Improvement of Lung Function by Micronutrient Supplementation in Patients with COPD: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Methods
2.2. Inclusion Criteria and Exclusion Criteria
2.3. Study Selection and Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
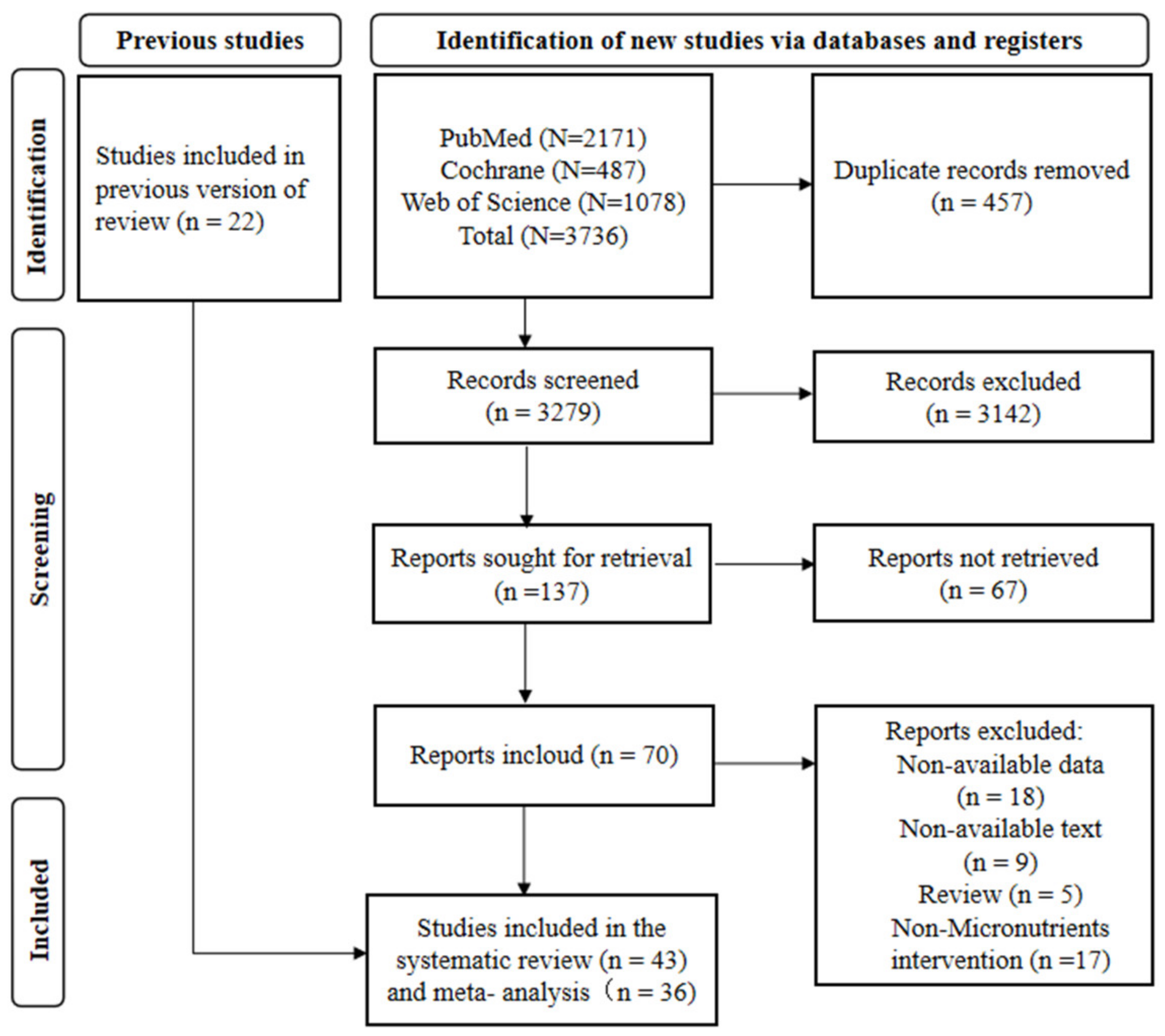
3.1. Study Screening and Results
3.2. Characteristics of Studies Included
3.3. Quality Assessment
3.4. Systematic Review and Meta-Analysis Results
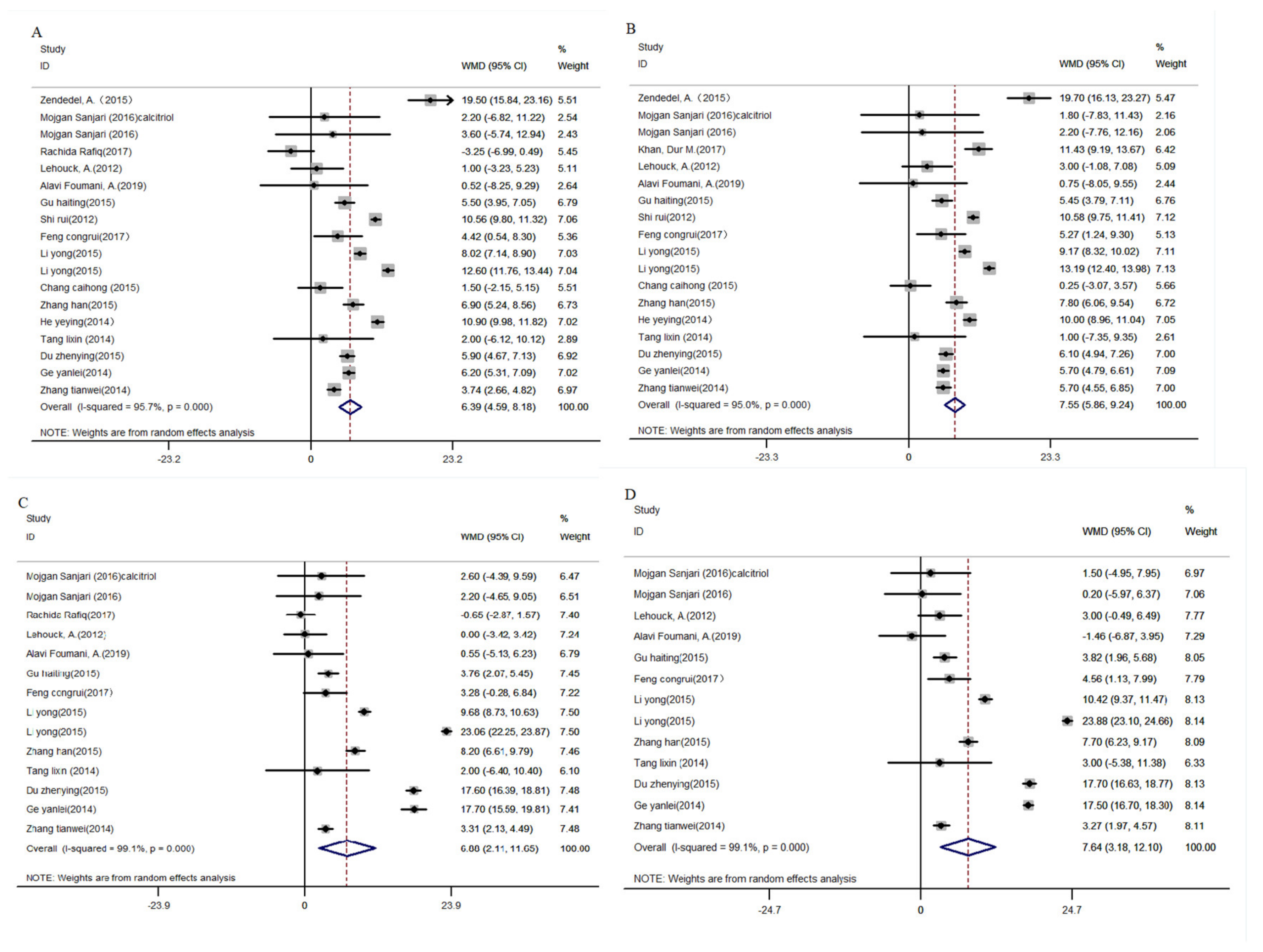
3.4.1. Vitamin D
FEV1 and FEV1/FVC%
Other Indicators Related to Lung Function and Disease Severity of COPD
T Cells Level
3.4.2. Vitamin C
3.4.3. Vitamin E
3.4.4. Magnesium
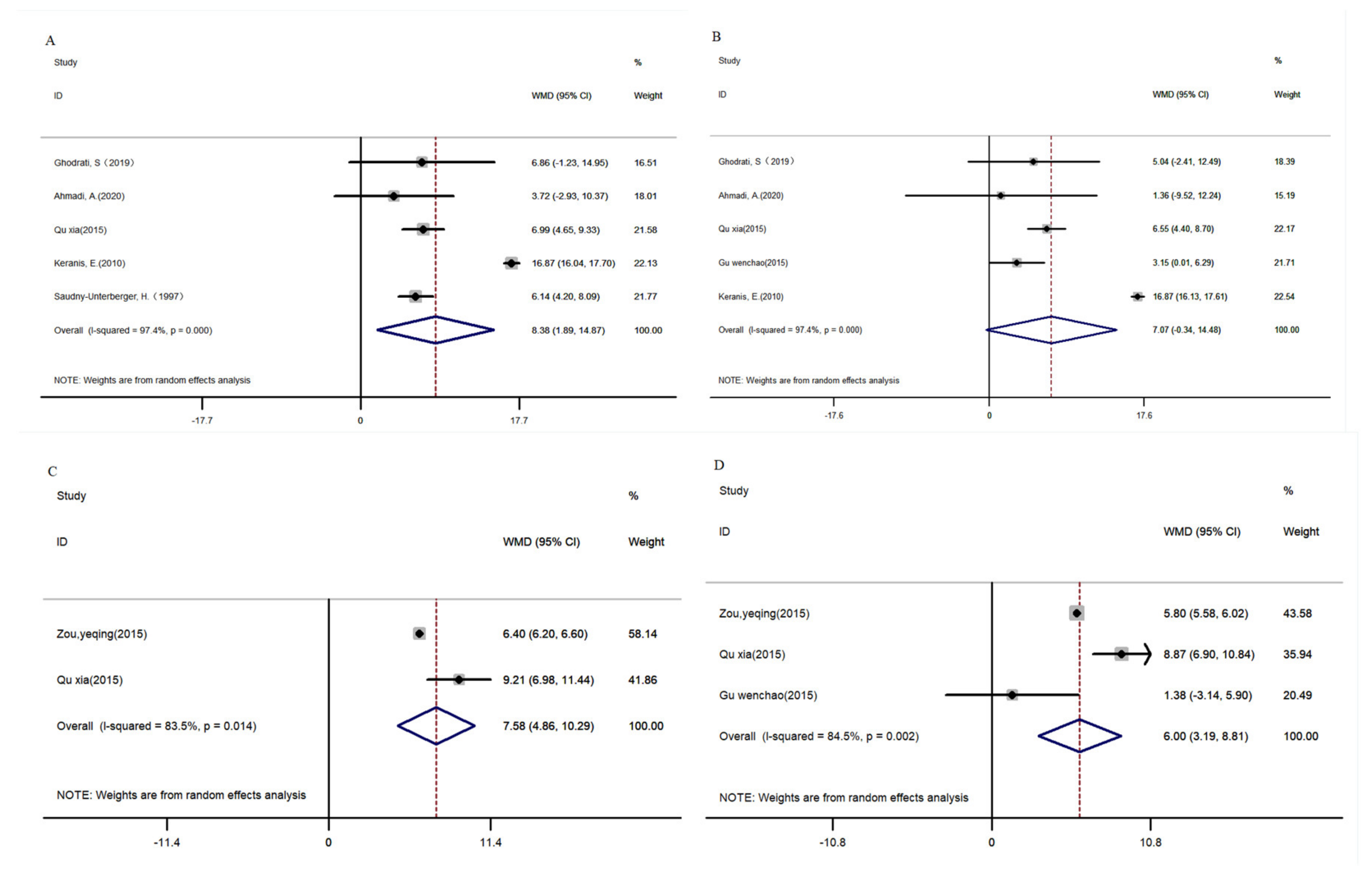
3.4.5. Compound Nutrients
FEV1 and FEV1/FVC%
Other Indicators Related to Lung Function and Disease Severity of COPD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Carson-Chahhoud, K.; Noori, M.; Nejadghaderi, S.A.; Sullman, M.J.M.; Ahmadian Heris, J.; Ansarin, K.; Mansournia, M.A.; Collins, G.S.; Kolahi, A.A.; et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study 2019. BMJ Clin. Res. Ed. 2022, 378, e069679. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P. GOLD COPD report: 2023 update. Lancet Respir. Med. 2023, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Vogelmeier, C.F.; Román-Rodríguez, M.; Singh, D.; Han, M.K.; Rodríguez-Roisin, R.; Ferguson, G.T. Goals of COPD treatment: Focus on symptoms and exacerbations. Respir. Med. 2020, 166, 105938. [Google Scholar] [CrossRef] [PubMed]
- Celli, B.R.; Cote, C.G.; Marin, J.M.; Casanova, C.; Montes de Oca, M.; Mendez, R.A.; Pinto Plata, V.; Cabral, H.J. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- den Otter, I.; Willems, L.N.; van Schadewijk, A.; van Wijngaarden, S.; Janssen, K.; de Jeu, R.C.; Sont, J.K.; Sterk, P.J.; Hiemstra, P.S. Lung function decline in asthma patients with elevated bronchial CD8, CD4 and CD3 cells. Eur. Respir. J. 2016, 48, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Nakagiri, T.; Warnecke, G.; Avsar, M.; Thissen, S.; Kruse, B.; Kühn, C.; Ziehme, P.; Knöfel, A.K.; Madrahimov, N.; Okumura, M.; et al. Lung function early after lung transplantation is correlated with the frequency of regulatory T cells. Surg. Today 2012, 42, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jin, F.; Wu, F. Clinical significance of changes in serum inflammatory factors in patients with chronic obstructive pulmonary disease and pulmonary infection. J. Int. Med. Res. 2021, 49, 3000605211013275. [Google Scholar] [CrossRef] [PubMed]
- Labaki, W.W.; Rosenberg, S.R. Chronic Obstructive Pulmonary Disease. Ann. Intern. Med. 2020, 173, Itc17–Itc32. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E.; Massaro, M.; Garbarino, S.; Toraldo, D.M. Role of Diet in Chronic Obstructive Pulmonary Disease Prevention and Treatment. Nutrients 2019, 11, 1357. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Tsuji, T.; Nemoto, K.; Nakamura, H.; Aoshiba, K. Undernutrition in patients with COPD and its treatment. Nutrients 2013, 5, 1316–1335. [Google Scholar] [CrossRef] [PubMed]
- Laudisio, A.; Costanzo, L.; Di Gioia, C.; Delussu, A.S.; Traballesi, M.; Gemma, A.; Antonelli Incalzi, R. Dietary intake of elderly outpatients with chronic obstructive pulmonary disease. Arch. Gerontol. Geriatr. 2016, 64, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.; Li, S.; Hu, W.; Li, D.; Leng, S. Potential Micronutrients and Phytochemicals against the Pathogenesis of Chronic Obstructive Pulmonary Disease and Lung Cancer. Nutrients 2018, 10, 813. [Google Scholar] [CrossRef] [PubMed]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef]
- Lei, T.; Lu, T.; Yu, H.; Su, X.; Zhang, C.; Zhu, L.; Yang, K.; Liu, J. Efficacy of Vitamin C Supplementation on Chronic Obstructive Pulmonary Disease (COPD): A Systematic Review and Meta-Analysis. Int. J. Chronic Obstr. Pulm. Dis. 2022, 17, 2201–2216. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, J.; Yu, M.; Sun, J. The efficacy of vitamin D therapy for patients with COPD: A meta-analysis of randomized controlled trials. Ann. Palliat. Med. 2020, 9, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zendedel, A.; Gholami, M.; Anbari, K.; Ghanadi, K.; Bachari, E.C.; Azargon, A. Effects of Vitamin D Intake on FEV1 and COPD Exacerbation: A Randomized Clinical Trial Study. Glob. J. Health Sci. 2015, 7, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Sanjari, M.; Soltani, A.; Habibi Khorasani, A.; Zareinejad, M. The effect of vitamin D on COPD exacerbation: A double blind randomized placebo-controlled parallel clinical trial. J. Diabetes Metab. Disord. 2016, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, R.; Prins, H.J.; Boersma, W.G.; Daniels, J.M.; den Heijer, M.; Lips, P.; de Jongh, R.T. Effects of daily vitamin D supplementation on respiratory muscle strength and physical performance in vitamin D-deficient COPD patients: A pilot trial. Int. J. Chronic Obstr. Pulm. Dis. 2017, 12, 2583–2592. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, R.; Aleva, F.E.; Schrumpf, J.A.; Daniels, J.M.; Bet, P.M.; Boersma, W.G.; Bresser, P.; Spanbroek, M.; Lips, P.; van den Broek, T.J.; et al. Vitamin D supplementation in chronic obstructive pulmonary disease patients with low serum vitamin D: A randomized controlled trial. Am. J. Clin. Nutr. 2022, 116, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; James, W.Y.; Hooper, R.L.; Barnes, N.C.; Jolliffe, D.A.; Greiller, C.L.; Islam, K.; McLaughlin, D.; Bhowmik, A.; Timms, P.M.; et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): A multicentre, double-blind, randomised controlled trial. The Lancet. Respir. Med. 2015, 3, 120–130. [Google Scholar] [CrossRef]
- Lehouck, A.; Mathieu, C.; Carremans, C.; Baeke, F.; Verhaegen, J.; Van Eldere, J.; Decallonne, B.; Bouillon, R.; Decramer, M.; Janssens, W. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: A randomized trial. Ann. Intern. Med. 2012, 156, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.M.; Ullah, A.; Randhawa, F.A.; Iqtadar, S.; Butt, N.F.; Waheed, K. Role of Vitamin D in reducing number of acute exacerbations in Chronic Obstructive Pulmonary Disease (COPD) patients. Pak. J. Med. Sci. 2017, 33, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Hornikx, M.; Van Remoortel, H.; Lehouck, A.; Mathieu, C.; Maes, K.; Gayan-Ramirez, G.; Decramer, M.; Troosters, T.; Janssens, W. Vitamin D supplementation during rehabilitation in COPD: A secondary analysis of a randomized trial. Respir. Res. 2012, 13, 84. [Google Scholar] [CrossRef] [PubMed]
- Bjerk, S.M.; Edgington, B.D.; Rector, T.S.; Kunisaki, K.M. Supplemental vitamin D and physical performance in COPD: A pilot randomized trial. Int. J. Chronic Obstr. Pulm. Dis. 2013, 8, 97–104. [Google Scholar] [CrossRef]
- Alavi Foumani, A.; Mehrdad, M.; Jafarinezhad, A.; Nokani, K.; Jafari, A. Impact of vitamin D on spirometry findings and quality of life in patients with chronic obstructive pulmonary disease: A randomized, double-blinded, placebo-controlled clinical trial. Int. J. Chronic Obstr. Pulm. Dis. 2019, 14, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.X.; Ye, Z.T.; Fu, W.Q. Effect of vitamin D on immunomodulatory function and quality of life in patients with copd. J. Clin. Pulmonol. 2016, 6, 1062–1066. (In Chinese) [Google Scholar]
- Gu, H.T.; Shao, H.Y.; Jing, X.H.; Mao, W.W. Clinical study of alfacalcidol soft capsule on immune function in patients with chronic obstructive pulmonary disease. Chin. J. Clin. Pharmacol. 2015, 31, 1373–1375. (In Chinese) [Google Scholar] [CrossRef]
- Shi, R.; Huang, H.; Fang, Z.Y.; Wang, J.; Gao, B. Study on impact of vitamin D supplementation on serum 25(OH) D and FEV1 in patients with chronic obstructive pulmonary disease. J. Clin. Exp. Med. 2012, 11, 1849–1850+1852. (In Chinese) [Google Scholar]
- Li, Y.; Chen, Z.L. Effects of vitamin D on acute exacerbation and mortality in patients with COPD. Mod. Pract. Med. 2016, 28, 314–315. (In Chinese) [Google Scholar] [CrossRef]
- Feng, C.R.; He, L.M.; Xu, G.; Li, B.K. Effect of vitamin D supplementation on COPD in elderly patients and its effect on serum IL-33 expression in patients. J. Pract. Med. 2017, 34, 609–612. (In Chinese) [Google Scholar] [CrossRef]
- Chang, C.H. Effects of vitamin D on chronic obstructive pulmonary disease. Chin. Community Physician 2015, 31, 15–17. (In Chinese) [Google Scholar]
- Zhang, H.; Gong, J.H.; Zhang, J.H.; Ma, J.P. The value of vitamin D in the treatment of stable patients with chronic obstructive pulmonary disease. Lab. Med. Clin. 2015, 12, 1304–1305. (In Chinese) [Google Scholar]
- Wang, Y.H. Effects of vitamin D on T lymphocyte subsets and lung function in patients with stable COPD. Zhejiang Med. Educ. 2017, 16, 52–54. (In Chinese) [Google Scholar]
- Wu, Y.P.; Hu, Q.M.; Liu, W.; Zhou, Z.H. Effects of vitamin D adjuvant therapy on quality of life in patients with chronic obstructive pulmonary disease. Clin. Meta-Anal. 2013, 28, 569–570. (In Chinese) [Google Scholar]
- Ma, Y.B. The adjunctive role of vitamin D in the treatment of COPD. Health All 2014, 8, 199. (In Chinese) [Google Scholar]
- He, Y.Y.; Geng, Y.D. The application value of vitamin D in the adjuvant therapy of chronic obstructive pulmonary disease. J. Pract. Cardiovasc. Cerebrovasc. Dis. 2014, 22, 24–26. (In Chinese) [Google Scholar]
- Tang, L.X.; Zhang, Y.; Yuan, Q.Y. The application of vitamin D in the treatment of patients with chronic obstructive pulmonary disease. Int. J. Lab. Med. 2014, 35, 2317–2318. (In Chinese) [Google Scholar]
- Du, Z.Y. Efficacy of vitamin D in the treatment of patients with acute exacerbation of chronic obstructive pulmonary disease complicated by hypocalcemia. Mod. Diagn. Treat. 2015, 26, 1763–1764. (In Chinese) [Google Scholar]
- Ge, Y.L.; Li, J.; Wang, H.Y.; Ge, X.L. Effect of vitamin D on hypocalcemia in patients with acute exacerbation of chronic obstructive pulmonary disease. Chin. J. Gerontol. 2014, 34, 2250–2251. (In Chinese) [Google Scholar]
- Zhang, T.W.; Fu, H.W.; Mao, L.Q.; Huang, M. Effects of vitamin D supplementation on bone mineral density and inflammatory cytokines in COPD patients. Clin. Medicat. J. 2014, 12, 37–40. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, W. The adjunctive role of vitamin D in the treatment of COPD. Health Today 2015, 14, 121. (In Chinese) [Google Scholar]
- Molmen, K.S.; Hammarstrom, D.; Pedersen, K.; Lian Lie, A.C.; Steile, R.B.; Nygaard, H.; Khan, Y.; Hamarsland, H.; Koll, L.; Hanestadhaugen, M.; et al. Vitamin D(3) supplementation does not enhance the effects of resistance training in older adults. J. Cachexia Sarcopenia Muscle 2021, 12, 599–628. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.C.; Huang, Y.C.; Hsu, S.Y.; Wang, Y.C.; Yeh, S.L. Vitamin E and Vitamin C Supplementation in Patients with Chronic Obstructive Pulmonary Disease. Int. J. Vitam. Nutr. Res. 2007, 77, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Ansari, S.; Memon, Z. Does antioxidant ascorbic acid supplementation delay lung function deterioration in stable patients with chronic obstructive? Pulmonary disease. Rawal Med. J. 2010, 35, 133–136. [Google Scholar]
- Chen, M.; Jin, Z.X.; Bi, H.; Du, J.Y. Research of vitamin C in the treatment of patients with AECOPD. J. Clin. Pulm. Med. 2016, 2016, 456–459. (In Chinese) [Google Scholar]
- Nadeem, A.; Raj, H.G.; Chhabra, S.K. Effect of vitamin E supplementation with standard treatment on oxidant-antioxidant status in chronic obstructive pulmonary disease. Indian J. Med. Res. 2008, 128, 705–711. [Google Scholar] [PubMed]
- Zanforlini, B.M.; Ceolin, C.; Trevisan, C.; Alessi, A.; Seccia, D.M.; Noale, M.; Maggi, S.; Guarnieri, G.; Vianello, A.; Sergi, G. Clinical trial on the effects of oral magnesium supplementation in stable-phase COPD patients. Aging Clin. Exp. Res. 2022, 34, 167–174. [Google Scholar] [CrossRef] [PubMed]
- van de Bool, C.; Rutten, E.P.A.; van Helvoort, A.; Franssen, F.M.E.; Wouters, E.F.M.; Schols, A. A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD. J. Cachexia Sarcopenia Muscle 2017, 8, 748–758. [Google Scholar] [CrossRef] [PubMed]
- van Beers, M.; Rutten-van Mölken, M.P.M.H.; van de Bool, C.; Boland, M.; Kremers, S.P.J.; Franssen, F.M.E.; van Helvoort, A.; Gosker, H.R.; Wouters, E.F.; Schols, A.M.W.J. Clinical outcome and cost-effectiveness of a 1-year nutritional intervention programme in COPD patients with low muscle mass: The randomized controlled NUTRAIN trial. Clin. Nutr. 2020, 39, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Saudny-Unterberger, H.; Martin, J.G.; Gray-Donald, K. Impact of nutritional support on functional status during an acute exacerbation of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1997, 156, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Ghodrati, S.; Ezzatpanah, A.; Asadi-Khiavi, M.; Alian Samakkah, S.; Esmaeilzadeh, A.; Pezeshgi, A. Administration of vitamin D to ameliorate dyspnea of chronic obstructive pulmonary disease patients: A randomized controlled trial. Immunopathol. Persa 2019, 5, e22. [Google Scholar] [CrossRef]
- Ahmadi, A.; Eftekhari, M.H.; Mazloom, Z.; Masoompour, M.; Fararooei, M.; Eskandari, M.H.; Mehrabi, S.; Bedeltavana, A.; Famouri, M.; Zare, M.; et al. Fortified whey beverage for improving muscle mass in chronic obstructive pulmonary disease: A single-blind, randomized clinical trial. Respir. Res. 2020, 21, 216. [Google Scholar] [CrossRef] [PubMed]
- Keranis, E.; Makris, D.; Rodopoulou, P.; Martinou, H.; Papamakarios, G.; Daniil, Z.; Zintzaras, E.; Gourgoulianis, K.I. Impact of dietary shift to higher-antioxidant foods in COPD: A randomised trial. Eur. Respir. J. 2010, 36, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Gouzi, F.; Maury, J.; Heraud, N.; Molinari, N.; Bertet, H.; Ayoub, B.; Blaquiere, M.; Bughin, F.; De Rigal, P.; Poulain, M.; et al. Additional Effects of Nutritional Antioxidant Supplementation on Peripheral Muscle during Pulmonary Rehabilitation in COPD Patients: A Randomized Controlled Trial. Oxid. Med. Cell Longev. 2019, 2019, 5496346. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.Q.; Zou, H.Y.; Jiang, Y.Q.; Lou, F.X.; Zou, T. Influence of antioxidant vitamins combined with moderate protein diet on lung function and quality of life of COPD patients in stable phase. Chin. Nurs. Res. 2015, 29, 1. [Google Scholar]
- Long, Z.Q. Chronic obstructive pulmonary disease patients breathing muscle recovery and vitamin C, E relationship. Natl. Med. Front. China 2013, 8, 25–26. (In Chinese) [Google Scholar]
- Qu, X.; Han, D.S.; Li, Y.P.; He, H.X.; Li, M. The adjuvant therapeutic effect of vitamin A and vitamin D on stable COPD patients. J. Pract. Med. 2015, 32, 995–996. (In Chinese) [Google Scholar] [CrossRef]
- Gu, W.C.; Qi, G.S.; Yuan, Y.P.; Yang, H.; Wu, H.; Tang, Z.J.; Wang, L.X.; Feng, H.L.; Wang, H.Z. Clinical study on the efficacy of vitamin D in delaying the progression of chronic obstructive pulmonary disease. Chin. Med. J. 2015, 17, 1124–1125. (In Chinese) [Google Scholar]
- Valle, M.S.; Russo, C.; Casabona, A.; Crimi, N.; Crimi, C.; Colaianni, V.; Cioni, M.; Malaguarnera, L. Anti-inflammatory role of vitamin D in muscle dysfunctions of patients with COPD: A comprehensive review. Minerva Medica 2022, 114, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Białek-Gosk, K.; Rubinsztajn, R.; Białek, S.; Paplińska-Goryca, M.; Krenke, R.; Chazan, R. Serum Vitamin D Concentration and Markers of Bone Metabolism in Perimenopausal and Postmenopausal Women with Asthma and COPD. Adv. Exp. Med. Biol. 2018, 1070, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Yang, J.; Hu, X.; Li, M.; Wang, Q.; Dancer, R.C.A.; Parekh, D.; Gao-Smith, F.; Thickett, D.R.; Jin, S. Vitamin D attenuates lung injury via stimulating epithelial repair, reducing epithelial cell apoptosis and inhibits TGF-β induced epithelial to mesenchymal transition. Biochem. Pharmacol. 2020, 177, 113955. [Google Scholar] [CrossRef] [PubMed]
- Schrumpf, J.A.; van der Does, A.M.; Hiemstra, P.S. Impact of the Local Inflammatory Environment on Mucosal Vitamin D Metabolism and Signaling in Chronic Inflammatory Lung Diseases. Front. Immunol. 2020, 11, 1433. [Google Scholar] [CrossRef]
- Higgins, M.R.; Izadi, A.; Kaviani, M. Antioxidants and Exercise Performance: With a Focus on Vitamin E and C Supplementation. Int. J. Environ. Res. Public Health 2020, 17, 8452. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants 2022, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, P.; Rahman, I. Oxidative stress in asthma and COPD: Antioxidants as a therapeutic strategy. Pharmacol. Ther. 2006, 111, 476–494. [Google Scholar] [CrossRef]
- Schols, A. Nutrition as a metabolic modulator in COPD. Chest 2013, 144, 1340–1345. [Google Scholar] [CrossRef]
- Park, H.J.; Byun, M.K.; Kim, H.J.; Kim, J.Y.; Kim, Y.I.; Yoo, K.H.; Chun, E.M.; Jung, J.Y.; Lee, S.H.; Ahn, C.M. Dietary vitamin C intake protects against COPD: The Korea National Health and Nutrition Examination Survey in 2012. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 2721–2728. [Google Scholar] [CrossRef] [PubMed]
- Hanson, C.; Lyden, E.; Furtado, J.; Campos, H.; Sparrow, D.; Vokonas, P.; Litonjua, A.A. Serum tocopherol levels and vitamin E intake are associated with lung function in the normative aging study. Clin. Nutr. 2016, 35, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Gozzi-Silva, S.C.; Teixeira, F.M.E.; Duarte, A.; Sato, M.N.; Oliveira, L.M. Immunomodulatory Role of Nutrients: How Can Pulmonary Dysfunctions Improve? Front. Nutr. 2021, 8, 674258. [Google Scholar] [CrossRef] [PubMed]
- Holford, P.; Carr, A.C.; Jovic, T.H.; Ali, S.R.; Whitaker, I.S.; Marik, P.E.; Smith, A.D. Vitamin C-An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients 2020, 12, 3760. [Google Scholar] [CrossRef] [PubMed]
- Talaei, M.; Hughes, D.A.; Mahmoud, O.; Emmett, P.M.; Granell, R.; Guerra, S.; Shaheen, S.O. Dietary intake of vitamin A, lung function and incident asthma in childhood. Eur. Respir. J. 2021, 58, 2004407. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaeizadeh, S.A. Zinc supplementation and COVID-19 mortality: A meta-analysis. Eur. J. Med. Res. 2022, 27, 70. [Google Scholar] [CrossRef] [PubMed]

| Author (Year) | Country | Blinding | Sample Size (I, C) | Sex | Age (y) | Patient Style | Intervention Group | Control Group | Duration | Outcome | Jadad Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dosage | Route | Composition (Dosage) | ||||||||||
| Vitamin D | ||||||||||||
| Zendedel, A. (2015) [18] | Iran | DB | 88 (44, 44) | M (60) F (28) | - | Severe and very severe COPD | Vitamin D (100,000 IU/m) | Oral | Placebo | 6 m | FEV1 (%), number of exacerbations | 4 |
| Mojgan Sanjari (2016) [19] | Iran | DB | 135 (IC: 39, IV: 39, 42) | M F | C: 58.4 ± 9.5 Vitamin D: 55.8 ± 9.5 Calcitriol: 55.6 ± 10.4 | Moderate to severe COPD and exacerbations | Calcitriol (0.25 μg/d) Vitamin D (50,000 IU/d) | Oral | Placebo (Similar to active drug) | 7 d | FEV1% | 6 |
| Rachida Rafiq (2017) [20] | Netherland | - | 50 (24, 26) | M (26) F (24) | I: 61 ± 5.92 C: 64 ± 3.7 | - | Vitamin D (1200 IU/d) | Oral | Placebo | 6 m | FEV1%, FEV1/FVC (%), number of exacerbations, 6MWD, MIP, MEP | 6 |
| Rachida Rafiq (2022) [21] | Netherland | DB | 155 (74, 81) | M F | I: 67 ± 9 C: 65 ± 9 | - | Vitamin D3 (16,800 IU/W) | Oral | Placebo | 1 y | Exacerbation rate in 1 y | 7 |
| Martineau, A.R. (2015) [22] | UK | DB | 240 (122, 118) | M F | I: 64.8 ± 7.9 C: 64.5 ± 9.2 | - | Vigantol oil with vitamin D3 (120,000 IU/2 m) | Oral | Placebo (Miglyol oil 6 mL) | 1 y (six 2-monthly) | Time to first moderate or severe COPD exacerbation | 7 |
| Lehouck, A. (2012) [23] | Belgium | DB | 182 (91, 91) | M (145) F (37) | I: 68 ± 9 C: 68 ± 8 | Moderate to very severe COPD | Vitamin D (100,000 IU/m) | Oral | Placebo (arachidis oleum 4 mL) | 1 y | FEV1%, FEV1/FVC | 6 |
| Khan, Dur M. (2017) [24] | Pakistan | - | 120 (60, 60) | M (78) F (42) | 46.28 ± 8.83 | - | Vitamin D (2000 IU/d) | Oral | - | 6 m | FEV1%, number of exacerbations | 2 |
| Hornikx, M. (2012) [25] | Belgium | DB | 50 (25, 25) | M (38) F (12) | I: 67 ± 8 C: 69 ± 6 | - | Vitamin D (100,000 IU/m) | Oral | Placebo (arachidis oleum 4 mL) | 1 y | MIP, MEP, 6MWD | 5 |
| Bjerk, S. M. (2013) [26] | USA | _ | 36 (18, 18) | M | I: 68 ± 8 C: 67.6 ± 7 | - | Cholecalciferol (2000 IU/d) | Oral | Placebo | 6 w | SGRQ | 3 |
| Alavi Foumani, A.(2019) [27] | Iran | DB | 63 (32, 31) | M (60) F (3) | I: 67.9 ± 7.9 C: 68.4 ± 7.8 | - | Vitamin D3 (50,000 IU/w) | Oral | Placebo | 6 m | FEV1%, FEV1/FVC%, number of exacerbations, CAT score | 6 |
| Tan, zhixiong (2016) [28] | China | - | 106 (53, 53) | M (61) F (55) | I: 53.9 ± 7.8 C: 54.3 ± 8.6 | - | Vitamin D3 (100,000 U/d) | intramuscular injection | Blank | 2 w | CD4+%, CD8+%, CD4+/CD4+%, CAT score | 3 |
| Gu haiting (2015) [29] | China | - | 172 (86, 86) | M (101) F (71) | I: 65.95 ± 7.56 C: 66.1 ± 7.62 | Stable COPD | Alfa-calciferol (0.25 μg/d) | Oral | Blank | 6 m | CD3+%, CD4+%, CD8+%, CD4+/CD8+%, FEV1%, FEV1/FVC% | 4 |
| Shi rui (2012) [30] | China | - | 72 (36, 36) | M | 65.23 ± 11.6 | Stable severe COPD | Alfa-calciferol (0.5 μg/d) | Oral | Blank | 3 m | FEV1% | 3 |
| Li yong (2016) [31] | China | - | 150 (IA:50, IB:50, 50) | M (84) F (66) | IA: 65.72 ± 4.98 IB: 65.66 ± 4.92 C: 65.6 ± 4.91 | Stable COPD | Alfa-calciferol (A: 400 U/d, B:1000 U/d) | Oral | Placebo (starch) | 2 m | FEV1%, FEV1/FVC% | 3 |
| Feng congrui (2017) [32] | China | - | 80 (Stable COPD: 20, 20) (AECOPD: 20, 20) | Stable COPD:M (29) F (11); AECOPD: M (31) F (9) | Stable COPD: I: 74.33 ± 6.43 C: 76.73 ± 5.92 AECOPD: I: 75.20 ± 5.31 C: 75.80 ± 4.86 | Stable COPD, Acute exacerbation | Alfa-calciferol (0. 25 μg/d) | Oral | Blank | 4 w | FEV1%, FEV1/FVC% | 3 |
| Chang caihong (2015) [33] | China | - | 80 (40, 40) | M (57) F (33) | I: 59.3 ± 1.2 C: 56.7 ± 0.8 | - | Vitamin D | Oral | Blank | 30 d | FEV1%, 6MWD | 3 |
| Zhang han (2015) [34] | China | - | 120 (60, 60) | M (78) F (42) | I: 71 ± 10 C: 73 ± 9 | Stable COPD | Alfa-calciferol 0.5 μg/d | Oral | Blank | 6 m | CD3+%, CD4+%, CD8+%, CD4+/CD8+, FEV1%, FEV1/FVC% | 3 |
| Wang yuehua (2017) [35] | China | - | 150 (IA: 50, IB: 50, 50) | M (99) F (51) | IA: 69.95 ± 3.05 IB: 70.12 ± 1.05 C: 67.77 ± 4.34 | Stable COPD | Alfa-calciferol (A:0.25 μg/d B:0.5 μg/d) | Oral | Placebo | 1 y | CD3+%, CD4+%, CD4+/CD8+% | 3 |
| Wu yunping (2015) [36] | China | - | 89 (44, 45) | M (52) F (37) | I: 53.6 ± 7.1 C: 54.1 ± 9.3 | Stable COPD, acute exacerbation | Vitamin D3 (100,000 U/d) | Intramuscular injection | Blank | 2 m | CAT score | 4 |
| Ma yinbo (2014) [37] | China | - | 292 (146, 146) | M (158) F (134) | 48.36 ± 6.0 | - | Vitamin D then Calcitriol (300,000 U/d + 0.25 μg/d) | Intramuscular injection | Blank | 3 m | FEV1%, CAT score | 3 |
| He yeying (2014) [38] | China | - | 120 (62, 58) | M (87) F (33) | 60.5 ± 5. 5 | - | Vitamin D then Calcitriol (300,000 U/d + 0.25 μg/d) | Intramuscular injection | Blank | 3 m | FEV1%, number of exacerbations | 3 |
| Tang lixin (2014) [39] | China | - | 60 (30, 30) | M (38) F (22) | 55–90 | - | Alfa-calciferol (0.5 μg/d) | Oral | Blank | 2 m | FEV1%, FEV1/FVC% | 3 |
| Du zhenying (2015) [40] | China | - | 58 (29, 29) | M (32) F (26) | I: 60.8 ± 11.9 C: 63.1 ± 12.6 | Acute exacerbation episode | Vitamin D (4 g/d) | Oral | Blank | 2 m | FEV1%, FEV1/FVC% | 4 |
| Ge yanlei (2014) [41] | China | - | 130 (68, 62) | - | - | Acute exacerbation episode | Vitamin D (800 U/w) | Oral | Blank | 2 m | FEV1%, FEV1/FVC% | 3 |
| Zhang tianwei (2014) [42] | China | - | 350 (175, 175) | M | I: 66.42 ± 7.20 C: 66.38 ± 7.15 | Stable COPD | Calcitriol (0.25 μg/d) | Oral | Blank | 3 m | FEV1%, FEV1/FVC% | 4 |
| Zhangwei (2015) [43] | China | - | 200 (100, 100) | M F | I: 45.3 ± 3.4 C: 45.2 ± 3.2 | - | Vitamin D (300,000 U/d) + Calcitriol (0.25 μg/d) | Oral | Blank | 3 m | FEV1% | 4 |
| Knut Sindre Mølmen (2021) [44] | Norway | DB | 78 (34, 44) | M F | C: 67 ± 4 I: 69 ± 5 | - | Vitamin D (10,000 IU/day, followed by 2000 IU/day) | Oral | placebo (Cold-pressed olive oil) | 12 M | Muscle strength, muscle mass, and endurance performance | 6 |
| Vitamin C | ||||||||||||
| Wu, T. C. (2007) [45] | China | - | 35 (9, 8) | M F | C: 65.5 (48,75) I: 68 (47, 89) | Stable COPD | Vitamin C (250 mg/d) | Oral | placebo | 12 w | FEV1%, FEV1/FVC% | 2 |
| Munawar A, A. (2010) [46] | Pakistan | SB | 45 (23, 22) | M | C: 55.33 ± 2.19 I: 53.46 ± 1.94 | - | Ascorbic acid (1000 mg/d) | Oral | - | 1.5 y | FEV1/FVC%, | 3 |
| Chen min (2016) [47] | China | - | 60 (30, 30) | M (27) F (33) | I: 71.27 ± 3.32 C: 71.57 ± 2.69 | Acute exacerbation episode | Vitamin C (500 mg/d) | Oral | Blank | 20 d | FEV1% | 2 |
| Vitamin E | ||||||||||||
| Nadeem, A. (2008) [48] | India | SB | 24 (10, 14) | M | C: 54.86 ± 7.13 I: 60.10 ± 1.16 | _ | Vitamin E (800 IU/d) | Oral | Blank | 8 w | FEV1% | 4 |
| Wu, T. C. (2007) [45] | China | - | 35 (I200: 9, I400: 9, 8) | M F | C: 65.5 (48, 75) I400: 71 (49, 84) I200: 72 (51, 86) | Stable COPD | Vitamin E (200 or 400 mg/d) | Oral | Placebo | 12 w | FEV1%, FEV1/FVC% | 2 |
| Magnesium | ||||||||||||
| Zanforlini, B. M. (2022) [49] | Italy | DB | 49 (25, 24) | M (38) F (11) | I: 73.0 ± 8.9 C: 72.2 ± 11.0 | Moderate–severe stable COPD | Magnesium citrate (300 mg/d) | Oral | Placebo (Maltodextrin, riboflavin, orange flavor, citric acid, sucrose, and sodium bicarbonate) | 6 m | FEV1%, FEV1/FVC%, 6MWD, SGRQ | 6 |
| Compound nutrients | ||||||||||||
| Van de Bool, Coby (2017) [50] | Netherland | DB | 81 (39, 42) | M (41) F (40) | 43–80 | - | Oral nutritional supplementation (9.4 g proteins, 28.1 g carbohydrates and 4.1 g fat, was enriched with leucine, n−3 PUFA and vitamin D) 2–3 portions | Oral | Placebo (non-caloric aqueous solution 2–3 portions) | 4 m | 6MWD | 7 |
| Martijn van Beer (2020) [51] | Netherland | DB | 81 (39, 42) | M (41) F (40) | C: 62.8 ± 1.3 I: 62.2 ± 1.3 | - | Oral nutritional supplementation (9.4 g proteins, 28.1 g carbohydrates and 4.1 g fat, was enriched with leucine, n-3 PUFA and vitamin D) (375 mL) | Oral | Placebo (non-caloric aqueous solution) (375 mL) | 4 m | SGRQ | 7 |
| Saudny-Unterberger, H. (1997) [52] | Canada | - | 24 (14, 10) | M (15) F (9) | 40–85 | - | Oral nutritional support | Oral | - | 2 w | FEV1% | 3 |
| Ghodrati, S (2019) [53] | Iran | - | 40 (20, 20) | M F | I: 62.05 ± 13.58 C: 54.25 ±14.34 | Vitamin D deficiency | Calcium-vitamin D (one calcium-vitamin D tablet/d+ vitamin D3 50,000 IU/w) | Oral | Placebo | 3 m | FEV1% | 2 |
| Ahmadi, A. (2020) [54] | Iran | SB | 44 (23, 21) | M | C: 63.47 ± 7.24 I: 62.08 ± 7.0 | - | Whey beverage magnesium and vitamin C (each 250 mL contained 275 mg elemental magnesium, 685 mg vitamin C, and 15.9 g whey protein) | Oral | Blank | 8 w | FEV1%, SGRQ | 5 |
| Keranis, E. (2010) [55] | Greece | - | 120 (60, 60) | M (105) F (15) | 68.1 ± 1.4 | - | Fruit and vegetables | oral | blank | 3 y | FEV1% | 3 |
| Gouzi, F (2019) [56] | France | - | 57 (31, 26) | M (28) F (29) | C: 61.1 ± 8.7 I: 62 4 ± 6.5 | Stable COPD | Antioxidant supplementation (α-tocopherol: 30 mg/day, ascorbate: 180 mg/day, zinc gluconate: 15 mg/day, and Selen methionine: 50 μg/day) | Oral | Placebo | 4 w | 6WMD | 4 |
| Zou,yeqing (2015) [57] | China | - | 117 (58, 59) | - | - | Stable COPD | Vitamin E (200 mg/d) + vitamin C (300–600 mg/d) + protein | Oral | Blank | 6 m | FEV1/FVC%, FEV1%, SGRQ | 3 |
| Long,zhuqing (2013) [58] | China | - | 45 (25, 20) | M (27) F (18) | I: 45.5 ± 13.2 C: 46.6 ± 3.6 | - | Vitamin E(8–10 IU/d) + vitamin C (400–800 IU/d) | Oral | Blank | 30 d | FEV1(L) | 2 |
| Qu xia (2015) [59] | China | - | 74 (37, 37) | M (44) F (30) | 49.8 | - | Vitamin D (0.25 μg/d) + vitamin A (5000 U/d) | Oral | Blank | 3 m | FEV1%, FEV1/FVC%, number of acute exacerbations | 3 |
| Gu wenchao (2015) [60] | China | - | 60 (30, 30) | M (58) F (12) | I: 65.37 ± 6.23 C: 65.13 ± 7.03 | Stable COPD | Puritan’s Pride liquid calcium (1000 U/d) + vitamin D (1200 mg) | Oral | Placebo | 12 m | FEV1%, FEV1/FVC%, 6MWD, SGRQ | 3 |
| Subgroup Analyses | FEV1% | FEV1/FVC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| WMD(de) (95%CI) | I2(%) | WMD(af) (95%CI) | I2(%) | WMD(de) (95%CI) | I2(%) | WMD(af) (95%CI) | I2(%) | ||
| Regional | Chinese | 6.39 (4.59, 8.18) | 95.7 | 7.55 (5.86, 9.24) | 95.0 | 6.88 (2.11, 11.65) | 99.1 | 7.64 (3.18, 12.10) | 99.1 |
| 6.97 (5.12, 8.82) | 96.4 | 7.21 (5.38, 9.05) | 96.1 | 10.05 (4.42, 15.68) | 99.3 | 10.44 (5.29, 15.59) | 99.3 | ||
| Other Asian countries | 6.91 (−4.47, 12.28) | 89.7 | 8.39 (1.71, 15.06) | 87.6 | 1.61 (−2.10, 5.32) | 0.0 | −0.10 (−3.54, 3.34) | 0.0 | |
| European countries | −1.24 (−5.4, 2.91) | 54.0 | 3.00 (−1.08, 7.08) | - | −0.46 (−2.32, 1.40) | 0.0 | 3.00 (−0.49, 6.49) | - | |
| The style of patients | Stable COPD | 3.09 (1.83, 4.35) | 98.0 | 8.70 (6.31, 11,09) | 96.8 | 9.61 (1.30, 17.92) | 99.6 | 9.83 (0.98, 18.69) | 99.6 |
| AECOPD | 1.28 (−0.02, 2.57) | 96.5 | 5.81 (5.10, 6.52) | 0.0 | 11.64 (6.60, 16.67) | 91.6 | 11.82 (8.06, 15,58) | 94.4 | |
| NA | 0.90 (0.12, 1.91) | 96.9 | 7.3 (3.02, 11.58) | 92.7 | −0.26 (−1.99, 1.47) | 0.0 | 1.83 (−0.93, 4.60) | 0.0 | |
| Both | 0.71 (0.07, 1.35) | - | 5.27 (1.24, 9.30) | - | 3.28 (−0.28, 6.84) | - | 4.56 (1.13, 7.99) | - | |
| Duration of intervention | ≤1 month | 2.87 (0.41, 5.33) | 0.0 | 2.35 (−0.46, 5.17) | 15.9 | 2.97 (0.10, 5.85) | 0.0 | 3.17 (0.45, 5.89) | 0.0 |
| 2–3 months | 7.98 (5.74, 10.22) | 97.3 | 8.3 (6.14, 10.46) | 97.0 | 12.56 (5.56, 19.57) | 99.4 | 13.06 (7.07, 19.05) | 99.4 | |
| ≥6 months | 5.35 (0.60, 10.09) | 94.1 | 8.55 (4.55, 12.56) | 92.6 | 2.59 (−1.17,6.35) | 92.1 | 3.90 (0.64, 7.16) | 85.0 | |
| Vitamin D supplement form | Vitamin D | 5.53 (3.05, 8.00) | 94.5 | 7.04 (4.70, 9.37) | 93.9 | 5.96 (−1.37, 13.29) | 98.2 | 7.58 (2.79, 12.37) | 97.3 |
| Calcitriol | 3.72 (2.65, 4.79) | 0.0 | 5.65 (4.50, 6.79) | 0.0 | 3.29 (2.13, 4.45) | 0.0 | 3.20 (1.93, 4.47) | 0.0 | |
| Alfa calciferol | 8.40 (4.99, 11.82) | 95.9 | 9.21 (6.02, 12.38) | 95.5 | 9.62 (1.33, 17.90) | 99.5 | 10.00 (1.28, 18.71) | 99.5 | |
| Vitamin D + Calcitriol | 10.90 (9.98, 11.82) | - | 10.00 (8.96, 11.04) | - | - | - | - | - | |
| Literature quality | High quality | 4.80 (1.91, 7.69) | 91.3 | 6.66 (3.94, 9.38) | 88.7 | 3.79 (−2.10, 9.69) | 98.3 | 4.19 (−2.53, 10.91) | 98.5 |
| Low quality | 7.83 (5.92, 9.73) | 95.3 | 8.17 (6.22, 10.13) | 95.6 | 11.00 (4.06, 17.93) | 99.2 | 11.62 (5.71, 17.53) | 99.2 | |
| Frequency of supplementation | One-time high-dose or spaced supplementation | 7.20 (−0.34, 14.74) | 94.7 | 7.68 (0.14, 15.12) | 95.0 | 6.21 (−7.23, 19.64) | 97.8 | 6.53 (−6.20, 19.25) | 98.1 |
| Continuous supplementation | 6.40 (4.39, 8.42) | 96.0 | 8.04 (6.32, 9.76) | 94.3 | 7.04 (1.60, 12.48) | 99.2 | 7.84 (2.17, 13.57) | 99.3 | |
| NA | 1.50 (−2.15, 5.15) | - | 0.25 (−0.37, 3.57) | - | - | - | - | - | |
| Dose | <10 μg/d | 6.26 (4.33, 8.20) | 93.9 | 7.09 (5.22, 8.96) | 90.1 | 6.79 (3.34, 10.24) | 96.3 | 6.76 (2.04, 11.49) | 98.6 |
| 10 μg–100 μg/d | 7.55 (−1.02, 16.12) | 97.2 | 10.61 (5.99, 15.23) | 97.9 | 7.51 (−10.96, 25.98) | 99.6 | 13.51 (−6.95, 33.97) | 99.2 | |
| >100 μg/d | 6.65 (2.42, 10.88) | 93.4 | 6.6 (3.20, 10.16) | 89.4 | 7.06 (−6.03, 20.13) | 96.0 | 5.68 (−9.02, 20.39) | 97.3 | |
| NA | 1.50 (−2.15, 5.15) | - | 0.25 (−0.37, 3.57) | - | - | - | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhao, L.; Hu, C.; Li, Y.; Yang, Y.; Zhang, X.; Li, Q.; Ma, A.; Cai, J. Improvement of Lung Function by Micronutrient Supplementation in Patients with COPD: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 1028. https://doi.org/10.3390/nu16071028
Li M, Zhao L, Hu C, Li Y, Yang Y, Zhang X, Li Q, Ma A, Cai J. Improvement of Lung Function by Micronutrient Supplementation in Patients with COPD: A Systematic Review and Meta-Analysis. Nutrients. 2024; 16(7):1028. https://doi.org/10.3390/nu16071028
Chicago/Turabian StyleLi, Mingxin, Liangjie Zhao, Chenchen Hu, Yue Li, Yang Yang, Xiaoqi Zhang, Quanguo Li, Aiguo Ma, and Jing Cai. 2024. "Improvement of Lung Function by Micronutrient Supplementation in Patients with COPD: A Systematic Review and Meta-Analysis" Nutrients 16, no. 7: 1028. https://doi.org/10.3390/nu16071028
APA StyleLi, M., Zhao, L., Hu, C., Li, Y., Yang, Y., Zhang, X., Li, Q., Ma, A., & Cai, J. (2024). Improvement of Lung Function by Micronutrient Supplementation in Patients with COPD: A Systematic Review and Meta-Analysis. Nutrients, 16(7), 1028. https://doi.org/10.3390/nu16071028






