A Low FODMAP Diet Supplemented with L-Tryptophan Reduces the Symptoms of Functional Constipation in Elderly Patients
Highlights
- A low-FODMAP diet relieves symptoms of IBS but not in all IBS patients, especially those with the IBS-C type.
- Dietary restrictions can reduce the levels of complete protein, including essential amino acids, such as tryptophan.
- Reduced tryptophan intake may, among other things, result in decreased serotonin secretion, which regulates gastrointestinal motility.
- Optimal tryptophan intake is desirable for various gastrointestinal disorders.
Abstract
1. Introduction
2. Material and Methods
2.1. Participants
2.2. Laboratory Tests
2.3. Nutritional Intervention
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barberio, B.; Judge, C.; Savarino, E.V.; Ford, A.C. Global prevalence of functional constipation according to the Rome criteria: A systemic review and meta-analysis. Lancet 2021, 6, 8. [Google Scholar] [CrossRef]
- Mari, A.; Mahamid, M.; Amara, H.; Baker, F.A.; Yaccob, A. Chronic Constipation in the Elderly Patient: Updates in Evaluation and Management. Korean J. Fam. Med. 2020, 41, 139. [Google Scholar] [CrossRef] [PubMed]
- Palsson, O.S.; Whitehead, W.; Törnblom, H.; Sperber, A.D.; Simren, M. Prevalence of Rome IV Functional Bowel Disorders Among Adults in the United States, Canada, and the United Kingdom. Gastroenterology 2020, 158, 1262–1273.e3. [Google Scholar] [CrossRef] [PubMed]
- Forootan, M.; Bagheri, N.; Darvishi, M. Chronic Constipation: A Review of Literature. Medicine 2018, 97, e10631. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Strisciuglio, C.; Scarpato, E.; Bruzzese, D.; Casertano, M.; Staiano, A. Functional Chronic Constipation: Rome III Criteria Versus Rome IV Criteria. J. Neurogastroenterol. Motil. 2019, 25, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, H.; Liu, J.; Han, L.; Kang, Z.; Liang, L.; Jianag, S.; Meng, N.; Chen, P.; Xu, Q.; et al. Factors Related to Irritable Bowel Syndrome and Differences Among Subtypes: A Cross-Sectional Study in the UK Biobank. Front. Pharmacol. 2022, 13, 905564. [Google Scholar] [CrossRef]
- Di Rosa, C.; Altomare, A.; Terrigno, V.; Carbpne, F.; Tack, J.; Cicala, M.; Guarino, M.P.L. Constipation-Predominant Irritable Bowel Syndrome(IBS-C): Effects of Different Nutritional Patterns on Intestinal Dysbiosis and Symptoms. Nutrients 2023, 15, 1647. [Google Scholar] [CrossRef]
- Ferrucci, L.; Gonzales-Freire, M.; Fabbri, E.; Simonsick, E.; Tanaka, T.; Moore, Z.; Salimi, S.; Sierra, F.; de Cabo, R. Measuring biological anging in Humans: A quest. Anging Cell 2020, 19, e13080. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, D.; Zhao, S.; Li, Z.; Wang, Y.; Qin, X. Deciphering the correlations between anging and constipation by metabolomics and network pharmacology. Anging 2021, 13, 3798–3818. [Google Scholar] [CrossRef]
- Gross, M.; Gros, B.; Mesonero, J.E.; Latorre, E. Naurotransmitter Dysfunction in Irritable Bowel Syndrome: Emerging Approaches for Manegement. J. Clin. Med. 2021, 10, 3429. [Google Scholar] [CrossRef]
- Dunlop, S.P.; Coleman, N.S.; Blackshaw, E.; Perkins, A.C.; Singh, G.; Marsden, C.A.; Spiller, R.C. Abnormalities of 5-Hydroxytryptamine Metabolism in Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2005, 3, 349–357. [Google Scholar] [CrossRef]
- Atkinson, W.; Lockhart, S.; Whorwell, P.J.; Keevil, B.; Houghton, L.A. Altered 5-Hydroxytryptamine Signaling in Patients with Constipation- and Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterology 2006, 130, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: Alteration in 5-HT signaling and metabolism in human diseases. Neurogastroenterol. Motil. 2007, 19 (Suppl. S2), 23–25. [Google Scholar] [CrossRef] [PubMed]
- You, F.Y.; Huang, S.G.; Zhang, H.Y.; Ye, H.; Chi, H.G.; Zou, Y.; Lv, R.X.; Zheng, X.B. Comparison of 5-hydroxytryptamine signaling pathway characteristics in diarrhea-predominant irritable bowel syndrome and ulcerative colitis. World J. Gastroenterol. 2016, 22, 3451–3459. [Google Scholar] [CrossRef] [PubMed]
- Klaessens, S.; Stroobant, V.; De Plaen, E.; Van den Eynde, B.J. Systemic Tryptophan Homeostasis. Front. Mol. Biosci. 2022, 9, 897929. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, Tryptophan Metabolism and the Brain-Gut-Microbiome Axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Mishima, Y.; Ishihara, S. Enteric Microbiota-Mediated Serotonergic Signalling in Pathogenesis of Irritable Bowel Syndrome. Int. J. Mol. Sci. 2021, 22, 10235. [Google Scholar] [CrossRef] [PubMed]
- Koopman, N.; Katsavelis, D.; Ten Hove, A.S.; Brul, S.; de Jonge, W.J.; Seppen, J. The Multifaceted Role of Serotonin in Intestinal Homeostasis. Int. J. Mol. Sci. 2021, 22, 9487. [Google Scholar] [CrossRef]
- Guzel, T.; Mirowska-Guzel, D. The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules 2022, 27, 1680. [Google Scholar] [CrossRef]
- Chojnacki, C.; Błońska, A.; Kaczka, A. Evaluation of serotonin and dopamine secretion and metabolism in patients with irritable bowel syndrome. Pol. Arch. Intern. Med. 2018, 128, 711. [Google Scholar] [CrossRef]
- Chojnacki, C.; Błońska, A.; Konrad, P.; Chojnacki, J.; Popławski, T.; Błasiak, J. The Usefulness of the low FODMAP Diet with Limited Tryptophan Intake in the Treatment of Diarrhoea-Predominant Irritable Bowel Syndrome. Nutrients 2023, 15, 1837. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Del Negro, V.; Angeletti, P.M.; Latella, G. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients 2017, 9, 940. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Tonarelli, S.; Barracca, F.; Morganti, R.; Pancetti, A.; Bertani, L.; de Bartoli, N.; Costa, F.; Mosca, F.; Marchi, M.; et al. A Low-FODMAP Diet for Irritable Bowel Syndrome: Some Answers to the Doubts from a Long-Term Follow –Up. Nutrients 2020, 12, 2360. [Google Scholar] [CrossRef] [PubMed]
- Sing, P.; Tuck, C.; Gibson, P.R.; Chey, W.D. The role of Food in the Treatment of Bowel Disorders: Focus on Irritable Bowel Syndrome and Functional Constipation. Am. J. Gastroenterol. 2022, 117, 947. [Google Scholar] [CrossRef]
- Van Lanen, A.S.; de Bree, A.; Greyling, A. Efficacy of a low-FODMAP diet in adult irritable bowel syndrome: A systemic review and meta-anlysis. Eur. J. Nutr. 2021, 60, 3505. [Google Scholar] [CrossRef]
- Morariu, I.D.; Avasilcani, L.; Vieriu, M.; Lupu, V.V.; Morariu, B.A.; Lupu, A.; Morariu, P.C.; Pop, O.L.; Staecea, L.M.; Trandafir, L. Effects of low-FODMAP Diet on Irritable Bowel Syndrome in Both Children and Adults—Narrative Review. Nutrients 2023, 15, 2295. [Google Scholar] [CrossRef]
- Nikolaki, M.D.; Kast, A.N.; Katsas, K.; Petsis, K.; Lambrinou, S.; Patsalidou, V.; Stamatppoulou, S.; Karlatira, K.; Kapolos, K.; Papadimitriou, K.; et al. The Low-FODMAP Diet, IBS, and BCFAs: Exploring the Positive, and Less Desirable Aspects—A Literature Review. Microorganisms 2023, 11, 2387. [Google Scholar] [CrossRef]
- Wang, J.; Yang, P.; Zhang, L.; Hou, X. A Low-FODMAP Diet Improves the Global Symptoms and Bowel Habits of Adult IBS Patients: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 683191. [Google Scholar] [CrossRef]
- Xie, C.R.; Tang, B.; Shi, Y.Z.; Peng, W.Y.; Ye, K.; Tao, Q.F.; Yu, S.G.; Zheng, H.; Vhen, M. Low FODMAP Diet and Probiotics in Irritable Bowel Syndrome: A Systematic Review With Network Meta-Analysis. Front. Pharmacol. 2022, 13, 853011. [Google Scholar] [CrossRef]
- Mitelmão, F.C.R.; Bergamaschi, C.D.C.; Gerenutti, M.; Hächel, K.; Silva, M.T.; Balcão, V.M.; Vila, M.M.D.C. The Effect of Probiotics on Functional Constipation in Adults: Double-Blind, Randomized, Placebo-Controlled Study. Medicine 2021, 100, E24938. [Google Scholar] [CrossRef]
- Araújo, M.M.; Botelho, P.B. Probiotics, Prebiotics, and Synbiotics in Chronic Constipation: Outstanding Aspects to Be Considered for the Current Evidence. Front. Nutr. 2022, 9, 935830. [Google Scholar] [CrossRef]
- He, Y.; Zhu, L.; Chen, J.; Tang, X.; Pan, M.; Yuan, W.; Wang, H. Efficacy of Probiotic Compounds in Relieving Constipation and Their Colonization in Gut Microbiota. Molecules 2022, 27, 666. [Google Scholar] [CrossRef]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv. Nutr. 2017, 8, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Martoni, C.J.; Evans, M.; Chow, C.E.T.; Chan, L.S.; Leyer, G. Impact of a Probiotic Product on Bowel Habits and Microbial Profile in Participants with Functional Constipation: A Randomized Controlled Trial. J. Dig. Dis. 2019, 20, 435–446. [Google Scholar] [CrossRef]
- Miller, L.E.; Ouwehand, A.C.; Ibarra, A. Effects of Probiotic-Containing Products on Stool Frequency and Intestinal Transit in Constipated Adults: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Ann. Gastroenterol. 2017, 30, 629–639. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, J.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Meta-Analysis of Randomized Controlled Trials of the Effects of Probiotics on Functional Constipation in Adults. Clin. Nutr. 2020, 39, 2960–2969. [Google Scholar] [CrossRef] [PubMed]
- Chumpitazi, B.P. The Gut Microbiome as a Predictor of lowe FODMAP Diet Efficacy in Functional Bowel Disorders. Curr. Opin. Gastroenterol. 2020, 36, 147. [Google Scholar] [CrossRef]
- Turan, B.; Bengi, G.; Cehreli, R.; Akpinar, H.; Soytürk, M. Clinical effectiveness of adding probiotics to a low FODMAP diet: Randomized double-blind controlled study. World J. Clin. Cases 2021, 9, 7417. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Loughman, A.; Staudacher, H.m. Wffects of a low FODMAP diet on the colonic microbiome in irritable bowel syndrome: A systematic review with meta-analysis. Am. J. Clin. Nutr. 2022, 116, 943. [Google Scholar] [CrossRef]
- Vandeputte, D.; Joossens, M. Effects of Low and High FODMAP Diets on Human Gastrointestinal Microbiota Composition in Adults with Intestinal Diseases: A Systematic Review. Microorganisms 2020, 8, 1638. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front.Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Choi, S.W. Dietary modulation of gut microbiota for the relief of irritable bowel syndrome. Nutr. Res. Pract. 2021, 15, 441. [Google Scholar] [CrossRef] [PubMed]
- Tennoune, N.; Andriamihaja, M.; Blachier, F. Production of Indole-Related Compounds by the Intestinal Microbiota and Consequence for the Host: The Good, The Bad, and the Ugly. Microorganisms 2022, 10, 930. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zhao, W.; DIng, C.; Tian, H.; Xu, L.; Wang, H.; Ni, L.; Jiang, J.; Gong, J.; Zhu, W.; et al. Potential Role of Fecal Microbiota from Patients with Slow Transit Constipation in the Regulation of Gastrointestinal Motility. Sci. Rep. 2017, 7, 441. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Camilleri, M.; Vijayvargiya, P.; Busciglio, I.; Burton, D.; Ryks, M.; Rhoten, D.; Lueke, A.; Saenger, A.; Girtman, A.; et al. Bowel Functions, Fecal Unconjugated Primary and Secondary Bile Acids, and Colonic Transit in Patients with Irritable Bowel Syndrome. Clin. Gastroenterol. Hepatol. 2013, 11, 1270–1275.e1. [Google Scholar] [CrossRef] [PubMed]
- Sahakian, A.B.; Jee, S.R.; Pimentel, M. Methane and the Gastrointestinal Tract. Dig Dis Sci. 2010, 55, 2135–2143. [Google Scholar] [CrossRef]
- Chatterjee, S.; Park, S.; Low, K.; Kong, Y.; Pimentel, M. The Degree of Breath Methane Production in IBS Correlates with the Severity of Constipation. Am. J. Gastroenterol. 2007, 102, 837–841. [Google Scholar] [CrossRef]
- Pimentel, M.; Kong, Y.; Park, S. IBS Subjects with Methane on Lactulose Breath Test Have Lower Postprandial Serotonin Levels than Subjects with Hydrogen. Dig. Dis. Sci. 2004, 49, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.; Shah, A.; Jones, M.P.; Koloski, N.; Talley, N.J.; Morrison, M.; Holtmann, G. Methane Positive Small Intestinal Bacterial Overgrowth in Inflammatory Bowel Disease and Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Gut Microbes 2021, 13, 1933313. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Chen, J.; Chen, X.; Chia, N.; O’Connor, H.M.; Wolf, P.G.; Gaskins, H.R.; Bharucha, A.E. Relationship Between Microbiota of the Colonic Mucosa vs Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients With Chronic Constipation. Gastroenterology 2016, 150, 367–379.e1. [Google Scholar] [CrossRef]
- Attaluri, A.; Jackson, M.; Valestin, J.; Rao, S.S.C. Methanogenic Flora Is Associated with Altered Colonic Transit but Not Stool Characteristics in Constipation without IBS. Am. J. Gastroenterol. 2010, 105, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-Gut Microbiota Metabolic Interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.K.; Yao, S.K. Roles of Gut Microbiota and Metabolites in Pathogenesis of Functional Constipation. Evid. Based Complement. Alternat. Med. 2021, 2021, 5560310. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, W.; Alkhouri, R.; Baker, R.D.; Bard, J.E.; Quigley, E.M.; Baker, S.S. Structural Changes in the Gut Microbiome of Constipated Patients. Physiol. Genomics. 2014, 46, 679–686. [Google Scholar] [CrossRef]
- Jouët, P.; Moussata, D.; Duboc, H.; Boschetti, G.; Attar, A.; Gorbatchef, C.; Sabaté, J.M.; Coffin, B.; Flourié, B. Effect of Short-Chain Fatty Acids and Acidification on the Phasic and Tonic Motor Activity of the Human Colon. Neurogastroenterol. Motil. 2013, 25, 943–949. [Google Scholar] [CrossRef]
- Hurst, N.R.; Kendig, D.M.; Murthy, K.S.; Grider, J.R. The Short Chain Fatty Acids, Butyrate and Propionate, Have Differential Effects on the Motility of the Guinea Pig Colon. Neurogastroenterol. Motil. 2014, 26, 1586–1596. [Google Scholar] [CrossRef]
- Wang, L.; Cen, S.; Wang, G.; Lee, Y.; Zhao, J.; Zhang, H.; Chen, W. Acetic Acid and Butyric Acid Released in Large Intestine Play Different Roles in the Alleviation of Constipation. J. Funct. Foods 2020, 69, 103953. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, C.; Gao, J. Extensive Summary of the Important Roles of Indole Propionic Acid, as Gut Microbial Metabolite in Host Health and Disease. Nutrients 2022, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, R.; Ono, S.; Karaki, S.; Kuwahara, A. Neural and Non-Neural Mediation of Propionate-Induced Contractile Responses in the Rat Distal Colon. Neurogastroenterol. Motil. 2005, 17, 585–594. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; An, Y.; Zhou, G.; Liu, Y.; Xu, M.; Dong, W.; Wang, S.; Yan, F.; Jiang, K.; et al. Dysbiosis Contributes to Chronic Constipation Development via Regulation of Serotonin Transporter in the Intestine. Sci. Rep. 2017, 7, 10322. [Google Scholar] [CrossRef]
- Shekhar, C.; Monaghan, P.J.; Morris, J.; Issa, B.; Whorwell, P.J.; Keevil, B.; Houghton, L.A. Rome III Functional Constipation and Irritable Bowel Syndrome with Constipation Are Similar Disorders within a Spectrum of Sensitization, Regulated by Serotonin. Gastroenterology 2013, 145, 749–757. [Google Scholar] [CrossRef]
- Costedio, M.M.; Coates, M.D.; Brooks, E.M.; Glass, L.M.; Ganguly, E.K.; Blaszyk, H.; Ciolino, A.L.; Wood, M.J.; Strader, D.; Hyman, N.H.; et al. Mucosal Serotonin Signaling Is Altered in Chronic Constipation but Not in Opiate-Induced Constipation. Am. J. Gastroenterol. 2010, 105, 1173–1180. [Google Scholar] [CrossRef]
- Zoppi, G.; Cinquetti, M.; Luciano, A.; Benini, A.; Muner, A.; Bertazzoni Minelli, E. The Intestinal Ecosystem in Chronic Functional Constipation. Acta Paediatr. 1998, 87, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Prichard, D.O.; Bharucha, A.E. Recent Advances in Understanding and Managing Chronic Constipation. F1000Research 2018, 7, 1640. [Google Scholar] [CrossRef] [PubMed]
- Ohkusa, T.; Koido, S.; Nishikawa, Y.; Sato, N. Gut Microbiota and Chronic Constipation: A Review and Update. Front. Med. 2019, 16, 19. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, R.; Li, D.; Zhao, L.; Zhu, L. Role of Gut Microbiota in Functional Constipation. Gastroenterol. Rep. 2021, 9, 392–401. [Google Scholar] [CrossRef]
- Biazzo, M.; Deidda, G. Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases. J. Clin. Med. 2022, 11, 4119. [Google Scholar] [CrossRef]
- Young, S.N. The effect of raising and lowering tryptophan levels on human mood and social behaviours. Trans. R. Soc. B 2013, 368, 20110375. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018, 23, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Palego, L.; Betti, L.; Rossi, A.; Giannacini, G. Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in Humans. J. Amino Acids 2016, 2016, 8952520. [Google Scholar] [CrossRef] [PubMed]

| Feature | Group I (n = 40) | Group II (n = 60) | p |
|---|---|---|---|
| Age (years) | 72.1 ± 8.6 | 73.3 ± 10.9 | 0.56 |
| Gender M/F | 9/31 | 15/45 | - |
| BMI (kg/m2) | 23.4 ± 2.1 | 23.9 ± 1.8 | 0.31 |
| GFR (ml/min) | 95.8 ± 4.9 | 92.1 ± 7.7 | 0.008 |
| ALT (U/L) | 18.6 ± 3.9 | 17.8 ± 4.6 | 0.37 |
| AST (U/L) | 16.2 ± 2.3 | 17.2 ± 2.6 | 0.052 |
| CRP (mg/L) | 3.8 ± 1.6 | 4.3 ± 2.8 | 0.31 |
| FC (µg/g) | 32.3 ± 19.8 | 36.9 ± 18.3 | 0.24 |
| Lab Test | Group I (n = 40) | Group II (n = 60) | p-Value |
|---|---|---|---|
| TRP | 13.7 ± 1.91 | 13.9 ± 12.1 | >0.05 |
| 5-HIAA | 3.68 ± 0.71 | 3.34 ± 1.21 | <0.05 |
| KYN | 0.46 ± 0.12 | 0.76 ± 0.13 | <0.001 |
| 3-IS | 35.2 ± 12.2 | 80.1 ± 12.9 | <0.001 |
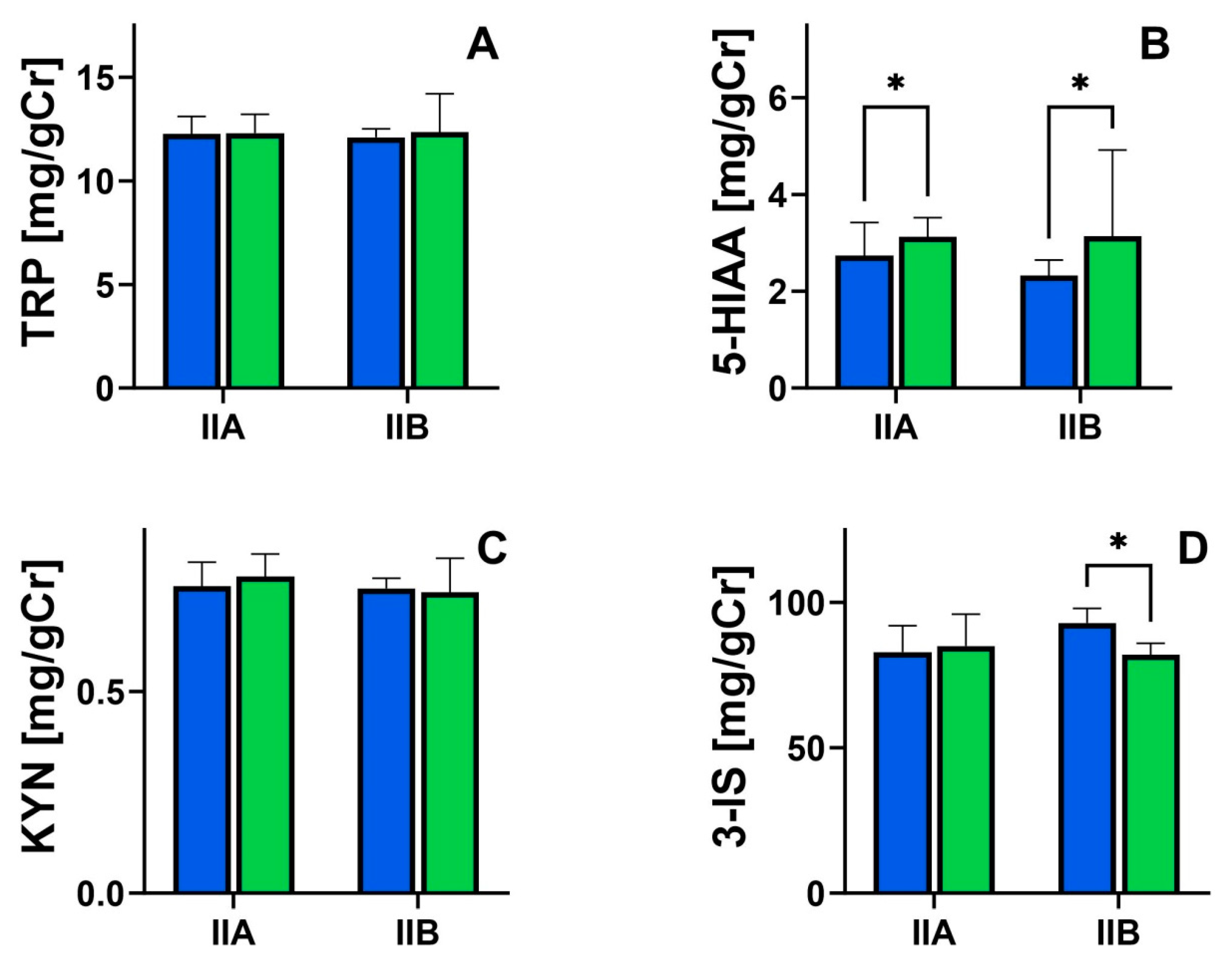
| Feature | Group IIa | Group IIb | p-Value |
|---|---|---|---|
| TRP (mg/gCr) | 11.9 ± 2.93 | 15.1 ± 14.9 | >0.05 |
| 5-HIAA (mg/gCr) | 3.04 ± 0.92 | 3.81 ± 1.38 | <0.05 |
| KYN (mg/gCr) | 0.97 ± 0.19 | 0.95 ± 0.24 | >0.05 |
| 3-IS (mg/gCr | 80.5 ± 13.8 | 74.9 ± 11.4 | >0.05 |
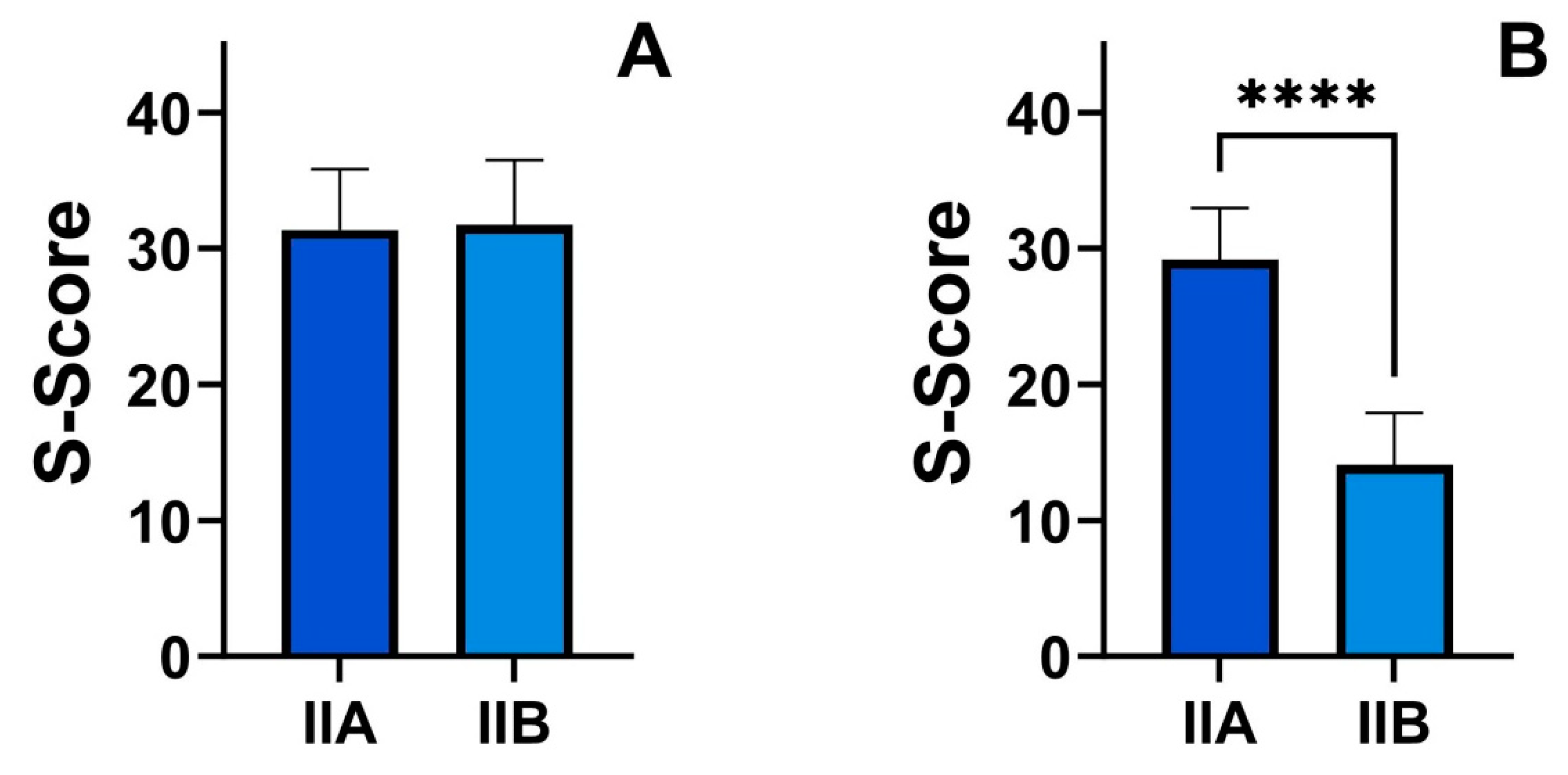
| S-score (points) | 29.2 ± 3.86 | 14.3 ± 3.81 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chojnacki, C.; Mędrek-Socha, M.; Błońska, A.; Błasiak, J.; Popławski, T.; Chojnacki, J.; Gąsiorowska, A. A Low FODMAP Diet Supplemented with L-Tryptophan Reduces the Symptoms of Functional Constipation in Elderly Patients. Nutrients 2024, 16, 1027. https://doi.org/10.3390/nu16071027
Chojnacki C, Mędrek-Socha M, Błońska A, Błasiak J, Popławski T, Chojnacki J, Gąsiorowska A. A Low FODMAP Diet Supplemented with L-Tryptophan Reduces the Symptoms of Functional Constipation in Elderly Patients. Nutrients. 2024; 16(7):1027. https://doi.org/10.3390/nu16071027
Chicago/Turabian StyleChojnacki, Cezary, Marta Mędrek-Socha, Aleksandra Błońska, Janusz Błasiak, Tomasz Popławski, Jan Chojnacki, and Anita Gąsiorowska. 2024. "A Low FODMAP Diet Supplemented with L-Tryptophan Reduces the Symptoms of Functional Constipation in Elderly Patients" Nutrients 16, no. 7: 1027. https://doi.org/10.3390/nu16071027
APA StyleChojnacki, C., Mędrek-Socha, M., Błońska, A., Błasiak, J., Popławski, T., Chojnacki, J., & Gąsiorowska, A. (2024). A Low FODMAP Diet Supplemented with L-Tryptophan Reduces the Symptoms of Functional Constipation in Elderly Patients. Nutrients, 16(7), 1027. https://doi.org/10.3390/nu16071027






