Improvement in Visceral Adipose Tissue and LDL Cholesterol by High PUFA Intake: 1-Year Results of the NutriAct Trial
Abstract
1. Introduction
2. Materials/Subjects and Methods
2.1. Study Design and Participants, Inclusion and Exclusion Criteria
2.2. Intervention
2.3. Anthropometric and Metabolic Assessment
2.4. Quantification of Adipose Tissue Depots and Intrahepatic Lipid Content
2.5. Biochemical Analyses
2.6. Outcomes and Statistical Analyses
2.7. Characteristics of the Study Group
3. Results
3.1. Baseline Associations between VAT, Dietary Intake and Cardiometabolic Ris Markers
3.2. Nutritional Changes within the First Year of Intervention
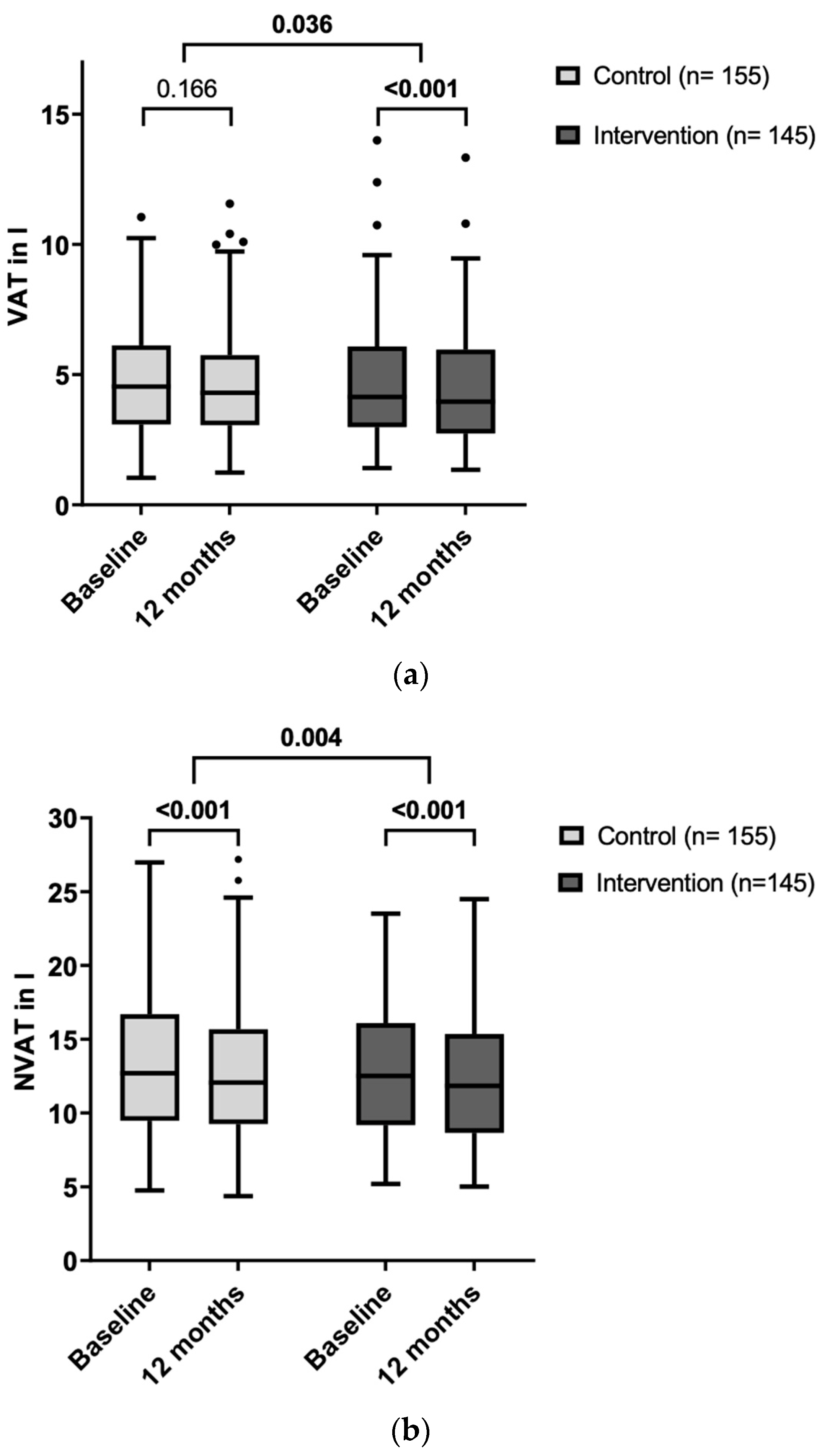
3.3. Effects of the Dietary Intervention on Adipose Tissue Compartments
3.4. Effects of the Dietary Intervention on Cardiometabolic Risk Factors
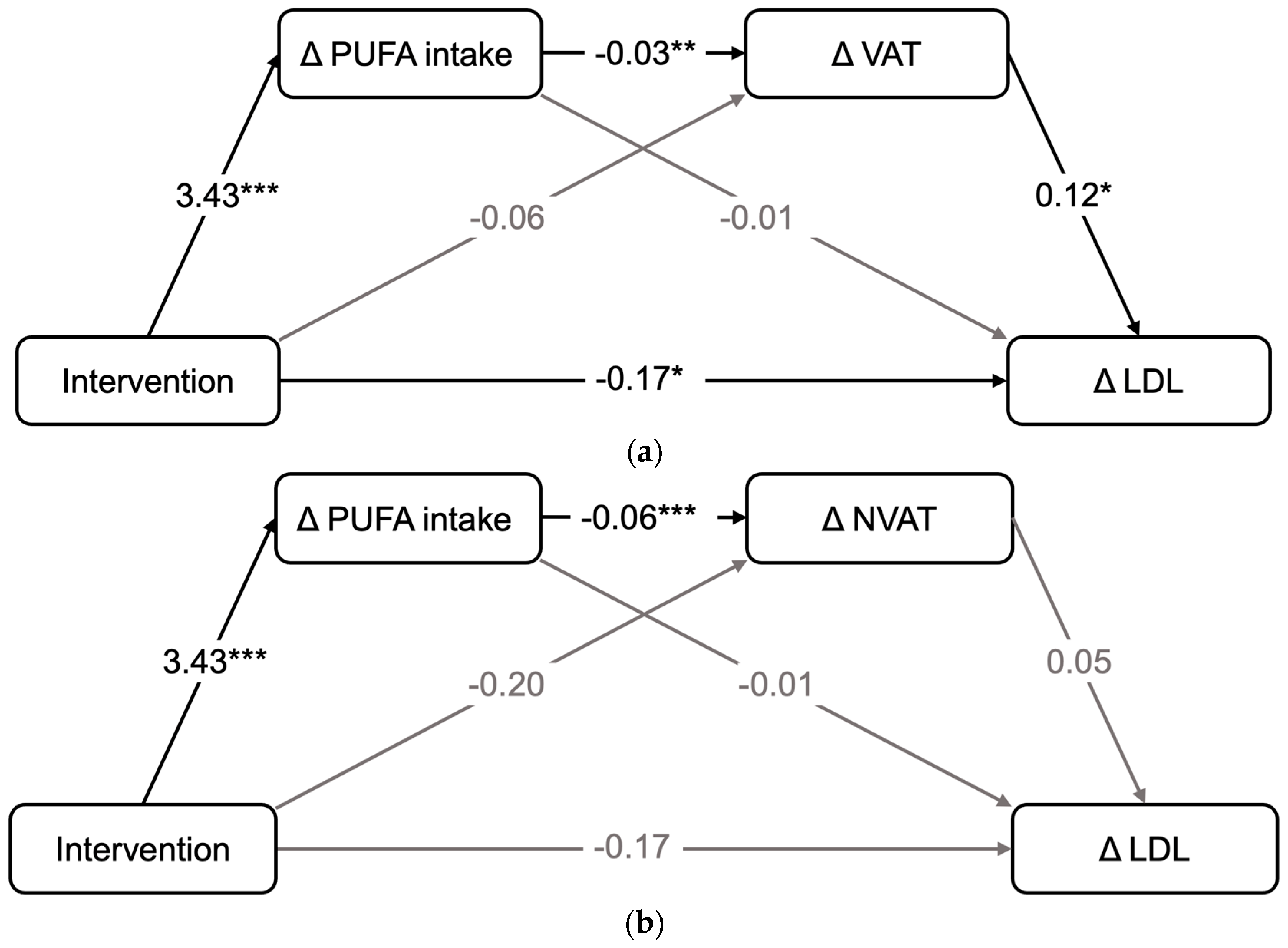
3.5. Relationship between Changes in Nutrient Intake, Adipose Tissue Compartments and Changes in LDL-C
4. Discussion
4.1. Effects on Adipose Tissue Compartments
4.2. Effects on Lipid and Glucose Metabolism
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CG | control group |
| CI | confidence interval |
| CV | coefficient of variance |
| CVD | cardiovascular diseases |
| DGE | German Nutrition Society |
| DIfE | German Institute of Human Nutrition Potsdam-Rehbruecke |
| eCRF | electronic case report form |
| % E | percent of total energy |
| FA | fatty acids |
| HbA1c | hemoglobin A1C |
| HDL-C | high-density lipoprotein cholesterol |
| 1H-MRS | proton magnetic resonance spectroscopy |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| IG | intervention group |
| IHL | intrahepatic lipids |
| IQR | interquartile range |
| IS | insulin sensitivity |
| LDL-C | low-density lipoprotein cholesterol |
| MD | mean difference |
| MeD | Mediterranean diet |
| MUFA | monounsaturated fatty acids |
| MWU | Mann–Whitney U Test |
| NVAT | non-visceral adipose tissue |
| n3 | omega-3 fatty acids |
| n6 | omega-6 fatty acids |
| oGTT | oral glucose tolerance test |
| PUFA | polyunsaturated fatty acids |
| RCT | randomized clinical trial |
| SCAT | subcutaneous adipose tissue |
| SFA | saturated fatty acids |
| TC | total cholesterol |
| TG | triacylglycerol |
| UFA | unsaturated fatty acids (poly- and monounsaturated) |
| VAT: | visceral adipose tissue |
References
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.F.; Schatzkin, A.; Harris, T.B.; Kipnis, V.; Mouw, T.; Ballard-Barbash, R.; Hollenbeck, A.; Leitzmann, M.F. Overweight, Obesity, and Mortality in a Large Prospective Cohort of Persons 50 to 71 Years Old. N. Engl. J. Med. 2009, 355, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Sam, S. Differential effect of subcutaneous abdominal and visceral adipose tissue on cardiometabolic risk. Horm. Mol. Biol. Clin. Investig. 2018, 33, 20180014. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.E.; Tchernof, A.; Després, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Chaston, T.B.; Dixon, J.B. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: Findings from a systematic review. Int. J. Obes. 2008, 32, 619–628. [Google Scholar] [CrossRef]
- Franco, M.; Bilal, U.; Orduñez, P.; Benet, M.; Morejón, A.; Caballero, B.; Kennelly, J.F.; Cooper, R.S. Population-wide weight loss and regain in relation to diabetes burden and cardiovascular mortality in Cuba 1980-2010: Repeated cross sectional surveys and ecological comparison of secular trends. BMJ 2013, 346, f1515. [Google Scholar] [CrossRef]
- Mai, K.; Brachs, M.; Leupelt, V.; Jumpertz-von Schwartzenberg, R.; Maurer, L.; Grüters-Kieslich, A.; Ernert, A.; Bobbert, T.; Krude, H.; Spranger, J. Effects of a combined dietary, exercise and behavioral intervention and sympathetic system on body weight maintenance after intended weight loss: Results of a randomized controlled trial. Metabolism 2018, 83, 60–67. [Google Scholar] [CrossRef]
- Villareal, D.T.; Apovian, C.M.; Kushner, R.F.; Klein, S. Obesity in Older Adults: Technical Review and Position Statement of the American Society for Nutrition and NAASO, The Obesity Society. Am. J. Clin. Nutr. 2005, 13, 1849–1863. [Google Scholar]
- Henkin, Y.; Kovsan, J.; Gepner, Y.; Shai, I. Diets and morbid tissues—History counts, present counts. Br. J. Nutr. 2015, 113, S11–S18. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity—A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Elhayany, A.; Lustman, A.; Abel, R.; Attal-Singer, J.; Vinker, S. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: A 1-year prospective randomized intervention study. Diabetes Obes. Metab. 2010, 12, 204–209. [Google Scholar] [CrossRef]
- Markova, M.; Pivovarova, O.; Hornemann, S.; Sucher, S.; Frahnow, T.; Wegner, K.; Machann, J.; Petzke, K.J.; Hierholzer, J.; Lichtinghagen, R.; et al. Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals With Type 2 Diabetes. Gastroenterology 2017, 152, 571–585.e8. [Google Scholar] [CrossRef] [PubMed]
- Vogtschmidt, Y.D.; Raben, A.; Faber, I.; de Wilde, C.; Lovegrove, J.A.; Givens, D.I.; Pfeiffer, A.F.; Soedamah-Muthu, S.S. Is protein the forgotten ingredient: Effects of higher compared to lower protein diets on cardiometabolic risk factors. A systematic review and meta-analysis of randomised controlled trials. Atherosclerosis 2021, 328, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Bjermo, H.; Iggman, D.; Kullberg, J.; Dahlman, I.; Johansson, L.; Persson, L.; Berglund, J.; Pulkki, K.; Basu, S.; Uusitupa, M.; et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Bendall, C.L.; Mayr, H.L.; Opie, R.S.; Bes-Rastrollo, M.; Itsiopoulos, C.; Thomas, C.J. Central obesity and the Mediterranean diet: A systematic review of intervention trials. Crit. Rev. Food Sci. Nutr. 2018, 58, 3070–3084. [Google Scholar] [CrossRef] [PubMed]
- Wernicke, C.; Apostolopoulou, K.; Hornemann, S.; Efthymiou, A.; Machann, J.; Schmidt, S.; Primessnig, U.; Bergmann, M.M.; Grune, T.; Gerbracht, C.; et al. Long-term effects of a food pattern on cardiovascular risk factors and age-related changes of muscular and cognitive function. Medicine 2020, 99, e22381. [Google Scholar] [CrossRef] [PubMed]
- Pletsch-Borba, L.; Wernicke, C.; Apostolopoulou, K.; Spira, D.; Pohrt, A.; Hornemann, S.; Gerbracht, C.; Pfeiffer, A.F.; Spranger, J.; Mai, K. Nutritional counseling frequency and baseline food pattern predict implementation of a high-protein and high-polyunsaturated fatty acid dietary pattern: 1-year results of the randomized NutriAct trial. Clin. Nutr. 2021, 40, 5457–5466. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Ernährung (DGE); Österreichische Gesellschaft für Ernährung (ÖGE); Schweizerische Gesellschaft für Ernährung (SGE). Referenzwerte für die Nährstoffzufuhr; DGE Medienservice: Budapest, Hungary, 2015. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Machann, J.; Thamer, C.; Stefan, N.; Schwenzer, N.F.; Kantartzis, K.; Häring, H.U.; Claussen, C.D.; Fritsche, A.; Schick, F. Follow-up whole-body assessment of adipose tissue compartments during a lifestyle intervention in a large cohort at increased risk for type 2 diabetes. Radiology 2010, 257, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Würslin, C.; Machann, J.; Rempp, H.; Claussen, C.; Yang, B.; Schick, F. Topography mapping of whole body adipose tissue using a fully automated and standardized procedure. J. Magn. Reson. Imaging 2010, 31, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.M.; Kenny, D.A. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986, 51, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Tiikkainen, M.; Bergholm, R.; Vehkavaara, S.; Rissanen, A.; Häkkinen, A.M.; Tamminen, M.; Teramo, K.; Yki-Järvinen, H. Effects of Identical Weight Loss on Body Composition and Features of Insulin Resistance in Obese Women with High and Low Liver Fat Content. Diabetes 2003, 52, 701–707. [Google Scholar] [CrossRef]
- Summers, L.K.M.; Fielding, B.A.; Bradshaw, H.A.; Ilic, V.; Beysen, C.; Clark, M.L.; Moore, N.R.; Frayn, K.N. Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia 2002, 45, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.; Skurnik, G.; Naour, N.; Pechtner, V.; Meugnier, E.; Rome, S.; Quignard-Boulangé, A.; Vidal, H.; Slama, G.; Clément, K.; et al. Treatment for 2 mo with n−3 polyunsaturated fatty acids reduces adiposity and some atherogenic factors but does not improve insulin sensitivity in women with type 2 diabetes: A randomized controlled study. Am. J. Clin. Nutr. 2007, 86, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.M.; Cohn, J.S.; O’connor, H.T.; Garg, M.L.; Caterson, I.D.; George, J.; Johnson, N.A. Effect of fish oil supplementation on hepatic and visceral fat in overweight men: A randomized controlled trial. Nutrients 2019, 11, 475. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Huang, C.J.; Xie, D. Anti-obesity effects of conjugated linoleic acid, docosahexaenoic acid, and eicosapentaenoic acid. Mol. Nutr. Food Res. 2008, 52, 631–645. [Google Scholar] [CrossRef] [PubMed]
- McMorrow, A.M.; Connaughton, R.M.; Lithander, F.E.; Roche, H.M. Adipose tissue dysregulation and metabolic consequences in childhood and adolescent obesity: Potential impact of dietary fat quality. Proc. Nutr. Soc. 2015, 74, 67–82. [Google Scholar] [CrossRef]
- Weisweiler, P.; Janetschek, P.; Schwandt, P. Influence of Polyunsaturated Fats and Fat Restriction on Serum Lipoproteins in Humans. Metabolism 1985, 34, 83–87. [Google Scholar] [CrossRef]
- Risérus, U.; Willett, W.C.; Hu, F.B. Dietary fats and prevention of type 2 diabetes. Prog. Lipid Res. 2009, 48, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Connor, W.E. Importance of n-3 fatty acids in health and disease. Am. J. Clin. Nutr. 2000, 71 (Suppl. 1), 171S–175S. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. The Effect of Diet on Cardiovascular Disease and Lipid and Lipoprotein Levels. In Endotext [Internet]; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Campos, R.M.d.S.; Masquio, D.C.L.; Corgosinho, F.C.; Caranti, D.A.; Ganen, A.d.P.; Tock, L.; Oyama, L.M.; Dâmaso, A.R. Effects of magnitude of visceral adipose tissue reduction: Impact on insulin resistance, hyperleptinemia and cardiometabolic risk in adolescents with obesity after long-term weight-loss therapy. Diabetes Vasc. Dis. Res. 2019, 16, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.J.; Lagerpusch, M.; Enderle, J.; Schautz, B.; Heller, M.; Bosy-Westphal, A. Beyond the body mass index: Tracking body composition in the pathogenesis of obesity and the metabolic syndrome. Obes. Rev. 2012, 13 (Suppl. 2), 6–13. [Google Scholar] [CrossRef] [PubMed]
- Pourhassan, M.; Glüer, C.C.; Pick, P.; Tigges, W.; Müller, M.J. Impact of weight loss-associated changes in detailed body composition as assessed by whole-body MRI on plasma insulin levels and homeostatis model assessment index. Eur. J. Clin. Nutr. 2017, 71, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Vessby, B.; Uusitupa, M.; Hermansen, K.; Riccardi, G.; Rivellese, A.A.; Tapsell, L.C.; Nälsén, C.; Berglund, L.; Louheranta, A.; Rasmussen, B.M.; et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU study. Diabetologia 2001, 44, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Rai-Ieja, B.S.; Sadikot, S.M.; Phatak, R.B.; Rao, M.B. Significance of the n-6/n-3 Ratio for Insulin Action in Diabetes and Biochemist. Ann. N. Y. Acad. Sci. 1993, 683, 258–271. [Google Scholar] [CrossRef]
- Javier Basterra-Gortari, F.; Ruiz-Canela, M.; Martínez-González, M.A.; Babio, N.; Sorlí, J.V.; Fito, M.; Ros, E.; Gómez-Gracia, E.; Fiol, M.; Lapetra, J.; et al. Effects of a Mediterranean Eating Plan on the Need for Glucose-Lowering Medications in Participants With Type 2 Diabetes: A Subgroup Analysis of the PREDIMED Trial. Diabetes Care 2019, 42, 1390–1397. [Google Scholar] [CrossRef]
- Weickert, M.O.; Roden, M.; Isken, F.; Hoffmann, D.; Nowotny, P.; Osterhoff, M.; Blaut, M.; Alpert, C.; Gögebakan, O.; Bumke-Vogt, C.; et al. Effects of supplemented isoenergetic diets differing in cereal fiber and protein content on insulin sensitivity in overweight humans. Am. J. Clin. Nutr. 2011, 94, 459–471. [Google Scholar] [CrossRef]
- Bello, O.; Ladwa, M.; Hakim, O.; Marathe, C.; Shojaee-Moradie, F.; Charles-Edwards, G.; Peacock, J.L.; Umpleby, A.M.; Amiel, S.A.; Goff, L.M. Differences in the link between insulin sensitivity and ectopic fat in men of Black African and White European ethnicity. Eur. J. Endocrinol. 2020, 182, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.K. Dietary intake—How do we measure what people are really eating? Obes. Res. 2002, 10 (Suppl. 1), 63S–68S. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.L.; Fitzpatrick, J.A.; Malik, S.J.; Taylor-Robinson, S.D.; Bell, J.D. Whole body fat: Content and distribution. Prog. Nucl. Magn. Reson. Spectrosc. 2013, 73, 56–80. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, G.; Bechthold, A.; Boeing, H.; Ellinger, S.; Hauner, H.; Kroke, A.; Leschik-Bonnet, E.; Linseisen, J.; Lorkowski, S.; Schulze, M.; et al. Evidence-Based Guideline of the German Nutrition Society: Fat Intake and Prevention of Selected Nutrition-Related Diseases. Ann. Nutr. Metab. 2015, 67, 141–204. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Control Group | Intervention Group | ||
|---|---|---|---|---|
| Value | N | Value | N | |
| Demographics | ||||
| Females (%) | 63.2 | 98 | 62.8 | 91 |
| Age (years) | 65 ± 7 | 155 | 66 ± 7 | 145 |
| <60 y. (%) | 20 | 31 | 16.6 | 24 |
| ≥60 and <65 y. (%) | 20 | 31 | 17.9 | 26 |
| ≥65 and <70 y. (%) | 34.2 | 53 | 36.6 | 53 |
| ≥70 and <75 y. (%) | 18.1 | 28 | 18.1 | 28 |
| ≥75 y. (%) | 7.7 | 12 | 11.0 | 16 |
| Patients with diabetes mellitus type 2 (%) | 11.0 | 17 | 9.7 | 14 |
| Patients with arterial hypertension (%) | 82.6 | 128 | 82.1 | 119 |
| Patients with hepatic steatosis (%) | 38.1 | 59 a | 35.9 | 52 b |
| Patients with metabolic syndrome (%) | 40.6 | 63 c | 44.1 | 64 d |
| Anthropometrics | ||||
| BMI (kg/m2) | 29.2 ± 4.7 | 155 | 29.1 ± 4.5 | 145 |
| Waist circumference (cm) | 98.7 ± 11.5 | 155 | 98.5 ± 11.2 | 145 |
| TAT (L) | 18.2 ± 6.2 | 155 | 18.1 ± 6.3 | 145 |
| VAT (L) | 4.7 ± 2.2 | 155 | 4.6 ± 2.3 | 145 |
| NVAT (L) | 13.3 ± 5.1 | 155 | 13.4 ± 5.1 | 145 |
| IHL (%) | 4.1 [1.6; 9.4] | 155 | 3.9 [1.5; 8.0] | 145 |
| Glycemic metabolism | ||||
| HOMA-IR | 1.8 [1.2; 2.9] | 155 | 1.6 [1.1; 2.7] | 145 |
| Lipid profile | ||||
| TC (mmol/L) | 5.4 ± 1.1 | 155 | 5.4 ± 1.1 | 145 |
| LDL-C (mmol/L) | 3.32 ± 1.0 | 155 | 3.4 ± 0.9 | 144 |
| HDL-C (mmol/L) | 1.4 ± 0.3 | 155 | 1.4 ± 0.3 | 145 |
| TG (mmol/L) | 1.2 [1.0; 1.6] | 155 | 1.2 [1.0; 1.6] | 145 |
| Nutritional data | ||||
| Energy intake (kcal·d−1) | 1967 ± 524 | 151 | 1988 ± 539 | 142 |
| Protein intake (g·kg−1·d−1) | 1.0 ± 0.3 | 151 | 1.0 ± 0.3 | 142 |
| Carbohydrates intake (% E·d−1) | 40.0 ± 6.8 | 151 | 40.8 ± 7.2 | 142 |
| Fiber intake (g·d−1) | 22.3 ± 7.9 | 151 | 23.2 ± 7.9 | 142 |
| Saturated fatty acids intake (% E·d−1) | 15.9 ± 3.3 | 151 | 15.7 ± 3.9 | 142 |
| MUFA intake (% E·d−1) | 13.2 ± 2.7 | 151 | 13.0 ± 2.8 | 142 |
| Intervention Group | Control Group | Intervention vs. Control Group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Mean ± SE | p-Value a | N | Mean ± SE | p-Value a | N | Mean Difference [95% CI a] | p-Value b | Partial η2 |
| ∆ Energy intake (kcal·d−1) | −122.4 ± 34.4 | 0.040 | 141 | −226.2 ± 33.5 | <0.001 | 149 | 103.8 [9.4; 198.3] | 0.031 | 0.016 |
| ∆ Protein intake (g·kg−1·d−1) | 0.11 ± 0.02 | <0.001 | 141 | −0.04 ± 0.02 | 0.017 | 149 | 0.16 [0.10; 0.22] c | <0.001 | 0.079 |
| ∆ Protein intake (% E·d−1) | 2.35 ± 0.29 | <0.001 | 141 | 0.88 ± 0.28 | 0.014 | 149 | 1.47 [0.67; 2.27] c | <0.001 | 0.044 |
| ∆ Carbohydrate intake (% E·d−1) | −3.58 ± 0.50 | <0.001 | 141 | −0.00 ± 0.48 | 0.362 | 149 | −3.58 [−4.95; −2.20] d | <0.001 | 0.084 |
| ∆ Fiber intake (g·d−1) | 3.63 ± 0.64 | <0.001 | 141 | −0.91 ± 0.63 | 0.158 | 149 | 4.55 [2.78; 6.32] c,d | <0.001 | 0.082 |
| ∆ Fatty acid intake (% E·d−1) | 1.76 ± 0.52 | 0.004 | 141 | −0.94 ± 0.51 | 0.048 | 149 | 2.70 [1.26; 4.13] | <0.001 | 0.045 |
| ∆ MUFA intake (% E·d−1) | 0.56 ± 0.23 | 0.013 | 141 | −0.67 ± 0.22 | 0.002 | 149 | 1.22 [.59; 1.86] | <0.001 | 0.048 |
| ∆ PUFA intake (% E·d−1) | 4.2 ± 0.28 | <0.001 | 141 | 0.72 ± 0.27 | 0.003 | 149 | 3.52 [2.75; 4.28] c,e | <0.001 | 0.221 |
| ∆ SFA intake (% E·d−1) | −3.28 ± 0.29 | <0.001 | 141 | −0.95 ± 0.28 | 0.001 | 149 | −2.33 [−3.12; −1.54] | <0.001 | 0.105 |
| Intervention Group | Control Group | Intervention vs. Control Group | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Mean ± SE | p-Value a | N | Mean ± SE | p-Value a | N | Mean Difference [95% CI a] | p-Value b | Partial η2 |
| ∆ BMI (kg/m2) | −0.71 ± 0.10 | <0.001 | 145 | −0.50 ± 0.09 | <0.001 | 155 | −0.22 [−0.48; 0.05] | 0.105 | 0.009 |
| ∆ waist circumference (cm) | −2.13 ± 0.63 | <0.001 | 145 | −1.73 ± 0.60 | 0.003 | 155 | −0.40 [−2.11; 1.31] | 0.643 | 0.001 |
| ∆ IHL (%) | −1.94 ± 0.25 | <0.001 | 132 | −1.58 ± 0.24 | <0.001 | 145 | −0.36 [−1.05; 0.33] | 0.302 | 0.004 |
| ∆ HOMA-IR | −0.56 ± 0.08 | <0.001 | 138 | −0.50 ± 0.08 | <0.001 | 149 | −0.06 [−0.27; 0.16] c | 0.597 | 0.001 |
| ∆ TC (mmol/L) | −0.39 ± 0.07 | <0.001 | 144 | −0.11 ± 0.07 | 0.114 | 155 | −0.29 [−0.47; −0.10] | 0.002 | 0.031 |
| ∆ LDL-C (mmol/L) | −0.28 ± 0.06 | <0.001 | 143 | −0.04 ± 0.06 | 0.311 | 155 | −0.24 [−0.40; −0.08] d | 0.004 | 0.028 |
| ∆ HDL-C (mmol/L) | −0.06 ± 0.01 | <0.001 | 144 | −0.02 ± 0.01 | 0.058 | 155 | −0.04 [−0.07; 0.00] d | 0.058 | 0.012 |
| ∆ TG (mmol/L) | −0.11 ± 0.04 | 0.004 | 144 | −0.09 ± 0.04 | 0.001 | 155 | −0.02 [−0.11; 0.08] | 0.753 | <0.001 |
| Metabolic Risk Factors | Pearson Correlation Coefficient | p-Value for Correlation with Delta VAT | N |
|---|---|---|---|
| (a) | |||
| ∆ HOMA-IR | 0.245 | <0.001 | 297 |
| ∆ TC (mmol/L) | 0.162 | 0.005 | 299 |
| ∆ LDL-C (mmol/L) | 0.151 | 0.009 | 298 |
| ∆ HDL-C (mmol/L) | −0.106 | 0.068 | 299 |
| ∆ TG (mmol/L) | 0.207 | <0.001 | 299 |
| (b) | |||
| ∆ HOMA-IR | 0.186 | 0.001 | 292 |
| ∆ TC (mmol/L) | 0.076 | 0.192 | 294 |
| ∆ LDL-C (mmol/L) | 0.088 | 0.135 | 293 |
| ∆ HDL-C (mmol/L) | −0.156 | 0.007 | 294 |
| ∆ TG (mmol/L) | 0.122 | 0.036 | 294 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, N.M.T.; Pohrt, A.; Wernicke, C.; Pletsch-Borba, L.; Apostolopoulou, K.; Haberbosch, L.; Machann, J.; Pfeiffer, A.F.H.; Spranger, J.; Mai, K. Improvement in Visceral Adipose Tissue and LDL Cholesterol by High PUFA Intake: 1-Year Results of the NutriAct Trial. Nutrients 2024, 16, 1057. https://doi.org/10.3390/nu16071057
Meyer NMT, Pohrt A, Wernicke C, Pletsch-Borba L, Apostolopoulou K, Haberbosch L, Machann J, Pfeiffer AFH, Spranger J, Mai K. Improvement in Visceral Adipose Tissue and LDL Cholesterol by High PUFA Intake: 1-Year Results of the NutriAct Trial. Nutrients. 2024; 16(7):1057. https://doi.org/10.3390/nu16071057
Chicago/Turabian StyleMeyer, Nina Marie Tosca, Anne Pohrt, Charlotte Wernicke, Laura Pletsch-Borba, Konstantina Apostolopoulou, Linus Haberbosch, Jürgen Machann, Andreas F. H. Pfeiffer, Joachim Spranger, and Knut Mai. 2024. "Improvement in Visceral Adipose Tissue and LDL Cholesterol by High PUFA Intake: 1-Year Results of the NutriAct Trial" Nutrients 16, no. 7: 1057. https://doi.org/10.3390/nu16071057
APA StyleMeyer, N. M. T., Pohrt, A., Wernicke, C., Pletsch-Borba, L., Apostolopoulou, K., Haberbosch, L., Machann, J., Pfeiffer, A. F. H., Spranger, J., & Mai, K. (2024). Improvement in Visceral Adipose Tissue and LDL Cholesterol by High PUFA Intake: 1-Year Results of the NutriAct Trial. Nutrients, 16(7), 1057. https://doi.org/10.3390/nu16071057






