain it and can we treat it? Mol. Biol. Rep. 2020, 47, 5587–5620. [Google Scholar] [CrossRef] [PubMed]
- Brocardo, P.S.; Budni, J.; Kaster, M.P.; Santos, A.R.; Rodrigues, A.L. Folic acid administration produces an antidepressant-like effect in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Neuropharmacology 2008, 54, 464–473. [Google Scholar] [CrossRef]
- Ferguson, S.A.; Berry, K.J.; Hansen, D.K.; Wall, K.S.; White, G.; Antony, A.C. Behavioral effects of prenatal folate deficiency in mice. Birth Defects Res. A Clin. Mol. Teratol. 2005, 73, 249–252. [Google Scholar] [CrossRef]
- Altaf, R.; Gonzalez, I.; Rubino, K.; Nemec, E.C., 2nd. Folate as adjunct therapy to SSRI/SNRI for major depressive disorder: Systematic review & meta-analysis. Complement. Ther. Med. 2021, 61, 102770. [Google Scholar]
- Crockett, M.J.; Clark, L.; Hauser, M.D.; Robbins, T.W. Serotonin selectively influences moral judgment and behavior through effects on harm aversion. Proc. Natl. Acad. Sci. USA 2010, 107, 17433–17438. [Google Scholar] [CrossRef] [PubMed]
- Ash, J.A.; Jiang, X.; Malysheva, O.V.; Fiorenza, C.G.; Bisogni, A.J.; Levitsky, D.A.; Strawderman, M.S.; Caudill, M.A.; Stover, P.J.; Strupp, B.J. Dietary and genetic manipulations of folate metabolism differentially affect neocortical functions in mice. Neurotoxicol. Teratol. 2013, 38, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Tchantchou, F.; Graves, V.; Rozen, R.; Shea, T.B. Dietary and genetic compromise in folate availability reduces acetylcholine, cognitive performance and increases aggression: Critical role of S-adenosyl methionine. J. Nutr. Health Aging 2008, 12, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Gesch, C.B.; Hammond, S.M.; Hampson, S.E.; Eves, A.; Crowder, M.J. Influence of supplementary vitamins, minerals and essential fatty acids on the antisocial behaviour of young adult prisoners. Randomised, placebo-controlled trial. Br. J. Psychiatry 2002, 181, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Zaalberg, A.; Nijman, H.; Bulten, E.; Stroosma, L.; van der Staak, C. Effects of nutritional supplements on aggression, rule-breaking, and psychopathology among young adult prisoners. Aggress. Behav. 2010, 36, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Schoenthaler, S.J.; Amos, W.; Doraz, M.A.; Kelly, G.; Muedeking, J.; Wakefield, J. The effect of randomized vitamin-mineral supplementation on violent and non-violent antisocial behavior among incarcerated juveniles. J. Nutr. Environ. Med. 1997, 7, 343–352. [Google Scholar] [CrossRef]
- Schoenthaler, S.; Gast, D.; Giltay, E.J.; Amos, S. The effects of vitamin-mineral supplements on serious rule violations in correctional facilities for young adult male inmates: A ran-domized controlled trial. Crime Delinq. 2023, 69, 822–840. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, H.Y.; Lee, H.J.; Kim, J.W.; Kang, H.J.; Kim, S.W.; Shin, I.S.; Chun, B.J.; Stewart, R. Prediction of Suicidality According to Serum Folate Levels in Depressive Patients Receiving Stepwise Pharmacotherapy. Front. Psychiatry 2021, 12, 747228. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, R.D.; Hur, K.; Lavigne, J.E.; Mann, J.J. Association Between Folic Acid Prescription Fills and Suicide Attempts and Intentional Self-harm Among Privately Insured US Adults. JAMA Psychiatry 2022, 79, 1118–1123. [Google Scholar] [CrossRef]
- Mann, J.J.; Hur, K.; Lavigne, J.E.; Gibbons, R.D. Folic acid prescription and suicide attempt prevention: Effect of past suicidal behaviour, psychiatric diagnosis and psychotropic medication. BJPsych Open 2023, 9, e159. [Google Scholar] [CrossRef]
- Dumais, A.; Lesage, A.D.; Lalovic, A.; Seguin, M.; Tousignant, M.; Chawky, N.; Turecki, G. Is violent method of suicide a behavioral marker of lifetime aggression? Am. J. Psychiatry 2005, 162, 1375–1378. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.; Turecki, G. Is Violent Suicide Molecularly Distinct? Am. J. Psychiatry 2022, 179, 180–181. [Google Scholar] [CrossRef] [PubMed]
- Punzi, G.; Ursini, G.; Chen, Q.; Radulescu, E.; Tao, R.; Huuki, L.A.; Di Carlo, P.; Collado-Torres, L.; Shin, J.H.; Catanesi, R.; et al. Genetics and Brain Transcriptomics of Completed Suicide. Am. J. Psychiatry 2022, 179, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Verwer, R.W.; van Heerikhuizen, J.; Balesar, R.; Correa-da-Silva, F.; Slabe, Z.; Lucassen, P.J.; Swaab, D.F. Stress-associated purinergic receptors code for fatal suicidality in the hippocampal-hypothalamic-prefrontal circuit. bioRxiv 2022. [Google Scholar] [CrossRef]
- Gajos, J.M.; Beaver, K.M. The effect of omega-3 fatty acids on aggression: A meta-analysis. Neurosci. Biobehav. Rev. 2016, 69, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Sublette, M.E.; Daray, F.M.; Gananca, L.; Shaikh, S.R. The role of polyunsaturated fatty acids in the neurobiology of major depressive disorder and suicide risk. Mol. Psychiatry 2023. [Google Scholar] [CrossRef] [PubMed]
- Ued, F.V.; Mathias, M.G.; Toffano, R.B.D.; Barros, T.T.; Almada, M.; Salomao, R.G.; Coelho-Landell, C.A.; Hillesheim, E.; Camarneiro, J.M.; Camelo-Junior, J.S.; et al. Vitamin B2 and Folate Concentrations are Associated with ARA, EPA and DHA Fatty Acids in Red Blood Cells of Brazilian Children and Adolescents. Nutrients 2019, 11, 2918. [Google Scholar] [CrossRef] [PubMed]
- Pita, M.L.; Delgado, M.J. Folate administration increases n-3 polyunsaturated fatty acids in rat plasma and tissue lipids. Thromb. Haemost. 2000, 84, 420–423. [Google Scholar] [PubMed]
- Durand, P.; Prost, M.; Blache, D. Pro-thrombotic effects of a folic acid deficient diet in rat platelets and macrophages related to elevated homocysteine and decreased n-3 polyunsaturated fatty acids. Atherosclerosis 1996, 121, 231–243. [Google Scholar] [CrossRef]
- Hirono, H.; Wada, Y. Effects of dietary folate deficiency on developmental increase of myelin lipids in rat brain. J. Nutr. 1978, 108, 766–772. [Google Scholar] [CrossRef]
- Umhau, J.C.; Dauphinais, K.M.; Patel, S.H.; Nahrwold, D.A.; Hibbeln, J.R.; Rawlings, R.R.; George, D.T. The relationship between folate and docosahexaenoic acid in men. Eur. J. Clin. Nutr. 2006, 60, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Kale, A.; Naphade, N.; Sapkale, S.; Kamaraju, M.; Pillai, A.; Joshi, S.; Mahadik, S. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: Implications for altered one-carbon metabolism. Psychiatry Res. 2010, 175, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Ravindran, A.; Yatham, L.N.; Marx, W.; Rucklidge, J.J.; McIntyre, R.S.; Akhondzadeh, S.; Benedetti, F.; Caneo, C.; Cramer, H.; et al. Clinician guidelines for the treatment of psychiatric disorders with nutraceuticals and phytoceuticals: The World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce. World J. Biol. Psychiatry 2022, 23, 424–455. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.C.; Venket Rao, A.; Irani, D. Chronic fatigue syndrome: Lactic acid bacteria may be of therapeutic value. Med. Hypotheses 2003, 60, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Logan, A.C.; Katzman, M. Major depressive disorder: Probiotics may be an adjuvant therapy. Med. Hypotheses 2005, 64, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Merlo, G.; Bachtel, G.; Sugden, S.G. Gut microbiota, nutrition, and mental health. Front. Nutr. 2024, 11, 1337889. [Google Scholar] [CrossRef]
- Martin, S.E.; Kraft, C.S.; Ziegler, T.R.; Millson, E.C.; Rishishwar, L.; Martin, G.S. The Role of Diet on the Gut Microbiome, Mood and Happiness. medRxiv 2023. [Google Scholar] [CrossRef]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Donoso, F.; Cryan, J.F.; Olavarria-Ramirez, L.; Nolan, Y.M.; Clarke, G. Inflammation, Lifestyle Factors, and the Microbiome-Gut-Brain Axis: Relevance to Depression and Antidepressant Action. Clin. Pharmacol. Ther. 2023, 113, 246–259. [Google Scholar] [CrossRef]
- Barone, M.; D’Amico, F.; Brigidi, P.; Turroni, S. Gut microbiome-micronutrient interaction: The key to controlling the bioavailability of minerals and vitamins? Biofactors 2022, 48, 307–314. [Google Scholar] [CrossRef]
- Aleman, R.S.; Moncada, M.; Aryana, K.J. Leaky Gut and the Ingredients That Help Treat It: A Review. Molecules 2023, 28, 619. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, T.; He, L.; Fu, J.Y.; Deng, H.X.; Xue, X.L.; Chen, B.T. Bacterial Translocation Associates With Aggression in Schizophrenia Inpatients. Front. Syst. Neurosci. 2021, 15, 704069. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zang, T.; Liu, J.; Wu, N.; Dai, J.; Bai, J.; Liu, Y. Changes in the gut microbiome in the first two years of life predicted the temperament in toddlers. J. Affect. Disord. 2023, 333, 342–352. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Wang, H.; Luo, J.; Wang, Z.; Chen, G.; Jiang, D.; Cao, R.; Huang, H.; Luo, D.; et al. Profiling the differences of gut microbial structure between schizophrenia patients with and without violent behaviors based on 16S rRNA gene sequencing. Int. J. Legal Med. 2021, 135, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Guimond, A.J.; Tworoger, S.S.; Huang, T.; Chan, A.T.; Liu, Y.Y.; Kubzansky, L.D. Gut feelings: Associations of emotions and emotion regulation with the gut microbiome in women. Psychol. Med. 2023, 53, 7151–7160. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, L.; Sellaro, R.; van Hemert, S.; Bosch, J.A.; Colzato, L.S. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav. Immun. 2015, 48, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Arteaga-Henriquez, G.; Rosales-Ortiz, S.K.; Arias-Vasquez, A.; Bitter, I.; Ginsberg, Y.; Ibanez-Jimenez, P.; Kilencz, T.; Lavebratt, C.; Matura, S.; Reif, A.; et al. Treating impulsivity with probiotics in adults (PROBIA): Study protocol of a multicenter, double-blind, randomized, placebo-controlled trial. Trials 2020, 21, 161. [Google Scholar] [CrossRef] [PubMed]
- Gulledge, L.; Oyebode, D.; Donaldson, J.R. The influence of the microbiome on aggressive behavior: An insight into age-related aggression. FEMS Microbiol. Lett. 2023, 370, fnac114. [Google Scholar] [CrossRef]
- Bruce-Keller, A.J.; Salbaum, J.M.; Luo, M.; Blanchard, E.T.; Taylor, C.M.; Welsh, D.A.; Berthoud, H.R. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol. Psychiatry 2015, 77, 607–615. [Google Scholar] [CrossRef]
- Li, N.; Wang, Q.; Wang, Y.; Sun, A.; Lin, Y.; Jin, Y.; Li, X. Fecal microbiota transplantation from chronic unpredictable mild stress mice donors affects anxiety-like and depression-like behavior in recipient mice via the gut microbiota-inflammation-brain axis. Stress 2019, 22, 592–602. [Google Scholar] [CrossRef]
- Wang, C.; Yan, J.; Du, K.; Liu, S.; Wang, J.; Wang, Q.; Zhao, H.; Li, M.; Yan, D.; Zhang, R.; et al. Intestinal microbiome dysbiosis in alcohol-dependent patients and its effect on rat behaviors. mBio 2023, 14, e0239223. [Google Scholar] [CrossRef] [PubMed]
- Ritz, N.L.; Brocka, M.; Butler, M.I.; Cowan, C.S.M.; Barrera-Bugueno, C.; Turkington, C.J.R.; Draper, L.A.; Bastiaanssen, T.F.S.; Turpin, V.; Morales, L.; et al. Social anxiety disorder-associated gut microbiota increases social fear. Proc. Natl. Acad. Sci. USA 2024, 121, e2308706120. [Google Scholar] [CrossRef]
- Uzan-Yulzari, A.; Turjeman, S.; Getselter, D.; Rautava, S.; Isolauri, E.; Khatib, S.; Elliott, E.; Koren, O. Aggression: A gut reaction? The effects of gut microbiome on aggression. bioRxiv 2023. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Liu, M.; Chen, J.; Pan, J.; Han, Y.; Liu, Y.; Cheng, K.; Zhou, C.; Wang, H.; et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 2019, 5, eaau8317. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Guo, R.; Wang, W.; Ju, Y.; Wang, Q.; Ma, Q.; Sun, Q.; Fan, Y.; Xie, Y.; Yang, Z.; et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol. Psychiatry 2020, 25, 2905–2918. [Google Scholar] [CrossRef]
- Li, D.; Liang, W.; Zhang, W.; Huang, Z.; Liang, H.; Liu, Q. Fecal microbiota transplantation repairs intestinal permeability and regulates the expression of 5-HT to influence alcohol-induced depression-like behaviors in C57BL/6J mice. Front. Microbiol. 2023, 14, 1241309. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, M.; Tong, C.; Wang, Y.; He, J.; Shao, Q.; Liu, Y.; Wu, Y.; Song, Y. Regulation of miRNA expression in the prefrontal cortex by fecal microbiota transplantation in anxiety-like mice. Front. Psychiatry 2024, 15, 1323801. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, S.; Sol Kim, D. Folate and vitamin B-12 deficiencies additively impaire memory function and disturb the gut microbiota in amyloid-beta infused rats. Int. J. Vitam. Nutr. Res. 2022, 92, 169–181. [Google Scholar] [CrossRef]
- Cheng, C.K.; Wang, C.; Shang, W.; Lau, C.W.; Luo, J.Y.; Wang, L.; Huang, Y. A high methionine and low folate diet alters glucose homeostasis and gut microbiome. Biochem. Biophys. Rep. 2021, 25, 100921. [Google Scholar] [CrossRef]
- Chen, S.; Yang, M.; Wang, R.; Fan, X.; Tang, T.; Li, P.; Zhou, X.; Qi, K. Suppression of high-fat-diet-induced obesity in mice by dietary folic acid supplementation is linked to changes in gut microbiota. Eur. J. Nutr. 2022, 61, 2015–2031. [Google Scholar] [CrossRef]
- Zhang, H.; Zuo, Y.; Zhao, H.; Zhao, H.; Wang, Y.; Zhang, X.; Zhang, J.; Wang, P.; Sun, L.; Zhang, H.; et al. Folic acid ameliorates alcohol-induced liver injury via gut-liver axis homeostasis. Front. Nutr. 2022, 9, 989311. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, J.; Liu, X.; Liu, R.; Wang, Y.; Huang, X.; Li, Y.; Liu, R.; Yang, X. Dietary folic acid addition reduces abdominal fat deposition mediated by alterations in gut microbiota and SCFA production in broilers. Anim. Nutr. 2023, 12, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Gobaud, A.N.; Mehranbod, C.A.; Dong, B.; Dodington, J.; Morrison, C.N. Absolute versus relative socioeconomic disadvantage and homicide: A spatial ecological case-control study of US zip codes. Inj. Epidemiol. 2022, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Stack, S. Contributing factors to suicide: Political, social, cultural and economic. Prev. Med. 2021, 152 Pt 1, 106498. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ales, G.; Jiang, T.; Keyes, K.M.; Gradus, J.L. The Recent Rise of Suicide Mortality in the United States. Annu. Rev. Public Health 2022, 43, 99–116. [Google Scholar] [CrossRef]
- Go, T.H.; Kim, M.H.; Choi, Y.Y.; Han, J.; Kim, C.; Kang, D.R. The short-term effect of ambient particulate matter on suicide death. Environ. Health 2024, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Carmichael, S.L.; Shaw, G.M. Folic acid fortification and prevalences of neural tube defects, orofacial clefts, and gastroschisis in California, 1989 to 2010. Birth Defects Res. A Clin. Mol. Teratol. 2016, 106, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.M.; Hughes, J.P.; Lacher, D.A.; Bailey, R.L.; Berry, R.J.; Zhang, M.; Yetley, E.A.; Rader, J.I.; Sempos, C.T.; Johnson, C.L. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988–2010. J. Nutr. 2012, 142, 886–893. [Google Scholar] [CrossRef]
- Petersen, J.M.; Smith-Webb, R.S.; Shaw, G.M.; Carmichael, S.L.; Desrosiers, T.A.; Nestoridi, E.; Darling, A.M.; Parker, S.E.; Politis, M.D.; Yazdy, M.M.; et al. Periconceptional intakes of methyl donors and other micronutrients involved in one-carbon metabolism may further reduce the risk of neural tube defects in offspring: A United States population-based case-control study of women meeting the folic acid recommendations. Am. J. Clin. Nutr. 2023, 118, 720–728. [Google Scholar]
- Wang, A.; Rose, C.E.; Qi, Y.P.; Williams, J.L.; Pfeiffer, C.M.; Crider, K.S. Impact of Voluntary Folic Acid Fortification of Corn Masa Flour on RBC Folate Concentrations in the U.S. (NHANES 2011–2018). Nutrients 2021, 13, 1325. [Google Scholar] [CrossRef]
- Murphy, R.; Marshall, K.; Zagorin, S.; Devarshi, P.P.; Hazels Mitmesser, S. Socioeconomic Inequalities Impact the Ability of Pregnant Women and Women of Childbearing Age to Consume Nutrients Needed for Neurodevelopment: An Analysis of NHANES 2007–2018. Nutrients 2022, 14, 3823. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, S.L.; Yang, W.; Herring, A.; Abrams, B.; Shaw, G.M. Maternal food insecurity is associated with increased risk of certain birth defects. J. Nutr. 2007, 137, 2087–2092. [Google Scholar] [CrossRef]
- Pruitt Evans, S.; Ailes, E.C.; Kramer, M.R.; Shumate, C.J.; Reefhuis, J.; Insaf, T.Z.; Yazdy, M.M.; Carmichael, S.L.; Romitti, P.A.; Feldkamp, M.L.; et al. Neighborhood Deprivation and Neural Tube Defects. Epidemiology 2023, 34, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Grinshteyn, E.; Hemenway, D. Violent Death Rates: The US Compared with Other High-income OECD Countries, 2010. Am. J. Med. 2016, 129, 266–273. [Google Scholar] [CrossRef]
- Grinshteyn, E.; Hemenway, D. Violent death rates in the US compared to those of the other high-income countries, 2015. Prev. Med. 2019, 123, 20–26. [Google Scholar] [CrossRef]
- Kose, S.; Sozlu, S.; Bolukbasi, H.; Unsal, N.; Gezmen-Karadag, M. Obesity is associated with folate metabolism. Int. J. Vitam. Nutr. Res. 2020, 90, 353–364. [Google Scholar] [CrossRef]
- Sutton, C.A.; Stratton, M.; L’Insalata, A.M.; Fazzino, T.L. Ultraprocessed, hyper-palatable, and high energy density foods: Prevalence and distinction across 30 years in the United States. Obesity 2024, 32, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.; Parekh, N.; Martinez-Steele, E.; Monteiro, C.A.; Chang, V.W. Ultra-processed food consumption among US adults from 2001 to 2018. Am. J. Clin. Nutr. 2022, 115, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Amaraggi, B.; Wood, W.; Guinovart Martin, L.; Gimenez Sanchez, J.; Fleta Sanchez, Y.; de la Garza Puentes, A. Ultra-processed food staples dominate mainstream US supermarkets. Americans more than Europeans forced to choose between health and cost. medRxiv 2024. [Google Scholar] [CrossRef]
- Leung, C.W.; Parnarouskis, L.; Slotnick, M.J.; Gearhardt, A.N. Food Insecurity and Food Addiction in a Large, National Sample of Lower-Income Adults. Curr. Dev. Nutr. 2023, 7, 102036. [Google Scholar] [CrossRef]
- Fazzino, T.L.; Jun, D.; Chollet-Hinton, L.; Bjorlie, K. US tobacco companies selectively disseminated hyper-palatable foods into the US food system: Empirical evidence and current implications. Addiction 2024, 119, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Logan, A.C. Each meal matters in the exposome: Biological and community considerations in fast-food-socioeconomic associations. Econ. Hum. Biol. 2017, 27 Pt B, 328–335. [Google Scholar] [CrossRef]
- Blumstein, A.; Rivara, F.P.; Rosenfeld, R. The rise and decline of homicide—And why. Annu. Rev. Public Health 2000, 21, 505–541. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoenthaler, S.J.; Prescott, S.L.; Logan, A.C. Homicide or Happiness: Did Folate Fortification and Public Health Campaigns Influence Homicide Rates and the Great American Crime Decline? Nutrients 2024, 16, 1075. https://doi.org/10.3390/nu16071075
Schoenthaler SJ, Prescott SL, Logan AC. Homicide or Happiness: Did Folate Fortification and Public Health Campaigns Influence Homicide Rates and the Great American Crime Decline? Nutrients. 2024; 16(7):1075. https://doi.org/10.3390/nu16071075
Chicago/Turabian StyleSchoenthaler, Stephen J., Susan L. Prescott, and Alan C. Logan. 2024. "Homicide or Happiness: Did Folate Fortification and Public Health Campaigns Influence Homicide Rates and the Great American Crime Decline?" Nutrients 16, no. 7: 1075. https://doi.org/10.3390/nu16071075
APA StyleSchoenthaler, S. J., Prescott, S. L., & Logan, A. C. (2024). Homicide or Happiness: Did Folate Fortification and Public Health Campaigns Influence Homicide Rates and the Great American Crime Decline? Nutrients, 16(7), 1075. https://doi.org/10.3390/nu16071075




